Introduction
Galectins have been characterized based on their
β-galactoside-binding affinity, and their proteins have an
evolutionarily conserved carbohydrate recognition domain (CRD)
(1,2). Galectin family proteins can be
classified by structure into three subtypes: chimeric galectin,
prototype galectin, and tandem-repeat galectin (3,4).
Galectin-9 (Gal-9), as a tandem-repeat galectin, was first
identified in a patient with Hodgkin’s disease (5). The structure of Gal-9 is similar to
that of other members of the galectin family, such as galectin-4
and galectin-8, which contained two homologous CRD domains
separated by a linker peptide (6,7).
There are three isoforms of Gal-9. Based on the size of the linker
peptide connecting two CRDs, they were named long-sized Gal-9
(Gal-9L), medium-sized Gal-9 (Gal-9M), and short-sized Gal-9
(Gal-9S) (8). All these isoforms
are transcriptionally active and protein-coding (9), but little is known about their
differences in cellular functions. Research has revealed that
overexpression of Gal-9L decreased E-selectin levels, while Gal-9M
or Gal-9S increased E-selectin levels in LoVo cells, suggesting
three different isoforms of Gal-9 might exert distinct biological
functions (10).
Increasing amounts of evidence suggest that Gal-9
has a variety of biological functions. Experimental results have
shown that Gal-9 induces apoptosis when added to various types of
cells, such as T-cells and different types of leukemia cells
(11,12). Gal-9-induced apoptosis may be
associated with the Ca2+-calpain-caspase-1 pathway and
Jun NH2-terminal kinase (JNK) and/or p38 MAPK signaling
pathways (13,14). Previous results have shown the
involvement of Gal-9 in tumor cell adhesion, aggregation, and
proliferation. For example, high levels of Gal-9 expression in
melanoma and MCF-7 cells causes formation of colonies and clusters,
but cells that lack Gal-9 expression do not (11,15).
Further studies have confirmed that abnormal expression of Gal-9 is
involved in the regulation of cell adhesion and aggregation. This
view was supported by findings reported by Zhang et al, who
observed that the loss of Gal-9 in hepatocellular carcinoma cells
in vitro increased the activity of adhesion and invasion of
these cells (16).
Marked differences were found between Gal-9
expression in cancer and in normal tissues. Differential Gal-9
protein expression was observed in different tumor tissues. Gal-9
protein was strongly and homogeneously expressed in melanocytic
nevi but downregulated in melanoma cells, especially in metastatic
lesions. High levels of Gal-9 expression are inversely correlated
with the progression of primary melanoma lesions (11). However, the lack of Gal-9
expression was correlated with distant metastasis in a majority of
patients with breast cancer (15).
Similarly, Gal-9 was evidently detected in normal epithelium and
endocervical glands, but those in cervical intraepithelial
neoplasia and cervical squamous cell carcinoma cells were
significantly faint (17). To be
clear, so far, the expression of Gal-9 in tumor tissues has only
been investigated in limited types of tumors, and all experiments
are based on histochemical staining to determine the expression
level of Gal-9 as an indicator. Here, we first measured Gal-9 gene
expression levels in clinically diagnosed primary gastric cancer
tissues by quantitative PCR (qPCR) and the relationship between
mRNA expression levels of Gal-9 and clinicopathological features in
gastric cancer was analyzed. The correlation between Gal-9 and
Tim-3 was also assessed in gastric cell lines.
Materials and methods
Tissue collection
Forty-four patients with primary gastric cancer were
enrolled in this study. All patients underwent radical surgery
between September 2008 and July 2013 at the First Hospital of Jilin
University and did not receive any adjuvant therapy before the
surgical operation. Gastric cancer and corresponding adjacent
(<2 cm away from the tumor area) and normal tissues (>5 cm
away from the tumor area) were collected from patients. The median
age of the patients was 64 years (range 44–84 years). Written
informed consent was obtained from all participants, and the study
was approved by the Institutional Ethics Board of the School of
Medicine, Jilin University. Medical records of the patients,
including age and gender, tumor staging, pathological diagnosis,
and surgical records were reviewed. Tumors were staged according to
the 2010 TNM classification system using the American Joint
Committee on Cancer (AJCC) stage grouping (18).
Antibodies
Anti-Galectin-9 (17938-1-AP) and anti-Tim-3
(11872-1-AP) polyclonal antibodies were purchased from Proteintech
Group (Wuhan, China). Anti-GAPDH was raised against bacterially
expressed proteins (Jilin University, Changchun, China).
Construction of galectin-9 (Gal-9)
expression plasmids
Expression vectors for human Gal-9 were constructed
by inserting cDNA into the XhoI/BamHI sites of
pcDNA3.1(-). The integrity of the DNA sequence was confirmed by DNA
sequencing (GenBank accession no. BAB83624).
Reverse transcription PCR (RT-PCR)
Total RNA from gastric cancer, adjacent, and normal
tissues was isolated using TRIzol® LS reagent
(Invitrogen, CA, USA). Then, 1 μg of total RNA from each sample was
used as a template to produce cDNA with a PrimeScript 1st Strand
cDNA Synthesis kit (Takara, Dalian, China). Human Gal-9, Tim3 and
GAPDH mRNA levels were analyzed using quantitative PCR (qPCR) with
an Eco Real-Time PCR system (Illumina, CA, USA). All PCR reactions
were finished as follows: initial denaturation step at 95°C for 30
sec, followed by 40 cycles of denaturation at 95°C for 5 sec,
annealing at 60°C for 30 sec, and extension at 72°C for 30 sec.
Primer sets used for PCR were as follows: Gal-9,
5′-CTTTCATCACCACCATTCTG-3′ (forward) and
5′-ATGTGGAACCTCTGAGCACTG-3′ (reverse). This pair of primers can
detect three different isoforms of Gal-9 and produce a 91-bp
product; β-actin, 5′-ATGGGTCAGAAGGATTCCTATGT-3′ (forward) and
5′-AGCCACACGCAGCTCATT-3′ (reverse) produce a 153-bp product. Tim-3,
5′-CAGATACTGGCTAAATGGG-3′ (forward) and 5′-CTTGGCTGGTTTGATGAC-3′
(reverse) produce a 160-bp product.
Cell culture and transient
transfection
Human gastric cancer cell lines SGC-7901 and MGC-803
were obtained from Department of Gastrointestinal Surgery, First
Hospital of Jilin University. Human gastric mucosal cell line GES-1
was provided by the Cancer Hospital of Beijing University. Cells
were cultured in Dulbecco’s modified Eagle’s medium (DMEM,
Sigma-Aldrich, St. Louis, MO, USA) with 5% glucose and 10% fetal
bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin
in 10-cm dishes at 37°C in a humidified atmosphere of 5%
CO2. For transient transfection, cells were cultured in
6-well tissue culture plates (~2.5×105 cells/well) in
DMEM medium containing 10% fetal bovine serum. Then cells were
transfected with Gal-9 cDNAs. After 48 h of transfection, cells
were harvested and lysed for western blotting and total RNA
isolation.
Preparation of whole cell extracts and
western blotting (WB)
First, 100 mg of gastric cancer, adjacent or normal
tissue samples were homogenized with liquid nitrogen and
solubilized in 200 μl cold PBS containing 1.0% Nonidet P-40, 0.5%
Na-deoxycholate, 0.1% SDS, 0.05 mM PMSF and protease inhibitor
cocktail. The homogenate was swirled and kept on ice for 30 min.
Whole-cell extracts were sonicated (Scientz-IID, Ningbo, China) for
10 sec with 50% initial cycle and centrifugation at 13,000 × g for
30 min. Equal total amounts of protein from tissue whole-cell
lysates were mixed with 4X SDS-containing sample buffer and boiled
for 5 min at 95°C. Proteins were then separated by 12% SDS-PAGE.
Specific proteins were detected by WB using Galectin-9, Tim-3, and
GAPDH polyclonal antibodies.
Statistical analysis
The western blot images were scanned and quantified
with Quantity One Basic software (Bio-Rad, USA). Differences in
gene and protein expression between tumor and normal tissues or
tumor and adjacent tissues or adjacent and normal tissues were
statistically analyzed using SPSS 17.0 (SPSS, Inc., Chicago IL,
USA). Statistical comparisons were analyzed using the Student’s
t-test. Values of p<0.05 were considered statistically
significant.
Results
Downregulation of Gal-9 gene expression
in gastric cancer
To investigate the involvement of Gal-9 gene
expression in the pathogenesis of primary gastric cancer, 44
clinical gastric cancer tissues and matched adjacent (<2 cm away
from the tumor) and normal (>5 cm away from the tumor) tissues
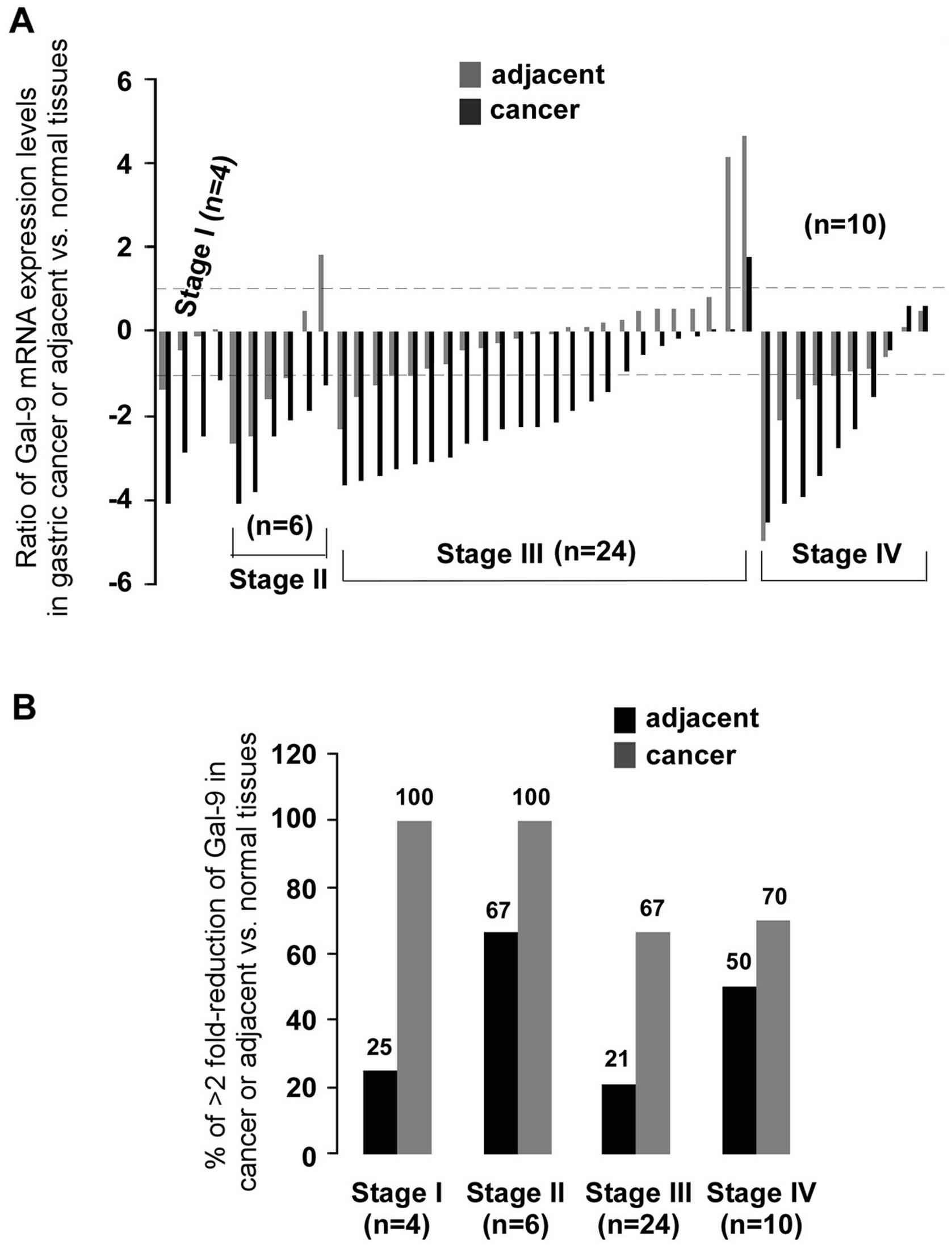
were used (Fig. 1A). Levels of
expression of Gal-9 were measured by qPCR. Compared to matched
normal or adjacent tissues, the gene expression of Gal-9 was
significantly decreased in gastric cancer tissues (p<0.001 both)
(Fig. 1C). Analysis of the mRNA
expression of 44 samples showed significant (>2-fold decreased)
downregulation of Gal-9 mRNA in 77% (34/44) of patients,
whereas 2% (1/44) of patients showed significant (>2-fold
increased) upregulation of Gal-9. Interestingly,
Gal-9 expression in adjacent tissues had also a reduction
(>2-fold decrease) in 34% (15/44) of samples (Fig. 1B). To determine whether the
reduction of Gal-9 mRNA expression resulted in decreased Gal-9
protein levels, aliquots of whole cell extract from eight selected
gastric cancer and corresponding normal or adjacent tissues were
analyzed by western blotting with the indicated antibodies
(Fig. 1D). As expected, there was
significantly less Gal-9 protein in gastric cancer samples than in
matched normal tissues (p<0.05). However, there was no
significant difference between gastric cancer tissues and adjacent
tissues (p>0.05).
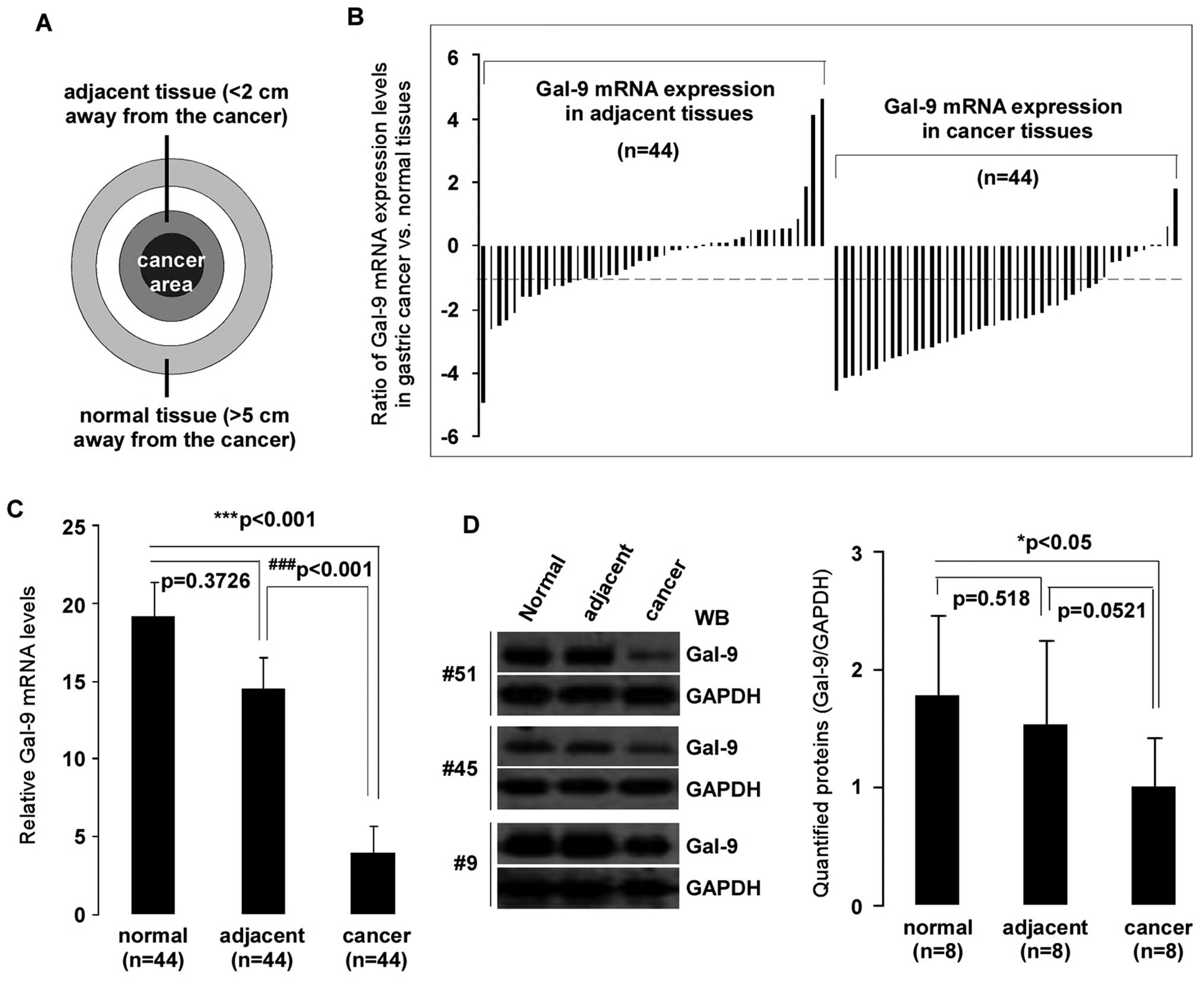 | Figure 1Downregulation of Gal-9 in
gastric cancer tissues. Total RNA was isolated using TRIzol from 44
corresponding gastric cancer, adjacent, and normal tissues. (A)
Tissue samples: cancer area, the site of pathologically diagnosed
gastric cancer; adjacent tissue, <2 cm away from the tumor in
which the cells were pathologically normal; normal tissue, >5 cm
away from the cancer. (B) Patterns of expression of Gal-9
mRNA in gastric cancer tissues. mRNA expression levels of
Gal-9 in clinically diagnosed gastric cancer and matched
adjacent/normal tissues were detected by qPCR. y-axis displays a
ratio of expression of Gal-9 in gastric cancer or adjacent
versus matched normal tissues. Each bar is the log2 value of the
ratio of Gal-9 expression levels between gastric cancer or
adjacent and matched normal tissues from the same patients. Bar
value >1 represents >2-fold increases, and bar value <-1,
represents >2-fold decreases. (C) Relative mRNA expression
levels of Gal-9 in gastric cancer. Each bar represents the
means of 2–3 independent replicates. The significant differences
between cancerous and normal tissues are expressed as
***p<0.001, and significant differences between
cancerous and adjacent tissues are expressed as
###p<0.001. (D) Declined in Gal-9 protein levels were
detected in selected gastric cancer tissues. Whole-cell extract was
prepared from 8 selected tissues, and equivalent total protein of
whole cell extract was subjected to SDS-PAGE in 12% gels. Proteins
were detected with western blotting with anti-Gal-9 and GAPDH
antibodies. The left panel shows representative examples of western
blot images were quantified using Quantity One software (Bio-Rad),
and normalized by GAPDH levels (right panel). The significant
difference is expressed as *p<0.05. |
Gal-9 gene expression and
clinicopathological features of gastric cancer
To expand upon the observations given above and to
determine the relationship between Gal-9 gene expression and
clinicopathological parameters, qPCR results were examined
according to the clinical characteristics of gastric cancer.
Gastric tumors were staged according to the 2010 TNM classification
system using American Joint Committee on Cancer (AJCC) stage
grouping (18). A summary of
clinical characteristics of the patients, including age, gender,
cell differentiation, and survival, is shown in Table I. Less Gal-9 expression was
observed in cancer tissues than in both normal and adjacent tissues
in both the >65 and ≤65 age groups, but the difference was
significantly less pronounced in patients ≤65 years than in
patients >65 years (p<0.05). However, there was no
significant difference by gender, cell differentiation, or survival
time.
 | Table IRelationship between hGal-9 gene
expression (qPCR) and clinicopathological characteristics of
gastric cancer. |
Table I
Relationship between hGal-9 gene
expression (qPCR) and clinicopathological characteristics of
gastric cancer.
| Factor | Case (n) | Normal mean ± SD | Adjacent mean ±
SD | Cancer mean ± SD | p-value nor vs.
adj | p-value nor vs.
can | p-value adj vs.
can |
|---|
| All | 44 | 19.1±2.23 | 14.5±2.07 | 3.99±1.69 | 0.373 | 0.000477c | 0.000753f |
| Age (years) |
| ≤65 | 21 | 23.3±2.23 | 14.4±2.11 | 5.35±1.67 | 0.303 | 0.0315a | 0.00389e |
| >65 | 23 | 15.3±2.22 | 14.6±2.05 | 2.75±1.70 | 0.909 | 0.00211b,g | 0.0189d |
| Gender |
| Male | 30 | 16.5±2.29 | 11.1±2.06 | 3.79±1.79 | 0.0739 | 1.35E-05c | 5.76E-05f |
| Female | 14 | 24.7±2.08 | 21.9±2.14 | 4.43±1.48 | 0.857 | 0.118 | 0.0415d |
|
Differentiation |
| Well | 2 | 10.3±1.95 | 10.6±1.66 | 25.1±13.0 | 0.982 | 0.518 | 0.503 |
| Moderate | 17 | 15.5±1.88 | 12.7±1.87 | 16.1±2.19 | 0.579 | 0.0083b | 0.519 |
| Poorly | 25 | 4.06±1.22 | 269±1.26 | 4.88±1.89 | 0.461 | 0.0199a | 0.0156d |
| Survival of
patients |
| >12 months | 15 | 15.0±2.17 | 13.9±1.99 | 4.03±1.43 | 0.836 | 0.00719b | 0.0195d |
| ≤12 months | 29 | 21.3±2.27 | 14.9±2.13 | 3.98±1.82 | 0.385 | 0.00797b | 0.00726e |
Detailed statistical analyses were performed in
order to further explore the correlation between Gal-9 expression
and clinical features. The correlation between Gal-9 expression and
clinical staging of cancer is shown in Fig. 2. There was significantly less
(>2-fold) Gal-9 mRNA than in normal tissues in 100% (4/4) of
stage I, in 100% (6/6) of stage II, in 67% (16/24) of stage III and
70% (7/10) of stage IV. After statistical analysis for clinical
staging (data not shown), significantly less Gal-9 mRNA than in
normal tissues was observed only in stage III gastric tumors (n=24,
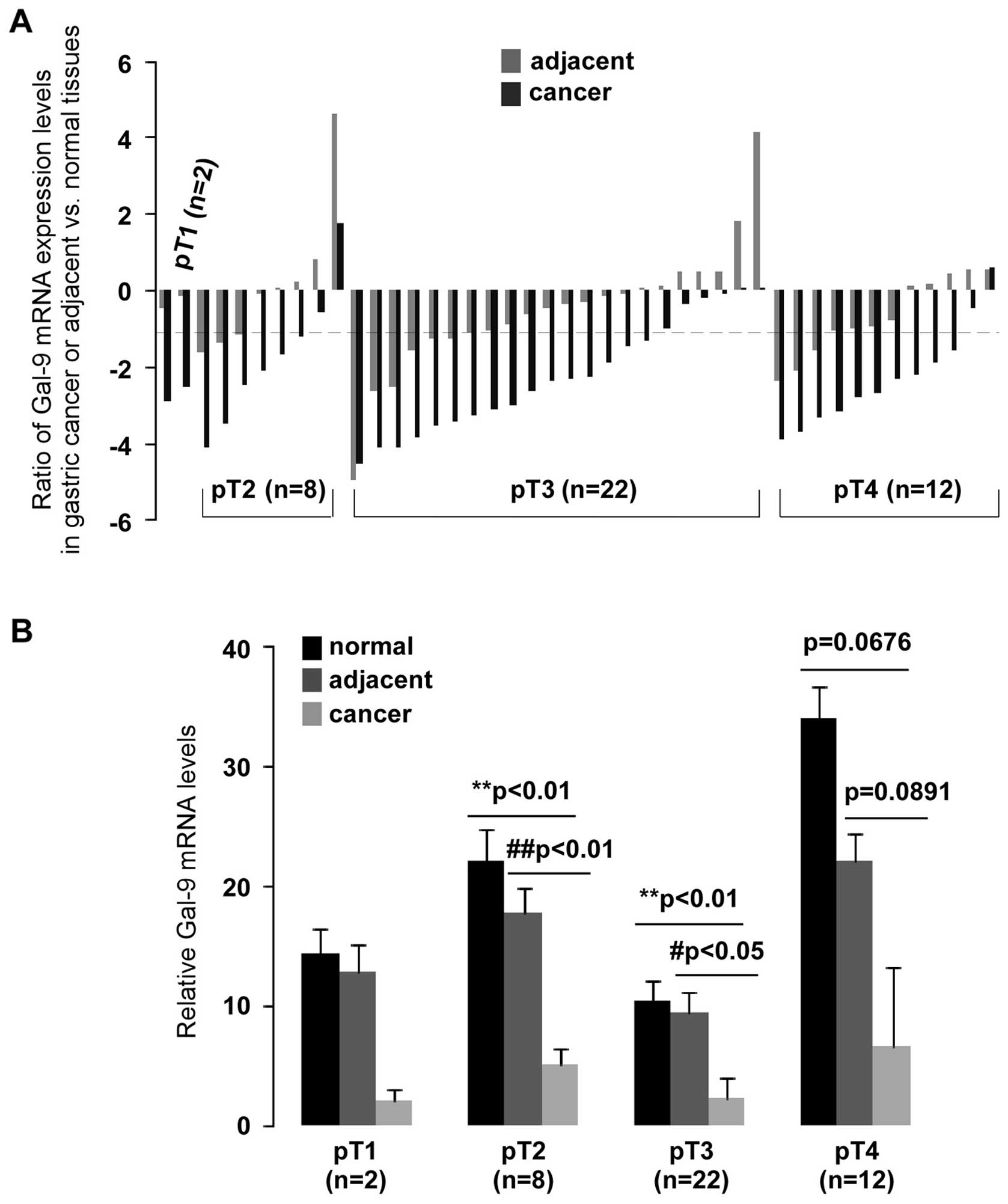
p<0.01). Analysis of the pathological stage showed significantly
low levels of Gal-9 expression in pT2- and pT3-stage gastric
cancer compared to normal (p<0.01) or adjacent (p<0.01 and
p<0.05, respectively) tissues (Fig.
3B). A >2-fold reduction of Gal-9 mRNA was found in 75%
(6/8) of pT2 and 73% (16/22) of pT3 (Fig. 3A). qPCR data were also analyzed
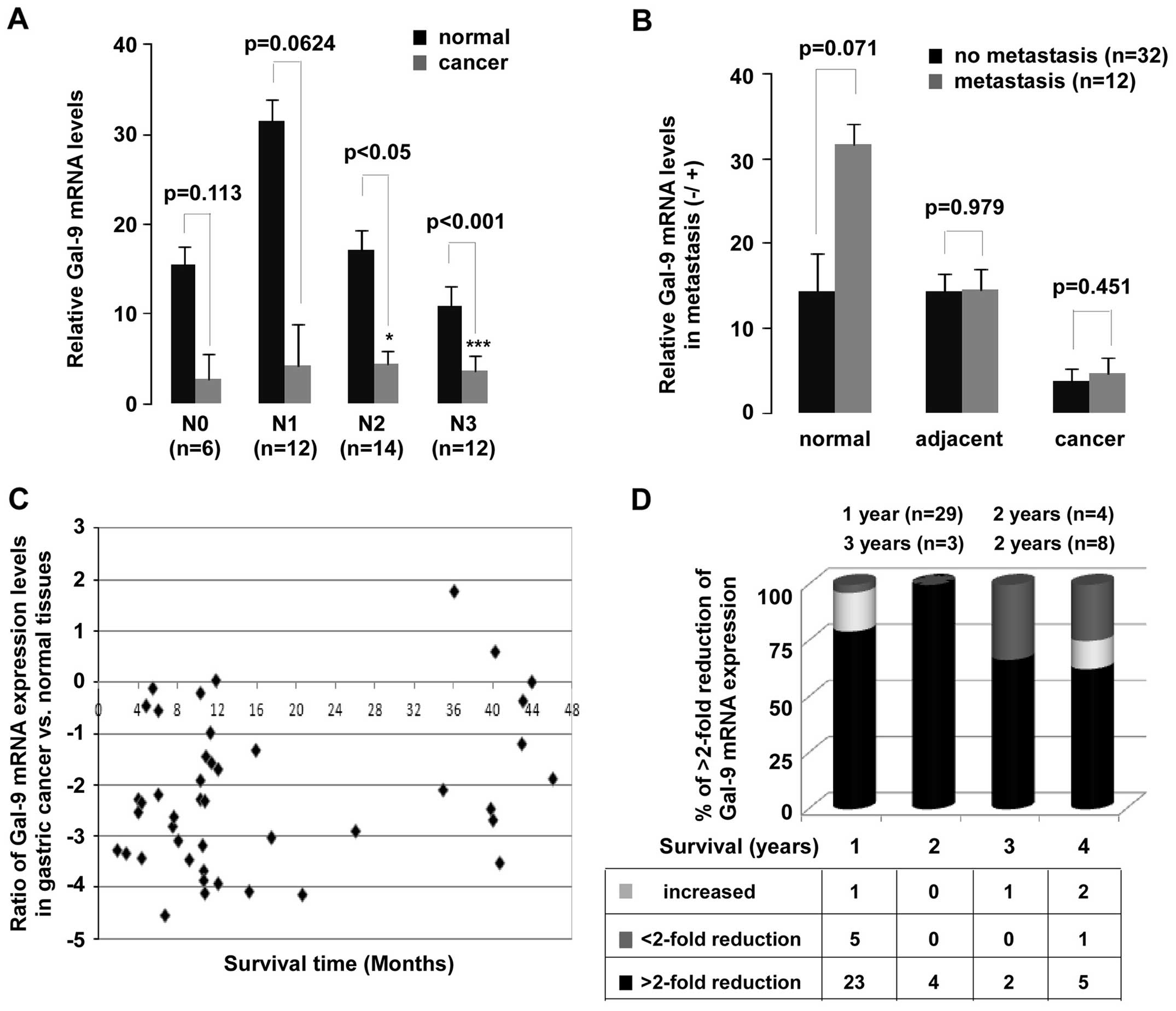
based on adjacent lymph node metastasis and distant metastasis. A
significant downregulation of Gal-9 mRNA in N2 (p<0.05) and N3
(p<0.001) of lymph node metastasis groups was observed (Fig. 4A). Despite the remarkably decrease
in Gal-9 expression in patients with gastric cancer, no
statistically significant difference was not found between with or
without metastasis groups (Fig.
4B). In addition, a significant observation was discovered in
the Gal-9 expression (Fig. 4C and
D). Low levels of Gal-9 mRNA expression were observed in
patients who survived for less (p<0.01) or more (p<0.01) than
one year. Of all patients evaluated here, 66% survived less than
one year.
Gal-9 and Tim-3 in gastric cancer
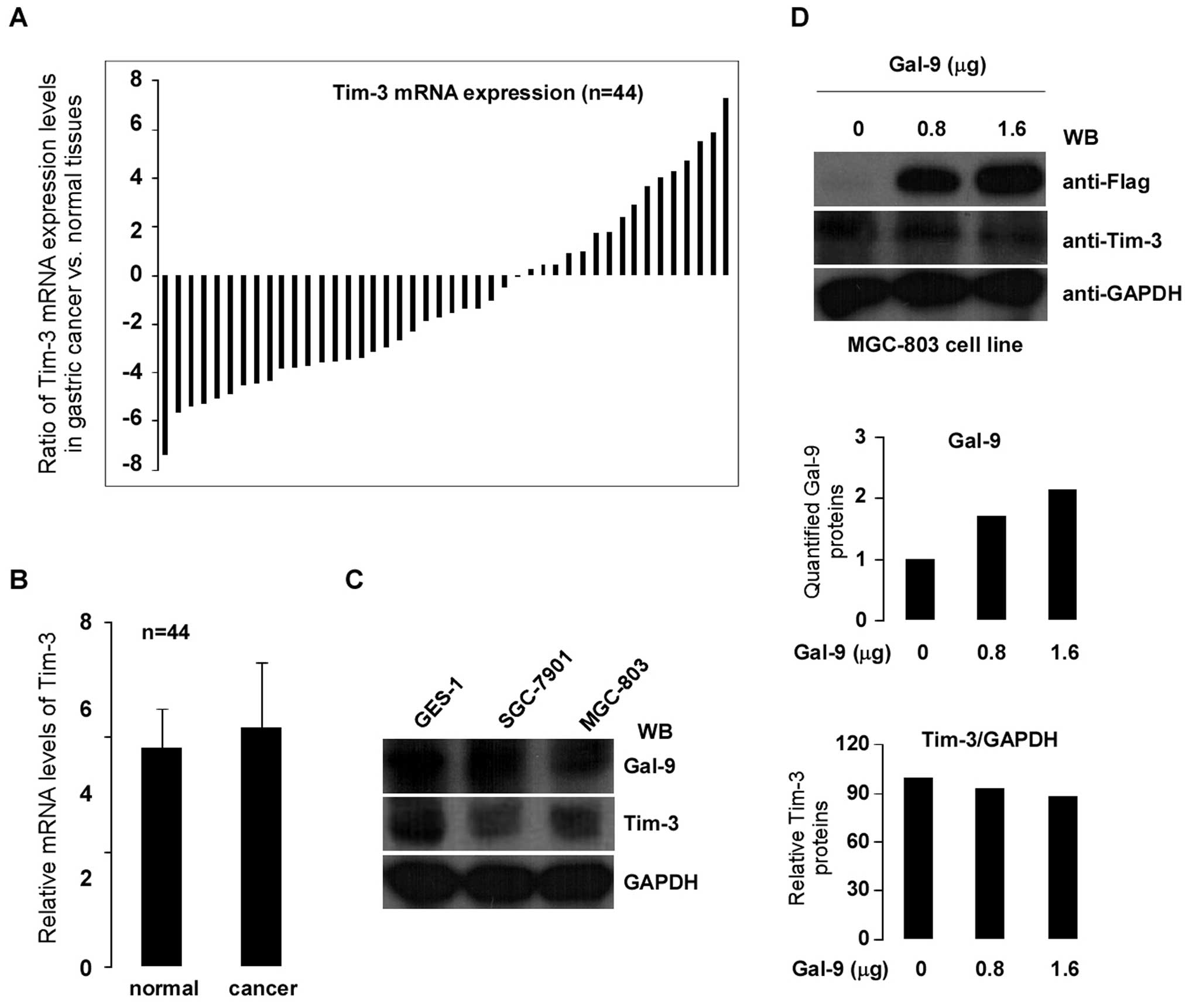
Gal-9 has been identified as a Tim-3 ligand and the
binding of Gal-9 to Tim-3 induces cell death in Th1 cells (19). In order to assess the correlation
between Gal-9 and Tim-3 in gastric cancer, Tim-3 gene expression
was examined in 44 paired clinically diagnosed primary gastric
cancer and matched normal tissues using qPCR. As shown in Fig. 5A, cancerous tissues showed less
expression of Tim-3 than in the matched normal tissues (>2-fold
decreased) in 59% of patients. However, Tim-3 expression was
significantly increased (>2-fold) in 25% of patients.
Statistical analysis confirmed the absence of any significant
difference between the tumor and matched normal tissues (p>0.05)
(Fig. 5B). To further determine
whether there is any regulatory relationship between Gal-9 and
Tim-3 in cells, experiments were performed using gastric cancer
cell lines as models. Gal-9 and Tim-3 protein expression levels
were evaluated in gastric cancer cells SGC-7901 and MGC-803 (GES-1
gastric mucosal cell as control). Fig.
5C shows the protein expression levels of Gal-9 and Tim-3 by
western blotting. To determine whether Tim-3 expression was
regulated by Gal-9, MGC-803 cells were transiently transfected with
0.8 and 1.6 μg of Gal-9 cDNA. Protein expression levels of Gal-9
increased dose-dependently (Fig.
5D top and middle). However, neither gene nor protein
expression of Tim-3 was affected by the transient transfection of
gastric cancer MGC-803 cells with Gal-9 cDNA (Fig. 5D, bottom; gene expression data not
shown).
Discussion
The galectin genes are evolutionarily conserved from
viruses to mammals (20). To date,
15 mammalian galectins have been identified, all of which contain
one or two CRDs. Prototype galectins such as galectin-1, -2, -5,
-7, -10, -11, -13 and -14 contain only one CRD. In contrast,
tandem-repeat type galectins including galectin-4, -6, -8, -9, and
-12 have two separate CRDs connected by linker peptides. Galectin-3
is the only chimeric type galectin. It contains a proline-glycine
rich N-terminal tail fused to a CRD (3,4). The
wide distribution of galectins and the variety of binding partners
led them to function in multiple biological reactions, including
mRNA splicing, cell apoptosis, cell cycle regulation, cell adhesion
and migration, and cell differentiation (21–24).
Galectin-9 (Gal-9) is widely distributed in tissues
involved in the immune system and in tissues of endodermal origin,
such as spleen, thymus, liver, intestine, and stomach tissues. Low
levels of Gal-9 expression were observed in breast, lung, renal,
adrenal, prostate, skin, cervical, oral, brain, ovarian, and liver
cancer cell lines, but not in leukemia or colon cancer cell lines
(4). The expression of Gal-9 in
tumor tissues has only been pathologically investigated in certain
limited tumor types. Research published by several groups show
levels of Gal-9 expression to be lower in cancer tissues than in
normal tissues (11,15). These findings were consistent with
the present results. In this study, the gene expression of Gal-9
was first investigated in clinically diagnosed primary gastric
cancer tissues using qRT-PCR. Significant (>2-fold decreased)
downregulation of Gal-9 mRNA was observed in 77% (34/44) of
patients with gastric cancer. It is noteworthy that the abnormal
expression of Gal-9 had already appeared in adjacent tissues (<2
cm away from the cancer tissue). Compared to matched normal
tissues, although no statistically significant difference was found
between adjacent and normal tissues, decreased expression of
Gal-9 (>2-fold) in adjacent tissues had already emerged
in 34% (15/44) of patients with gastric cancer. Loss of
Gal-9 expression was found to be consistently correlated
with distant metastasis (11,15).
The results of the present study show low levels of expression of
Gal-9 mRNA to be associated with clinical staging, tumor pT stage,
cell differentiation, lymph node metastasis, and patient survival.
However, no significant association was observed between
Gal-9 expression and distant metastasis (p=0.0616,
p>0.05). Recently, Jiang et al reported that although the
Gal-9 shows pathologically higher levels of expression in gastric
cancer than in normal tissues, low levels of Gal-9 expression are
correlated with poor cancer prognosis (25). Combined with our findings,
suggesting that low expression of Gal-9 is involved in
tumorigenesis of gastric cancer.
Tim-3, a member of the T cell Ig and mucin domain
(Tim) family, was found to be specifically expressed on terminally
differentiated CD4+ Th1 cells, but not on Th2 cells
(26). Cumulative findings
indicate that the interaction of Tim-3 and its natural ligand
Gal-9, exerts a crucial role in immune regulation. A recent study
shows that the Tim-3/Gal-9 signaling pathway plays a critical role
in the homeostasis of hepatic NKT cells through activation-induced
apoptosis and secondary proliferation (27). In the present study, significantly
less Tim-3 mRNA expression (>2-fold) was observed in gastric
cancer tissues in 59% of patients, but higher than normal
expression of Tim-3 (>2-fold) was also observed in 25% of
the patients. Cell experiments showed no correlation between Gal-9
and Tim-3 in gastric cancer. Further investigation might be needed
to determine the functional mechanism of Tim-3/Gal-9 in gastric
cancer.
In conclusion, downregulation of Gal-9 mRNA
was observed in gastric cancer tissues, and statistical analysis
demonstrated the molecular mechanism underlying this process. These
results showed that the loss of Gal-9 expression may be involved in
the progression of gastric cancers. Although the Gal-9 gene was
found to be involved in gastric cancer, further studies are
required to address the many remaining questions, such as the
effects of alternative splicing of Gal-9 in different tumor
models.
Acknowledgements
This study was supported by the Key Scientific and
Technological Project of Jilin Province Science and Technology
Development Program (20130206005YY).
References
|
1
|
Barondes SH, Castronovo V, Cooper DN,
Cummings RD, Drickamer K, Felzi T, Gitt MA, Hirabayashi J, Hughes
C, Kasai K, Leffler H, Liu FT, Lotan R, Mercurio AM, Monsigny M,
Pillai S, Poirer F, Raz A, Rigby PW, Rini JM and Wang JL:
Galectins: a family of animal β-galactoside-binding lectins. Cell.
76:597–598. 1994.
|
|
2
|
Leffler H, Carlsson S, Hedlund M, Qian Y
and Poirier F: Introduction to galectins. Glycoconj J. 19:433–440.
2004. View Article : Google Scholar
|
|
3
|
Hirabayashi J and Kasai K: The family of
metazoan metal-independent beta-galectoside-binding lectins:
structure, function and molecular evolution. Glycobiology.
3:297–304. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heusschen R, Griffioen AW and Thijssen VL:
Galectin-9 in tumor biology: a jack of multiple trades. Biochim
Biophys Acta. 1836:177–185. 2013.PubMed/NCBI
|
|
5
|
Türeci O, Schmitt H, Fadle N, Pfreundschuh
M and Sahin U: Molecular definition of a novel human galectin which
is immunogenic in patients with Hodgkin’s disease. J Biol Chem.
272:6416–6422. 1997.PubMed/NCBI
|
|
6
|
Oda Y, Herrmann J, Gitt MA, Turck CW,
Burlingame AL, Barondes SH and Leffler H: Soluble lactose-binding
lectin from rat intestine with two different carbohydrate-binding
domains in the same peptide chain. J Biol Chem. 268:5929–5939.
1993.PubMed/NCBI
|
|
7
|
Hadari YR, Paz K, Dekel R, Mestrovic T,
Accili D and Zick Y: Galectin-8. A new rat lectin, related to
galectin-4. J Biol Chem. 27:3447–3453. 1995.PubMed/NCBI
|
|
8
|
Sato M, Nishi N, Shoji H, Seki M,
Hashidate T, Hirabayashi J, Kasai Ki K, Hata Y, Suzuki S, Hirashima
M and Nakamura T: Functional analysis of the carbohydrate
recognition domains and a linker peptide of galectin-9 as to
eosinophil chemoattractant activity. Glycobiology. 12:171–197.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oh JH, Yang JO, Hahn Y, Kim MR, Byun SS,
Jeon YJ, Kim JM, Song KS, Noh SM, Kim S, Yoo HS, Kim YS and Kim NS:
Transcriptome analysis of human gastric cancer. Mamm Genome.
16:942–954. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang F, Zheng M, Qu Y, Li J, Ji J, Feng
B, Lu A, Li J, Wang M and Liu B: Different roles of galectin-9
isoforms in modulating E-selectin expression and adhesion function
in LoVo colon carcinoma cells. Mol Biol Rep. 36:823–830. 2009.
View Article : Google Scholar
|
|
11
|
Kageshita T, Kashio Y, Yamaguchi A, Seki
M, Abedin MJ, Nishi N, Shoji H, Nakamura T, Ono T and Hirashima M:
Possible role of galectin-9 in cell aggregation and apoptosis of
human melanoma cell lines and its clinical significance. Int J
Cancer. 99:809–816. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu LH, Nakagawa R, Kashio Y, Ito A, Shoji
H, Nishi N, Hirashima M, Yamaguchi A and Nakamura T:
Charaterization of galectin-9-induced death of Jurkat T cells. J
Biochem. 141:157–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi T, Kuroda J, Ashihara E, Oomizu
S, Terui Y, Taniyama A, Adachi S, Takagi T, Yamamoto M, Sasaki N,
Horiike S, Hatake K, Yamauchi A, Hirashima M and Taniwaki M:
Galectin-9 exhibits anti-myeloma activity through JNK and p38 MAP
kinase pathways. Leukemia. 24:843–850. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kashio Y, Nakamura K, Abedin MJ, Seki M,
Nishi N, Yoshida N, Nakamura T and Hirashima M: Galectin-9 induces
apoptosis through the calcium-calpain-caspase-1 pathway. J Immunol.
170:3631–3636. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Irie A, Yamauchi A, Kontani K, Kihara M,
Liu D, Shirato Y, Seki M, Nishi N, Nakamura T, Yokomise H and
Hirashima M: Galectin-9 as a prognostic factor with antimetastatic
potential in breast cancer. Clin Cancer Res. 11:2962–2968. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang ZY, Dong JH, Chen YW, Wang XQ, Li
CH, Wang J, Wang GQ, Li HL and Wang XD: Galectin-9 acts as a
prognostic factor with antimetastatic potential in hepatocellular
carcinoma. Asian Pac J Cancer Prev. 13:2503–2509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang M, Ueno M, Oomizu S, Arikawa T,
Shinonaga R, Zhang S, Yamauchi A and Hirashima M: Galectin-9
expression links to malignant potential of cervical squamous cell
carcinoma. J Cancer Res Clin Oncol. 134:899–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; Chicago, IL: 2010
|
|
19
|
Zhu C, Anderson AC, Schubart A, Xiong H,
Imitola J, Khoury SJ, Zheng XX, Strom TB and Kuchroo V: The Tim-3
ligand galectin-9 negatively regulates T helper type 1 immunity.
Nat Immunol. 6:1245–1252. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cooper DN and Barondes SH: God must love
galectins; he made so many of them. Glycobiology. 9:979–984. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vyakarnam A, Dagher SF, Wang JL and
Patterson RJ: Evidence for a role for galectin-1 in pre-mRNA
splicing. Mol Cell Biol. 17:4730–4737. 1997.PubMed/NCBI
|
|
22
|
Kuwabara I, Kuwabara Y, Yang RY, Schuler
M, Green DR, Zuraw BL, Hsu DK and Liu FT: Galectin-7 (PIG1)
exhibits pro-apoptotic function through JNK activation and
mitochondrial cytochrome c release. J Biol Chem. 277:3487–3497.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang RY, Hsu DK, Yu L, Ni J and Liu FT:
Cell cycle regulation by galectin-12, a new member of the galectin
superfamily. J Biol Chem. 276:20252–20260. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elola MT, Wolfenstein-Todel C, Troncoso
MF, Vasta GR and Rabinovich GA: Galectins: matricellular
glycan-binding proteins linking cell adhesion, migration, and
survival. Cell Mol Life Sci. 64:1679–1700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang J, Jin MS, Kong F, Cao D, Ma HX, Jia
Z, Wang YP, Suo J and Cao X: Decreased galectin-9 and increased
Tim-3 expression are related to poor prognosis in gastric cancer.
Plos One. 8:e817992013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Monney L, Sabatos CA, Gaglia JL, Ryu A,
Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel
RA, Freeman GJ and Kuchroo VK: Th1-specific cell surface protein
Tim-3 regulates macrophage activation and severity of an autoimmune
disease. Nature. 415:536–541. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang ZH, Liang S, Potter J, Jiang X, Mao
HQ and Li Z: Tim-3/galectin-9 regulate the homeostasis of hepatic
NKT cells in a murine model of nonalcoholic fatty liver disease. J
Immunol. 190:1788–1796. 2013. View Article : Google Scholar : PubMed/NCBI
|