Introduction
The majority (85–90%) of patients with pancreatic
adenocarcinoma have unresectable disease at diagnosis. Gemcitabine
is a palliative treatment option. Median survival of gemcitabine
treated patients is only 6 months (1). In patients with resectable disease,
postoperative gemcitabine therapy significantly delayed time to
recurrence (2). However,
irrespective of treatment regimens, survival of pancreatic cancer
patients remains poor and new therapeutic strategies are
needed.
During the past years introduction of targeted
therapeutics has been tested in pancreatic adenocarcinoma (1,3,4).
Therapeutic cancer vaccines (TCV) is such an approach. Chemotherapy
in combination with TCV might add to the immunological and clinical
effects of TCV. Gemcitabine may augment immune responses by
increasing the amounts of antigens loaded onto antigen-presenting
cells (APC) (5) and downregulate
T-regulatory cells (6). Patients
with pancreatic carcinoma receiving chemoradiotherapy were capable
of mounting a humoral and cellular response to tetanus toxoid,
pneumococcal and hemophilus vaccines indicating a functionally
preserved immune system (7).
Administration of gemcitabine did not significantly decrease the
number of T and B cells or APC and enhanced the immune response
against cancer vaccine (8).
Telomerase is expressed in 85–90% of pancreatic
adenocarcinomas (9). GV1001 is a
telomerase derived peptide vaccine (hTERT: 611–626) consisting of
16 amino acids (10). This
multiepitope peptide vaccine binds to various DP, DR and DQ HLA
class II molecules. GV 1001 vaccination in non-small cell
lung-cancer patients induced a cellular immune response in 85% of
the patients (11). In a dose
escalation study of GV1001 in pancreatic cancer patients, the
vaccine was safe and induced a telomerase specific T cell response
in 63%. Patients mounting a specific immune response had a better
survival than immune non-responders (12). In advanced melanoma, treatment with
GV1001 in combination with temozolomide was safe. Those patients
developing a GV1001 specific long-term T-cell memory response
survived longer than those rapidly losing the T-cell immunity
(10).
In this explorative investigation we studied
immunogenicity and safety of GV1001 in combination with different
schedules of GM-SCF and gemcitabine as first line treatment of
patients with advanced pancreatic adenocarcinoma.
Materials and methods
Patients
The report includes the results of two studies.
Study 1 consists of groups A and B, and study 2 of groups C and D
(see below). In total 28 patients were enrolled. Evaluable patients
for immunogenicity per protocol were those who had completed immune
testing at weeks 0 and 6/7. Eligibility criteria included
histologically confirmed diagnosis of non-resectable pancreatic
adenocarcinoma and a life expectancy of at least three months.
Patients were required to have a Karnofsky performance status ≥70
and adequate bone marrow, cardiac, renal and hepatic functions.
Exclusion criteria included chemotherapy or other potentially
immune-suppressive therapy within 4 weeks prior to start of
treatment including chronic use of systemic anti-histamines,
corticosteroids or high-doses of NSAIDs.
Prior to entry, a complete case history, physical
examination and blood tests including haemoglobin, WBC with a
differential and platelet counts, electrolytes, liver function
tests, standard urine analysis and serum tumor marker (CA 19-9) as
well as an abdominal CT or MRI scan was performed. Chest X-ray was
done on clinical request. During the study, patients were checked
at regular intervals for performance status, routine blood
hematology and chemistry analyses, and the serum tumor marker.
Adverse events (AE) were assessed according to the
National Cancer Institute Common Toxicity Criteria versions 2.0
(groups A/B) or 3.0 (groups C/D), respectively, and considered
related to treatment if a relationship was reported as possible or
probable. AEs related to gemcitabine were observed for 8 weeks in
groups A/B, and in groups C/D at all administrations. The primary
endpoint was induction of an immune response against the vaccine as
well as safety evaluation.
Patients were treated according to the Declaration
of Helsinki ethical principles for medical research involving human
subjects. The trial was performed according to GCP guidelines and
approved by the Regional Ethics Review Board in Stockholm, Sweden
[dnr: 03-145 (groups A/B) and 2006/1491-32 (groups C/D)] and by the
Medical Products Agency in Uppsala, Sweden [dnr; 151:2003/25888
(groups A/B) and 151:2006/45316 (groups C/D)]. All patients
provided a signed informed consent prior to study entry.
Vaccine
The GV1001 peptide vaccine was supplied as a
freeze-dried product in sterile vials. The 16-amino acid
hTERT-peptide (EARPALLTSRLRFIPK) covers positions 611–626; 300
nmole (560 μg) of GV1001 in 0.10 ml saline (groups A/B) and in 0.20
ml saline (group C) was administered. The dose of GV1001 was based
on studies in non-small cell lung cancer (11) and pancreatic carcinoma patients
(12). Isopharma AS, Norway
manufactured GV1001 and the supplier was GemVax AS, Norway for
group A and B patients. GV1001 was manufactured by Laboratoire
Elaiapharm, France and supplied by Penn Pharmaceutical Services
Ltd, UK for group C patients.
Vaccination schedule
Group A patients received GV1001 (560 μg)
intradermally (i.d) days 1, 3 and 5 during the first week followed
by a once weekly schedule in weeks 2, 3, 4 and 6. At each
vaccination, the patients also received 150 μg GM-CSF (Leukine,
Berlex Laboratories, Seattle, WA, USA) i.d., 15 min prior to GV1001
at the site of vaccination. All injections were given in the lower
abdominal wall. Group B received the vaccine schedule as in group A
with the exception that GM-CSF (150 μg) was given i.d. for five
consecutive days the first week (days 1–5), and in weeks 2, 3, 4
and 6, GM-CSF (150 μg) for four consecutive days starting on the
day of the peptide vaccination. Gemcitabine 1,000 mg/m2
was administered intravenously (i.v.) once weekly for seven
consecutive weeks in both groups. When gemcitabine and vaccine were
given the same day, the vaccine was administered first. In groups
A/B, after the initial seven weeks, gemcitabine was continued until
progression at the clinicians’ discretion. In group C GV1001 (560
μg) plus GM-CSF (75 μg) i.d., (Leukine, Berlex Laboratories) was
administered as in group A. Gemcitabine (1,000 mg/m2)
was added at the time of progression and continued at the
clinicians discretion. In group D gemcitabine (1,000
mg/m2) alone was given weekly for the first 7 weeks, and
then in 4-week cycles with 3 consecutive weekly administrations of
gemcitabine followed by a rest for 1 week.
Clinical evaluation criteria
Progressive disease (PD) was defined by radiological
measurements, supplemented by serum tumor marker and/or at clinical
progression as determined by the investigator. Computer tomography
(CT) or magnetic resonance imaging (MRI) was performed at the end
of vaccination to assess tumor burden in groups A/B and every 8th
week in groups C/D to assess time to progression (TTP).
Disappearance of all radiographic evidence was considered a
complete response (CR), while 30% or more decrease in the size of
the tumor was considered to be a partial response (PR). At least
20% increase or appearance of new lesions was considered
progressive disease (PD). Neither sufficient shrinkage to qualify
for PR nor sufficient increase to qualify for PD was considered
stable disease (SD). Level of serum CA19-9 was considered to be
stable if the increase or decrease was ≤50%.
Immune tests
Collection of blood samples as well as delayed type
hypersensitivity (DTH) test against GV1001 were done prior to start
of vaccine treatment. During treatment and follow-up, DTH was
performed at weeks 4 and 7 in groups A/B and at weeks 2, 3, 4, 6,
10 in group C.
Blood T-cell responses against GV1001 were evaluated
at week 7 and then every 8th week until two consecutive negative
tests were noted (groups A/B). The corresponding time points in
groups C/D were weeks 6, 12, 20 and 28. T-cell phenotyping (flow
cytometry) was performed at the same time points.
Delayed type hypersensitivity (DTH)
GV1001 (0.112 mg) in 0.1 ml saline (groups A/B) was
injected intradermally in the volar part of the forearm. GV1001
(0.105 mg) in 0.22 ml saline (group C) was injected intradermally
in the lower abdominal wall. The skin test was read after 48 h by
measuring the diameter of induration (mm). A positive DTH response
was defined as ≥5 × 5 mm of induration.
In vitro immune responses
In vitro immune responses were analyzed
against GV1001 (immunizing peptide) and a ras-derived peptide
(KLVVVGAAGVGKSALTI) (manufactered by Merck KGaA, Darmstadt, Germany
and supplied by Avencia LSM, UK) [>90% of the pancreatic
carcinoma patients express the ras-oncoprotein (13)]. As a negative control, a peptide
corresponding to HIV reverse transcriptase (KEPIVGAETFYVDGA)
(Thermo Fisher Scientific, Waltham, MA, USA) was used.
Proliferation assay
The proliferation assay (3H-thymidine
incorporation) has been described previously (14). Peripheral blood mononuclear cells
(PBMC) were isolated. A total of 105 cells/well were
incubated for 6 days with GV1001, the ras-peptide and the
HIV-peptide (1 and 10 μg/ml), respectively. Phytohemagglutinin
(PHA) (10 μg/ml) (Sigma) and purified protein derivative of
tuberculin (PPD) (10 μg/ml) (Statens Seruminstitut, Copenhagen,
Denmark) were used as controls.
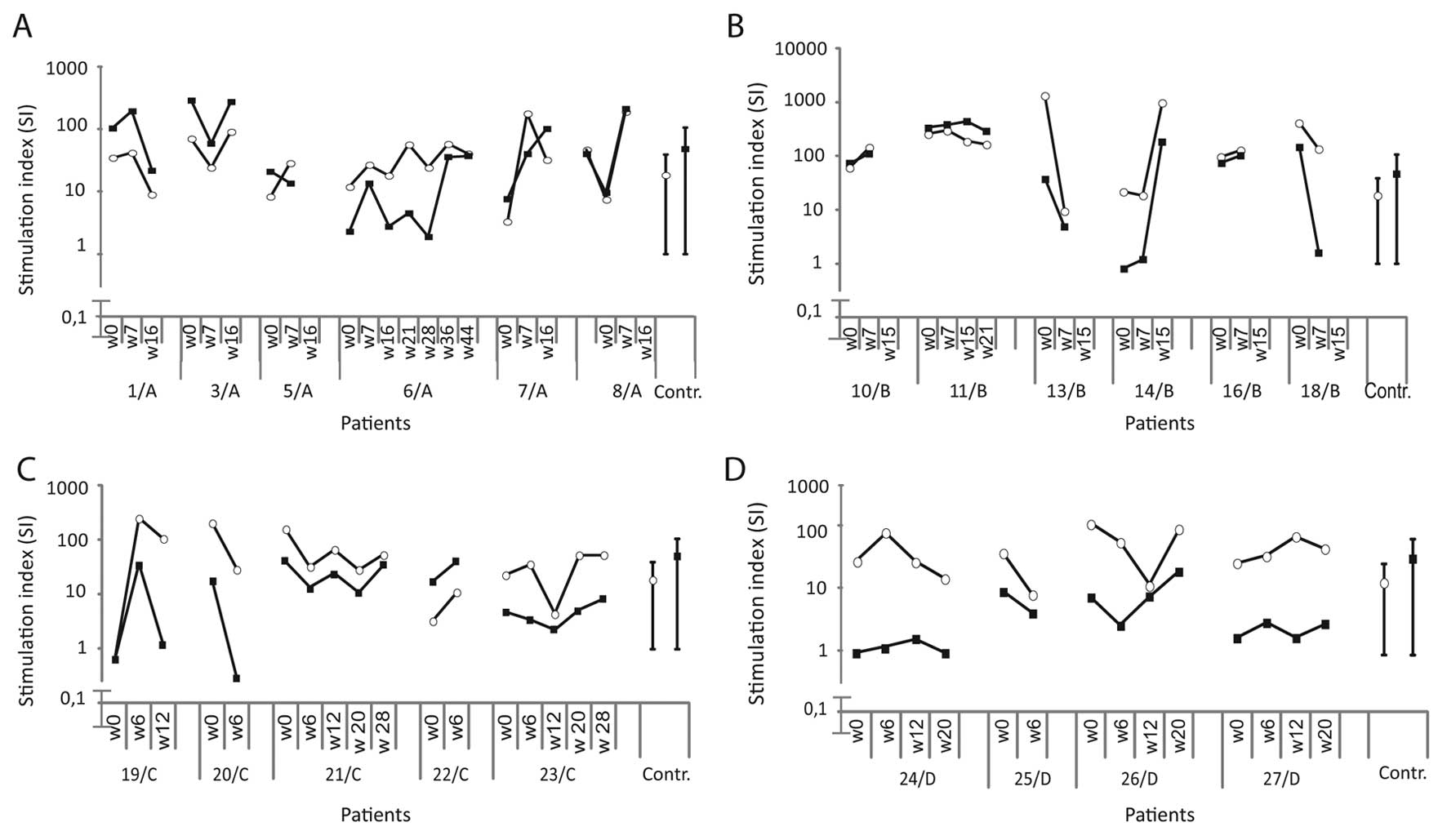
A stimulation index (SI) was calculated by dividing
mean radioactivity (cpm) of 6 replicates of experimental wells by
that of the background value (cells in medium alone) (15). SI values (mean ± 2SD) of healthy
donors (n=9) against GV1001, ras- and HIV-derived peptides were
1.12±0.75, 1.24±0.79 and 1.18±0.74, respectively. The cut-off level
for a proliferative T cell response was set to ≥2.0.
A positive telomerase (T)/ras (R) proliferative T
cell response was defined if all of the following criteria were
met: i) an SI in experimental wells ≥2.0; ii) an SI of cells
stimulated with the control peptide <2.0; iii) a vaccine induced
SI at least twice that of the pre-vaccination value. All patients
had a positive response to PPD and PHA prior to, during and after
vaccination.
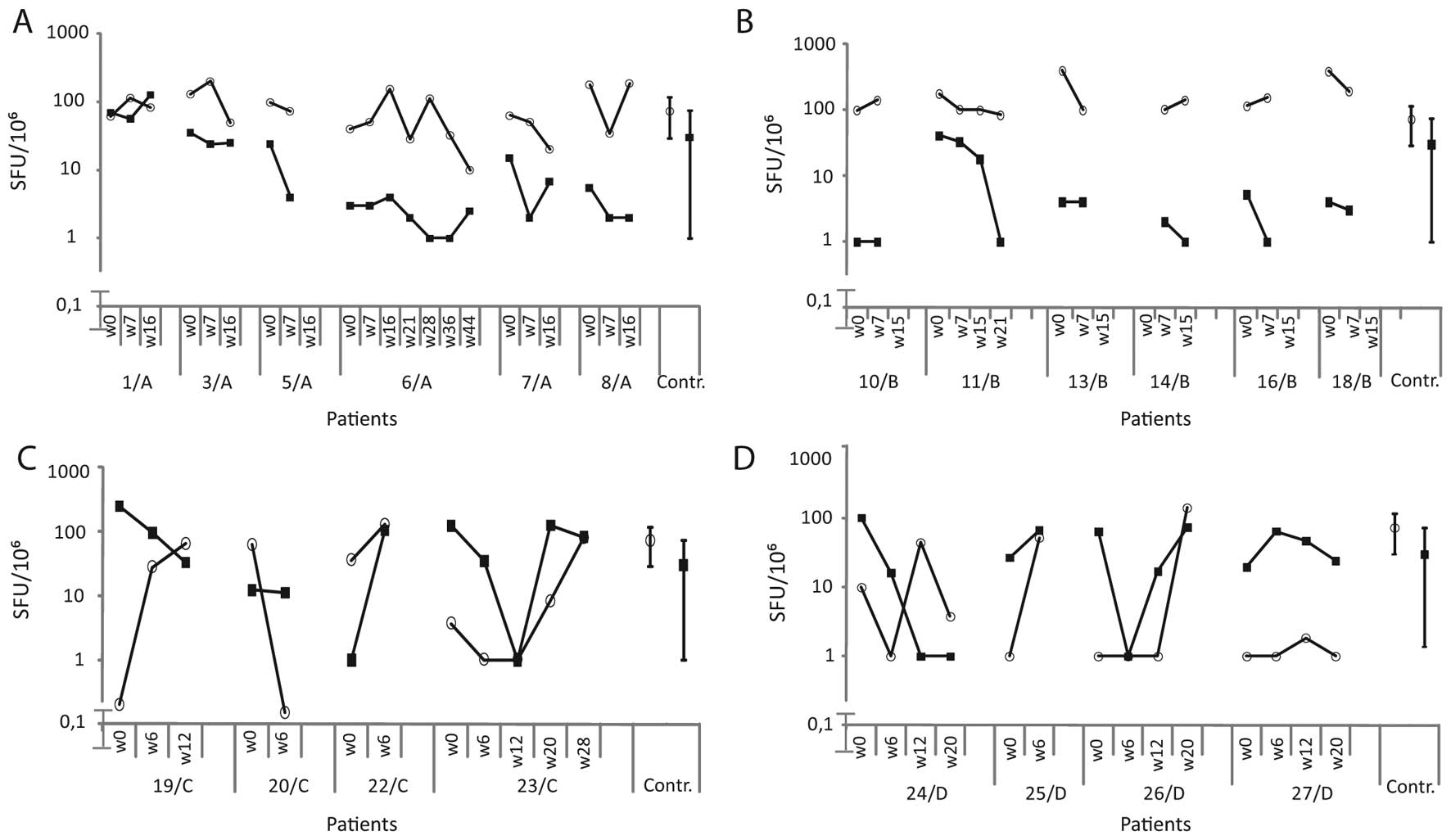
ELISPOT (IFN-γ)
ELISPOT was performed as previously described
(15). PBMC (2×105
cells/well) were cultured in 48-well plates with GV1001, ras and
HIV peptides resp. (1 and 10 μg/ml), PHA (5 μg/ml) or PPD (2.5
μg/ml) for 5 days in 6 replicates. A millipore 96-well filter plate
was coated with anti-IFN-γ antibody (10 μg/ml) (Mabtech, Stockholm,
Sweden). Cultured PBMC were transferred to the coated plate and
incubated for 20 h with the antigens as above. Cells were washed
and incubated with a secondary biotinylated anti-IFN-γ antibody (1
μg/ml) (Mabtech, San Jose, CA, USA) for 2 h at room temperature.
After washing, streptavidin-ALP conjugate (1:1,000) (Mabtech, San
Jose, CA, USA) was added to the cells and incubated for 1 h at room
temperature. Cells secreting IFN-γ were developed by adding
substrate BCIP/NBT (Mabtech, San Jose, CA, USA) and incubated at
room temperature for 5 min. The reaction was stopped at the
appearance of dark spots. Spots were counted by an automatic
ELISPOT assay reader (AID, Strassberg, Germany).
A vaccine induced IFN-γ response was defined if all
of the following criteria were fulfilled: i) spot forming units
(SFU) of stimulated (GV1001) cells significantly higher (p<0.05)
than that of unstimulated cells (background) and at least twice
that of the background; ii) SFU of cells stimulated with the
control peptides not significantly (p>0.05) higher than that of
the background; iii) SFU of a post-vaccination test at least twice
that of the pre-vaccination test (15).
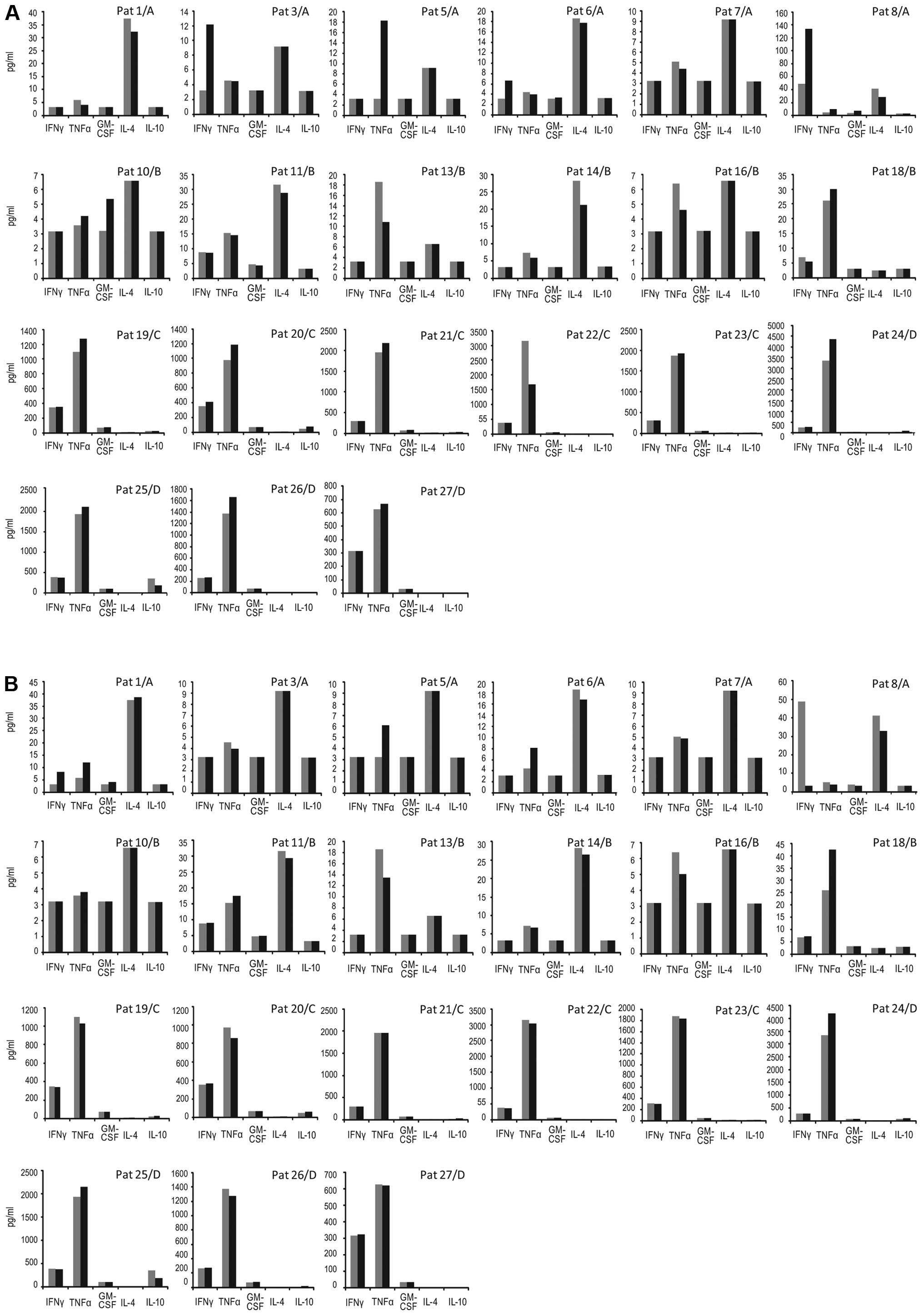
Cytokine secretion assay
Supernatants were collected (20 μl/well) after 24
and 120 h of incubation from the proliferation assay. The volume
was replaced with complete medium.
In groups A/B, IL-4, IL-10, IFN-γ, TNF-α and GM-CSF
were analyzed using the Luminex technology (LINCOplex Kit, Linco
Research Inc., St. Charles, MO, USA) according to the
manufacturer’s instruction. In groups C/D, IL-4, IL-10, IFN-γ,
TNF-α and GM-CSF were analyzed using a human 8-plex cytokine
reagent kit (171-304000) and the Bio-Plex instrument (Bio-Rad,
Hercules, CA, USA) according to manufacturer’s instruction.
Standard concentration curves were generated. The coefficient of
variation of PHA stimulated cells (n=5) was 12±10% (mean ± SD).
Cytokine concentration (pg/ml) in supernatants of
antigen stimulated cells divided by that of cells alone using the
highest value at 24 or 120 h culture periods respectively was used.
The post-vaccination ratio divided by pre-vaccination ratio at
different time points is shown. A ratio ≥2 (relative increase) was
considered an antigen-induced specific response (Table II). The absolute concentrations of
the different cytokines over time are also shown.
 | Table IITreatment induced immune responses
against telomerase (T)/ras (R). |
Table II
Treatment induced immune responses
against telomerase (T)/ras (R).
| Week 6/7 | Week 12/15 | Week 20/21 | | | | |
|---|
|
|
|
| | | | |
|---|
| Patient
no./group | Prolif.a (SI) | Elispotb SFU/106 cells | Cytokine secretion
(ratio)c | Prolif.a (SI) | Elispotb SFU/106 cells | Cytokine secretion
(ratio)c | Prolif.a (SI) | Elispotb SFU/106 cells | Cytokine secretion
(ratio)c | Week 28d | DTH response at
week | STIR response | SIR response |
|---|
| | |
|
|---|
| IFN-γ | TNF-α | GM-CSF | IFN-γ | TNF-α | GM-CSF | IFN-γ | TNF-α | GM-CSF | Prolif.a | Elispotb |
|---|
| T/R | T/R | T/R | T/R | T/R | T/R | T/R | T/R | T/R | T/R | T/R | T/R | T/R | T/R | T/R | T/R | T/R | T/R | T/R |
|---|
| 1/A | - | - | 3.8/− | −/5.6 | - | - | - | −/2.9 | - | - | - | - | - | - | - | - | - | - | +/+ | −/+ |
| 3/A | - | - | 12/− | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4 | +/− | +/− |
| 5/A | −/2.5 | - | 13/− | 3.5/− | - | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | - | +/+ | - |
| 6/A | −/3.7 | - | - | - | - | −/30 | - | - | - | - | - | - | - | - | - | - | - | - | −/+ | −/+ |
| 7/A | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 8/A | - | - | 2.8/− | 2.3/− | 2.9/− | - | - | - | - | - | - | - | - | - | - | - | - | - | +/− | - |
| 10/B | 6.1/− | - | - | - | 2.3/− | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | - | +/− | - |
| 11/B | - | - | - | - | - | - | - | - | - | - | - | - | 2.5/− | - | - | - | - | - | +/− | - |
| 13/B | - | - | - | - | - | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | - | - | - |
| 14/B | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 7 | +/− | - |
| 16/B | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 18/B | 3.8/4.5 | - | - | 2.6/3.7 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | +/+ | - |
| 19/C | - | - | - | - | 8.8/− | - | - | - | - | - | - | - | - | - | - | - | - | - | +/− | - |
| 20/C | - | - | - | - | - | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | - | - | - |
| 21/C | - | - | - | - | - | - | −/58 | - | - | - | - | - | - | - | - | - | - | - | −/+ | - |
| 22/C | - | - | - | - | - | - | - | - | - | - | ND | ND | ND | ND | ND | ND | ND | - | - | - |
| 23/C | - | - | - | - | - | - | 58/− | - | - | - | - | - | - | - | - | - | - | - | +/− | - |
| 24/D | - | - | - | - | - | - | - | - | - | - | - | - | 2.8/2.9 | - | - | ND | ND | ND | +/+ | - |
| 25/D | - | - | - | - | - | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | - | - |
| 26/D | - | - | - | - | - | - | - | - | 3.3/− | - | - | - | - | - | - | ND | ND | ND | +/− | - |
| 27/D | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ND | ND | ND | - | - |
A single time point-induced immune
response (STIR) post-vaccination
A patient was considered to have a single time
point-induced immune response if a response in one of the assays
(DTH, proliferation, ELISPOT, cytokine secretion) was noted at one
time point only.
A sustained immune response (SIR)
post-vaccination
A patient was considered to be a sustained immune
responder if an immune response was noted in at least one of the
assays (DTH, proliferation assay, ELISPOT, cytokine secretion) at
two time points or more.
Flow cytometry
Peripheral blood mononuclear cells were analyzed for
T cell subsets by flow cytometry as previously described (16). Flurochrome-conjugated antibodies
(CD3, CD4, CD8, CD25, CD45RA) (Becton Dickinson Biosciences, San
Jose, CA, USA), CCR7 (R&D Systems, Minneapolis, MN, USA) and
Foxp3 staining kit from e-Biosciences Inc. (San Diego, CA, USA) was
used. A minimum of 20,000 events were collected using a FACSCalibur
(BD) and analyzed by the Cellquest® Software (BD).
Statistical analysis
The non-parametric Mann-Whitney two-tailed rank sum
test for comparison of independent variables and the two-tailed
non-parametric Wilcoxon signed rank test for dependent
observations, were applied.
Results
Patients
Twenty-one out of initially 28 enrolled patients
completed immune testing at weeks 0 and 6/7 and were considered
evaluable for an induced immune response. Clinical characteristics
of the 21 patients are shown in Table
I. Eight patients were initially enrolled in group A but two
were withdrawn, one not fulfilling inclusion criteria and one with
disease progression at week one. Two patients in group B withdrew
informed consent at week 3 and 4, respectively. One was withdrawn
due to progression at week 3. One patient in group B succumbed
before study completion and was replaced. Six evaluable patients in
group A and B, respectively, completed at least 7 weeks of study.
All five patients enrolled in group C were immunologically
evaluable. Five patients were enrolled in group D. One was
withdrawn during the first week due to disease progression.
 | Table IClinical characteristics of
pancreatic adenocarcinoma patients. |
Table I
Clinical characteristics of
pancreatic adenocarcinoma patients.
| Patient
no./group | Gender/age
(years) | Tumor
localization | Site of metastasis
at inclusion | Previous
treatment | Time from diagnosis
to start of vaccination (weeks) | No. of
immunisations | No. of Gemcitabine
administrations (n) | Second line
treatment | Time to
progressiona (weeks) | Overall
survivala (weeks) |
|---|
| 1/A | M/60 | PAC | Liver, lung | None | 10 | 7 | 18 | None | 33 | 35 |
| 3/A | F/79 | LR | None | op Whipple | 8 | 7 | 9 | None | 26 | 34 |
| 5/A | M/62 | PAC | Liver | None | 3 | 7 | 8 | None | 8 | 18 |
| 6/A | M/72 | PAC | None | None | 11 | 7 | 7 | None | 8 | 73 |
| 7/A | F/59 | LR | Liver, lung | op Whipple | 12 | 7 | 11 | None | 17 | 35 |
| 8/A | M/76 | LR | None | op Whipple | 7 | 7 | 10 | None | 43 | 49 |
| 10/B | M/60 | PAC | Liver | None | 4 | 7 | 6 | None | 10 | 12 |
| 11/B | M/72 | LR | Lung, distant
nodes | op Whipple | 7 | 7 | 8 | None | 53 | 73 |
| 13/B | M/73 | LR | Liver | op Whipple | 6 | 7 | 9 | None | 17 | 20 |
| 14/B | M/73 | PAC | Liver | None | 5 | 7 | 9 | None | 23 | 42 |
| 16/B | M/75 | PAC | None | None | 8 | 7 | 11 | 5-Fu | 104 | 166 |
| 18/B | F/66 | PAC | None | None | 8 | 7 | 6 | None | 32 | 36 |
| 19/C | F/70 | PAC | Liver | None | 12 | 7 | 3 | None | 8 | 13 |
| 20/C | F/57 | PAC | None | None | 5 | 6 | 0 | None | 10b | 15 |
| 21/C | F/74 | PAC | Lung, nodes | None | 5 | 10 | 2 | None | 24 | 37 |
| 22/C | F/58 | PAC | Liver | None | 13 | 5 | 0 | None | 8 | 22 |
| 23/C | M/58 | PAC | None |
Gastroenterostomy | 10 | 14 | 14 | None | 8 | 38 |
| 24/D | M/64 | PAC | Lung, nodes | None | 10 | NA | 22 | None | 32 | 33 |
| 25/Dc | M/52 | PAC | Nodes | None | 8 | NA | 21 | 5-Fu-Oxa | 40 | 70 |
| 26/D | M/61 | PAC | Liver | None | 5 | NA | 19 | None | 32 | 36 |
| 27/D | M/66 | PAC | Lung, nodes | None | 4 | NA | 19 | None | 31 | 42 |
Immune responses
In vivo immune response
Delayed type hypersensitivity (DTH)
No patient had a DTH response prior to vaccination.
One patient, no. 3 (group A), developed a DTH at week 4, which
disappeared at week 7. Patient no. 14 (group B) showed a DTH
response at week 7 (Table
II).
In vitro immune responses
Proliferation assay
Proliferative responses post-vaccination against
telomerase (T) or ras (R) are shown in Table II. One patient (no. 3, group A)
had a GV1001-specific proliferative response prior to vaccination
but not at subsequent testing (data not shown). A GV1001-specific
response was induced in two patients in group B. Two patients in
group A, and one patient in group B had a ras-induced specific
response at week 7. In one patient, the ras-induced immune response
was sustained. No proliferative responses against telomerase or ras
were detected in groups C and D. The proliferative response against
PHA and PPD over time are shown in Fig. 1. The PHA and PPD responses of the
patients were within the range of healthy donors.
ELISPOT (IFN-γ)
Prior to vaccination no IFN-γ ELISPOT response was
seen in any patient. No IFN-γ ELISPOT responses against telomerase
or ras were induced at any time points in patients of groups A and
B. A GV1001-specific IFN-γ response was evoked at week 12 in one
patient (no. 23) in group C and a ras-specific response in patient
21 at week 12 (Table II). IFN-γ T
cell responses against PHA and PPD over time for patients are shown
in Fig. 2. The PHA and PPD
responses of patients were within the range of healthy control
donors.
Cytokine secretion assay
The relative increase of induced antigen specific
cytokine secretion is shown in Table
II. A telomerase response was noted in 4 patients in group A. A
Th1-like (IFN-γ, TNF-α, GM-CSF) cytokine response pattern was seen
but no Th2 cytokines (IL-4, IL-10) (data not shown). One patient
had a ras response. In group B three patients mounted a telomerase
response and one a ras response. Again, the response pattern was
Th1 cytokines, but no Th2. One patient in group C developed a
Th1-like cytokine response. In group D, two patients developed a
telomerase Th1-like cytokine response and one a ras response. The
absolute cumulative concentrations (pg/ml) of cytokines (IFN-γ,
TNF-α, GM-CSF, IL-4, IL-10) for all individual patients are shown
in Fig. 3.
Single time point immune responders and
sustained immune responder
Four out of the 6 patients (67%) in group A
developed a single time point induced immune response against
telomerase and 3 (50%) against ras. In group B, 4 out of 6 (67%)
patients mounted a telomerase response and one (17%) against ras. A
telomerase response in group C was noted in 2/5 (40%) patients and
a ras response in 1/5 (20%) patients. In group D, a telomerase
response was recorded in 2/4 (50%) patients and a ras response in
1/4 (25%) patients.
A sustained induced immune response (an immune
response at at least two different time points) was only seen in
group A patients, in one patient against telomerase and in two
patients against ras.
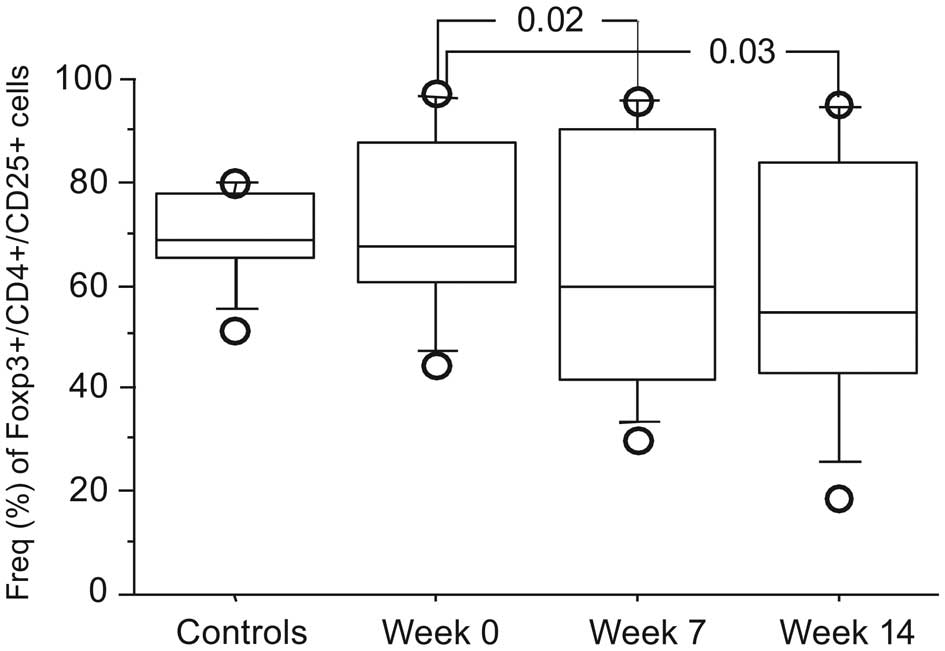
Lymphocyte subsets
There was no significant difference in
Treg cells at baseline comparing patients with healthy
controls (n=9) (data not shown). A significant decrease in the
frequency of Treg
(CD4+CD25+Foxp3+) cells was noted
for patients in groups A and B over time (Fig. 4), but no significant change in
Treg cells in groups C and D patients (data not shown).
No change over time was seen in CD4+ or CD8+
T cells or in NK cells in any of the groups (data not shown).
Adverse events
Immunization was done on an out-patient basis. AEs
associated with GV1001 and GM-CSF was generally mild. No grades 4/5
AEs were seen. The most common AEs considered to be related to
immunization are presented in Table
III and were most commonly noted during the first 6 weeks (data
not shown). Injection site reactions and influenza-like symptoms
were most pronounced in group B. In group B, one injection of
GM-CSF in two patients and two injections in two patients were
omitted due to a strong skin reaction as well as one injection in
one patient in group C.
 | Table IIIFrequency of adverse events (AE)
related to GV1001 and GM-CSF. Highest grade (1–5)a reported once for each
patient. |
Table III
Frequency of adverse events (AE)
related to GV1001 and GM-CSF. Highest grade (1–5)a reported once for each
patient.
| Group A (n=6) | Group B (n=6) | Group C (n=5) | Total (n=17) |
|---|
|
|
|
|
|
|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | Grades 1–3 |
|---|
| Adverse events
(AE) | no. of pts (%) | no. of pts (%) | no. of pts (%) | no. of pts (%) | no. of pts (%) | no. of pts (%) | no. of pts (%) | no. of pts (%) | no. of pts (%) | no. of pts (%) |
|---|
| Local AE | | | | | | | | | | |
| Injection site
reaction | | | | 3 (50) | | 3 (50) | 4 (80) | | | 10 (59) |
| Urticaria
local | | | | | 1 (17) | | | | | 1 (6) |
| Pruritus | | | | | 1 (17) | | | | | 1 (6) |
| Systemic AE | | | | | | | | | | |
| Fatigue | 1 (17) | 1 (17) | | 1 (17) | 4 (67) | | 1 (20) | | 1 (20) | 9 (53) |
| Chills | 1 (17) | | | 4 (67) | | | 1 (20) | | | 6 (35) |
| Fever | 1 (17) | | 1 (17) | 1 (17) | 1 (17) | | 2 (40) | | | 6 (35) |
| Leg pain | | | | 1 (17) | 1 (17) | | | | | 2 (12) |
| Myalgia | | | | 1 (17) | 1 (17) | | | | | 2 (12) |
| Elevated liver
enzymes | | | | | | | | | 2 (40) | 2 (12) |
| Urticaria | | | | 1 (17) | | | | | | 1 (6) |
| Abdominal pain | | | | | | | | 1 (20) | | 1 (6) |
Side-effects related to gemcitabine were as expected
with predominantly hematological and infectious grade 1–4 AEs. The
most frequent grade 3 (n=3) or grade 4 (n=5) AE was neutropenia.
Grade 3 infections were noted in two patients and trombocytopenia
grade 3 and 4 in one patient, respectively. Four patients,
respectively, had a grade 3 anorexia, nausea, fever or liver
abscess (data not shown).
In total, 32 serious adverse events (SAE) were
reported. In group A, 2 SAE in 2 patients; in group B, 6 SAE in 5
patients; in group C, 12 SAE in 4 patients; and in group D, 12 SAE
in 2 patients. The majority was disease or gemcitabine related. One
SAE was initially suspected to be related to GV1001 or GM-CSF in a
patient in group C, who developed hepatic dysfunction (grade 3).
Immunization was stopped. Liver metastasis obstructing the bile
ducts was later diagnosed and considered to be responsible for the
reported SAE.
Clinical efficacy
None of the vaccinated patients achieved CR or PR.
In group A (n=6) four patients had SD and two patients PD at week 8
after start of treatment. A decline of >50% in CA 19-9 was
observed in one patient in group A with a radiological SD (pat. no.
7). All patients in group B had SD at week 8 after start of
treatment. One patient in group C who received only vaccination had
SD for 24 weeks. The remaining patients in group C progressed
rapidly. In group D, the four patients had SD for 31–40 weeks. In
patient no. 25 gemcitabine was switched to 5-Fu/Oxa at week 12 due
to the patient’s own decision. There was no sign of progression
until week 40.
Median time to progression (TTP) in group A was 22
weeks (range, 8–43), in group B 27 weeks (range, 10–104), in group
C 8 weeks (range, 8–24) and in group D 32 weeks (range, 31–40).
Median OS from start of study treatment to death was 35 weeks in
group A (range, 18–73), 39 weeks in group B (range, 12–166), 22
weeks in group C (range, 13–38) and 39 weeks (range, 33–70) in
group D (Table I).
Discussion
The combination of GV1001, gemcitabine and GM-CSF
appeared to be safe and well tolerated. Vaccine related AEs were
mild and transient. A higher dose of GM-CSF induced a higher
frequency and severity of injection site reactions. Gemcitabine
related side-effects were as expected (17) and without overlapping toxicity with
the vaccine treatment.
Similar criteria for mounting a single time point
induced immune response was applied as in the study of Bernhardt
et al (12) immunizing
non-resectable pancreatic carcinoma patients with GV1001 and GM-CSF
but without gemcitabine where 75% of the patients mounted an immune
response. In groups A and B, differing only in the dose of GM-CSF,
a total of 67% of the patients showed an induced telomerase
response. The results might indicate that concomitant treatment
with gemcitabine may not hamper the induction of an immune response
but delayed administration of gemcitabine might reduce the capacity
to mount an immune response and may favor tumor progression (group
C). The study might also support the notion that multiple immune
read-out systems may increase the sensitivity to detect antigen
specific immune responses (18,19).
In the cytokine secretion assay, IFN-γ and TNF-α
were the most prevalent cytokines in post-vaccination T cell
cultures, indicating the induction of a Th1-like response. Vetsika
et al (20), also noted a
TERT-specific Th1 skewed response in 69% of TERT vaccinated NSCLC
patients. A Th-2-like response could not be detected in our
study.
GV1001 is capable of binding to molecules encoded by
multiple alleles of all the three loci of HLA class II (11) and also to be endogenously processed
in APC of the skin (21) and as
such able to induce CD4+ and CD8+ T cells
(22). These characteristics of
the hTERT peptide might enable all patients, irrespective of
HLA-type, to present one or more GV1001 epitopes to immune effector
cells. Moreover, cytokine secretion of activated CD4 T-cells may
stimulate CD8 and NK cells to increased infiltration in the tumor
as well as upregulation of MHC class I molecules, which might be
downregulated in advanced cancer (4,23).
Such an orchestration of cellular immune responses should be of
therapeutic advantage.
In advanced colorectal cancer patients vaccinated
with a multipeptide cancer vaccine containing both class I and II
peptides, a class I response was noted in 43% of the patients, and
a class II response in 65%. A total of 34% of the patients had both
a class I and II response which was associated with a significantly
higher disease control rate (24).
To improve the efficacy of cancer vaccines,
adjuvants should be added. GM-CSF may be an appropriate choice. The
dose and schedule of GM-CSF is however not clearly established. In
a study by Faries et al (25), patients with resected melanoma
received a melanoma vaccine with a high dose of GM-CSF (>300–400
μg/d for 5 days) compared to no GM-CSF. An enhanced
antigen-specific response was seen in the GM-CSF group. However,
early death and a trend towards worse survival was noted in the
GM-CSF group. Slingluff et al (26) studied the effect of a low dose of
GM-CSF (<20 μg/d once a week) together with a melanoma vaccine.
There was a significantly lower rate of CD8+ T-cell
responses in the GM-CSF group (34%) compared to the control group
(73%). We have previously analyzed the humoral and T-cell responses
in CRC patients vaccinated with a recombinant CEA with or without
concomitant GM-CSF (80 μg/d) for four consecutive days. GM-CSF
significantly increased the humoral anti-CEA response as well as
the T-cell response (27–29). In follicular lymphoma patients, a
customized idiotype vaccine together with GM-CSF induced an
anti-idiotypic T-cell response. The dose of GM-CSF was important. A
total of 50,000 units of GM-CSF were less effective than 10,000
units (30). Parmiani et al
(31) showed that relatively low
doses of GM-CSF (40–80 μg for 1–5 days together with a cancer
vaccine) augmented a tumor specific immune response, while higher
doses (100–500 μg) had no advantage, or even induced immune
suppression. Doses exceeding 80 μg/day for more than 3–5 days have
been shown to induce mobilization of myeloid-derived suppressor
cells (32–34), which might inhibit tumor-specific
and non-specific T-cell responses (35). In the present study, the different
doses and schedules of GM-CSF did not permit firm conclusions due
to the small number of patients but only in the low dose of GM-CSF
group a sustained induced immune response was seen.
Chemotherapeutics may also augment a tumor vaccine
specific cellular response by several mechanisms. The T cell
repertoire might be skewed towards the tumor antigen during
recovery from chemotherapy induced lymphopenia (36). Chemotherapeutics may augment a T
cell response by reducing the number of Treg cells
(37). Gemcitabine has previously
been shown to reduce the frequency of Treg in man
(6) and in an animal model to act
synergistically with a tumor-vaccine to improve the therapeutic
antitumor effect (5,38). The limited number of patient
receiving gemcitabine only did not permit conclusions but in
patients receiving gemcitabine and GV1001 concomitantly (groups A
and B) a significant reduction in the number of Treg
cells was seen. Antigen presenting cells may cross-present tumor
antigens induced by chemotherapy-mediated tumor lysis (36). We also observed induction of
reactivity against a ras derived peptide. Epitope spreading could
be due to tumor lysis induced by gemcitabine or by the vaccine as
HER2 alone vaccinated breast cancer patients also showed antigenic
spreading which was an independent predictor of survival (39).
Treatment with GV1001, GM-CSF and gemcitabine seemed
to be safe. An immune response against telomerase in the best
schedule was noted in approximately two thirds of the patients,
similar to other studies but the immune response was weak and
transient. It should be noted that the patients did not seem to be
immune hyporesponsive as evaluated by PHA and PPD responses.
A multicenter study (Primovax) (http://www.clinicaltrials.gov/ct2/results?term=primovax&Search=Search)
in advanced pancreatic cancer was early closed due to lack of
effect. In another phase III study (Telovac study) chemotherapy ±
GV1001 vaccination could not show a significant difference in
overall survival (40). Based on
the experience of the present study and those of others including
immune responses and clinical efficacy, measures have to be taken
to augment the magnitude and duration of the immune response to
GV1001. Maybe, the GV1001 vaccine is not an optimal telomerase
vaccine candidate, although it has been shown that immune
responders to GV1001 vaccination survived longer than non-immune
responders (12) and CLL patients
exhibited spontaneously T cells recognizing GV1001, which T cells
could lyse autologous telomerase expressing leukemic cells
(41). Treatment strategies to
downregulate immune suppression as anti-CTLA-4 or anti-PD1
monoclonal antibodies might be of importance to add (42,43).
Advanced pancreatic carcinoma patients may not be a preferred
clinical setting for vaccine treatment, as is the case for other
TCVs in several other advanced tumors (44) but the combination of gemcitabine
and a cancer vaccine may be a beneficial approach as shown in a
pancreatic carcinoma animal model (45).
Acknowledgements
This study was supported by the Swedish Cancer
Society, the Cancer Society in Stockholm, the Cancer and Allergy
Foundation, the King Gustav V Jubilee Fund, The Torsten and Ragnar
Söderberg Foundation, The Karolinska Institute Foundation and the
Stockholm County Council. The skillful technical assistance of Lena
Virving, Ann Svensson, Barbro Näsman-Glaser, and Ingrid Eriksson is
highly appreciated. We thank Ms. Leila Relander for excellent
secretarial help. H.M. is an advisor of KAEL-Gemvax. The other
authors have no conflict of interest to disclose.
References
|
1
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar
|
|
2
|
Oettle H, Post S, Neuhaus P, et al:
Adjuvant chemotherapy with gemcitabine vs observation in patients
undergoing curative-intent resection of pancreatic cancer: a
randomized controlled trial. JAMA. 297:267–277. 2007. View Article : Google Scholar
|
|
3
|
Diaz-Rubio E: New chemotherapeutic
advances in pancreatic, colorectal, and gastric cancers.
Oncologist. 9:282–294. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shaw VE, Naisbitt DJ, Costello E, et al:
Current status of GV1001 and other telomerase vaccination
strategies in the treatment of cancer. Expert Rev Vaccines.
9:1007–1016. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nowak AK, Lake RA, Marzo AL, et al:
Induction of tumor cell apoptosis in vivo increases tumor antigen
cross-presentation, cross-priming rather than cross-tolerizing host
tumor-specific CD8 T cells. J Immunol. 170:4905–4913. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Correale P, Cusi MG, Tsang KY, et al:
Chemo-immunotherapy of metastatic colorectal carcinoma with
gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte
macrophage colony-stimulating factor and interleukin-2 induces
strong immunologic and antitumor activity in metastatic colon
cancer patients. J Clin Oncol. 23:8950–8958. 2005.
|
|
7
|
Tseng JF, Willett CG, Fernandez-del
Castillo C, et al: Patients undergoing treatment for pancreatic
adenocarcinoma can mount an effective immune response to
vaccinations. Pancreatology. 5:67–74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Plate JM, Plate AE, Shott S, Bograd S and
Harris JE: Effect of gemcitabine on immune cells in subjects with
adenocarcinoma of the pancreas. Cancer Immunol Immunother.
54:915–925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hiyama E, Kodama T, Shinbara K, et al:
Telomerase activity is detected in pancreatic cancer but not in
benign tumors. Cancer Res. 57:326–331. 1997.PubMed/NCBI
|
|
10
|
Kyte JA, Gaudernack G, Dueland S, Trachsel
S, Julsrud L and Aamdal S: Telomerase peptide vaccination combined
with temozolomide: a clinical trial in stage IV melanoma patients.
Clin Cancer Res. 17:4568–4580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brunsvig PF, Aamdal S, Gjertsen MK, et al:
Telomerase peptide vaccination: a phase I/II study in patients with
non-small cell lung cancer. Cancer Immunol Immunother.
55:1553–1564. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bernhardt SL, Gjertsen MK, Trachsel S, et
al: Telomerase peptide vaccination of patients with non-resectable
pancreatic cancer: A dose escalating phase I/II study. Br J Cancer.
95:1474–1482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Almoguera C, Shibata D, Forrester K,
Martin J, Arnheim N and Perucho M: Most human carcinomas of the
exocrine pancreas contain mutant c-K-ras genes. Cell. 53:549–554.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ullenhag GJ, Frodin JE, Mosolits S, et al:
Immunization of colorectal carcinoma patients with a recombinant
canarypox virus expressing the tumor antigen Ep-CAM/KSA (ALVAC-KSA)
and granulocyte macrophage colony- stimulating factor induced a
tumor-specific cellular immune response. Clin Cancer Res.
9:2447–2456. 2003.
|
|
15
|
Mosolits S, Markovic K, Frodin JE, et al:
Vaccination with Ep-CAM protein or anti-idiotypic antibody induces
Th1-biased response against MHC class I- and II-restricted Ep-CAM
epitopes in colorectal carcinoma patients. Clin Cancer Res.
10:5391–5402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mozaffari F, Hansson L, Kiaii S, et al:
Signalling molecules and cytokine production in T cells of multiple
myeloma-increased abnormalities with advancing stage. Br J
Haematol. 124:315–324. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Herrmann R, Bodoky G, Ruhstaller T, et al:
Gemcitabine plus capecitabine compared with gemcitabine alone in
advanced pancreatic cancer: a randomized, multicenter, phase III
trial of the Swiss Group for Clinical Cancer Research and the
Central European Cooperative Oncology Group. J Clin Oncol.
25:2212–2217. 2007. View Article : Google Scholar
|
|
18
|
Clay TM, Hobeika AC, Mosca PJ, Lyerly HK
and Morse MA: Assays for monitoring cellular immune responses to
active immunotherapy of cancer. Clin Cancer Res. 7:1127–1135.
2001.PubMed/NCBI
|
|
19
|
Abdalla AO, Hansson L, Eriksson I, et al:
Idiotype protein vaccination in combination with adjuvant cytokines
in patients with multiple myeloma - evaluation of T-cell responses
by different read-out systems. Haematologica. 92:110–114. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vetsika EK, Konsolakis G, Aggouraki D, et
al: Immunological responses in cancer patients after vaccination
with the therapeutic telomerase-specific vaccine Vx-001. Cancer
Immunology Immunother. 61:157–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gunturu KS, Rossi GR and Saif MW:
Immunotherapy updates in pancreatic cancer: are we there yet? Ther
Adv Med Oncol. 5:81–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu MH, Liao ZL, Zhao XY, et al:
hTERT-based therapy: a universal anticancer approach (Review).
Oncol Rep. 28:1945–1952. 2012.PubMed/NCBI
|
|
23
|
Seliger B: Molecular mechanisms of MHC
class I abnormalities and APM components in human tumors. Cancer
Immunol Immunother. 57:1719–1726. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuttruff S: Immune responses and
association with clinical outcome of advanced colorectal cancer
patients treated with the multi-peptide vaccine IMA910. J Clin
Oncol. 30(Suppl): abs 147. 2012.
|
|
25
|
Faries MB, Hsueh EC, Ye X, Hoban M and
Morton DL: Effect of granulocyte/macrophage colony-stimulating
factor on vaccination with an allogeneic whole-cell melanoma
vaccine. Clin Cancer Res. 15:7029–7035. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Slingluff CL Jr, Petroni GR, Olson WC, et
al: Effect of granulocyte/macrophage colony-stimulating factor on
circulating CD8+ and CD4+ T-cell responses to
a multipeptide melanoma vaccine: outcome of a multicenter
randomized trial. Clin Cancer Res. 15:7036–7044. 2009.PubMed/NCBI
|
|
27
|
Ullenhag GJ, Frodin JE, Jeddi-Tehrani M,
et al: Durable carcinoembryonic antigen (CEA)-specific humoral and
cellular immune responses in colorectal carcinoma patients
vaccinated with recombinant CEA and granulocyte/macrophage
colony-stimulating factor. Clin Cancer Res. 10:3273–3281. 2004.
View Article : Google Scholar
|
|
28
|
Ullenhag GJ, Frodin JE, Strigard K,
Mellstedt H and Magnusson CG: Induction of IgG subclass responses
in colorectal carcinoma patients vaccinated with recombinant
carcinoembryonic antigen. Cancer Res. 62:1364–1369. 2002.
|
|
29
|
Staff C, Magnusson CG, Hojjat-Farsangi M,
et al: Induction of IgM, IgA and IgE antibodies in colorectal
cancer patients vaccinated with a recombinant CEA protein. J Clin
Immunol. 32:855–865. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kwak LW, Young HA, Pennington RW and Weeks
SD: Vaccination with syngeneic, lymphoma-derived immunoglobulin
idiotype combined with granulocyte/macrophage colony-stimulating
factor primes mice for a protective T-cell response. Proc Natl Acad
Sci USA. 93:10972–10977. 1996. View Article : Google Scholar
|
|
31
|
Parmiani G, Castelli C, Pilla L, Santinami
M, Colombo MP and Rivoltini L: Opposite immune functions of GM-CSF
administered as vaccine adjuvant in cancer patients. Ann Oncol.
18:226–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dillman RO, Wiemann M, Nayak SK, DeLeon C,
Hood K and DePriest C: Interferon-gamma or granulocyte-macrophage
colony-stimulating factor administered as adjuvants with a vaccine
of irradiated autologous tumor cells from short-term cell line
cultures: a randomized phase 2 trial of the cancer biotherapy
research group. J Immunother. 26:367–373. 2003. View Article : Google Scholar
|
|
33
|
Marshall JL, Gulley JL, Arlen PM, et al:
Phase I study of sequential vaccinations with
fowlpox-CEA(6D)-TRICOM alone and sequentially with
vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage
colony-stimulating factor, in patients with carcinoembryonic
antigen-expressing carcinomas. J Clin Oncol. 23:720–731. 2005.
View Article : Google Scholar
|
|
34
|
Ramanathan RK, Potter DM, Belani CP, et
al: Randomized trial of influenza vaccine with
granulocyte-macrophage colony-stimulating factor or placebo in
cancer patients. J Clin Oncol. 20:4313–4318. 2002. View Article : Google Scholar
|
|
35
|
Vergati M, Schlom J and Tsang KY: The
consequence of immune suppressive cells in the use of therapeutic
cancer vaccines and their importance in immune monitoring. J Biomed
Biotechnol. 2011:1824132011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Emens LA and Jaffee EM: Leveraging the
activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res.
65:8059–8064. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang G, Sun Y, Ji C, et al: Correlation
between the CD4+CD25high regulatory T cells and the
outcome of chemotherapy in advanced esophageal carcinoma.
Hepatogastroenterology. 60:704–708. 2013.
|
|
38
|
Hou JM, Liu JY, Yang L, et al: Combination
of low-dose gemcitabine and recombinant quail vascular endothelial
growth factor receptor-2 as a vaccine induces synergistic antitumor
activities. Oncology. 69:81–87. 2005. View Article : Google Scholar
|
|
39
|
Salazar LG GV, O’Meara M, et al:
Persistent immunity and survival after immunization with a Her2/neu
vaccine. J Clin Oncol. 27(Suppl): abs 15. 2009.
|
|
40
|
Middleton GW, Valle JW, Wadsley J, et al:
A phase III randomized trial of chemoimmunotherapy comprising
gemcitabine and capecitabine with or without telomerase vaccine
GV1001 in patients with locally advanced or metastatic pancreatic
cancer. J Clin Oncol. 31(Suppl): abs LBA4004. 2013.
|
|
41
|
Kokhaei P, Palma M, Hansson L, Osterborg
A, Mellstedt H and Choudhury A: Telomerase (hTERT 611–626) serves
as a tumor antigen in B-cell chronic lymphocytic leukemia and
generates spontaneously antileukemic, cytotoxic T cells. Exp
Hematol. 35:297–304. 2007.
|
|
42
|
Hodi FS, O’Day SJ, McDermott DF, et al:
Improved survival with ipilimumab in patients with metastatic
melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar
|
|
43
|
Topalian SL, Weiner GJ and Pardoll DM:
Cancer immunotherapy comes of age. J Clin Oncol. 29:4828–4836.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hale DF, Clifton GT, Sears AK, et al:
Cancer vaccines: should we be targeting patients with less
aggressive disease? Expert Rev Vaccines. 11:721–731. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bauer C, Sterzik A, Bauernfeind F, et al:
Concomitant gemcitabine therapy negatively affects DC
vaccine-induced CD8(+) T-cell and B-cell responses but improves
clinical efficacy in a murine pancreatic carcinoma model. Cancer
Immunol Immunother. 63:321–333. 2014. View Article : Google Scholar
|