Introduction
Pancreatic cancer is a lethal disease with only a 6%
of overall 5-year survival rate. Surgical resection remains the
only cure option, which improves the 5-year survival rate to 20%;
however, frequent recurrence is recorded after surgery (1). Most pancreatic cancer patients are
diagnosed at advanced stages of the disease, making curable surgery
impossible. During disease progression, the blood supply is
necessary for tumor growth, invasion and metastasis (2,3),
thus, neoangiogenesis is the key for cancer development and
progression. It was thought that formation of new blood vessels in
tumor lesions depends on vascular endothelial cells. However, in
1999, Maniotis et al (4)
reported that there was a ring-shaped loop interconnecting network
from extracellular matrix and melanoma cells to facilitate
neoangiogenesis in skin or liver metastasis. Under a scanning
electron microscope, red cells were observed in this network. Both
indocyanine green angiography and in vitro microinjection
demonstrated that the networks are similar to the artery with
vascular lumen tissue perfusion effects. This novel network, which
is independent from endothelial cells, was referred to as
vasculogenic mimicry (VM). The level of VM was associated with poor
prognosis of patients (4). As a
part of the classic tumor vascular endothelium-dependent
complement, VM may provide a reasonable explanation of ineffective
anti-angiogenesis therapy for cancer patients. VM has been observed
in several other aggressive tumor types, such as laryngeal squamous
cell carcinoma, ovarian cancer, breast cancer, osteosarcoma,
astrocytoma and gallbladder cancer (5–12).
Most recent studies have shown that vascular endothelial-cadherin
(VE-cadherin), epithelial cell kinase (EphA2), and matrix
metalloproteinase (MMPs) play a crucial role in VM formation
(13–21). Thus, regulation of VM formation
could be a novel cancer therapy strategy against human cancers,
including pancreatic cancer.
Ginseng is an oriental medicine used for thousand
years and possesses immunomodulatory, ‘qi’ and anti-aging effects
(22). Ginsenoside Rg3 (Rg3) is a
trace tetracyclic triterpenoid saponin extracted from ginseng and
can induce tumor cell apoptosis, but inhibits tumor cell
proliferation, adhesion, invasion and metastasis as well as tumor
angiogenesis (23–29). Rg3 adjuvant therapy synergies the
effects of chemotherapy drugs and enhances host immune function
(23–29). Since the last decade,
anti-angiogenesis therapy has been widely accepted as a means for
tumor therapy, mainly to control the growth of vascular endothelial
cells. However, in recent studies (30,31),
anti-angiogenesis therapy using angiostatin or endostatin to target
endothelial cells showed to have little effect on regulating the
progression of tumors with VM formation. This may be because VM
does not involve endothelial cells, and thus does not respond to
anti-angiogenesis therapy (30,31).
Moreover, van der Schaft et al (32) reported that Anginex, TNP-470, and
endostatin inhibit growth of vascular endothelial cells, but did
not prevent melanoma cells to form VM. Further research on VM
inhibition could yield a better antitumor activity (33). Indeed, Wang et al (34) demonstrated that Rg3 could inhibit
tube-like structure formation in a human nasopharyngeal carcinoma
cell line in vitro.
In this study, we assessed VM formation in
pancreatic cancer tissues ex vivo and then investigated
correlations between the expression of VE-cadherin, EphA2 and MMP
protein and VM formation. In addition, we explored the effects of
Rg3 on the regulation of VM formation in vitro and in
vivo nude mouse xenografts.
Materials and methods
Patients and tissue specimens
A total of 117 patients with pancreatic cancer and
62 patients with benign pancreatic disease were recruited from The
Second Affiliated Hospital, Wenzhou Medical University (Wenzhou,
China) and First Affiliated Hospital, Zhejiang University School of
Medicine, (Hangzhou, China) between 2007, and 2012. Our
institutional review board approved this study and a written
informed consent form was obtained from each patient. All patients
were diagnosed histologically and confirmed by an experienced
pathologist. Paraffin-embedded tissue specimens were retrieved from
the Pathology Department for immunohistochemistry and PAS
staining.
Immunohistochemistry
Paraffin sections (4-μm thick) of pancreatic tissue
specimens were prepared for immunohistochemistry. Briefly, the
sections were heated in an oven at 65°C for 60 min and then
deparaffinized in xylene and rehydrated in series of ethanol. The
sections were then subjected to high boiling antigen retrieval in a
pressure cooker and washed with phosphate-buffered saline (PBS) 3
times, 5 min each. Next, the sections were treated with 3% hydrogen
peroxide for 20 min at room temperature to inactivate peroxidase
and then rinsed with PBS and blocked subsequently with 5% normal
goat serum. Next, the sections were incubated with the primary
antibody (i.e., the anti-CD31 at a dilution of 1:100,
anti-VE-cadherin at a dilution of 1:100, anti-EphA2 at a dilution
of 1:50, anti-MMP-2 at a dilution of 1:100, or anti-MMP-9 at a
dilution of 1:200) in a moist chamber overnight at 4°C. A mouse
monoclonal anti-CD31 antibody was purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA), mouse anti-MMP-2 and rabbit
anti-VE-cadherin antibodies were purchased from Abgent (San Diego,
CA, USA), a mouse anti-EphA2 was purchased from R&D Systems
(Boston, MA, USA), and a rabbit anti-MMP-9 was obtained from Abcam
(Cambridge, MA, USA). The next day, the sections were rinsed with
PBS for three times and further incubated with a horseradish
peroxidase (hRP)-conjugated secondary antibody (Beyotime
Biotechnology, Haimen, China) at room temperature for 30 min. Then,
peroxidase labeling was developed by incubating the sections with
diaminobenzidine tetrahydrochloride (DAB) solution for 3 min,
counterstained with hematoxylin, and then mounted and evaluated
under a light microscope (Olympus BX51, Japan). Negative control
sections were incubated with PBS instead of the specific primary
antibody.
CD31 and PAS double-staining
Sections were first stained for CD31
immunohistochemistry and then stained with 0.5%
periodic-acid-Schiff (PAS) solution for 10 min and rinsed with
distilled water for 2–3 min. In a dark chamber, these sections were
further stain treated with Schiff solution for 15 min and then
rinsed with distilled water, dehydrated and mounted. Normal
pancreatic tissues were used as a positive control. CD31 staining
was used to visualize blood vessels, helping to distinguish the
PAS-positive network of VM from endothelium-lined microvessel. PAS
staining was used to identify matrix-associated vascular channels
in pancreatic cancer tissues. Levels of VM were quantified
according to a previous study (35). Specifically, the stained sections
were scored under a microscope for 10 randomly chosen fields at
×400. The vessels lined by endothelial cells, regardless of the
presence of basement membrane, were counted as
endothelium-dependent vessels. In contrast VM was defined as
enclosed pancreatic cancer cells with PAS-positive material. The
average number of VM channels was determined for each section.
Cell line and culture
Human pancreatic cancer cell lines (PANC-1 and
SW1990) were obtained from Shanghai Cell Bank (Shanghai, China).
Human pancreatic cancer cell lines (Bxpc-3 and MiaPaCa-2) were
obtained from American Type Culture Collection (Manassas, VA, USA).
All the cell lines were cultured in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100
U/ml penicillin, and 100 μg/ml streptomycin (all from
Gibco-BRL/Invitrogen, Grand Island, NY, USA) at 37°C in a
humidified incubator with 5% CO2. Cells were passaged at
70–80% confluence. For Rg3 treatment, ginsenoside Rg3 standard with
a purity ≥98% was purchased from Shanghai Bo Yun Biotechnology
(Shanghai, China) and dissolved in dimethylsulfoxide (DMSO,
Invitrogen, Carlsbad, CA, USA) at the concentration of 200×10
μmol/l. The solution was then diluted with DMEM to the desired
concentration before use. The cells were grown overnight and then
treated with Rg3 at different concentrations, while the medium
containing 0.1% DMSO served as a negative control.
Tumor cell three-dimension culture and
PAS staining
Three-dimensional type I collagen gels were prepared
as described previously (19). A
total of 25 μl of rat-tail type I collagen (average 3 mg/ml; from
BD Biosciences, Bedford, MA, USA) were dropped onto 18-mm glass
coverslips in 12-well culture plates and polymerized 5 min at room
temperature. After washing with PBS for 5 min, 5×105
tumor cells were seeded onto the three-dimensional type I collagen
gel and treated with Rg3 at 0, 25, 50, 100 and 200 μmol/l for 72 h
to analyze the ability of tumor cells to form VM. At the end of the
experiments, the cells were fixed with 4% formaldehyde in PBS for
10 min and washed with PBS. The cells were then stained with
PAS.
Animal experiments
A protocol of animal experiments was approved by
Wenzhou Medical University Experimental Animal Center (Wenzhou,
China). Briefly, 28 six-week old, male, athymic, BaLB/c nu/nu mice
were purchased from the Shanghai Cancer Institute (Shanghai, China)
and were maintained in a specific-pathogen-free environment in our
animal center. The housing temperature was maintained at 25±1°C and
relative humidity was controlled at 40–60%. SW-1990 cells in the
log-growth phase were detached with 0.05% trypsin and re-suspended
with serum-free culture medium. The cells were then subcutaneously
injected into the right flank with 5×106 SW-1990 cells
per injection (36). Three days
later, the mice were randomly assigned into control and ginsenoside
Rg3 groups. The control mice (n=7) were treated by intraperitoneal
injection with 0.9% sodium chloride once every other day and three
groups of ginsenoside Rg3-treated mice (n=7, each group) were
intraperitoneally injected with 5, 10 or 20 mg/kg/day ginsenoside,
respectively. The treatment was continued every other day for 28
days. At the end of the experiments, the mice were sacrificed and
tumor xenografts were resected, weighed and then fixed in 10%
neutral buffered formalin and embedded in paraffin.
Paraffin-embedded tissue blocks were cut into 4-μm thick sections
for immunohistochemistry and PAS staining.
RNA isolation and qRT-PCR
Total cellular RNA from cell lines or tissues was
isolated using TRIzol reagent (Invitrogen) according to the
manufacturer’s protocol. RNA was then reverse transcribed into cDNA
using RevertAid First Strand cDNA Synthesis Kit (Fermentas, South
Logan, UT, USA) according to the manufacturer’s instructions. PCR
amplification was performed using gene-specific primers (Table I) in a Roche real-time PCR machine
in a total of 10 μl reaction mixture that contained 1 μl cDNA, 5 μl
SYBR-Green real-time PCR master mix-plus (Toyobo, Japan), and 1 μl
primer each. The PCR conditions were set to an initial denaturation
at 95°C for 90 sec and 40 cycles of 95°C for 5 sec, 60°C for 30
sec, and 72°C for 45 sec. GAPDH mRNA was used as a loading control.
The experiments were performed in triplicates and repeated three
times with independently derived samples. The data were analyzed
using LightCycler 480 software (Roche, Switzerland).
 | Table IPrimer sequences and PCR product
size. |
Table I
Primer sequences and PCR product
size.
| Gene | Primers | Size of PCR
products (bp) |
|---|
| VE-cadherin |
5′-aagcgtgagtcgcaa-3′
5′-tctccaggttttcgc-3′ | 179 |
| EphA2 |
5′-gagggcgtcatctccaaata-3′
5′-tcagacaccttgcagaccag-3′ | 236 |
| MMP-2 |
5′-gatacccctttgacggtaagga-3′
5′-ccttctcccaaggtccatagc-3′ | 112 |
| MMP-9 |
5′-ttgacagcgacaagaagtgg-3′
5′-gccattcacgtcgtccttat-3′ | 179 |
| GAPDH |
5′-gagtcaacggatttggtcgt-3′
5′-ttgattttggagggatctcg-3′ | 238 |
Protein extraction and western blot
analysis
Total cellular protein was extracted from cultured
cells or tissue samples using a radioimmunoprecipitation assay
(RIPA) buffer (Pierce, Rockford, IL, USA). After centrifugation at
12,000 × g for 20 min at 4°C, the supernatant was collected and
protein concentration was measured using the BCA Protein Assay Kit
(Pierce) according to the manufacturer’s instructions. Samples
containing 40 μg of protein from cell culture and 60 μg of protein
from tissue samples were subjected to 8% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred electrophoretically on to polyvinylidene fluoride
(PVDF) membranes (Invitrogen). Equal protein loading was confirmed
by Coomassie staining (Bio-Rad, Hercules, CA, USA) of the gel.
After blocking with 5% bovine serum albumin (BSA), the membrane was
incubated with the primary antibodies followed by incubation with
the secondary antibodies. Immunoreactivity was detected using the
Enhanced Chemiluminescence Kit (Pierce) according to the
manufacturer’s instructions. Each experiment was repeated three
times and the data were analyzed using AlphaEaseFC 4.0 software
(San Leandro, CA, USA).
Statistical analysis
Data are summarized as mean ± SD. Statistical
analysis was performed using SPSS 17.0. (SPSS, Chicago, IL, USA)
and differences between ginsenoside Rg3 and DMSO-treated (control)
groups were analyzed with an unpaired Student’s t-test or ANOVA
analysis. Association of clinicopathological data from pancreatic
cancer cases or between groups was analyzed by the χ2
test. p<0.05 was considered statistically significant.
Results
Induction of VM in pancreatic cancer
tissues
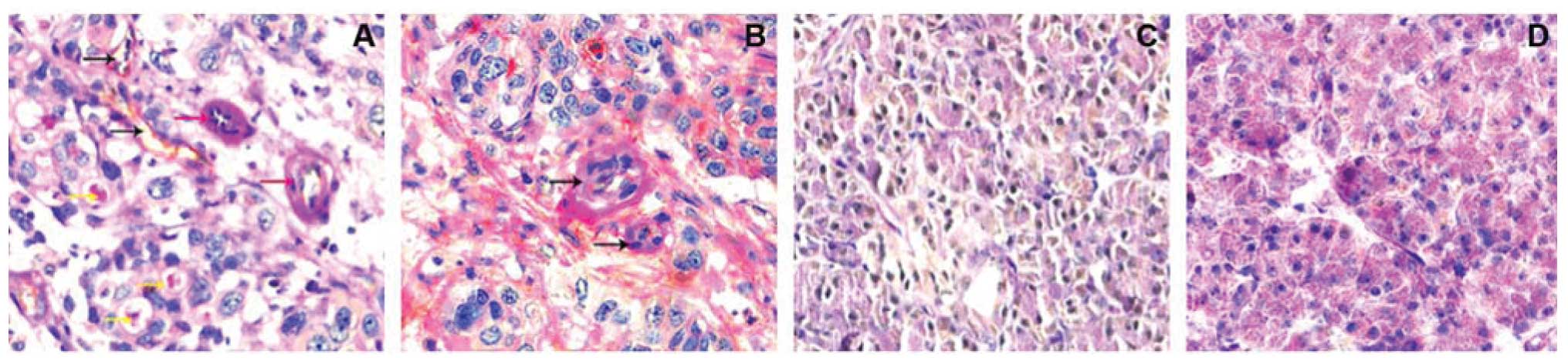
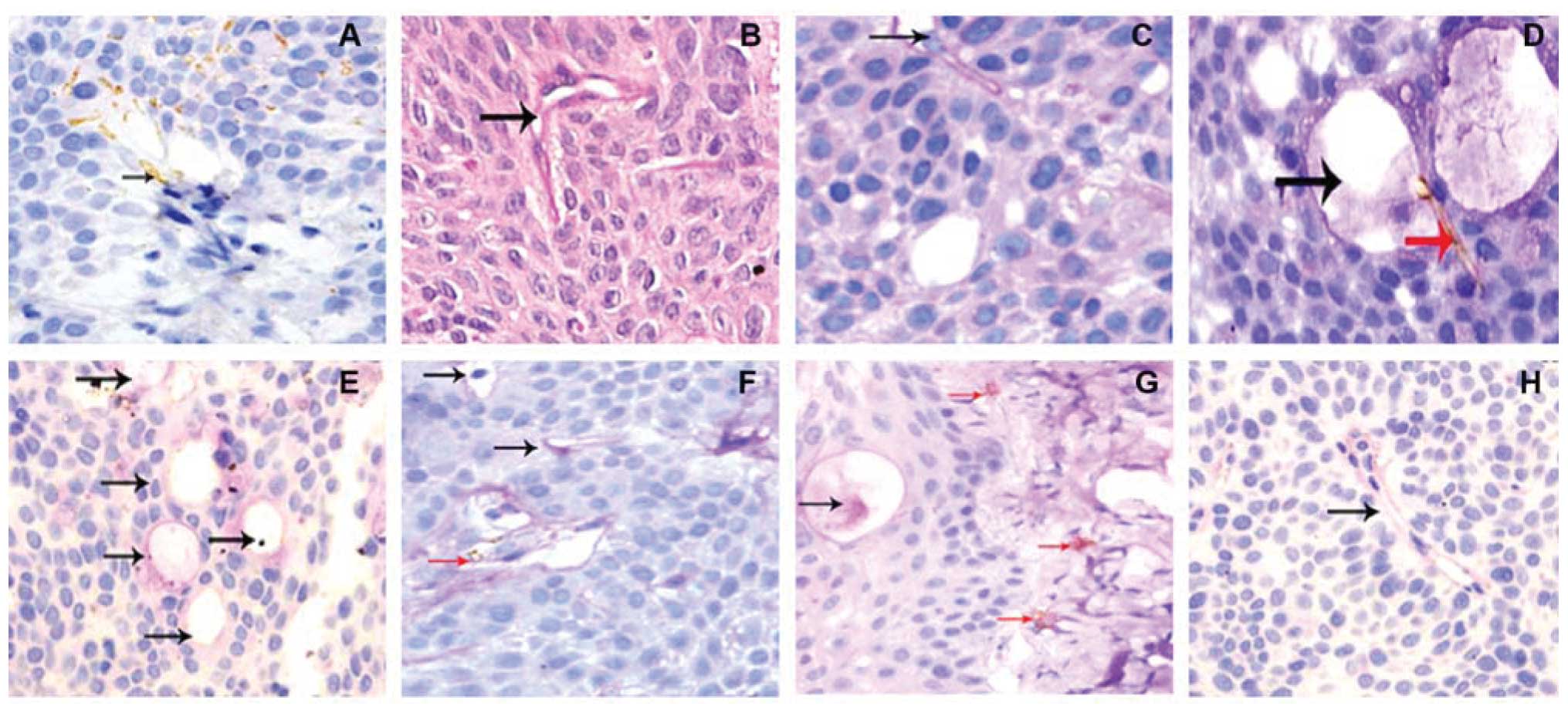
Endothelial structure has stained brown by an
anti-CD31 antibody, while VM pipe and extracellular matrix were
stained red color by PAS staining. Based on CD31 and PAS staining,
CD31-negative, PAS-positive vascular-like structures were VM. In
these 117 cases of pancreatic cancer tissues, VM was shown for
71.79% (84/117) of pancreatic cancer cases, while all 53 benign
pancreatic disease cases had no VM (0%, 0/53) (Fig. 1).
Association of VM with the expression of
VE-cadherin, EphA2, MMP-2 and MMP-9 proteins in pancreatic cancer
tissues
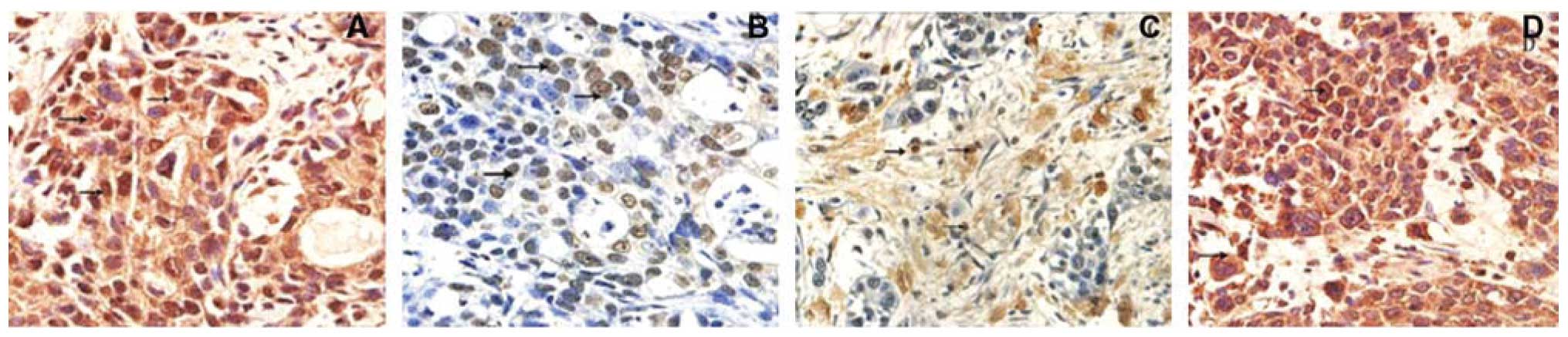
We then assessed the expression of VE-cadherin,
EphA2, MMP-2 and MMP-9 proteins in pancreatic tissues for
association with VM. The data showed that expression of these
proteins was associated with VM formation of pancreatic cancer
tissues compared to those of benign pancreatic tissues (Fig. 2 and Table II).
 | Table IIAssociation of VE-cadherin, EphA2,
MMP-2 and MMP-9 proteins with VM. |
Table II
Association of VE-cadherin, EphA2,
MMP-2 and MMP-9 proteins with VM.
| VM (+) | VM (−) | p-value |
|---|
| VE-cadherin
(+) | 78 | 2 | <0.05 |
| VM (−) | 0 | 4 | |
| EphA2 (+) | 68 | 8 | <0.05 |
| EphA2 (−) | 1 | 7 | |
| MMP-2 (+) | 77 | 4 | <0.05 |
| MMP-2 (−) | 0 | 3 | |
| MMP-9 (+) | 70 | 3 | <0.05 |
| MMP-9 (−) | 3 | 8 | |
Different levels of VM in pancreatic
cancer cell lines
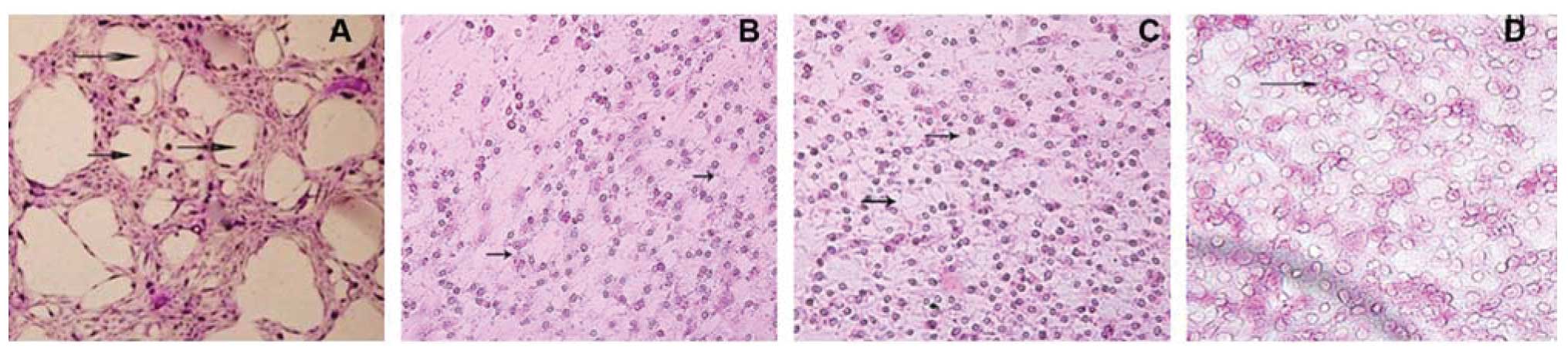
We then detected VM in pancreatic cancer cell lines
using 3D cultures and found that SW-1990 cells formed circular
channel features, while Panc-1, Bxpc-3 and MiaPaCa-2 did not
(Fig. 3).
Effects of ginsenoside Rg3 on the
regulation of VM levels in vitro
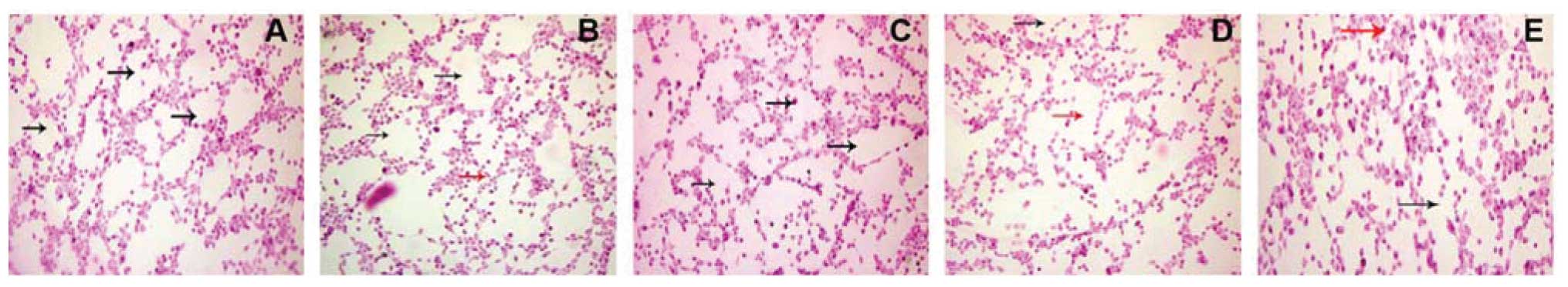
Since SW-1990 cells can form VM in a 3D culture, we
utilized this cell line for further study of the effects of Rg3 on
the regulation of VM formation in vitro. We found that
SW-1990 cells treated with 25 μmol/l ginsenoside Rg3 began to form
irregular VM, while 50 μmol/l concentrations led more SW-1990 cells
to form irregular vascular mimicry. Ginsenoside Rg3 (200 μmol/l)
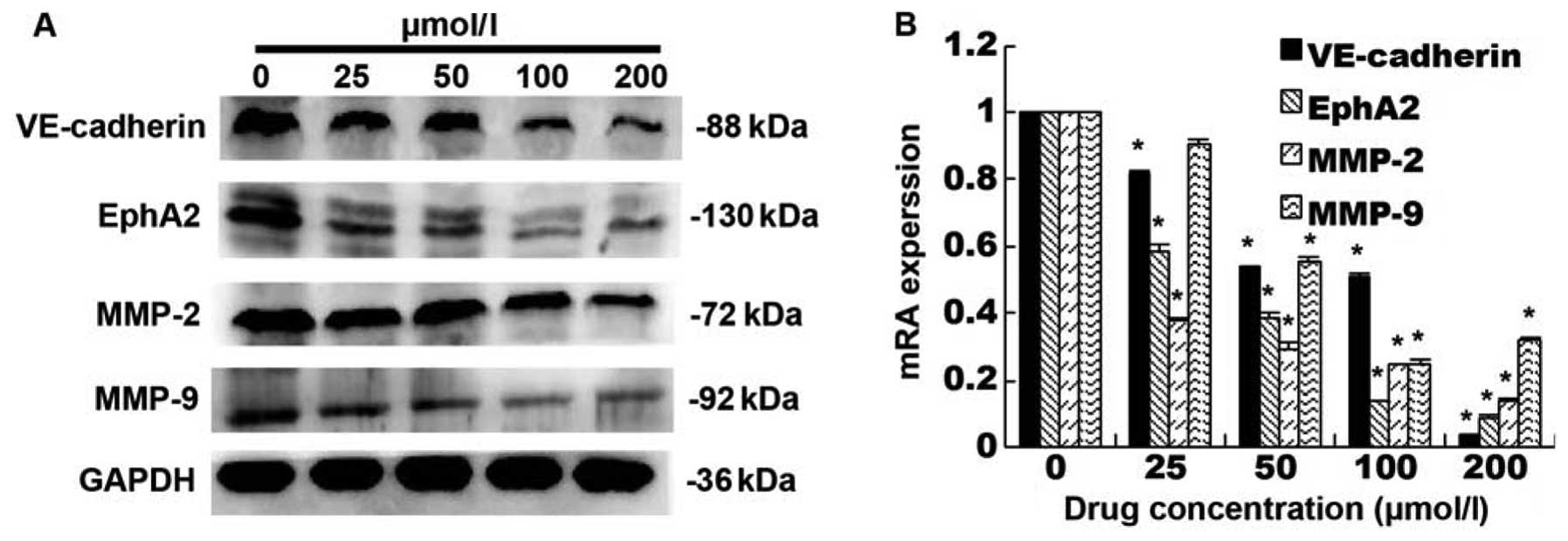
totally inhibited SW-1990 cells to form VM (Fig. 4). We then analyzed the expression
of VE-cadherin, EphA2, MMP-2 and MMP-9 protein and mRNA in SW-1990
cells. We found that ginsenoside Rg3 dose-dependently reduced
expression of these proteins in SW-1990 cells (p<0.05, Fig. 5A) and levels of their mRNA
(Fig. 5B).
Effects of ginsenoside Rg3 on the
regulation of tumor growth and VM formation in vivo
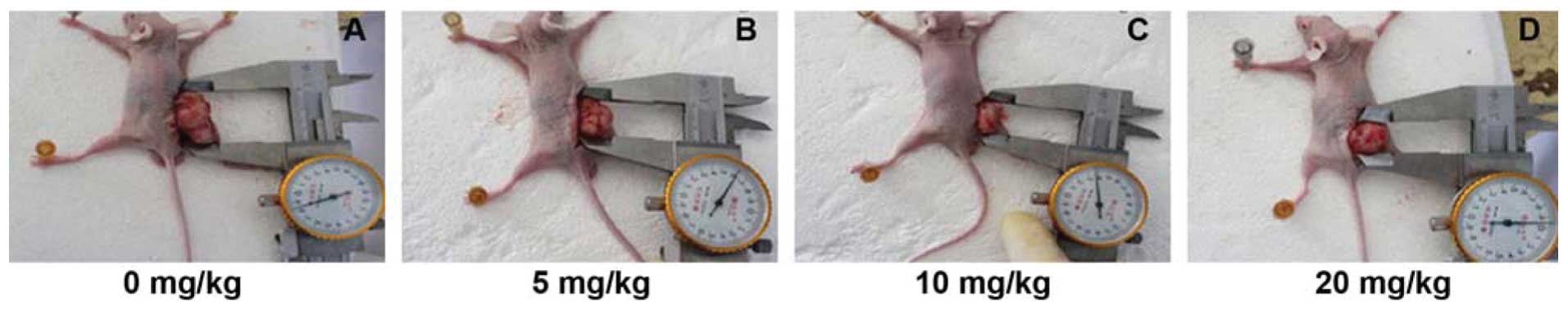
Next, we assessed the effects of Ginsenoside Rg3 on
the regulation of tumor growth and VM formation in vivo in a
nude mouse model. The data showed that Ginsenoside Rg3
dose-dependently suppressed tumor growth in nude mice (Fig. 6 and Table III). Similarly, ginsenoside Rg3
treatment of mice dose-dependently suppressed VM formation
(Fig. 7 and Table IV).
 | Table IIIEffect of ginsenoside Rg3 on
regulation of pancreatic cancer cell xenograft growth in nude
mice. |
Table III
Effect of ginsenoside Rg3 on
regulation of pancreatic cancer cell xenograft growth in nude
mice.
| Treatment | Tumor weight
(g) | Tumor volume
(mm3) |
|---|
| 0 mg/kg | 1.48±0.130 | 662.78±12.91 |
| 5 mg/kg | 1.11±0.455 |
414.64±13.46a |
| 10 mg/kg | 0.95±0.317 |
351.43±20.65a |
| 20 mg/kg | 0.58±0.236a |
300.33±14.71a |
 | Table IVEffects of ginsenoside Rg3 on the
regulation of tumor xenograft VM formation in vivo. |
Table IV
Effects of ginsenoside Rg3 on the
regulation of tumor xenograft VM formation in vivo.
| VM (+) | p-value |
|---|
| 0 mg/kg | 2.3±1.159 | |
| 5 mg/kg | 1.6±0.843 | 0.563 |
| 10 mg/kg | 0.5±0.572 | 0.004 |
| 20 mg/kg | 0.3±0.483 | 0.002 |
Effects of ginsenoside Rg3 on the
regulation of gene expression in tumor xenografts in vivo
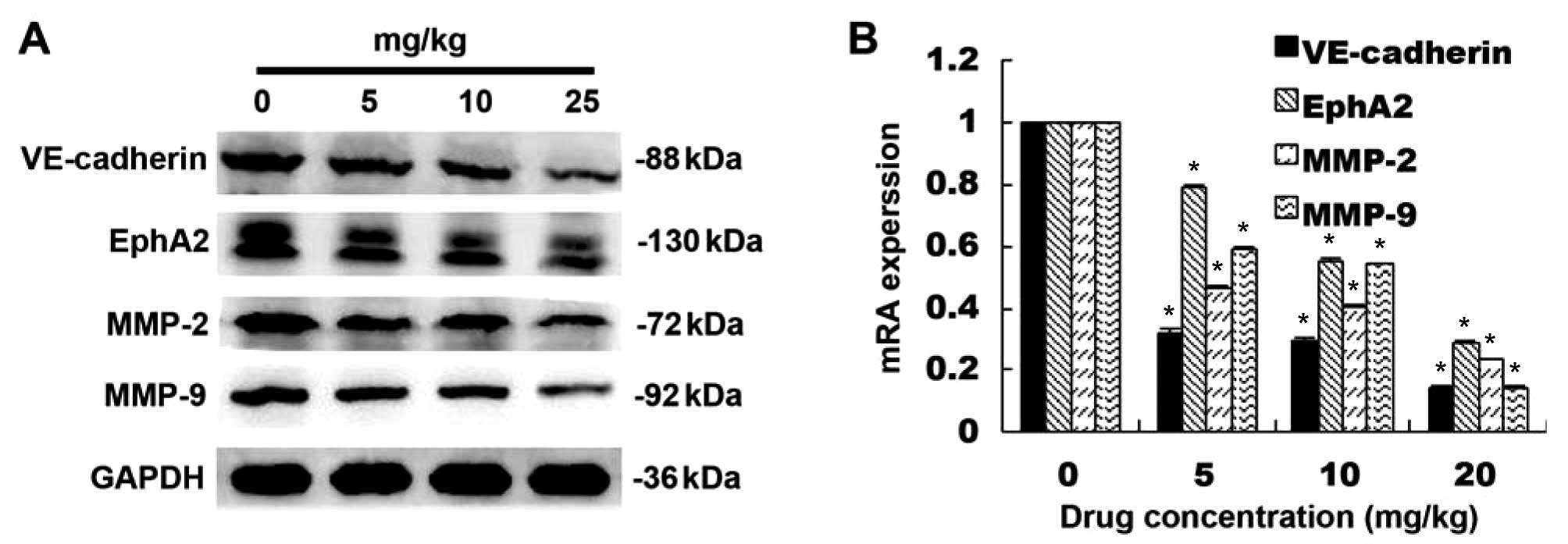
Ginsenoside Rg3 treatment of nude mice also showed a
dose-dependent inhibition of VE-cadherin, EphA2, MMP-2 and MMP-9
proteins (p<0.05; Fig. 8A) and
mRNA in pancreatic cancer cell xenografts (p<0.05; Fig. 8B).
Discussion
VM was first reported by Maniotis et al
(4) in 1999 as a ring-shaped loop
interconnecting network, which is made of extracellular matrix and
melanoma tumor cells. This structure can transport erythrocytes and
plays an important role in tumor progression. As a novel tumor
microcirculation system, VM differs from classically described
endothelium-dependent angiogenesis. In addition, VM has been
observed in several other tumor types, such as laryngeal squamous
cell carcinoma, ovarian cancer, breast cancer, osteosarcoma,
astrocytoma and gallbladder cancer (5–10).
Thus, more recently, VM has been targeted as a novel strategy to
treat solid tumors (32,37). However, not all tumor cells can
form VM. Histologically, VM channels are patterned networks of
interconnected loops of PAS-positive extracellular matrix formed by
highly malignant melanoma cells, but not by endothelia cells
(4). Other studies have
demonstrated that VM levels are associated with a poor prognosis in
certain tumor patients (4,38–40).
In the current study, we confirmed VM in pancreatic cancer tissues
and cell lines, even though we did not provide patient survival
data. In the 117 cases of pancreatic cancer tissues in this study,
VM was shown to be expressed in 71.79% (84/117) of pancreatic
cancer cases.
Moreover, previous studies have shown that VM
formation is associated with the expression of particular genes,
such as VE-cadherin, EphA2, MMP-2 and MMP-9. VE-cadherin belongs to
the cadherin family and is specifically expressed in endothelial
cells. VE-cadherin is a transmembrane protein and functions to
mainly mediate adhesion between cells (41), while EphA2 is a tyrosine kinase
receptor and can regulate angiogenesis. VE-cadherin protein is
highly expressed in high-grade malignant melanoma cells, but is not
expressed in low-grade malignant melanoma cells (41). Inhibition of VE-cadherin expression
using thiosulfate-modified oligonucleotides blocks vasculogenic
mimicry formation in high-grade malignant melanoma (13). Similarly, immunofluorescence
staining showed that the tube-like network channels in vitro
expressed phosphorylated tyrosine kinase and EphA2 proteins,
whereas tyrosine kinase inhibitor and/or knockdown of EphA2
expression suppressed CM formation (15). VE-cadherin co-localizes with EphA2
at areas of cell-cell contact and directly interact during VM
(14). Furthermore, matrix
metalloproteinases are a group of zinc-dependent endopeptidases
that degrade extracellular matrix. Seftor et al (19) reported that the expression of
MMP-2, MMP-9, MMP-14 and tumor cell surface laminin receptor is
significantly increased in high-grade invasive melanoma tissues.
Activated MMP decomposition can cleave laminin into multiple
short-chains, promoting the formation of VM. Sood et al
(21) demonstrated that the
expression of MMP-1, MMP-2, MMP-9, MT1-MMP and laminin is
significantly increased in 3D culture of invasive ovarian cancer
cells. Interestingly, they showed that the metalloproteinase
inhibitor Metastat in the 3D culture could inhibit VM. Transfection
with extracellular matrix metalloproteinase CD147 CDNA into low
invasive ovarian cancer cells leads to the formation of VM in 3D
culture. In addition, MMP-2 and MMP-9 protein levels and their
activity are significantly increased, and this promoted formation
of vasculogenic mimicry (6). Taken
together, these proteins promote VM formation in different tumor
cell lines and inhibition or knockdown of these proteins suppresses
VM formation. Indeed, our current study also confirmed these
studies ex vivo.
Classic tumor angiogenesis theory believes that
tumor lesions greater than 1–2 mm will activate and promote
endothelial cells to build new blood vessels for tumor cell growth.
Thus, tumor growth, invasion, metastasis and recurrence are
dependent on the blood supply (2,3).
Anti-angiogenesis therapy could be a useful treatment strategy for
cancer therapy. The traditional anti-angiogenesis therapies mainly
target vascular endothelial cells. Liu et al (42) showed that melanin anti-angiogenesis
therapy has little effect on a patient’s prognosis. Van der Schaft
et al (32) reported that
angiogenesis inhibitors (Anginex, TNP-470 and endostatin) inhibit
angiogenesis, but cannot prevent melanoma cells forming VM. In this
regard, VM formation may provide a reasonable explanation for
ineffective clinical anti-angiogenesis therapy against human
cancers. In the current study, we assessed ginsenoside Rg3 as an
alternative strategy to inhibit VM formation for adjuvant treatment
of pancreatic cancer. Indeed, previous studies reported by Shin
et al (43) and Xu et
al (44) showed that
Ginsenoside Rg3 was able to inhibit MMP-9 expression in cultured
mammalian and ovarian cancer cells and metastasis of ovarian cancer
cells. Chen et al (45)
revealed that Ginsenoside Rg3 inhibits MMP-2 expression in a human
lung adenocarcinoma cell line. Our current study showed that
Ginsenoside Rg3 treatment reduced tumor xenograft weigh and tumor
size in vivo in nude mice. This was associated with the
inhibition of VM formation and downregulation of VE-cadherin,
EphA2, MMP-9 and MMP-2 expression.
In summary, our current study demonstrated increased
VM formation in pancreatic cancer tissues when compared to benign
pancreatic diseases. VM formation was associated with the
expression of cell adhesion and MMP proteins. Furthermore,
ginsenoside Rg3 effectively inhibited VM formation of pancreatic
cancer cells in vivo and in vitro. At the gene level,
ginsenoside Rg3-inhibited VM formation was associated with the
downregulation of VE-cadherin, EphA2, MMP-9 and MMP-2 protein
expression. Thus, our present study provides preliminary evidence
for the use of Rg3 for the treatment of pancreatic cancer.
Acknowledgements
We would like to thank Dr Liwei Xie and Dr Qiaoqiao
Hua of The Second Affiliated Hospital, Wenzhou Medical University
(Wenzhou, China) and The Pathology Department of First Affiliated
Hospital, Zhejiang University School of Medicine (Hangzhou, China)
for providing help in immunohistochemistry. We are grateful for
funding support from: the Administration of Traditional Chinese
Medicine of Zhengjing Province, China (grant no. 2011ZZ010),
Zhejiang Provincial Science Fund for Distinguished Young Scholars
(grant no. LR12H280001) and the National Natural Science Foundation
of China (grant no. 81173606).
References
|
1
|
Saif M, Lee Y and Kim R: Harnessing
gemcitabine metabolism: a step towards personalized medicine for
pancreatic cancer. Ther Adv Med Oncol. 4:341–346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar
|
|
4
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe’er J, Trent JM, Meltzer PS, et al: Vascular channel
formation by human melanoma cells in vivo and in vitro:
vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang W, Lin P, Han C, Cai W, Zhao X and
Sun B: Vasculogenic mimicry contributes to lymph node metastasis of
laryngeal squamous cell carcinoma. J Exp Clin Cancer Res. 60:2–9.
2010.PubMed/NCBI
|
|
6
|
Millimaggi D, Marl M, D’Ascenzo S, Giusti
I, Pavan A and Dolo V: Vasculogenic mimicry of human ovarian cancer
cells: Role of CDl47. Int J Oncol. 35:1423–1428. 2009.PubMed/NCBI
|
|
7
|
Clemente M, Pérez-Alenza MD, Illera JC,
Illera JC and Peña L: Histological, immunohistological, and
ultrastructural description of vasculogenic mimicry in canine
mammary cancer. Vet Pathol. 47:265–274. 2010. View Article : Google Scholar
|
|
8
|
Cai XS, Jia YW, Jiong M and Tang RY: Tumor
blood vessels formation in osteosarcoma: vasculogenesis mimicry.
Chin J Med. 117:94–98. 2004.PubMed/NCBI
|
|
9
|
Yue WY and Chen ZP: Does vasculogenic
mimicry exist in astrocytoma? J Histochem Cytochem. 539:997–1002.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun W, Fan YZ, Zhang WZ and Ge CY: A pilot
histomorphology and hemodynamic of vasculogenic mimicry in
gallbladder carcinomas in vivo and in vitro. J Exp Clin Cancer Res.
46:2–11. 2011.
|
|
11
|
Yue WY and Chen ZP: Vasculogenic mimicry -
potential target for tumor therapy. Ai Zheng. 25:914–916. 2006.(In
Chinese).
|
|
12
|
Shirakawa K, Kobayashi H, Heike Y,
Kawamoto S, Brechbiel MW, Kasumi F, Iwanaga T, et al: Hemodynamics
in vasculogenic mimicry and angiogenesis of inflammatory breast
cancer xenograft. Cancer Res. 62:560–566. 2002.PubMed/NCBI
|
|
13
|
Hendrix MJ, Seftor EA, Meltzer PS, Gardner
LM, Hess AR, Kirschmann DA, Schatteman GC, et al: Expression and
functional signiicance of VE-cadherin in aggressive human melanoma
cells: role in vasculogenic mimicry. Proc Natl Acad Sci USA.
98:8018–8023. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hess AR, Seftor EA, Gruman LM, Kinch MS,
Seftor RE and Hendrix MJ: VE-cadherin regulates EphA2 in aggressive
melanoma cells through a novel signaling pathway: implications for
vasculogenic mimicry. Cancer Biol Ther. 5:228–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hess AR, Seftor EA, Gardner LM,
Carles-Kinch K, Schneider GB, Seftor RE, Kinch MS, et al: Molecular
regulation of tumor cell vasculogenic mimicry by tyrosine
phosphorylation: role of epithelial cell kinase (Eck/EphA2). Cancer
Res. 61:3250–3255. 2001.PubMed/NCBI
|
|
16
|
Margaryan NV, Strizzi L, Abbott DE, Seftor
EA, Rao MS, Hendrix MJ and Hess AR: EphA2 as a promoter of melanoma
tumorigenicity. Cancer Biol Ther. 8:279–288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hess AR, Margaryan NV, Seftor EA and
Hendrix MJ: Deciphering the signaling events that promote melanoma
tumor cell vasculogenic mimicry and their link to embryonic
vasculogenesis: role of the Eph receptors. Dev Dyn. 236:3283–3296.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seftor RE, Seftor EA, Koshikawa N, Meltzer
PS, Gardner LM, Bilban M, Stetler-Stevenson WG, et al: Cooperative
interactions of laminin 5 gamma 2 chain, matrix
metalloproteinase-2, and membrane type-1-matrix/metalloproteinase
are required for mimicry of embryonic vasculogenesis by aggressive
melanoma. Cancer Res. 61:6322–6327. 2001.
|
|
19
|
Seftor RE, Seftor EA, Kirschmann DA and
Hendrix MJ: Targeting the tumor microenvironment with chemically
modified tetracyclines: inhibition of laminin 5 gamma 2 chain
promigratory fragments and vasculogenic mimicry. Mol Cancer Ther.
1:1173–1179. 2002.
|
|
20
|
Hess AR, Seftor EA, Seftor RE and Hendrix
MJ: Phosphoinositide 3-kinase regulates membrane type 1-matrix
metalloproteinase (MMP) and MMP-2 activity during melanoma cell
vasculogenic mimicry. Cancer Res. 63:4757–4762. 2003.PubMed/NCBI
|
|
21
|
Sood AK, Fletcher MS, Cofin JE, Yang M,
Seftor EA, Gruman LM, Gershenson DM, et al: Functional role of
matrix metalloproteinases in ovarian tumor cell plasticity. Am J
Obstet Gynecol. 190:899–909. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yue PY, Wong DY and Wu PK: The
angiosuppressive effects of 20(R)-ginsenoside Rg3. Biochem
Pharmacol. 72:437–445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han J, Hao F, Hao F, An Y, Xu Y, Xiaokaiti
Y, Pan Y, et al: Ginsenoside Rg3 attenuates cell migration via
inhibition of aquaporin 1 expression in PC-3M prostate cancer
cells. Eur J Pharmacol. 683:27–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JW, Jung SY, Kwon YH, Lee JH, Lee YM,
Lee BY and Kwon SM: Ginsenoside Rg3 attenuates tumor angiogenesis
via inhibiting bioactivities of endothelial progenitor cells.
Cancer Biol Ther. 13:504–515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang C, Liu L, Yu Y, Chen B, Chen B, Tang
C and Li X: Antitumor effects of ginsenoside Rg3 on human
hepatocellular carcinoma cells. Mol Med Rep. 5:1295–1298.
2012.PubMed/NCBI
|
|
26
|
Lee CK, Park KK, Chung AS and Chung WY:
Ginsenoside Rg3 enhances the chemosensitivity of tumors to
cisplatin by reducing the basal level of nuclear factor erythroid
2-related factor 2-mediated heme oxygenase-1/NAD(P)H quinone
oxidoreductase-1 and prevents normal tissue damage by scavenging
cisplatin-induced intracellular reactive oxygen species. Food Chem
Toxicol. 50:2565–2574. 2012.
|
|
27
|
Liu JP, Lu D, Nicholson RC, Li PY and Wang
F: Toxicity of a novel anti-tumor agent 20(S)-ginsenoside Rg3: a
26-week intramuscular repeated administration study in Beagle dogs.
Food Chem Toxicol. 49:1718–1727. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan HD, Quan HY, Zhang Y, Kim SH and
Chung SH: 20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon
cancer cells is associated with AMPK signaling pathway. Mol Med
Rep. 3:825–831. 2010.PubMed/NCBI
|
|
29
|
Chen XP, Qian LL, Jiang H and Chen JH:
Ginsenoside Rg3 inhibits CXCR4 expression and related migrations in
a breast cancer cell line. Int J Clin Oncol. 16:519–523. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Greenberg E, Hershkovitz L, Itzhaki O,
Hajdu S, Nemlich Y, Ortenberg R, Gefen N, et al: Regulation of
cancer aggressive features in melanoma cells by microRNAs. PLoS
One. 6:e189362011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Folkman J: Tumor angiogenesis therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van der Schaft DW, Seftor RE, Seftor EA,
Hess AR, Gruman LM, Kirschmann DA, Yokoyama Y, et al: Effects of
angiogenesis inhibitors on vascular network formation by human
endothelial and melanoma cells. J Natl Cancer Inst. 96:1473–1477.
2004.PubMed/NCBI
|
|
33
|
Chen LX, He YJ, Zhao SZ, Wu JG, Wang JT,
Zhu LM, Lin TT, et al: Inhibition of tumor growth and vasculogenic
mimicry by curcumin through downregulation of the EphA2/PI3K/MMP
pathway in a murine choroidal melanoma model. Cancer Biol Ther.
11:229–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang HB, Lin YC, Zeng DE, Lin W, Hong CQ,
Lin WZ and Chen JY: Inhibitory effect of ginsenoside Rg3 on the
tube-like structure formation in human nasopharyngeal carcinoma
HNE-1 cell line in vitro. Zhonghua Zhong Liu Za Zhi. 32:739–742.
2010.(In Chinese).
|
|
35
|
Sun B, Zhang S, Zhang D, Yin X, Wang S, Gu
Y and Wang Y: Doxycycline influences microcirculatin patterns in
B16 melanoma. Exp Biol Med (Maywood). 232:1300–1307. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo HC, Bu HQ, Luo J, Wei WT, Liu DL, Chen
H, Tong HF, et al: Emodin potentiates the antitumor effects of
gemcitabine in PANC-1 pancreatic cancer xenograft model in
vivo via inhibition of inhibitors of apoptosis. Int J Oncol.
40:1849–1857. 2012.PubMed/NCBI
|
|
37
|
Ruf W, Seftor EA, Petrovan RJ, Weiss RM,
Gruman LM, Margaryan NV, Seftor RE, et al: Differential role of
tissuefactor pathway inhibitors 1 and 2 in melanoma vasculogenic
mimicry. Cancer Res. 63:5381–5389. 2003.PubMed/NCBI
|
|
38
|
Kirschmann DA, Seftor EA, Hardy KM, Seftor
RE and Hendrix MJ: Molecular pathways: vasculogenic mimicry in
tumor cells: diagnostic and therapeutic implications. Clin Cancer
Res. 18:2726–2732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hendrix MJ, Seftor EA, Hess AR and Seftor
RE: Vasculogenic mimicry and tumour-cell plasticity: lessons from
melanoma. Nat Rev Cancer. 3:411–421. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Folberg R, Arbieva Z, Moses J, Hayee A,
Sandal T, Kadkol S, Lin AY, et al: Tumor cell plasticity in uveal
melanoma: microenvironment directed dampening of the invasive and
metastatic genotype and phenotype accompanies the generation of
vasculogenic mimicry patterns. Am J Pathol. 169:1376–1389. 2006.
View Article : Google Scholar
|
|
41
|
Fan YZ and Sun W: Molecular regulation of
vasculogenic mimicry in tumors and potential tumor-target therapy.
World J Gastrointest Surg. 2:117–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu R, Cao Z, Tu J, Pan Y, Shang B, Zhang
G, Bao M, et al: Lycorine hydrochloride inhibits metastatic
melanoma cell-dominant vasculogenic mimicry. Pigment Cell Melanoma
Res. 25:630–638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shin YM, Jung HJ, Choi WY and Lim CJ:
Antioxidative, anti-inflammatory, and matrix metalloproteinase
inhibitory activities of 20(S)-ginsenoside Rg3 in cultured
mammalian cell lines. Mol Biol Rep. 40:269–279. 2013. View Article : Google Scholar
|
|
44
|
Xu TM, Cui MH, Xin Y, Gu LP, Jiang X, Su
MM, Wang DD, et al: Inhibitory effect of ginsenoside Rg3 on ovarian
cancer metastasis. Chin Med J. 121:1394–1397. 2008.PubMed/NCBI
|
|
45
|
Chen MW, Ni L, Zhao XG and Niu XY: The
inhibition of 20(R)-ginsenoside Rg3 on the expressions of
angiogenesis factors proteins in human lung adenocarcinoma cell
line A549 and HUVEC304 cell. Zhongguo Zhong Yao Za Zhi. 30:357–360.
2005.(In Chinese).
|