Introduction
Ovarian cancer still represents one of the most
lethal gynecologic malignancies. Within this type of gynecological
cancer the small cell ovarian carcinoma of the hypercalcemic type
(SCCOHT) is defined as a rare form of an aggressive ovarian tumor
predominantly affecting young women between ages of 13 to 35 which
is mostly associated with paraendocrine hypercalcemia (1–3).
Following the initial histopathological evaluation of several
clinical cases, the SCCOHT has been classified as a separate
pathological entity (2). Recent
studies revealed a mutation in the SMARCA4 gene as a
potential marker for the SCCOHT (4–6).
The SCCOHT tumor disease is associated with poor
prognosis and appears different and clearly distinguishable from
other ovarian cancer types such as ovarian epithelial tumors and
ovarian germ cell tumors (7).
Initial immunohistochemical analysis of the SCCOHT postulated a
germ cell-derived tumor (8).
Another study reported SCCOHT as an epithelial-like originating
tumor (3). In fact, some cells
stained positive for epithelial cell markers, however, the
intermediate filament protein vimentin predominantly associated
with cells of a mesenchymal phenotype has been identified in the
majority of cells in the SCCOHT (9). Further investigations using
additional genetic analysis of SCCOHT tumor specimen suggested a
heterogeneous tumor entity but did not confirm a germ cell-derived
or an epithelial cell-derived tumor origin (9–11).
The heterogeneity of these data may be explained in part by the
rare and limited tumor material from patients. Considering these
controversial reports, the histogenesis of SCCOHT and the mechanism
of the development and physiological role of an accompanying
hypercalcemia still remain unclear. Likewise, reasonable approaches
for a sufficient (chemo)therapeutic management to treat SCCOHT
patients are completely unknown. Although a multi-modality platform
is suggested including surgery followed by chemotherapy and
radiotherapy (12,13), only very few patients survived
longer than the following two years (14–17).
Recently, we developed a cellular model for the
SCCOHT and the resulting SCCOHT-1 tumor cells were derived from a
primary culture of biopsy material from a 31-year-old patient with
recurrent SCCOHT. In vivo studies with these primary cells
substantiated a SCCOHT phenotype with histopathological
similarities between the mouse xenograft-developed tumors and the
original patient tumor. Moreover, development of SCCOHT-1-induced
tumor xenografts displayed an accompanying hypercalcemia in
NOD/scid mice with serum calcium levels above 3.5 mmol/l (1).
Using this unique cellular model, we examined in the
present study the effects of exogenous calcium representing a
hypercalcemia on SCCOHT-1 in comparison to established human
ovarian adenocarcinoma cell lines including NIH:OVCAR-3 and SK-OV-3
cells. Moreover, different calcium-mediated signaling pathways were
analysed in these ovarian cancer cells, which may be supportive in
search of an appropriate therapeutic approach, particularly in
SCCOHT.
Materials and methods
Cell culture
Primary human SCCOHT-1 cells
SCCOHT-1 cells were derived as a spontaneous,
permanently growing primary culture from a tumor biopsy after
surgery of a 31-year-old patient with recurrent SCCOHT (1). Informed written consent was obtained
from the patient for the use of this material and the study was
approved by the Ethics Committee of Hannover Medical School,
Project #3916, June 15, 2005. The SCCOHT-1 cells were cultured in
RPMI-1640 supplemented with 1 or 10% (v/v) fetal calf serum, 100
U/ml L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin.
The tissue culture was performed at 37°C in a humidified atmosphere
of 5% (v/v) CO and the medium was changed at intervals of 3 to 4
days. For subculture, the cells were centrifuged (320 g/6 min) and
resuspended in growth medium and the proliferative capacity at
various conditions and the population doublings in parallel to the
cell viability during culture was determined in a hemocytometer
using the trypan blue exclusion test. In an alternative
fluorescence-based proliferation assay the SCCOHT-1 cells have been
transduced with a 3rd generation lentiviral SIN vector containing
the eGFP gene (SCCOHT-1GFP) as previously described for
these cells (1).
Human ovarian adenocarcinoma cell
lines
Human NIH:OVCAR-3 ovarian cancer cells
(ATCC® #HTB-161™) were commercially obtained in passage
76 (P76) from the Institute for Applied Cell Culture (IAZ), Munich,
Germany. The SK-OV-3 ovarian cancer cells (ATCC®
#HTB-77™) were commercially obtained in P25 from the ATCC,
Manassas, VA, USA. These ovarian adenocarcinoma cell lines were
originally established from the malignant ascites of a patient with
progressive adenocarcinoma of the ovary, respectively. The cells
were cultivated at about 1,750 cells/cm2 in RPMI-1640
supplemented with 1 or 10% (v/v) fetal calf serum, 100 U/ml
L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin.
Subculture was performed by trypsin/EDTA (Biochrom GmbH, Berlin,
Germany) treatment for 5 min at 37°C. For the experiments
NIH:OVCAR-3 cells were used in P86 to P118 and SK-OV-3 cells were
used in P37 to P39. For fluorescence measurement in an appropriate
proliferation assay the NIH:OVCAR-3 as well as the SK-OV-3 cells
were also transduced with a eGFP gene vector
(NIH:OVCAR-3GFP and SK-OV-3GFP) similar to
SCCOHT-1GFP cells.
Human breast cancer cell line
Human MDA-MB-231 breast cancer cells (MDA) were
obtained from the ATCC (#HTB-26). This cell line was analyzed in a
short tandem repeat (STR)-based authentication by the Institute for
Legal Medicine at the University Hospital Schleswig-Holstein as
recently documented (18). MDA
cells were cultivated at about 1,500 cells/cm2 in
Leibovitz’s L-15-medium (Invitrogen) with 10% (v/v) FCS, 2 mM
L-glutamin and 1 mM penicillin/streptomycin. For fluorescence
measurement MDA-MB-231GFP cells were also generated
after transduction with the eGFP gene vector.
Cell line authentication
Authentication of SCCOHT-1, NIH:OVCAR-3, SK-OV-3,
and MDA-MB-231 cells was performed by short tandem repeat (STR)
fragment analysis using the GenomeLab human STR primer set (Beckman
Coulter Inc., Fullerton, CA, USA). Following DNA isolation of the
cell lines and amplification by polymerase chain reaction (PCR)
with the STR primer set, the appropriate PCR products were
sequenced in the CEQ8000 Genetic Analysis System (Beckman Coulter)
using the GenomeLab DNA size standard kit-600 (Beckman Coulter).
Comparison of the sequencing results from SCCOHT-1 were similar to
the original SCCOHT patient cells cultured in our lab. Moreover,
the NIH:OVCAR-3, SK-OV-3 and MDA-MB-231 cell lines demonstrated a
similar STR pattern according to the STR database provided by the
Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ,
Braunschweig, Germany) for these cell lines.
Proliferation and cell cycle
analysis
For fluorescence measurement the different
eGFP-transduced ovarian cancer populations were cultured in flat
bottom 96-well plates (Nunc/ThermoFischer, Roskilde, Denmark) and
incubated with 1.6, 3.2 and 6.4 mM Ca2+, respectively,
for 24 to 72 h. At different time points, the medium was removed
and the cells were lysed with 5% (w/v) sodium dodecylsulfate (SDS).
Thereafter, the fluorescence intensities of GFP in the cell
homogenate which corresponded to the appropriate cell number of
ovarian cancer cells, were measured at excitation 485 nm/emission
520 nm using the Fluoroscan Ascent Fl (Thermo Fisher Scientific)
fluorescence plate reader.
To substantiate these results in an alternative
assay, wild-type ovarian cancer populations were incubated
similarly with 1.6, 3.2 and 6.4 mM Ca2+, respectively,
for 24 to 72 h and the cells were counted at the appropriate time
points in a hemocytometer following trypan blue staining.
The cell cycle analysis was performed as described
previously (19) using untreated
compared to 1.6 mM Ca2+- and 6.4 mM
Ca2+-stimulated SCCOHT-1GFP,
NIH:OVCAR-3GFP and SK-OV-3GFP ovarian cancer
cells after 48 h. Briefly, 5×105 cells were fixed in 70%
(v/v) ice-cold ethanol at 4°C for 24 h. Thereafter, the fixed cells
were stained with CyStain DNA 2 step kit (Partec GmbH, Münster,
Germany) and filtered through a 50 μm filter. The samples were then
analyzed in a Galaxy flow cytometer (Partec) using the MultiCycle
cell cycle software (Phoenix Flow Systems Inc., San Diego, CA,
USA).
Immunoblot analysis
Following culture of SCCOHT-1GFP cells in
culture medium with 1% FCS, untreated control cells and
Ca2+-stimulated cells were washed three times in
ice-cold PBS and lysed in a reswelling buffer containing 8 M urea
(Carl Roth GmbH Co KG, Karlsruhe, Germany), 1% CHAPS
(3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate) (Carl
Roth GmbH Co KG), 0.5% (v/v) pharmalyte 3–10 (GE Healthcare Europe
GmbH, Freiburg, Germany), 0.002% (w/v) bromophenol blue (Serva
Electrophoresis GmbH, Heidelberg, Germany) and freshly prepared
0.4% (w/v) dithiothreitol (DTT) (Carl Roth GmbH Co KG). Protein
concentration was adjusted using the colorimetric BCA-assay (Perbio
Science Deutschland, Bonn, Germany), subjected to
SDS-polyacrylamide gel electrophoresis and transferred to a Hybond
C Extra Nitrocellulose membrane (GE Healthcare Life Science). The
membranes were blocked with PBS containing 5% FCS and 0.05%
Tween-20 (PBS/Tween). After washing four times with PBS/Tween, the
membranes were incubated with the primary antibodies (polyclonal
anti-phospho-p44/42 MAPKThr202/Tyr204 (Cell Signaling
Technology Inc.); polyclonal anti-Stim-1 (clone D88E10; Cell
Signaling Technology Inc.); polyclonal anti-IP3 receptor (clone
D53A5; Cell Signaling Technology Inc.); monoclonal anti-GAPDH
(clone 6C5, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C
overnight. Thereafter, the membranes were washed four times with
PBS/Tween and incubated with the appropriate horseradish
peroxidase-conjugated secondary antibody (all from Santa Cruz
Biotechnology) for 1 h/25°C. The membranes were washed with
PBS/Tween and visualized by autoradiography using the ECL-detection
kit (GE Healthcare Europe GmbH).
Prostaglandin E2 (PGE2) ELISA
SCCOHT-1WT, SK-OV-3WT and
NIH:OVCAR-3WT cells were seeded in 24-well plates at
106 cells/well (Nunc/ThermoFischer, Roskilde, Denmark)
with 500 μl culture medium per well. In comparison to untreated
control cells, the populations were stimulated with 1.6, 3.2 and
6.4 mM Ca2+, respectively, in the absence or presence of
a 1-h pre-incubation with 50 μM of the MAP kinase inhibitor PD98059
(Cell Signaling Technology Inc.). The conditioned medium was
collected after 12 and 24 h, respectively, and centrifuged at 1,000
rpm/10 min. Thereafter, 50 μl aliquots of the supernatant were
applied to the appropriate PGE2 measurements which were performed
in an ELISA system according to the manufacturer’s recommendation
(R&D Systems Ltd., Abingdon, UK).
Results
Proliferation
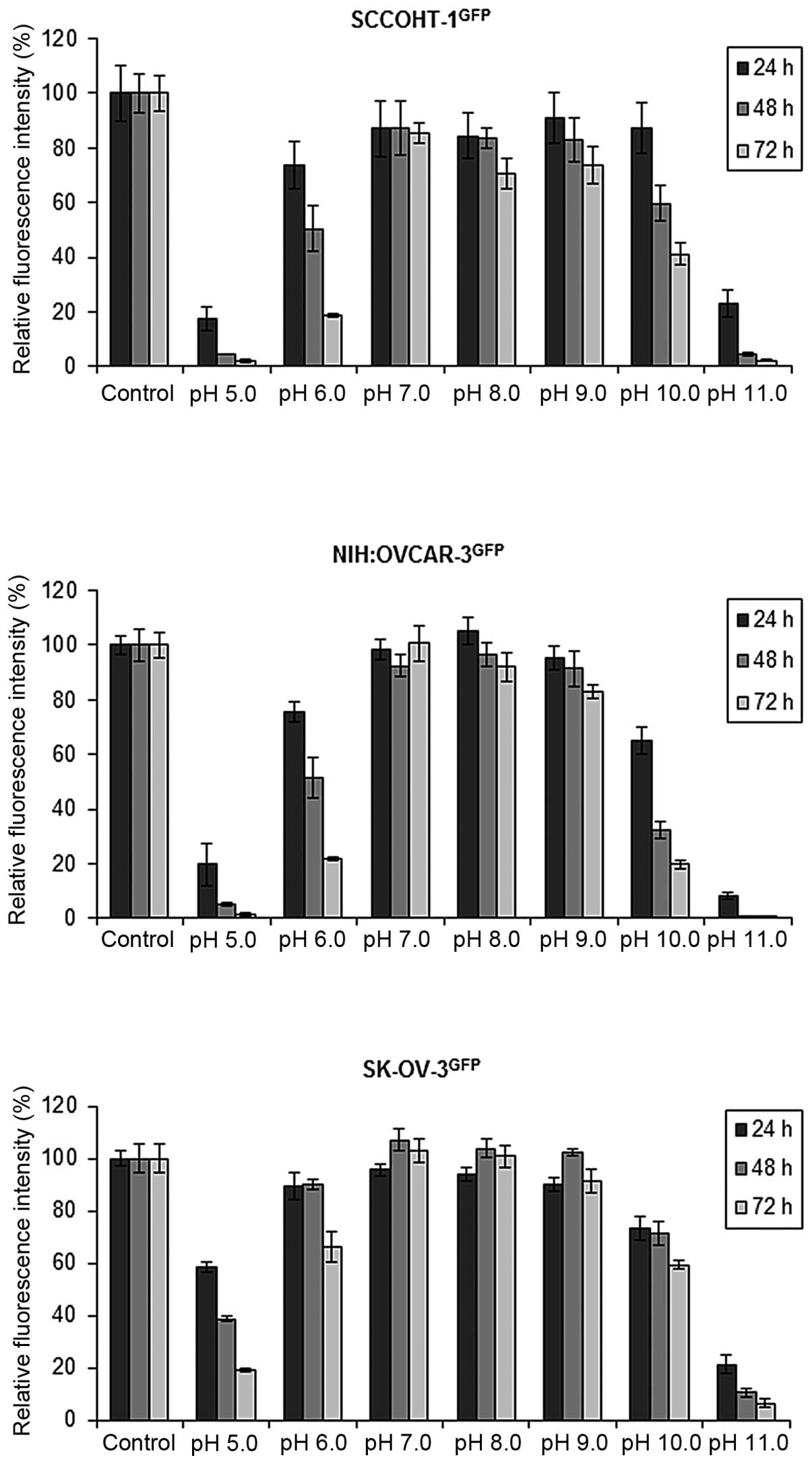
All three ovarian cancer cell types exhibited
sensitivity for an acidic culture milieu and continued maximal
proliferation in alkaline medium of approximately pH 9.0 (Fig. 2). The proliferative capacity of
SCCOHT-1 and NIH:OVCAR-3 cells was progressively inhibited by about
80% at pH 6.0 within 72 h (n=5) whereas SK-OV-3 cells demonstrated
more stability with a growth reduction of about 30% (n=6). At pH
10.0 the proliferation progressively declined by 59±4% (n=5) in
SCCOHT-1 cells after 72 h. A higher sensitivity with 80±2% (n=5)
was observed in NIH:OVCAR-3 cells at pH 10.0 after 72 h and SK-OV-3
cells revealed 41±6% (n=5) growth inhibition at similar conditions
(Fig. 1).
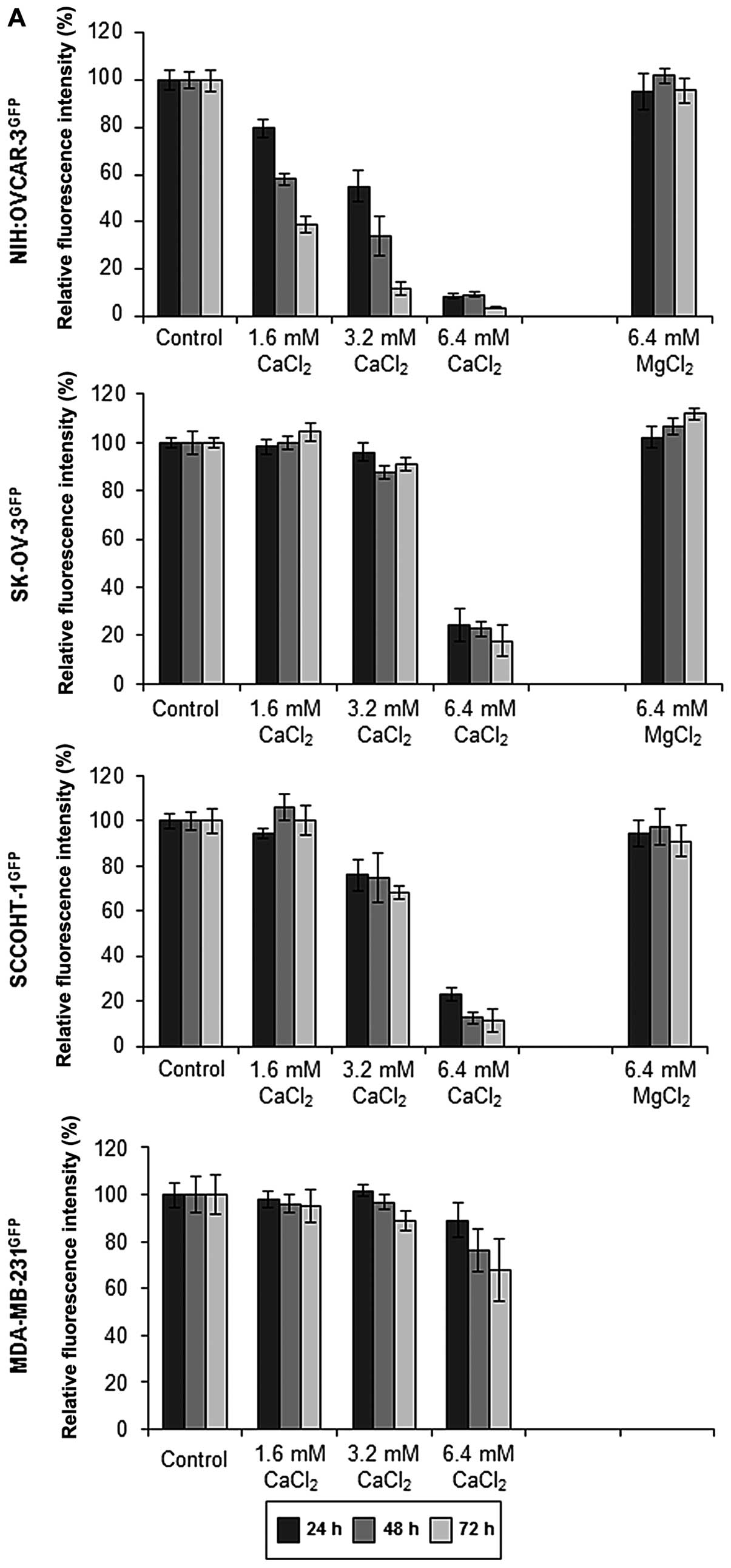
According to the hypercalcemia associated with
SCCOHT, exogenous stimulation with calcium was tested and revealed
a significant growth inhibition in all three ovarian carcinoma cell
types in a concentration- and time-dependent manner. Whereas the
culture medium constitutively contained about 0.8 mM
Ca2+ and Mg2+ during steady-state culture
conditions, the proliferative capacity of SK-OV-3 cells after
exogenous addition of 1.6 mM Ca2+ up to 6.4 mM
Ca2+ was progressively reduced to 17.8±6.2% (n=10)
within 72 h. These growth-inhibitory effects of 6.4 mM
Ca2+ were even more pronounced in SCCOHT-1 with growth
reduction down to 11.4±5.0% (n=9) and were maximal in NIH:OVCAR-3
cells reaching only 3.8±0.5% (n=10) of proliferative capacity after
72 h as compared to control cells in normal culture medium
(Fig. 2A). In contrast to these
significant growth-inhibitory effects of Ca2+,
incubation of the three ovarian carcinoma cell populations with 6.4
mM Mg2+ demonstrated little if any effect on the cell
growth and remained at a normal growth rate of approximately 100%
within 72 h (Fig. 2A). Moreover,
culture of the breast cancer cell line MDA-MB-231 in the presence
of 6.4 mM Ca2+ was associated with a growth rate of
68.1±13.2% (n=6) compared to a control culture after 72 h (Fig. 2A). Similar results of a marked
Ca2+-mediated concentration- and time-dependent growth
inhibition were also obtained with the appropriate wild-type
ovarian cancer cell populations by cell counting in a trypan blue
exclusion assay (Fig. 2B). The
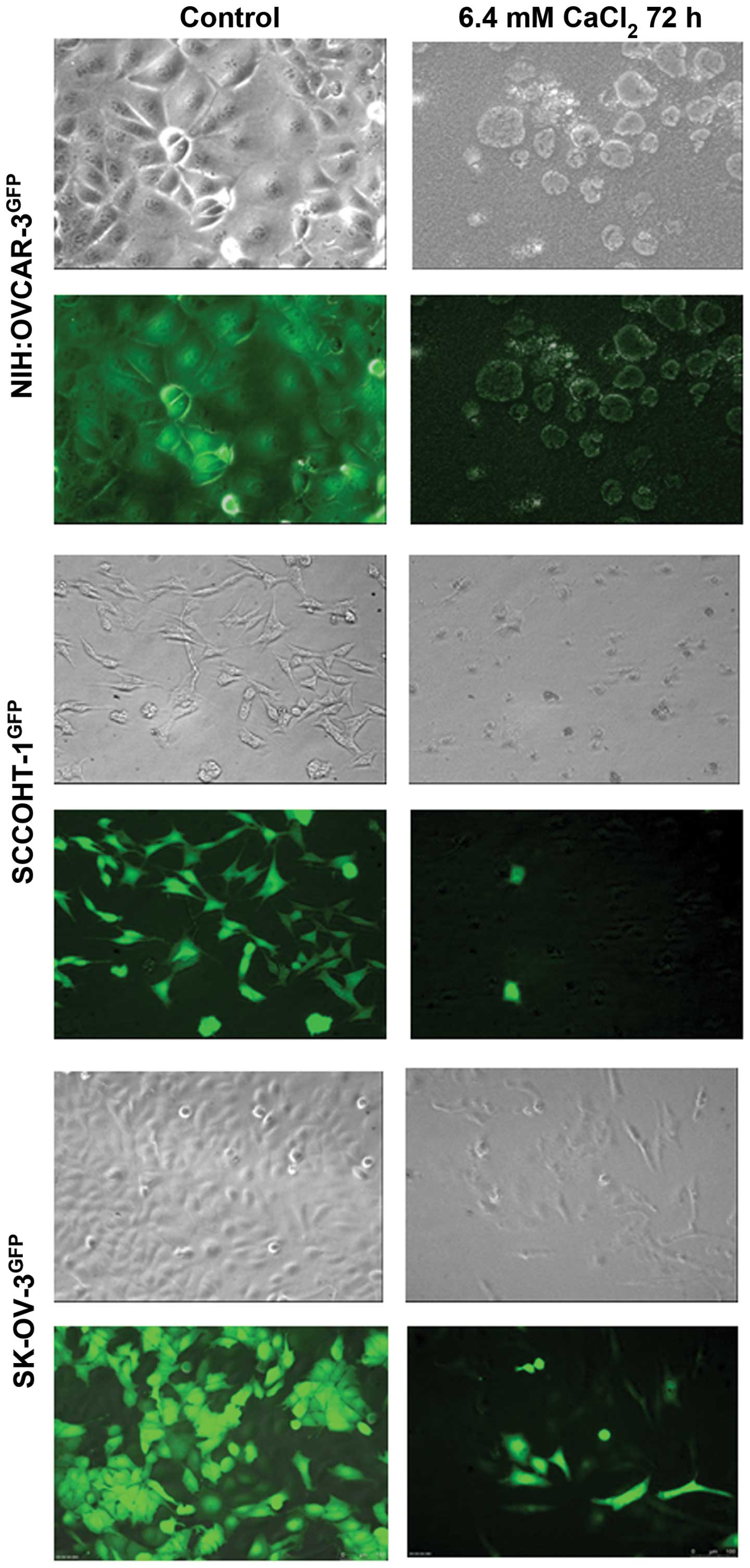
results from the proliferation assays were also accompanied by
appropriate morphological changes. Whereas the different ovarian
cancer cell types demonstrated their typical morphology in phase
contrast microscopy of the control cultures together with a GFP
expression of the lentiviral eGPF-transduced cultures, a
significant cell death with rounded and granulated cell bodies was
observed in NIH:OVCAR-3GFP cells following exposure to
6.4 mM Ca2+ for 72 h (Fig.
3, upper panel). Moreover, little if any fluorescence was
detectable anymore in NIH:OVCAR-3GFP cells. Only few
GFP-expressing viable cells remained in the SCCOHT-1GFP
culture after incubation with 6.4 mM Ca2+ for 72 h
(Fig. 3, middle panel).
SK-OV-3GFP cells also exhibited a significant
granulation after incubation with exogenous Ca2+ with
some more GFP-positive viable cells which substantiated the results
of the proliferation assay (Fig.
3, lower panel).
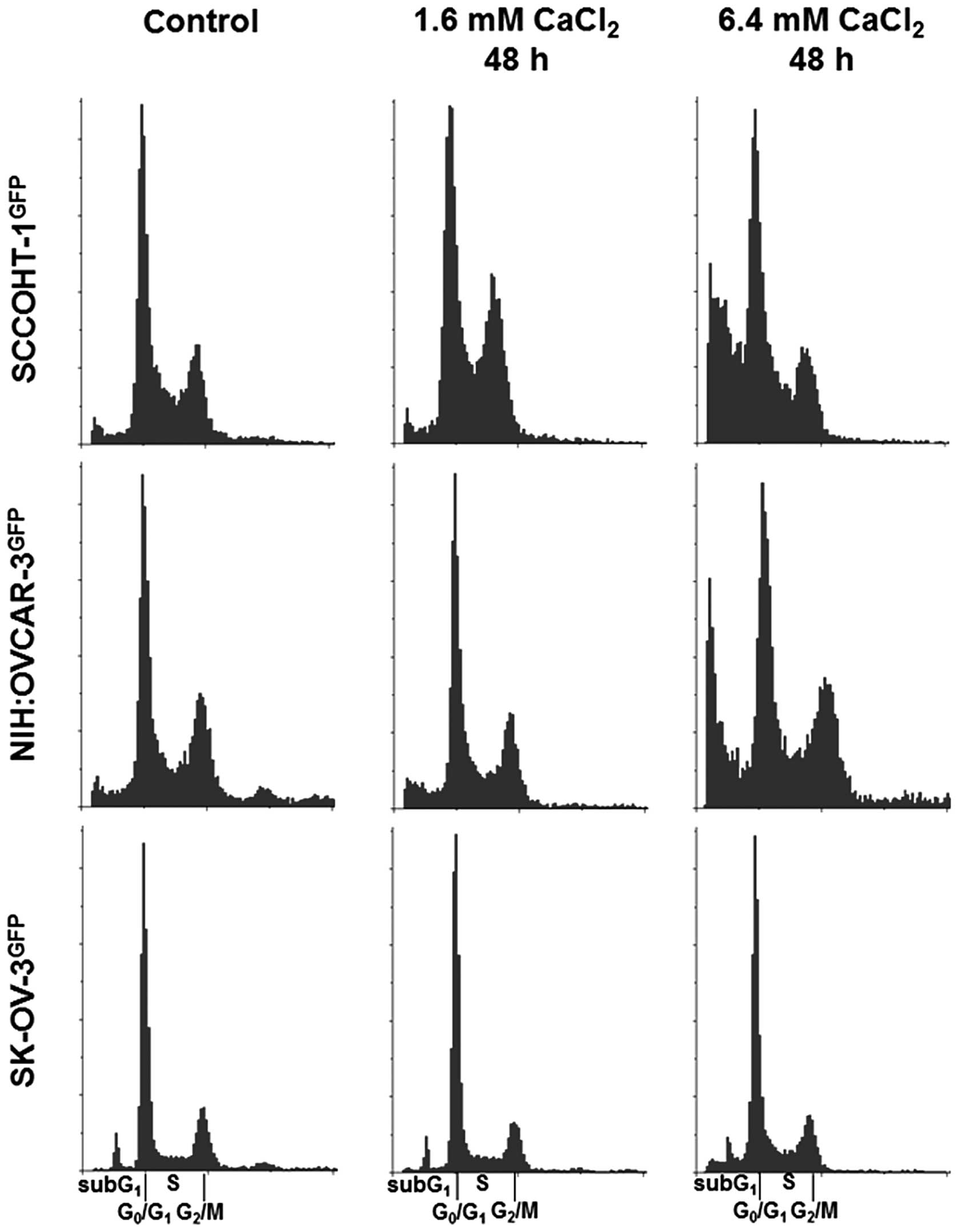
Cell cycle analysis revealed a significant arrest of
SCCOHT-1GFP cells in the G phase after 48 h in the
presence of 1.6 mM Ca2+. An elevation to 6.4 mM
Ca2+ was associated with increased cell death by an
accumulation of SCCOHT-1GFP cells in the subG phase.
Similar findings were observed in 6.4 mM Ca2+-exposed
NIH:OVCAR-3GFP cells with significantly elevated levels
of cells in the subG phase after 48 h whereas the cell cycle of the
lesser Ca2+-sensitive SK-OV-3GFP cells still
remained unaltered (Fig. 4).
Together, these findings suggested an optimal growth
of the different ovarian cancer cells in a neutral to alkaline pH
range whereby enhanced exogenous Ca2+ significantly
reduced the proliferative capacity and tumor cell viability.
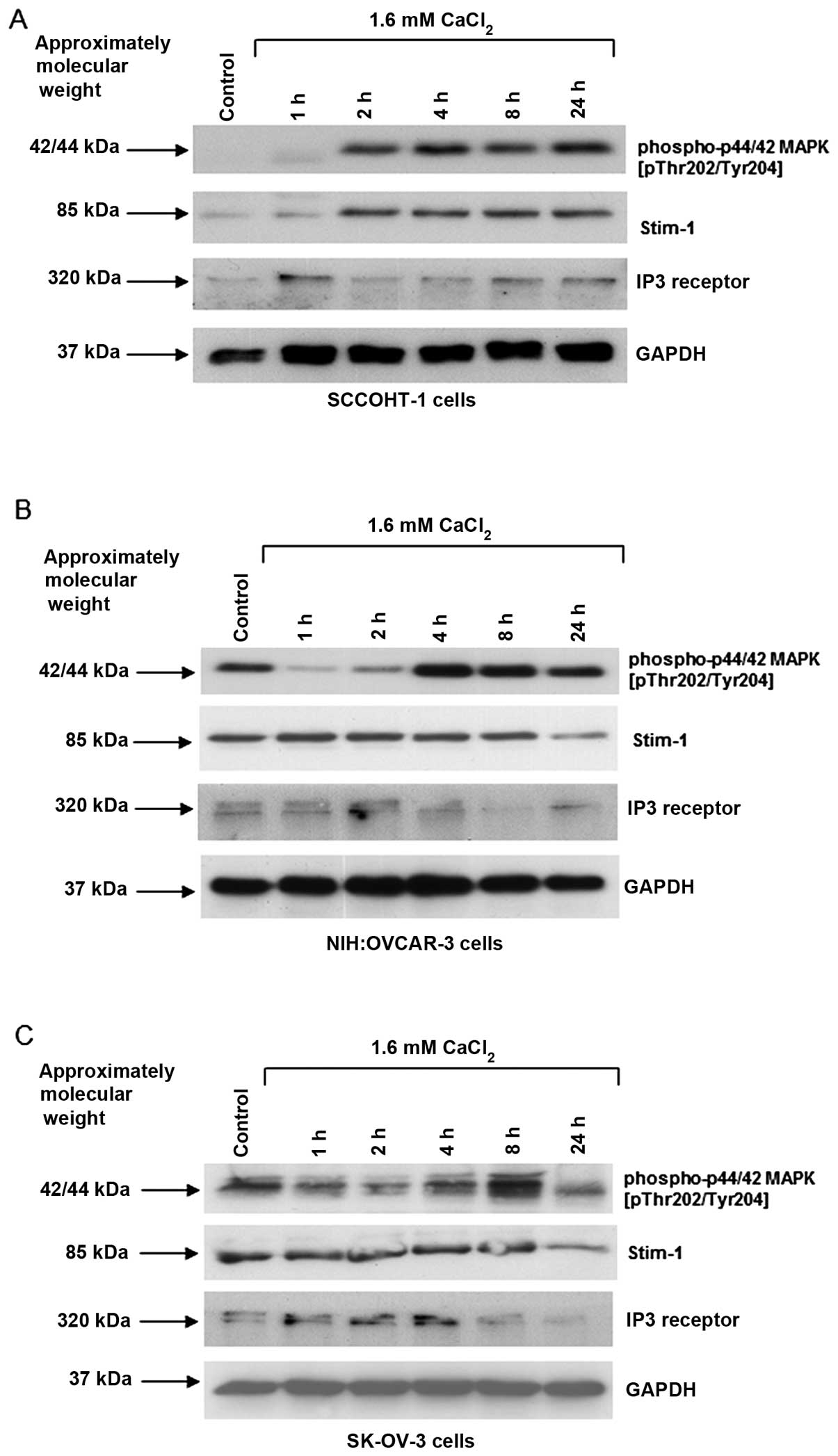
Western blot analysis was performed to further investigate specific
signaling effects of Ca2+ in the different ovarian
cancer cells. Exposure to 1.6 mM Ca2+ revealed a marked
appearance of phosphorylated p42/44 MAP kinase (Thr202/Tyr204)
within 2 h in SCCOHT-1 cells and this phosphorylation signal
sustained for at least 24 h (Fig.
5A). A constitutive p42/44 MAP kinase phosphorylation in
NIH:OVCAR-3 and SK-OV-3 cells was initially reduced by exogenous
Ca2+ and significantly increased after 4 to 8 h before
this signal was markedly reduced again within 24 h (Fig. 5B and C).
Ca2+-sensitizing proteins were also investigated,
including stromal interaction molecule-1 (Stim-1) which determines
differences in [Ca2+] in the endoplasmic reticulum and
can oscillate for stimulatory interactions with the ORAI1 calcium
ion channels to the plasma membrane (20). The Stim-1 expression was enhanced
between 1 and 2 h of 1.6 mM Ca2+ treatment of SCCOHT-1
cells (Fig. 5A), whereas little,
if any, different Stim-1 protein levels were observed in
NIH:OVCAR-3 and SK-OV-3 cells until a decrease was observed after
24 h (Fig. 5B and C). With respect
to the IP3 receptor which releases Ca2+ from endoplasmic
storage compartments upon phospholipase C-mediated PI-breakdown and
inositol trisphosphate generation, there was only marginal if any
change in the IP3 receptor protein levels following Ca2+
stimulation of the ovarian cancer cells. The unaltered GAPDH
expression served as loading control (Fig. 5A).
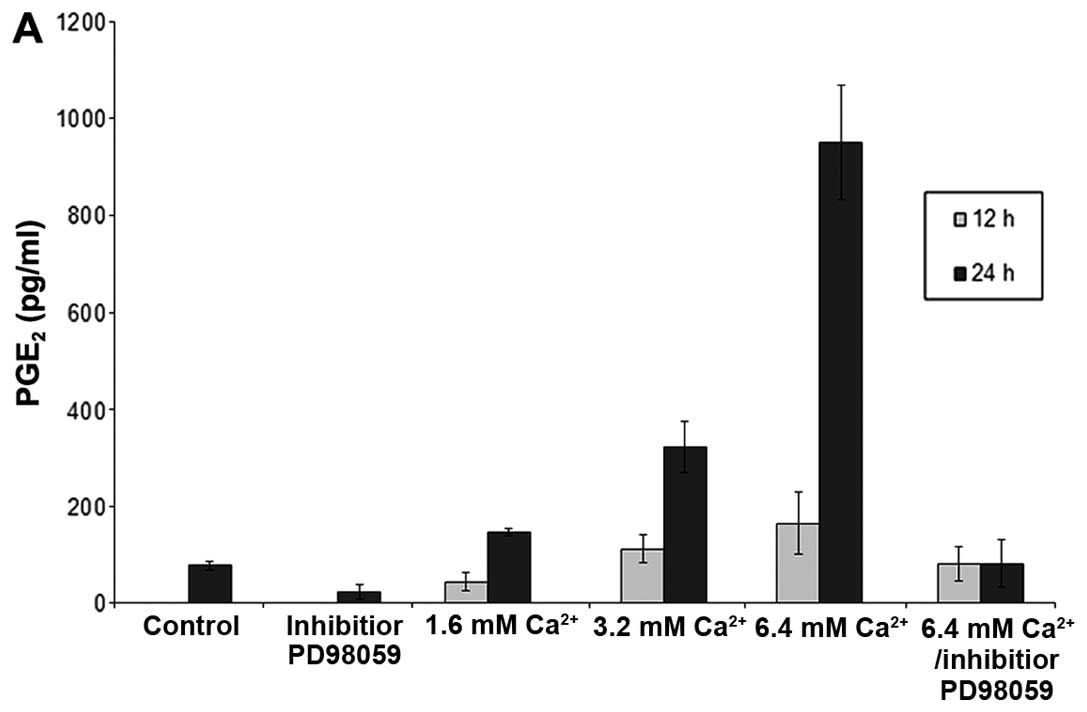
Ca2+-mediated phosphorylation of p42/44
MAP kinase (Thr202/Tyr204) was associated with enhanced PGE2
production in the ovarian cancer cells. Thus, stimulation of
SK-OV-3 cells with increasing [Ca2+] between 1.6 and 6.4
mM exhibited progressively increasing PGE2 release after 12 h,
which was significantly further elevated after 24 h following
Ca2+ stimulation (Fig.
6A). Moreover, pre-treatment with the p42/44 MAP kinase
inhibitor PD98059 completely abolished even the highest levels of
Ca2+-mediated PGE2 production (Fig. 6A). Similar results were obtained in
SCCOHT-1 cells with 40.4±24.1 pg/ml PGE2 after 1.6 mM
Ca2+ and 232.5±37.9 pg/ml PGE2 after 6.4 mM
Ca2+ stimulation for 12 h. Likewise, NIH:OVCAR-3 cells
produced 41.2±0.1 pg/ml PGE2 after 1.6 mM Ca2+ and
48.4±0.1 pg/ml PGE2 after 6.4 mM Ca2+ incubation within
24 h whereas non-stimulated control cells displayed 12.1±0.1 pg/ml
PGE2 and the PGE2 concentrations in the presence of the MAP kinase
inhibitor PD98059 were below detection limit. To test any potential
growth-inhibitory effects of PGE2 on the different ovarian cancer
populations, SCCOHT-1, NIH:OVCAR-3 and SK-OV-3 cells were incubated
with various PGE2 concentrations between 1 pg/ml to 10 ng/ml and
revealed little if any effect on the proliferative capacity of the
tumor cells (Fig. 6B). Together,
these findings suggested that the Ca2+-mediated p42/44
MAP kinase phosphorylation and subsequent stimulation of PGE2
production was independent of the Ca2+-mediated growth
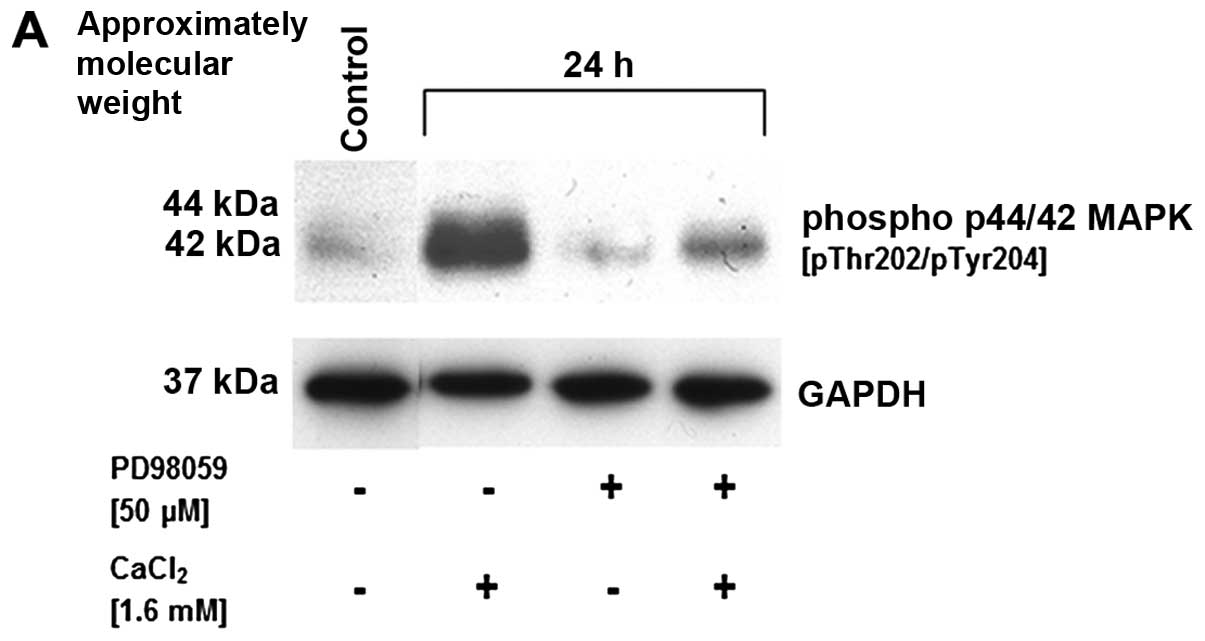
inhibition. Indeed, this suggestion was substantiated by the MAP
kinase inhibitor PD98059 which completely abolished the
Ca2+-mediated p42/44 MAP kinase phosphorylation in
SCCOHT-1 cells (Fig. 7A).
Moreover, MAP kinase inhibition did not demonstrate any effect on
the increased G arrest by 1.6 mM Ca2+ nor on the
pronounced cell death of SCCOHT-1 cells in subG1 phase by 6.4 mM
Ca2+ after 48 h similar to the results in Fig. 4 (Fig.
7B).
Discussion
Ovarian cancer represents the predominant cause of
gynecological cancer-related deaths affecting approximately 65,000
females in economically-developed countries in 2011 (21). As a rare form and special kind of
ovarian cancer, the SCCOHT represents an aggressive tumor with poor
prognosis and characteristics as compared to other ovarian
carcinoma types remain unclear. The in vitro results in this
study revealed common pH sensitivity in acidic milieu and
continuous proliferation in neutral/low alkaline environment.
Whereas young patients diagnosed with SCCOHT often present with a
concomitant serum hypercalcemia, it was of interest to focus on
calcium effects in ovarian cancer cells.
According to normal serum calcium levels of 2 to 2.5
mmol/l, hypercalcemia is considered as a mild type at
concentrations between 2.5 to 3.0 mmol/l serum calcium and as a
moderate type at concentrations between 3.0 to 3.5 mmol/l serum
calcium. Patients with serum calcium levels above 3.5 mmol/l are
diagnosed with a hypercalcemic crisis. Of interest, a recent study
in a variety of ovarian cancer patients reports elevated blood
calcium levels whereby [Ca2+] was proposed a potential
predictive marker for ovarian cancer (22).
To test different levels of hypercalcemia in
vitro, calcium concentrations of 1.6, 3.2 and 6.4 mmol/l were
applied to the various ovarian cancer cells and revealed already
significant growth-inhibitory effects between mild and moderate
hypercalcemia. These growth-inhibitory effects were
calcium-specific, since none of these results were obtained with
similar concentrations of Mg2+ or further cations.
Moreover, other tumor types such as breast cancer cells
demonstrated much less responsiveness to high Ca2+
concentrations as compared to the different ovarian cancer cells.
This calcium sensitivity of ovarian cancer cells suggested that
elevated [Ca2+] is supportive for a therapeutic approach
particularly in SCCOHT. Additional examination of these
growth-inhibitory effects of high [Ca2+] in vitro
demonstrated a morphological disintegration of the ovarian cancer
cells. This was associated with increased cell death as revealed by
cell cycle analysis. Interference with the calcium homeostasis can
induce cell damage and eventually initiate cell death (23), whereby recent studies proposed a
process of programmed necrosis (necroptosis) upon cytosolic calcium
accumulation in mouse xenografts of human neuroblastoma (24). These findings further substantiate
our hypothesis that suitable chemotherapeutic compounds in
combination with increased calcium levels contribute to an enhanced
promotion of tumor cell death particularly in SCCOHT-1 cells. In
this context, the hypercalcemia associated with SCCOHT may reflect
a physiological anti-tumor response. However, due to a certain
protection of the tumor cells within the tumor microenvironment and
potential interactions with other cell types including immune cells
and mesenchymal stem/stroma cells as documented for other tumor
types such as breast cancer (18,25,26),
the hypercalcemic effects achieve only a limited threshold and
therefore, remain unresponsive and inefficient without further
support to directly target the SCCOHT cancer cells.
At the molecular level, high [Ca2+] was
associated with increased activation/phosphorylation of the p42/p44
MAPK in the ovarian cancer cells. Activated p42/p44 MAPK can
further relay phosphorylation signals to eventually stimulate
phospholipase A2 (27). Upon
cleavage of polyunsaturated fatty acids including arachidonic acid
from the C2-position of membrane phospholipids by activated
phospholipase A2, the elevated levels of arachidonic acid can be
further metabolized via cyclooxygenase isoforms (COX-1, COX-2) into
prostanoids and predominantly PGE2 (28,29).
Indeed, the data obtained in the present study substantiated such a
signaling pathway, whereby stimulation of the ovarian cancer cells
with increasing calcium concentration resulted in appropriately
increasing PGE2 production both, in a concentration- and
time-dependent manner. Enhanced PGE2-synthesis accompanied by an
increased expression of COX-1 and COX-2 has been documented in
certain epithelial ovarian cancer (30) indicating potential metabolic
alterations with the malignant transformation and progression. PGE2
can mediate suppressive effects via ligation to the E prostanoid
receptors EP2 and EP4 followed by enhanced production of cyclic
AMP. However, PGE2 binding to EP3 can also exert immune-stimulatory
properties by decreasing cAMP levels. Therefore, PGE2-mediated
immune-modulation by tumors can alter immune surveillance by
re-educating the infiltrating inflammatory and immune cells to
support tumorigenesis (31).
Whereby no direct proliferative effects of PGE2 on the ovarian
cancer cells were detectable, our work also demonstrated that the
calcium-mediated PGE2 production was p42/p44 MAPK-dependent since
MAPK inhibition abolished the PGE2 production in the different
ovarian cancer cells.
Together, increased calcium concentrations can
specifically stimulate PGE2 production via p42/p44 MAPK activation
and in parallel, contribute to the induction of cell death in
ovarian cancer cells, whereby these calcium-mediated effects are
relayed via different signaling pathways. Although the appearance
of a serum hypercalcemia in SCCOHT patients and a variety of other
ovarian cancer patient exhibit only a limited and insufficient
threshold, the present findings indicate that elevated
Ca2+ levels can enhance a physiological antitumor
strategy for SCCOHT in support of a combined therapeutic approach
against this rare but severe type of ovarian cancer.
Acknowledgements
This study was supported by a grant from the
Niedersächsische Krebsgesellschaft e.V. to R.H.
References
|
1
|
Otte A, Gohring G, Steinemann D, et al: A
tumor-derived population (SCCOHT-1) as cellular model for a small
cell ovarian carcinoma of the hypercalcemic type. Int J Oncol.
41:765–775. 2012.
|
|
2
|
Dickersin GR, Kline IW and Scully RE:
Small cell carcinoma of the ovary with hypercalcemia: a report of
eleven cases. Cancer. 49:188–197. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Young RH, Oliva E and Scully RE: Small
cell carcinoma of the hypercalcemic type in the ovary. Gynecol
Oncol. 57:7–8. 1995.PubMed/NCBI
|
|
4
|
Jelinic P, Mueller JJ, Olvera N, et al:
Recurrent SMARCA4 mutations in small cell carcinoma of the ovary.
Nat Genet. 46:424–426. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Witkowski L, Carrot-Zhang J, Albrecht S,
et al: Germline and somatic SMARCA4 mutations characterize small
cell carcinoma of the ovary, hypercalcemic type. Nat Genet.
46:438–443. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramos P, Karnezis AN, Craig DW, et al:
Small cell carcinoma of the ovary, hypercalcemic type, displays
frequent inactivating germline and somatic mutations in SMARCA4.
Nat Genet. 46:427–429. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scully RE: Atlas of Tumor Pathology:
Tumors of the Ovary and Maldeveloped Gonads. Armed Forces Institute
of Pathology; Washington, DC: 1979
|
|
8
|
Ulbright TM, Roth LM, Stehman FB, Talerman
A and Senekjian EK: Poorly differentiated (small cell) carcinoma of
the ovary in young women: evidence supporting a germ cell origin.
Hum Pathol. 18:175–184. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aguirre P, Thor AD and Scully RE: Ovarian
small cell carcinoma. Histogenetic considerations based on
immunohistochemical and other findings. Am J Clin Pathol.
92:140–149. 1989.PubMed/NCBI
|
|
10
|
Walt H, Hornung R, Fink D, et al:
Hypercalcemic-type of small cell carcinoma of the ovary:
characterization of a new tumor line. Anticancer Res. 21:3253–3259.
2001.PubMed/NCBI
|
|
11
|
McCluggage WG, Oliva E, Connolly LE,
McBride HA and Young RH: An immunohistochemical analysis of ovarian
small cell carcinoma of hypercalcemic type. Int J Gynecol Pathol.
23:330–336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harrison ML, Hoskins P, du Bois A, et al:
Small cell of the ovary, hypercalcemic type - analysis of combined
experience and recommendation for management. A GCIG study. Gynecol
Oncol. 100:233–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shrimali RK, Correa PD and Reed NS:
Dose-dense and dose-intense chemotherapy for small cell ovarian
cancer: 2 cases and review of literature. Med Oncol. 28:766–770.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benrubi GI, Pitel P and Lammert N: Small
cell carcinoma of the ovary with hypercalcemia responsive to
sequencing chemotherapy. South Med J. 86:247–248. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reed WC: Small cell carcinoma of the ovary
with hypercalcemia: report of a case of survival without recurrence
5 years after surgery and chemotherapy. Gynecol Oncol. 56:452–455.
1995.PubMed/NCBI
|
|
16
|
Dykgraaf RH, de Jong D, van Veen M,
Ewing-Graham PC, Helmerhorst TJ and van der Burg ME: Clinical
management of ovarian small-cell carcinoma of the hypercalcemic
type: a proposal for conservative surgery in an advanced stage of
disease. Int J Gynecol Cancer. 19:348–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barondeau J, Rodgers M, Braun L, Azarow K,
Forouhar M and Faucette K: Small cell ovarian carcinoma: a rare,
aggressive tumor masquerading as constipation in a teenager with a
fatal outcome. J Pediatr Hematol Oncol. 32:e139–e141. 2010.
View Article : Google Scholar
|
|
18
|
Mandel K, Yang Y, Schambach A, Glage S,
Otte A and Hass R: Mesenchymal stem cells directly interact with
breast cancer cells and promote tumor cell growth in vitro and in
vivo. Stem Cells Dev. 22:3114–3127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bertram C and Hass R: Cellular senescence
of human mammary epithelial cells (HMEC) is associated with an
altered MMP-7/HB-EGF signaling and increased formation of
elastin-like structures. Mech Ageing Dev. 130:657–669. 2009.
View Article : Google Scholar
|
|
20
|
Putney JW: Capacitative calcium entry:
from concept to molecules. Immunol Rev. 231:10–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
22
|
Schwartz GG and Skinner HG: Prospective
studies of total and ionized serum calcium in relation to incident
and fatal ovarian cancer. Gynecol Oncol. 129:169–172. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Trump BF and Berezesky IK:
Calcium-mediated cell injury and cell death. FASEB J. 9:219–228.
1995.PubMed/NCBI
|
|
24
|
Nomura M, Ueno A, Saga K, Fukuzawa M and
Kaneda Y: Accumulation of cytosolic calcium induces necroptotic
cell death in human neuroblastoma. Cancer Res. 74:1056–1066. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ungefroren H, Sebens S, Seidl D, Lehnert H
and Hass R: Interaction of tumor cells with the microenvironment.
Cell Commun Signal. 9:182011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hass R and Otte A: Mesenchymal stem cells
as all-round supporters in a normal and neoplastic
microenvironment. Cell Commun Signal. 10:262012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Van Rossum GS, Klooster R, van den Bosch
H, Verkleij AJ and Boonstra J: Phosphorylation of p42/44(MAPK) by
various signal transduction pathways activates cytosolic
phospholipase A(2) to variable degrees. J Biol Chem.
276:28976–28983. 2001.
|
|
28
|
Koehler L, Hass R, DeWitt DL, Resch K and
Goppelt-Struebe M: Glucocorticoid-induced reduction of prostanoid
synthesis in TPA-differentiated U937 cells is mainly due to a
reduced cyclooxygenase activity. Biochem Pharmacol. 40:1307–1316.
1990. View Article : Google Scholar
|
|
29
|
Rehfeldt W, Hass R and Goppelt-Struebe M:
Characterization of phospholipase A2 in monocytic cell lines.
Functional and biochemical aspects of membrane association. Biochem
J. 276:631–636. 1991.PubMed/NCBI
|
|
30
|
Rask K, Zhu Y, Wang W, Hedin L and
Sundfeldt K: Ovarian epithelial cancer: a role for PGE2-synthesis
and signalling in malignant transformation and progression. Mol
Cancer. 5:622006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Medeiros A, Peres-Buzalaf C, Fortino
Verdan F and Serezani CH: Prostaglandin E2 and the suppression of
phagocyte innate immune responses in different organs. Mediators
Inflamm. 2012:3275682012. View Article : Google Scholar : PubMed/NCBI
|