Introduction
Cholangiocarcinoma (CCA) is a lethal malignancy that
originates from the biliary epithelium. The worldwide incidence of
CCA is relatively rare but the incidence and mortality have both
gradually increased over the past decades (1–4). CCA
is an aggressive cancer, with a poor survival rate and lack of
effective medical treatment (4,5). Up
to date, surgical resection is the only potentially curative
treatment for CCA, nevertheless, the 5-year survival rate is dismal
(6–8). In addition, the majority of CCA
patients presents at an advanced stage when surgical therapy is not
feasible (5,9,10).
Chemotherapy is considered as a reasonable treatment
option for unresectable and advanced CCA (9). However, the clinical benefit of this
treatment regime is very low due to remarkably resistant of CCA to
common chemotherapy (6,9). The use of several chemotherapeutic
drugs such as gemcitabine, cisplatine, leucovorrin-mediated
5-fluorouracil or capectabine has been considered as the active
regimens in CCA (11–13). Nevertheless, neither single drug
nor combination treatment has prolonged the median survival time to
exceed 12 months (14).
Radiotherapy is regarded as an alternative therapy
for patients with unresectable CCA and as an adjuvant or
neoadjuvant treatment (15–20).
Most studies have demonstrated the benefit of radiotherapy in terms
of survival outcomes. However, the efficacy of this modality
remains very low due to the resistance of CCA cells to the fatal
effects of radiation. The responsiveness of cancer cells to
radiotherapy is influenced by many factors. This includes such
biologic factors as intrinsic radiosensitivity, proliferation
status, and genetic alterations in DNA damage checkpoints, DNA
repair and cell death pathways (21–24).
The DNA damage checkpoints are cardinal factors in
response to radiation-induced DNA damage or DNA damaging
chemotherapy. The function of these checkpoints is to facilitate
DNA repair and promote cell death in unrepaired cells (25). Most cancer cells encompass multiple
defects in DNA damage checkpoints and cell death pathways leading
to resistance to radiation-induced cell death (26). Cancer cells with a defect in G1
checkpoint depend on S and G2 checkpoints to repair
radiation-induced DNA damage (27). Since cells with damaged DNA can
only be transiently arrested in the S phase, G2 checkpoints are key
guardians to prevent these cells from attempting the complex
process of mitosis (25). It has
been proposed that abrogation of G2 checkpoints in cancer cells
could prevent completion of DNA repair. Therefore, cancer cells
with damaged DNA are forced to enter the M phase and subsequently
die while attempting to divide (27,28).
Enhancing radioresponsiveness of tumors by using
checkpoint kinase inhibitors is theoretically a promising approach
to increase efficacy of radiotherapy (29). Thus, several studies have analyzed
the effects of combined applications of checkpoint kinase
inhibitors and ionizing radiation (30–33).
Nevertheless, the enhancement of radioresponsiveness of CCA cells
by checkpoint kinase inhibitors has not been investigated.
In this study, we examined the efficiency of G1 and
G2 checkpoints of three human CCA cell lines: KKU-100, KKU-M214,
and KKU-M055 and correlated existing G1 and/or G2 checkpoint
defects with the radiosensitivity of CCA cell lines. Furthermore,
we evaluated the potential of checkpoint kinase Chk1/2 inhibition
to enhance the radiosensitivity of CCA cell lines. Thus, this study
provides useful information for improving radiotherapeutic outcome
of CCA patients.
Materials and methods
Cell culture
The human CCA cell lines established from primary
tumor of CCA patients with different histological types: KKU-100
and KKU-M055 (poorly differentiated adenocarcinoma), and KKU-M214
(moderately differentiated adenocarcinoma) were obtained from the
Liver Fluke and Cholangiocarcinoma Research Center, Khon Kaen
University. MMNK1, an immortalized human cholangiocyte cell line
was a gift from Dr N. Kobayashi (34). Cells were cultured at 37°C, 5%
CO2 in DMEM/F12, containing 2.5 mM L-glutamine, 10%
fetal bovine serum, 0.25% sodium bicarbonate, 40 U/ml penicillin G
and 40 μg/ml streptomycin.
Cell irradiation and treatments
Approximately 5×104 cells/well of
exponentially growing cells were seeded into 6-well plates. Cells
were irradiated at room temperature with a single dose of 0, 2, 4
or 6 Gy at a dose rate of 2.2 Gy/min (Cobalt-60 source; Theratron
Phoenix). The source to sample distance was 80 cm. After
irradiation, cells were incubated at 37°C in a 5% CO2
humidified atmosphere. For G1 checkpoint analyses, the cells were
cultivated with growth medium containing 10% fetal bovine serum for
12 h. Then, the cells were washed with PBS, cultivated in
serum-free medium for 24 h and irradiated with a single dose of 6
Gy. Subsequently, cells were released from starvation by
replacement the medium with fresh growth medium containing 10%
fetal bovine serum. The cells were collected at different time
points for further analysis. For G2 checkpoint, CCA cells were
gamma-irradiated with a single dose of 6 Gy and subsequently, the
irradiated cells were collected at different time points for cell
cycle and western blot analysis. For checkpoint kinase inhibitor
treatment, cells were treated with 50 or 100 nM of
1-(2-((S)-piperidin-3-ylcarbamoyl)-5-(3-fluorophenyl)thiophen-3-yl)
urea (AZD7762) (Selleck Chemicals, USA) for 2 h. Then, the cells
were irradiated with a single dose of 2, 4 or 6 Gy, and were
collected at different time points for further analysis.
Clonogenic cell survival assays
Cells were seeded in duplicate into a 6-well plate.
The cell number seeded per plate varied with the radiation dose, so
that colonies could be counted conveniently. The cells were
irradiated with a single dose of 0, 2, 4 or 6 Gy. The cells were
allowed to grow for 10–14 days until the surviving cells produced
macroscopically visible colonies. The cells were fixed with 95%
ethanol for 10 min and then stained with Giemsa for 10 min.
Colonies containing more than 50 cells were counted, and survival
fractions were calculated as ratios of the amount of colonies
formed from treated cells and untreated cells, corrected for
plating efficiency.
Cell cycle analysis
Approximately 8×104 cells/well were
seeded into a 6-well plate and cultivated for 12 h. Two hours prior
to irradiation, the cells were treated with or without 50 or 100 nM
of AZD7762. The treated cells were irradiated with a single dose of
6 Gy and collected 24 h after irradiation. Propidium iodide (PI)
staining of isolated nuclei for cell cycle analysis was performed
as described previously (35).
Briefly, the cell pellet was mildly resuspended in a solution
containing 584 μg/ml NaCl, 1,000 μg/ml Na-citrate, 10 μg/ml RNase
A, 0.3 μg/ml Nonidet P-40 and 50 μg/ml PI. The cell suspensions
were incubated for 30 min in the dark at room temperature, followed
by the addition of a solution containing 15 mg/ml citric acid, 0.25
mM sucrose and 50 μg/ml PI. The suspension of PI-stained isolated
nuclei was analyzed with a FACScan (Becton-Dickinson) flow
cytometer.
Terminal deoxynucleotidyltransferase dUTP
nick end labeling (TUNEL) assay
Cells were seeded onto sterile glass cover slips and
cultured in 6-well plates overnight. The cells were treated with
100 nM μg/ml of AZD7762 or 6 Gy of gamma-irradiation alone or with
a combination of both treatments. Forty-eight hours after
irradiation, a TUNEL assay was performed according to the
manufacturer’s protocol (Click-iT® TUNEL Alexa
Fluor® 647 Imaging Assay, Invitrogen). The stained cells
were visualized under fluorescent microscope. For each treatment
condition, the number of TUNEL-positive stained cells was counted
from 30 randomly selected fields and expressed as percentage of the
total number of nucleated cells.
Western blot analysis
Total protein was extracted from cells at indicated
time points after each treatment as described (35). A total of 30 μg of protein from
each sample were separated by SDS-polyacrylamide gels
electrophoresis and electro-blotted onto PVDF membranes. The
membranes were blocked in TBS-T containing 5% non-fat skim milk for
1 h at room temperature. Then, the membranes were probed with a
primary antibody diluted in 3% BSA in TBS-T overnight at 4°C. After
washing thrice with TBS-T, the membranes were incubated with a
horseradish peroxidase-labeled secondary antibody diluted in
blocking buffer for 1 h. The membranes were washed thrice with
TBS-T and the immunoreactivity was detected by chemiluminescence
(GE Healthcare, Buckinghamshire, UK) on X-ray film.
Statistical analyses
Data are shown as the mean ± SD or SE of three
independent experiments. For survival analysis, survival fractions
SF were normalized to the survival observed in the treatment
groups that received no irradiation. Survival curves were fitted to
a linear quadratic model of the form SF =
e−αd−βd2
for the cell lines KKU-M214 and KKU-M055, where d is the
radiation dose and α and β are constants. Survival of
the cell line KKU-100 was fitted to a linear model of the form
SF = e−αd.
D37 values were calculated from three independent
experiments and tested for significant differences by ANOVA
analysis followed by Tukey’s post hoc testing.
Results
Human CCA cells possess different
radiosensitivities
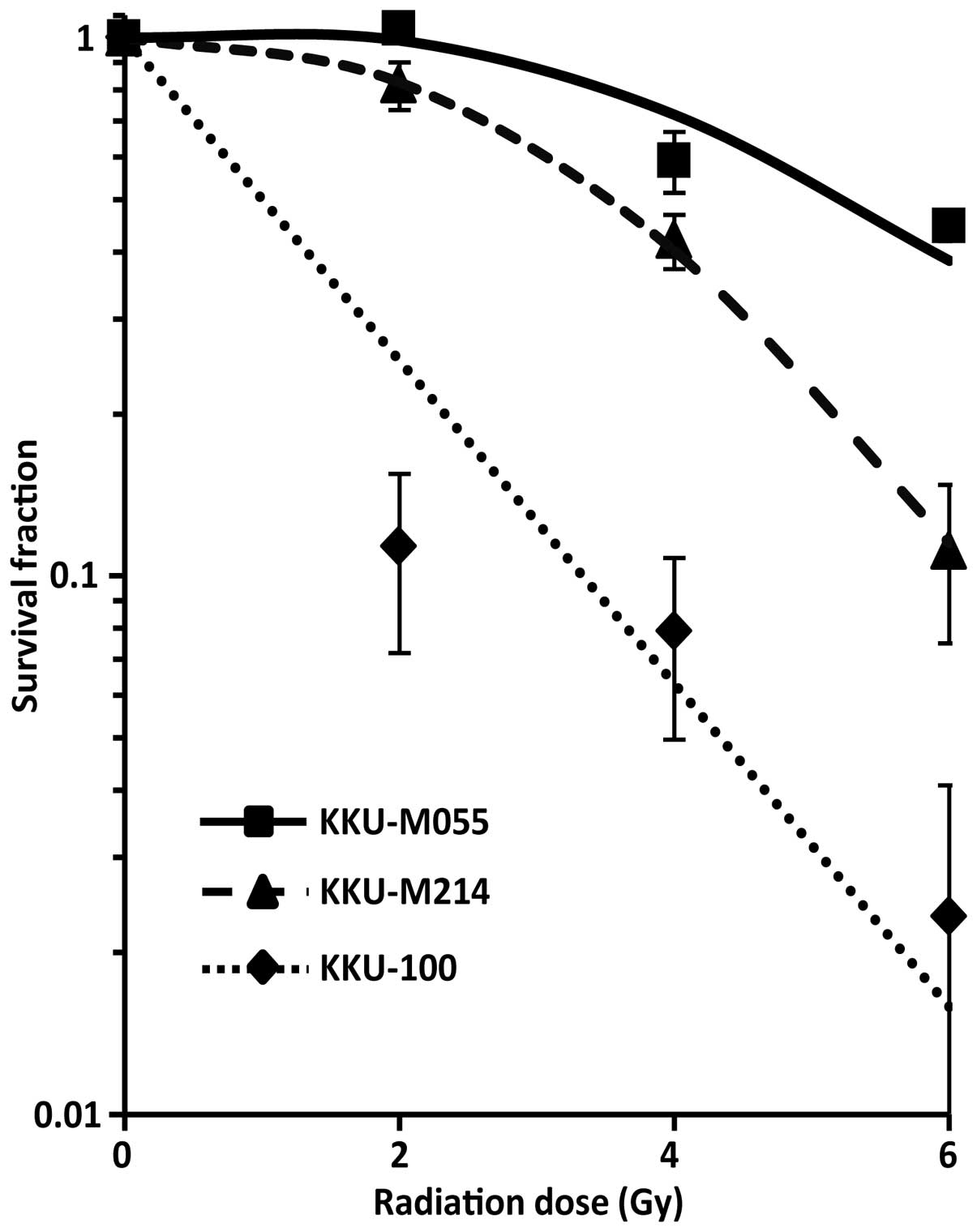
We first assessed the radiosensitivity of each CCA
cell line. Clonogenic survival assays were performed after gamma
irradiated with the dose rate of 2.2 Gy/min. Cell survival curves
were plotted and the D37 values were calculated. As
shown in Fig. 1, the
radiosensitivity of CCA cell lines varied from the most
radiosensitive to the most radioresistant cell lines KKU-100
>KKU-M214 >KKU-M055 with the D37 values of
1.5±0.2, 4.2±0.2 and 6.1±0.2 Gy, respectively.
P53 status of the human CCA cells in
response to radiation damage
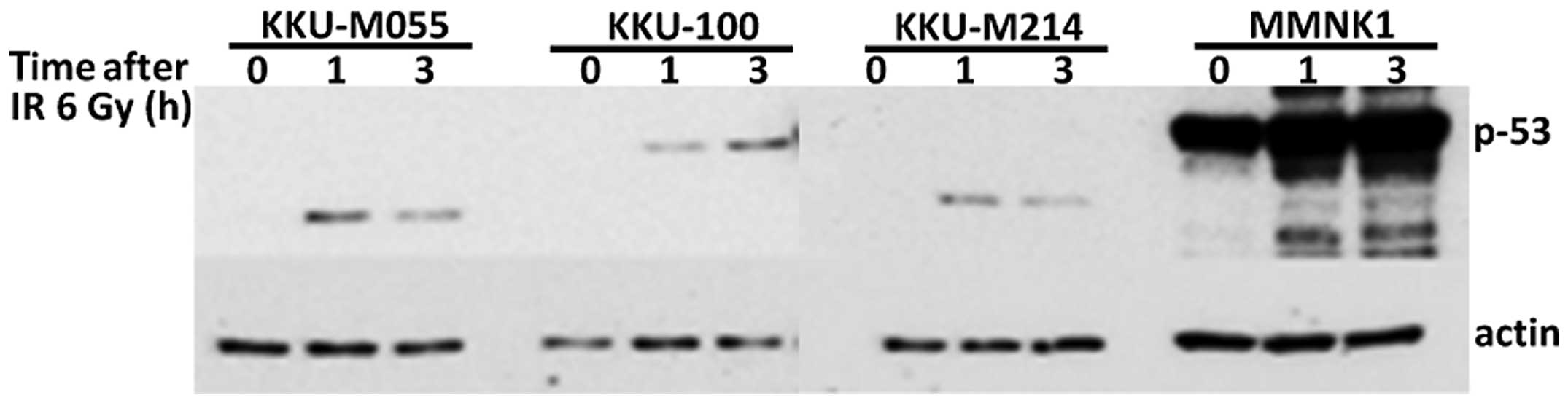
As the accumulation of p53 protein following
irradiation is an important determinant of cellular
radiosensitivity, we further determined p53 levels of each cell
line after exposure to radiation. The levels of p53 proteins in CCA
cell lines observed as full length in KKU-100 or truncated as
observed in KKU-M214 and KKU-M055 and the non-cancerous MMNK1 cell
were increased with time of irradiation (Fig. 2). The levels of p53 protein were
significantly lower in all CCA cell lines as compared to that of
the immortalized cholangiocyte MMNK1 cells.
Radiation-induced apoptosis in human CCA
cells
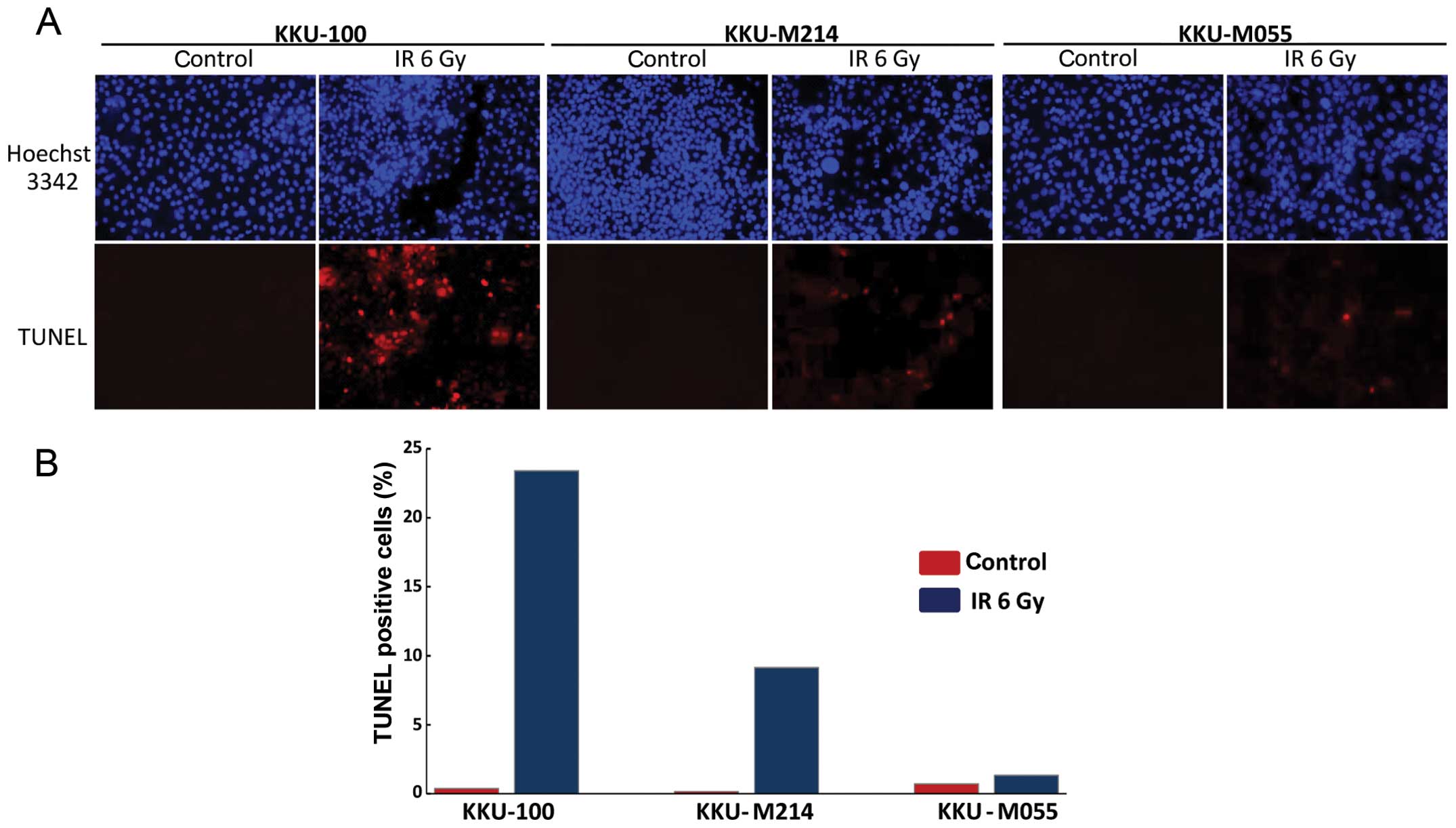
Apoptosis is a common mechanism of cell death in
tumors induced by ionizing radiation. Therefore, apoptosis
induction in CCA cell lines was determined in this study (Fig. 3). In the most radiosensitive cell
line (KKU-100), apoptosis was strongly induced from 0.37% apoptotic
cells in the control cultures to 23.40% in the irradiated cell
cultures at 48 h after irradiation. Apoptosis induction in KKU-M214
cell line was moderate. The amount of apoptotic cells increased
from 2.14 to 9.15%. Remarkably, radiation increased the amount of
apoptotic cells only slightly from 0.71 to 1.43% in KKU-M055 cells,
which is the most radioresistant cell line.
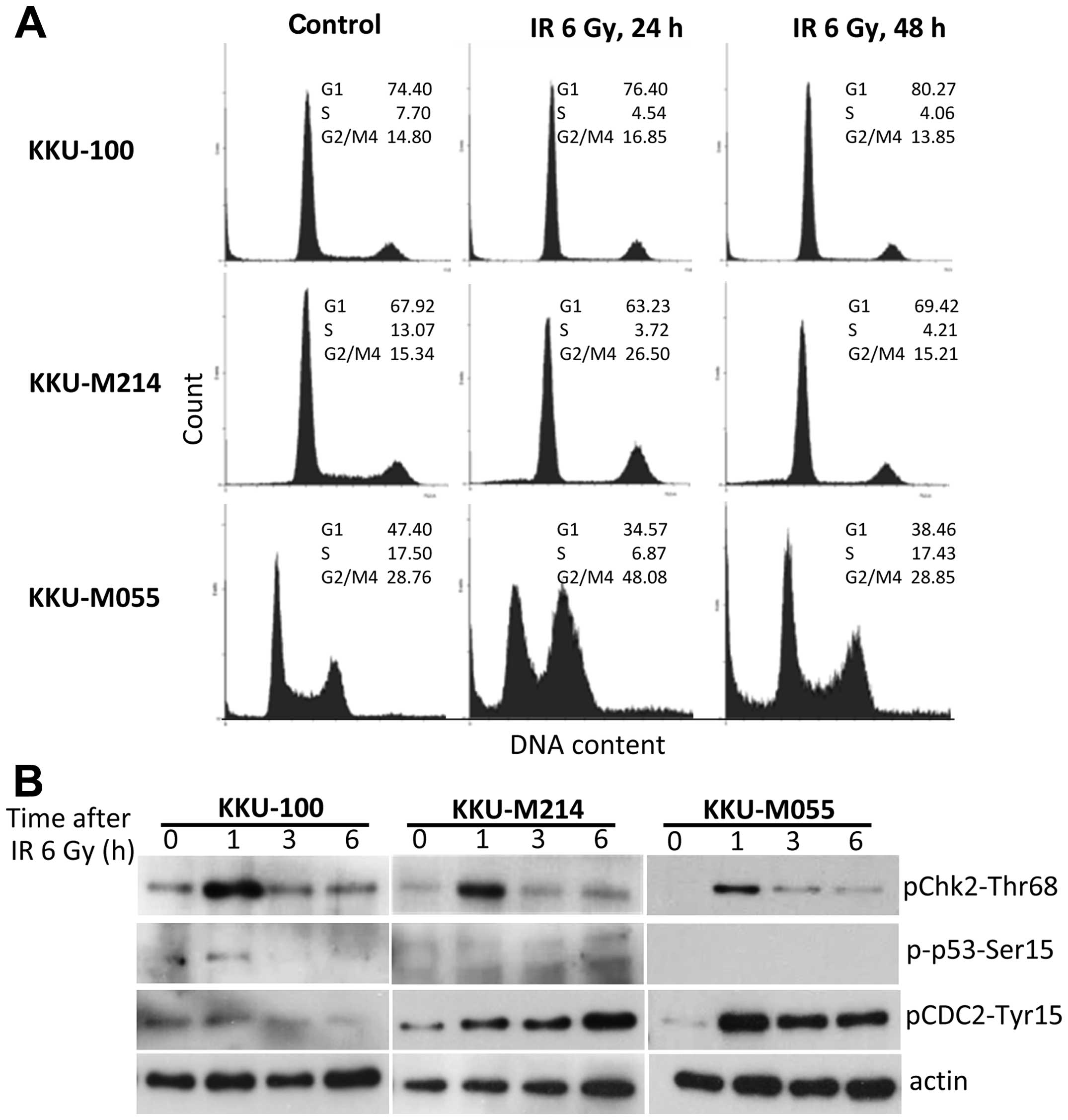
G1 checkpoint is defective in
radioresistant CCA cells
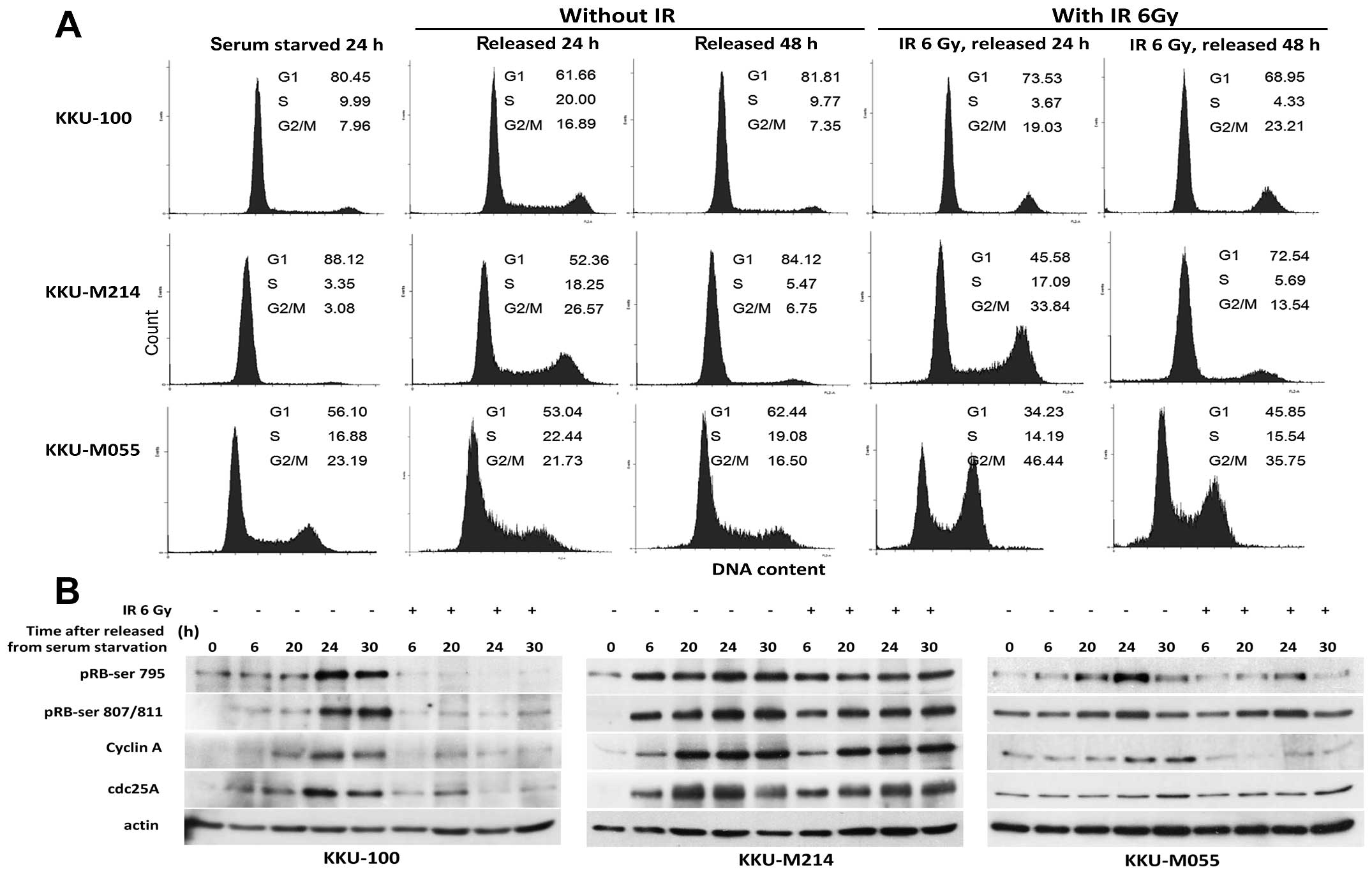
To assess whether CCA cell lines had a defect in G1
checkpoint, the cells were synchronized at early G1 phase by serum
deprivation, then were irradiated and released from starvation as
described in Materials and methods. Representative results of cell
cycle analyses are shown in Fig.
4A. After release from serum starvation, the proportions of the
cells in S phase were significantly increased in all CCA cell lines
as observed at 24 h then declined to nearly basal level within 48
h. However, in the cells that were irradiated prior to release from
serum starvation, only KKU-100 cells were persisted at the G1 phase
while KKU-M214 and KKU-M055 cells entered S and G2/M phase
dramatically. These results indicate that G1 checkpoint was
effective in KKU-100 cells, but defective in M055 and KKU-M214
cells. The observations were further underlined by detection of
proteins involved in the cell cycle and checkpoint control during
G1/S transition. As shown in Fig.
4B, after release from serum starvation, levels of pRb-Ser795,
pRb-Ser807/811, cdc25A and cyclin A were clearly induced in all
unirradiated CCA cells. Remarkably, these proteins were not induced
in KKU-100 cells that were irradiated prior to release from serum
starvation. This finding indicated no progression of KKU-100 cells
from G1 to S phase in response to radiation-induced damage. In
contrast, induction of pRb-Ser795 and pRb-Ser807/811 was observed
in irradiated KKU-M214 and KKU-M055 cells. In addition, cdc25A, and
cyclin A were increased with time after irradiation in KKU-M214
cells. From these results, it can be assumed that KKU-M214 and
KKU-M055 cells were not halted at G/S phase in response to
radiation-induced damage.
Transient activation of the G2 checkpoint
in radioresistant CCA cells
To investigate the efficiency of the G2 checkpoint
of CCA cells, cell cycle and protein levels of phospho-p53,
phospho-Chk2 kinase and phospho-Cdc2 kinase were analyzed. Cell
cycle distributions were analyzed 24 and 48 h after irradiation
(Fig. 5A). A radiation-induced
G2/M block was clearly demonstrated in KKU-M055 cells by an
increase of the G2/M population from 29 to 48% at 24 h after
irradiation. However, the G2/M population of KKU-M055 cells
decreased to base line level within 48 h as compared to that of
unirradiated control cells. The G2/M population of KKU-M214 cells
was moderately increased from 15 to 27% at 24 h after irradiation
and decreased to 15% within 48 h after irradiation. These results
indicate that the G2 checkpoints of KKU-M055 and KKU-M214 cells
were transiently activated but cells failed to prolong the G2
arrest after gamma-irradiation. Remarkably, no activation of the G2
checkpoint was found in KKU-100 cells. These findings are
consistent with the rapid induction of phospho-Chk2 and
phospho-Cdc2 without induction of phospho-p53 in response to
radiation damage in KKU-M055 and KKU-M214 cells. In KKU-100 cells,
only phospho-Chk2 levels were strongly increased after irradiation,
while phospho-Cdc2 and phospho-p53 were slightly elevated (Fig. 5B).
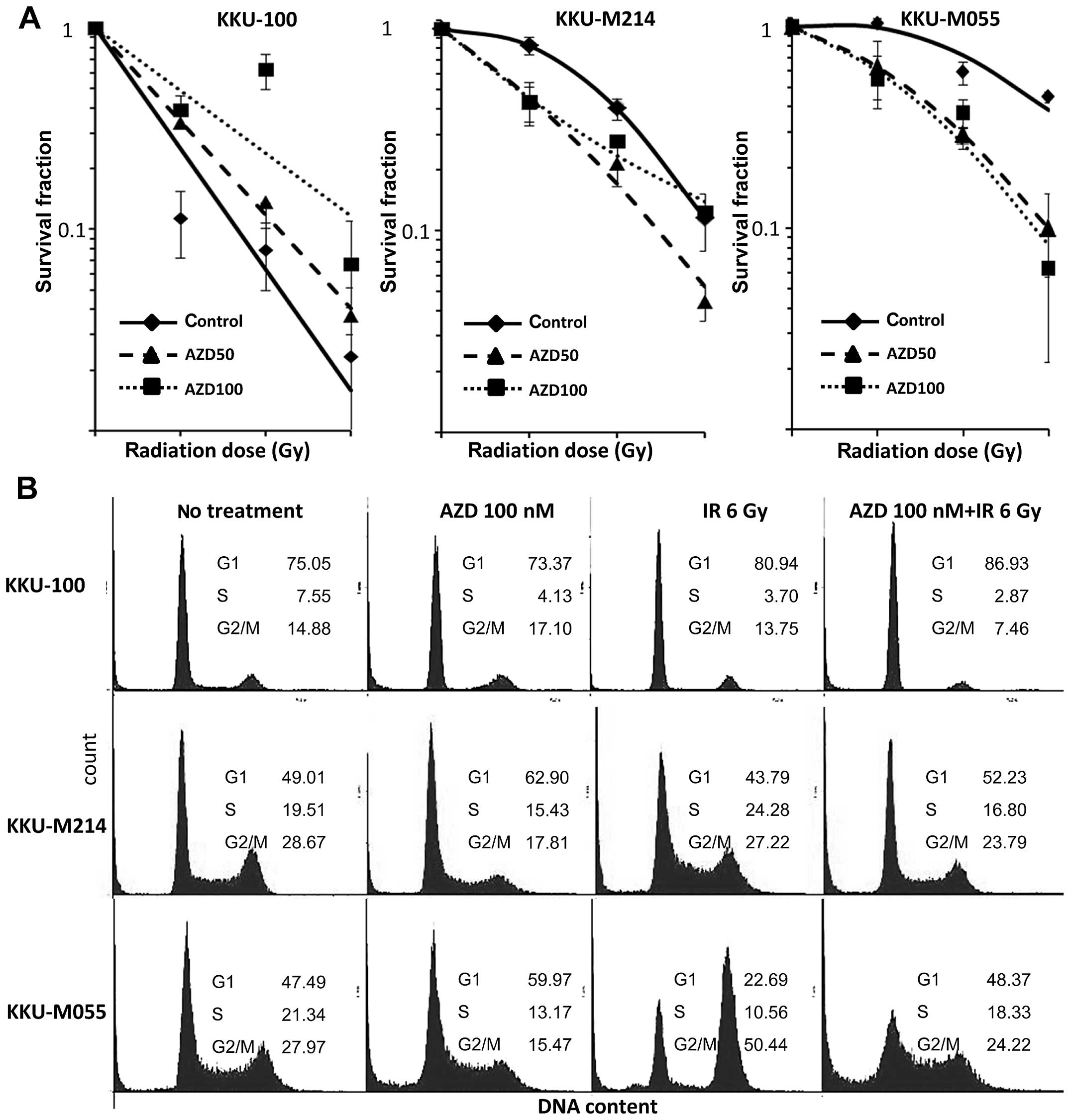
Chk1/2 inhibitor enhances the
radiosensitivity of radioresistant CCA cells
The inhibition of Chk1 and Chk2 has been reported to
enhance the radiosensitivity of several cancer cell lines.
Therefore, the potential of Chk1/2 inhibitor (AZD7762) to enhance
radiosensitivity of CCA cells was investigated. In all three cell
lines, AZD7762 treatment significantly impacted cellular
radiosensitivity (Fig. 6A and
Table I). Treatment of the cells
with AZD7762 significantly enhanced radiosensitivity of KKU-M055
and KKU-M214 cells. The radiation enhancement ratios for KKU-M055
cells, calculated from D37 values are 1.8 and 2.0 for 50
nM and 100 nM AZD7762, respectively. The radiation enhancement
ratios for KKU-M214 cells are 1.7 and 1.6 for 50 nM and 100 nM
AZD7762, respectively. However, a contradictory effect of AZD7762
on the radiosensitvity of KKU-100 cells was found. AZD7762
treatment significantly reduced radiosensitivity of KKU-100 cells,
reflected by an increase of the D37 value from 1.5 to
1.9 and 2.7 for the concentration of 50, and 100 nM AZD7762,
respectively. This result indicates antagonistic effect of AZD7762
on radiation-induced cell killing in KKU-100 cells.
 | Table ID37 values observed in
different cell lines after AZD7762 treatment. |
Table I
D37 values observed in
different cell lines after AZD7762 treatment.
| Cell line | AZD (nM) | D37 (Gy)
± SD | p-value (vs. no AZD
treatment) | p-value (vs. 100 nM
AZD treatment) |
|---|
| KKU-100 | 0 | 1.5±0.2 | | |
| 50 | 1.9±0.1 | 0.338 | |
| 100 | 2.7±0.5 | 0.006a | 0.034b |
| KKU-M214 | 0 | 4.1±0.2 | | |
| 50 | 2.4±0.5 | 0.010a | |
| 100 | 2.6±0.6 | 0.015a | 0.928 |
| KKU-M055 | 0 | 6.1±0.2 | | |
| 50 | 3.4±0.8 | 0.004a | |
| 100 | 3.1±0.7 | 0.002a | 0.827 |
Chk1/2 inhibitor abrogates
radiation-induced G2 arrest of CCA cells
Inhibition of Chk1/2 kinases has been shown to
abrogate G2 checkpoint in response to radiation-induced DNA damage
(36). To investigate the effect
of AZD7762 on the radiation-induced G2/M arrest of CCA cells, the
cell cycle distribution profiles were analyzed for irradiated cells
with or without AZD7762 pretreatment (Fig. 6B).
Radiation-mediated G2 arrest and abrogation of G2
arrest by AZD7762 was merely observed in KKU-100 and KKU-M214
cells. By contrast, G2 arrest was strongly induced by radiation
treatment in KKU-M055 cells and treatment of the cells with AZD7762
significantly abrogated G2 arrest.
Discussion
Cellular radiosensitivity is influenced by several
factors of DNA damage response and repair processes, cell cycle
regulation and cell death pathways (21,23,24).
In this study, we demonstrated that three CCA cell lines possessed
remarkably different radiosensitivity. The differences were shown
partly due to intrinsic factors including the intact p53 and DNA
damage checkpoints. Based on this context, KKU-100 cells, shown to
be chemotherapeutic resistant (37), were sensitive to radiation. In
contrast, chemosensitive cell, KKU-M055, were resistant to
irradiation. Moreover, we showed that Chk1/2 inhibitor could
abrogate radiation-induced G2 arrest and enhanced radiosensitivity
of KKU-M055.
Radiotherapy is regarded as a promising approach to
control CCA either as monotherapy or combined with surgical
intervention (9,14,19).
Although most studies have demonstrated a survival benefit of
radiotherapy, the effectiveness of this treatment modality remains
very low due to the resistance of CCA cells to the fatal effects of
radiation (15,19,20,38).
The variation of the radiation responsiveness of individual cancer
cells is a key factor that influences radiation treatment outcome
(22,39,40).
In this study, we demonstrated that the radiosensitivity of CCA
cell lines-KKU-100, KKU-M214 and KKU-M055 was considerably
different, with D37 values of 1.5 Gy of the most
radiosensitive cells (KKU-100) versus 6.1 Gy of the most
radioresistant cells (KKU-M055). Interestingly, among three CCA
cell lines, KKU-100 has been reported to be the most resistant cell
line towards chemotherapeutic drugs (37). Therefore, it would be possible that
radiotherapy could be an effective treatment in chemotherapy
resistant CCA.
The p53 tumor suppressor protein plays an important
role in the regulation of the above described processes (41). It has been reported that cells
containing wild-type p53 are more sensitive to radiation than
mutant p53-expressing cells (42).
In this study, an accumulation of p53 protein following
radiation-induced DNA damage was found in all three CCA cell lines.
Nevertheless, expression of full length p53 protein was found in
KKU-100 cells only, whereas KKU-M055 and KKU-M214 cells expressed
truncated p53. Thus, it can be speculated that the different
radiosensitivities of the three CCA cell lines may result from
alterations of p53 functions in these cells.
Efficiency of DNA damage checkpoints has a major
impact on cellular radiosensitivity. However, p53 mediates cellular
radiosensitivity only in the G1-phase of the cell cycle (42). We found that KKU-100, the most
radiosensitive cell line, expressed full-length p53 and possessed
an intact G1 checkpoint. Therefore, radiosensitivity of CCA cells
might rely on the function of p53 and the efficiency of the G1
checkpoint. Since various p53 mutations may result in different
phenotypes, the impact of p53 and the G1 checkpoint on the
radiosensitivity of CCA cells needs to be further investigated.
Evidence of several studies reveals a strong
relationship between apoptotic cell death and radiosensitivity of
tumors (23). In the present
study, radiation is a potent inducer of apoptosis in the most
radiosensitive cell line (KKU-100). Conversely, the potential of
radiation to induce apoptosis is very weak in the most
radioresistant cells (KKU-M055). Therefore, it can be speculated
that cell death by apoptosis might determine overall
radiosensitivity of CCA cells. Frequently, aberrations in apoptosis
are found in cancer cells carrying p53 mutations and result in a
radioresistant phenotype (43). In
agreement with our findings, KKU-M055 and M214 cells, expressing
truncated p53 proteins are more resistant to radiation as compared
to KKU-100 cells. However, the role of p53 and apoptosis for
determining the radiosensitivity of CCA cells remains to be further
clarified.
Several studies revealed that Chk1/2 inhibition
offers significant and selective tumor radiosensitization (29). In this study, a radiosensitization
effect of the Chk1/2 inhibitor AZD7762 was found in two CCA cell
lines (KKU-M055 and M214). These cells express a truncated p53
protein and are defective in the G1 checkpoint. On the other hand,
the Chk1/2 inhibitor AZD7762 treatment failed to enhance
radiosensitivity of KKU-100 cells. Since AZD7762 abrogates
radiation-induced G2 arrest in KKU-M055 cells only, it is unlikely
that the abrogation of the G2 checkpoint primarily contributes to
AZD7762-mediated radiosensitization of CCA cells. Recently, AZD7762
was reported to radiosensitize p53 mutant breast cancer cells by
inhibiting DNA damage repair and promoting radiation-induced
apoptosis and mitotic catastrophe (30). Thus, it is possible that the
radiosensitization effect of AZD7762 in CCA cells is mediated via
DNA damage repair process and/or cell death pathways.
In conclusion, the benefit of radiotherapy in terms
of survival outcomes of CCA patients could be improved by
technological advancement of radiation therapy delivery and by
selecting tumor entities that respond well to this treatment
option. Thus, identification of molecular markers for predicting
radiation response of this disease is required. In this study, we
demonstrated that the variation of radiosensitivity of CCA cells is
correlated with their p53 status and existing G1 and/or G2
checkpoint defects. We also demonstrated the potential of
checkpoint kinase Chk1/2 inhibition on the enhancement of the
radiosensitivity of CCA cells. Thus, this study provides useful
information for predicting radiation response and provides evidence
for the enhancement of radiotherapeutic efficiency by targeting
checkpoint kinase Chk1/2 in some subpopulations of CCA
patients.
Acknowledgements
This research was supported by Khon Kaen University
Research grant and the Higher Education Research Promotion and
National Research University Project of Thailand, Office of the
Higher Education Commission, through the Health cluster (SHeP-GMS)
to S.W. We thank the radiation therapy department, Buddchachinaraj
Hospital, Phitsanulok for providing the radiation source.
References
|
1
|
Welzel TM, McGlynn KA, Hsing AW, O’Brien
TR and Pfeiffer RM: Impact of classification of hilar
cholangiocarcinomas (Klatskin tumors) on the incidence of intra-
and extrahepatic cholangiocarcinoma in the United States. J Natl
Cancer Inst. 98:873–875. 2006. View Article : Google Scholar
|
|
2
|
Luke C, Price T and Roder D: Epidemiology
of cancer of the liver and intrahepatic bile ducts in an Australian
population. Asian Pac J Cancer Prev. 11:1479–1485. 2010.PubMed/NCBI
|
|
3
|
Khan SA, Taylor-Robinson SD, Toledano MB,
Beck A, Elliott P and Thomas HC: Changing international trends in
mortality rates for liver, biliary and pancreatic tumours. J
Hepatol. 37:806–813. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patel T: Worldwide trends in mortality
from biliary tract malignancies. BMC Cancer. 2:102002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel T: Cholangiocarcinoma -
controversies and challenges. Nat Rev Gastroenterol Hepatol.
8:189–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arrington AK, Nelson RA, Falor A, Luu C,
et al: Impact of medical and surgical intervention on survival in
patients with cholangiocarcinoma. World J Gastrointest Surg.
5:178–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
DeOliveira ML, Cunningham SC, Cameron JL,
et al: Cholangiocarcinoma: thirty-one-year experience with 564
patients at a single institution. Ann Surg. 245:755–762.
2007.PubMed/NCBI
|
|
8
|
Hemming AW, Reed AI, Fujita S, Foley DP
and Howard RJ: Surgical management of hilar cholangiocarcinoma. Ann
Surg. 241:699–702. 2005. View Article : Google Scholar
|
|
9
|
Ramirez-Merino N, Aix SP and Cortes-Funes
H: Chemotherapy for cholangiocarcinoma: An update. World J
Gastrointest Oncol. 5:171–176. 2013. View Article : Google Scholar
|
|
10
|
Zografos GN, Farfaras A, Zagouri F,
Chrysikos D and Karaliotas K: Cholangiocarcinoma: principles and
current trends. Hepatobiliary Pancreat Dis Int. 10:10–20. 2011.
View Article : Google Scholar
|
|
11
|
Alberts SR, Al-Khatib H, Mahoney MR, et
al: Gemcitabine, 5-fluorouracil, and leucovorin in advanced biliary
tract and gallbladder carcinoma: a North Central Cancer Treatment
Group phase II trial. Cancer. 103:111–118. 2005. View Article : Google Scholar
|
|
12
|
Patt YZ, Hassan MM, Aguayo A, et al: Oral
capecitabine for the treatment of hepatocellular carcinoma,
cholangiocarcinoma, and gallbladder carcinoma. Cancer. 101:578–586.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Woo SM, Lee WJ, Han SS, et al:
Capecitabine plus cisplatin as first-line chemotherapy for advanced
biliary tract cancer: a retrospective single-center study.
Chemotherapy. 58:225–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maithel SK, Gamblin TC, Kamel I,
Corona-Villalobos CP, Thomas M and Pawlik TM: Multidisciplinary
approaches to intrahepatic cholangiocarcinoma. Cancer.
22:3929–3942. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barney BM, Olivier KR, Miller RC and
Haddock MG: Clinical outcomes and toxicity using stereotactic body
radiotherapy (SBRT) for advanced cholangiocarcinoma. Radiat Oncol.
7:672012. View Article : Google Scholar
|
|
16
|
Gonzalez Gonzalez D, Gouma DJ, Rauws EA,
van Gulik TM, Bosma A and Koedooder C: Role of radiotherapy, in
particular intraluminal brachytherapy, in the treatment of proximal
bile duct carcinoma. Ann Oncol. 10(Suppl 4): 215–220.
1999.PubMed/NCBI
|
|
17
|
Isayama H, Tsujino T, Nakai Y, et al:
Clinical benefit of radiation therapy and metallic stenting for
unresectable hilar cholangiocarcinoma. World J Gastroenterol.
18:2364–2370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McMasters KM, Tuttle TM, Leach SD, Rich T,
Cleary KR, Evans DB and Curley SA: Neoadjuvant chemoradiation for
extrahepatic cholangiocarcinoma. Am J Surg. 174:605–608. 1997.
View Article : Google Scholar
|
|
19
|
Brunner TB and Eccles CL: Radiotherapy and
chemotherapy as therapeutic strategies in extrahepatic biliary duct
carcinoma. Strahlenther Onkol. 186:672–680. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ghafoori AP, Nelson JW, Willett CG, et al:
Radiotherapy in the treatment of patients with unresectable
extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys.
81:654–659. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mladenov E, Magin S, Soni A and Iliakis G:
DNA double-strand break repair as determinant of cellular
radiosensitivity to killing and target in radiation therapy. Front
Oncol. 3:1132013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baumann M, Krause M and Hill R: Exploring
the role of cancer stem cells in radioresistance. Nat Rev Cancer.
8:545–554. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Balcer-Kubiczek EK: Apoptosis in radiation
therapy: a double-edged sword. Exp Oncol. 34:277–285.
2012.PubMed/NCBI
|
|
24
|
Shimura T: Acquired radioresistance of
cancer and the AKT/GSK3beta/cyclin D1 overexpression cycle. J
Radiat Res. 52:539–544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K
and Linn S: Molecular mechanisms of mammalian DNA repair and the
DNA damage checkpoints. Annu Rev Biochem. 73:39–85. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmitt CA: Senescence, apoptosis and
therapy - cutting the lifelines of cancer. Nat Rev Cancer.
3:286–295. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawabe T: G2 checkpoint abrogators as
anticancer drugs. Mol Cancer Ther. 3:513–519. 2004.PubMed/NCBI
|
|
28
|
Bucher N and Britten CD: G2 checkpoint
abrogation and checkpoint kinase-1 targeting in the treatment of
cancer. Br J Cancer. 98:523–528. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lapenna S and Giordano A: Cell cycle
kinases as therapeutic targets for cancer. Nat Rev Drug Discov.
8:547–566. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma Z, Yao G, Zhou B, Fan Y, Gao S and Feng
X: The Chk1 inhibitor AZD7762 sensitises p53 mutant breast cancer
cells to radiation in vitro and in vivo. Mol Med Rep.
6:897–903. 2012.PubMed/NCBI
|
|
31
|
Riesterer O, Matsumoto F and Wang L: A
novel Chk inhibitor, XL-844, increases human cancer cell
radiosensitivity through promotion of mitotic catastrophe. Invest
New Drugs. 29:514–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tandle AT, Kramp T, Kil WJ, et al:
Inhibition of polo-like kinase 1 in glioblastoma multiforme induces
mitotic catastrophe and enhances radiosensitisation. Eur J Cancer.
49:3020–3028. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang Y, Dai Y, Grant S and Dent P:
Enhancing CHK1 inhibitor lethality in glioblastoma. Cancer Biol
Ther. 13:379–388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maruyama M, Kobayashi N, Westerman KA, et
al: Establishment of a highly differentiated immortalized human
cholangiocyte cell line with SV40T and hTERT. Transplantation.
77:446–451. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hematulin A, Sagan D, Eckardt-Schupp F and
Moertl S: NBS1 is required for IGF-1 induced cellular proliferation
through the Ras/Raf/MEK/ERK cascade. Cell Signal. 20:2276–2285.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morgan MA, Parsels LA, Zhao L, et al:
Mechanism of radiosensitization by the Chk1/2 inhibitor AZD7762
involves abrogation of the G2 checkpoint and inhibition of
homologous recombinational DNA repair. Cancer Res. 70:4972–4981.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tepsiri N, Chaturat L, Sripa B, Namwat W,
Wongkham S, Bhudhisawasdi V and Tassaneeyakul W: Drug sensitivity
and drug resistance profiles of human intrahepatic
cholangiocarcinoma cell lines. World J Gastroenterol. 11:2748–2753.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Deodato F, Clemente G, Mattiucci GC, et
al: Chemoradiation and brachytherapy in biliary tract carcinoma:
long-term results. Int J Radiat Oncol Biol Phys. 64:483–488. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bonkhoff H: Factors implicated in
radiation therapy failure and radiosensitization of prostate
cancer. Prostate Cancer. 2012:5932412012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Coventry BJ and Ashdown ML: Complete
clinical responses to cancer therapy caused by multiple divergent
approaches: a repeating theme lost in translation. Cancer Manag
Res. 4:137–149. 2012. View Article : Google Scholar
|
|
41
|
Fei P and El-Deiry WS: P53 and radiation
responses. Oncogene. 22:5774–5783. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mazzatti DJ, Lee YJ, Helt CE, O’Reilly MA
and Keng PC: p53 modulates radiation sensitivity independent of p21
transcriptional activation. Am J Clin Oncol. 28:43–50. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu C and El-Deiry WS: Targeting p53 for
enhanced radio- and chemo-sensitivity. Apoptosis. 14:597–606. 2009.
View Article : Google Scholar : PubMed/NCBI
|