Introduction
Mycosis fungoides (MF) is the most common form of
cutaneous T-cell lymphomas (CTCL), which is a heterogeneous group
of non-Hodgkin’s lymphomas thought to result from malignancies of
skin homing T cells (1) and
presenting with patches, plaques and tumors.
While the early stage shows a wide clinical spectrum
that overlaps with several inflammatory dermatoses, clinical as
well as pathological findings indicate various overlaps between MF
and inflammatory diseases, with epidermotropism disproportionate to
the degree of spongiosis being one of the most useful pathological
distinguishing features.
Adult T-cell leukemia/lymphoma (ATLL) is a human
malignancy associated with human T-cell lymphotropic virus-type I
(HTLV-1). Cutaneous lesions of ATLL consist of papules and
nodules/tumors. While survival of ATLL cases with skin
manifestations is reportedly significantly shorter than that of MF
cases (2), histological findings
for the two diseases are similar.
The mechanism of epidermotrophism seen in MF and
ATLL has not yet been clearly identified. What is known is that
chemokines regulate multiple cell functions, including cell
chemotaxis, proliferation and apoptosis, and are involved in
leukocyte transendothelial migration and homing to tissues.
Previous studies have reported that positivity for CCR4 is
significantly associated with skin involvement in MF and ATLL, and
CCR10 is expressed by malignant cells in MF and Sézary syndrome
(SS) (3). Malignant MF and SS
cells also show high expression of CCR7. The cutaneous
lymphocyte-associated antigen (CLA) recognized by the HECA-452
antibody is an adhesion molecule selectively expressed by a subset
of circulating memory T-cells, normal T cells in inflamed skin and
by the vast majority of CTCL (4–6). A
previous study found that CLA was expressed on epidermotrophic
lymphoma cells in all early stages MF. As for ATLL, CLA+
cases showed a significant preference for skin involvement when
compared with the CLA− cases as also previously reported
by other investigators (7).
Complementary DNA (cDNA) microarray technology is a
powerful tool for gaining insight into the molecular complexity and
pathogenesis of various diseases and makes it possible to identify
differences in numerous gene expressions.
Since the skin consists of epidermis [including
keratinocytes and dendritic cells (DCs)], dermis (stroma and
vessels) and subcutaneous tissue with or without inflamed and/or
inflammatory cells, gene expression of epidermis and dermis should
be analyzed separately to gain a better understanding of the
contribution made by each component of a tissue to the pathogenesis
of epidermotrophism. To this end, laser capture microdissection
(LMD) was used in our study to allow us to focus on the differences
in gene expression levels between epidermis and dermis in MF and
ATLL and to subsequently determine these expressions
immunohistochemically in patients.
It is believed that this technique, which combines
LMD and microarray technology for the analysis of CTCL gene
expression in epidermis and dermis, can improve our understanding
of epidermotrophism at the molecular level.
Materials and methods
Eighteen skin samples obtained from patients treated
at the Department of Dermatology, Kurume University School of
Medicine consisted of five epidermal and three dermal samples each
from MF and ATLL as well as two epidermal samples from dermatitis
patients used as controls. Skin biopsies were performed after
informed consent had been obtained from all patients or their
guardians in accordance with the Declaration of Helsinki. Both
paraffin-embedded and frozen tissues were used. Histopathological
diagnoses were based on the World Health Organization
classification (WHO) and performed by five pathologists (K.H.,
J.K., Y.K., H.S. and K.O.). The clinical data for all patients
obtained from the medical records are summarized in Table I, which shows that both epidermal
and dermal samples were harvested from two MF (E-M3/D-M1 and
E-M5/D-M2) and two ATLL (E-A2/D-A1 and E-A3/D-A2) patients. The
other samples were either epidermal or dermal. This study was
approved by the Kurume University Institutional Review Board.
 | Table ISummary of patients enrolled in this
study: number, age, gender and stage. |
Table I
Summary of patients enrolled in this
study: number, age, gender and stage.
| Sample no. | Age (years) | Gender | TNM | Stage |
|---|
| MF |
| E-M1 | 78 | Male | T2aN0M0 | IB |
| E-M2 | 62 | Female | T2bN0M0 | IB |
| E-M3,D-M1 | 57 | Male | T1bN0M0 | IA |
| E-M4 | 22 | Male | T1bN0M0 | IA |
| E-M5, D-M2 | 64 | Male | T2bN0M0 | IB |
| D-M3 | 61 | Male | T2bN0M0 | IB |
| ATLL |
| E-A1 | 56 | Male | T2aN2M0 | IIA |
| E-A2, D-A1 | 70 | Male | T2cN0M0 | IB |
| E-A3, D-A2 | 32 | Female | T2cN0M0 | IB |
| E-A4 | 63 | Male | T3aNxM0 | IIB |
| E-A5 | 79 | Male | T3bN0M0 | IIB |
| D-A3 | 54 | Male | T3aN1M0 | IIB |
| Control |
| N1 | 66 | Male | | |
| N2 | 71 | Female | | |
Laser microdissection
The samples were embedded in an optical cutting
temperature compound (Sakura Finetechnical, Tokyo, Japan),
immediately frozen in liquid nitrogen, and stored at −80°C for
microdissection. A series of 10-μm thick sections were cut from
frozen tissue specimens at −20°C, and mounted on 2.0-μm-thick
PEN-Membrane slides (MicroDissect GmbH, Herborn, Germany). The
epidermal and/or dermal regions were microdissected from the about
70 cryosections by means of LMD. After fixation in 100% ethanol,
the slides were stained in turn with Mayer hematoxylin and eosin,
washed in diethylpyrocarbonate-treated water at each phase of the
process and then air-dried with a fan. The frozen sections were
microdissected with a Leica LMD6000 laser microdissection system
(Leica, Wetzlar, Germany) in accordance with the company’s
protocol. Finally, the microdissected fragments were dropped into
0.5 ml tube caps containing 50 μl lysis buffer for RNA extraction
(8).
RNA extraction and biotinylated cRNA
amplification
Total RNA was extracted from the samples collected
by means of LMD and with an RNAqueous-Micro kit (AM1931; Ambion,
Austin, TX) according to the manufacturer’s instructions.
Complementary RNA (cRNA) amplification and labeling with biotin
were used for gene expression profiling by microarray analysis.
Briefly, 500 ng total RNA was amplified overnight (14 h) with the
Illumina Total Prep RNA Amplification kit (AMIL1791; Ambion)
according to the manufacturer’s protocol. Reaction cRNA was
biotinylated during in vitro transcription.
Illumina BeadChips microarray
Sentrix Human WG-6 v3.0 Expression BeadChips were
purchased from Illumina, Inc. (San Diego, CA). More than 48,000
different bead types, each with a 50-base gene-specific probe, are
represented on a single Beadchip. For each probe represented on the
array, beads are assembled with an average 30-fold redundancy. A
hybridization mixture containing 1.5 μg biotinylated cRNA was
hybridized to the beadchips at 58°C overnight (18 h) before being
washed and stained with streptavidin-Cyanine-3 (Cy3) (PA23031; GE
Healthcare, Buckinghamshire, UK) according to the manufacturer’s
protocol. Beadchips were scanned on Illumina BeadStation 500 and
fluorescent hybridization signals were assessed with Illumina
Beadstudio software.
The data discussed in this publication have been
deposited in National Center for Biotechnology Information (NCBIs)
Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) and are accessible
through GEO Series accession number GSE40639.
Statistical analysis
For the pre-processing step, variance in the data
was first stabilized with the variance stabilizing transform method
(9) and then normalized with a
robust spline normalization method, both of which are used with the
Lumi BioConductor package (Illumina) (10). Effectively absent transcripts were
filtered out to reduce false positives. Detection of transcripts
was considered to be achieved if the detection p-value calculated
from the background with the Illumina BeadStudio was <0.05 for
all hybridizations. The Significance Analysis of the Microarrays
statistical test (11), which is
used as part of the bioconductor ‘samr’ package and takes multiple
testing into account by estimating the false discovery rate, was
used to identify differentially expressed transcripts for a
comparison of MF and ATLL patients. If a transcript was up- or
downregulated by a factor of ≥2 and had a Significance Analysis of
Microarrays q-value (false discovery rate) of <0.001, we
regarded the expression of this transcript in MF patients as
different from that in ATLL patients. To obtain reproducible
clusters for classifying the 16 samples (all samples detailed above
except for the two control samples), expression data were analyzed
with GeneSpring 7.2 software (Silicon Genetics, Redwood City, CA),
which was also used to generate heatmaps of certain genes of
interest.
Immunohistochemistry
The paraffin-embedded specimens were used for manual
immunohistochemical analysis of CCR4 (Pharmingen, San Diego, CA),
CCR7, CCR6 and CCR10 (all from Medical and Biological Laboratories
Co., Ltd., Nagoya, Japan), CCL20, CCL27, lymphotoxin (LT) β and TNF
receptor (TNFR) 2 (all from R&D Systems, Minneapolis, MN),
CCL21 (Santa Cruz Biotechnology, Santa Cruz, CA), β-defensin (BDF)
1 (Phoenix Pharmaceuticals, Inc., Burlingame, CA) as previously
described (12). Appropriate
positive and negative control experiments were run simultaneously.
Heat-mediated antigen retrieval was used for all analyses except
those of CCR6 and BDF1. The staining results were evaluated
semiquantitatively by three independent observers, and scored
comprehensively in view of intensity, expression pattern and number
of positive cells, and so on. Images were visualized with an
Olympus AX80 microscope (Olympus, Tokyo, Japan), equipped with an
Olympus Planapo 40x/0.95 numerical aperture objective. Images were
captured with an Olympus DP70 camera and Olympus DP controller
software, and were processed with Olympus DP manager software.
Results
Following appropriate normalization and
standardization procedures, the data on each chip were compared
with each other by using a hierarchical clustering method (Fig. 1). Genes differentially expressed in
MF and ATLL were organized by means of Ingenuity Pathway Analysis
into an interactome network, which was then used for an comparison
of the gene expression (transcriptional profile) of epidermal or
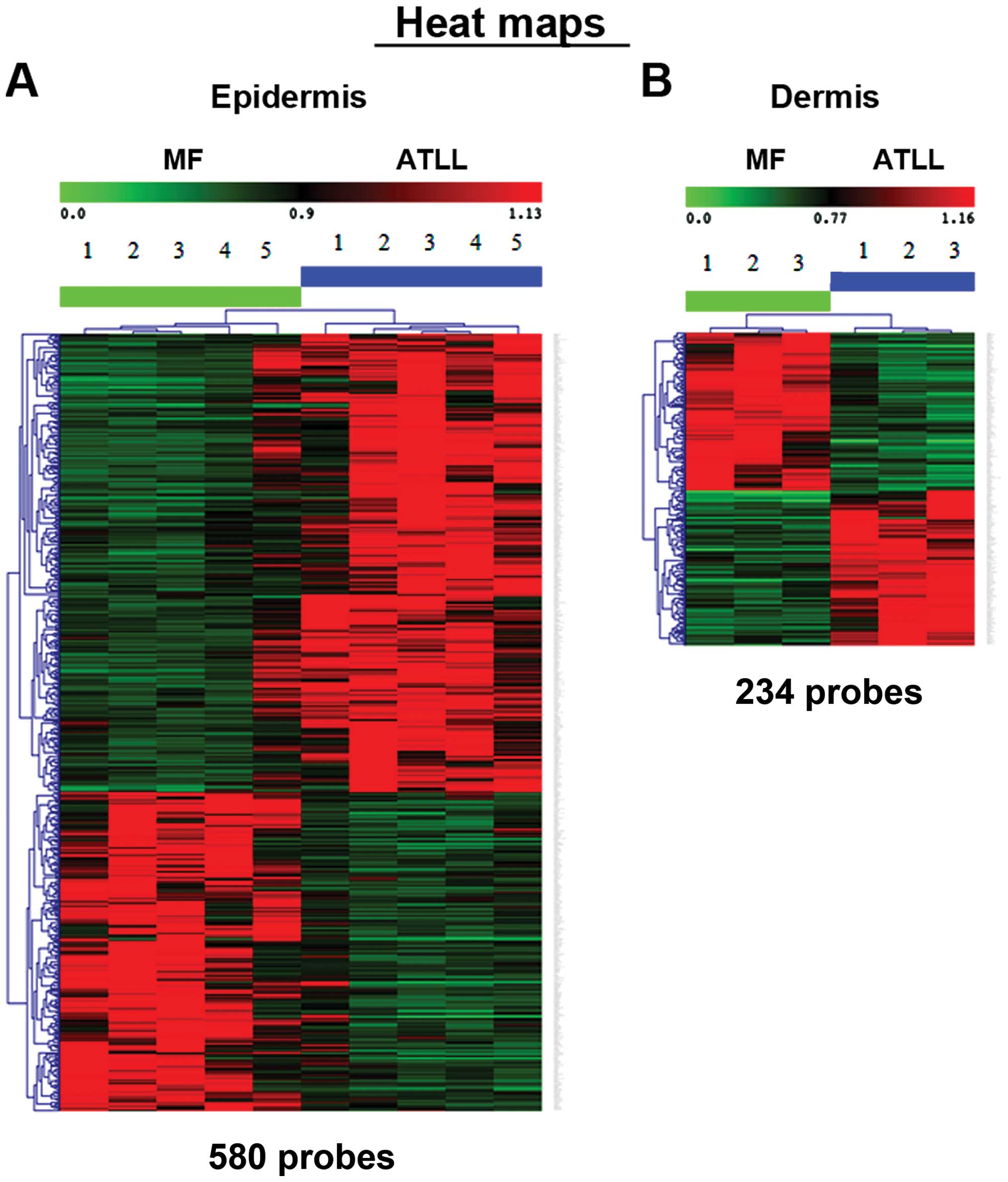
dermal MF with that of ATLL. In the epidermis, 580 probe sets were
identified as upregulated or downregulated on a heat map (Fig. 1A), while in the dermis, 234 probe
sets were found to be up- or downregulated (Fig. 1B). The first 10 genes showing the
highest upregulation are listed in Table II. In the dermis, LTβ and CCL21 in
particular showed different expressions in MF and ATLL.
 | Table IIGenes differentially regulated in MF
and ATLL. |
Table II
Genes differentially regulated in MF
and ATLL.
| A, Upregulated
genes in MF compared with genes in ATLL |
|---|
|
|---|
| Epidermis | Gene symbol | Gene name | Exp. value |
|---|
| 1 | ZSCAN18 | Zinc finger and
SCAN domain containing 18 | 1.392 |
| 2 | COL16A1 | Collagen | 1.217 |
| 3 | EID2B | EP300 interacting
inhibitor of differentiation 2B | 1.167 |
| 4 | HSPA1A | Heat shock 70 kDa
protein 1A | 1.046 |
| 5 | GGA1 | Golgi
associated | 1.038 |
| 6 | LRIG1 | Leucine-rich
repeats and immunoglobulin-like domains 1 | 1.010 |
| 7 | XPNPEP3 | X-prolyl
aminopeptidase (aminopeptidase P) 3 | 1.003 |
| 8 | SMARCA2 | SWI/SNF
related | 0.979 |
| 9 | ID2 | Inhibitor of DNA
binding 2 | 0.968 |
| 10 | ARL6IP5 |
ADP-ribosylation-like factor 6 interacting
protein 5 | 0.950 |
|
| Dermis | Gene symbol | Gene name | Exp. value |
|
| 1 | LTB | Lymphotoxin β | 2.946 |
| 2 | CCL21 | Chemokine (C-C
motif) ligand 21 | 2.189 |
| 3 | IFITM3 | Interferon induced
transmembrane protein 3 | 1.666 |
| 4 | THY1 | Thy-1 cell surface
antigen | 1.594 |
| 5 | ACTN1 | Actinin | 1.585 |
| 6 | CHST15 | Carbohydrate | 1.449 |
| 7 | PON2 | Paraoxonase 2 | 1.447 |
| 8 | CYR61 | Cysteine-rich | 1.436 |
| 9 | TAGLN | Transgelin | 1.436 |
| 10 | PRRX1 | Paired related
homeobox 1 | 1.412 |
|
| B, Downregulated
genes in MF compared with genes in ATLL |
|
| Epidermis | Gene symbol | Gene name | Exp. value |
|
| 1 | GPX3 | Glutathione
peroxidase 3 | −2.097 |
| 2 | PSMC1 | Proteasome | −1.668 |
| 3 | CALML3 | Calmodulin-like
3 | −1.587 |
| 4 | NDRG4 | NDRG family member
4 | −1.169 |
| 5 | PRSS3 | Protease | −1.113 |
| 6 | ADM | Adrenomedullin | −1.063 |
| 7 | SMOX | Spermine
oxidase | −1.056 |
| 8 | CBX3 | Chromobox homolog
3 | −1.044 |
| 9 | WBP5 | WW domain binding
protein 5 | −0.970 |
| 10 | LRRC20 | Leucine rich repeat
containing 20 | −0.967 |
|
| Dermis | Gene symbol | Gene name | Exp. value |
|
| 1 | TOX2 | TOX high mobility
group box family member 2 | −2.925 |
| 2 | ADAP1 | ArfGAP with dual PH
domains 1 | −2.848 |
| 3 | AKAP7 | A kinase anchor
protein 7 | −2.449 |
| 4 | CADM1 | Cell adhesion
molecule 1 | −2.293 |
| 5 | ZNF365 | Zinc finger protein
365 | −2.157 |
| 6 | RGS2 | Regulator of
G-protein signaling 2 | −2.129 |
| 7 | MTX3 | Metaxin 3 | −2.080 |
| 8 | SH3KBP1 | SH3-domain kinase
binding protein 1 | −1.871 |
| 9 | CHST7 | Carbohydrate
sulfotransferase 7 | −1.792 |
| 10 | CST7 | Cystatin F
(leukocystatin) | −1.739 |
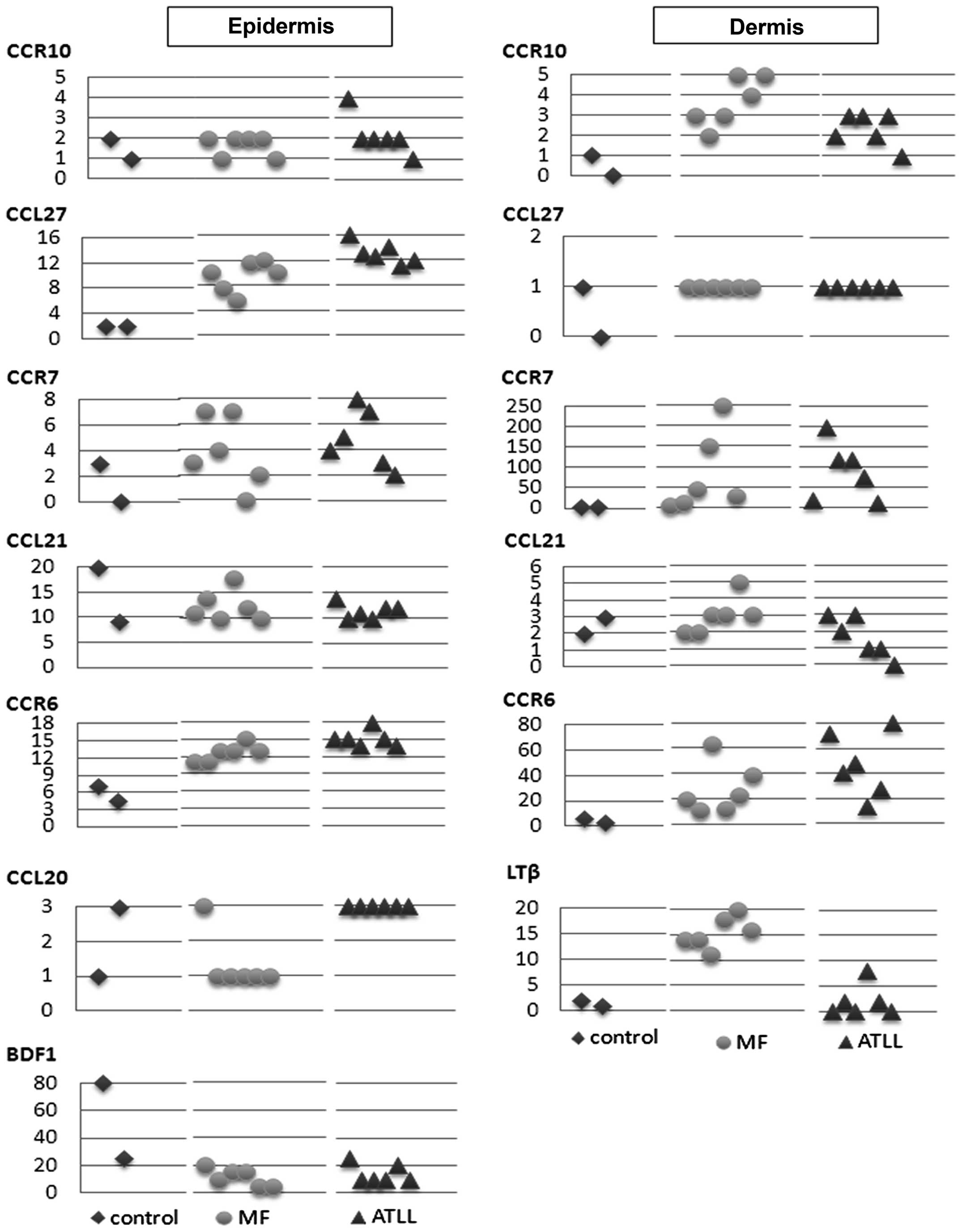
Next, we focused on the array data that could
identify the possible involvement of specific chemokine receptors
and ligands, including CCR4, CCR6, CCR7 and CCR10 and others, in
the pathophysiology of CTCL, MF and ATLL. CCR4 expression was
especially high in ATLL, while CCL27 expression in the epidermis
was high in both MF and ATLL, and CCR10, a receptor of CCL27
detected in the dermis, was more highly expressed in MF than in
ATLL. Also in the dermis, expression of the LN homing molecule CCR7
was high in both MF and ATLL, while a close correlation between
CCR7 and CCL21, a chemokine receptor and its ligand, was observed
in MF. CCR6 expression was elevated in the epidermis and dermis of
both MF and ATLL, while expression of CCL20, a CCR6 ligand, showed
no correlation with CCR6. BDFs were identified as not only
antibacterial peptides but also as CCR6 ligands. BDF 1 expression
was reduced in the epidermis of MF and ATLL, while BDF 3 expression
in the epidermis of MF was higher than in controls, which was
similar to the finding of a previous study (13).
We also focused on CLA, which is recognized as an
adhesion molecule selectivity expressed by a subset of circulating
memory T-cells, normal T cells in inflamed skin and by the vast
majority of CTCL (4–6). CLA was expressed in the dermis of
both MF and ATLL, with a particularly high expression in ATLL.
In order to confirm the expression levels of the
proteins, CCR6, CCR7, CCR10, CCL20, CCL21, CCL27, BDF1, LTβ and
TNFR2 on MF and ATLL tissue sections were subjected to
immunohistochemistry. The findings of the immunohistochemical
analysis were basically consistent with the results of the
microarray analysis. A representative stain is shown in Fig. 2 and the immunohistochemical scores
are presented graphically in Fig.
3.
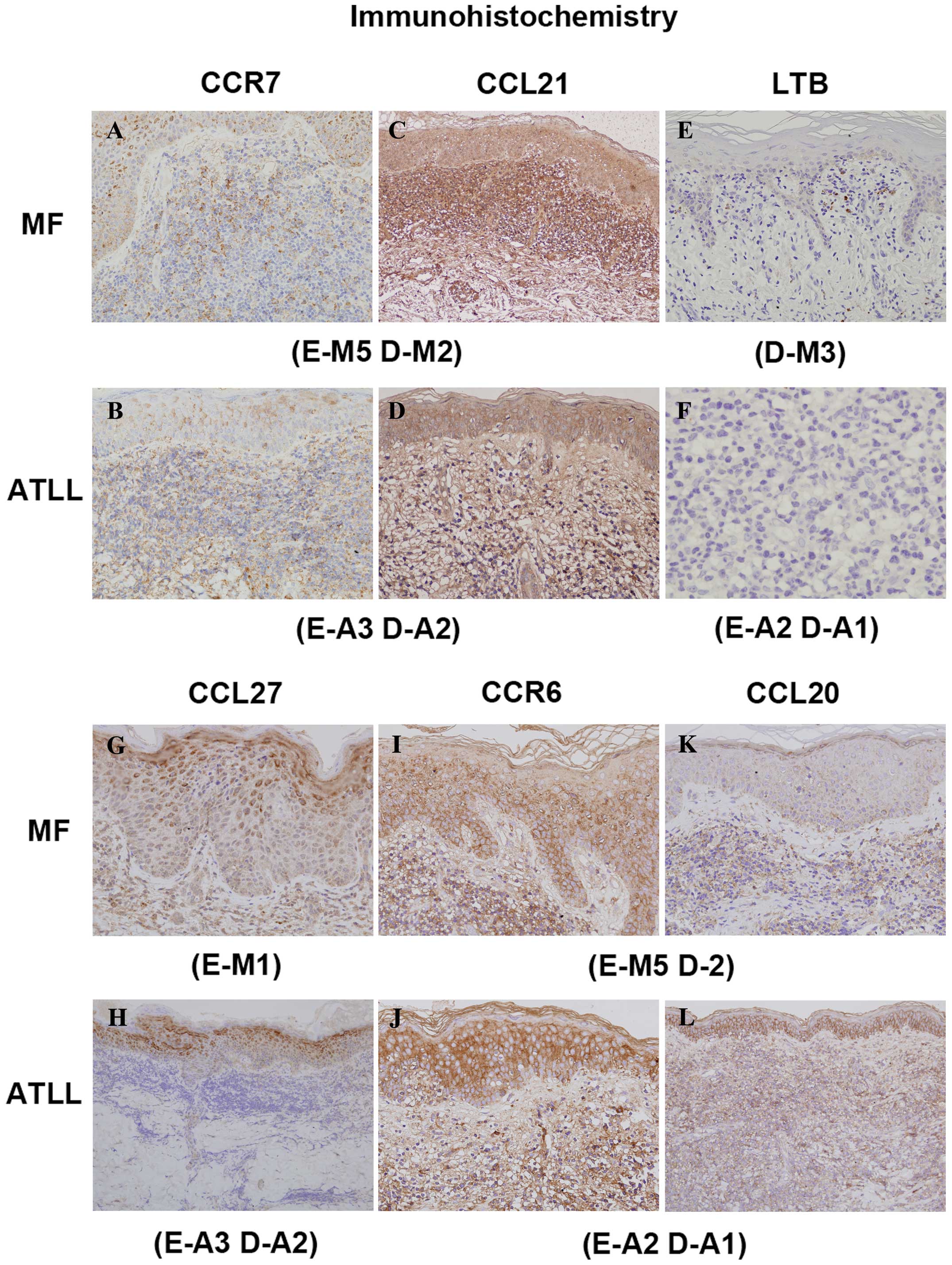 | Figure 2Representative immunohistochemical
stain. CCR7 was expressed in the lymphoma cells (A, B) and CCL21 in
the extracellular matrix (stroma) (C, D). CCL21 and LTβ were higher
in the dermis in MF (C, E) than in ATLL (D, F). CCL27 (G–H) and
CCL20 (K–L) were expressed in keratinocytes. CCR6 expression in the
epidermis and dermis was elevated in both MF and ATLL (I–J). CCL27,
CCR6 and CCL20 in the epidermis were relatively higher in ATLL than
in MF. (Original magnification ×100). (A, C, I, K) were from sample
no. E-M5 D-M2 and (B, D, H) from E-A3 D-A2, (E) was from D-M3, (F,
J, L) were from E-A2 D-A1 and (G) was from E-M1. |
In principle, CCR4, CCR6, CCR7, CCR10 and/or CLA
were all expressed in the lymphoma cells. CCR4, CCR6 and CCR10 were
identified in both dermis and epidermis, while CCL27 and CCL20 were
expressed in keratinocytes and CCL21 was expressed in the
extracellular matrix (stroma). In addition, CCL27 was also stained
in dermis released from keratinocytes.
In controls, epidermal keratinocytes weakly
expressed CCL27 within their cytoplasm, and mainly in the spinous
layers of the epidermis. However, positive lesions of epidermis
keratinocytes were stronger in thicker layers in most MF and ATLL
patients than in controls. Moreover, CCL27 was expressed not only
in cytoplasm but also occasionally in membrane of MF and ATLL, so
that there was in fact not much difference between these two types
of CTCL. Faint CCL27 expression in dermis was detected in MF, ATLL
and in one of the controls. The differences in CCL27 expression
levels in dermis were immunohistochemically imperceptible. CCR10
was stained weakly in both epidermis and dermis, but in the dermis
usually more strongly in MF than in ATLL cells, which correlated
moderately with the overall gene expression profile. CCR6
expression was more elevated in the epidermis and dermis of both MF
and ATLL than in controls, and the immunohistochemical score
correlated with the result of the microarray analysis. CCL20 was
expressed very weakly in the cytoplasm of keratinocytes, stained in
the granular layer of all samples and in all layers of the
epidermis of some samples.
Although each one of the controls and MF samples
showed high levels of CCL20, all ATLL samples displayed high
expression in the epidermis. Expression of BDF1 in nucleus and/or
nuclear membrane was diminished in the epidermis of MF and ATLL.
Because the stain was quite faint, it was difficult to determine
even whether CCL20 and BDF1 in the dermis were positive or
negative. The cell membrane of infiltrating lymphoma cells stained
for CCR7, while staining for CCL21 was observed within the
cytoplasm of epidermal keratinocytes and was diffusely distributed
on the dermal extracellular matrix. The intensity and density of
CCR7+ infiltrating lymphoma cells correlated with the
intensity of CCL21 in stroma in the dermis of MF cases. LTβ
positive cells were scattered throughout the dermis and their
expression was homogeneous in the nucleus or the surface of the
nucleus of lymphoma cells, while LTβ expression levels in the
dermis were higher for MF than for ATLL. No correlation with LTβ
was observed for TNFR2, which is one of the soluble secreted LT
homotrimers (LTα3) that trigger TNFRs.
Discussion
The microarray and immunohistochemistry findings of
our study showed high expression of LTβ and CCL21, and correltion
between CCL21 and CCR7, a receptor of CCL21 which is the LN homing
molecule, was observed in the dermis of MF. CCR7 expression was
also high in the dermis of ATLL.
LTβ, which was indentified in the dermis of MF
samples in our study, is a member of the tumor necrosis factor
superfamily. LTβ in human cells contains a transmembrane domain,
and the surface LT form is most likely a trimer with an LTα1β2
stoichiometry, while the LTα1β2 heterotrimer binds the LTβ receptor
(LTβR) (14).
LTs are expressed by activated T-, B-, NK- and
lymphoid tissue inducer cells (15–17)
and are crucial for organogenesis and maintenance of lymphoid
tissues (18,19). LTβR, on the other hand, is
expressed on many different cell types including cells of
epithelial lineages, while ligation of LTβR results in NF-κB
activation (20–22) and leads to secondary lymphoid
organogenesis and homeostasis. Signaling via LTβR is involved not
only in host defense and autoimmune diseases, but also in tumor
cell proliferation (23).
Furthermore, NF-κB stimulates proliferation and blocks programmed
cell death (apoptosis) in various cell types (24,25).
Previous reports have suggested that the activation
of the NF-κB signaling pathway via LTβR results in the
proliferation of melanoma (26)
and hepatocellular carcinoma (27). NF-κB activation was also detected
in lymphoid malignancies (28,29).
In this connection, it was found that peripheral T cell
lymphomas/not otherwise specified (PTCLs/NOS) with NF-κB inactivity
showed better survival (30–32).
Another study reported that LTβR organizes the
recruitment and survival of CD4 T cells through the induction of
CCL21 expression which is produced exclusively by stroma (33). In our study we found that
expression levels of both the LTβ and CCL21 gene are high in the
dermis of MF. These findings seem to suggest that LTβR, which can
be expressed in epidermis, organizes the recruitment and survival
of CD4-positive MF cells through the induction of CCL21.
CCR7 (the receptor of CCL21) physiologically
regulates the lymphoid homing of T-cells and probably plays an
important role in the tropism of CTCL cells to peripheral LNs,
which constitutively synthesize CCR7 ligands, CCL19 and CCL21
(34). It has been further
hypothesized that CCR7, which is expressed at fairly high levels in
SS cells (35), may function as a
mediator of LN infiltration of CTCL. In our study, CCR7 expression
was detected in the dermis of MF and ATLL. Interaction between CCR7
and CCL21 in the dermis of MF probably functions to maintain
localization to the skin for a long time. On the other hand, T
cells infected by HTLV-1 circulate in the bloodstream from the
outset, while ATLL cells, expressing the skin-homing properties of
CCR4, CLA, CCR6 and CCR10, may affect cutaneous involvement in
smorderling or cutaneous type of ATLL patients.
While T cells infected by HTLV-1 are circulating in
the peripheral blood, they can spontaneously encounter CCL21
produced by LNs, and CCR7+ ATLL cells can be trapped in
the LNs. Migration to the LNs can thus occur at an earlier stage in
ATLL than in MF.
CTCL is characterized by clonal expansion of a
mature CD4-positive clone of the Th phenotype, putatively from a
skin homing subset of memory T cells (36) whose migration to respective tissues
is tightly regulated by adhesion molecules and chemokine receptors.
For example, memory T cells that infiltrate the skin express a
unique adhesion molecule known as CLA. Chemokines and their
receptors have also been associated with tumor metastasis (37–39)
and possibly trafficking of lymphoma cells (40). In addition, chemokines are produced
by not only malignant T cells but also epidermal keratinocytes, DCs
and dermal vessels. We therefore did not use fractionated malignant
T cells, but the microdissected epidermal and dermal affected
regions in MF and ATLL to investigate the expression profiles of
CCR4, CCR6, CCR7, CCR10, CCL20, CCL21, CCL27, BDFs and CLA by means
of microarray analysis and/or immunohistochemistry. We found that
CCL27 expression in the epidermis was high in both MF and ATLL.
CCR10 expression observed in the dermis was higher in MF than ATLL,
and the microarray analysis showed that CCR4 expression was
especially high in ATLL. Previous studies have reported that CCR4
expression was detected much more frequently in both MF and ATLL
than in healthy controls (41,42).
In addition, findings of a previous study indicated
that DCs synthesize CCR4 ligands, which rapidly stimulate
chemotaxis of, or conjugate formation with, normal T cells
(43,44). A characteristic histopathological
marker, Pautrier’s microabscesses of CTCL, which are known to be
DC-malignant T cell conjugates (45), may be initiated by DC-derived
chemokines. Other data indicated that preferential expression of
CCR4 constitutes a sign of worse prognosis, and CCR4 expression was
especially high in ATLL in our study. This finding corresponds to
the pathological feature of more visible and larger Pautrier’s
microabscesses as well as the clinical feature of worse prognosis
for ATLL than for MF.
CCR10 is enriched in CLA+ skin-homing T
cells of psoriasis, atopic dermatitis and CTCL patients, while it
is only rarely expressed on peripheral blood T cells and skin
samples from healthy persons (3,46).
Moreover, CCL27 (CCR10 ligand) is constitutively present in
epidermal keratinocytes (basal layer) under basal, non-inflammatory
conditions and can be rapidly released from activated keratinocytes
(46). CCL27-CCR10 interactions
thus play an early role in the pathophysiology of MF from the patch
stage (47), and an increase in
CCL27 in the serum of MF patients was observed in previous studies
(48). Our data for CCL27-CCR10
interactions agreed with the pathophysiology outlined above.
Our findings also indicate that CCR4 in ATLL as well
as CCR10 in MF may play a much more prominent role in
epidermotrophism. However, the origin of malignant T cells in MF
and ATLL remains to be fully elucidated. High-level expressions of
CCR4, CLA and CCR6 indicate the possibility of not only Th but also
Treg origin especially in ATLL. Treg action suppresses activation
of the immune system and tolerance for self-antigens. The phenotype
of CD4+CD25+ and the expression of Foxp3, a
master transcriptional regulator of Treg development and function,
suggest that human skin-resident T cells appear to represent Tregs.
Tregs also express functional skin-homing receptors. Although CCR6
does not seem to be a functional marker for Treg, it may facilitate
CLA+ Treg migration to skin. As for Th, there are
conflicting reports about Th1 and Th2 profiles of CCR4 in MF.
Berger et al demonstrated that malignant CD4+ T
cells can be induced to express a CD25+ Treg phenotype
(49). Our analyses, however,
disclosed that CCR4 and CCR6 in both epidermis and dermis and CLA
in dermis were expressed in MF and ATLL, with the expression being
especially high in ATLL. Our data therefore support the possibility
that the origin of CTCL is Treg (particularly in ATLL) as also
previously reported (50). Further
investigation will be required to determine whether CTCL shows
Th1/Th2/Treg-type polarization in lesional skin.
While the mechanisms involved in tumor homing have
not yet been fully identified, it has been suggested that
chemokines and chemokine receptors are involved in the pathogenesis
of tumor homing. There are no previous reports on the use of the
microdissection method for a comparison of epidermal and dermal
gene expression levels in MF and ATLL by means of microarray
analysis, a procedure which has made it possible to observe and
evaluate the regional environment of MF and ATLL, including
malignant and inflamed T-cells, DCs, keratinocytes and dermal
vessels. DNA microarray analysis enabled us to comprehensively
identify differences and patterns in gene expression in MF and
ATLL, while our findings support the notion that CCR10 and its
ligand CCL27 may contribute to the skin infiltration of malignant
T-cells in MF and ATLL. However, further studies are needed to
clarify the mechanism for epidermotrophism because of the
complexity of its interactions.
Acknowledgements
The authors would like to thank Konomi Takasu,
Mayumi Miura and Kanoko Miyazaki for their technical support.
References
|
1
|
Willemze R, Jaffe ES, Burg G, et al:
WHO-EORTC classification for cutaneous lymphomas. Blood.
105:3768–3785. 2009. View Article : Google Scholar
|
|
2
|
Kikuchi A, Ohata Y, Matsumoto H, Sugiura M
and Nishikawa T: Anti-HTLV-1 antibody positive cutaneous T-cell
lymphoma. Cancer. 79:269–274. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Notohamiprodjo M, Segerer S, Huss R, et
al: CCR10 is expressed in cutaneous T-cell lymphoma. Int J Cancer.
115:641–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Picker LJ, Terstappen LW, Rott LS,
Streeter PR, Stein H and Butcher EC: Differential expression of
homing-associated adhesion molecules by T-cell subsets in man. J
Immunol. 145:3247–3255. 1990.PubMed/NCBI
|
|
5
|
Picker LJ, Michie SA, Rott LS and Butcher
EC: A unique phenotype of skin-associated lymphocytes in humans:
preferential expression of the HECA-452 epitope by benign and
malignant T cells at cutaneous sites. Am J Pathol. 136:1053–1068.
1990.PubMed/NCBI
|
|
6
|
Berg EL, Yoshino T, Rott LS, et al: The
cutaneous lymphocyte antigen is a skin lymphocyte homing receptor
for the vascular lectin endothelial cell leukocyte adhesion
molecule 1. J Exp Med. 174:1461–1466. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka Y, Wake A, Horgan KJ, et al:
Distinct phenotype of leukemic T cells with various tissues
tropisms. J Immunol. 158:3822–3829. 1997.PubMed/NCBI
|
|
8
|
Rubin MA: Use of laser capture
microdissection, cDNA microarrays, and tissue microarrays in
advancing our understanding of prostate cancer. J Pathol.
195:80–86. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin SM, Du P, Huber W and Kibbe WA:
Model-based variance-stabilizing transformation for Illumina
microarray data. Nucleic Acids Res. 36:e112008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du P, Kibbe WA and Lin SM: lumi: a
pipeline for processing Illumina microarray. Bioinformatics.
24:1547–1548. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tusher VG, Tibshirani R and Chu G:
Significance analysis of microarrays applied to the ionizing
radiation response. Proc Natl Acad Sci USA. 98:5116–5121. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohshima K, Kawasaki C, Muta H, et al: CD10
and Bcl10 expression in diffuse large B-cell lymphoma: CD10 is a
marker of improved prognosis. Histopathology. 39:156–162. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gambichler T, Skrygan M, Appelhans C, et
al: Expression of human beta-defensins in patients with mycosis
fungoides. Arch Dermatol Res. 299:221–224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Browning JL, Dougas I, Ngam-ek A, et al:
Characterization of surface lymphotoxin forms. Use of specific
monoclonal antibodies and soluble receptors. J Immunol. 154:33–46.
1995.PubMed/NCBI
|
|
15
|
Ware CF, Crowe PD, Grayson MH, Androlewicz
MJ and Browning JL: Expression of surface lymphotoxin and tumor
necrosis factor on activated T, B, and natural killer cells. J
Immunol. 149:3881–3888. 1992.PubMed/NCBI
|
|
16
|
Fu YX, Huang G, Wang Y and Chaplin DD: B
lymphocytes induce the formation of follicular dendritic cell
clusters in a lymphotoxin alpha-dependent fashion. J Exp Med.
187:1009–1018. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ware CF: Network communications:
lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 23:787–819. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rennert PD, Browning JL, Mebius R, Mackay
F and Hochman PS: Surface lymphotoxin alpha/beta complex is
required for the development of peripheral lymphoid organs. J Exp
Med. 184:1999–2006. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tumanov AV, Kuprash DV and Nedospasov SA:
The role of lymphotoxin in development and maintenance of secondary
lymphoid tissues. Cytokine Growth Factor Rev. 14:275–288. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dejardin E, Droin NM, Delhase M, et al:
The lymphotoxin-beta receptor induces different patterns of gene
expression via two NF-kappaB pathways. Immunity. 17:525–535. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dejardin E: The alternative NF-kappaB
pathway from biochemistry to biology: pitfalls and promises for
future drug development. Biochem Pharmacol. 72:1161–1179. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsushima A, Kaisho T, Rennert PD, et al:
Essential role of nuclear factor (NF)-kappaB-inducing kinase and
inhibitor of kappaB (IkappaB) kinase alpha in NF-kappaB activation
through lymphotoxin beta receptor, but not through tumor necrosis
factor receptor I. J Exp Med. 193:631–636. 2001. View Article : Google Scholar
|
|
23
|
Hehlgans T, Stoelcker B, Stopfer P, et al:
Lymphotoxin-beta receptor immune interaction promotes tumor growth
by inducing angiogenesis. Cancer Res. 62:4034–4040. 2002.PubMed/NCBI
|
|
24
|
Barkett M and Gilmore TD: Control of
apoptosis by Rel/NF-kappaB transcription factors. Oncogene.
18:6910–6924. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Biswas DK, Martin KJ, McAlister C, et al:
Apoptosis caused by chemotherapeutic inhibition of nuclear
factor-kappaB activation. Cancer Res. 63:290–295. 2003.PubMed/NCBI
|
|
26
|
Dhawan P, Su Y, Thu YM, et al: The
lymphotoxin-beta receptor is an upstream activator of
NF-kappaB-mediated transcription in melanoma cells. J Biol Chem.
283:15399–15408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haybaeck J, Zeller N, Wolf MJ, et al: A
lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer
Cell. 16:295–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gilmore TD: Multiple mutations contribute
to the oncogenicity of the retroviral oncoprotein v-Rel. Oncogene.
18:6925–6937. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Häcker H and Karin M: Is NF-kappaB2/p100 a
direct activator of programmed cell death? Cancer Cell. 2:431–433.
2002.PubMed/NCBI
|
|
30
|
Martinez-Delgado B, Meléndez B, Cuadros M,
et al: Expression profiling of T-cell lymphomas differentiates
peripheral and lymphoblastic lymphomas and defines survival related
genes. Clin Cancer Res. 10:4971–4982. 2004. View Article : Google Scholar
|
|
31
|
Martinez-Delgado B, Cuadros M, Honrado E,
et al: Differential expression of NF-kappaB pathway genes among
peripheral T-cell lymphomas. Leukemia. 19:2254–2263. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ballester B, Ramuz O, Gisselbrecht C, et
al: Gene expression profiling identifies molecular subgroups among
nodal peripheral T-cell lymphomas. Oncogene. 25:1560–1570. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bekiaris V, Gaspal F, Kim MY, et al: CD30
is required for CCL21 expression and CD4 T cell recruitment in the
absence of lymphotoxin signals. J Immunol. 182:4771–4775. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kallinich T, Muche JM, Qin S, Sterry W,
Audring H and Kroczek RA: Chemokine receptor expression on
neoplastic and reactive T cells in the skin at different stages of
mycosis fungoides. J Invest Dermatol. 121:1045–1052. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sokolowska-Wojdylo M, Wenzel J, Gaffal E,
et al: Circulating clonal CLA(+) and CD4(+) T cells in Se’zary
syndrome express the skin-homing chemokine receptors CCR4 and CCR10
as well as the lymph node-homing chemokine receptor CCR7. Br J
Dermatol. 152:258–264. 2005.PubMed/NCBI
|
|
36
|
Willemze R, Kerl H, Sterry W, et al: EORTC
classification for primary cutaneous lymphomas: a proposal from the
Cutaneous Lymphoma Study Group of the European Organization for
Research and Treatment of Cancer. Blood. 90:354–371. 1997.
|
|
37
|
Müller A, Homey B, Soto H, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001.PubMed/NCBI
|
|
38
|
von Andrian UH and Mempel TR: Homing and
cellular traffic in lymph nodes. Nat Rev Immunol. 3:867–878.
2003.PubMed/NCBI
|
|
39
|
Balkwill F: Cancer and the chemokine
network. Nat Rev Cancer. 4:540–550. 2004. View Article : Google Scholar
|
|
40
|
Jones D, O’Hara C, Kraus MD, et al:
Expression pattern of T-cell-associated chemokine receptors and
their chemokines correlates with specific subtypes of T-cell
non-Hodgkin lymphoma. Blood. 96:685–690. 2000.PubMed/NCBI
|
|
41
|
Ferenczi K, Fuhlbrigge RC, Pinkus J,
Pinkus GS and Kupper TS: Increased CCR4 expression in cutaneous T
cell lymphoma. J Invest Dermatol. 119:1405–1410. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Narducci MG, Scala E, Bresin A, et al:
Skin homing of Se’zary cells involves SDF-1-CXCR4 signaling and
down-regulation of CD26/dipeptidylpeptidase IV. Blood.
107:1108–1115. 2006.
|
|
43
|
Tang HL and Cyster JG: Chemokine
up-regulation and activated T cell attraction by maturing dendritic
cells. Science. 284:819–822. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu M, Fang H and Hwang ST: Cutting edge:
CCR4 mediates antigen-primed T cell binding to activated dendritic
cells. J Immunol. 167:4791–4795. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Edelson RL: Cutaneous T cell lymphoma: the
helping hand of dendritic cells. Ann N Y Acad Sci. 941:1–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Homey B, Alenius H, Müller A, et al:
CCL27-CCR10 interactions regulate T cell-mediated skin
inflammation. Nat Med. 8:157–165. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fujita Y, Abe R, Sasaki M, et al: Presence
of circulating CCR10+ T cells and elevated serum
CTACK/CCL27 in the early stage of mycosis fungoides. Clin Cancer
Res. 12:2670–2675. 2006.PubMed/NCBI
|
|
48
|
Kagami S, Sugaya M, Minatani Y, et al:
Elevated serum CTACK/CCL27 levels in CTCL. J Invest Dermatol.
126:1189–1191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Berger CL, Tigelaar R, Cohen J, et al:
Cutaneous T-cell lymphoma: malignant proliferation of T-regulatory
cells. Blood. 105:1640–1647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Karube K, Aoki R, Sugita Y, et al: The
relationship of FOXP3 expression and clinicopathological
characteristics in adult T-cell leukemia/lymphoma. Mod Pathol.
21:617–625. 2008. View Article : Google Scholar : PubMed/NCBI
|