1. TRAP1 milestones
Although twenty years have passed since TRAP1/HSP75
was firstly identified, only during recent years some light has
been shed on its molecular functions. The cloning of tumor necrosis
factor receptor-associated protein 1 (TRAP1) as a type I tumor
necrosis factor receptor-associated protein, and the identification
of HSP75 as a retinoblastoma protein (Rb)-binding protein, were
independently performed by two different groups, and it was
immediately clear that they had identified the same protein
(1,2). TRAP1 belongs to the HSP90 chaperone
family (3), sharing 26% identity
and 45% similarity with cytosolic HSP90 (4). Bioinformatic analysis and microscopic
observations suggest that TRAP1 is mostly localized to mitochondria
and is targeted to the organelle by its N-terminal presequence
(5). Interestingly, the ATPase
activity of TRAP1 is inhibited by both geldanamycin and radicicol,
which have been shown to block specifically HSP90 function;
however, in vitro experiments showed that TRAP1 does not
bind and fold HSP90 client proteins, suggesting distinct functional
properties. Quantitative immunogold electron microscopy and
biochemical analysis confirmed the mitochondrial distribution of
TRAP1 in rat tissues, but additionally revealed a number of
non-mitochondrial locations, including nuclei (6).
Following its identification, TRAP1 attracted
increasing interest for its homology to HSP90, which has long been
pursued for novel cancer therapeutics, coupled with a distinct
subcellular localization. Consequently, the majority of the studies
focused on expression of this protein in tumors:
immunohistochemical staining revealed that TRAP1 is strongly
expressed in tumor cells of adenocarcinomas of pancreas, breast,
colon, and lung. Conversely, normal matched epithelia contain very
low levels of TRAP1 (7). We
strongly contributed to these studies, demonstrating that TRAP1
expression is upregulated in approximately 60% of human colorectal
cancers and correlates with a multi-drug resistant phenotype in
colon carcinoma cells (8).
Our group was among the first to demonstrate TRAP1
involvement in stress-adaptive response of cancer cells: high
levels of both TRAP1 mRNA and protein were found in Saos-2
osteosarcoma cells chronically adapted to mild oxidative conditions
(9). Even more interestingly, we
identified TRAP1 as a key target in the previously hypothesized
correlations between resistance to antitumor agents and adaptation
to oxidative stress (OS), since very high levels of this protein
were analogously found in tumor cells resistant to 5-fluorouracil
and to platin derivatives. However, the most striking data came
from the observation that TRAP1 interference, as well as the use of
dominant negative mutants of TRAP1, sensitized OS/chemoresistant
cells to cell death inducers, thus supporting the hypothesis of
common mechanisms shared by chemoresistance and adaptation to OS
and providing the first evidence that TRAP1 is an important player
in the development and the maintenance of these phenotypes. Indeed,
TRAP1 hyperexpressing cells show a decreased cleavage of the
apoptotic markers Caspase 3 and PARP, and increased levels of the
scavenging tripeptide GSH. Hence, TRAP1 may be considered a
reliable tool to investigate the correlations between oxidative
stress, resistance to apoptosis and chemoresistance (8,9).
A role of TRAP1 in the protection from apoptosis and
its consequent involvement in the onset and maintenance of tumor
phenotypes was firstly and elegantly described by Kang et al
(7). These authors discovered that
only tumor cells organize a mitochondrial chaperone network, which
involves HSP90, its homolog TRAP1 and the immunophilin cyclophilin
D (CypD) in a physical complex that regulates permeability
transition pore (PTP) opening, maintaining mitochondrial
homeostasis and antagonizing the function of CypD in permeability
transition. Considering the high ‘druggability’ of HSP90 ATPase
pocket, Kang and colleagues developed a mitochondria-directed
variant of 17-AAG carrying the Antennapedia peptide (called
Shepherdin) that efficiently accumulates inside the mitochondria,
binds mitochondrial TRAP1 and HSP90, and inhibits their chaperone
activity via an ATP competition mechanism, thus resulting in
CypD-mediated cell death. These observations labelled TRAP1 as an
essential controller of mitochondrial homeostasis in tumor cells,
conferring them resistance to apoptosis and a survival advantage
over the normal counterpart (7).
The cytoprotective effect of TRAP1 was further investigated by our
group through the identification and functional characterization of
TRAP1 interaction with the novel mitochondrial isoform of Sorcin,
this providing the first demonstration of a new antiapoptotic
complex (10).
TRAP1 role in cell death protection, beyond the
direct control of PTP opening in concert with HSP90 and CypD even
further characterized by other studies (11,12),
may involve some other more general homeostatic mechanisms, linking
the control of proteostasis inside mitochondria to the activation
of extramitochondrial survival pathways. In this context, we have
shown that TRAP1 function could be relevant in crosstalk between
mitochondria and other subcellular compartments (3). Accordingly, the HSP90 mitochondrial
network contributes to the folding of mitochondrial proteins;
however, when this process is dysregulated, a series of
cytoprotective/adaptive cellular responses occurs, including
activation of autophagy, inter-organelle stress response and
induction of gene expression modifications, that lead to the
impairment of tumor cell bioenergetics (13). These processes may further increase
the apoptotic threshold in tumor cells undergoing mitochondriotoxic
stress, ending up with better survival. This adaptive model is
highly suitable to enhance the protein buffering capacity of
transformed cells, which are especially at risk of proteotoxic
stress for their high biosynthetic requirements.
2. TRAP1 in cancers
In previous years, substantial data from several
groups confirmed the involvement of TRAP1 in tumor biology. A
general overview supports the role of TRAP1 in a wide range of
cancer types: Zhang et al (14) analyzed the effect of polyphenols of
green tea extract (GTE), which exhibit multiple antitumor
activities in various cancers, on pancreatic ductal adenocarcinoma
and found that GTE inhibited molecular chaperones HSP90, TRAP1 and
HSP27 concomitantly in HPAF-II cells. In colorectal carcinomas
(CRC) Gao et al (15) found
that the increase in TRAP1 expression level was significantly
correlated to increased lymph node metastases, advanced tumor stage
and reduced overall survival. This suggests that TRAP1 plays an
important role in the progression of CRC from a localized to a
locoregional metastatic disease and makes TRAP1 a candidate
biomarker, predictive for the poor outcome of CRC patients.
Consistently, Han et al (16) found that, in patients with
metastatic CRC who received first-line oxaliplatin/5-fluorouracil
therapy, lower TRAP1 levels correlated with increased overall
survival. Furthermore, involvement of TRAP1 in neuroblastoma was
recently suggested by Zhan et al (17). Interestingly, TRAP1 was found to be
upregulated in hepatocellular carcinoma (HCC) (18), thus the chaperone could have the
potential to act as diagnostic HCC biomarker candidate.
Analogously, Im and Seo (19)
showed that TRAP1 is highly expressed and the mitochondrial mass is
decreased in lung carcinoma cell line A549 compared with a normal
lung fibroblast. TRAP1 knockdown also reduces cell growth and
clonogenic cell survival in non-small cell lung cancer (NSCLC)
cells, impairing ATP production and mitochondrial membrane
potential. Accordingly, immunohistochemical analysis, performed to
evaluate the prognostic potential of TRAP1 expression in NSCLCs,
revealed that high TRAP1 expression was associated with increased
risk of disease recurrence (20).
Altogether, these data further support the
involvement of TRAP1 in biogenesis and progression of several kinds
of tumors. Most cancers exhibit an antiapoptotic threshold higher
than controls, which contributes to disease progression.
Hyperexpression of TRAP1 could contribute to this phenotype, thus
assigning to this chaperone a leading role in malignancies and an
increasing value as a biomarker. The significance of TRAP1
hyperexpression in tumor cells is provided by the evidence that the
mitochondrial pool of HSP90s has evolved to face stress conditions
and to maintain mitochondrial proteostasis and energetic balance
almost exclusively in tumor cells, thus providing a survival
advantage. This issue has been recently addressed by Chae et
al (21): a proteomic
analysis, comparing cells treated with the mitochondrial inhibitor
of HSP90s (Gamitrinib) with control cells, showed a modulation in
the expression levels of proteins involved in essential
mitochondrial processes, such as ribosomal proteins, components of
mitochondrial translation apparatus, regulators of purine
biosynthesis and the methyl cycle, effectors of oxidative
phosphorylation, thus leading to global defects in tumor cell
metabolism. Gamitrinib-treated cells, as far as TRAP1 KD cells,
exhibited aberrant accumulation of citric acid cycle metabolites,
such as succinate, fumarate and malate, and loss of ATP production.
A deeper analysis showed that TRAP1 binds the electron transport
chain complex II subunit succinate dehydrogenase-B (SDHB), whose
solubility is highly decreased in tumor cells upon treatment with
Gamitrinib. Accordingly, it was shown that mitochondrial
chaperones’ function is crucial in maintaining cellular respiration
under low-nutrient conditions, protecting complex II in conditions
of oxidative stress, and contributing to hypoxia-inducible
factor-1α (HIF-1α)-mediated tumorigenesis in patients carrying SDHB
mutations. This pathway may be ideally suited to buffer the risk of
proteotoxic stress in transformed cells with high biosynthetic
need, preserve organelle integrity against CypD-dependent apoptosis
and maintain multiple sources of energy production, especially
under stress conditions, such as hypoxia and nutrient deprivation.
Thus, HSP90-directed proteostasis in mitochondria regulates tumor
cell metabolism, and is gaining increasing importance as a
mechanistic explanation for cancer homeostatic regulation,
potentially contributing to disease maintenance.
3. TRAP1 as an oncogene?
Other outstanding breakthroughs in TRAP1 biology
come from evidence by Sciacovelli et al (22): this elegant recent study provides
the first demonstration that TRAP1 may behave as an oncogene able
to promote neoplastic transformation in different cell systems. The
most striking data demonstrated that TRAP1 knock-down (KD) by RNA
interference abrogates the transforming potential of different
cancer cell lines in in vitro focus forming and soft agar
assays, as well as after cell injection in nude mice. Conversely,
overexpression of TRAP1 in non-transformed fibroblasts led to cell
transformation in analogous assays. Interestingly, overexpression
of a TRAP1 deletion mutant, unable to localize into mitochondria,
was ineffective, thus suggesting that TRAP1 transforming potential
is due to its mitochondrial localization/function. Remarkably,
these authors demonstrated that TRAP1 controls mitochondrial
metabolism, contributing to the Warburg phenotype. Accordingly,
data showed that TRAP1 directly binds complex II and IV of the
electron transport chain and inhibits succinate dehydrogenase (SDH)
activity in Saos-2 osteosarcoma cells, without affecting the
cytochrome oxidase enzymatic activity of complex IV, complex II
protein levels or mitochondrial mass. The use of the TRAP1
inhibitor 17AAG in isolated mitochondria restored SDH activity,
independently of HSP90. These results were supported by evidence
that TRAP1 expression and SDH activity are inversely correlated in
colon cancer specimens previously characterized for higher levels
of TRAP1 expression compared to surrounding healthy mucosa
(8). Strikingly, TRAP1
downregulation markedly increased mitochondrial-dependent
respiration in Saos-2 cells in oxygen consumption rate (OCR)
experiments, whereas a high proportion of the intracellular ATP
content in control cells is provided by glycolysis. Symmetrically,
TRAP1 expression in non-transformed fibroblast reduced
mitochondrial respiration, thus mimicking the respiratory pattern
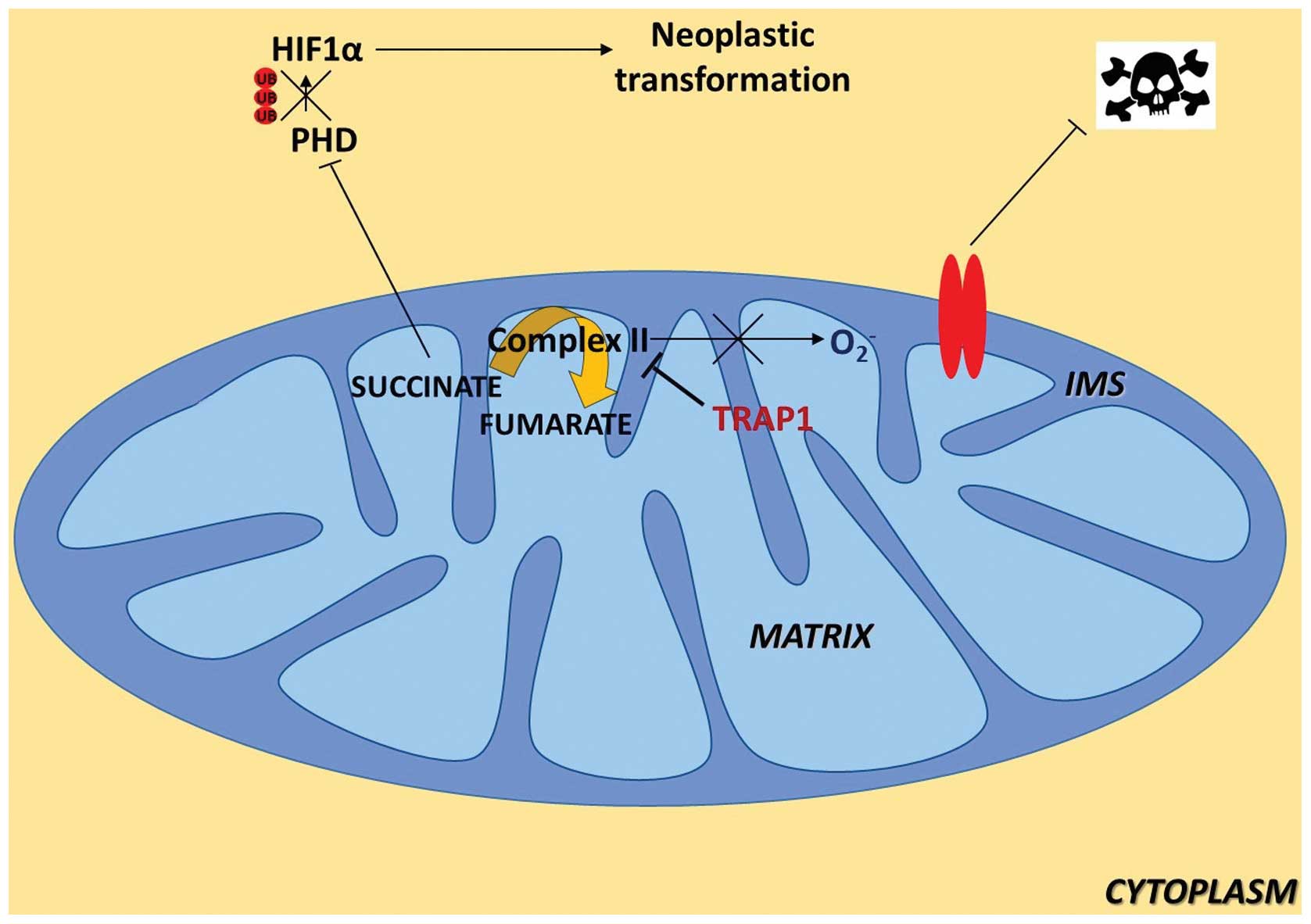
of cancer cells. It is known that succinate contributes to HIF1α
stability through inhibition of prolyl-hydroxylases (PHDs), whose
activity leads to HIF1α ubiquitin-dependent degradation; therefore,
these authors analyzed HIF1α expression levels during the focus
forming assay and found that only TRAP1-expressing cells
accumulated succinate during the assay, and consequently that HIF1α
was exclusively detectable in this cell population. Consistently,
HIF1α was clearly detected in the majority of cells from tumor
samples obtained from nude mice xenografted with TRAP1-expressing
Saos-2 cells, whereas HIF1α downregulation fully abolished the
formation of foci in TRAP1-expressing tumor cells and in MEFs
transfected with a TRAP1 cDNA. Taken together, the authors propose
that TRAP1 induces neoplastic growth through a succinate-dependent
stabilization of HIF1α. This novel and fascinating hypothesis is
schematically summarized in Fig.
1.
4. TRAP1 as a tumor suppressor?
Consistently with the observation by Sciacovelli
et al (22), Yoshida et
al (23) showed that TRAP1
downregulates mitochondrial respiration and ATP production. TRAP1
KO mouse adult fibroblasts (MAFs) displayed a higher basal OCR and
a significantly higher maximum respiratory capacity than wild-type
(WT) cells, accompanied by decreased glycolysis. This effect was
TRAP1-dependent, since re-introduction of TRAP1 in MAFs was
sufficient to restore basal OCR levels, confirming a preference for
oxidative phosphorylation over aerobic glycolysis in TRAP1 KO
cells. In agreement, TRAP1 KO cells showed reduced levels of
glycolytic metabolites, increased levels of TCA cycle intermediates
and anaplerotic substrates, and increased fatty acid oxidation and
NAD+/NADH ratio, confirming a metabolic flux through the
TCA cycle independent of glucose metabolism. Increased complex IV
enzymatic activity was found in TRAP1 KO cells as well as
steady-state ATP levels that was significantly higher than in WT
cells. These results were confirmed in HeLa and HCT116 human cancer
cell lines. In agreement with previous reports, TRAP1-dependent
inhibition of mitochondrial respiration resulted in reduced ROS
levels in both MAFs and HCT116, which proves that TRAP1 KO cells
are constitutively exposed to elevated oxidative stress. Since
elevated ROS favor cell invasion, the authors showed that indeed
TRAP1 KO or transient suppression dramatically enhance cell
invasiveness, both in mouse fibroblasts and in a variety of human
cell lines. Importantly, this phenotype is sensitive to c-Src
inhibition and ROS scavenging, supporting a direct link between
TRAP1 deficiency, elevated ROS, and c-Src activation in mediating
this process. These authors postulated that the impact of TRAP1 on
mitochondrial respiration is mediated, at least in part, by its
direct regulation of mitochondrial c-Src. In fact, a reciprocal
regulation of the two proteins was observed, which led to increased
Tyr-416 phosphorylation of mitochondrial c-Src upon TRAP1
suppression, paired with a preferential interaction of TRAP1 with
the inactive form of c-Src.
The impact of reduced TRAP1 expression on in
vitro cell invasion led Yoshida and colleagues to hypothesize,
contrary to other previously presented findings, that certain more
aggressive, metastatic, or late-stage cancer types may express
lower TRAP1 levels than less advanced tumors. In particular, they
confirmed an inverse correlation between TRAP1 expression and tumor
stage in cervical, bladder, and clear cell renal cell carcinoma.
Intriguingly, cervical carcinoma is among those cancers whose
predominant energy metabolism is obtained via oxidative
phosphorylation, not glycolysis. Therefore, the authors suggested a
re-evaluation of the assumption that TRAP1 is uniformly
pro-oncogenic, and to consider that TRAP1 inhibition alone is
unlikely to be a viable treatment strategy for cancers that use
oxidative phosphorylation.
The intriguing hypothesis that, in some settings and
depending on the cell type context, TRAP1 may act as a tumor
suppressor is partially supported by a study on 208 patients
affected by ovarian cancer. Immunohistochemical analyses showed
that high TRAP1 expression correlated significantly with favorable
chemotherapy-response and showed a significantly positive impact on
overall survival (24). The
authors also found that high TRAP1 expression correlated
significantly with estrogen receptor α levels. Consistently, the
expression of TRAP1 was previously shown as significantly increased
in letrozole-responsive cancers compared to letrozole-insensitive
(25). Considering that TRAP1 has
been reported as an estrogen-regulated gene (26), it will be interesting, in future
studies, to analyze the relationship between TRAP1 and tumor
outcome in gynaecological malignancies.
5. TRAP1 outside the mitochondria: quality
control of mitochondrial proteins
Although original reports already suggested evidence
of extra-mitochondrial localizations of TRAP1, for a long time this
has been substantially ignored by the scientific community and
TRAP1 has been only considered ‘the mitochondrial HSP90’, whose
role was restricted to the maintenance of organelle homeostasis
through the regulation of the mitochondrial transition pore (MTP)
as a scavenger of mitochondrial ROS. However, our group started few
years ago to ‘look’ outside the mitochondria: taking advantage of
proteomic profiles, mass spectra data, as well as biochemical and
microscopic observations, we provided the first demonstration of an
extramitochondrial localization of TRAP1 linked to a specific
function. This novel and interesting field to explore allowed us to
identify a novel interaction of TRAP1 with the proteasomal particle
TBP7 on the outer side of the endoplasmic reticulum (ER), whose
function controls the fate of two mitochondrial proteins, Sorcin
isoform B and F1ATPase β subunit, through the regulation of their
ubiquitination (27). However,
even though the structural characterization of TRAP1/TBP7
interaction and their functional role in the quality control of
mitochondria destined proteins was deeply explored in our studies,
a question remained unanswered: how can TRAP1 take care of
mitochondrial proteins even before they reach their destination?
Excluding the hypothesis of a folding control, which is known to
occur inside mitochondria, and after the demonstration that TRAP1
does not influence the half-life of substrates (28), we hypothesized a role in the
quality control at early steps of protein synthesis. It is now
accepted that up to 30% of neo-synthesized proteins undergo
ubiquitination to be cotranslationally degraded if they are damaged
(29). Very recent reports support
this hypothesis: many groups demonstrated the presence of chaperone
complexes and ubiquitination machinery that regulate the first
steps of protein synthesis, to ensure that damaged polypeptides are
immediately degraded before the complete folding, in many cases
while they are still bound to ribosomes (30). These observations open a new
scenario in which ribosome-associated chaperones act as key
regulators of cellular proteostasis through direct or indirect
modulation of protein synthesis, folding, assembly and transport.
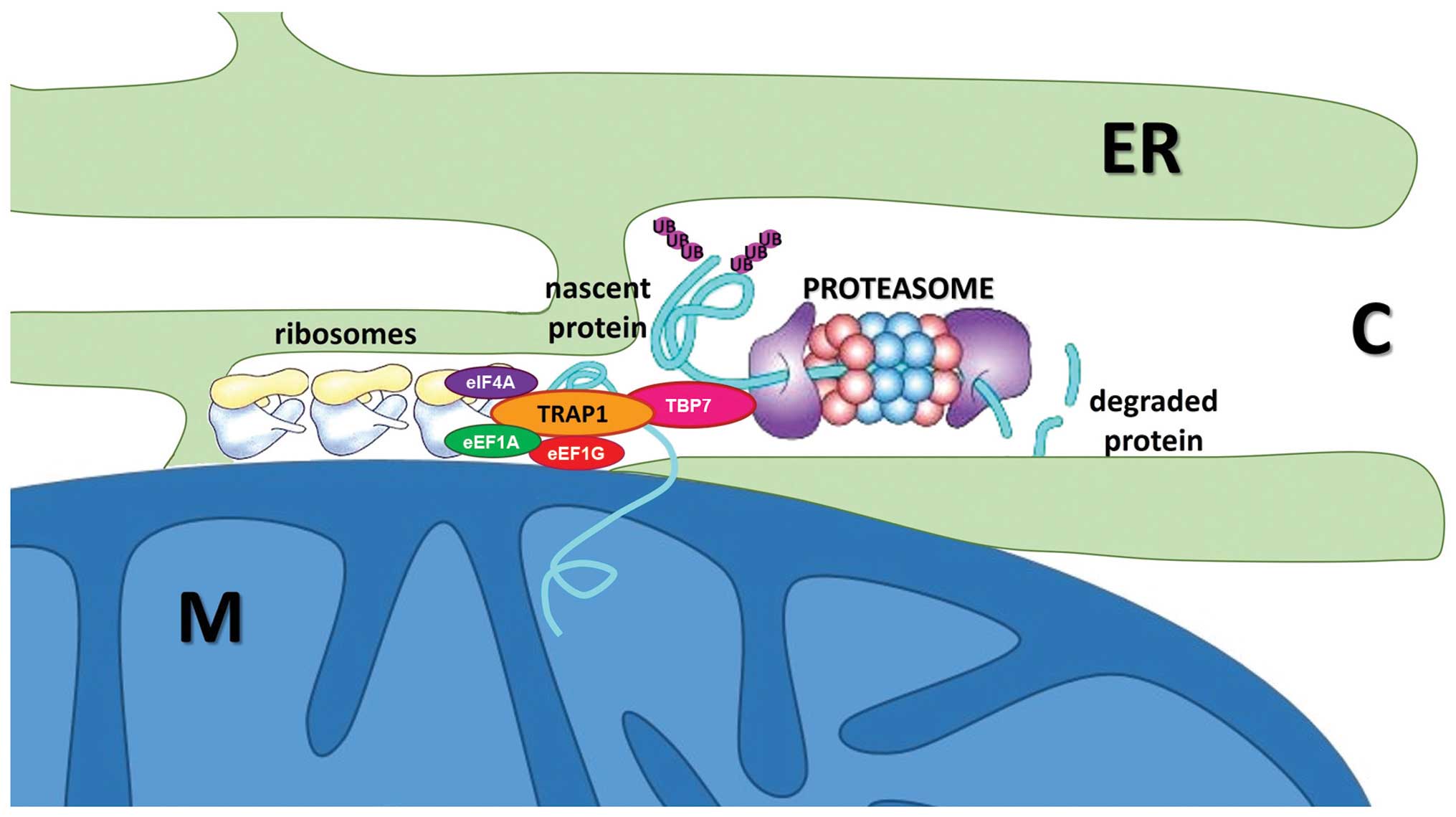
Consistently, we found TRAP1 associated to actively translating
polysomes and to three translation factors, namely eIF4A, eEF1A,
eEF1G (28) in HCT116 CRC cells.
This suggests novel roles for TRAP1 in translational processes,
which explains the mechanism of quality control on its
mitochondrial substrates. At early stages of protein synthesis,
TRAP1 substrates are rapidly accumulated but more rapidly degraded
in TRAP1 KO cells, indicating that this chaperone regulates the
fate of its target proteins through a cotranslational mechanism,
controlling the balance between synthesis and degradation. TRAP1
control involves the ubiquitin-proteasome system (UPS): TRAP1
silencing causes an increase in total ubiquitination levels, and
this phenotype is rescued by the transfection of an
extramitochondrial TRAP1 mutant (27). Consistently, TRAP1 regulation of
ubiquitination occurs during translation: recovery of total
ubiquitinated proteins by immunoprecipitation, following a brief
pulse with radiolabelled amino acids, showed that TRAP1-stable
interfered CRC cells accumulate more than double amount of
ubiquitinated proteins during protein synthesis. TRAP1-dependent
regulation of protein synthesis/ubiquitination is schematically
represented in Fig. 2.
Recently our group unraveled that this regulatory
mechanism is quite complex and involves the modulation of
phosphorylation levels, either in basal conditions or under stress,
of the translation factor eIF2α, whose phosphorylation results in
the attenuation of cap-dependent translation, while favoring the
IRES-dependent one. TRAP1 slows the rate of protein synthesis
through the eIF2α pathway, favoring the activation of GCN2 and PERK
kinases, with consequent phosphorylation of eIF2α and attenuation
of cap-dependent translation. This enhances the synthesis of
selective stress-responsive proteins, as the transcription factor
ATF4 and its downstream effectors BiP/Grp78 and the cystine
antiporter system xCT, thereby providing protection toward ER
stress, oxidative damage and nutrient deprivation. Accordingly,
TRAP1 silencing sensitizes cells to apoptosis induced by novel
antitumoral drugs that inhibit cap-dependent translation, such as
Ribavirin or 4EGI-1, and reduces the ability of cells to migrate
through the pores of transwell filters in the presence of these
drugs (28). The relevance of
these findings is supported by evidence that translational control
is a crucial component of cancer development and progression,
involved in the regulation of both global protein synthesis and
translation of selective mRNAs species that promote tumor cell
survival, angiogenesis, transformation, invasion and metastasis
(31).
Translational control of cancer is multifaceted,
involving alterations in translation factor expression levels and
activities unique to different types of cancers, disease stages and
the tumor microenvironment (32,33).
This process allows cells to respond swiftly to a changing
environment, and becomes very important during tumorigenesis, when
cells have to face stress conditions resulting from reduced oxygen
and nutrient availability, and have to reprogram their metabolism.
We suggest that TRAP1 could be involved in this adaptation process.
Indeed, TRAP1 is hyperexpressed in colorectal and breast carcinoma
specimens, and it is co-upregulated with members of translational
machinery and with stress-responsive chaperones such as BiP/Grp78.
In this perspective, the role of TRAP1 becomes relevant in
resistance to antiapoptotic agents that involve ER stress
activation, condition often found in tumors. We have shown that
TRAP1 CRC interfered cells are more sensitive to thapsigargin (an
ER-stress inducer), and very recently confirmed these data in a
breast cancer cell model, demonstrating that TRAP1 also confers
resistance to paclitaxel, a microtubule stabilizing/ER stress
inducer agent widely used in breast cancer therapy (34), and to genotoxic agents, as
anthracyclins (35). Accordingly,
paclitaxel- and anthracyclin-resistant cell lines show higher
levels of TRAP1 compared to the non-resistant counterpart. In this
context, the extramitochondrial TRAP1 antiapoptotic activity
represents a mechanism responsible for the protection against ER
stress, ER stress inducers and a variety of chemotherapeutics
causing ER stress: indeed, ER-associated TRAP1 regulates
mitochondrial apoptosis by controlling the expression of specific
client proteins and, among others, Sorcin, thus suggesting that
TRAP1 may be a key player in coupling organelle proteostasis,
adaptation to stress and cell survival, through the regulation of
the mitochondrial apoptotic pathway. These data are further
confirmed by the finding that an extramitochondrial TRAP1 mutant is
still able to provide resistance against ER stress-induced cell
death and to maintain high levels of mitochondrial cytoprotective
proteins such as Sorcin. Thus, TRAP1 is emerging as a key regulator
of bidirectional crosstalk between ER and mitochondria, and
ER-localized TRAP1 is becoming relevant in the balance of cellular
homeostasis since it controls protein synthesis, contributes to
cell’s proper response to stress conditions, and regulates pathways
involved in cytoprotection and drug resistance. In such a
perspective, cytoprotective activities of both intramitochondrial
and extramitochondrial TRAP1 are involved in the resistance to
apoptosis of tumor cells and in protection from traditional
chemotherapeutics (8,10,34,35).
These new findings confirm that TRAP1 network could be an
attractive target to develop novel anticancer strategies aimed at
inhibiting TRAP1 function in cell compartments other than
mitochondria, in order to disrupt protein quality control
mechanisms, lower the threshold of MTP opening selectively in
cancer cells and revert the drug-resistant phenotype of human
malignancies. In such a perspective, further studies are urgently
needed to identify specific subsets of human tumors, which are
dependent for their survival on this extramitochondrial quality
control network and are, thus, suitable for a TRAP1-targeted
therapy.
6. TRAP1 in health and disease
Beyond the widely described roles of TRAP1 in the
etiopathogenesis of a multifactorial disease as cancer, in the past
10 years very sophisticated and focused studies have unveiled an
important involvement of this chaperone in the genesis and
development of some human diseases. These include several human
neurodegenerative diseases, such as Alzheimer’s disease,
Parkinson’s disease (PD) and Huntington’s disease, all considered
as ‘mitochondropathies’, as well as other genetic, and not
necessarily mitochondrial, kidney and cardiovascular diseases. Due
to our limited contribution and knowledge of these studies the most
recent achievements will be briefly summarized, and we apologize to
all the authors for not giving enough ‘space’ to their outstanding
results.
TRAP1 in the maintenance of mitochondrial
integrity
Changes in mitochondrial morphology, which is
regulated by continuous fusion and fission to form highly connected
networks or fragmented units, may lead to the degradation of
mitochondria via autophagy (so-called mitophagy) and often cause
neuronal synaptic loss and cell death in several human
neurodegenerative diseases. Therefore, cells have developed complex
quality control mechanisms to cope with the different challenges
constantly imposed on the integrity of mitochondria. Pridgeon et
al (36) have indirectly
linked TRAP1 to Parkinson’s disease, demonstrating that PINK1, a
major kinase whose mutations are involved in the development of
autosomic recessive forms of PD, is a binding partner of TRAP1,
phosphorylates it upon induction of oxidative stress, and that
TRAP1 is required for PINK1-mediated protection against
oxidative-stress-induced apoptosis.
Takamura et al (37) showed that TRAP1 KD in neuroblastoma
cells and glioma cancer cell lines of neuronal derivation induced
an abnormal mitochondrial morphology, through a significant
decrease in dynamin-related protein 1 (Drp1) and mitochondrial
fission factor (Mff), affecting mitochondrial function. These
observations confirm a role of TRAP1 in maintaining mitochondrial
morphology.
Some other studies performed in Drosophila
systems by Zhang et al (38) demonstrated that human TRAP1 is able
to rescue phenotypes in PINK1 loss-of-function flies, but has only
minor effects on phenotypes of flies deficient of PARKIN, another
protein associated with autosomal recessive, early-onset PD. In
addition, detrimental effects observed after RNAi-mediated
silencing of complex I subunits were rescued by TRAP1 in
Drosophila. Remarkably, these studies confirm the metabolic
control by TRAP1, albeit in different experimental systems: in
fact, the lack of functional mitochondria obviously coincides with
reduced levels of complex I subunits, impaired complex I activity
and a decline in ATP content. TRAP1 protective effects require its
ATP binding properties, as ATP-binding deficient TRAP1 mutants were
unable to rescue this activity. Considering that functional ETC
causes polarization of the mitochondrial membrane and that
depolarization of mitochondria is required to initiate mitophagy,
it can be speculated that TRAP1 might negatively regulate mitophagy
by maintaining the ETC in a functional state (38).
Accordingly, Costa et al (39) characterized Drosophila TRAP1
null mutants, showing that loss of TRAP1 results in a decrease in
mitochondrial function and increased sensitivity to stress. These
findings demonstrated that TRAP1 works downstream of PINK1 and in
parallel with PARKIN in Drosophila, and that enhancing its
function may ameliorate mitochondrial dysfunction and rescue
neurodegeneration in PD.
Moreover, TRAP1 protein has been identified as a
novel modifier of the mitochondrial toxicity induced by
[A53T]α-Synuclein in fruitfly models of PD. Cell culture
experiments further demonstrated that [A53T]α-Synuclein directly
interferes with a number of mitochondrial functions, including
complex I ATP production, mitochondrial fragmentation, and
sensitivity to oxidative stress. These effects could be blocked by
TRAP1 overexpression (40).
As mitochondrial dysfunction has been previously
linked to mutations in several other genes associated with genetic
PD, all the described data provide further evidence of a common
mitochondrial-centric mechanism of PD pathogenesis, that probably
involves TRAP1 function, its interaction with PINK1 and the
regulation of ETC function.
The genetics of TRAP1
In last two years, few pioneering studies suggested
an involvement of TRAP1 in renal diseases. Fismen et al
(41) analyzed the coregulation
between TRAP1 and DNaseI in glomerulonephritis. Previous data
indicate that TRAP1 and DNaseI genes overlap; recent findings,
moreover, show that transformation of mild glomerulonephritis into
end-stage disease coincides with shutdown of renal DNaseI
expression in mouse models, damaging glomerular basement membranes.
Translating the observations obtained in mice to human lupus
nephritis, they observed that DNaseI shutdown coincides with TRAP1
overexpression, with a still unclear, but structured molecular
mechanism, that could probably involve transcriptional
interference, thus providing to TRAP1 a new role in the progression
of this disease.
Our group contributed to a partial characterization
of a novel role of TRAP1 in kidney abnormalities of genetic origin.
Saisawat et al (42) shed
new light on the etiology of congenital anomalies of the kidney and
urinary tract (CAKUT) and vertebral anomalies, anal atresia,
cardiovascular anomalies, tracheoesophageal fistula, renal and/or
radial anomalies, limb defects (VACTERL) association, rare
pathologies that affect kidney and urinary tract. Recessive
mutations of TRAP1 were found by deep sequencing analysis of the
genome from affected patients, eliciting some questions about the
role of TRAP1 in the genesis of these pathologies and its
involvement in embryonic development of the kidney. In normal
tissues, TRAP1 was found to be expressed in renal epithelia of
developing mouse kidney and in the kidney of adult rats. Likely,
the antiapoptotic role of TRAP1 is important in the correct
development of the renal apparatus, and its loss of function due to
mutations in its functional domains could be disease-causing. It is
worth noting that there are six case reports published of
individuals with VACTERL association in conjunction with
mitochondrial dysfunction, as summarized recently by Siebel and
Solomon (43); this could be
consistent with the well known role of TRAP1 in control of
mitochondrial integrity and metabolism, thus suggesting a possible
relationship between TRAP1 inactivation and the pathogenesis of the
disease.
From the overall data, it would not be surprising to
hypothesize a role for TRAP1 in normal cellular and tissue
development. Indeed, it is well known that HSPs are related to
development; in addition, HSP genes are shown to be phase- and
tissue-specific during embryogenesis. It was found that 19 HSPs of
the HSP70, HSP90, and HSP110 families hold different expression and
characteristic correlations with the developmental phases in
embryonic forelimb tissue of normal mice. Among those, TRAP1 may be
considered as a limb-development-related gene (44). Moreover, in vivo experiments
showed that TRAP1 expression could be closely associated with
normal palate development and cleft palate in mouse embryo,
probably through participating in the stress response process
and/or the antiapoptotic processes (45).
7. Concluding remarks and future
perspectives
The interest for HSP90 as a therapeutic target of
several human diseases, from neurodegeneration to cancers, is still
very high. However, very few data are available on the isoform
specificity profiles of the HSP90 inhibitors. The crystal structure
of full-length TRAP1, very recently presented (46), will certainly open a new scenario
on the: i) selectivity to target only TRAP1 among all the other
members of HSP90 family; ii) possibility to design novel, specific
TRAP1 inhibitors to be directed either inside or outside
mitochondria.
This review aims at presenting an updated view of
this important protein mainly in tumor cell pathophysiology. In our
opinion, the core result of recent advances in TRAP1 biology lies
in two items of evidence: TRAP1 crucially influences the switch
from oxidative metabolism to glycolysis, and therefore contributes
to tumorigenesis by inhibiting mitochondrial respiration, and
simultaneously affects global protein quality control by
contributing to protein synthesis regulation in the ER. This is
realized through another focused metabolic switch between prevalent
cap- to IRES-dependent translation and the balance between protein
synthesis and cotranslational degradation.
TRAP1 seems therefore to be a central regulatory
protein with homeostatic roles at the crossroad between different
kinds of cell functions/metabolism during the transformation
process or, possibly, during normal development, as suggested by
the novel and emerging evidence on its involvement in the etiology
of human pathologies. Strikingly, TRAP1 function in normal cells is
almost unexplored. In this context, the recent findings of TRAP1
involvement in mouse and human embryonic development could
stimulate an interesting study of the physiological role of this
chaperone.
As widely addressed in this review, TRAP1 has long
been considered an attractive biomarker and/or target for future
development of novel therapeutic strategies. The apparent
discrepancies on several data recently obtained by different
outstanding scientists, i.e. stabilization/inhibition of the ETC
component activity, HIF-mediated tumorigenesis, TRAP1 as a tumor
suppressor or TRAP1 as an oncogene, further reinforce the interest
in TRAP1 ‘life’. In addition, the apparently opposite/contrasting
function of TRAP1 in ovarian cancers, compared to its very
conserved uniform trend in all other tumor types, including
colorectal cancer, suggests that further studies are required to
shed light on the role of TRAP1 in different types of human
cancers.
Undoubtedly, traditional anticancer
chemotherapeutics, targeting DNA replication and cell division,
have proved to be highly successful in the treatment of some
tumors. However, they have severe side effects and limitations,
above all the development of resistance. Now, a new wave of
anticancer agents is emerging, targeting complex multicomponent
cellular machineries, including heat shock protein chaperones and
the proteasome, which thus interfere with those support systems
that are more essential for cancer cells than for normal cells
(47).
As one of the ‘oldest’ TRAP1 study groups, we
strongly hope that both the continuously emerging findings on new
TRAP1 features together with the reading of the present review on
the ‘revisited’ TRAP1 could contribute to identify this chaperone
as an important novel target in future anticancer, and not only,
drug development.
Acknowledgements
This study was supported by the Associazione
Italiana per la Ricerca sul Cancro (AIRC) (Grant IG13128 to M.L.
and F.E.), by the Italian Ministry of Health (Grant
GR-2010-2310057), by POR Campania FSE 2007-2013, Projects CRÈME and
STRAIN.
Abbreviations:
|
CAKUT
|
congenital anomalies of the kidney and
urinary tract
|
|
CRC
|
colorectal carcinoma
|
|
CypD
|
cyclophilin D
|
|
ER
|
endoplasmic reticulum
|
|
ETC
|
electron transport chain
|
|
HSP
|
heat shock protein
|
|
KO
|
knock-out
|
|
KD
|
knock-down
|
|
MTP
|
mitochondrial transition pore
|
|
OCR
|
oxygen consumption rate
|
|
PD
|
Parkinson’s disease
|
|
PTP
|
permeability transition pore
|
|
TRAP1
|
tumor necrosis factor
receptor-associated protein 1
|
|
VACTERL
|
vertebral anomalies, anal atresia,
cardiovascular anomalies, tracheoesophageal fistula, renal and/or
radial anomalies, limb defects
|
|
OS
|
oxidative stress
|
References
|
1
|
Song HY, Dunbar JD, Zhang YX, Guo D and
Donner DB: Identification of a protein with homology to hsp90 that
binds the type 1 tumor necrosis factor receptor. J Biol Chem.
270:3574–3581. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen CF, Chen Y, Dai K, Chen PL, Riley DJ
and Lee WH: A new member of the hsp90 family of molecular
chaperones interacts with the retinoblastoma protein during mitosis
and after heat shock. Mol Cell Biol. 16:4691–4699. 1996.PubMed/NCBI
|
|
3
|
Matassa DS, Amoroso MR, Maddalena F,
Landriscina M and Esposito F: New insights into TRAP1 pathway. Am J
Cancer Res. 2:235–248. 2012.PubMed/NCBI
|
|
4
|
Chen B, Piel WH, Gui L, Bruford E and
Monteiro A: The HSP90 family of genes in the human genome: insights
into their divergence and evolution. Genomics. 86:627–637. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Felts SJ, Owen BA, Nguyen P, Trepel J,
Donner DB and Toft DO: The hsp90-related protein TRAP1 is a
mitochondrial protein with distinct functional properties. J Biol
Chem. 275:3305–3312. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cechetto JD and Gupta RS: Immunoelectron
microscopy provides evidence that tumor necrosis factor
receptor-associated protein 1 (TRAP-1) is a mitochondrial protein
which also localizes at specific extramitochondrial sites. Exp Cell
Res. 260:30–39. 2000. View Article : Google Scholar
|
|
7
|
Kang BH, Plescia J, Dohi T, Rosa J, Doxsey
SJ and Altieri DC: Regulation of tumor cell mitochondrial
homeostasis by an organelle-specific Hsp90 chaperone network. Cell.
131:257–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Costantino E, Maddalena F, Calise S,
Piscazzi A, Tirino V, Fersini A, Ambrosi A, Neri V, Esposito F and
Landriscina M: TRAP1, a novel mitochondrial chaperone responsible
for multi-drug resistance and protection from apoptotis in human
colorectal carcinoma cells. Cancer Lett. 279:39–46. 2009.
View Article : Google Scholar
|
|
9
|
Montesano Gesualdi N, Chirico G, Pirozzi
G, Costantino E, Landriscina M and Esposito F: Tumor necrosis
factor-associated protein 1 (TRAP-1) protects cells from oxidative
stress and apoptosis. Stress. 10:342–350. 2007.PubMed/NCBI
|
|
10
|
Landriscina M, Laudiero G, Maddalena F,
Amoroso MR, Piscazzi A, Cozzolino F, Monti M, Garbi C, Fersini A,
Pucci P and Esposito F: Mitochondrial chaperone Trap1 and the
calcium binding protein Sorcin interact and protect cells against
apoptosis induced by antiblastic agents. Cancer Res. 70:6577–6586.
2010. View Article : Google Scholar
|
|
11
|
Ghosh JC, Siegelin MD, Dohi T and Altieri
DC: Heat shock protein 60 regulation of the mitochondrial
permeability transition pore in tumor cells. Cancer Res.
70:8988–8993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rasola A, Sciacovelli M, Pantic B and
Bernardi P: Signal transduction to the permeability transition
pore. FEBS Lett. 584:1989–1996. 2010. View Article : Google Scholar
|
|
13
|
Altieri DC: Hsp90 regulation of
mitochondrial protein folding: from organelle integrity to cellular
homeostasis. Cell Mol Life Sci. 70:2463–2472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Pang E, Loo RR, Rao J, Go VL, Loo
JA and Lu QY: Concomitant inhibition of HSP90, its mitochondrial
localized homologue TRAP1 and HSP27 by green tea in pancreatic
cancer HPAF-II cells. Proteomics. 11:4638–4647. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao JY, Song BR, Peng JJ and Lu YM:
Correlation between mitochondrial TRAP-1 expression and lymph node
metastasis in colorectal cancer. World J Gastroenterol.
18:5965–5971. 2012. View Article : Google Scholar
|
|
16
|
Han JJ, Baek SK, Lee JJ, Kim GY, Kim SY
and Lee SH: Combination of TRAP1 and ERCC1 expression predicts
clinical outcomes in metastatic colorectal cancer treated with
oxaliplatin/5-fluorouracil. Cancer Res Treat. 46:55–64. 2014.
View Article : Google Scholar
|
|
17
|
Zhan Q, Tsai S, Lu Y, Wang C, Kwan Y and
Ngai S: RuvBL2 is involved in histone deacetylase inhibitor
PCI-24781-induced cell death in SK-N-DZ neuroblastoma cells. PLoS
One. 8:e716632013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Megger DA, Bracht T, Kohl M, Ahrens M,
Naboulsi W, Weber F, Hoffmann AC, Stephan C, Kuhlmann K, Eisenacher
M, Schlaak JF, Baba HA, Meyer HE and Sitek B: Proteomic differences
between hepatocellular carcinoma and nontumorous liver tissue
investigated by a combined gel-based and label-free quantitative
proteomics study. Mol Cell Proteomics. 12:2006–2020. 2013.
View Article : Google Scholar
|
|
19
|
Im CN and Seo JS: Overexpression of tumor
necrosis factor receptor-associated protein 1 (TRAP1), leads to
mitochondrial aberrations in mouse fibroblast NIH/3T3 cells. BMB
Rep. Dec 1–2013.(Epub ahead of print). pii: 2469.
|
|
20
|
Agorreta J, Hu J, Liu D, Delia D, Turley
H, Ferguson DJ, Iborra F, Pajares MJ, Larrayoz M, Zudaire I, Pio R,
Montuenga LM, Harris AL, Gatter K and Pezzella F: TRAP1 regulates
proliferation, mitochondrial function and has prognostic
significance in NSCLC. Mol Cancer Res. 3–June;2014.(Epub ahead of
print).
|
|
21
|
Chae YC, Angelin A, Lisanti S, Kossenkov
AV, Speicher KD, Wang H, Powers JF, Tischler AS, Pacak K, Fliedner
S, Michalek RD, Karoly ED, Wallace DC, Languino LR, Speicher DW and
Altieri DC: Landscape of the mitochondrial Hsp90 metabolome in
tumours. Nat Commun. 4:21392013.PubMed/NCBI
|
|
22
|
Sciacovelli M, Guzzo G, Morello V, Frezza
C, Zheng L, Nannini N, Calabrese F, Laudiero G, Esposito F,
Landriscina M, Defilippi P, Bernardi P and Rasola A: The
mitochondrial chaperone TRAP1 promotes neoplastic growth by
inhibiting succinate dehydrogenase. Cell Metab. 17:988–999. 2013.
View Article : Google Scholar
|
|
23
|
Yoshida S, Tsutsumi S, Muhlebach G,
Sourbier C, Lee MJ, Lee S, Vartholomaiou E, Tatokoro M, Beebe K,
Miyajima N, Mohney RP, Chen Y, Hasumi H, Xu W, Fukushima H,
Nakamura K, Koga F, Kihara K, Trepel J, Picard D and Neckers L:
Molecular chaperone TRAP1 regulates a metabolic switch between
mitochondrial respiration and aerobic glycolysis. Proc Natl Acad
Sci USA. 110:E1604–E1612. 2013. View Article : Google Scholar
|
|
24
|
Aust S, Bachmayr-Heyda A, Pateisky P, Tong
D, Darb-Esfahani S, Denkert C, Chekerov R, Sehouli J, Mahner S, Van
Gorp T, Vergote I, Speiser P, Horvat R, Zeillinger R and Pils D:
Role of TRAP1 and estrogen receptor alpha in patients with ovarian
cancer - a study of the OVCAD consortium. Mol Cancer. 11:692012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Walker G, MacLeod K, Williams AR, Cameron
DA, Smyth JF and Langdon SP: Estrogen-regulated gene expression
predicts response to endocrine therapy in patients with ovarian
cancer. Gynecol Oncol. 106:461–468. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
O’Donnell AJ, Macleod KG, Burns DJ, Smyth
JF and Langdon SP: Estrogen receptor-alpha mediates gene expression
changes and growth response in ovarian cancer cells exposed to
estrogen. Endocr Relat Cancer. 12:851–866. 2005.
|
|
27
|
Amoroso MR, Matassa DS, Laudiero G,
Egorova AV, Polishchuk RS, Maddalena F, Piscazzi A, Paladino S,
Sarnataro D, Garbi C, Landriscina M and Esposito F: TRAP1 and the
proteasome regulatory particle TBP7/Rpt3 interact in the
endoplasmic reticulum and control cellular ubiquitination of
specific mitochondrial proteins. Cell Death Differ. 19:592–604.
2012. View Article : Google Scholar
|
|
28
|
Matassa DS, Amoroso MR, Agliarulo I,
Maddalena F, Sisinni L, Paladino S, Romano S, Romano MF, Sagar V,
Loreni F, Landriscina M and Esposito F: Translational control in
the stress adaptive response of cancer cells: a novel role for the
heat shock protein TRAP1. Cell Death Dis. 4:e8512013. View Article : Google Scholar
|
|
29
|
Wang F, Durfee LA and Huibregtse JM: A
cotranslational ubiquitination pathway for quality control of
misfolded proteins. Mol Cell. 50:368–378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brandman O, Stewart-Ornstein J, Wong D,
Larson A, Williams CC, Li GW, Zhou S, King D, Shen PS, Weibezahn J,
Dunn JG, Rouskin S, Inada T, Frost A and Weissman JS: A
ribosome-bound quality control complex triggers degradation of
nascent peptides and signals translation stress. Cell.
151:1042–1054. 2012. View Article : Google Scholar
|
|
31
|
Ruggero D: Translational control in cancer
etiology. Cold Spring Harb Perspect Biol. 5:pii a012336. 2013.
View Article : Google Scholar
|
|
32
|
Silvera D, Formenti SC and Schneider RJ:
Translational control in cancer. Nat Rev Cancer. 10:254–266. 2010.
View Article : Google Scholar
|
|
33
|
Liu B, Han Y and Qian SB: Cotranslational
response to proteotoxic stress by elongation pausing of ribosomes.
Mol Cell. 49:453–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maddalena F, Sisinni L, Lettini G,
Condelli V, Matassa DS, Piscazzi A, Amoroso MR, La Torre G,
Esposito F and Landriscina M: Resistance to paclitxel in breast
carcinoma cells requires a quality control of mitochondrial
antiapoptotic proteins by TRAP1. Mol Oncol. 7:895–906. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sisinni L, Maddalena F, Lettini G,
Condelli V, Matassa DS, Esposito F and Landriscina M: TRAP1 role in
endoplasmic reticulum stress protection favors resistance to
anthracyclins in breast carcinoma cells. Int J Oncol. 44:573–582.
2014.PubMed/NCBI
|
|
36
|
Pridgeon JW, Olzmann JA, Chin LS and Li L:
PINK1 protects against oxidative stress by phosphorylating
mitochondrial chaperone TRAP1. PLoS Biol. 5:e1722007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takamura H, Koyama Y, Matsuzaki S, Yamada
K, Hattori T, Miyata S, Takemoto K, Tohyama M and Katayama T: TRAP1
controls mitochondrial fusion/fission balance through Drp1 and Mff
expression. PLoS One. 7:e519122012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang L, Karsten P, Hamm S, Pogson JH,
Müller-Rischart AK, Exner N, Haass C, Whitworth AJ, Winklhofer KF,
Schulz JB and Voigt A: TRAP1 rescues PINK1 loss-of-function
phenotypes. Hum Mol Genet. 22:2829–2841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Costa AC, Loh SH and Martins LM:
Drosophila Trap1 protects against mitochondrial dysfunction
in a PINK1/parkin model of Parkinson’s disease. Cell Death Dis.
4:e4672013. View Article : Google Scholar
|
|
40
|
Butler EK, Voigt A, Lutz AK, Toegel JP,
Gerhardt E, Karsten P, Falkenburger B, Reinartz A, Winklhofer KF
and Schulz JB: The mitochondrial chaperone protein TRAP1 mitigates
α-Synuclein toxicity. PLoS Genet. 8:e10024882012.PubMed/NCBI
|
|
41
|
Fismen S, Thiyagarajan D, Seredkina N,
Nielsen H, Jacobsen S, Elung-Jensen T, Kamper AL, Johansen SD,
Mortensen ES and Rekvig OP: Impact of the tumor necrosis factor
receptor-associated protein 1 (Trap1) on renal DNaseI shutdown and
on progression of murine and human lupus nephritis. Am J Pathol.
182:688–700. 2013. View Article : Google Scholar
|
|
42
|
Saisawat P, Kohl S, Hilger AC, Hwang DY,
Yung Gee H, Dworschak GC, Tasic V, Pennimpede T, Natarajan S,
Sperry E, Matassa DS, Stajić N, Bogdanovic R, de Blaauw I, Marcelis
CL, Wijers CH, Bartels E, Schmiedeke E, Schmidt D, Märzheuser S,
Grasshoff-Derr S, Holland-Cunz S, Ludwig M, Nöthen MM, Draaken M,
Brosens E, Heij H, Tibboel D, Herrmann BG, Solomon BD, de Klein A,
van Rooij IA, Esposito F, Reutter HM and Hildebrandt F: Whole-exome
resequencing reveals recessive mutations in TRAP1 in individuals
with CAKUT and VACTERL association. Kidney Int. 85:1310–1317. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Siebel S and Solomon BD: Mitochondrial
factors and VACTERL association-related congenital malformations.
Mol Syndromol. 4:63–73. 2013. View Article : Google Scholar
|
|
44
|
Zhu Y, Zhou H, Zhu Y, Wan X, Zhu J and
Zhang T: Gene expression of Hsp70, Hsp90, and Hsp110 families in
normal and abnormal embryonic development of mouse forelimbs. Drug
Chem Toxicol. 35:432–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu Y, Ren C, Wan X, Zhu Y, Zhu J, Zhou H
and Zhang T: Gene expression of Hsp70, Hsp90 and Hsp110 families in
normal palate and cleft palate during mouse embryogenesis. Toxicol
Ind Health. 29:915–930. 2013. View Article : Google Scholar
|
|
46
|
Lavery LA, Partridge JR, Ramelot TA,
Elnatan D, Kennedy MA and Agard DA: Structural asymmetry in the
closed state of mitochondrial Hsp90 (TRAP1) supports a two-step ATP
hydrolysis mechanism. Mol Cell. 53:330–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dobbelstein M and Moll U: Targeting
tumour-supportive cellular machineries in anticancer drug
development. Nat Rev Drug Discov. 13:179–196. 2014. View Article : Google Scholar : PubMed/NCBI
|