Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant neoplasms in humans and is more prevalent among
Asian and African populations (1,2).
Molecular analyses have shown that HCC pathogenesis is a
multifactorial and multistep process reflecting alterations derived
from epigenetic instability, chromosomal instability and
deregulated transcription (3).
Risk factors for HCC development include viral infections such as
hepatitis B or C, exposure to aflatoxin B, chronic alcohol
ingestion and cirrhosis from variable causes (4–6).
This advanced or relapsing disease has a poor prognosis due to
underlying liver disease and lack of effective systemic treatment.
Moreover, conventional chemotherapy has not been demonstrated to
prolong survival of patients with advanced HCC (7,8).
However, recent advances in our knowledge of HCC biology have led
to the development of efficient agents to treat HCC (9).
Sorafenib (Nexavar, BAY 43-9006), an orally active
multikinase inhibitor, has been used to treat several malignant
neoplasms such as HCC and renal cell carcinoma (10). Although monotherapy with sorafenib
has been shown to significantly prolong overall survival and delay
time to progression in patients with advanced HCC, the recent
reports have disappointingly been unable to substantiate a
reduction of tumor burden in patients with advanced HCC (11–13).
Sorafenib is currently the only accepted systemic chemotherapeutic
used in the treatment of HCC, and as such, there is an urgent need
to elucidate the genes that diminish its effectiveness.
RNA interference (RNAi) is a powerful new tool for
performing loss-of-function genetic screens in lower organisms and
can greatly facilitate the identification of components of cellular
signaling pathways (14,15). By suppressing gene expression and
therefore, protein function to a certain extent, RNAi models the
pharmacological inhibition of a target protein and can be an
effective tool for proof-of-principle experiments to identify and
validate cancer drug targets (16).
We performed an unbiased genetic screen on Hep3B
human HCC cancer cell lines using a short hairpin RNA (shRNA)
library that identified SMG-1 as a modulator of sorafenib
sensitivity in vitro and executed the validation procedures
to confirm whether the resultant sequenced target gene can be a
significant molecular target for resistance to sorafenib treatment
of HCC.
Materials and methods
Cell culture
Hep3B, HepG2 and Huh7 cells (American Tissue Culture
Collection) were seeded at a density of 1×106
cm2 and routinely cultured in Dulbecco’s modified
Eagle’s medium (DMEM, Invitrogen Life Technology Inc.) supplemented
with 10% fetal bovine serum plus penicillin-streptomycin (10 IU/ml)
and fungizone (2.5 μg/ml). Cultures were maintained at 37°C in a
humidified 5% CO2 atmosphere.
Establishment of the retroviral vector
(pRS)- and pRS shRNA-Hep3B cell line
For viral production, 2 μg of retroviral vector pRS
and pRS shRNA library were transfected into 293 Ampho cell lines
(1×106). After 4 days of transfection their supernatants
were infected into Hep3B cell lines and selected with 1.0 μg/ml
puromycin as previously described (17).
siRNA transfection
Three Stealth™ RNAi duplexes were synthesized
commercially by Invitrogen Life Technologies Inc. with the help of
tools available online (http://www.invitrogen.com). Stealth RNAi lines
(HSS118096, HSS118097, HSS11808) were designed to target different
coding regions of the human SMG-1 mRNA sequence (NCBI Reference
Sequence: NM_015092.3). Hep3B, HepG2 and Huh7 cells were seeded at
4×105 cells per well in a 60-mm dish in DMEM (Invitrogen
Life Technology Inc.) containing 10% fetal bovine serum (Invitrogen
Life Technology Inc.). On the following day, cells were transfected
with the three Stealth RNAi duplexes as directed by the
manufacturer’s protocol for the Hiperfect transfection reagent
(Qiagen). The final concentration of Stealth RNAi was 50 nM. A
control also contained siRNA Negative Control Med GC (Invitrogen
Life Technology Inc.).
Reverse transcription and real-time
qPCR
Total RNA was extracted from Hep3B using NucleoSpin
RNA II (Macherey-Nagel, Düren, Germany). The 1 μg of total RNA was
reverse-transcribed using Reverse Transcriptase Premix (Elpis
Biotech, Daejeon, Korea) according to the manufacturer’s
instructions. The probe for TaqMan PCR was purchased from
Invitrogen and real-time PCR was performed using the 7500 Fast
Real-Time PCR System (Applied Biosystems, Foster City, CA).
Glyceraldehyde-3-phosphate dehydrogenase was used as an internal
standard and used to normalize the Ct values.
Western blot analysis
Cells were washed with PBS and harvested in lysis
buffer. Proteins were separated using NuPAGE Novex 3–8% Tri-Acetate
(Invitrogen Life Technology Inc.). All primary antibodies were
diluted in 3% BSA in Tris-buffered saline with 0.1% Tween (TBS-T)
at the following dilutions: anti-β-actin (1:1,000, Cell Signaling
Technology), anti-SMG-1 (1:500, Cell Signaling Technology) and
secondary HRP conjugated antibodies (Cell Signaling Technology).
HRP was detected using an Immunobilon Western Chemiluminescent HRP
substrate kit (Millipore). Images were scanned using a Bio-Rad
ChemiDoc XRS System.
Cell proliferation assay
The cells were treated with 0–10 μmol of sorafenib.
Following a 48-h incubation period, cellular proliferation was
assessed using the EZ-CyTox cell viability assay kit (Daeillab,
Korea). EZ-CyTox solution (10 μl) was added to each cell cultured
96-well, and the mixtures were incubated for 2 h at 37°C.
Absorbance was then measured using an ELISA reader at 450 nm
(microplate reader, Bio-Rad). Cellular proliferation was expressed
as a percentage of vehicle-treated cells, which were defined as
100% viable.
Immunohistochemical staining
To investigate the expression of SMG-1 in 20 human
hepatocellular carcinoma tissue samples, we performed
immunohistochemical staining by using anti-SMG-1 (1:200, Cell
Signaling Technology) to tissues that had been surgically resected
or biopsied after informed consents. Enrolled patients had no
previous sorafenib treatment. We designated the expression
strengths as 1 (weakest staining), 2, 3 and 4 (strongest staining)
after meticulous investigation of tissue slides and then compared
the expression strengths of the cancer region with non-cancerous
regions of resected tissue samples.
Statistical analysis
Single group comparisons were done with two-tailed
Student’s t-tests and one-way Anova.
Results
Establishment of Hep3B cell lines
containing the shRNA screening library
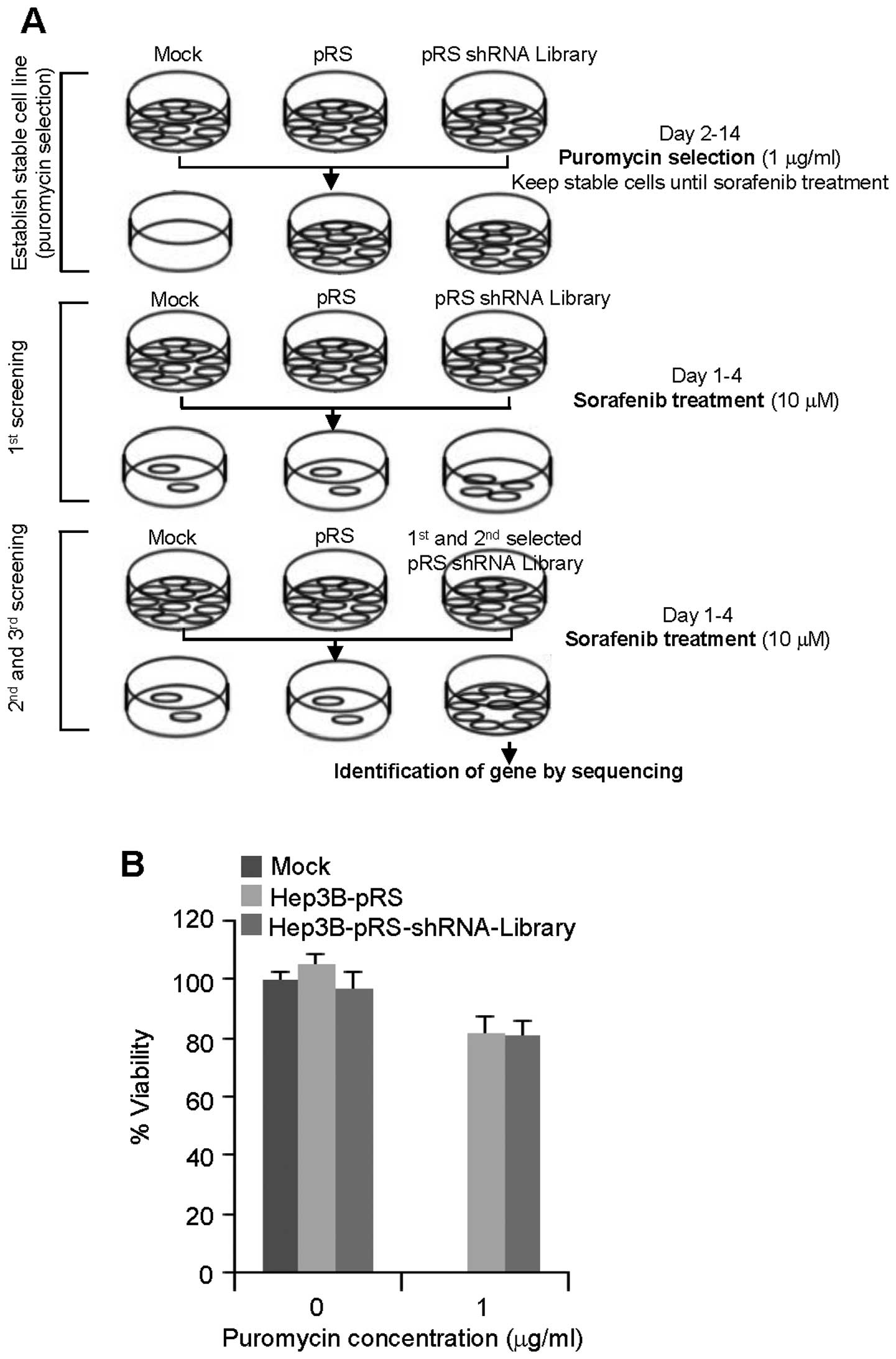
A schematic diagram shows the screening strategy
employed to identify sorafenib-resistant genes from the stage of
puromycin selection to sequencing of target genes (Fig. 1A). Hep3B cells known to have high
sorafenib sensitivity (18) were
infected with the empty retrovirus-based pRS vector or the
pRS-shRNA library that can suppress 8,000 human genes (19) and selected with puromycin (1.0
μg/ml) for 2 weeks. During puromycin selection, mock cells were all
dead, whereas more than 80% of the cells containing pRS vector and
pRS-shRNA library survived (Fig.
1B).
shRNA screen identifies genes involved in
sorafenib resistance
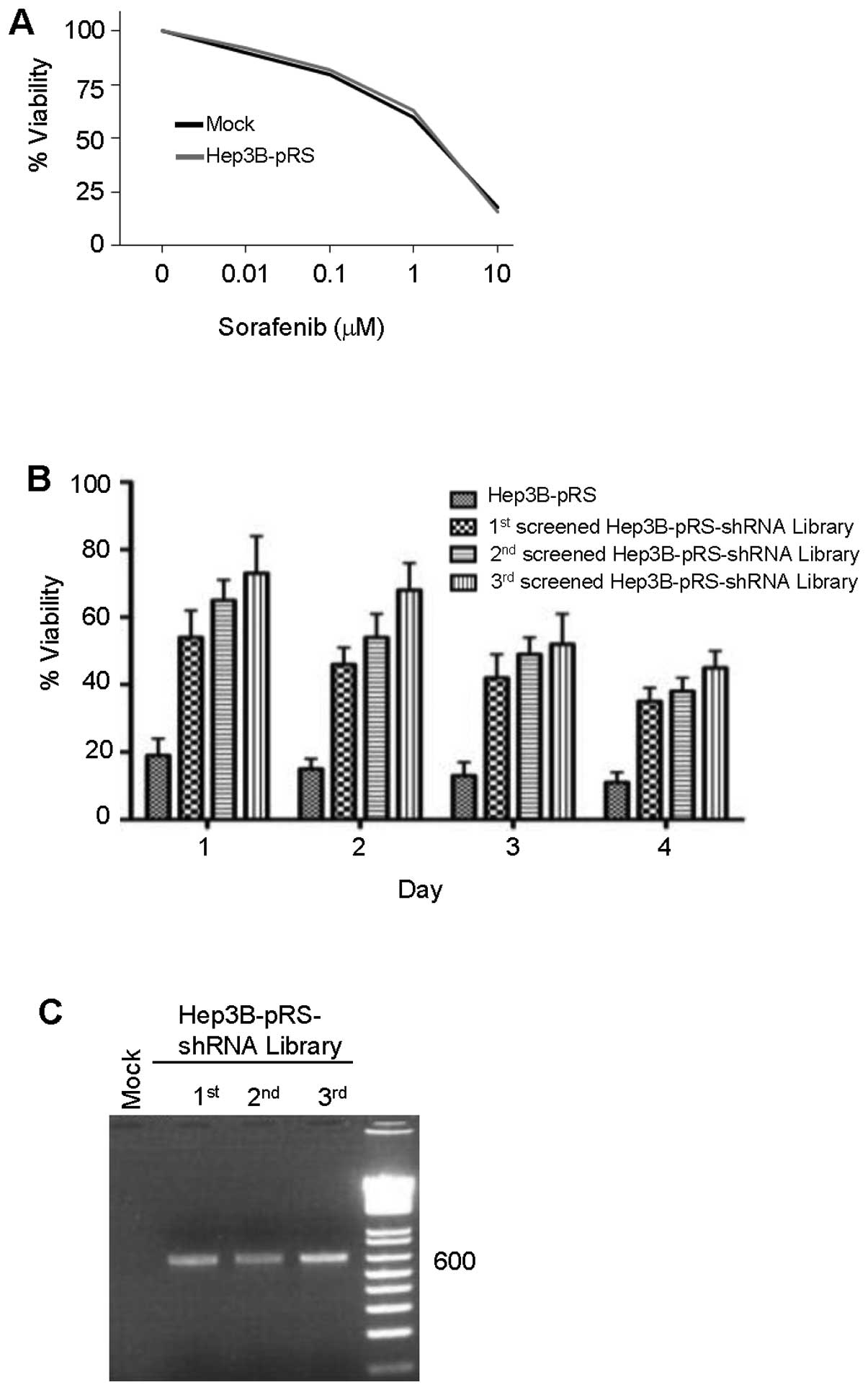
To determine the optimal dose for shRNA library
selection, Hep3B mock or Hep3B-pRS cells were treated with
sorafenib (0 to 10 μM) for 4 days. The effect of sorafenib on cell
viability was measured by the WST-1 assay. Sorafenib inhibited cell
viability dose-dependently (Fig.
2A). The maximal effect of sorafenib on the viability of Hep3B
or Hep3B-pRS cells was shown at the concentration of 10 μM. This
dose was also shown to be the highest concentration producing
acquired resistance to sorafenib in HCC (20). Based on these findings, the
pRS-shRNA library or pRS vector-infected Hep3B cells were cultured
in the presence of sorafenib (10 μM) for 1 to 4 days. An increase
in viability of shRNA library-infected cells was observed with 3-
and 4-day sorafenib treatments after the third screening using the
WST assay (Fig. 2B). Three
successive rounds of screening led to the identification of shRNA
for SMG-1 (shSMG-1) which was present in sorafenib-resistant third
screened shRNA library-infected cells after each round (Fig. 2C) and also identified five other
genes for investigation.
Suppression of SMG-1 leads to sorafenib
resistance in HCC cell lines
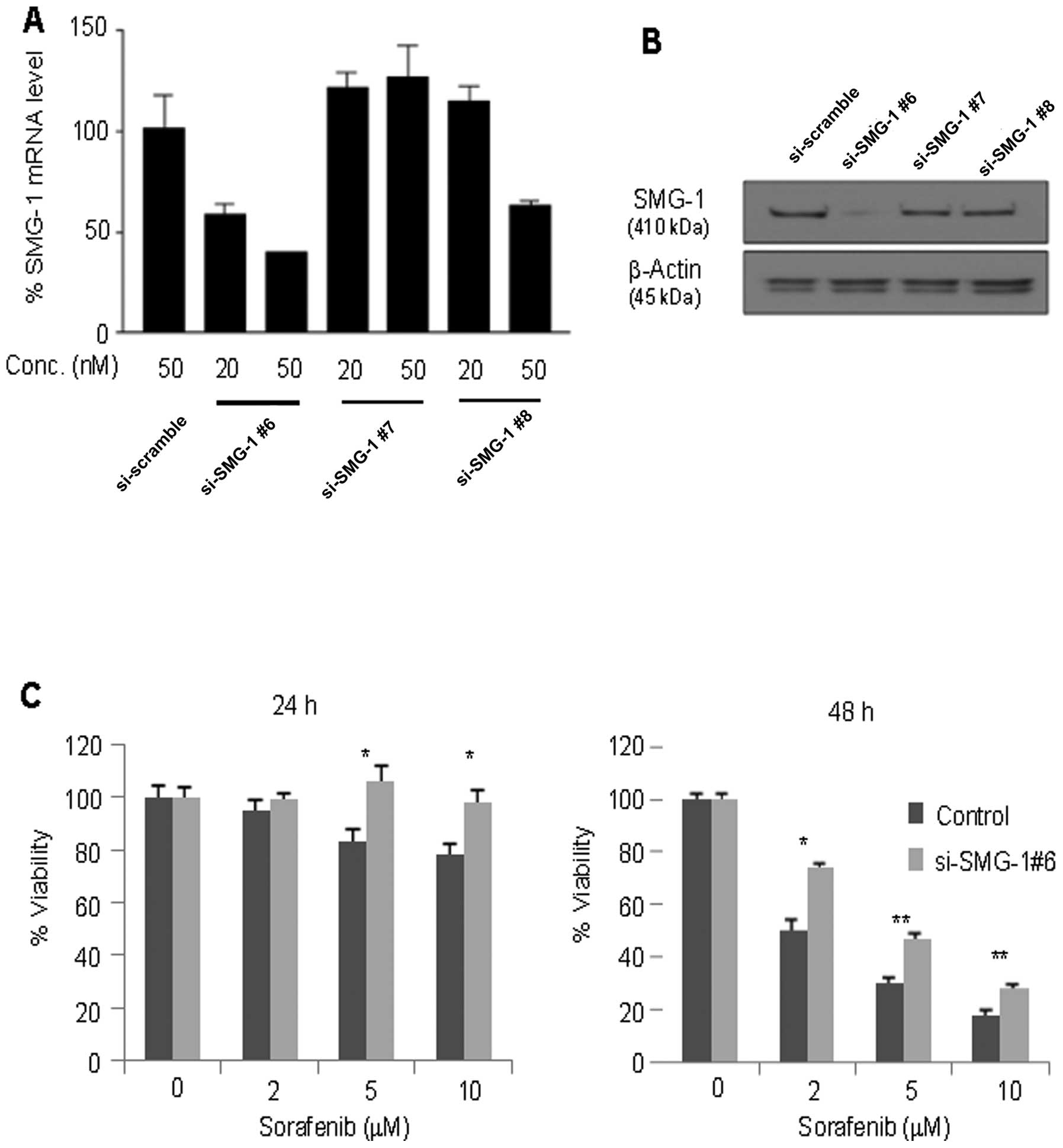
To confirm the effect of suppressed SMG-1 expression
on sorafenib resistance, three specific RNAi oligonucleotides of
SMG-1 were transfected into the Hep3B cell line. Among them, the
cells transfected with si-SMG-1 #6 effectively suppressed the SMG-1
mRNA transcripts (Fig. 3A) and
proteins (Fig. 3B) at the
concentration of 50 nM. After the transfection with si-SMG-1 #6,
the cells were treated with sorafenib at the indicated doses for 24
and 48 h and assayed for cell viability. Significantly higher
viability at day 1 (p<0.05) and day 2 (p<0.01) was shown in
the si-SMG-1-transfected cells compared to the control at the 5 and
10 μM concentrations of sorafenib treatment in Hep3B cells.
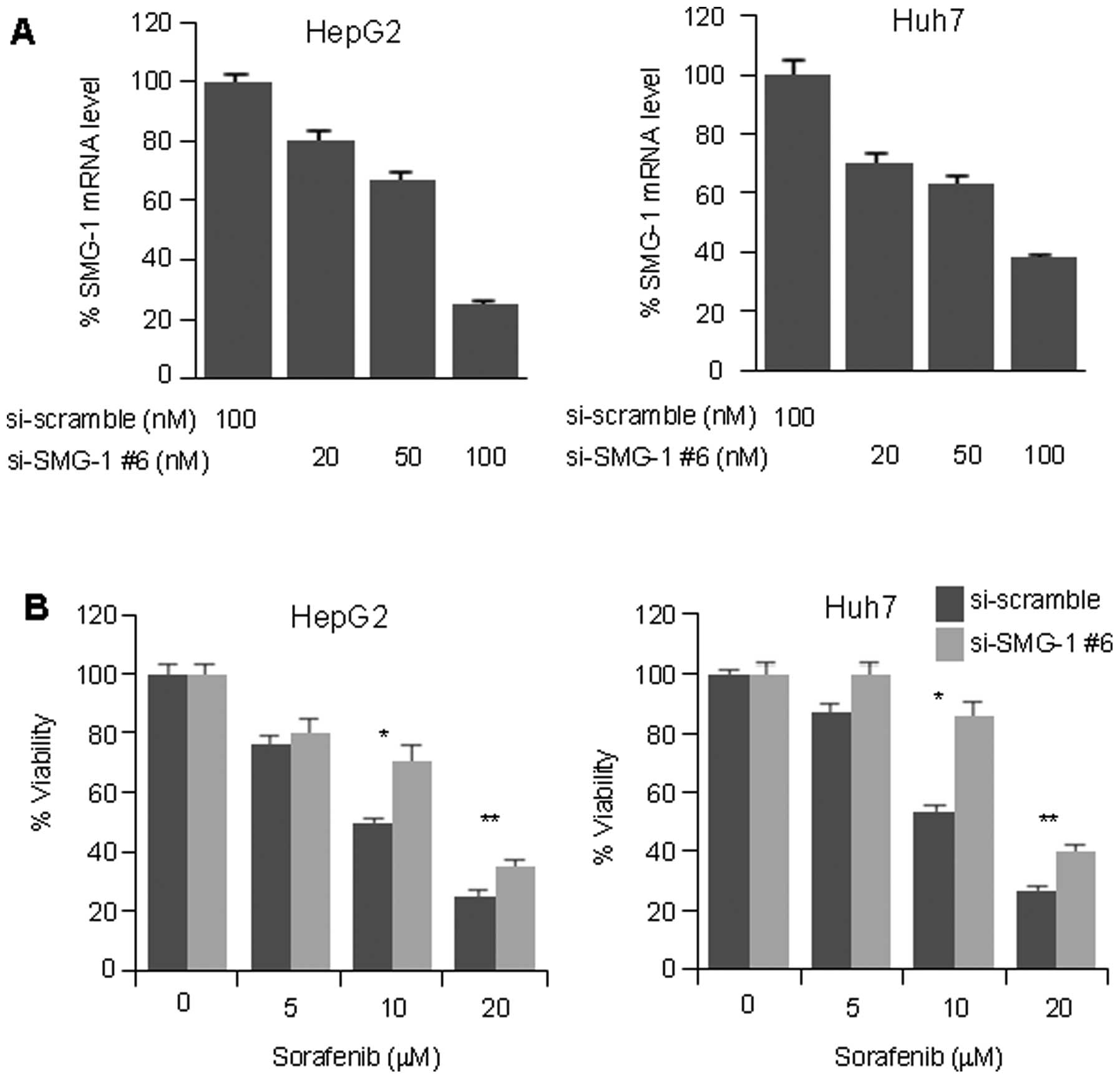
To investigate these results in other HCC cell
lines, HepG2 and Huh7 cell lines were transfected with either
si-SMG-1 #6 or si-scramble. SMG-1 RNAi suppressed the SMG-1 mRNA
level to 80 and 70% at the 100 μM concentration, as seen in the
HepG2 and Huh7 cell lines, respectively (Fig. 4A). Similarly, suppression of the
SMG-1 resulted in significantly higher cell viability at the 10 μM
(p<0.05) and 20 μM (p<0.01) concentrations of sorafenib in
both cell lines (Fig. 4B). Our
data suggest that the suppression of SMG-1 may increase sorafenib
resistance in HCC.
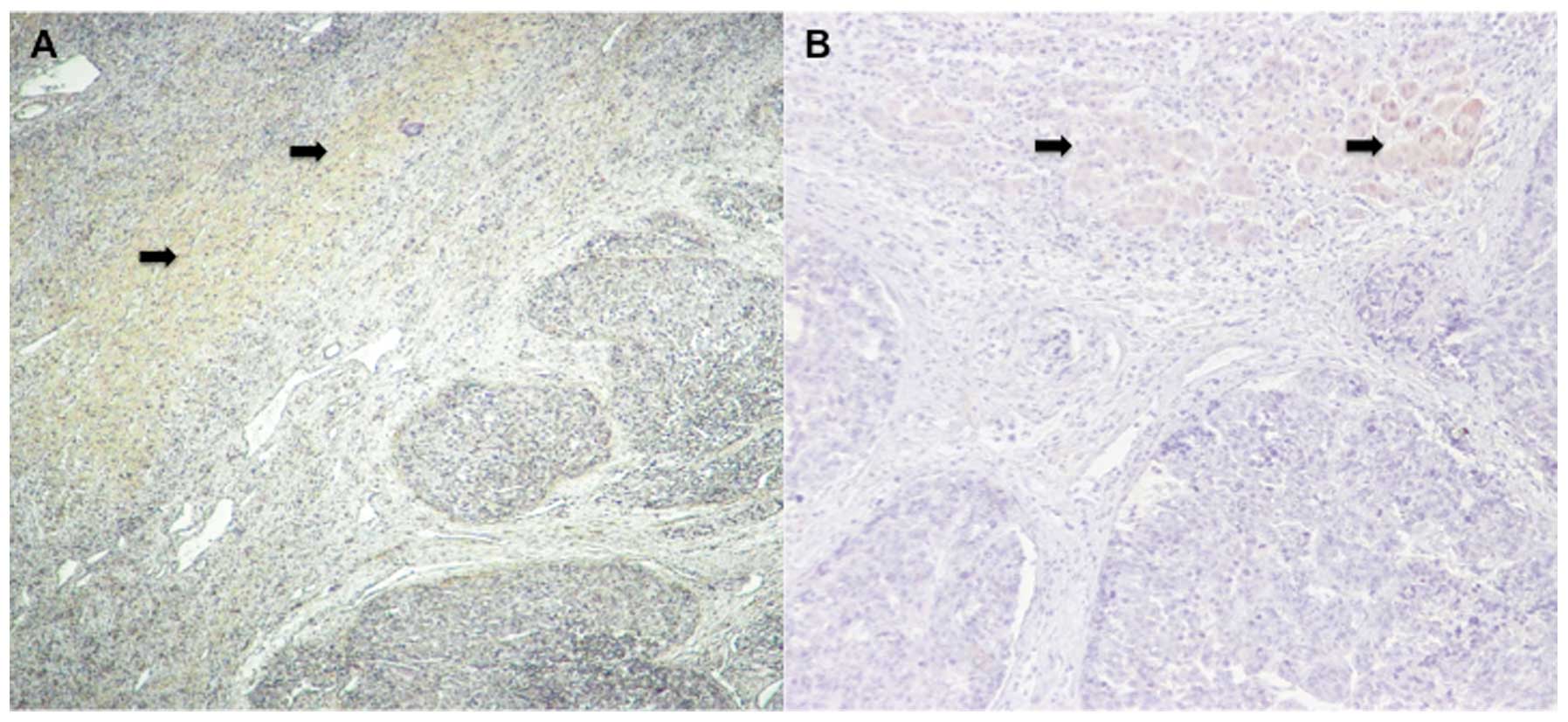
Immunohistochemical staining results show
reduced SMG-1 expression in cancerous regions
Immunohistochemical staining of SMG-1 in cancerous
and non-cancerous regions of surgically resected tissues (Fig. 5A and B) showed reduced expression
in cancerous regions compared to non-cancerous regions and
controls. On the other hand, there was stronger cytoplasmic
granular staining in non-cancerous regions (p<0.01).
Discussion
We applied the shRNA library-based genetic approach
to identify genes related to the sorafenib resistance of human HCC
cell lines. As expected, an increase in the viability of shRNA
library-infected cells was observed with 3- and 4-day sorafenib
treatments after the third screening compared to controls. The
value of this approach is that it allows the least biased
identification of new and unanticipated tumor suppressors. In this
regard, our study provides significant evidence for the
identification of the novel tumor suppressor SMG-1. Successive
validation experiments using commercial siRNA of SMG-1 have
revealed sorafenib resistance in siRNA-transfected hepatocellular
cell lines compared to controls.
Hepatocarcinogenesis is a multistep process often
initiated by external insults that lead to genetic aberrations in
hepatocytes or stem cells, resulting in proliferation, apoptosis,
dysplasia and neoplasia (4,21,22).
Significant progress in cancer molecular research technology has
led to the development of various molecular approaches for the
treatment of HCC. The identification of molecular targets involved
in HCC pathogenesis and drug response continues to be an important
area of active research.
Sorafenib is a potent antitumor agent that displays
clinical activity against several solid tumors and is licensed for
the treatment of patients with unresectable HCC. Its cytotoxic mode
of action in HCC is mediated by inhibition of the RAS/MEK/ERK
pathway, inhibition of tumor angiogenesis and induction of tumor
cell apoptosis (10,23). The effect of sorafenib on tumor
size reduction was lower than anticipated, and it has shown only a
minimal effect on survival and reduced disease progression in
patients with advanced HCC (11,12,24).
Hence, the importance of discovering genes related to resistance or
non-responsiveness to sorafenib treatment in the hope of developing
novel means of potentiating the drug’s antitumor properties.
RNA interference (RNAi) is a naturally occurring
mechanism that modulates gene expression at the
post-transcriptional level and is a powerful tool to perform
loss-of-function genetic screens for the identification of
components of cellular signaling pathways (25–27).
In eukaryotes, double-stranded interfering RNAs target
complementary mRNAs for degradation, which results in the selective
silencing of specific proteins. This characteristic of RNAi makes
it an important laboratory work tool and has led to the
characterization of novel oncogenes (28,29).
With the development of genome-wide RNA interference (RNAi)
approaches, the cost and time that are involved in target
identification, validation and other aspects of drug discovery have
been significantly reduced.
SMG-1 is a serine-threonine kinase which plays a
conserved role in nonsense-mediated mRNA decay (NMD) in worms and
mammals, and human SMG-1 has also been implicated in the
p53-mediated response to genotoxic stress (30,31).
In addition, SMG-1 negatively regulates hypoxia-inducible factor-1
(HIF-1) (32,33) activity, which plays a central role
in tumor progression by regulating genes involved in proliferation,
glycolysis, angiogenesis and metastases, in hypoxia through MAPK
activation (34). HIF-1 is one of
the primary transcription factors responsible for increased gene
expression in hypoxia, and vascular endothelial growth factor
(VEGF), erythropoietin, and glycolytic enzymes are all targets of
HIF-1 involved in the adaptive response of cells to hypoxia
(35,36). The hypervascularized nature of HCC
implies a particular reliance on factors related to angiogenesis
(37,38). In several recent studies, VEGF was
overexpressed in HCC, and HIF-1 played a central role in HCC
progression and angiogenesis (39–41).
Consequently, despite the complicated nature of tumor proliferation
and the existence of multiple regulatory checkpoints, we
hypothesize that knockdown of SMG-1 may potentiate angiogenesis or
metastasis of HCC due to disinhibition of HIF-1.
Nenasheva et al (42) previously reported that defective
SMG-1 kinase function might contribute to the process of malignant
transformation in B cell lymphomas of different origins. Xia et
al (43) recently reported
that human SMG-1 was involved in gemcitabine-induced primary
microRNA-155/BIC upregulation in a human pancreatic cancer cell
line. Though little information about SMG-1 has been elucidated to
date, further investigations of the precise action of SMG-1 in
oncogenesis or chemotherapeutic responsiveness may be meaningful.
In our present study, knockdown by RNAi of SMG-1 led to higher cell
viability in sorafenib-treated Hep3B cells.
We performed RNAi knockdown on other HCC cell lines
such as HepG2 and Huh7 cells, which resulted in a similarly
increased cell viability. Although there were significant
differences in cell viability between RNAi transfected and
non-transfected cell lines, the survival rate of sorafenib-treated
cancer cells was lower than anticipated. This may be due to the
short reaction time and lower transfection efficiency of commercial
RNAi itself, which is less of a consideration in the stable shRNA
platform.
Immunohistochemical staining of SMG-1 in HCC tissues
showed strong and diffuse cytoplasmic expression in non-cancerous
cirrhotic region compared with cancerous region. These results
suggest that SMG-1 expression is reduced during neoplastic
transformation and also implies its role as a tumor suppressor
gene. Loss of SMG-1 function may accelerate or convert malignant
transformation potential to neoplasmic formation from uncertain
causes. Therefore, we can postulate that SMG-1 modulates the
response of HCC to sorafenib through effects on the RAS/MEK/ERK
pathway, PI3K/Akt/mTOR pathway or HIF-1 (34,44,45).
There are several limitations to consider in our
experiment. Firstly, we could not determine anything about the
underlying mechanisms or related signal transduction of SMG-1.
Secondly, we could not show whether the potential regulation of
SMG-1 by sorafenib is indirect or direct. Thirdly, we could not
perform apoptotic analysis or other experiments to evaluate the
role of SMG-1. Further molecular studies will be needed to
investigate these properties.
In conclusion, this is the first report that
utilizes the unbiased genetic approach of shRNA library screening
to identify genes related to sorafenib resistance in HCC. These
results show that using such a screening method is successful in
identifying targets that have biological relevance to the
pathogenesis and disease progressions of HCC. A potential
regulator, SMG-1, was validated, and future studies can investigate
its potential as an adjunctive clinical target to improve sorafenib
therapy in patients with HCC.
Acknowledgements
This study was supported in part by grants from the
Alberta Cancer Research Institute (23123), Canada Research Chair
Program (95-203751), CIHR (MOP97962) and a fund (CMCDJ-2011-P-007)
of the Clinical Medical Research Institute from Daejeon St. Mary’s
Hospital, the Medical School of the Catholic University of
Korea.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: from genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fattovich G, Stroffolini T, Zagni I and
Donato F: Hepatocellular carcinoma in cirrhosis: incidence and risk
factors. Gastroenterology. 127:S35–S50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen CJ, Yang HI and Iloeje UH: Hepatitis
B virus DNA levels and outcomes in chronic hepatitis B. Hepatology.
49:S72–S84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forner A, Reig ME, de Lope CR and Bruix J:
Current strategy for staging and treatment: the BCLC update and
future prospects. Semin Liver Dis. 30:61–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-Serag HB, Marrero JA, Rudolph L and
Reddy KR: Diagnosis and treatment of hepatocellular carcinoma.
Gastroenterology. 134:1752–1763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wysocki PJ: Targeted therapy of
hepatocellular cancer. Expert Opin Investig Drugs. 19:265–274.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu L, Cao Y, Chen C, et al: Sorafenib
blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and
induces tumor cell apoptosis in hepatocellular carcinoma model
PLC/PRF/5. Cancer Res. 66:11851–11858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng AL, Kang YK, Chen Z, et al: Efficacy
and safety of sorafenib in patients in the Asia-Pacific region with
advanced hepatocellular carcinoma: a phase III randomised,
double-blind, placebo-controlled trial. Lancet Oncol. 10:25–34.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trojniak MP, Palozzo AC, Mazurek M and
Jirillo A: Sorafenib in hepatocellular carcinoma - a post marketing
evaluation. Immunopharmacol Immunotoxicol. 34:419–422. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lum L, Yao S, Mozer B, et al:
Identification of Hedgehog pathway components by RNAi in
Drosophila cultured cells. Science. 299:2039–2045. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ashrafi K, Chang FY, Watts JL, et al:
Genome-wide RNAi analysis of Caenorhabditis elegans fat
regulatory genes. Nature. 421:268–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iorns E, Lord CJ, Turner N and Ashworth A:
Utilizing RNA interference to enhance cancer drug discovery. Nat
Rev Drug Discov. 6:556–568. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahn BY, Elwi AN, Lee B, et al: Genetic
screen identifies insulin-like growth factor binding protein 5 as a
modulator of tamoxifen resistance in breast cancer. Cancer Res.
70:3013–3019. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang TC, Man S, Xu P, et al: Development
of a resistance-like phenotype to sorafenib by human hepatocellular
carcinoma cells is reversible and can be delayed by metronomic UFT
chemotherapy. Neoplasia. 12:928–940. 2010.PubMed/NCBI
|
|
19
|
Berns K, Hijmans EM, Mullenders J, et al:
A large-scale RNAi screen in human cells identifies new components
of the p53 pathway. Nature. 428:431–437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen KF, Chen HL, Tai WT, et al:
Activation of phosphatidylinositol 3-kinase/Akt signaling pathway
mediates acquired resistance to sorafenib in hepatocellular
carcinoma cells. J Pharmacol Exp Ther. 337:155–161. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thorgeirsson SS and Grisham JW: Molecular
pathogenesis of human hepatocellular carcinoma. Nat Genet.
31:339–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Villanueva A, Newell P, Chiang DY,
Friedman SL and Llovet JM: Genomics and signaling pathways in
hepatocellular carcinoma. Semin Liver Dis. 27:55–76. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JE, Ryoo BY, Ryu MH, et al: Sorafenib
for hepatocellular carcinoma according to Child-Pugh class of liver
function. Cancer Chemother Pharmacol. 68:1285–1290. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature.
391:806–811. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chapman EJ and Carrington JC:
Specialization and evolution of endogenous small RNA pathways. Nat
Rev Genet. 8:884–896. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kamath RS, Fraser AG, Dong Y, et al:
Systematic functional analysis of the Caenorhabditis elegans
genome using RNAi. Nature. 421:231–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan Q, Bao LW, Kleer CG, et al: Protein
kinase C epsilon is a predictive biomarker of aggressive breast
cancer and a validated target for RNA interference anticancer
therapy. Cancer Res. 65:8366–8371. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Z, Jiang G, Yang F and Wang J:
Knockdown of mutant K-ras expression by adenovirus-mediated siRNA
inhibits the in vitro and in vivo growth of lung cancer cells.
Cancer Biol Ther. 5:1481–1486. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamashita A, Ohnishi T, Kashima I, Taya Y
and Ohno S: Human SMG-1, a novel phosphatidylinositol
3-kinase-related protein kinase, associates with components of the
mRNA surveillance complex and is involved in the regulation of
nonsense-mediated mRNA decay. Genes Dev. 15:2215–2228. 2001.
View Article : Google Scholar
|
|
31
|
Masse I, Molin L, Mouchiroud L, et al: A
novel role for the SMG-1 kinase in lifespan and oxidative stress
resistance in Caenorhabditis elegans. PLoS One. 3:e33542008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Keith B and Simon MC: Hypoxia-inducible
factors, stem cells, and cancer. Cell. 129:465–472. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaelin WG Jr and Ratcliffe PJ: Oxygen
sensing by metazoans: the central role of the HIF hydroxylase
pathway. Mol Cell. 30:393–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen RQ, Yang QK, Chen YL, et al: Kinome
siRNA screen identifies SMG-1 as a negative regulator of
hypoxia-inducible factor-1alpha in hypoxia. J Biol Chem.
284:16752–16758. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci
USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mottet D, Michel G, Renard P, Ninane N,
Raes M and Michiels C: ERK and calcium in activation of HIF-1. Ann
NY Acad Sci. 973:448–453. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pang R and Poon RT: Angiogenesis and
antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett.
242:151–167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kuboki S, Shimizu H, Mitsuhashi N, et al:
Angiopoietin-2 levels in the hepatic vein as a useful predictor of
tumor invasiveness and prognosis in human hepatocellular carcinoma.
J Gastroenterol Hepatol. 23:e157–e164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Torimura T, Sata M, Ueno T, et al:
Increased expression of vascular endothelial growth factor is
associated with tumor progression in hepatocellular carcinoma. Hum
Pathol. 29:986–991. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakamura K, Zen Y, Sato Y, et al: Vascular
endothelial growth factor, its receptor Flk-1, and hypoxia
inducible factor-1alpha are involved in malignant transformation in
dysplastic nodules of the liver. Hum Pathol. 38:1532–1546. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu XZ, Xie GR and Chen D: Hypoxia and
hepatocellular carcinoma: The therapeutic target for hepatocellular
carcinoma. J Gastroenterol Hepatol. 22:1178–1182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nenasheva VV, Nikolaev AI, Martynenko AV,
et al: Differential gene expression in HIV/SIV-associated and
spontaneous lymphomas. Int J Med Sci. 2:122–128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xia QS, Ishigaki Y, Zhao X, et al: Human
SMG-1 is involved in gemcitabine-induced primary microRNA-155/BIC
up-regulation in human pancreatic cancer PANC-1 cells. Pancreas.
40:55–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|