Introduction
Apoptosis (programmed cell death) plays an important
role in a variety of biological phenomena including tissue
homeostasis, morphogenesis and tumorigenesis (1,2).
There are two common pathways for initiation of apoptosis, i.e.,
the intrinsic (mitochondrial) and extrinsic (or death receptor)
pathways. The intrinsic pathway is closely regulated by proteins of
the Bcl-2 family. This family consists of two main groups, namely
the pro-apoptotic proteins, i.e., Bak, Bax, Bad, Bcl-Xs, Bid, Bik,
Bim and Hrk and the anti-apoptotic proteins, i.e., Bcl-2, Bcl-xL,
Bcl-W, Bfl-1 and Mcl-1. The anti-apoptotic proteins regulate
apoptosis by blocking the release of some mitochondrial molecules,
such as cytochrome c, whereas the pro-apoptotic proteins
promote their release from mitochondria to the cytoplasm. Caspases
are activated by cytochrome c in the cytoplasm (3,4).
Alteration of the apoptotic pathways results in a
variety of pathological tissue processes including cancer,
autoimmune disorders and neurodegenerative diseases. In cancer,
there is a loss of balance between cell division and cell death
that seems to play a pivotal role in carcinogenesis (2,5,6). It
has been reported that the expression of the anti-apoptotic Bcl-2
gene is increased in prostate cancer cells and is associated with a
hormone-insensitive, metastatic phenotype of prostate cancer
(7–9). The high expression of Bcl-2 protein
in surgical specimens of prostate cancer was demonstrated in
response to androgen deprivation therapy (ADT) applied prior to
radical prostatectomy (10).
Whereas the expression of the anti-apoptotic Bcl-2 protein was
significantly increased in prostate cancer tissues along the
increasing Gleason score, expression of the pro-apoptotic Bak and
Bax molecules remained similar in these tissues (11).
The anti-apoptotic Bcl-2 and Bcl-x proteins can
interact structurally with the voltage dependent anion channel
(VDAC), especially with the N-terminal region of VDAC isoform 1
(12). The VDACs are found mostly
in the outer mitochondrial membrane (OMM), which are active during
the movement of various substances into and out of the mitochondria
(reviewed in ref. 13). The VDACs
play an essential role in the release of cytochrome c during
the apoptotic process (14–16).
Moreover, some publications reported that the family of the
apoptotic Bcl-2 proteins interact the outer mitochondrial membrane
protein (TOM) (17,18). Protein complexes such as TOM and
VDAC were proposed to act similarly to Bax receptors in regulation
of apoptosis (18). Bellot et
al (17) demonstrated that TOM
is a receptor for the pro-apoptotic Bax protein. Expression of
TOMM22 gene resulted in inhibition of the association between Bax
and mitochondria preventing Bax-dependent apoptosis (17). It remains unclear, how VDACs or
TOMs interact with the other apoptotic proteins, such as Bax, Bak
and Bcl-2 molecules to induce apoptosis. However, the latter one is
inhibited during prostate tumorigenesis.
In this context, we investigated both the level of
expression of protein complexes and their association with the
expression of Bcl-2 family in prostate cancer tissue. We measured
the transcript (mRNA) expression level of three members of the
Bcl-2 family, i.e., the pro-apoptotic Bak and Bid protein, as well
as anti-apoptotic Bcl-2 protein; three isoforms of VDAC, i.e.,
VDAC1, VDAC2 and VDAC3; and three member genes of TOM complexes,
i.e., TOMM20, TOMM22 and TOMM40 in prostate cancer or normal
prostate tissues. Based on the transcript expression level, we
evaluated in the same tissues the expression level of the family of
Bcl-2 proteins, i.e., anti-apoptotic Bcl-2 and pro-apoptotic Bax,
as well as outer mitochondrial membrane protein of VDAC1.
Materials and methods
Samples
We analyzed two groups of tissues, i.e., prostate
cancers with Gleason score 6 to 10 and normal prostates. Fourteen
paraffin-embedded prostate cancers classified as patterns Gleason 6
to 10. All of them were obtained from the Tissue Archives at the
Department of Anatomical Pathology, Faculty of Medicine, University
of Indonesia during a period of 2007–2010. These samples were
obtained from the prostate cancer patients who underwent
prostatectomy. Five normal prostates were obtained from adult human
male cadavers at Department of Forensic Medicine, Faculty of
Medicine, University of Indonesia using the following criteria:
tissues were taken from the deceased within 0 to 12 h from death,
age between 15–25 years, who had no health problems including
hypogonadism. A pathologist consultant verified the diagnosis of
prostate cancer in the appropriate tissues as well as normal
prostate tissue structure in the control group. The study was
approved by the local ethics committee and informs consent was
obtained from the patients or the family of the deceased.
Tissue processing, RNA extraction and
cDNA synthesis
Tumor areas were identified in the 4 μm-thick,
formalin-fixed and paraffin-embedded prostate sections. Normal
prostate tissue were put into fixative, embedded in paraffin and
cut into 4 μm-sections for routine histology examination. Total RNA
from both kinds of tissues was extracted using High Pure RNA
Paraffin Kit (Roche, Mannheim, Germany) according to the
manufacturer’s protocol. In the protocol, DNase and Proteinase K
were added to improve RNA quality. Concentration of total RNA in
the samples was quantified. RNA purity was determined using
Nanodrop (Thermo Scientific, Langenselbold, Germany). Subsequently,
integrity of RNA was evaluated using Experion (Bio-Rad, Munich,
Germany). cDNA was synthesized from 500 ng of total RNA using the
RT2 Formalin Fix Paraffin-Embedded (FFPE) PreAMP cDNA synthesis kit
(SABiosciences, MD, USA).
PCR array
Quantitative mRNA expressions of three members of
the family of Bcl-2 genes, i.e., Bak, Bid and Bcl-2; three isoforms
of VDAC, i.e., VDAC1, VDAC2 and VDAC3; and three member genes of
TOM complexes, i.e., TOMM20, TOMM22 and TOMM40 were analysed in
custom designed PCR array plate for human genes according to the
manufacturer’s protocol (SABiosciences). As a control, expression
of three housekeeping genes GAPDH, ACTB and HPRT1 were investigated
in this study. The reaction was performed using commercially
available RT2 Nano PreAMP cDNA Synthesis Primer Mix for Human
Custom PCR array (SABiosciences). The following conditions were
applied: 95°C for 10 min, 8 cycles of 95°C for 15 sec, 60°C for 2
min. The cDNA was amplified by RT2 Real Time Master Mix SYBR-Green
(SABiosciences) using iCycler (Bio-Rad) under the following
conditions: 95°C for 10 min, 40 cycles of 95°C for 15 sec, 60°C for
60 sec and 72°C for 20 sec.
Immunohistochemical staining of the
Bcl-2, Bax and VDAC1 proteins
Based on the mRNA expression results, the expression
of the anti-apoptotic Bcl-2 and VDAC1 proteins in both kinds of
tissues were evaluated. For the expression in pro-apoptotic protein
level, we evaluated pro-apoptotic Bax, instead of the Bak molecule.
The formalin fixed paraffin-embedded tissue of prostate cancer and
normal prostate samples were cut into 4 μm-thick histological
sections. The samples were placed on the positively charged
microscope slides, dried at 37°C for 30 min and incubated at 60°C
for 60 min using the slide warmer (Premiere, Atlanta, GA, USA). The
sections were deparaffinised in Xylene 3 times for 3 min of each
and dehydrated in the alcohol series and tap water. Endogenous
peroxidase was blocked by submerging the slides in 0.5%
H2O2 in methanol for 5 min. Subsequently, the
slides were washed in tap water and rinsed in distilled water,
heated in TE solution using microwave (Electrolux, Italy) power
level 9 for 3 min and power level 1 for 10 min; and allowed to cool
down at room temperature for 30 min. Staining was performed using
the Starr Trek Universal HRP Detection System (Biocare, CA, USA) as
follows: the slides were placed in PBS pH 7.2 for 5 min, dried and
dropped by the protein blocker of background sniper (Biocare) for
15 min at room temperature and probed with the primary monoclonal
antibody of Bcl-2 or Bax (Santa Cruz Biotechnology, CA, USA) or
anti-VDAC1 antibody (19).
Extensive washing in PBS was carried out before the sections were
incubated with Trek Avidin-HRP label (Biocare) for 20 min at room
temperature. The samples were washed again three times. Betazoid
diaminobenzidine chromogen (Biocare) was then added to the sections
which were incubated in the dark for 5 min before being counter
stained in haematoxylin for 60 sec, washed in tap water, dehydrated
in ascending grades of alcohol before cleaning with xylene and
mounting under a cover slip.
Semi-quantitative analysis of expression
of Bcl-2, Bax and VDAC1 antigen in tissue sections
Histological slides of normal prostate or prostate
cancers stained with antibodies for Bcl-2 or VDAC1 were digitalized
using both the microscope Olympus BX51TR-32FB3F01 with a lamp house
for 100W halogen and the camera DP25-SET. The images were
digitalized at magnification, ×1,000 and snap resolution 2,650 ×
1,920. They were saved in the jpg format. The images were analyzed
by the semiquantitative algorithm DAB Immune Membrane written as
the Image J plugin by Vilppu Tuominen and Jorma Isola of the Cancer
Biology Research Group, Institute of Biosciences and Medical
Technology (BioMediTech), University of Tampere, Finland. This
plugin is a part of the open-source software package (Image J, NIH,
USA). The plugin evaluates both intensity and completeness of
staining of prostate glands or cell infiltrates. First, it segments
diaminobenzidine-stained (DAB) regions of the image. Then,
classifies the image based on the staining completeness and
intensity. One calibrates the algorithm for the first measurement
by defining contrast, intensity range and scale in pixels/μm.
Subsequently, the algorithm was tested and tuned up against the
images representing both a positive control, i.e., a strong
PSA-positive prostate cancer tissue and a negative control, i.e., a
PSA-negative colonic mucosa.
Statistical analysis
The number of cycles required for the fluorescent
signal to cross the threshold in qPCR method (cycle threshold/Ct)
values for each qPCR product, where the amplification curves
corrected against the background crossed a threshold value, were
applied in the statistical analysis. Some candidate reference genes
were selected using GeNorm software (20). Applying that software, ACTB and
HPRT1 were found to be the best combination of two reference genes.
For further analysis of the real-time RT-PCR data, we combined ACTB
and HPRT1 as the reference genes for normalization of gene
expression. Relative expression levels were calculated using the
software GenEx Pro 4.3.7 (MultiD Analyses AB Copyright©,
Weihenstephan, Germany). The mean expression of mRNA corresponding
to nine selected genes was analyzed in the group of prostate
cancers or in normal prostates using Student’s t-test at
p<0.05.
Results
Transcript expressions of
mitochondrial-apoptotic molecules (Bak, Bid, Bcl-2), -transport
molecules (VDAC1, VDAC2, VDAC3, TOM20, TOM22 and TOM40)
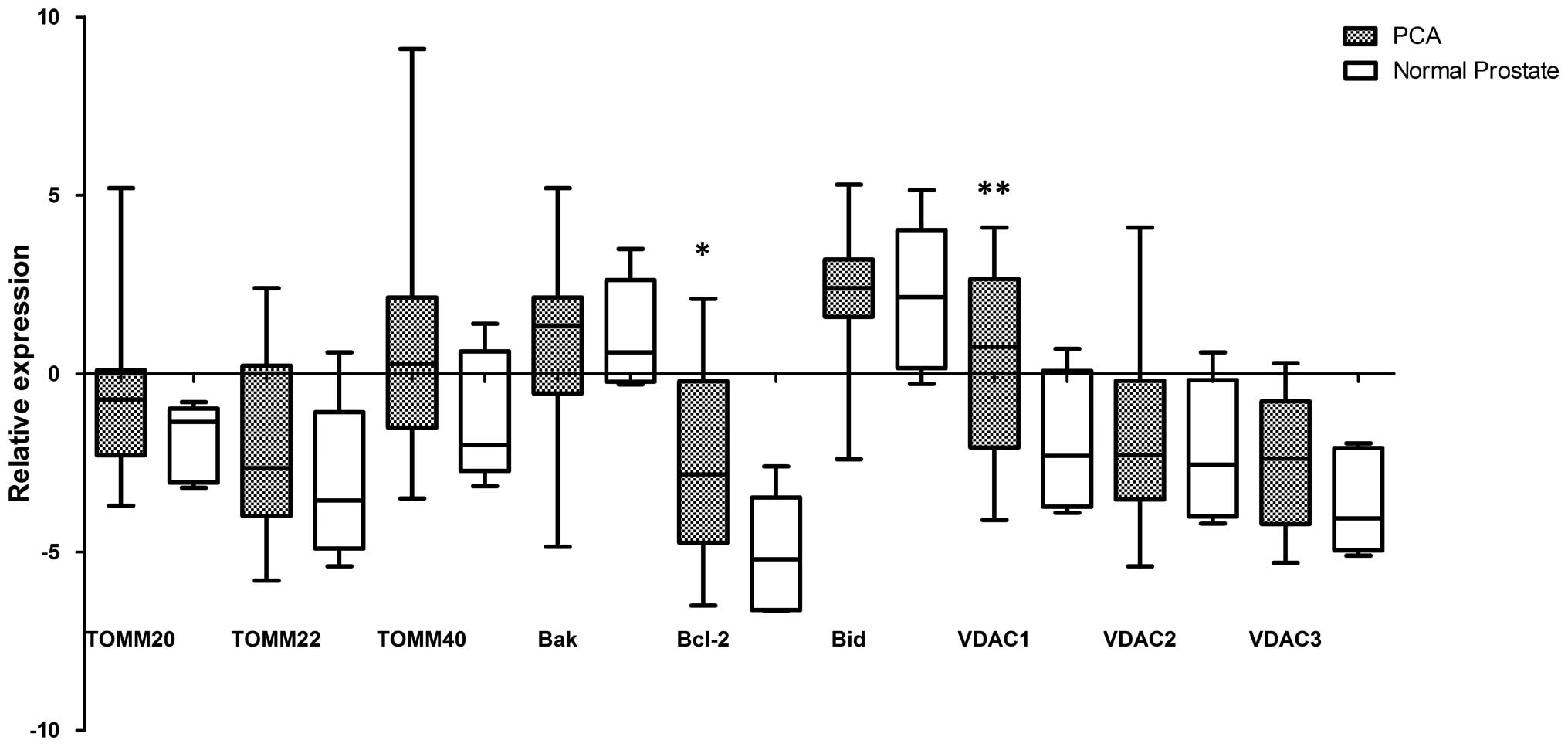
Quantitative PCR array demonstrated a significant
increase in mRNA expression of anti-apoptotic Bcl-2 gene (p=0.038)
and VDAC1 gene (p=0.034) in all 14 prostate cancer tissues compared
with 5 normal tissue samples. No significant difference in mRNA
expression was found between the pro-apoptotic Bak and Bid, VDAC2
and VDAC3 genes as well as TOMM20, TOMM22, TOMM40 genes in prostate
cancer tissue samples and normal tissues (p>0.05) (Fig. 1).
Expression of Bcl-2, Bax and VDAC1 at the
protein level in prostate tissues
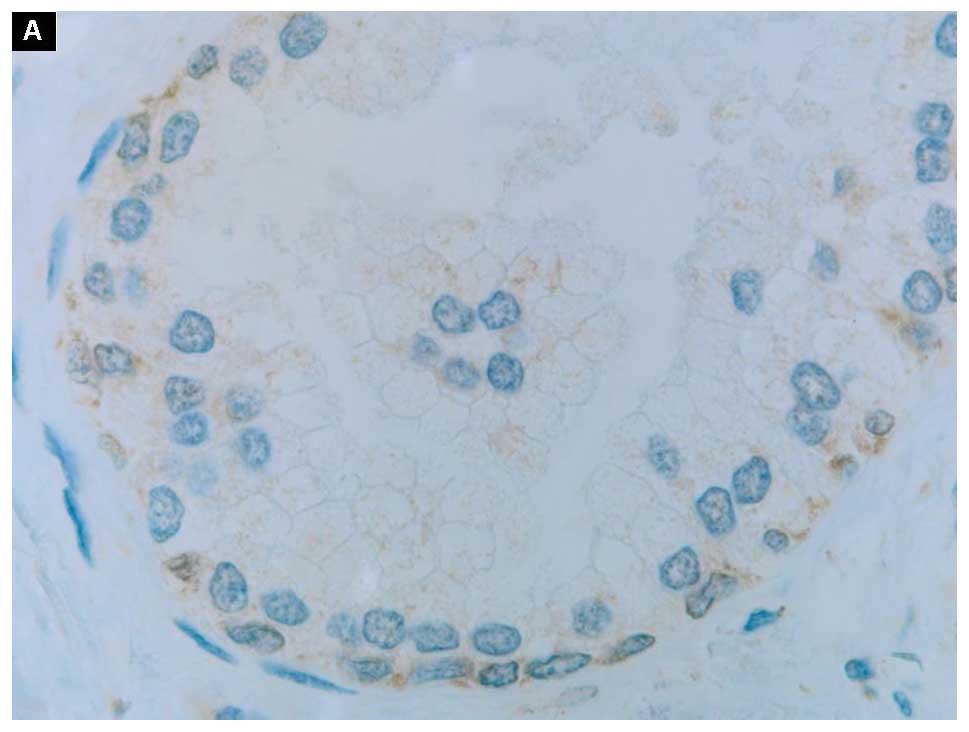
The expression of Bcl-2, Bax and VDAC1 at the
protein level in normal prostate and cancer prostate tissues can be
appreciated in Fig. 2: A and B, C and
D, E and F, respectively. All three proteins were dispersed in
a form of the cytoplasmic grains with a faint membrane staining. In
particular, the positive signals of the anti-apoptotic Bcl-2
protein appeared in basal epithelial cells rather than in secretory
ones (Fig. 2A). Those proteins
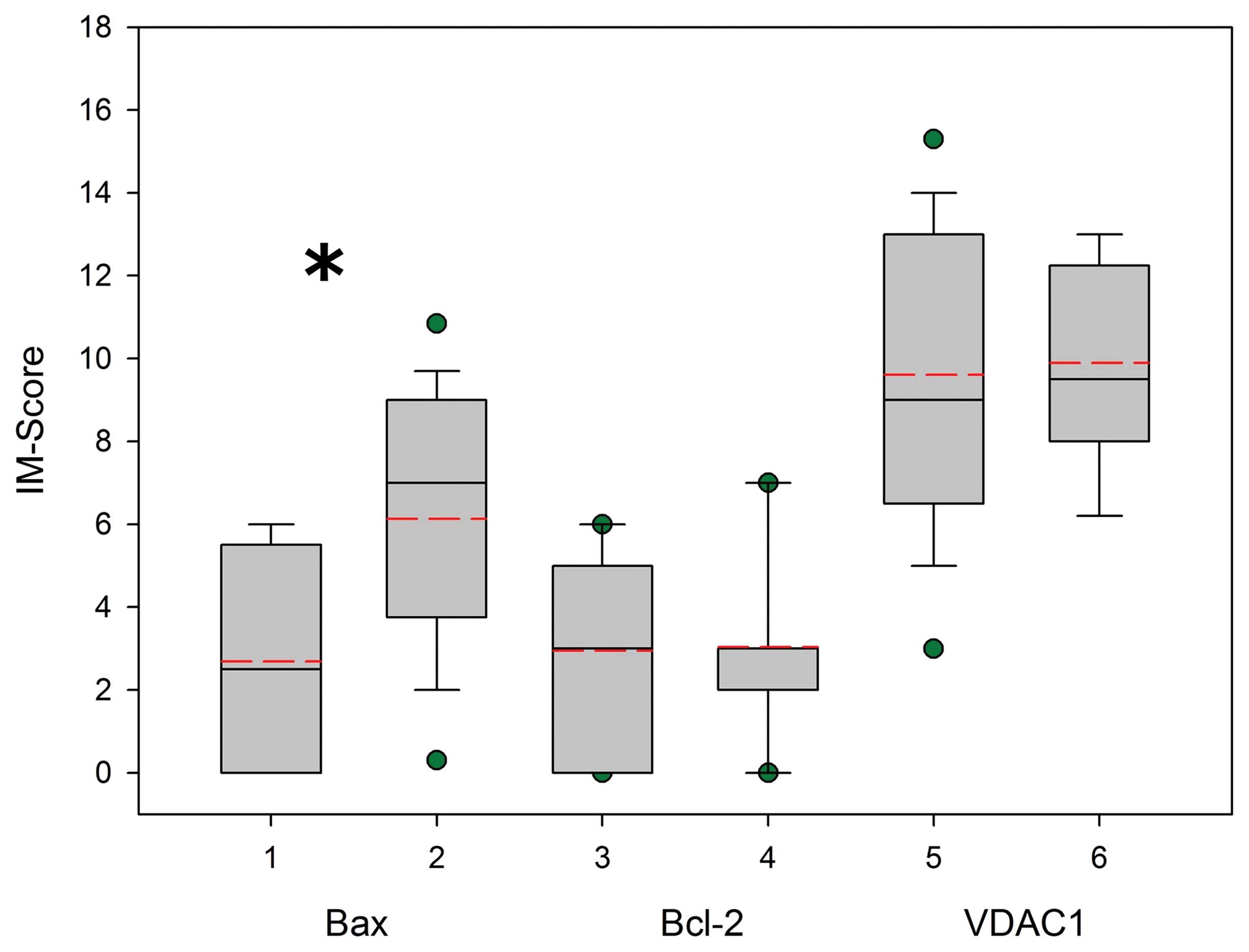
were expressed in cells in a relatively broad range as measured by
intensity and completeness, i.e., IM-score (Fig. 3). There was a significant
difference in translation of Bax, but not in translation of Bcl-2
and VDAC1 (p<0.05, p=0.887 and p=0.814, respectively). The
semiquantitative algorithm indicates that Bcl-2 and VDAC1 in
prostate cancer cells has a tendency to increased expression in
comparison with normal prostate. However, this difference is not
statistically significant. On the contrary, there was a significant
decrease in Bax expression in prostate cancer tissues as compared
to normal prostate (Fig. 3).
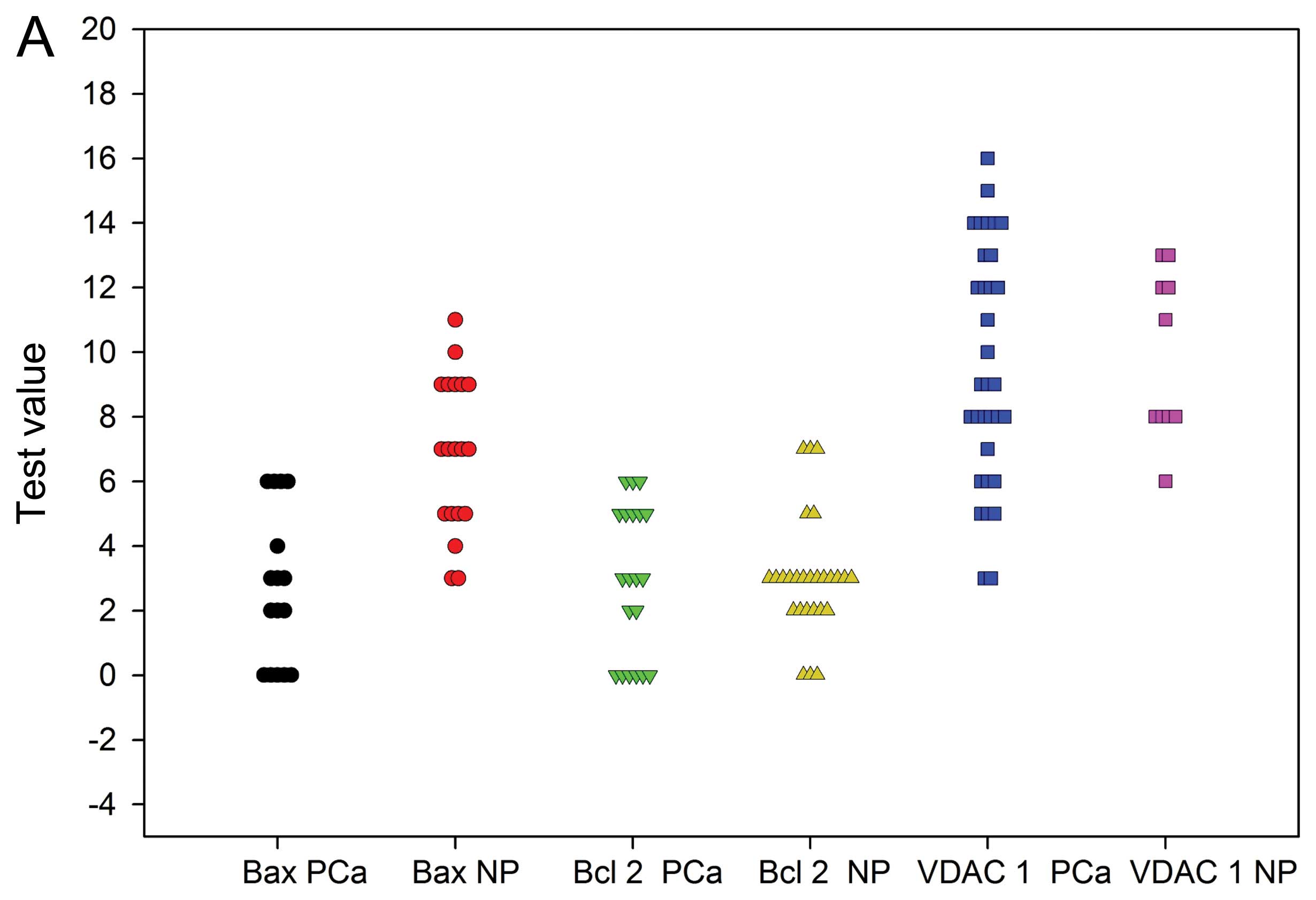
In the ROC analysis, Bax is shown as the only marker
with sufficient statistical power to distinguish between normal
prostate (NP) and prostate cancer (PCa) (Fig. 4A). Bax protein sensitivity was 100%
and specificity 64% at the cut-off value 6.60. The other two
markers were not useful producing AUC of about 0.5 or lower
(Fig. 4B).
Discussion
By using PCR array method, this study showed the
transcript expression of three kinds of apoptotic proteins of the
Bcl-2 family, i.e., pro-apoptotic Bak and Bid, anti-apoptotic Bcl-2
and protein subunits of the outer mitochondrial membrane transport
complexes, i.e., three isoforms of voltage dependent anion channels
(VDAC1, VDAC2 and VDAC3) as well as three isoforms of the
translocase of the outer mitochondrial membrane (TOM20, TOM22 and
TOM40) in prostate cancer tissues compared to normal prostate
tissues. We found that the transcript expression of the
anti-apoptotic Bcl-2 protein and VDAC1 increased significantly in
prostate cancer tissues compared to normal ones. When we compared
the transcript expression of Bcl-2 and VDAC1 between normal tissues
and prostate cancer with Gleason score >7 (10 samples) the
increase was even more significant (p=0.028). Unfortunately, we
were not able to analyze the differences of the level expression of
both genes in normal tissues and prostate cancer tissues with
Gleason score <7 due to lack of sufficient amount of samples (4
samples). The transcript expression of VDAC1 might be related to
the increased expression of anti-apoptotic Bcl-2 in cancer cells.
Both proteins would inhibit apoptosis of prostate cancer cells.
Hence, we conclude that the increased Bcl-2 mRNA
expression is associated with progression of prostate cancer. Our
finding is in concert with the previous observations that Bcl-2
gene may be involved in progression of prostate cancer (21). The increased amount of the Bcl-2
transcript in prostate cancer tissue was found also in prostatic
intra-epithelial neoplasia. Overexpression of the anti-apoptotic
Bcl-2 protein causes effects similar to those of an oncogene
(22).
A relationship between gene expression measured as
mRNA amount of Bcl-2 as well as VDAC1 and the amount of the
appropriate protein is non-linear. The mean amounts of Bcl-2 or
VDAC1 mRNA are greater in prostate cancer than in normal prostate.
Those differences disappear at the protein level. In addition,
translation of the genes in normal prostate does not produce
different amounts of the proteins to the extent of that in prostate
cancer and results in greater values of standard deviation. Other
researchers have found a reverse relationship. For example, Fuzio
et al demonstrated that the Bcl-2 mRNA level was
significantly decreased in prostate cancer in comparison with
normal prostate tissues. Androgen deprivation therapy increased the
Bcl-2 mRNA level. However, this difference was not significant
(10).
Immunostaining demonstrated in normal prostate
tissues positive signals of the anti-apoptotic Bcl-2 protein in
basal epithelial cells (Fig. 2A).
This finding is in line with the results of Hockenbery et al
showing that this anti-apoptotic protein was confined to the basal
cells of the prostate epithelium, and was undetectable in secretory
cells (23). The disparity between
these two cell types is marked by their different capacity to
express the anti-apoptotic proteins (20).
Proteins of the Bcl-2 family play a pivotal role in
the mitochondrial apoptotic pathway. The ratio between
pro-apoptotic and anti-apoptotic molecules determines whether or
not apoptosis will occur (5,6). In
this study, we analyzed the transcript expression of two
pro-apoptotic molecules, Bak and Bid, resulting in no significant
transcript expression of both genes in prostate cancer tissue in
comparison to normal prostate. However, expression of Bax at the
protein level decreased significantly in prostate cancer tissues in
comparison to normal prostate tissues. Since Bax is the
pro-apoptotic protein, one can expect that the decline of its
expression be entangled in prostate tumorigenesis. Consequently,
the ratio of the anti-apoptotic Bcl-2 to the pro-apoptotic Bax is
expected to be increased during tumorigenesis. This study suggests
that the high Bcl-2/Bax ratio may contribute to the emergence of
prostate cancer by disrupting apoptosis cascade.
Furthermore, the ROC analysis in this study showed
the normal prostate and prostate cancer tissue can be separated
using Bax in protein level as a marker with sensitivity 100% at the
cut-off value 6.6. However, VDAC1 and Bcl-2 proteins were not
informative. These findings suggest the evaluation of Bax in
protein level with immunostaining can be used as diagnostic method
to determine prostate cancer tissue.
We also evaluated the transcript expression of the
mitochondrial transport system, especially in the outer membrane
that mediates the cytochrome c release in order to initiate
apoptosis. There are at least two main transport systems in the
outer mitochondrial membrane that co-operate with Bcl-2 family
proteins, porin, known also as voltage dependent anion channel
(VDAC) and the translocase of the outer mitochondrial membrane
(TOM) complex (24). It has been
reported that Bax/Bak and Bcl-xL, but not Bik and Bid, can bind
directly to the voltage-dependent anion channel (VDAC) and modulate
its activity to induce apoptosis in mammalian cells (14,15).
It was shown that the N-terminal region of VDAC1 isoform
structurally interacts with the Bcl-2 and Bcl-xL to mediate
anti-apoptotic effects (12,16).
The immunoblot study with isolated rat liver mitochondria was
performed to elucidate which receptor could bind to the
pro-apoptotic Bax. This study showed that Bax was associated with
TOM complexes, especially TOM22. Furthermore, this analysis
demonstrated that Bax could not associate with VDAC1. It was
suggested that formation of the mitochondrial permeability pore was
involved in the interaction between Bax and TOM22 while triggering
apoptosis (17). Working with
isolated mitochondria from yeast, Ott et al demonstrated
that TOM40 played a role in the Bax translocation into the outer
mitochondrial membrane and its action to release cytochrome
c (18). However, Ross
et al described that there was no direct dependence of Bax
and Bak insertion and oligomerization in the outer mitochondrial
membrane on the import and assembly machineries of TOM in HeLa
cells (25). The use of different
cell types in these studies is most likely responsible for these
differences. Indeed, apoptosis in yeast and mammalian cells depends
on different molecular events. For example, yeast lacks Bcl-2-like
proteins. Although we did not analyze the interaction between the
family of Bcl-2 proteins, i.e. the pro-apoptotic Bak and Bid as
well as the anti-apoptotic Bcl-2 and proteins in the mitochondrial
transport complexes, i.e., VDAC and TOM isoforms, we could show
that there was no significant differences in the expression of
pro-apoptotic Bak and Bid or TOMM20, TOMM22 and TOMM40 transcripts
in prostate cells with the altered apoptotic process. This finding
suggests that the activities of the TOM complexes do not play any
essential role in development of prostate cancer.
Furthermore, we found a significant difference in
transcript expression of VDAC1 isoform in prostate cancer cells
compared to normal prostate cells, but not in VDAC2 and VDAC3
expressions. As a channel protein, VDAC is located not only in the
mitochondrial membrane of eukaryotes but also in the
extra-mitochondrial regions of eukaryotic cells, e.g., in the
plasmalemma (26–29). In mammals there are three isoforms
of VDAC protein. The distribution, characterization and function of
the three isoforms of VDAC differ and the transcript level of VDAC
isoforms varies in different tissues and species (reviewed in ref.
13). In higher eukaryotes, VDAC1
is the most predominant isoform. Several studies demonstrated
differences in the expression levels of VDACs in cancer cells and
normal cells, most of them showed alteration in VDAC1 expression
(30). In a study with 11 human
cancer cell lines, Simamura et al reported that cancer cells
expressed higher levels of VDAC1 than normal cells such as WI-38
fibroblasts (31). The study,
combining affinity labeling of cell surface molecules, avidin
affinity chromatography and mass spectrometry analyses of cell
membrane proteins of normal and cancerous cells originating from
one prostate cancer patient, revealed reduced expression level for
type-1 (VDAC1) and type-2 (VDAC2) porins in the mitochondrial
membrane of cancer cells (32).
Several studies demonstrated VDAC1 upregulation in various cancer
cell lines, which is induced by different treatments, i.e., in
three acute lymphoblastic leukemia (ALL) cell lines after
prednisolone treatment (33), in
cervix squamous cell carcinoma cell line (A431) after cisplatin
treatment (34), and in human
malignant melanoma cell line (A375) after treatment with tyrosinase
inhibitor arbutin (35). In
prostate cancer cell lines, it has been reported that the VDAC1
expression in LNCaP cell line was approximately two times higher
than its expression in PC-3 and DU 145 cell lines. Increased
expression in prostate cancer cell lines was also induced by G3139,
the 18-meric phosphorothionate antisense oligonucleotide; an
inducer of caspase-dependent apoptosis (36). Since cells cultured in vitro
represent a supramolecular system pre-selected in very specific
conditions, their profile of gene expression will not overlap with
the profile of cells living in vivo. Hence, the conclusive
power of such analyses is limited, and their results may not
reflect the biological reality. Looking from that perspective, our
results seem to be more realistic for tissue systems, such as
prostate cancer.
The VDAC1 was also proposed to be involved in the
extrinsic apoptotic pathway. This channel appears to interact with
different modulators such as sigma-1 receptor and estrogen receptor
ERα in the cell membrane. For example, it can induce the extrinsic
apoptotic pathway in LNCaP cell line (37). For the reasons stated above, it is
unknown if this effect takes place in prostatic tissue in
vivo.
We demonstrated the level of expression and the
localization of VDAC1 in prostate cancer tissues (Fig. 2F) and normal prostate tissues
(Fig. 2E). The strong positive
signal was found in both basal epithelial and secretory cells of
normal prostate gland. This finding denotes that both cell types
need this protein for regulation of both apoptosis and cell growth.
Indeed, VDAC plays an important role in energy production via
controlling metabolite traffic across the outer mitochondrial
membrane (38). It has been
reported that the downregulation of VDAC1 expression would result
in decreased energy production and, thus, may affect cell vitality.
The decrease in hVDAC1 levels resulted in a dramatic decrease in
cell growth, a 5-fold decrease in ATP synthesis, as well as a
decrease of about 50% in the cellular levels of ATP and ADP
(39). The channel of VDAC
regulates ATP flux from mitochondria (40). The closure state of VDAC channel
shows a higher permeability to Ca2+, reduces metabolite
flux, and thus sensitizes mitochondria to apoptotic signals
(41). The positive signal of
VDAC1 expression was found mainly in secretory cells of the
prostate cancer gland.
Our study demonstrates that VDAC1, not TOM complex,
together with the anti-apoptotic protein, Bcl-2 plays a role in
prostate tumorigenesis. However, it remains unclear how VDAC1 is
involved in development of prostate cancer. The level of
interference may not primarily be gene expression but
posttranslational complex assembly, since in the context of
apoptosis VDAC may be induced to form the oligomeric complexes. Of
course, this is the case with the composition of TOM isoforms.
Expression of the protein subunits is one aspect, but formation of
the complexes that would be active in the physiological, impaired
or pathological way is the other one. Pro- or anti-apoptotic
proteins of the Bcl-2 family may interfere with either the level of
biosynthesis, gene expression and complex assembly. This issue
remains beyond the scope of this article.
Acknowledgements
This study was supported by International
Collaborative Research Fund from University of Indonesia. The
authors are grateful to Dr Dwi Ari Pujianto, Faculty of Medicine
University of Indonesia, for his help in editing the
manuscript.
References
|
1
|
Adams JM: Ways of dying: multiple pathways
to apoptosis. Genes Dev. 17:2481–2495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bidere N, Su HC and Lenardo MJ: Genetic
disorders of programmed cell death in the immune system. Annu Rev
Immunol. 24:321–352. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Danial NN and Korsmeyer SJ: Cell death:
critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong RBY: Apoptosis in cancer: from
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McDonnell TJ, Troncoso P, Brisbay SM,
Logothetis C, Chung LW, Hsieh JT, et al: Expression of the
protooncogene BCL-2 in the prostate and its association with
emergence of androgen-independent prostate cancer. Cancer Res.
52:6940–6944. 1992.PubMed/NCBI
|
|
8
|
Raffo AJ, Perlman H, Chen MW, Day ML,
Streitman JS and Buttyan R: Overexpression of BCL-2 protects
prostate cancer cells from apoptosis in vitro and confers
resistance to androgen depletion in vivo. Cancer Res. 55:4438–4445.
1995.PubMed/NCBI
|
|
9
|
McConkey DJ, Greene G and Pettaway CA:
Apoptosis resistance increase with metastatic potential in cells of
the human LNCaP prostate carcinoma line. Cancer Res. 56:5594–5599.
1996.PubMed/NCBI
|
|
10
|
Fuzio P, Ditonno P, Lucarelli G, Battaglia
M, Bettocchi C, Senia T and Perlino E: Androgen deprivation therapy
affects BCL-2 expression in human prostate cancer. Int J Oncol.
39:1233–1242. 2011.PubMed/NCBI
|
|
11
|
Yoshino T, Shiina H, Urakami S, Kikuno N,
Yoneda T, Shigeno K and Igawa M: Bcl-2 expression as a predictive
marker of hormone-refractory prostate cancer treated with
taxane-based chemotherapy. Clin Cancer Res. 12:6116–6124. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geula S, Ben-hail D and Shoshan-Barmartz
V: Structure-based analysis of VDAC1: N-terminus location,
translocation, channel gating and association with anti-apoptotic
protein. Biochem J. 444:475–485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Messina A, Reina S, Guarino F and De Pinto
V: VDAC isoforms in mammals. Biochim Biophys Act. 1818:1466–1476.
2012. View Article : Google Scholar
|
|
14
|
Shimizu S, Matsuoka Y, Shinohara Y, Yoneda
Y and Tsujimoto Y: Essential role of voltage-dependent anion
channel in various forms of apoptosis in mammalian cells. J Cell
Biol. 152:237–250. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shore GC: Apoptosis: it’s Bak to VDAC.
EMBO Rep. 10:1311–1313. 2009.
|
|
16
|
Abu-Hamad S, Arbel N, Calo D, Arzoine L,
Israelson A, Keinan N, Ben-Romano R, Friedman O and Shoshan-Barmatz
V: The VDAC1 N-terminus is essential both for apoptosis and the
protective effect of anti-apoptotic proteins. J Cell Sci.
122:1906–1916. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bellot G, Cartron PF, Er E, Oliver L, Juin
P, Armstrong LC, Bornstein P, Mihara K, Manon S and Vallette FM:
TOM22, a core component of the mitochondria outer membrane protein
translocation pore, is a mitochondrial receptor for the
proapoptotic protein Bax. Cell Death Differ. 14:785–794. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ott M, Norberg E, Zhivotovsky B and
Orrenius S: Mitochondrial targeting of tBid/Bax: a role for the TOM
complex? Cell Death Differ. 16:1075–1078. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cassará MC, Menzel VA, Hinsch KD,
Wrenzycki C and Hinsch E: Voltage-dependent anion channels 1 and 2
are expressed in porcine oocytes. Biosci Rep. 30:193–200.
2009.PubMed/NCBI
|
|
20
|
Vandesompele J, De Preter K, Pattyn F,
Poppe B, Van Roy N, De Paepe A and Speleman F: Accurate
normalization of the real-time quantitative RT-PCR data by
geometric averaging of multiple internal control genes. Genome
Biol. 3:RESEARCH00342002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Catz SD and Johnson JL: Bcl-2 in prostate
cancer: a minireview. Apoptosis. 8:29–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lemelynova AA, Grygorenko VM, Cheremuha SV
and Romanenko АМ: Correlation between histological type and
immunohistochemical profile of prostate cancer and Gleason scale
gradation. J Exp Oncol. 31:246–249. 2009.PubMed/NCBI
|
|
23
|
Hockenbery DM, Zutter M, Hickey W, Nahm M
and Korsmeyer SJ: Bcl2 protein is topographically restricted in
tissues characterized by apoptotic cell death. Proc Natl Acad Sci
USA. 88:6961–6965. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Motz C, Martin H, Krimmer T and Rassow J:
Bcl-2 and porin follow different pathways of TOM-dependent
insertion into the mitochondrial outer membrane. J Mol Biol.
323:729–738. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ross K, Rudel T and Kozjak-Pavlovic V:
TOM-independent complex formation of Bax and Bak in mammalian
mitochondria during TNFalpha-induced apoptosis. Cell Death Differ.
16:697–707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schein SJ, Colombini M and Finkelstein A:
Reconstitution in planar lipid bilayers of a voltage-dependent
anion-selective channel obtained from Paramecium
mitochondria. J Membr Biol. 30:99–120. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Benz R: Permeation of hydrophilic solutes
through mitochondrial outer membranes: review on mitochondrial
porins. Biochim Biophys Acta. 1197:167–196. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thinnes FP: Evidence for
extra-mitochondrial localization of the VDAC/porin channel in
eukaryotic cells. J Bioenerg Biomembr. 24:71–75. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
De Pinto V, Messina A, Lane DJ and Lawen
A: Voltage-dependent anion-selective channel (VDAC) in the plasma
membrane. FEBS Lett. 584:1793–1799. 2010.
|
|
30
|
Shoshan-Barmatz V and Mizrachi D: VDAC1:
from structure to cancer therapy. Front Oncol. 2:1642012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simamura E, Hirai K, Shimada H, Koyama J,
Niwa Y and Shimizu S: Furanonaphthoquinones cause apoptosis of
cancer cells by inducing the production of reactive oxygen species
by the mitochondrial voltage-dependent anion channel. Cancer Biol
Ther. 5:1523–1529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hastie C, Saxton M, Akpan A, Cramer R,
Master JR and Naaby-Hansen S: Combined affinity labeling and mass
spectrometry analysis of differential cell surface protein
expression in normal and prostate cancer cells. Oncogene.
24:5905–5913. 2005. View Article : Google Scholar
|
|
33
|
Jiang N, Kham SK, Koh GS, Suang Lim JY,
Ariffin H, Chew FT and Yeoh AE: Identification of prognostic
protein biomarkers in childhood acute lymphoblastic leukemia (ALL).
J Proteomics. 74:843–857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Castagna A, Antonioli P, Astner H, Hamdan
M, Righetti SC, Perego P, Zunino F and Righetti PG: A proteomic
approach to cisplatin resistance in the cervix squamous cell
carcinoma cell line A431. Proteomics. 4:3246–3267. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nawarak J, Huang-Liu R, Kao SH, Liao HH,
Sinchaikul S, Chen ST and Cheng SL: Proteomic analysis of A375
human malignant melanoma cells in response to arbutin treatment.
Biochim Biophys Acta. 1792:159–167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lai JC, Tan W, Benimetskaya L, Miller P,
Colombini M and Stein CA: A pharmacologic target of G3139 in
melanoma cells may be the mitochondrial VDAC. Proc Natl Acad Sci
USA. 103:7494–7499. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thinnes FP: Neuroendocrine differentiation
of LNcaP cells suggests: VDAC in the cell membrane is involved in
the extrinsic apoptotic pathway. Mol Genet Metab. 97:241–243. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shoshan-Barmatz V, Keinan N and Zaid H:
Uncovering the role of VDAC in the regulation of cell life and
death. J Bioenerg Biomembr. 40:183–191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Abu-Hamad S, Sivan S and Shoshan-Barmatz
V: The expression level of the voltage-dependent anion channel
controls life and death of the cell. Proc Natl Acad Sci USA.
103:5787–5792. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rostovtseva T and Colombini M: VDAC
channels mediate and gate the flow of ATP: implications for the
regulation of mitochondrial function. Biophys J. 72:1954–1962.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tan W and Colombini M: VDAC closure
increases calcium ion flux. Biochim Biophys Acta. 1768:2510–2515.
2007. View Article : Google Scholar : PubMed/NCBI
|