Introduction
Lung cancer is the leading cause of cancer-related
death worldwide (1). Non-small
cell lung cancer (NSCLC) accounts for ~80% of all lung cancers with
long-term survival restricted to a small subset of patients.
Chemotherapy has a modest but significant impact on survival and
quality of life in patients with NSCLC (2). Malignant mesothelioma is a relatively
rare malignancy with a generally poor outcome. The median survival
is currently 9–12 months after diagnosis. At this time, there are
few effective chemotherapeutic options for treatment of malignant
mesothelioma. They include cisplatin, vinorelbine, and gemcitabine
(3). New strategies based on a
better understanding of tumor biology may help to maximize the
efficacy of current treatments.
Pemetrexed, a multitargeted antifolate cytotoxic
agent, is a chemotherapeutic agent used in malignant mesothelioma
and NSCLC (mostly used in non-squamous cell carcinomas) (4–6).
Pemetrexed primarily inhibits thymidylate synthase (TS),
dihydrofolate reductase (DHFR), and glycinamide ribonucleotide
formyltransferase (GARFT), these are all enzymes in
folate-dependent metabolic processes (7,8).
Previous studies have reported that pemetrexed-induced apoptosis is
associated with upregulation of p53 and inactivation of Bcl-2
(9,10), and inhibition of the intrinsic
apoptosis pathway has been shown to suppress the cytotoxicity of
pemetrexed (11).
Statins are a class of drugs that inhibit the
rate-limiting step of the mevalonate pathway, which is catalyzed by
3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (12). Besides their lipid-lowering effect,
statins have been studied for their antineoplastic properties in
many solid tumor cells, including NSCLC (13,14).
Statins have been also shown to sensitize cancer cell lines to
cytotoxic drugs such as 5-fluorouracil (5-FU), taxol, etoposide,
doxorubicin, and cisplatin (15–18).
We recently demonstrated that the combination of sulindac and
simvastatin augmented their apoptotic potential above that is seen
with either drug alone in A549 lung cancer cells. These effects
were mediated via reactive oxygen species (ROS)-dependent
mitochondrial dysfunction (19).
Although pemetrexed has generally been a
well-tolerated drug, its toxicity profile is not trivial. The most
frequently observed adverse effects include myelosuppression,
fatigue, hepatotoxicity, nephrotoxicity, pneumonitis, and mucositis
(20,21). Until now, the mechanism by which
pemetrexed and statin combines to induce apoptosis and inhibit the
growth of mesothelioma and NSCLC cells has not been elucidated. In
this study, we demonstrated the synergistic interaction of
pemetrexed and simvastatin and explored the mechanisms underlying
this synergy.
Materials and methods
Materials
Roswell Park Memorial Institute medium-1640
(RPMI-1640), fetal bovine serum (FBS), and antibiotics (penicillin
and streptomycin) were obtained from Gibco BRL Co. (Grand Island,
NY, USA). Pemetrexed was purchased from Toronto Research Chemicals,
Inc. (Toronto, Ontario, Canada). Simvastatin,
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT), propidium iodide (PI), dimethyl sulfoxide (DMSO) and
N-acetylcysteine (NAC) were purchased from Sigma-Aldrich (St.
Louis, MO, USA). JC-1, a lipophilic, fluorescent dye used to detect
mitochondrial membrane depolarization was obtained from Molecular
Probes Co. Primary antibodies against the following targets:
caspase-3, -8 and -9, poly(ADP-ribose) polymerase (PARP), Puma,
Bim, Mcl-1, Bcl-XL, and XIAP were obtained from Santa Cruz
Biotechnology (Santa Cruz, CA, USA); antibodies against heme
oxygenase-1 (HO-1), MnSOD, survivin, VDAC, and β-actin were
purchased from Cell Signaling Technology (Beverly, MA, USA).
Antibodies to cytochrome c were obtained from Pharmingen
(San Diego, CA, USA). Anti-rabbit IgG-conjugated horseradish
peroxidase (HRP) antibodies and enhanced chemiluminescence (ECL)
kits were purchased from Amersham Pharmacia Biotech
(Buckinghamshire, UK).
Cell culture and viability test
MSTO-211 cells were purchased from the American Type
Culture Collection (Manassas, VA, USA), and A549 human lung cancer
cells were obtained from the Korean Cell Line Bank (Seoul, Korea).
These cell lines were grown in RPMI-1640 containing 100 U/ml
penicillin, 0.1 mg/ml streptomycin, and 10% FBS. The cells were
incubated in a humidified atmosphere of 5% CO2 in air at
37°C and maintained in log phase growth.
Cell viability was determined by measuring the
mitochondrial conversion of MTT to formazan, which was measured
spectrophotometrically. After cells were treated with the specified
study drugs, MTT was added to the cell suspension for 4 h. After
three washes with phosphate-buffered saline (PBS; pH 7.4), the
insoluble formazan product was dissolved in dimethyl sulfoxide
(DMSO). The optical density (OD) of each well was measured using a
microplate reader (Titertek Multiskan; Flow Laboratories, North
Ryde, New South Wales, Australia) at 590 nm. The OD resulting from
formazan production in control cells was considered as 100% cell
viability, and all other measurements were expressed as a
percentage of the control cell value.
Annexin V assay for the assessment of
apoptosis
MSTO-211 and A549 cells undergoing early/late
apoptosis were analyzed by Annexin V-FITC and PI staining. Cells in
the exponential growth phase (2.5×105 cells) were seeded
in 35-mm2 dishes. Cells were left untreated or incubated
with specified drugs for the indicated times at 37°C. Both adherent
and floating cells were collected and analyzed by the Annexin V
assay, according to the manufacturer’s instructions. Pelleted cells
were briefly washed with PBS and resuspended in annexin binding
buffer. Cells were then incubated with Annexin V-FITC and PI for 15
min at room temperature. After incubation, the stained cells were
analyzed using a fluorescence-activated cell sorting (FACS) Calibur
system equipped with CellQuest software (Becton-Dickinson, San
Jose, CA, USA). Cells with no drug treatment were used as
controls.
Measurement of the mitochondrial membrane
potential (ΔΨm)
MSTO-211 and A549 cells were harvested at the
indicated treatment times, washed with PBS, and then stained with
10 μg/ml JC-1 at 37°C for 30 min. After a brief wash with PBS,
cells were immediately analyzed using a FACSCalibur system equipped
with CellQuest software. At low concentrations, JC-1 exists mainly
in a monomeric form, emitting green fluorescence (emission maximum
at ~530 nM), whereas at higher concentrations it forms aggregates,
known as J-aggregates, which emit orange-red fluorescence (emission
maximum at ~590 nM).
Measurement of reactive oxygen species
(ROS)
To measure intracellular ROS, cells were incubated
with 10 μmol/l 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein
diacetate (carboxy-H2DCFDA, Molecular Probes, Eugene,
OR, USA) at 37°C for 30 min. Cells were then washed, scraped
gently, resuspended in PBS, and kept on ice for immediate analysis
by FACSCalibur flow cytometry using an argon laser (488 nm) for
excitation. Green fluorescence due to trapped DCF inside the cells
was collected and plotted on a log scale. Data were acquired and
analyzed with the CellQuest program. To measure
mitochondria-derived ROS, the mitochondria-targeted, peroxide ion
(O2−) sensitive, hydroethidine analog probe
MitoSOX (Invitrogen Life Technologies, M36008) was used to
determine relative O2− levels. Cells were
incubated with 5 μM MitoSOX for 10 min in RPMI-1640, washed twice
with PBS, and analyzed with FACSCalibur flow cytometry.
Western blotting
Cells were harvested and lysed using
radio-immunoprecipitation assay buffer [50 mM Tris-Cl (pH 7.4), 1%
NP40, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1
μg/ml each of aprotinin and leupeptin and 1 mM
Na3VO4]. After centrifugation at 12,000 × g
for 30 min, the supernatant was collected, and the protein
concentration was determined by the method of Bradford (Bio-Rad
protein assay). Equal amounts of protein were separated by 12%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) under reducing conditions and subsequently transferred
to nitrocellulose membranes. Membranes were blocked with 5% skim
milk in TBS-T [25 mM Tris (pH 7.6), 138 mM NaCl, and 0.05%
Tween-20] for 1 h and probed with primary antibodies (at
1:1,000–1:5,000). After a series of washes, membranes were further
incubated with secondary antibody (at 1:2,000–1:10,000) conjugated
with HRP. Detection of the immunoreactive signals was carried out
using an ECL detection system.
Preparation of cytosolic and
mitochondrial fractions
Cytosolic and mitochondrial fractions were prepared
as described previously (22) with
modifications. Cells were harvested, washed with ice-cold PBS, and
then incubated with 500 μM buffer A [250 mM sucrose, 20 mM HEPES
(pH 7.5), 10 mM KCl, 1.5 mM MgCl2, 1 mM EGTA, 1 mM EDTA,
1 mM DTT, 1 mM PMSF and 10 μg/ml each of leupeptin, aprotinin, and
pepstatin A] on ice for 30 min. Cells were then disrupted by 20
passages through a 26-gauge needle and centrifuged at 750 × g for
10 min. The supernatant was centrifuged at 10,000 × g for 25 min.
After centrifugation, the cytosolic fraction was frozen at 70°C.
The pellet containing mitochondria was washed with ice-cold buffer
A and then resuspended with cell lysis buffer. The resuspended
pellet was incubated on ice for 30 min and then centrifuged at
10,000 × g for 25 min. The supernatant thus collected represented
the mitochondrial fraction of cells.
Gene silencing
Transcriptional expression of Bim was specifically
suppressed by the introduction of 21-nucleotide duplex small
interfering RNA (siRNA), which targets nucleotides of Bim mRNA
coding sequence (23). Cells
(105 cells/well) were plated in 6-well plates and
transiently transfected with 50 nM per well of Bim siRNA (Cell
Signaling Technology) mixed with the X-tremeGENE siRNA transfection
reagent (Roche Applied Science, Penzberg, Germany) according to the
manufacturer’s instructions. Silencer Negative Control siRNA (Roche
Applied Science) was used as a negative control and introduced into
the cells using the same protocol.
Statistical analysis
Each experiment was performed at least 3 times, and
all values were expressed as the mean ± SD of triplicate samples.
The Student’s t-test was used to determine the statistical
significance of the results. Values of p<0.05 were considered
statistically significant.
Results
Effect of pemetrexed and simvastatin,
alone and in combination on the growth of malignant mesothelioma
and lung cancer cells
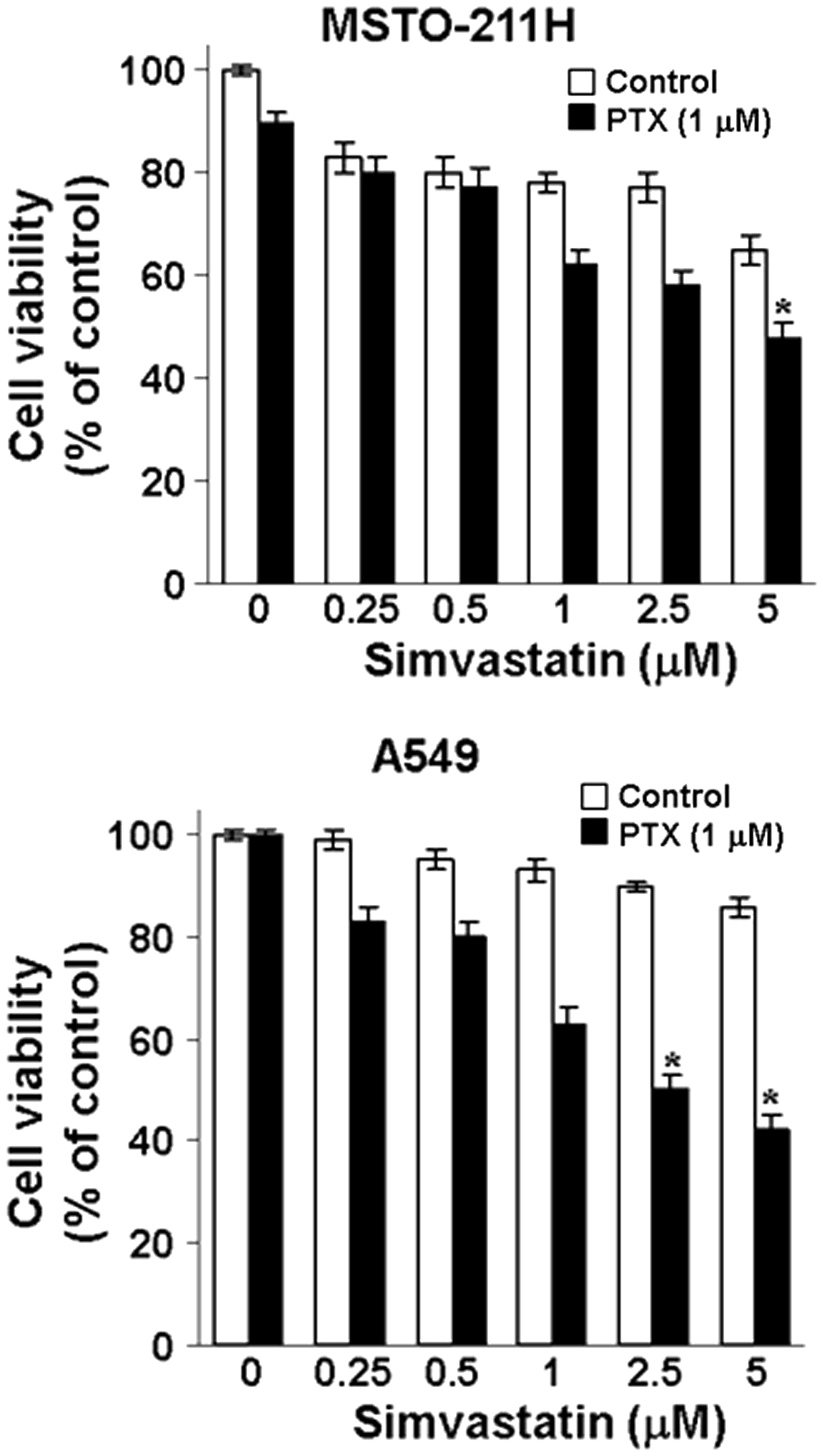
MSTO-211 and A549 cells were treated with different
concentrations of simvastatin in the absence or presence of
pemetrexed, and viability was measured by the MTT assay. As shown
in Fig. 1, the combination of
pemetrexed and simvastatin produced a synergistic inhibitory effect
on the growth of both MSTO-211 and A549 cells. Simvastatin
inhibited cell growth in a dose-dependent fashion in the presence
of pemetrexed.
Combination of pemetrexed and simvastatin
enhances caspase-dependent apoptosis
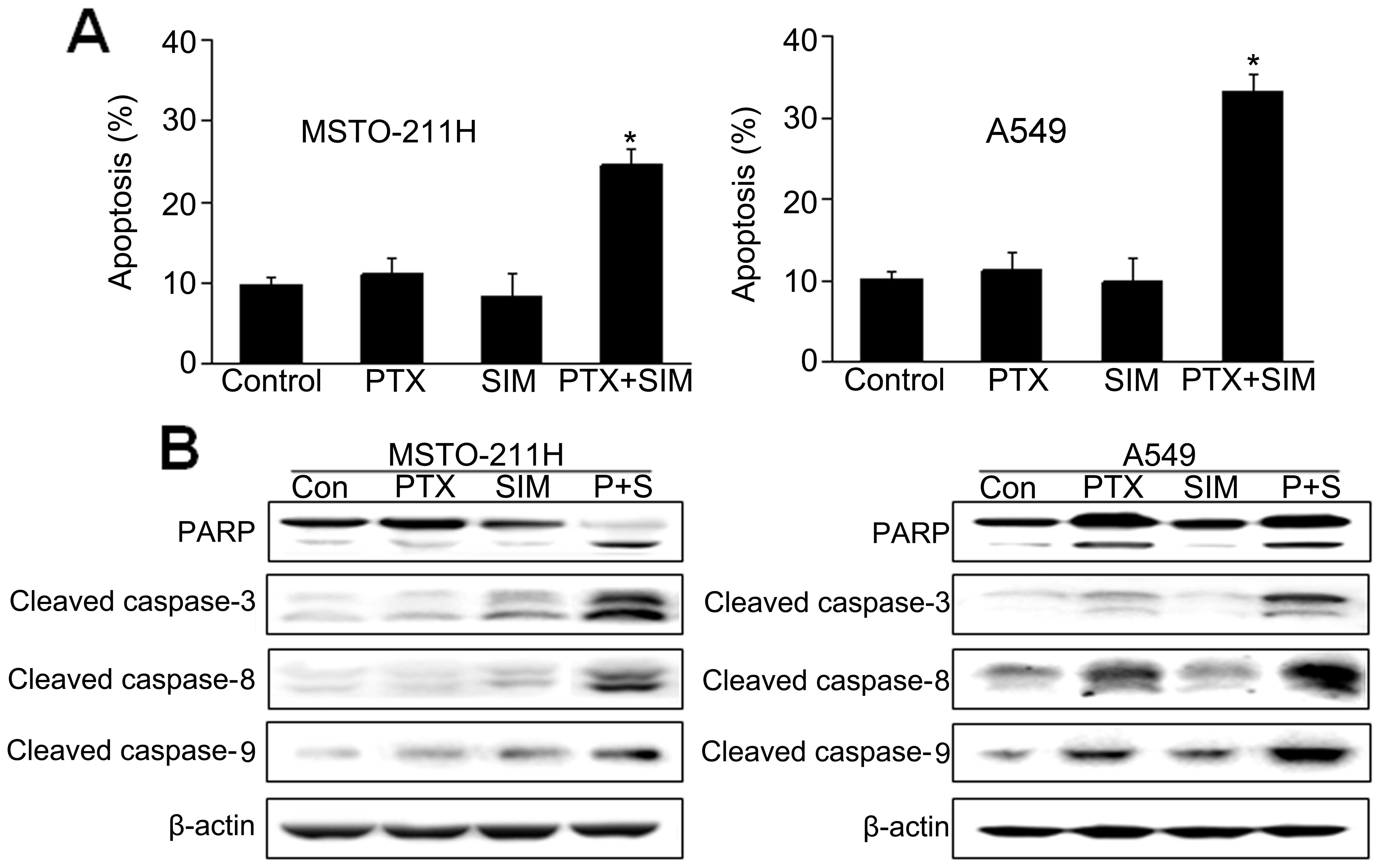
To examine whether the observed growth inhibition
was due to enhanced apoptosis, the proportion of apoptotic cells
was determined using Annexin V-PI staining. Annexin V staining
showed that the combination of pemetrexed and simvastatin
significantly enhanced apoptosis compared with either drug alone in
MSTO-211 and A549 cells (Fig.
2A).
To further elucidate the mechanism of apoptosis
induced by pemetrexed and simvastatin, cell lysates were evaluated
by immunoblotting (Fig. 2B). Our
results showed that the combination of pemetrexed and simvastatin
enhanced the expression of the processed 85-kDa isoform of PARP,
which is known to play a major role in circumventing the apoptosis
process. Moreover, the combination of pemetrexed and simvastatin
led to a marked increase in the expression of caspase-3, -8 and -9.
These results indicate that pemetrexed and simvastatin enhanced
caspase-dependent apoptosis in MSTO-211 and A549 cells.
Combination of pemetrexed and simvastatin
enhances intracellular ROS production
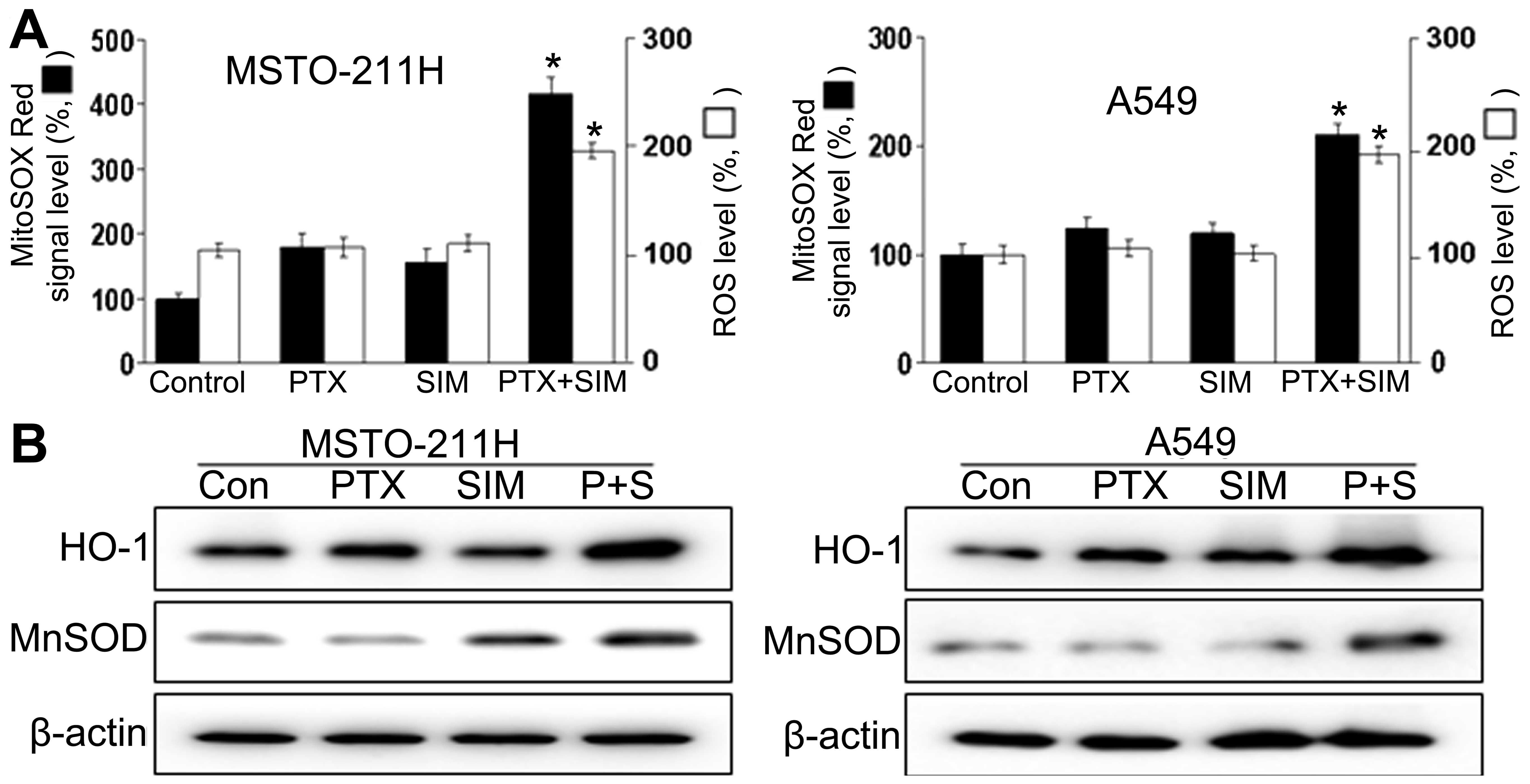
We also investigated the upstream regulatory
mechanisms leading to the induction of apoptosis by the combination
of pemetrexed and simvastatin. Intracellular ROS generation was
assessed by flow cytometry using the total ROS marker
H2DCFDA and the mitochondrial superoxide marker MitoSOX
RED. The results demonstrate that ROS generation in MSTO-211 and
A549 cells treated with both pemetrexed and simvastatin increased
markedly compared to ROS generation in cells treated with
pemetrexed or simvastatin alone (Fig.
3A). FACS analysis using MitoSOX revealed that intracellular
O2− levels increased significantly, which
correlated well with the onset of total ROS production. It is
possible that the combination treatment increased cellular
oxidative stress. We then investigated whether combined treatment
with pemetrexed and simvastatin affected two markers for oxidative
stress: inducible HO-1 and MnSOD (Fig.
3B). The combination of pemetrexed and simvastatin resulted in
enhanced expression of HO-1 and MnSOD compared to MSTO-211 and A549
cells treated with either pemetrexed or simvastatin alone.
Combination of pemetrexed and simvastatin
leads to mitochondrial dysfunction
We also investigated components upstream of
caspase-3 in apoptotic signaling. Markers of mitochondrial
dysfunction, including ΔΨm transition and cytosolic
release of cytochrome c, were evaluated in cells treated
with pemetrexed and simvastatin. JC-1 has been widely used for the
detection of apoptosis by measuring mitochondrial depolarization.
As shown in Fig. 4A, JC-1 monomer
level was enhanced in MSTO-211 and A549 cells treated with the drug
combination. Since the loss of ΔΨm results in cytochrome
c release into the cytosol, cytochrome c levels were
evaluated by western blotting in both mitochondrial and cytosolic
fractions (Fig. 4B). Combination
treatment with pemetrexed and simvastatin was associated with an
increased level of cytochrome c in the cytosolic fraction
over that seen with either agent alone and a corresponding decrease
in levels in the mitochondrial fraction.
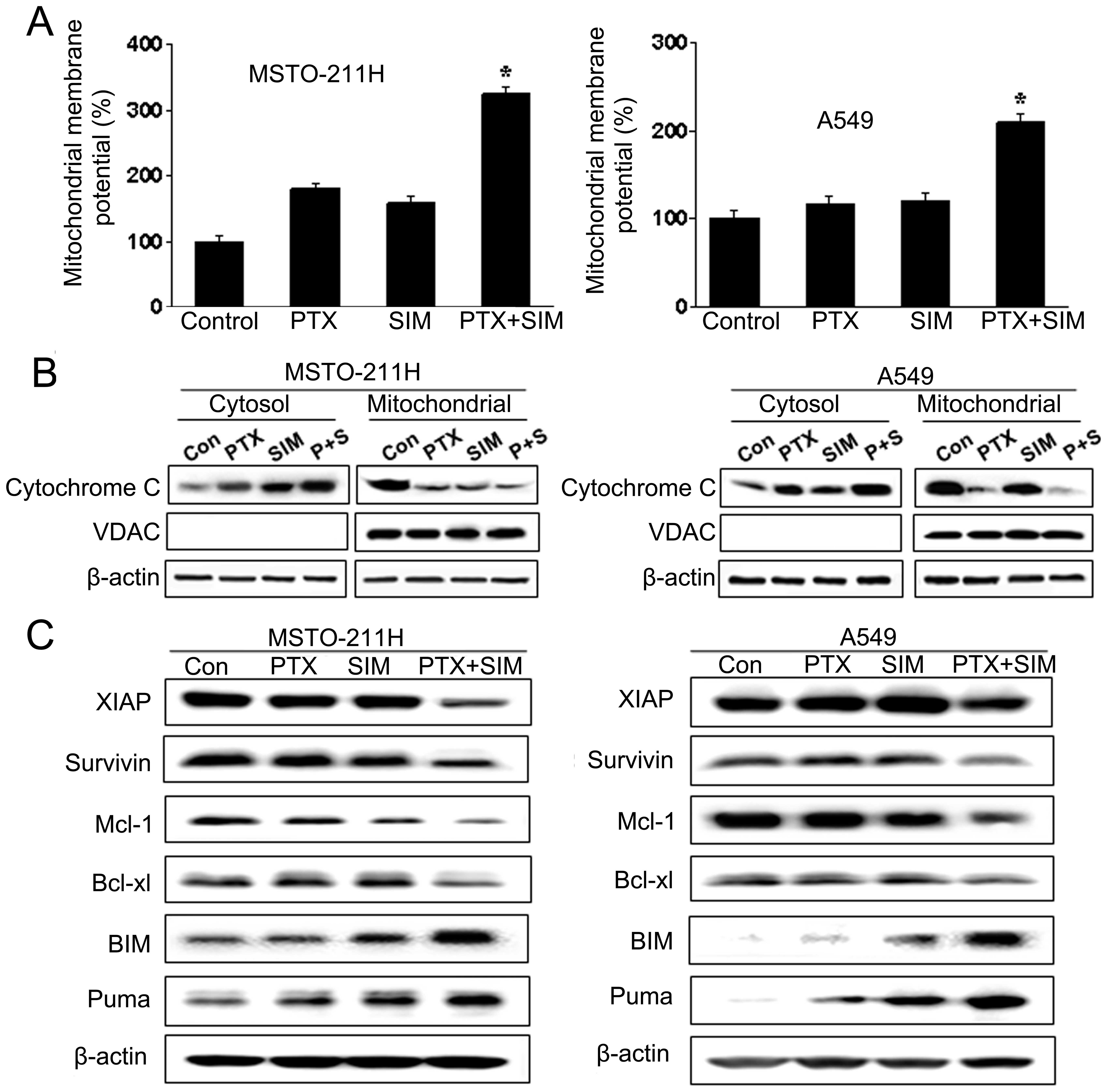 | Figure 4Mitochondrial dysfunction induced by
treatment with pemetrexed and/or simvastatin in MSTO-211 and A549
cells. (A) Cells were treated with 1 μM pemetrexed and/or 5 μM
simvastatin for 24 h, then stained with 10 μg/ml of JC-1, and
analyzed by flow cytometry. Percentage of JC-1 monomer in control
cells was set at 100%, and ΔΨm transition relative to
that of the control is presented. Each panel is representative of
three identical experiments. (B) Cells were incubated with 1 μM
pemetrexed and/or 5 μM simvastatin for 36 h. The cell lysate was
fractionated into cytosolic and mitochondrial portions, and
proteins were separated on 15% SDS-PAGE for cytochrome c
immunoblotting. The purity of mitochondrial fraction was verified
by western blotting with anti-VDAC antibody. (C) Cells were treated
with pemetrexed and simvastatin, alone and in combination, for 48
h, and the cell lysate was subjected to 15% SDS-PAGE to measure the
expression of the IAP (XIAP and survivin), anti-apoptotic (Mcl-1,
Bcl-xL), and pro-apoptotic (Bim, Puma and Bid) Bcl-2 families.
Immunoblots are representative of at least two independent
experiments. |
Combination of pemetrexed and simvastatin
induces changes in IAP and Bcl-2 families
Members of the IAP and Bcl-2 families are important
regulators of the mitochondrial apoptotic pathway. To identify the
molecular mechanism underlying apoptosis induced by combined
treatment with pemetrexed and simvastatin, we examined the
expression levels of the IAP (XIAP and survivin), anti-apoptotic
(Mcl-1 and Bcl-xL), and pro-apoptotic (Bim and Puma) Bcl-2
families, by immunoblot analysis in MSTO-211 and A549 cells treated
with pemetrexed and/or simvastatin for 36 h. As shown in Fig. 4C, treatment of MSTO-211 and A549
cells with pemetrexed and simvastatin resulted in a significant
decrease in XIAP and survivin levels relative to treatment with
either drug alone. In addition, combination treatment with
pemetrexed and simvastatin decreased the expression of the
anti-apoptotic factors Mcl-1 and Bcl-xL, and increased the
expression of pro-apoptotic factors Bim and Puma.
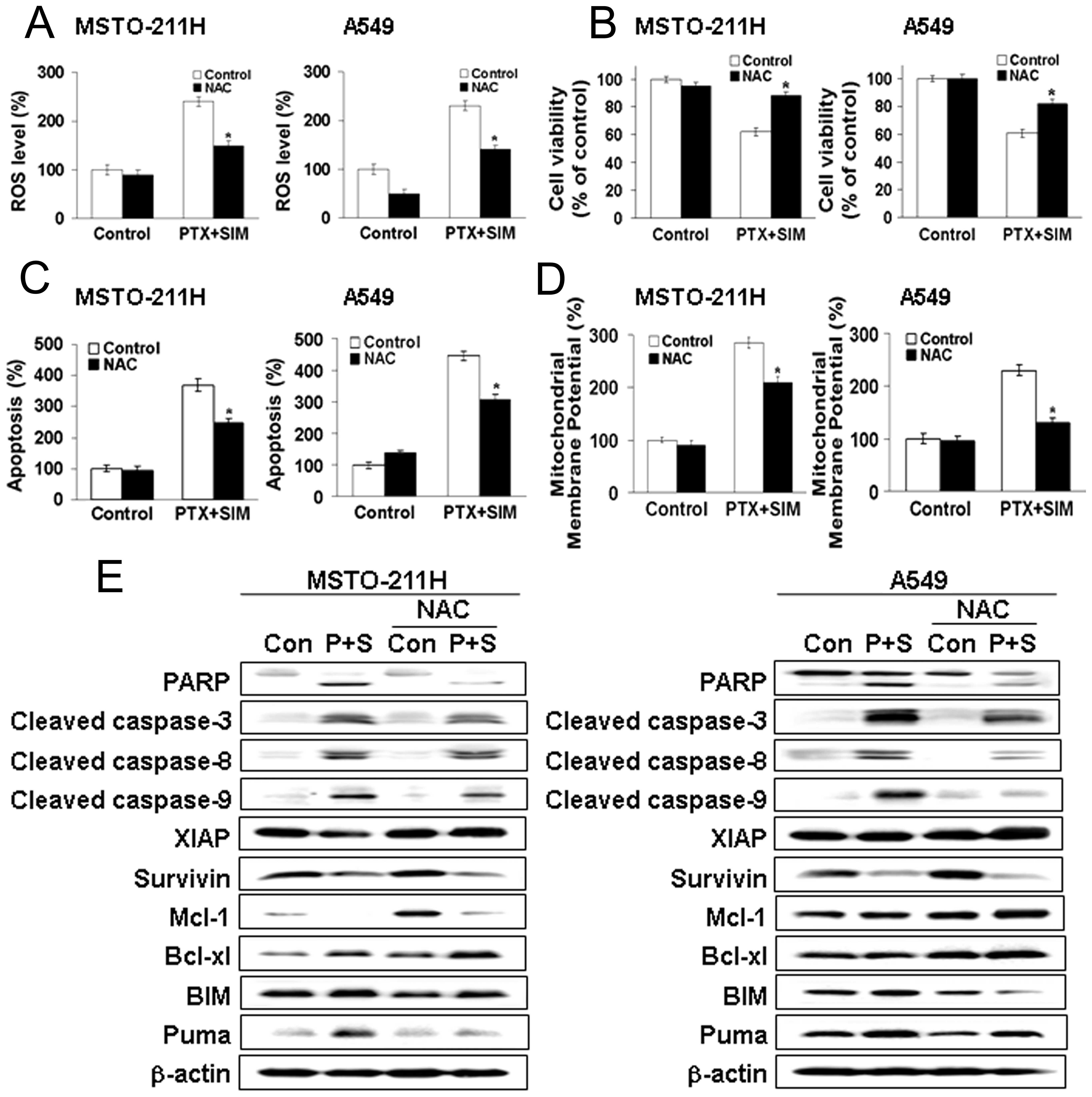
Pretreatment with NAC prevents apoptosis
induced by pemetrexed and simvastatin
We next tested the effect of the free radical
scavenger NAC in pemetrexed and simvastatin-treated MSTO-211 and
A549 cells. Cells were pretreated with NAC, followed by the
addition of pemetrexed and simvastatin for 24 h. As shown in
Fig. 5A, the enhancement of ROS
generation by combination treatment with pemetrexed and simvastatin
was abrogated by NAC. Moreover, NAC markedly inhibited the effects
of combination therapy on cell viability, as evaluated by the MTT
assay (Fig. 5B).
Our results indicate that elevated ROS may be
necessary for the potentiation of cell death in pemetrexed plus
simvastatin-treated cells. To determine whether elevated ROS
participated in the apoptosis induced by the combination of
pemetrexed and simvastatin, the proportion of apoptotic cells was
determined by Annexin V-PI staining (Fig. 5C). Annexin V-positive cells were
increased in MSTO-211 and A549 cells treated with the drug
combination. Pretreatment with NAC markedly reduced this
increase.
We also observed that JC-1 monomers were increased
in MSTO-211 and A549 cells treated with the drug combination, and
the loss of ΔΨm was significantly reduced in cells
pretreated with NAC (Fig. 5D).
Western blot analysis of MSTO-211 and A549 cell lysates (Fig. 5E) showed that the combination of
pemetrexed and simvastatin enhanced the expression of cleaved PARP,
caspase proteins, Bim, and Puma, decreased the expression of XIAP,
survivin, Mcl-1, and Bcl-xL. Pretreatment with NAC blocked these
effects. Together, these findings indicate that ROS generation
played a primary role in apoptosis induced by pemetrexed and
simvastatin.
Combination of pemetrexed and simvastatin
induces apoptosis by upregulation of Bim expression
Previous studies revealed that the expression of
Bim, a pro-apoptotic protein, was significantly induced by statins
or gefitinib in lung cancer (24,25).
To determine the role of Bim in apoptosis induced by pemetrexed and
simvastatin, we decreased the level of Bim expression by
introducing siRNA for Bim. We then examined the effect of Bim
siRNA-transfected cells on apoptosis induced by pemetrexed and
simvastatin. We spread an equal number of viable Bim
siRNA-transfected and non-silencing siRNA-transfected cells at 48 h
after siRNA transfection. After an additional 48-h incubation, the
cells were treated with pemetrexed and simvastatin for 36 h, and
the cell lysate was used to carry out western blotting. Both Bim
siRNA and the pemetrexed-simvastatin combination decreased the
expression of cleaved PARP, caspase-3, -8 and -9. Together, these
data indicate that the induction of apoptosis by pemetrexed and
simvastatin is due, at least in part, to the upregulation of
Bim.
Discussion
In the present study, we demonstrated the
synergistic effect of the combination of pemetrexed and simvastatin
on apoptosis of MSTO-211 malignant mesothelioma cells and A549 lung
cancer cells compared to the use of either agent alone. These
findings suggest that a combination of these two agents can
potentially kill malignant mesothelioma and lung cancer cells more
effectively and with fewer side effects than either drug alone,
thereby providing a rationale for combining these drugs for the
treatment of malignant mesothelioma and lung cancer.
Previous studies have examined the effects of
pemetrexed on various human tumor cells including malignant
mesothelioma and NSCLC. Pemetrexed has demonstrated clinical
activity, either alone or in combination with the platinum
compounds, vinorelbine, and gemcitabine, in a broad array of solid
tumors (26). Studies on the
mechanism of action of pemetrexed have shown that it inhibits cell
proliferation and induces apoptosis in cancer cells (9). One of the most important approaches
for developing improved cancer therapies is to understand the
mechanisms by which successful therapies induce apoptosis. To our
knowledge, no mechanistic studies have been conducted on the
combination treatment of pemetrexed and simvastatin in malignant
mesothelioma and lung cancer cells.
Mitochondria play a central role in cellular
metabolism and are a major source of ROS in cells (27,28).
Several studies have reported that mitochondrial morphology changes
during apoptosis, resulting in the appearance of small, round
mitochondrial fragments (29,30).
Mitochondria play a major role in many apoptotic responses by
coordinating caspase activation through cytochrome c and
Bcl-2 family proteins (31–33).
Our results demonstrate the release of cytochrome c from the
mitochondria into the cytosol. This leads to activation of
caspase-9, and subsequently, the activation of caspase-3. In
addition, cleavage of PARP, a downstream target in this pathway,
occurs during pemetrexed and simvastatin-induced apoptosis in
malignant mesothelioma and lung cancer cells.
Although ROS are essential to cell survival,
elevated levels of ROS result in slowed growth, cell cycle arrest,
and apoptosis (34). Many
chemotherapeutic strategies have been designed to significantly
increase cellular ROS levels in order to induce irreparable tumor
cell damage and death. In our earlier study, we investigated the
role of simvastatin-induced apoptosis in lung A549 cells by
mitochondrial ROS production (19). Buque et al (35) reported that an increase in
intracellular ROS and p53 was required for pemetrexed-induced
cytotoxicity in melanoma cells.
Increased ROS initiates a wide range of irreversible
oxidative damage in the mitochondria. This in turn can lead to
alteration in mitochondrial membrane potential (36). Accordingly, we investigated the
possibility that ROS plays a role in pemetrexed and
simvastatin-induced ROS generation in malignant mesothelioma and
lung cancer cells. We demonstrated that, compared to individual
treatments, combination treatment with pemetrexed and simvastatin
increased ROS levels, suggesting that the combination of these
drugs produces higher ROS levels.
If ROS were indeed involved in apoptosis, ROS
quenchers, such as antioxidants, would be anticipated to prevent
apoptosis. Moreover, we found that pemetrexed and
simvastatin-induced apoptosis, mitochondrial dysfunction, and
caspase activation were greatly reduced by pretreatment with NAC.
These results suggest that, in this model system, ROS generation
has a primary role in the induction of apoptosis by pemetrexed and
simvastatin.
ΔΨm occurred during pemetrexed and
simvastatin-induced apoptosis that also resulted in several changes
in IAP and Bcl-2 family proteins that may promote apoptosis. To
better understand the contribution of these proteins to the
sensitivity of malignant mesothelioma and lung cancer to pemetrexed
and simvastatin-induced apoptosis, we compared their expression
levels. Our study revealed that pemetrexed and simvastatin induced
downregulation of XIAP, survivin, Mcl-1 and Bcl-xL and upregulation
of Bim and Puma. We also found that siRNA-mediated knockdown of Bim
reduced the expression of apoptotic related proteins. Taken
together, our results indicate that the upregulation of Bim may
have contributed, at least in part, to pemetrexed and
simvastatin-induced apoptosis.
In conclusion, we demonstrated that combination
treatment with pemetrexed and simvastatin potentiated their
apoptotic activity over that seen with either drug alone in
malignant mesothelioma and lung cancer cells. These effects were
mediated through mitochondrial dysfunction, by triggering ROS
production, and by Bim induction. Taken together, these results
indicate that the combination of pemetrexed and simvastatin may be
a clinically promising therapy for the treatment of malignant
mesothelioma or NSCLC. Our study elucidated a possible mechanism of
action for the pemetrexed and simvastatin combination in effecting
cell death in malignant mesothelioma and lung cancer cells.
However, further studies are required including in vivo
xenograft models of malignant mesothelioma and lung cancer before
embarking on human studies.
Acknowledgements
This study was supported by a grant of the Korean
Health Technology R&D Project, Ministry of Health &
Welfare, Republic of Korea (A120152).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Hotta K, Matsuo K, Ueoka H, Kiura K,
Tabata M and Tanimoto M: Meta-analysis of randomized clinical
trials comparing cisplatin to carboplatin in patients with advanced
non-small-cell lung cancer. J Clin Oncol. 22:3852–3859. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Belli C, Fennell D, Giovannini M, Gaudino
G and Mutti L: Malignant pleural mesothelioma: current treatments
and emerging drugs. Expert Opin Emerg Drugs. 14:423–437. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S,
Manegold C, Niyikiza C and Paoletti P: Phase III study of
pemetrexed in combination with cisplatin versus cisplatin alone in
patients with malignant pleural mesothelioma. J Clin Oncol.
21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S,
Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP and Gandara
D: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naïve patients with
advanced-stage non-small-cell lung cancer. J Clin Oncol.
26:3543–3551. 2008.PubMed/NCBI
|
|
6
|
Hong J, Kyung SY, Lee SP, Park JW, Jung
SH, Lee JI, Park SH, Sym SJ, Park J, Cho EK, Shin DB and Lee JH:
Pemetrexed versus gefitinib versus erlotinib in previously treated
patients with non-small cell lung cancer. Korean J Intern Med.
25:294–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shih C, Chen VJ, Gossett LS, Gates SB,
MacKellar WC, Habeck LL, Shackelford KA, Mendelsohn LG, Soose DJ,
Patel VF, Andis SL, Bewley JR, Rayl EA, Moroson BA, Beardsley GP,
Kohler W, Ratnam M and Schultz RM: LY231514, a
pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple
folate-requiring enzymes. Cancer Res. 57:1116–1123. 1997.
|
|
8
|
Park CK, Kim KS, Oh IJ, Tseden-Ish M, Choi
YD, Kwon YS, Kim YI, Lim SC and Kim YC: Efficacy of pemetrexed in
relapsed non-small cell lung cancer and thymidylate synthase
expression. Tuberc Respir Dis. 67:191–198. 2009. View Article : Google Scholar
|
|
9
|
Ramirez JM, Ocio EM, San Miguel JF and
Pandiella A: Pemetrexed acts as an antimyeloma agent by provoking
cell cycle blockade and apoptosis. Leukemia. 21:797–804.
2007.PubMed/NCBI
|
|
10
|
Lu X, Errington J, Curtin NJ, Lunec J and
Newell DR: The impact of p53 status on cellular sensitivity to
antifolate drugs. Clin Cancer Res. 7:2114–2123. 2001.PubMed/NCBI
|
|
11
|
Vandermeers F, Hubert P, Delvenne P,
Mascaux C, Grigoriu B, Burny A, Scherpereel A and Willems L:
Valproate, in combination with pemetrexed and cisplatin, provides
additional efficacy to the treatment of malingnant mesothelioma.
Clin Cancer Res. 15:2818–2828. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldstein JL and Brown MS: Regulation of
the mevalonate pathway. Nature. 343:425–430. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan KK, Oza AM and Siu LL: The statins as
anticancer agents. Clin Cancer Res. 9:10–19. 2003.
|
|
14
|
Demierre MF, Higgins PD, Gruber SB, Hawk E
and Lippman SM: Statins and cancer prevention. Nat Rev Cancer.
5:930–942. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Holstein SA and Hohl RJ: Synergistic
interaction of lovastatin and paclitaxel in human cancer cells. Mol
Cancer Ther. 1:141–149. 2001.PubMed/NCBI
|
|
16
|
Feleszko W, Mlynarczuk I, Olszewska D,
Jalili A, Grzela T, Lasek W, Hoser G, Korczak-Kowalska G and
Jakobisiak M: Lovastatin potentiates antitumor activity of
doxorubicin in murine melanoma via an apoptosis-dependent
mechanism. Int J Cancer. 100:111–118. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khanzada UK, Pardo OE, Meier C, Downward
J, Seckl MJ and Arcaro A: Potent inhibition of small-cell lung
cancer cell growth by simvastatin reveals selective functions of
Ras isoforms in growth factor signaling. Oncogene. 25:877–887.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kozar K, Kaminski R, Legat M, Kopec M,
Nowis D, Skierski JS, Koronkiewicz M, Jakobisiak M and Golab J:
Cerivastatin demonstrates enhanced antitumor activity against human
breast cancer cell lines when used in combination with doxorubicin
or cisplatin. Int J Oncol. 24:1149–1157. 2004.
|
|
19
|
Hwang KE, Park C, Kwon SJ, Kim YS, Park
DS, Lee MK, Kim BR, Park SH, Yoon KH, Jeong ET and Kim HR:
Synergistic induction of apoptosis by sulindac and simvastatin in
A549 human lung cancer cells via reactive oxygen species-dependent
mitochondrial dysfunction. Int J Oncol. 43:262–270. 2013.PubMed/NCBI
|
|
20
|
Sun JM, Lee KW, Kim JH, Kim YJ, Yoon HI,
Lee JH, Lee CT and Lee JS: Efficacy and toxicity of pemetrexed as a
third-line treatment of non-small cell lung cancer. Jpn J Clin
Oncol. 39:27–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim KH, Song SY, Lim KH, Han SS, Kim SH,
Cho JH, Park CW, Lee S and Lee HY: Interstitial pneumonitis after
treatment with pemetrexed for non-small cell lung cancer. Cancer
Res Treat. 45:74–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wolf CM and Eastman A: The temporal
relationship between protein phosphatase, mitochondrial cytochrome
c release. Exp Cell Res. 247:505–513. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elbashir SM, Harborth J, Lendeckel W,
Yalcin A, Weber K and Tuschi T: Duplexes of 21-nucleotide RNAs
mediate RNA interference in cultured mammalian cells. Nature.
411:494–498. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prevost GP, Pradines A, Brezak MC,
Lonchampt MO, Viossat I, Ader I, Toulas C, Kasprzyk P, Gordon T,
Favre G and Morgan B: Inhibition of human tumor cell growth in vivo
by an orally bioavailable inhibitor of human farnesyltransferase,
BIM-46228. Int J Cancer. 91:718–722. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song JY, Kim CS, Lee JH, Jang SJ, Lee SW,
Hwang JJ, Lim C, Lee G, Seo J, Cho SY and Choi J: Dual inhibition
of MEK 1/2 and EGFR synergistically induces caspase-3-dependent
apoptosis in EGFR inhibitor-resistant lung cancer cells via BIM
upregulation. Invest New Drugs. 31:1458–1465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schulze-Bergkamen H and Krammer PH:
Apotosis in cancer-implications for therapy. Semin Oncol.
31:90–119. 2004. View Article : Google Scholar
|
|
27
|
Copeland WC, Wachsman JT, Johnson FM and
Penta JS: Mitochondrial DNA alterations in cancer. Cancer Invest.
20:557–569. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim HR, Yang SH and Jeong ET: Combination
treatment with arsenic trioxide and sulindac induces apoptosis of
NCI-H157 human lung carcinoma cells via ROS generation with
mitochondrial dysfunction. Tuberc Respir Dis. 59:30–38. 2005.
|
|
29
|
Frank S, Gaume B, Bergmann-Leitner ES,
Leitner WW, Robert EG, Catez F, Smith CL and Youle RJ: The role of
dynamic-related protein 1, a mediator of mitochondrial fission, in
apoptosis. Dev Cell. 1:515–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karbowski M, Lee YJ, Gaume B, Jeong SY,
Frank S, Nechushtan A, Santel A, Fuller M, Smith CL and Youle RJ:
Spatial and temporal association of Bax with mitochondrial fission
sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 159:931–938.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar
|
|
32
|
Li LY, Luo X and Wang X: Endonuclease G is
an apoptotic DNase when released from mitochondria. Nature.
412:95–99. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tournier C, Hess P, Yang DD, Xu J, Turner
TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA and Davis RJ:
Requirement of JNK for stress-induced activation of the cytochrome
c-mediated death pathway. Science. 288:870–874. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Burdon RH: Control of cell proliferation
by reactive oxygen species. Biochem Soc Trans. 24:1028–1032.
1996.PubMed/NCBI
|
|
35
|
Buque A, Muhialdin JSh, Munoz A, Calvo B,
Carrera S, Aresti U, Sancho A, Rubio I and Lopez-Vivinco G:
Molecular mechanism implicated in pemetrexed-induced apoptosis in
human melanoma cells. Mol Cancer. 11:252012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fiers W, Beyaert R, Declercq W and
Vandenabeele P: More than one way to die: apoptosis, necrosis and
reactive oxygen damage. Oncogene. 18:7719–7730. 1999. View Article : Google Scholar
|