Introduction
Docetaxel, the most potent taxane, is derived from
extracts of European yew needles. Docetaxel has been shown to have
significant antitumor activity. The main mechanism of action is
through stabilization of tubulin, arresting cells in the
G2/M phase of the cell cycle (1). In head and neck cancer patients,
docetaxel is now widely applied as first- and second-line induction
chemotherapy or used in combination with other anticancer drugs or
radiation (2–7). However, some patients develop
resistance to docetaxel. Although the causes and mechanisms of
docetaxel resistance are still unknown, activation of the redox
system is thought to be involved (8). Thioredoxin (TRX), a small
redox-active multifunctional protein, acts as a potent antioxidant
and redox regulator in signal transduction (9). TRX has been reported to be
overexpressed in various types of cancers (10–12)
and its overexpression is associated with a poor prognosis in
patients (13,14). Indeed, Kim et al (15) reported that breast tumors with high
TRX expression show a significantly lower response rate to
docetaxel treatment than those with low TRX expression.
d-allose is a rare sugar that is
found only in very small quantities in nature. In recent studies,
we reported that d-allose inhibited the growth of
head and neck cancer cells by inducing of cell cycle arrest,
apoptosis and competition with glucose uptake (16). In addition, d-allose stimulates the
overexpression of TRX-interacting protein (TXNIP) and enhances the
effects of radiation (17). TXNIP
is known to interact with TRX and is involved in the regulation of
the cellular redox state (18).
Moreover, the TXNIP gene is a tumor suppressor (19) and metastasis suppressor (20) and its expression is lower in
various cancer cells when compared to normal cells (21–23).
Therefore, we speculated that the induction of TXNIP expression by
d-allose treatment
may enhance the anticancer effects of docetaxel.
In the present study, we evaluated the combined
effects and mechanisms of docetaxel and d-allose in head and neck cancer
in vitro and in vivo.
Materials and methods
Cell culture
The human head and neck carcinoma cell line HSC3
(tongue carcinoma) was obtained from the Health Science Research
Resources Bank, Osaka, Japan. HSC3 cells were cultured at 37°C in
an atmosphere containing 5% CO2 in Eagle’s minimal
essential medium (EMEM), 10% heat-inactivated fetal bovine calf
serum and 1% penicillin-streptomycin.
Determination of cell survival
d-allose was kindly supplied by
Dr K. Izumori at the Department of Biochemistry and Food Science,
Faculty of Agriculture, Kagawa University, Kagawa, Japan. Docetaxel
was obtained from Sanofi (Paris, France) and stored in frozen
aliquots. Before use, docetaxel was thawed and diluted to the
desired concentrations in the cell culture medium. The growth
inhibitory effects of docetaxel plus d-allose were compared with those
of docetaxel or d-allose alone. The cells were
seeded in 96-well plates at a density of 2.5×103
cells/100 μl (n=5 wells/treatment) and were cultured for 24 h.
Medium was then removed, and fresh medium containing 0.1 ng/ml
docetaxel, 10 mM d-allose or 0.1 ng/ml docetaxel
plus 10 mM d-allose
were added. Cells were incubated for an additional 24–96 h. The net
number of viable cells was then determined using a Cell Counting
Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) according to
the manufacturer’s instructions. The absorbance was measured by a
microplate reader at 450 nm after 2 h of incubation.
To investigate the enhancement of
docetaxel-dependent anticancer effects by d-allose, 5×103
cells/500 μl were plated into 6-well plates and cultured for 24 h
(n=3 wells/treatment). The cells were treated with 0, 10 or 25 mM
d-allose and various
concentrations of docetaxel and were incubated at 37°C for 96 h.
Colonies were fixed with 3:1 methanol/acetic acid and stained with
0.5% crystal violet in methanol. Colonies were counted under a
microscope, with a cut-off of 50 viable cells. The survival
fraction was calculated as (mean colonies)/(cells inoculated) ×
(plating efficiency). The docetaxel dose enhancement ratio (DER)
was calculated as the dose (ng/ml) for docetaxel alone divided by
the dose (ng/ml) for docetaxel plus d-allose for a survival fraction
of 0.25.
For three-dimensional (3D) culture experiments, the
3D culture BME cell proliferation assay (Trevigen, Gaithersburg,
MD, USA) was used. Each well of a 96-well plate was coated with 35
μl of 3D Culture BME, and plates were then incubated at 37°C for 1
h to promote gel formation. Cells were then seeded in the coated
96-well plates at a density of 5×103 cells per 100 μl
and cultured for 48 h. After the establishment of 3D structures,
100 μl of fresh medium containing 0.1 ng/ml docetaxel, 25 mM
d-allose or 0.1 ng/ml
docetaxel plus 25 mM d-allose was added. Cells were
then incubated at 37°C for an additional 5 days. Colonies growing
>5 fold were scored as survivors. Error bars indicate the
standard deviation calculated after pooling the results of 3
independent experiments.
Measurement of apoptosis
TUNEL assays were performed using the Apoptosis
Detection System Fluorescein kit (Promega, Madison, WI, USA).
Briefly, treated HSC-3 cells were spread on a poly-l-lysine slide
(Sigma, St. Louis, MO, USA), fixed with 4% paraformaldehyde and
permeabilized with 0.2% Triton X-100. Cells were incubated in 50 μl
TdT incubation buffer [nucleotide mix (fluorescein-12-dUTP) and TdT
enzyme prepared according to the manufacturer’s protocol] for 60
min at 37°C in a humidified chamber. The reaction was terminated by
washing the cells in 2X SSC followed by 2 washes in PBS. Cells were
counterstained with 1 μg/ml propidium iodide and then washed in
distilled water. Staining was observed under a fluorescence
microscope. Green fluorescence indicated DNA fragmentation due to
fluorescein-12-dUTP labeling. For each experimental time point, 10
fields, each containing 100 cells, were scored for the appearance
of apoptosis; two chambers were scored in this manner for each time
point.
Cell cycle analysis
Flow cytometry was performed using a FACSEpics XL
flow cytometer (Beckman Coulter, Fullerton, CA, USA). Cells were
washed twice with PBS, fixed in 1 ml of 70% ethanol for 2 h at 4°C,
treated with 200 g RNase A, and stained with 50 μg propidium
iodide. Cell cycle distribution was analyzed using System II
software (Beckman Coulter).
Analysis of mRNA expression
To investigate the effects of docetaxel,
d-allose and
docetaxel plus d-allose on the expression of
TXNIP and TRX transcripts, cells were cultured in
6-cm dishes with 0.1 ng/ml docetaxel, 25 mM d-allose or 0.1 ng/ml docetaxel
plus 25 mM d-allose
and incubated for an additional 24 h. Real-time polymerase chain
reaction (PCR) was carried out using TaqMan gene expression assay
primers and an ABI7700 real-time PCR system. Each reaction was
performed in duplicate. The GAPDH gene was used to normalize
expression across assays and runs, and a threshold value (Ct) for
each sample was used to determine the expression level of the
gene.
Western blot analysis
After treatment with 0.1 ng/ml docetaxel, 25 mM
d-allose or 0.1 ng/ml
docetaxel plus 25 mM d-allose for 24 h, cells were
scraped into lysis buffer (1% NP40, 150 mM NaCl, 50 mM NaF, 20 mM
Tris-HCl, pH 7.5, 1 mM Na3VO4, 10 μM
Na2MnO4, 1 mM PMSF, 10 μg/ml leupeptin, 1%
aporotinin) with protease inhibitors and sonicated. Samples were
centrifuged for 10 min at 14,000 rpm and supernatants were
collected. For western blot analyses, proteins were separated on
10% SDS-PAGE gels, transferred to nitrocellulose membranes, blocked
with 5% (w/v) non-fat dried milk in PBS and incubated with
anti-TXNIP (MBL, Nagoya, Japan), anti-TRX (MBL) or anti-β-actin
antibodies (Sigma). Membranes were probed with a horseradish
peroxidase-conjugated anti-mouse IgG (Amersham, Tokyo, Japan), and
signals were detected with an enhanced chemiluminescence system
(Amersham).
Detection of reactive oxygen species
(ROS) detection
Intracellular ROS generation was measured using the
Total ROS Detection kit (Enzo Life Sciences, Plymouth Meeting, PA,
USA) according to the manufacturer’s instructions. Cells were
incubated with dye from the kit at 37°C for 1 h. Immediately after
staining, the cells were analyzed using a fluorescence microscope
(Olympus BX-51, Tokyo, Japan) equipped with a standard green filter
(490/525 nm).
In vivo xenograft experiment
HSC3 cells were used in a xenograft model with
female athymic nude mice (BALB/c nu/nu, 5–6 weeks old). A
suspension of 1×106 cells in 0.1 ml volume was injected
subcutaneously into the posterior flanks of mice using a 1-cc
syringe with a 27-G needle. Tumors were grown for 14 days until
attaining an average size of 100–150 mm3 (Day 0).
Treatment groups were as follows: a) control; b) treatment with 500
mM d-allose; c) 12
mg/kg docetaxel; and d) 12 mg/kg docetaxel plus 500 mM d-allose. Each treatment group
contained 6 mice. d-allose was prepared by
dissolving compound in normal saline to reach a final concentration
of 500 mM and aliquots (0.2 ml) were injected around tumors 5
times/week for 3 weeks. Docetaxel was diluted in normal saline to
reach a final concentration of 1 mg/ml, and aliquots (0.2 ml) were
administered by intraperitoneal injection on Days 0 and 7. Mice
from the control group were injected with 0.2 ml normal saline at
the same time points. This research was approved by the Animal Care
and Use Committee of Kagawa University.
Statistical analysis
Comparisons between groups were carried out using
the Student’s t-test. Differences with P-values of <0.05 were
considered statistically significant.
Results
Effects of d-allose in combination with
docetaxel
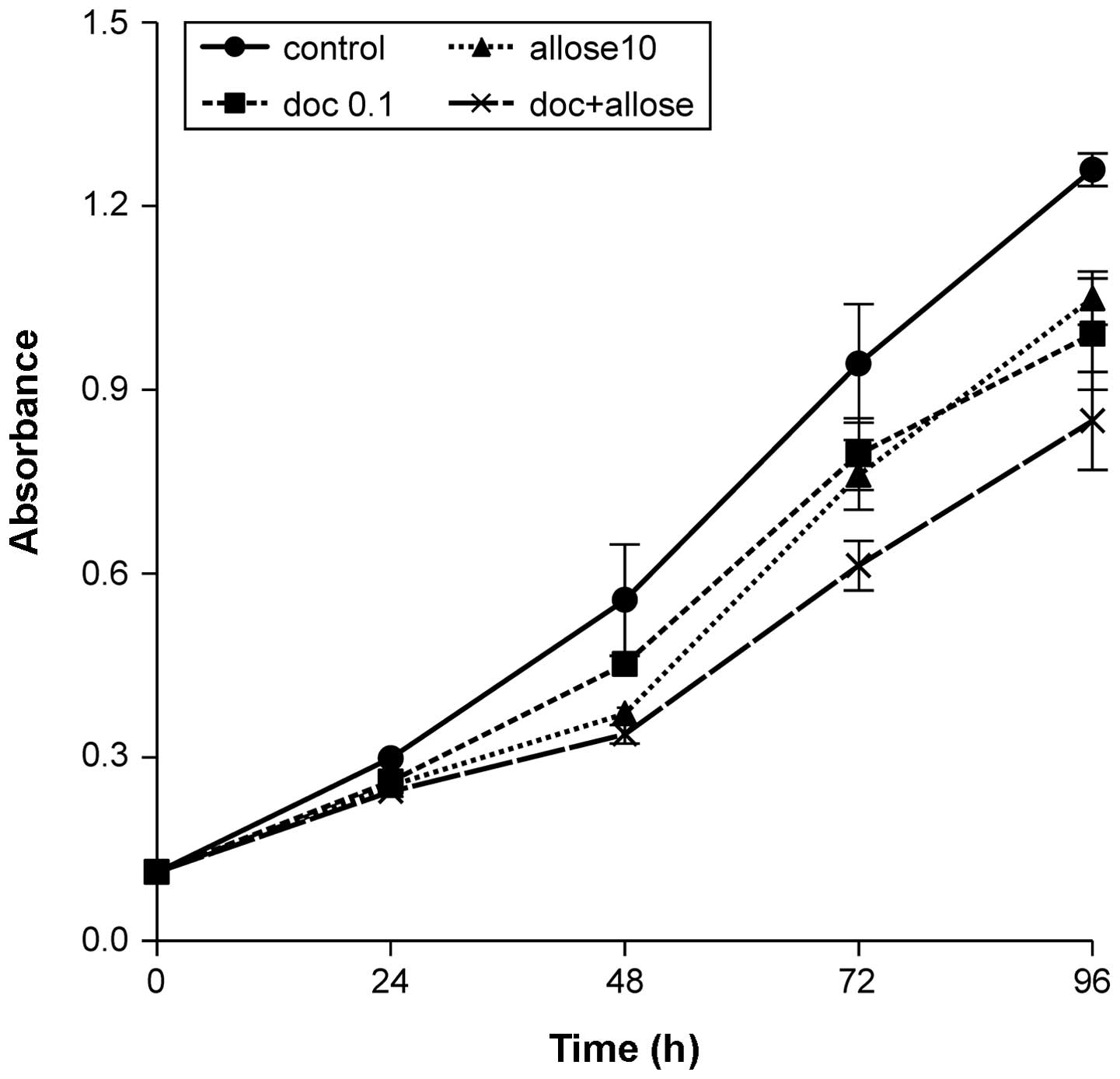
Ninety-six hours after treatment with docetaxel,
d-allose or docetaxel
plus d-allose, growth
of HSC3 cells was decreased to 78.7, 83.3 and 67.4% that of the
control, respectively. The combination of docetaxel plus
d-allose
significantly inhibited cell proliferation when compared to
treatment with docetaxel or d-allose alone (P<0.001 and
P<0.001, respectively; Fig. 1).
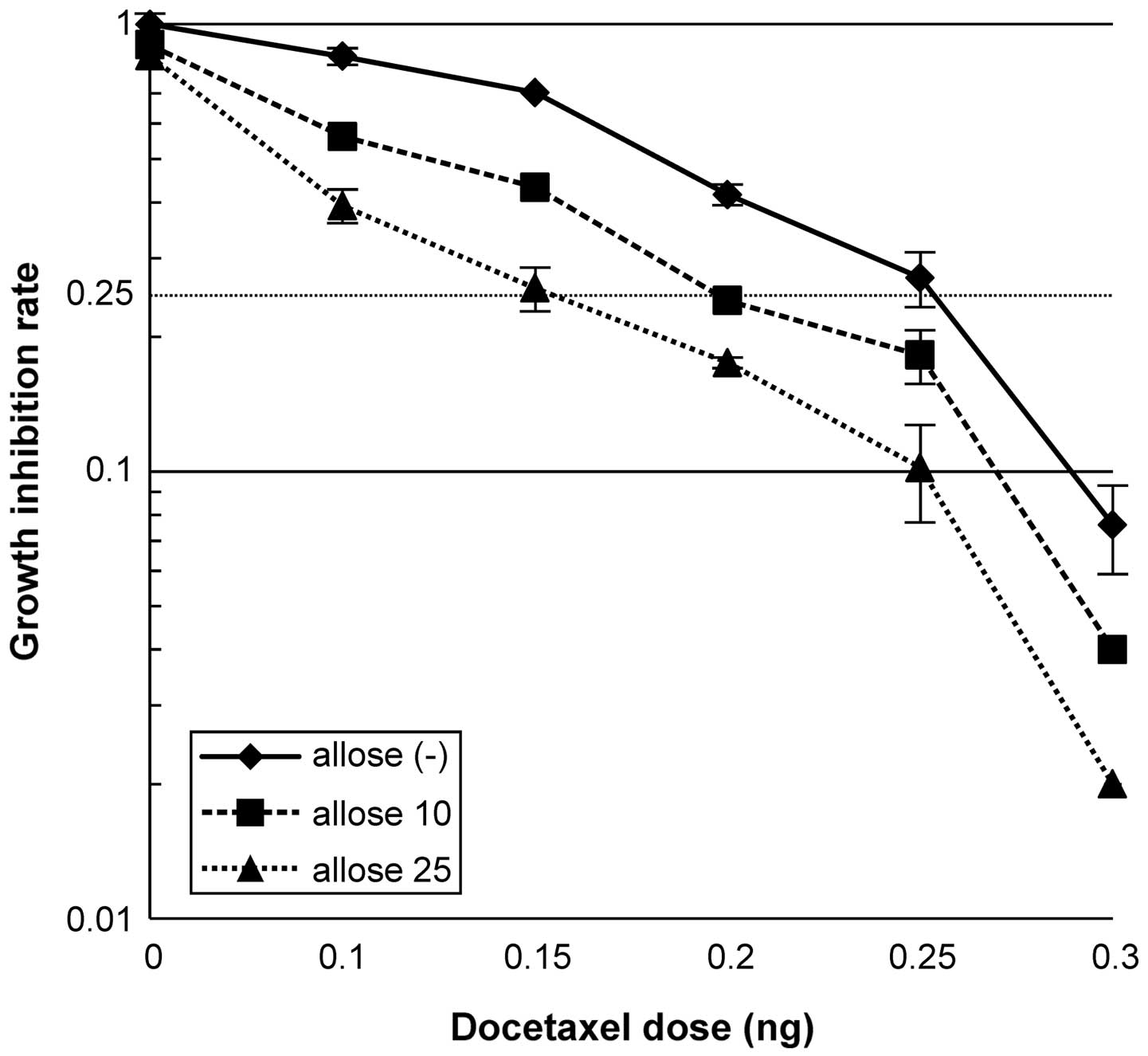
As shown in Fig. 2, treatment of
cells with 10 mM d-allose resulted in a docetaxel
dose enhancement ratio (DER) of 1.3, while treatment with 25 mM
d-allose resulted in
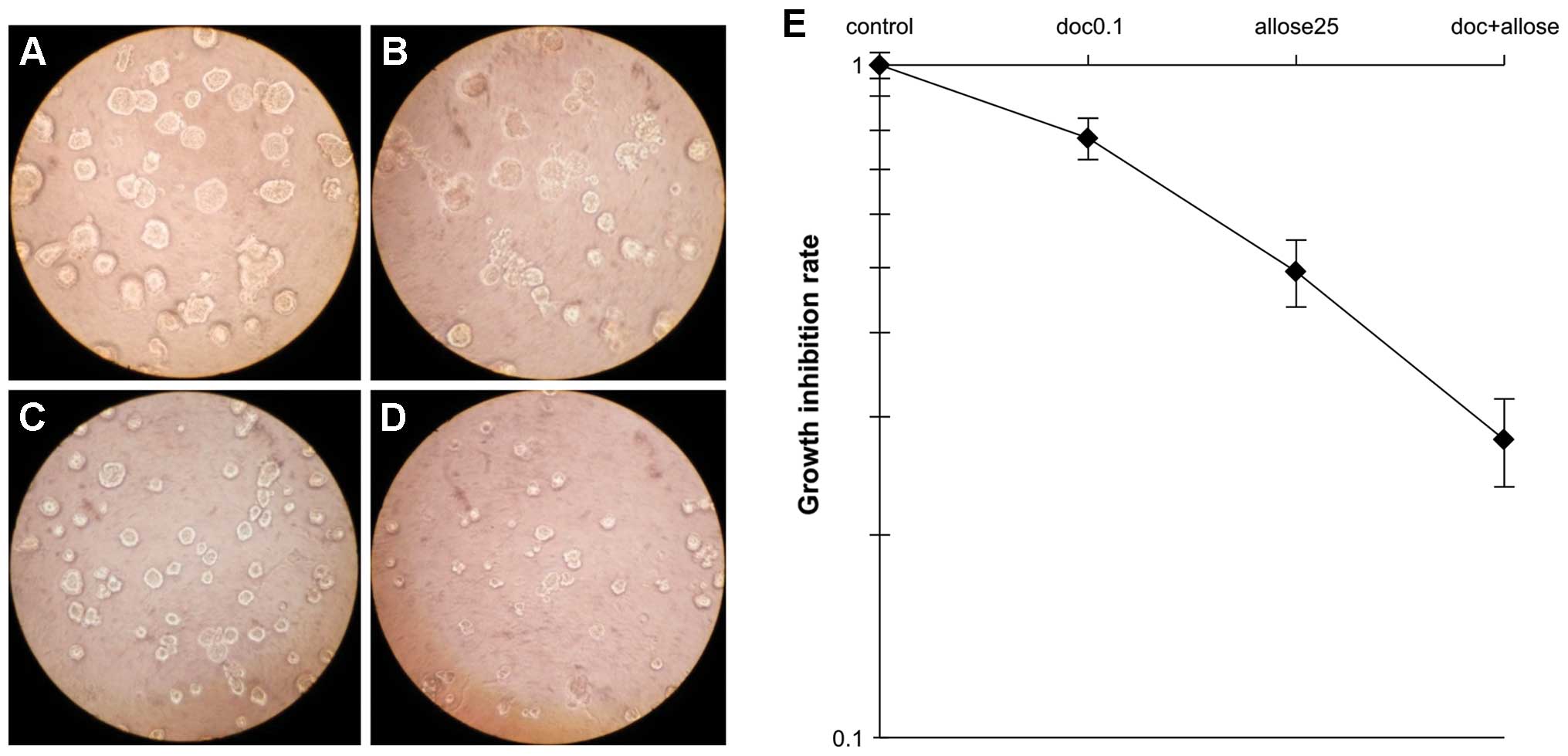
a DER of 1.71. Analysis of the morphology and growth of cells in 3D
cultures, as shown in Fig. 3,
revealed that treatment with docetaxel alone, d-allose alone or docetaxel plus
d-allose reduced cell
survival to 78, 49 and 28% that of the control group, respectively.
Moreover, the combination of docetaxel and d-allose also induced the highest
percentage of apoptosis in comparison to either docetaxel alone or
d-allose alone
(P<0.0001; Table I).
 | Table IDocetaxel dose enhancement ratios and
percent apoptosis induced by each treatment. |
Table I
Docetaxel dose enhancement ratios and
percent apoptosis induced by each treatment.
| Treatment | Ratio to
docetaxel | % Apoptosis |
|---|
| No treatment | 0.78 | 0.55±0.1 |
| Docetaxel | 1.00 | 1.71±0.22 |
| Allose | 1.58 | 1.22±0.27 |
| Docetaxel +
allose | 2.81 | 4.25±0.54 |
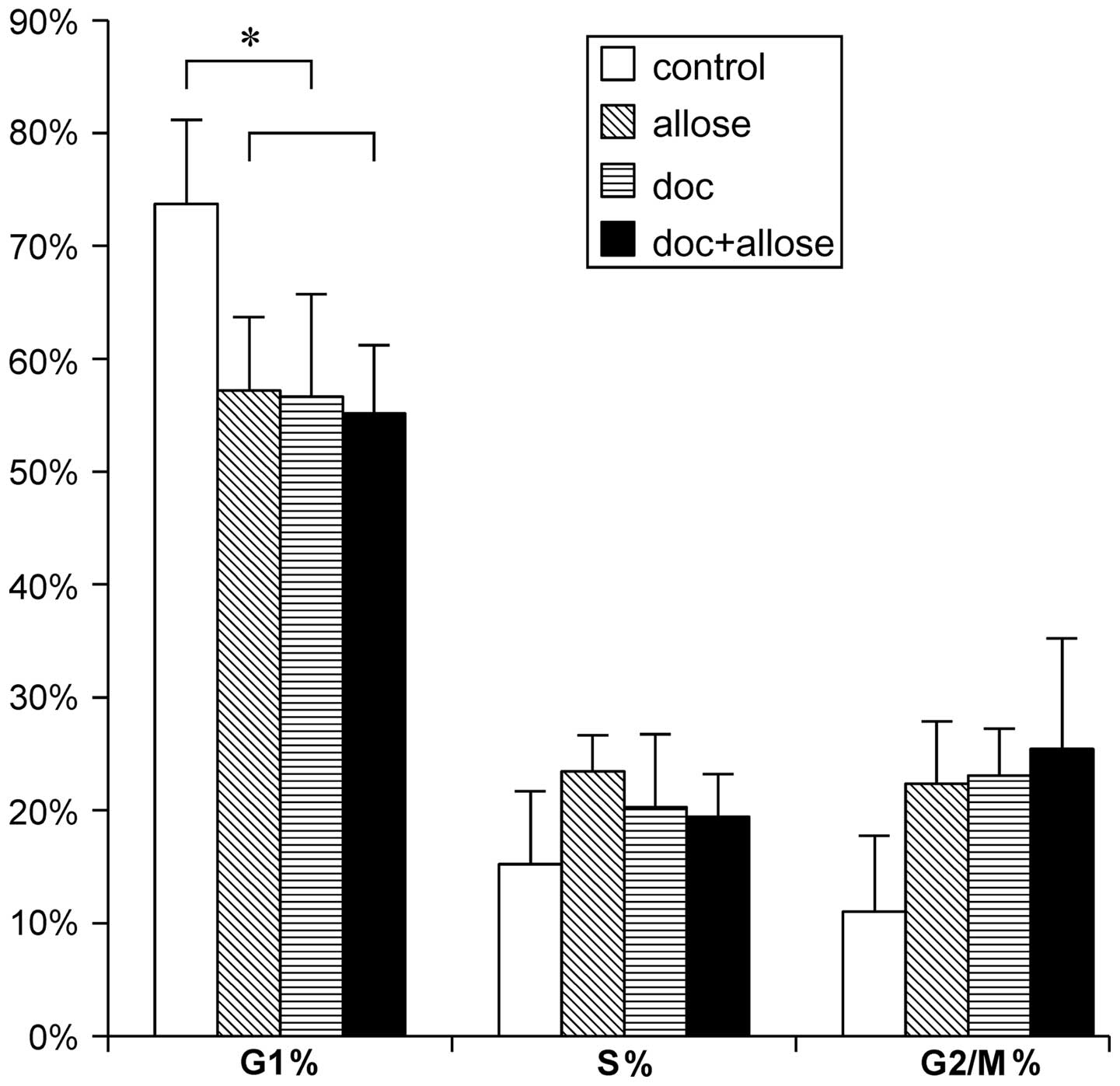
Modification of the cell cycle
Cell cycle modification by docetaxel and
d-allose treatment
was analyzed by flow cytometry. Accumulation of cells in the
G1 phase of the cell cycle was significantly decreased
after treatment with docetaxel alone, d-allose alone or docetaxel plus
d-allose as compared
to that of control group. Although the G2/M-phase cell
populations tended to increase after treatment, no significant
differences were found (Fig.
4).
Regulation of mRNA and protein
expression
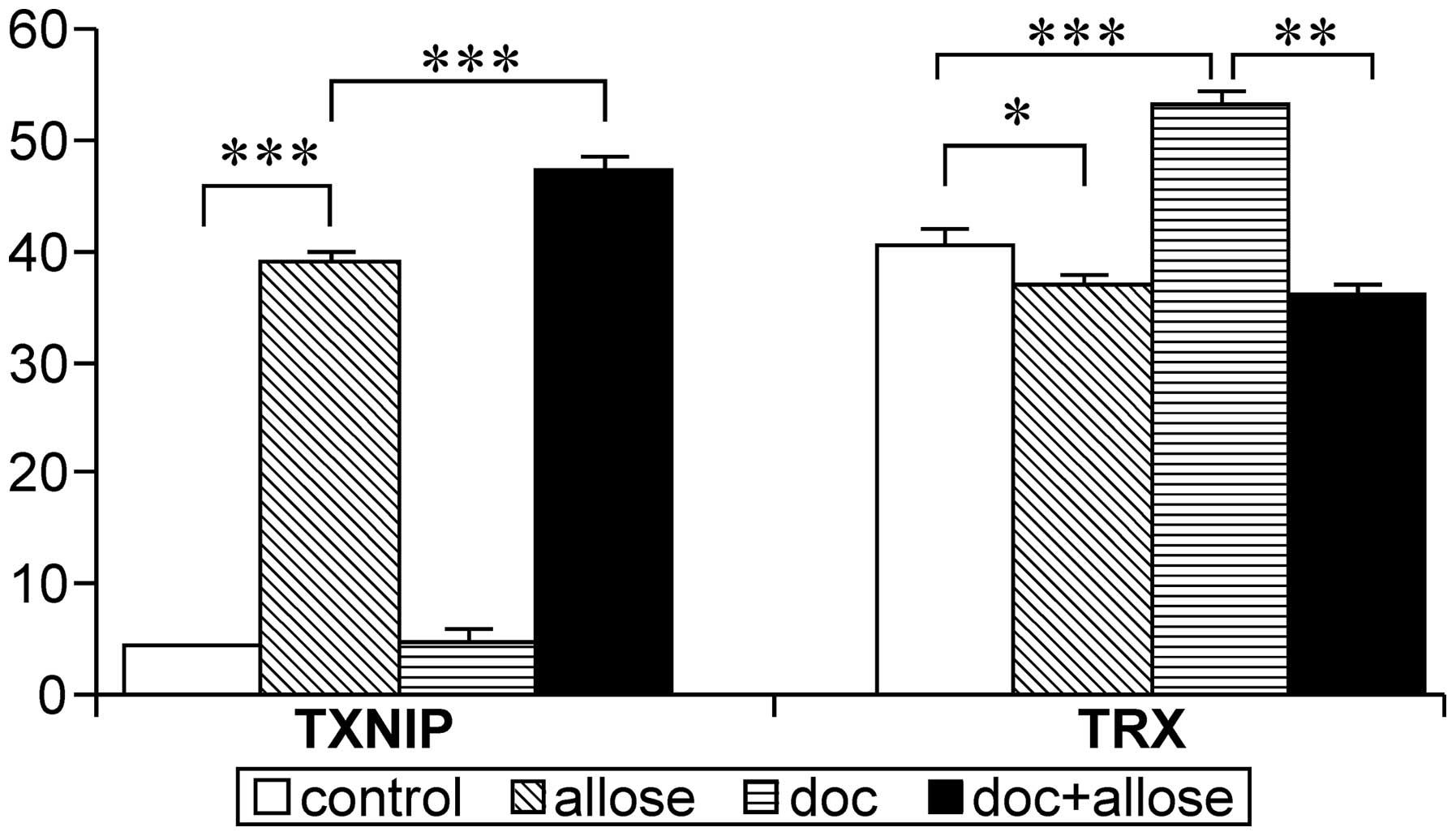
The mRNA expression of TXNIP was markedly
increased in HSC3 cells following treatment with d-allose. Additionally, the
expression of TXNIP mRNA was enhanced after treatment with
the combination of docetaxel plus d-allose treatment, while no
significant increase was observed following treatment with
docetaxel alone. Although the mRNA expression of TRX was
increased by docetaxel treatment, combined treatment with
d-allose and
docetaxel significantly suppressed the expression of TRX
mRNA (Fig. 5).
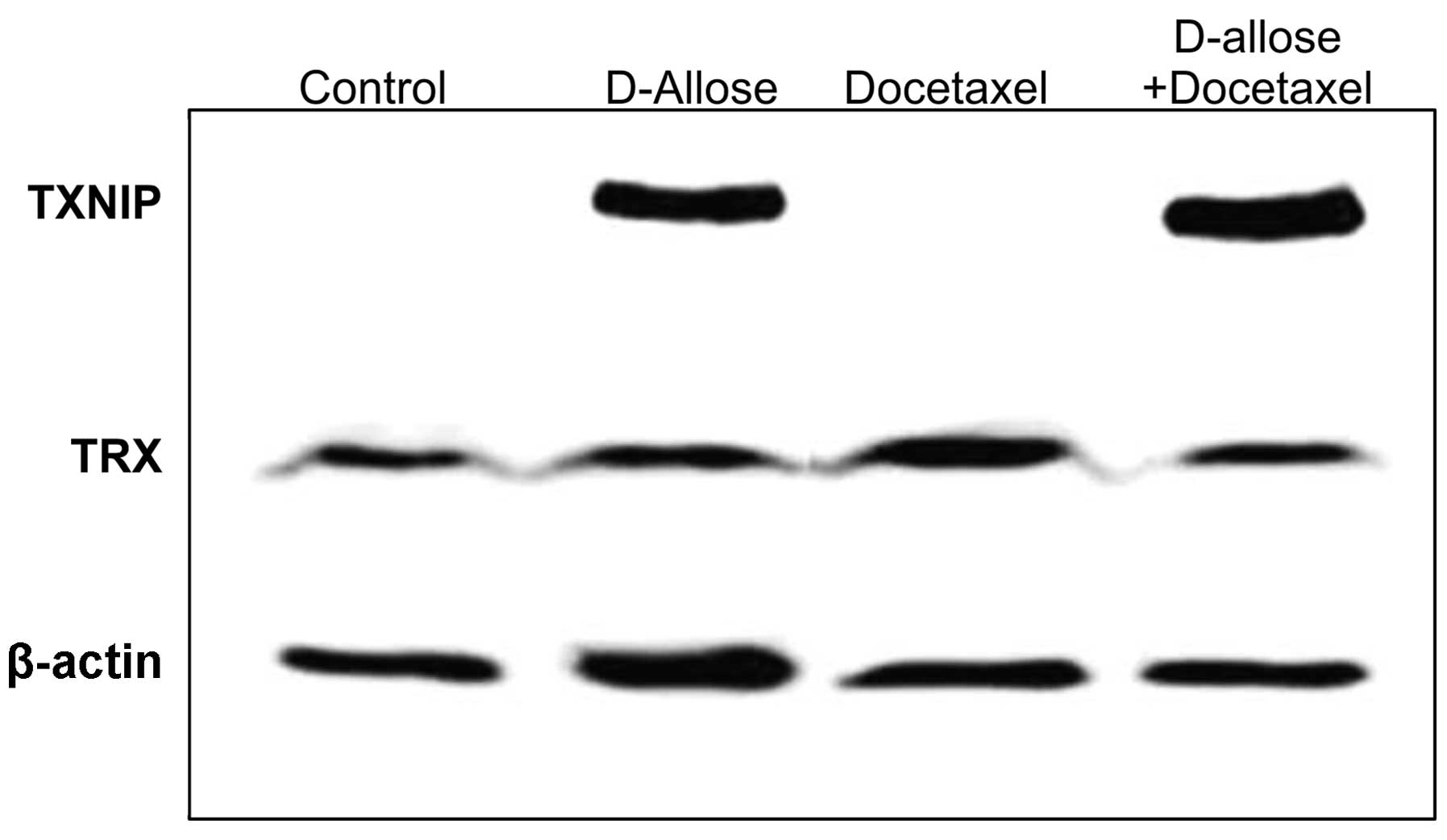
TXNIP and TRX protein levels were also evaluated by
western blot analysis (Fig. 6).
The expression of TNNIP was significantly increased by d-allose treatment, while
docetaxel had no effect on TXNIP expression. Although no apparent
changes were observed by docetaxel plus d-allose treatment, the protein
expression levels of TXNIP and TRX were comparable to the
expression levels of their corresponding genes.
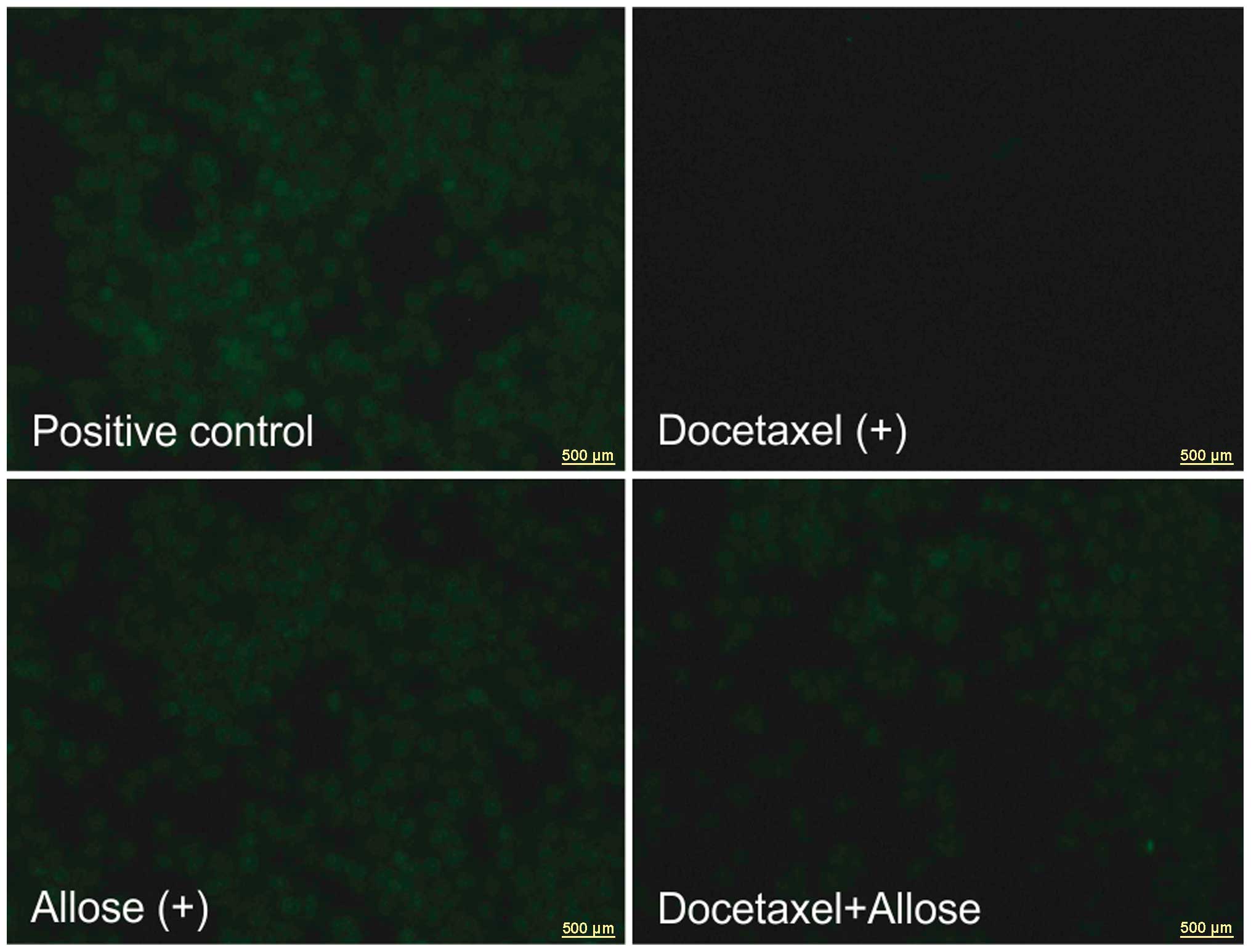
Effects of docetaxel and d-allose on ROS production
The intracellular ROS levels following treatment
with d-allose were
the same as those of the positive control. No excitation emission
was observed by exposure to docetaxel in the HSC3 cells. Compared
with docetaxel treatment alone, the addition of d-allose induced ROS generation
(Fig. 7).
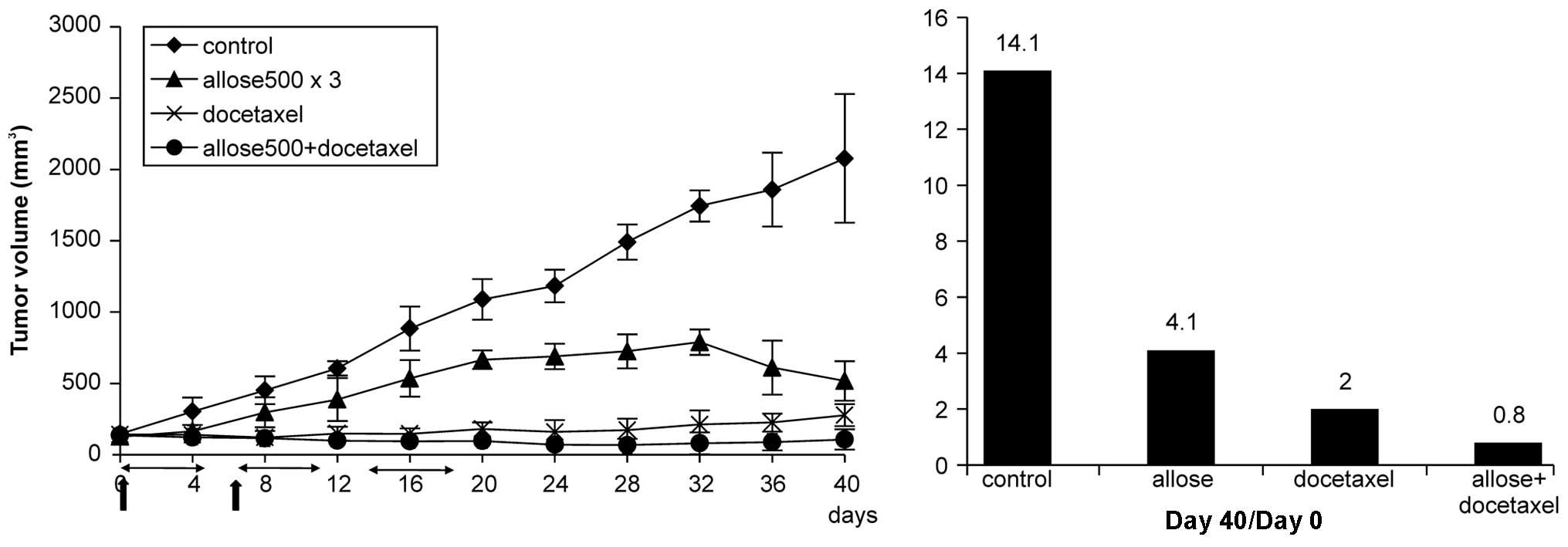
Effects of d-allose on cell proliferation in
vivo
Results of tumor growth assays in vivo are
presented in Fig. 8.
Administration of 500 mM d-allose for 3 weeks resulted in
a significant inhibition of tumor growth compared with that of the
control group at Day 20 (p<0.005). Moreover, docetaxel treatment
also strongly inhibited tumor growth, and combined treatment with
d-allose and
docetaxel markedly reduced tumor volumes compared to tumor volumes
at the beginning of the treatment. No significant tissue damage
(such as skin erythema or inflammation) was observed in the
treatment groups.
Discussion
Docetaxel is a common anticancer drug used in a
variety of cancers. In this study, we investigated whether
d-allose, a rare
sugar that possesses diverse biological effects in cells, could
enhance the anticancer effects of docetaxel in head and neck cancer
cells. Indeed, our results supported that combined treatment with
d-allose plus
docetaxel inhibited cell growth and survival to a greater extent
than treatment with either compound alone.
The results of the present study showed that
docetaxel treatment induced G2/M-phase cell cycle arrest
and enhanced activation of the apoptotic pathway in head and neck
cancer cells. Stabilization of microtubules by taxanes results in
phosphorylation and inactivation of Bcl-2, leading to increases in
Bax levels and a consequent increase in apoptosis (24). Naha et al (25) reported that d-allose induces apoptosis by
altering the expression of Bcl-2/Bax. In a previous study and in
the present study, we clarified that d-allose modulates cell cycle
regulatory proteins, G2/M cell cycle arrest and
apoptosis. These results suggested that the induction of
G2/M cell arrest and enhancement of the apoptotic
pathway by combined treatment of docetaxel plus d-allose promoted the inhibition
of cell growth.
TRX expression has been shown to be increased after
docetaxel therapy and is thought to protect cells against docetaxel
(14). Therefore, tumors showing
increased TRX expression in response to docetaxel are expected to
be more resistant to docetaxel than those showing no increase in
TRX. Consistent with this, we observed an increase in TRX
mRNA expression following docetaxel treatment, without an apparent
increase in the generation of ROS. Thus, cancer cells may prevent
ROS generation by upregulation of TRX expression. On the other
hand, combined use of d-allose and docetaxel resulted
in upregulation of TXNIP expression and downregulation of TRX
expression compared to treatment with docetaxel alone. These
results suggested that induction of TXNIP and suppression in TRX
following d-allose
treatment may prevent HSC3 cells from becoming resistant to
docetaxel.
The radiosensitizing potential of docetaxel has been
explored in vitro, in vivo and in clinical trials
(5,6,26,27).
However, several reports have shown that docetaxel resistance
depends on redox regulation, as mediated by increased expression of
TRX and a reduction in ROS generation (28). Khan and Ludueña (29) have shown that TRX can reduce a
disulfide bridge within the tubulin dimer and inhibit microtubule
assembly in vitro. These antioxidant molecules are also
thought to contribute to radiation resistance (30–33).
Therefore, regulation of the redox state is one of the key
mechanisms maintaining radiosensitivity. Recently, we demonstrated
that the induction of TXNIP by d-allose can enhance the effects
of radiation by increasing both the intracellular ROS level and
radiation-induced apoptosis (17).
In addition, if d-allose inhibits the attenuation
of docetaxel toxicity in cancer cells, we can expect to observe
highly enhanced effects by a 3-drug combined therapy.
Docetaxel should be administered 24 h before
irradiation to achieve optimal enhancement of the effects of
radiation (27). This is because
accumulation in the radiosensitive phase of the cell cycle, i.e.,
the G2/M phase, is most likely observed after 24 h with
docetaxel administration. However, one report demonstrated that
preradiation in head and neck cancer cells significantly enhanced
docetaxel cytotoxicity by arresting cells in the S phase (34). They concluded that irradiation
followed by docetaxel may be the most effective sequence for head
and neck cancer therapy. On the other hand, overexpression of TXNIP
occurs at 6 h after d-allose treatment and persists
for 24–48 h after treatment (35).
Further studies are needed to clarify the most effective sequence
of combined treatment with docetaxel, d-allose and irradiation.
In the present study, our in vivo experiment
revealed that 500 mM d-allose injection for 3 weeks
prolonged the tumor-suppressive effect after d-allose treatment was completed.
In our previous study, tumor regrowth was observed after the
completion of d-allose administration for 2
weeks (36). These results
suggested that the dosing period may be more important than the
application of high doses of d-allose. Tumor volumes in the
docetaxel treatment group doubled at 40 days after the initiation
of treatment, while those in the control group grew up to 14 times
their original size. Moreover, tumor volumes in the combined
treatment group were markedly smaller than those at the initiation
of treatment. These results suggested that d-allose treatment enhanced the
anticancer effects of docetaxel and may reduce the side effects of
the chemotherapeutic drug by reducing the total dose of docetaxel
required. Major toxicities of docetaxel are neutropenia, mucositis,
peripheral neuropathy and pulmonary disorders (37). Concurrent radiation therapy may
increase docetaxel toxicity. In particular, radiation-induced
mucositis can result in interruption of radiation therapy. However,
some reports have shown that d-allose protects the retina and
neurons against ischemia-induced damage by attenuating oxidative
stress (38,39). It is unknown whether such
contradictory responses occur in normal tissue and malignant
tumors. However, combined treatment with d-allose may be helpful to
prevent radiation-induced mucositis if d-allose suppresses oxidative
stress in normal mucosa surrounding the tumor. The mechanism of the
redox regulation in normal mucosa by d-allose remains to be
elucidated. Further studies are needed to evaluate whether
d-allose acts as an
antioxidant to protect against radiation and anticancer drugs in
normal mucosa.
In conclusion, d-allose enhanced the anticancer
effects of docetaxel by inducing changes in the cell cycle and
stimulation of apoptotic pathways. Control of the redox state by
d-allose may
strengthen the radiosensitivity of docetaxel.
Acknowledgements
This study was supported in part by a Grant-in-Aid
for General Scientific Research (Grant 20592019) from the Ministry
of Education, Culture, Sports, Science and Technology, Japan.
References
|
1
|
Schiff PB and Horwitz SB: Taxol stabilizes
microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA.
77:1561–1565. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Catimel G, Verweij J, Mattijssen V,
Hanauske A, Piccart M, Wanders J, Franklin H, Le Bail N, Clavel M
and Kaye SB: Docetaxel (taxotere): an active drug for the treatment
of patients with advanced squamous cell carcinoma of the head and
neck. Ann Oncol. 5:533–537. 1994.PubMed/NCBI
|
|
3
|
Dreyfuss AI, Clark JR, Norris CM, Rossi
RM, Lucarini JW, Busse PM, Poulin MD, Thornhill L, Costello R and
Posner MR: Docetaxel: an active drug for squamous cell carcinoma of
the head and neck. J Clin Oncol. 14:1672–1678. 1996.PubMed/NCBI
|
|
4
|
Baur M, Kienzer HR, Schweiger J, DeSantis
M, Gerber E, Pont J, Hudec M, Schratter-Sehn AU, Wicke W and
Dittrich C: Docetaxel/cisplatin as first-line chemotherapy in
patients with head and neck carcinoma: a phase II trial. Cancer.
94:2953–29582. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tishler RB, Posner MR, Norris CM Jr,
Mahadevan A, Sullivan C, Goguen L, Wirth LJ, Costello R, Case M,
Stowell S, Sammartino D, Busse PM and Haddad RI: Concurrent weekly
docetaxel and concomitant boost radiation therapy in the treatment
of locally advanced squamous cell cancer of the head and neck. Int
J Radiat Oncol Biol Phys. 15:1036–1044. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Katori H, Tsukuda M and Watai K:
Comparison of hyper-fractionation and conventional fractionation
radiotherapy with concurrent docetaxel, cisplatin and
5-fluorouracil (TPF) chemotherapy in patients with locally advanced
squamous cell carcinoma of the head and neck (SCCHN). Cancer
Chemother Pharmacol. 60:399–406. 2007. View Article : Google Scholar
|
|
7
|
Lorch JH, Goloubeva O, Haddad RI, Cullen
K, Sarlis N, Tishler R, Tan M, Fasciano J, Sammartino DE and Posner
MR; TAX 324 Study Group. Induction chemotherapy with cisplatin and
fluorouracil alone or in combination with docetaxel in locally
advanced squamous-cell cancer of the head and neck: long-term
results of the TAX 324 randomised phase 3 trial. Lancet Oncol.
12:153–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwao-Koizumi K, Matoba R, Ueno N, Kim SJ,
Ando A, Miyoshi Y, Maeda E, Noguchi S and Kato K: Prediction of
docetaxel response in human breast cancer by gene expression
profiling. J Clin Oncol. 20:422–431. 2005.PubMed/NCBI
|
|
9
|
Holmgren A: Thioredoxin. Annu Rev Biochem.
54:237–271. 1985. View Article : Google Scholar
|
|
10
|
Miyazaki K, Noda N, Okada S, Hagiwara Y,
Miyata M, Sakurabayashi I, Yamaguchi N, Sugimura T, Terada M and
Wakasugi H: Elevated serum level of thioredoxin in patients with
hepatocellular carcinoma. Biotherapy. 11:277–288. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura H, Bai J, Nishinaka Y, Ueda S,
Sasada T, Ohshio G, Imamura M, Takabayashi A, Yamaoka Y and Yodoi
J: Expression of thioredoxin and glutaredoxin, redox-regulating
proteins, in pancreatic cancer. Cancer Detect Prev. 24:53–60.
2000.PubMed/NCBI
|
|
12
|
Grogan TM, Fenoglio-Prieser C, Zeheb R,
Bellamy W, Frutiger Y, Vela E, Stemmerman G, Macdonald J, Richter
L, Gallegos A and Powis G: Thioredoxin, a putative oncogene
product, is overexpressed in gastric carcinoma and associated with
increased proliferation and increased cell survival. Hum Pathol.
31:475–481. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kakolyris S, Giatromanolaki A, Koukourakis
M, Powis G, Souglakos J, Sivridis E, Georgoulias V, Gatter KC and
Harris AL: Thioredoxin expression is associated with lymph node
status and prognosis in early operable non-small cell lung cancer.
Clin Cancer Res. 7:3087–3091. 2001.PubMed/NCBI
|
|
14
|
Raffel J, Bhattacharyya AK, Gallegos A,
Cui H, Einspahr JG, Alberts DS and Powis G: Increased expression of
thioredoxin-1 in human colorectal cancer is associated with
decreased patient survival. J Lab Clin Med. 142:46–51. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim SJ, Miyoshi Y, Taguchi T, Tamaki Y,
Nakamura H, Yodoi J, Kato K and Noguchi S: High thioredoxin
expression is associated with resistance to docetaxel in primary
breast cancer. Clin Cancer Res. 11:8425–8430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mitani T, Hoshikawa H, Mori T, Hosokawa T,
Tsukamoto I, Yamaguchi F, Kamitori K, Tokuda M and Mori N: Growth
inhibition of head and neck carcinomas by D-allose. Head Neck.
31:1049–1055. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hoshikawa H, Indo K, Mori T and Mori N:
Mori, enhancement of the radiation effects by D-allose in head and
neck cancer cells. Cancer Lett. 306:60–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chung JW, Jeon JH, Yoon SR and Choi I:
Vitamin D3 upregulated protein 1 (VDUP1) is a regulator for redox
signaling and stress-mediated diseases. J Dermatol. 33:662–669.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han SH, Jeon JH, Ju HR, Jung U, Kim KY,
Yoo HS, Lee YH, Song KS, Hwang HM, Na YS, Yang Y, Lee KN and Choi
I: VDUP1 upregulated by TGF-beta1 and 1,25-dihydorxyvitamin D3
inhibits tumor cell growth by blocking cell-cycle progression.
Oncogene. 22:4035–4046. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohta S, Lai EW, Pang AL, Brouwers FM, Chan
WY, Eisenhofer G, de Krijger R, Ksinantova L, Breza J, Blazicek P,
Kvetnansky R, Wesley RA and Pacak K: Downregulation of metastasis
suppressor genes in malignant pheochromocytoma. Int J Cancer.
114:139–143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Butler LM, Zhou X, Xu WS, Scher HI,
Rifkind RA, Marks PA and Richon VM: The histone deacetylase
inhibitor SAHA arrests cancer cell growth, up-regulates
thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc
Natl Acad Sci USA. 99:11700–11705. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ikarashi M, Takahashi Y, Ishii Y, Nagata
T, Asai S and Ishikawa K: Vitamin D3 up-regulated protein 1 (VDUPI)
expression in gastrointestinal cancer and its relation to stage of
disease. Anticancer Res. 22:4045–4048. 2002.PubMed/NCBI
|
|
23
|
de Vos S, Hofmann WK, Grogan TM, Krug U,
Schrage M, Miller TP, Braun JG, Wachsman W, Koeffler HP and Said
JW: Gene expression profile of serial samples of transformed B-cell
lymphomas. Lab Invest. 83:271–285. 2003.PubMed/NCBI
|
|
24
|
Haldar S, Basu A and Croce CM: Bcl2 is the
guardian of microtubule integrity. Cancer Res. 57:229–233.
1997.PubMed/NCBI
|
|
25
|
Naha N, Lee HY, Jo MJ, Chung BC, Kim SH
and Kim MO: Rare sugar D-allose induces programmed cell death in
hormone refractory prostate cancer cells. Apoptosis. 13:1121–1134.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pradier O, Rave-Fränk M, Lehmann J, Lücke
E, Boghun O, Hess CF and Schmidberger H: Effects of docetaxel in
combination with radiation on human head and neck cancer cells
(ZMK-1) and cervical squamous cell carcinoma cells (CaSki). Int J
Cancer. 91:840–845. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mason KA, Kishi K, Hunter N, Buchmiller L,
Akimoto T, Komaki R and Milas L: Effect of docetaxel on the
therapeutic ratio of fractionated radiotherapy in vivo. Clin Cancer
Res. 5:4191–4198. 1999.PubMed/NCBI
|
|
28
|
Mizumachi T, Suzuki S, Naito A,
Carcel-Trullols J, Evans TT, Spring PM, Oridate N, Furuta Y, Fukuda
S and Higuchi M: Increased mitochondrial DNA induces acquired
docetaxel resistance in head and neck cancer cells. Oncogene.
27:831–838. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khan IA and Ludueña RF: Possible
regulation of the in vitro assembly of bovine brain tubulin by the
bovine thioredoxin system. Biochim Biophys Acta. 1076:289–297.
1991. View Article : Google Scholar
|
|
30
|
Hirose K, Longo DL, Oppenheim JJ and
Matsushima K: Overexpression of mitochondrial manganese superoxide
dismutase promotes the survival of tumor cells exposed to
interleukin-1, tumor necrosis factor, selected anticancer drugs,
and ionizing radiation. FASEB J. 7:361–368. 1993.
|
|
31
|
Lee HC, Kim DW, Jung KY, Park IC, Park MJ,
Kim MS, Woo SH, Rhee CH, Yoo H, Lee SH and Hong SI: Increased
expression of antioxidant enzymes in radioresistant variant from
U251 human glioblastoma cell line. Int J Mol Med. 13:883–887.
2004.PubMed/NCBI
|
|
32
|
Mirkovic N, Voehringer DW, Story MD,
McConkey DJ, McDonnell TJ and Meyn RE: Resistance to
radiation-induced apoptosis in Bcl-2-expressing cells is reversed
by depleting cellular thiols. Oncogene. 15:1461–1470. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun J, Chen Y, Li M and Ge Z: Role of
antioxidant enzymes on ionizing radiation resistance. Free Rad Biol
Med. 24:586–593. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Furuse S, Adachi M, Ijichi K, Ohta S,
Torigoe S, Nakazawa M, Miura S, Mitsudo K and Tohnai I:
Pre-radiation enhances the cytotoxicity of docetaxel in head and
neck squamous cell carcinoma cells. Oncol Rep. 23:1339–1343.
2010.PubMed/NCBI
|
|
35
|
Yamaguchi F, Takata M, Kamitori K, Nonaka
M, Dong Y, Sui L and Tokuda M: Rare sugar D-allose induces specific
up-regulation of TXNIP and subsequent G1 cell cycle arrest in
hepatocellular carcinoma cells by stabilization of p27kip1. Int J
Oncol. 32:377–385. 2008.PubMed/NCBI
|
|
36
|
Hoshikawa H, Mori T and Mori N: In vitro
and in vivo effects of D-allose: up-regulation of
thioredoxin-interacting protein in head and neck cancer cells. Ann
Otol Rhinol Laryngol. 119:567–571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Adachi I, Watanabe T, Takashima S,
Narabayashi M, Horikoshi N, Aoyama H and Taguchi T: A late phase II
study of RP56976 (docetaxel) in patients with advanced or recurrent
breast cancer. Br J Cancer. 73:210–216. 1996. View Article : Google Scholar
|
|
38
|
Hirooka K, Miyamoto O, Jinming P, Du Y,
Itano T, Baba T, Tokuda M and Shiraga F: Neuroprotective effects of
D-allose against retinal ischemia-reperfusion injury. Invest
Ophthalmol Vis Sci. 47:1653–1657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mizote M, Hirooka K, Fukuda K, Nakamura T,
Itano T and Shiraga F: D-allose as ischemic retina injury inhibitor
during rabbit vitrectomy. Jpn J Ophthalmol. 55:294–300. 2011.
View Article : Google Scholar : PubMed/NCBI
|