Introduction
The extracellular matrix and integrins, their
cellular receptors, play key roles in tumor progression and tumor
angiogenesis, and blocking antibodies against αvβ3, αvβ5 and α5β1
are currently under development as potential cancer therapies
(1,2). The α6 integrin subunit is expressed
by endothelial cells, platelets, monocytes/macrophages,
neutrophils, epithelial cells and Schwann cells (3–6). It
can associate with β1 or β4 subunits to form receptors for
laminins, which are major components of the extracellular matrix
and endothelial basement membrane (7). A role of α6 integrin subunit has been
demonstrated in the progression of several malignancies such as
breast cancer, prostate cancer, glioblastoma and pancreatic cancer.
α6 integrin overexpression or de novo expression by tumor
cells (depending on the type of cancer) is associated with a poor
prognosis. Indeed, α6 expression increases cell motility and
adhesion, which confers invasive properties to tumor cells
(8–12). α6 is also strongly expressed on
endothelial cells, and its expression is enhanced by proangiogenic
growth factors such as VEGF and FGF2 (13–17).
We have previously shown that α6 is involved in endothelial cell
adhesion, migration, pseudotube formation and post-ischemic
vascular repair (13,14,18,19).
Moreover, α6 binds laminin 411 (laminin 8) and laminin 511 (laminin
10) (7), expression of which is
upregulated in the basement membrane of tumor blood vessels in
invasive brain (20) and breast
(21) carcinomas. α6 targeting
could potentially have therapeutic benefits by disrupting tumor
angiogenesis and growth, but the role of α6 in tumor angiogenesis
is controversial. In a breast carcinoma xenograft model
(MDA-MB-231), Lee et al (16) found that an anti-α6 blocking
antibody reduced tumor volume, tumor weight and blood vessel
abundance. However, these effects might have resulted from either
an antitumoral effect or anti-angiogenic activity or both effects.
To resolve this issue, Primo et al (17) used Rip-Tag2 mice, which
spontaneously develop pancreatic tumors that do not express α6.
They found that α6 expression on tumor blood vessels was increased
during the angiogenic stage and that administration of an anti-α6
blocking antibody reduced tumor vascularization. However, other
authors obtained opposite results with a genetically manipulated
mouse model. Germain et al (22) generated mice in which α6 gene
deletion was restricted to endothelial cells, by using the cre-lox
system under the control of the Tie1 promoter. However,
Tie1-dependent α6 deletion was counterbalanced by VEGFR2
overexpression, resulting in enhanced tumor growth and
angiogenesis. Further investigation was thus required to clarify
the role for α6 in tumor angiogenesis.
To understand the discrepancy in the results
reported in the different studies cited above we generated a mouse
model in which the gene coding for α6 was deleted by using the
cre-lox system under the control of the Tie2 promoter, leading to
α6 gene deletion only in Tie2-lineage cells (endothelial cells,
subsets of hematopoietic stem cells, pericytes and
monocytes/macrophages). We investigated the effect of this
Tie2-dependent α6 gene deletion on B16F10 melanoma growth, tumor
angiogenesis, macrophage infiltration and pericyte coverage by
comparing α6fl/fl-Tie2Cre+ and
α6fl/fl-Tie2Cre− mice.
Materials and methods
Animals
We generated α6 floxed mice [Itga6tm2Egl,
MGI:4439081] as previously described and bred them with mice
expressing Cre recombinase under the control of the Tie2 promoter
[B6.Cg-Tg(Tek-cre)12Flv/J], purchased from Jackson Laboratory (Bar
Harbor, ME, USA), in order to generate α6fl/fl-Tie2Cre+
and α6fl/fl-Tie2Cre− mice (on a C57BL/6 background)
(19). All the protocols were
approved by the Regional Ethics Committee on Animal Experimentation
(protocol CEEA34.CB.041.11) and all the experiments complied with
European Parliament Directive 2010/63/EU. The adequacy of
anesthesia was confirmed by the lack of the tail pinch
response.
Tumor growth assay
One million B16F10 melanoma cells (syngeneic to
C57BL/6 mice) were suspended in 100 μl of PBS and injected
subcutaneously in the right flank of 8-week-old
α6fl/fl-Tie2Cre+ and α6fl/fl-Tie2Cre− male
mice. Tumor growth was quantified by Vernier caliper measurements
every 2 or 3 days. Tumor volume was calculated as 0.5 × length ×
width2. At the end of the experiment the mice were
anesthetized with a single intraperitoneal injection of ketamine
(80 mg/kg) and xylazine (16 mg/kg), then sacrificed by cervical
dislocation. Tumors were harvested, weighed and frozen in
isopentane solution cooled in liquid nitrogen before being stored
at −80°C until immunohistological analysis. Two different
independent experiments were performed. For one experiment all the
tumors were harvested at Day 12 (n=7 to 9 mice/genotype), and for
another experiment the tumors were harvested at different time
points in order to analyze size-matched tumors (n=10 per
genotype).
Immunohistological tumor analysis
For all immunofluorescence experiments, frozen
10-μm-thick sections of tumors from α6fl/fl-Tie2Cre+ and
α6fl/fl-Tie2Cre− mice were fixed in ice-cold acetone for
10 min, stained as described below and examined by an observer in a
blinded manner using a confocal microscope (TCS SP2, Leica,
Wetzlar, Germany).
Study of α6 integrin subunit
co-expression with laminin chains α4 and α5
Tumor sections were incubated for 1 h with the
following primary antibodies: rabbit anti-mouse laminin α4
(23), rabbit anti-mouse laminin
α5 (24) (both generous gifts from
Sorokin LM, University of Muenster, Germany) or rat anti-human α6
(clone GoH3, BD Biosciences, Franklin Lakes, NJ, USA), then further
incubated for 1 h with the following secondary antibodies: goat
anti-rabbit Alexa555 (Invitrogen, Carlsbad, CA, USA) or goat
anti-rat FITC (Abcam, Cambridge, MA, USA). Nuclei were stained with
TOPRO3-Iodide (Thermo Fischer Scientific, Waltham, MA, USA).
Analysis of tumor vascularization
Tumor sections were incubated for 1 h with a rat
anti-mouse CD31 monoclonal antibody (clone MEC 13.3, BD
Biosciences), then with a goat anti-rat secondary antibody coupled
to FITC (Abcam). Ten fields were examined per section. The vessel
surface area, the number of vessels and vessel diameter were
quantified with Histolab™ software (Microvision Instruments, Evry,
France). Results were expressed as the vessel surface area (%), the
number of vessels/mm2 and mean vessel diameter.
Quantification of Tie2-expressing
macrophages
Tumor sections were sequentially incubated with the
following antibodies: rat anti-mouse F4/80 (Abd Serotec,
Düsseldorf, Germany), goat anti-rat FITC, biotinylated rat
anti-mouse Tie2 (eBiosciences, San Diego, CA, USA) and
streptavidin-Alexa 555 (Invitrogen). Ten fields were examined per
section and the numbers of Tie2-expressing macrophages (TEMs)
(F4/80+ Tie2+) and total macrophages
(F4/80+) were determined with Image J and Histolab™
(Microvision) software. Results were expressed as the number of
TEMs and total macrophages/mm2 and as the percentage of
TEMs in the total macrophage population.
Analysis of pericyte coverage
Tumor sections were incubated with the following
primary antibodies: biotinylated mouse anti-smooth muscle actin
(αSMA) (Clone 1A4, Thermo Fischer Scientific), rat anti-mouse CD31
(clone mec13.3, BD Biosciences), and then with streptavidin Cy3
(Amersham, Little Chalfont, UK) and goat anti-rat Alexa 488
(Invitrogen). Ten fields were examined per section. The number of
blood vessels positive for αSMA, and the surface areas stained for
CD31 (endothelial cells) and αSMA (pericytes) were determined with
Image J software. Results were expressed as a percentage of blood
vessels positive for αSMA and as a ratio of αSMA/CD31 surface
areas.
Blood sampling and analysis of monocyte
α6 expression
Eight-week-old α6fl/fl-Tie2Cre+ and
α6fl/fl-Tie2Cre− mice were anesthetized with a single
intraperitoneal injection of ketamine (80 mg/kg) and xylazine (16
mg/kg). Peripheral blood was collected by cardiac puncture and the
mice were sacrificed by cervical dislocation. Peripheral blood
samples were incubated with CD45-PerCP and CD49f-PE (clone GoH3) or
isotype-matched irrelevant antibodies (BD Biosciences). After a
lysis step (FACS lysing solution, BD Biosciences), monocyte α6
expression was analyzed on a FACS Calibur flow cytometer using Cell
Quest Pro software (BD Biosciences). A SSC/CD45-PerCP dot plot was
used for monocyte gating. Results were expressed as the percentage
of monocytes positive or negative for α6 expression among the total
monocyte population.
Statistical analysis
Results are expressed as means ± SEM. Data were
analyzed using ANOVA followed by Fisher’s PLSD post hoc test
implemented with StatView software (SAS, Cary, NC, USA).
Significance was assumed at P<0.05.
Results
Tie2-dependent deletion of α6 integrin
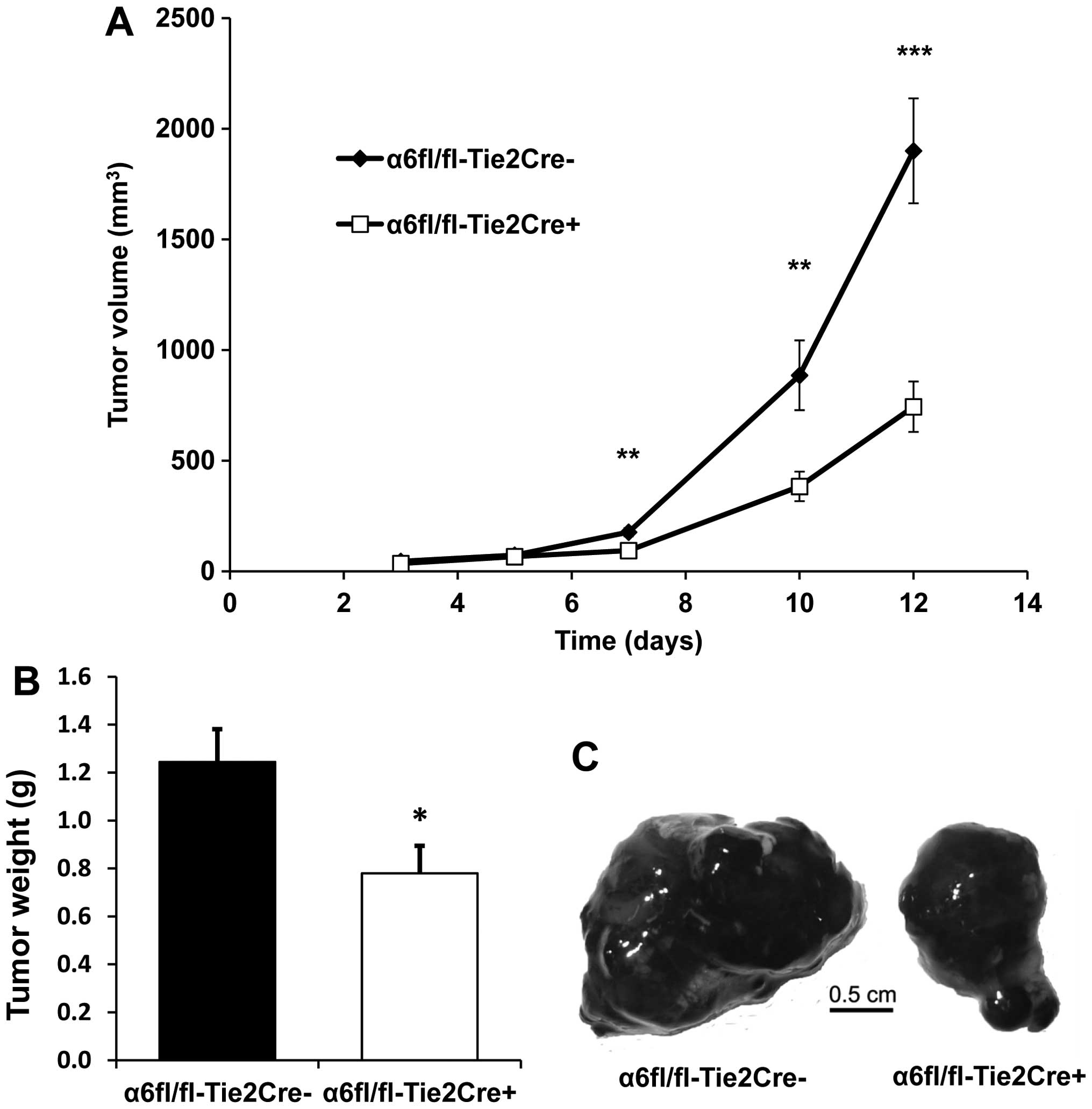
subunit reduces tumor growth
The growth of subcutaneous B16F10 tumors was
significantly reduced in α6fl/fl-Tie2Cre+ mice (n=9)
compared to α6fl/fl-Tie2Cre− mice (n=7): tumor volume
was reduced by 56% at Day 10 (P<0.01) and by 60% at Day 12
(P<0.001) (Fig. 1A), while
tumor weight was reduced by 37% at Day 12 (P<0.05) (Fig. 1B).
Tie2-dependent deletion of α6 integrin
subunit reduces tumor angiogenesis
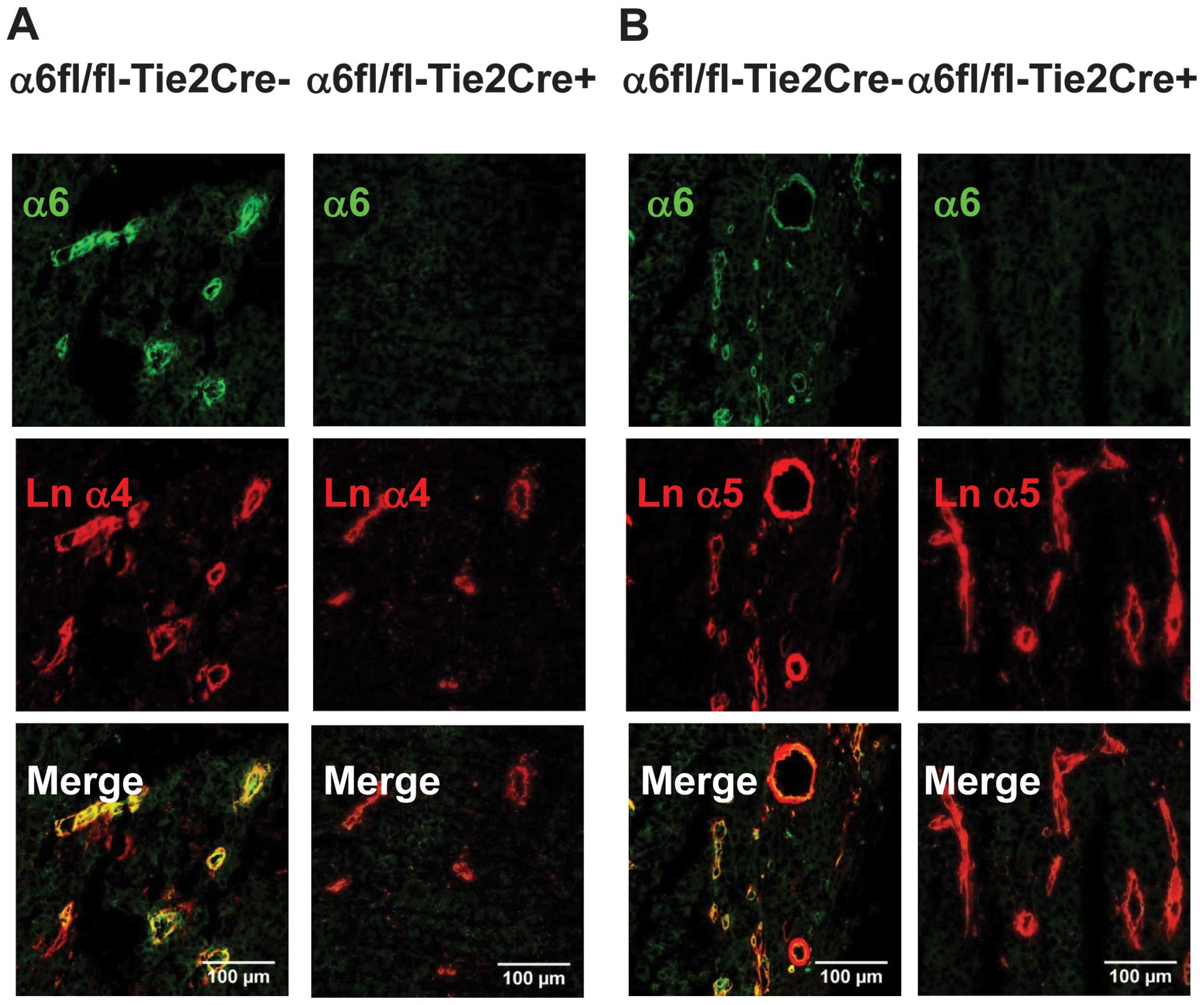
Immunofluorescence experiments showed that α6 was
expressed on tumor blood vessels from
α6fl/fl-Tie2Cre−mice and was associated with laminin α4
and α5 chains. Endothelial α6 deletion was complete in
α6fl/fl-Tie2Cre+ mice (Fig.
2).
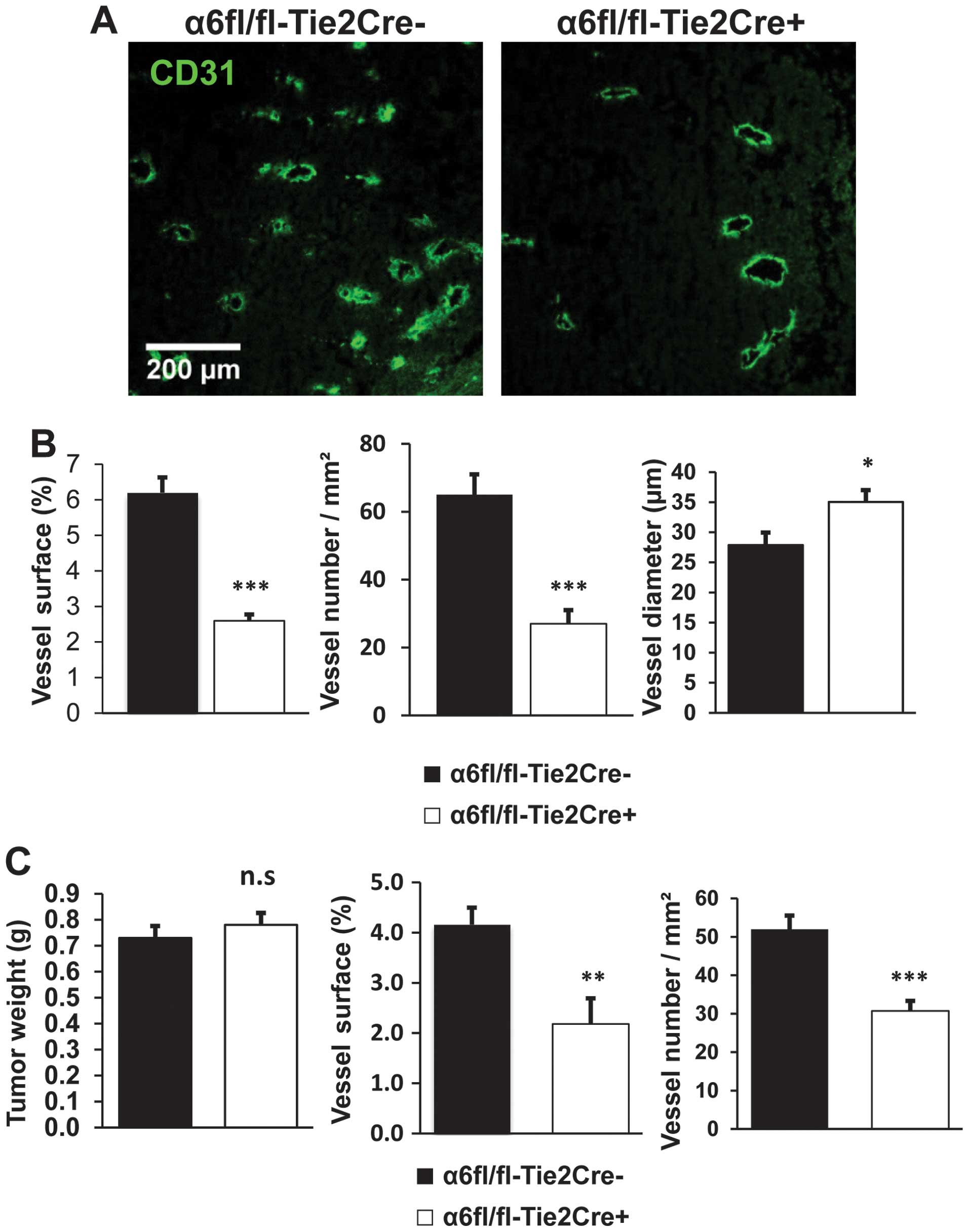
For tumors harvested at Day 12, vessel density was
reduced in tumors from α6fl/fl-Tie2Cre+ mice (n=9)
compared to α6fl/fl-Tie2Cre− mice (n=7): both vessel
number/mm2 and vessel surface area were reduced by 58%
(P<0.001). The average diameter of tumor blood vessels was
significantly larger in α6fl/fl-Tie2Cre+ mice than in
α6fl/fl-Tie2Cre− mice (P<0.05) (Fig. 3A and B).
To rule out an effect of tumor size on angiogenesis
we also analyzed tumor vascularization in size-matched tumors from
α6fl/fl-Tie2Cre+ and α6fl/fl-Tie2Cre− mice,
harvested at varying time points. Vessel density was reduced in
tumors from α6fl/fl-Tie2Cre+ mice (n=10) compared to
α6fl/fl-Tie2Cre−mice (n=10): the number of
vessels/mm2 was reduced by 41% (P<0.001) and vessel
surface area by 48% (P<0.01) (Fig.
3C).
Tie2-dependent deletion of α6 integrin
subunit reduces infiltration by Tie2-expressing macrophages
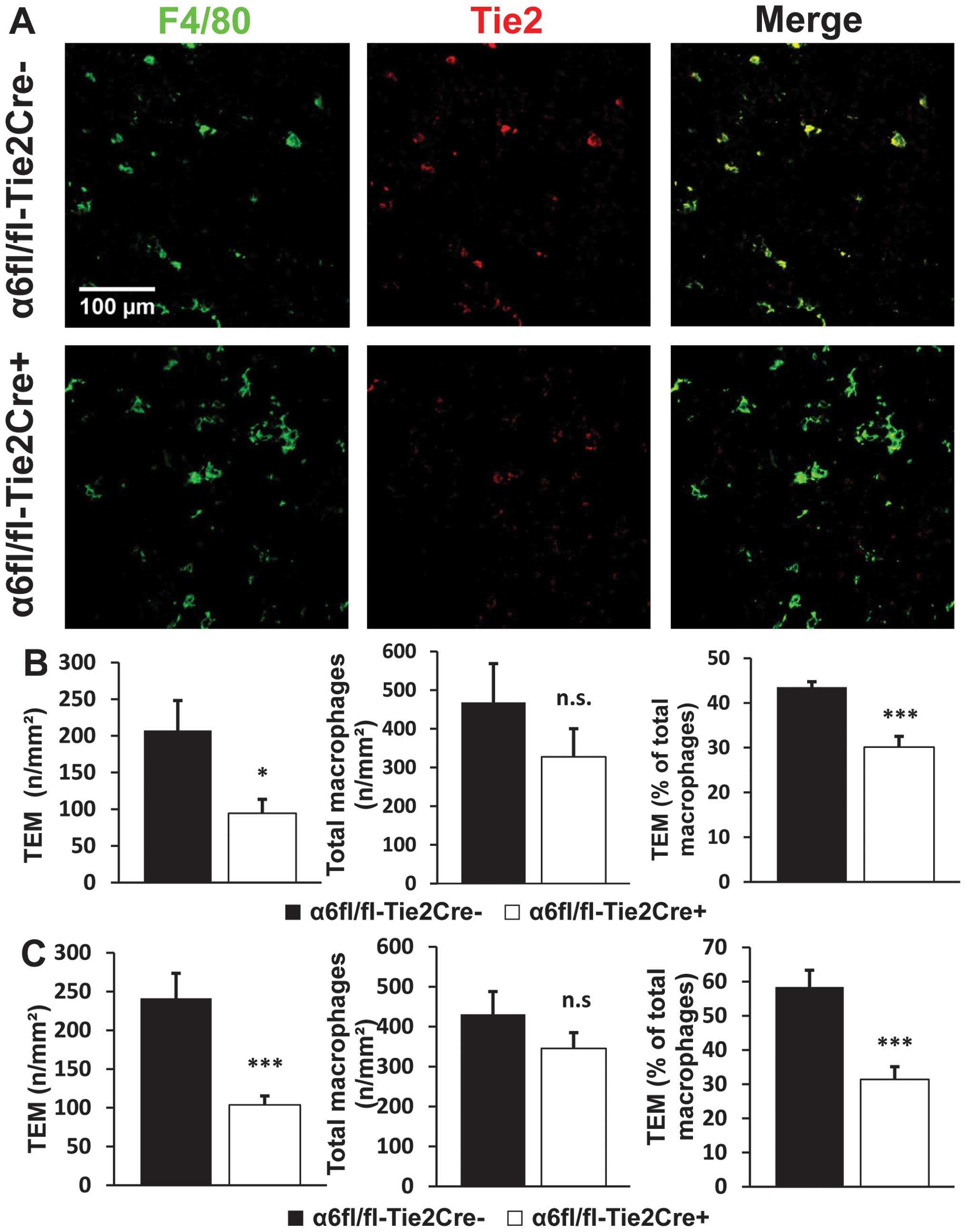
For tumors harvested at Day 12, the number of TEMs
per square millimeter was reduced by 55% in tumors from
α6fl/fl-Tie2Cre+ mice (n=9) compared to
α6fl/fl-Tie2Cre− mice (n=7) (P<0.05). Tie2-dependent
deletion of α6 also led to a 32% reduction in the percentage of
TEMs among the total macrophage population (P<0.001). The total
number of macrophages in tumors from α6fl/fl-Tie2Cre+
mice was also reduced, but the difference with
α6fl/fl-Tie2Cre− mice was not statistically significant
(Fig. 4A and B). The analysis of
size-matched tumors (n=10 mice/genotype) led to the same results
with no significant difference observed between size-matched tumors
and tumors harvested at Day 12 (Fig.
4C).
Tie2-dependent deletion of α6 integrin
subunit on peripheral blood monocytes
The proportion of peripheral blood monocytes
positive for α6 was 52.5±7.6% in α6fl/fl-Tie2Cre− mice
and only 1.85±0.60% in α6fl/fl-Tie2Cre+ mice (mean ±
SEM, n=5 mice/genotype, P<0.01).
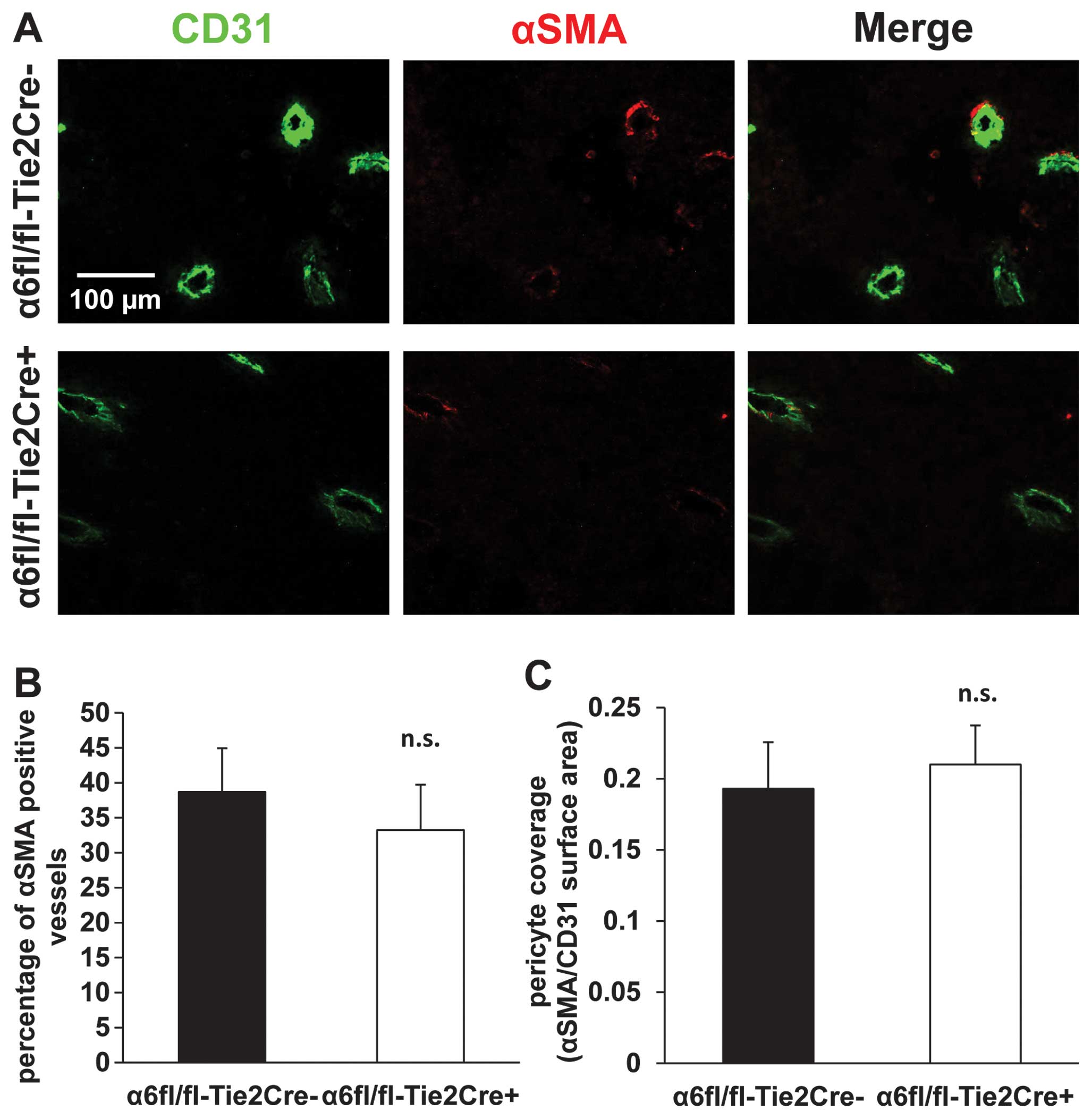
Tie2-dependent deletion of α6 integrin
subunit does not significantly change pericyte coverage of tumor
blood vessels
The percentage of blood vessels that were positive
for αSMA was 38.7±6.2% in α6fl/fl-Tie2Cre− mice (n=5)
and 33.25±6.5% in α6fl/fl-Tie2Cre+ mice (n=8) (mean ±
SEM) (Fig. 5A and B). The ratio of
αSMA/CD31 surface areas was 0.19±0.03 in
α6fl/fl-Tie2Cre− mice (n=5) and 0.21±0.03 in
α6fl/fl-Tie2Cre+ mice (n=8) (mean ± SEM) (Fig. 5A and C). There was no statistically
significant difference between the two genotypes.
Discussion
Tie2-dependent α6 deletion significantly reduced
tumor angiogenesis and, consequently, tumor growth in a B16F10
mouse melanoma model. The number of tumor blood vessels was
significantly lower in α6fl/fl-Tie2Cre+ mice than in
α6fl/fl-Tie2Cre− mice. This difference is not due to a
difference in tumor size as the analysis of size-matched tumors led
to the same conclusion. These latter results are in keeping with
those reported by Lee et al (16) and Primo et al (17), who used an anti-α6 blocking
antibody (GoH3), and also with our previous results on
post-ischemic angiogenesis (19).
Indeed, α6 is required for endothelial cell adhesion and migration
and for pseudotube formation in vitro (13,16,18).
In contrast, however, Germain et al (22) observed enhanced neovessel formation
in α6fl/fl-Tie1Cre+ mice compared to
α6fl/fl-Tie1Cre−mice. The most likely explanation for
these conflicting results is VEGFR2 overexpression on endothelial
cells from α6fl/fl-Tie1Cre+ mice compared to
α6fl/fl-Tie1Cre− mice, and the lack of this compensatory
mechanism in α6fl/fl-Tie2Cre+ mice. Indeed, we have
shown that the loss of α6 expression on endothelial cells isolated
from α6fl/fl-Tie2Cre+ was not counterbalanced by other
integrins or VEGFR2 overexpression (19). Another difference between the
Tie1Cre and Tie2Cre models is that Tie1 is restricted to
endothelial cells, whereas Tie2 is expressed on endothelial cells,
pericyte precursors of mesenchymal origin, subsets of hematopoietic
stem cells, and also a subset of monocytes/macrophages (25). Angiopoietin 2 release by
endothelial cells in tumors is upregulated by hypoxia, and its
receptor, Tie2, is strongly upregulated when monocytes are
recruited into the tumor and differentiate into perivascular
macrophages (26). These
Tie2-expressing macrophages (TEMs) are highly proangiogenic: they
secrete growth factors such as VEGF and matrix metalloproteinases
such as MMP9 and thereby promote neovessel formation in
endometriotic lesions and tumors (26–28).
TEM targeting might thus enhance anti-angiogenic therapy efficiency
(29–31). About 50% of peripheral blood
monocytes were positive for α6 in our α6fl/fl-Tie2Cre−
mice, whereas nearly all were negative for α6 in
α6fl/fl-Tie2Cre+ mice, suggesting that Tie2-dependent α6
deletion also occurred efficiently in monocytes. Consequently, α6
would also be deleted from TEMs in α6fl/fl-Tie2Cre+
mice, and we found that TEM tumor infiltration was significantly
reduced compared to α6fl/fl-Tie2Cre− mice. In
macrophages, α6 ligation on laminin triggers intracellular
phosphorylation and cytoskeleton rearrangement (6). This would explain why α6 deletion in
TEMs inhibits their infiltration, as TEM tumor infiltration
involves laminin interaction and transendothelial migration steps.
Administration of α6 blocking antibodies might also reduce TEM
infiltration, a possibility not investigated by Lee et al
(16) and Primo et al
(17).
Interestingly, we also found fewer microvessels in
tumors from α6fl/fl-Tie2Cre+ mice, resulting in a
slightly increased average vessel diameter. This could stem from a
decreased sprouting capacity, as we have previously found that
neovessel outgrowth from preexisting blood vessels in the ex
vivo aortic ring assay is reduced in
α6fl/fl-Tie2Cre+ mice (19). In addition, it has been
demonstrated using a three dimensional in vitro co-culture
model, that α6 expression increases on both endothelial cells and
pericytes when they are cultured together, and that the addition of
an anti-α6 blocking antibody leads to an increased vessel width in
pericyte-endothelial cell cocultures but not in endothelial cell
monocultures (32). This suggests
that α6 could play a role in pericyte-endothelial cell interactions
and therefore in vessel morphogenesis and maturation. However, we
did not find any significant genotype-dependent difference in the
pericyte coverage of the blood vessels of B16F10 tumors from
α6fl/fl-Tie2Cre+ mice compared to
α6fl/fl-Tie2Cre− mice, suggesting that pericyte
recruitment is not impaired in this model.
Endothelial progenitor cells (EPCs) can also
participate in tumor angiogenesis (33). We have previously shown that α6 is
involved in EPC mobilization from bone marrow after ischemia
(19). We used flow cytometry to
determine the number of circulating EPCs in the peripheral blood of
tumor-bearing mice, as previously described (19), and found no genotype-dependent
difference (data not shown). However, it is possible that EPC
recruitment to tumors may be reduced in α6fl/fl-Tie2Cre+
mice compared to α6fl/fl-Tie2Cre−, as we have previously
shown that α6 is required for EPC recruitment to ischemic tissues
(18).
This study highlights differences between Tie1Cre
and Tie2Cre conditional knockout models. Our results confirm that
α6 plays an important role in tumor growth and angiogenesis, by
promoting neovessel formation and tumor infiltration by
proangiogenic TEMs. Therapeutic targeting of α6 might affect the
invasive properties of tumor cells, endothelial cells and TEMs, and
could thereby reduce tumor growth and invasiveness.
Acknowledgements
This study would not have been possible without the
contribution of E.G.-L. who provided α6-floxed mice. Sadly, E.G.-L.
passed away prematurely on July 21, 2012. We thank Mevyn Nizard and
Eric Tartour for the generous gift of the B16F10 melanoma cell line
and Lydia Sorokin for the antibodies against laminin α4 and α5
chains. We thank the staff of the Institut Médicament, Toxicologie,
Chimie, Environnement animal facility and imaging platform for
their help and advice. Claire Bouvard was supported by grants from
Ministère de l’Enseignement Supérieur et de la Recherche and the
French Society of Haematology.
References
|
1
|
Avraamides CJ, Garmy-Susini B and Varner
JA: Integrins in angiogenesis and lymphangiogenesis. Nat Rev
Cancer. 8:604–617. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garmy-Susini B and Varner JA: Roles of
integrins in tumor angiogenesis and lymphangiogenesis. Lymphat Res
Biol. 6:155–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hogervorst F, Admiraal LG, Niessen C, et
al: Biochemical characterization and tissue distribution of the A
and B variants of the integrin alpha 6 subunit. J Cell Biol.
121:179–191. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Terpe HJ, Stark H, Ruiz P and Imhof BA:
Alpha 6 integrin distribution in human embryonic and adult tissues.
Histochemistry. 101:41–49. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sonnenberg A, Linders CJ, Daams JH and
Kennel SJ: The alpha 6 beta 1 (VLA-6) and alpha 6 beta 4 protein
complexes: tissue distribution and biochemical properties. J Cell
Sci. 96:207–217. 1990.PubMed/NCBI
|
|
6
|
Shaw LM, Messier JM and Mercurio AM: The
activation dependent adhesion of macrophages to laminin involves
cytoskeletal anchoring and phosphorylation of the alpha 6 beta 1
integrin. J Cell Biol. 110:2167–2174. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hallmann R, Horn N, Selg M, Wendler O,
Pausch F and Sorokin LM: Expression and function of laminins in the
embryonic and mature vasculature. Physiol Rev. 85:979–1000. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chung J and Mercurio AM: Contributions of
the alpha6 integrins to breast carcinoma survival and progression.
Mol Cells. 17:203–209. 2004.PubMed/NCBI
|
|
9
|
Zhu GH, Huang C, Qiu ZJ, et al: Expression
and prognostic significance of CD151, c-Met, and integrin
alpha3/alpha6 in pancreatic ductal adenocarcinoma. Dig Dis Sci.
56:1090–1098. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rabinovitz I, Nagle RB and Cress AE:
Integrin alpha 6 expression in human prostate carcinoma cells is
associated with a migratory and invasive phenotype in vitro and in
vivo. Clin Exp Metastasis. 13:481–491. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Delamarre E, Taboubi S, Mathieu S, et al:
Expression of integrin alpha6beta1 enhances tumorigenesis in glioma
cells. Am J Pathol. 175:844–855. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Colomiere M, Findlay J, Ackland L and
Ahmed N: Epidermal growth factor-induced ovarian carcinoma cell
migration is associated with JAK2/STAT3 signals and changes in the
abundance and localization of α6β1 integrin. Int J Biochem Cell
Biol. 41:1034–1045. 2009.PubMed/NCBI
|
|
13
|
Chabut D, Fischer AM, Colliec-Jouault S,
et al: Low molecular weight fucoidan and heparin enhance the basic
fibroblast growth factor-induced tube formation of endothelial
cells through heparan sulfate-dependent alpha6 overexpression. Mol
Pharmacol. 64:696–702. 2003. View Article : Google Scholar
|
|
14
|
Zemani F, Benisvy D, Galy-Fauroux I, et
al: Low-molecular-weight fucoidan enhances the proangiogenic
phenotype of endothelial progenitor cells. Biochem Pharmacol.
70:1167–1175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smadja DM, Bieche I, Helley D, et al:
Increased VEGFR2 expression during human late endothelial
progenitor cells expansion enhances in vitro angiogenesis with
up-regulation of integrin alpha(6). J Cell Mol Med. 11:1149–1161.
2007. View Article : Google Scholar
|
|
16
|
Lee TH, Seng S, Li H, Kennel SJ, Avraham
HK and Avraham S: Integrin regulation by vascular endothelial
growth factor in human brain microvascular endothelial cells: role
of alpha6beta1 integrin in angiogenesis. J Biol Chem.
281:40450–40460. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Primo L, Seano G, Roca C, et al: Increased
expression of alpha6 integrin in endothelial cells unveils a
proangiogenic role for basement membrane. Cancer Res. 70:5759–5769.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bouvard C, Gafsou B, Dizier B, et al:
Alpha6-integrin subunit plays a major role in the proangiogenic
properties of endothelial progenitor cells. Arterioscler Thromb
Vasc Biol. 30:1569–1575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bouvard C, De Arcangelis A, Dizier B, et
al: Tie2-dependent knockout of α6 integrin subunit in mice reduces
post-ischaemic angiogenesis. Cardiovasc Res. 95:39–47. 2012.
|
|
20
|
Ljubimova JY, Fujita M, Khazenzon NM,
Ljubimov AV and Black KL: Changes in laminin isoforms associated
with brain tumor invasion and angiogenesis. Front Biosci. 11:81–88.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujita M, Khazenzon NM, Bose S, et al:
Overexpression of beta1-chain-containing laminins in capillary
basement membranes of human breast cancer and its metastases.
Breast Cancer Res. 7:R411–R421. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Germain M, De Arcangelis A, Robinson SD,
et al: Genetic ablation of the alpha 6-integrin subunit in Tie1Cre
mice enhances tumour angiogenesis. J Pathol. 220:370–381. 2010.
|
|
23
|
Ringelmann B, Roder C, Hallmann R, et al:
Expression of laminin alpha1, alpha2, alpha4, and alpha5 chains,
fibronectin, and tenascin-C in skeletal muscle of dystrophic 129ReJ
dy/dy mice. Exp Cell Res. 246:165–182. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sorokin LM, Pausch F, Frieser M, Kroger S,
Ohage E and Deutzmann R: Developmental regulation of the laminin
alpha5 chain suggests a role in epithelial and endothelial cell
maturation. Dev Biol. 189:285–300. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Palma M, Venneri MA, Galli R, et al:
Tie2 identifies a hematopoietic lineage of proangiogenic monocytes
required for tumor vessel formation and a mesenchymal population of
pericyte progenitors. Cancer Cell. 8:211–226. 2005.PubMed/NCBI
|
|
26
|
Coffelt SB, Tal AO, Scholz A, et al:
Angiopoietin-2 regulates gene expression in TIE2-expressing
monocytes and augments their inherent proangiogenic functions.
Cancer Res. 70:5270–5280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Capobianco A, Monno A, Cottone L, et al:
Proangiogenic Tie(2) macrophages infiltrate human and murine
endometriotic lesions and dictate their growth in a mouse model of
the disease. Am J Pathol. 179:2651–2659. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mazzieri R, Pucci F, Moi D, et al:
Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis
by impairing angiogenesis and disabling rebounds of proangiogenic
myeloid cells. Cancer Cell. 19:512–526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Squadrito ML and De Palma M: Macrophage
regulation of tumor angiogenesis: implications for cancer therapy.
Mol Aspects Med. 32:123–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Welford AF, Biziato D, Coffelt SB, et al:
TIE2-expressing macrophages limit the therapeutic efficacy of the
vascular-disrupting agent combretastatin A4 phosphate in mice. J
Clin Invest. 121:1969–1973. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang H, Lai JY, Do J, et al: Specifically
targeting angiopoietin-2 inhibits angiogenesis, Tie2-expressing
monocyte infiltration, and tumor growth. Clin Cancer Res.
17:1001–1011. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stratman AN, Malotte KM, Mahan RD, Davis
MJ and Davis GE: Pericyte recruitment during vasculogenic tube
assembly stimulates endothelial basement membrane matrix formation.
Blood. 114:5091–5101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nolan DJ, Ciarrocchi A, Mellick AS, et al:
Bone marrow-derived endothelial progenitor cells are a major
determinant of nascent tumor neovascularization. Genes Dev.
21:1546–1558. 2007. View Article : Google Scholar : PubMed/NCBI
|