Introduction
According to statistics of 2012 International Agency
for Research on Cancer (IARC), almost one million new cases of
gastric cancer were estimated to have occurred in 2012 (952,000
cases, 6.8% of the total), making it the fifth most common
malignancy in the world, after cancers of the lung, breast,
colorectum and prostate. More than 70% of cases (677,000 cases)
occur in developing countries (456,000 in men, 221,000 in women),
and half the world total occurs in Eastern Asia (mainly in China).
Gastric cancer is the third leading cause of cancer death in both
genders worldwide (723,000 deaths, 8.8% of the total), and the
third leading cause of cancer death in both males and females in
China (1). The poor prognosis of
gastric cancer is due to its metastasis and relapse. Metastasis, as
a result of dissemination and growth of cancer cells, represents
the most common cause of death in cancer patients. Therefore, it is
necessary to characterize the molecular mechanism of gastric cancer
metastasis.
Circulating tumor cells (CTCs) are cells that leave
the primary tumor and circulate in the periphery blood. CTCs are
both prognostic and predictive marker for cancer patients (2–4).
Previous studies also demonstrated that CTCs could help clinicians
learn more about tumor biological behavior, such as,
chemo-sensitivity or resistance and metastatic ability, and CTCs
could provide for screening for adjuvant therapy patients, and
tumor pharmacokinetics, and provide new targets for treatment
(5). It is reported that CTCs
participated in the initial process of metastasis (6). Thus, CTCs may have a potential
utility as less invasive than standard biopsies to further
understand the mechanism of metastasis.
Epithelial-mesenchymal transition (EMT) plays an
important role in tumor cell invasion and metastasis (7). EMT is a complex process that refers
to the transformation of epithelial cells to mesenchymal cells, in
which the polarity of epithelial cells is lost, accompanied with
enhanced migration and invasion (8). The characteristics of EMT is the loss
of expression of epithelial cell markers (E-cadherin) and
overexpression of mesenchymal cell markers (e.g., α-smooth muscle
actin protein, α-SMA, N-cadherin, vimentin) (8–10).
Recently, Yu et al reported that circulating breast tumor
cells exhibited dynamic changes in epithelial and mesenchymal
characteristics during treatment of breast cancer patients
(11). In addition, miR-200b has
been reported to inhibit EMT in prostate cancer cells (12). Virtakoivu et al found that
inhibited Akt2 could induce overexpression of miR-200s and then
regulated invasion and migration of prostate cancer cells (13). Our previous study confirmed that
gastric CTCs had stronger capacity of migration, invasion,
metastasis, and radiation-resistance than human gastric cancer cell
lines SGC-7901 and MKN-45. Thus, this study aimed to detect whether
EMT occurred in gastric CTCs and participated in the process of
human gastric cancer metastasis, and then to find the mechanism of
EMT in human gastric CTCs.
Materials and methods
Cell lines
GES, SGC-7901, MKN-45 cell lines were purchased from
Chinese Academy of Medical Sciences Cancer Cell Bank (Beijing,
China). The human gastric circulating tumor cells (CTC-105,
CTC-141, CTC-1, CTC-12) used in this study were previously
established by CD44+/CD45− isolation. Cells
were cultured in Dulbecco’s modified Eagle’s medium (DMEM;
Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS; Hyclone, Beijing, USA). All of the cell lines were
maintained in a humidified atmosphere containing 5%
CO2.
Western blot analysis
Protein extracts were resolved by 12% SDS-PAGE
electrophoresis (Bio-Rad, USA), and transferred to PVDF membranes
(Millipore, MA, USA), and probed with antibodies against human
N-cadherin (1:4,000, Epitomics, USA), E-cadherin (1:1,000, Abcam,
USA), vimentin (1:4,000, Epitomics), Akt (1:1,000, CST, USA),
phospho-Akt (473) (1:4,000, Epitomics), anti-zeb1 antibody
(1:1,000, Abcam) or GAPDH (1:1,000, Sigma, USA).
Fluorescence-conjugated anti-mouse or rabbit IgG (1:10,000, Sigma)
were used as the secondary antibodies, and the antigen-antibody
reactions were visualized using LI-COR Odyssey Infrared Imaging
System (USA). Triple tests were replicated.
Triple staining of immunofluorescence
analysis
Cells were fixed with 4% paraformaldehyde for 20 min
and blocked in 1X PBS (pH 7.4) solution with 1% BSA. The
anti-N-cadherin (1:400, Epitomics), anti-E-cadherin (1:300, Abcam),
anti-vimentin (1:400, Epitomics), or anti-pan-CK (1:300, Abcam),
were added and incubated overnight at 4°C in a humidified box.
After washing, the fluorescent secondary antibody (Epitomics) was
added at a dilution of 1:400 and incubated for 1.5 h. The cells
were then washed three times with PBS, and counter-stained with
DAPI (Sigma) for 2 min. Fluorescence was analyzed using a
fluorescent microscope (Zeiss, Germany). Triple tests were
replicated.
Quantitative real-time PCR
Total RNA was isolated with the TRIzol reagent
(Invitrogen, USA) from cells according to the manufacturer’s
instructions. Single-strand cDNA was synthesized from 1 μg of total
RNA by reverse transcription according to the manufacturer’s
instructions (Takara, China). Single-strand cDNA for miRNA was
synthesized by reverse transcription using miRNA cDNA kit according
to the manufacturer’s instructions (CW Biotech, China).
Quantitative real-time PCR was used to measure the mRNA levels of
E-cadherin, N-cadherin, vimentin, snail1, twist1, zeb1 and miRNA
levels of miR-200a, b and c in gastric circulating tumor cells and
gastric cancer cell lines. Quantitative PCR was performed on
Bio-Rad CFX manager (USA). Amplification was carried out in a 20-μl
volume in triplicate for 40 cycles and the product was detected
using SYBR Green fluorochrome. The geometric average Ct value was
used to calculate relative expression of the above genes using the
method 2−ΔΔCT. U6 and GADPH were used as endogenous
control. Triple tests were replicated. The primers were synthesized
by Genwiz Co. (Suzhou, China). The primers are listed in Table I).
 | Table IPrimer sequences of related
genes. |
Table I
Primer sequences of related
genes.
| Gene | Primers |
|---|
| E-cadherin | Forward:
5′-CGAGAGCTACACGTTCACGG-3′
Reverse: 5′-GGGTGTCGAGGGAAAAATAGG-3′ |
| N-cadherin | Forward:
5′-AGCCAACCTTAACTGAGGAGT-3′
Reverse: 5′-GGCAAGTTGATTGGAGGGATG-3′ |
| Vimentin | Forward:
5′-GACGCCATCAACACCGAGTT-3′
Reverse: 5′-CTTTGTCGTTGGTTAGCTGGT-3′ |
| snail1 | Forward:
5′-TCGGAAGCCTAACTACAGCGA-3′
Reverse: 5′-AGATGAGCATTGGCAGCGAG-3′ |
| twist1 | Forward:
5′-GTCCGCAGTCTTACGAGGAG-3′
Reverse: 5′-GCTTGAGGGTCTGAATCTTGCT-3′ |
| zeb1 | Forward:
5′-TTACACCTTTGCATACAGAACCC-3′
Reverse: 5′-TTTACGATTACACCCAGACTGC-3′ |
| GADPH | Forward:
5′-CTGCACCACCAACTGCTTAG-3′
Reverse: 5′-TGAAGTCAGAGGAGACCACC-3′ |
| U6 | miRNA qPCR Primer
Set (RiboBio, MQP-0201) |
| miR-200a | miRNA qPCR Primer
Set (Tiangen, CD201-0022) |
| miR-200b | miRNA qPCR Primer
Set (Tiangen, CD201-0023) |
| miR-200c | miRNA qPCR Primer
Set (Tiangen, CD201-0024) |
Migration and invasion assay
Cell migration and invasion assays were performed as
follows. For invasion assay, 1×104 cells were seeded on
an 8-μm-pore size Transwell insert (BD, USA) coated with
extracellular matrix (ECM) (1:4) (BD), while in the migration assay
ECM was not used. After 48 h of incubation at 37°C and 5%
CO2, cells adherent to the upper surface of the filter
were removed. Cells were stained with hematoxylin-eosin, and the
number of cells on the bottom were counted under a microscope.
Triple tests were replicated.
Hsa-miR-200s mimic transfection
Cells (20×104) were seeded in 6-well
plates and grown to 50–60% confluence. Human hsa-miR-200b and c
(RiboBio, Guangzhou, China) or its negative control (RiboBio,
Guangzhou, China) was directly transfected into circulating gastric
tumor cells in free of serum Opti-MEM (Invitrogen) at a final
concentration of 50 nmol, according to the manufacturer’s
protocol.
Statistical analysis
The quantitative data are presented as mean values ±
SD from three independent experiments. One-way analysis of variance
(ANOVA) was used to analyze differences among groups. In addition,
the LSD multiple comparison test was used to identify differences
among means of two different groups. Test level of α was 0.05,
P-values <0.05 were considered statistically significant.
Results
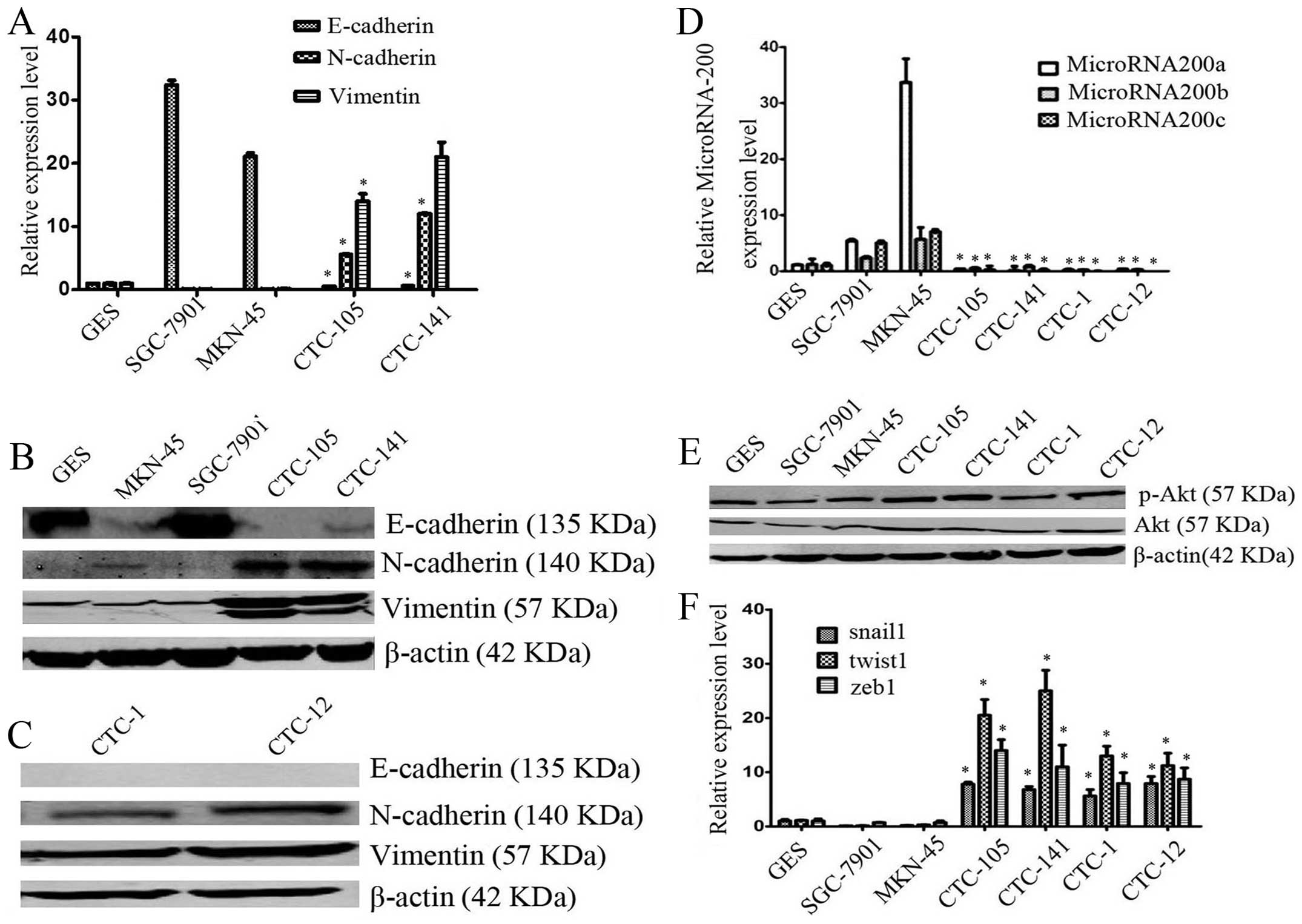
Gastric CTCs exhibit remarkable EMT
process
To examine the differences of EMT phenotype between
gastric circulating tumor cells and gastric cancer cell lines
SGC-7901 and MKN-45, the mRNA and protein levels of EMT markers
were measured. The relative mRNA levels of mesenchymal markers
N-cadherin, and vimentin were significantly overexpressed in
circulating gastric tumor cells, compared with gastric cancer cell
lines (*P<0.05 for all comparation, Fig. 1A), whereas the relative mRNA level
of E-cadherin was significantly decreased (*P<0.05
for all comparisons, Fig. 1A), as
assessed by real-time PCR assay. The results from western blot
analysis demonstrated that the relative protein levels of
N-cadherin, and vimentin were overexpressed (Fig. 1B and C), whereas expression of
E-cadherin was low in the circulating gastric tumor cells, as
compared with gastric cancer cell lines, SGC-7901and MKN-45
(Fig. 1B and C). These results
indicated that the expression of mesenchymal biomarkers was
elevated, while expression of epithelial biomarkers was decreased,
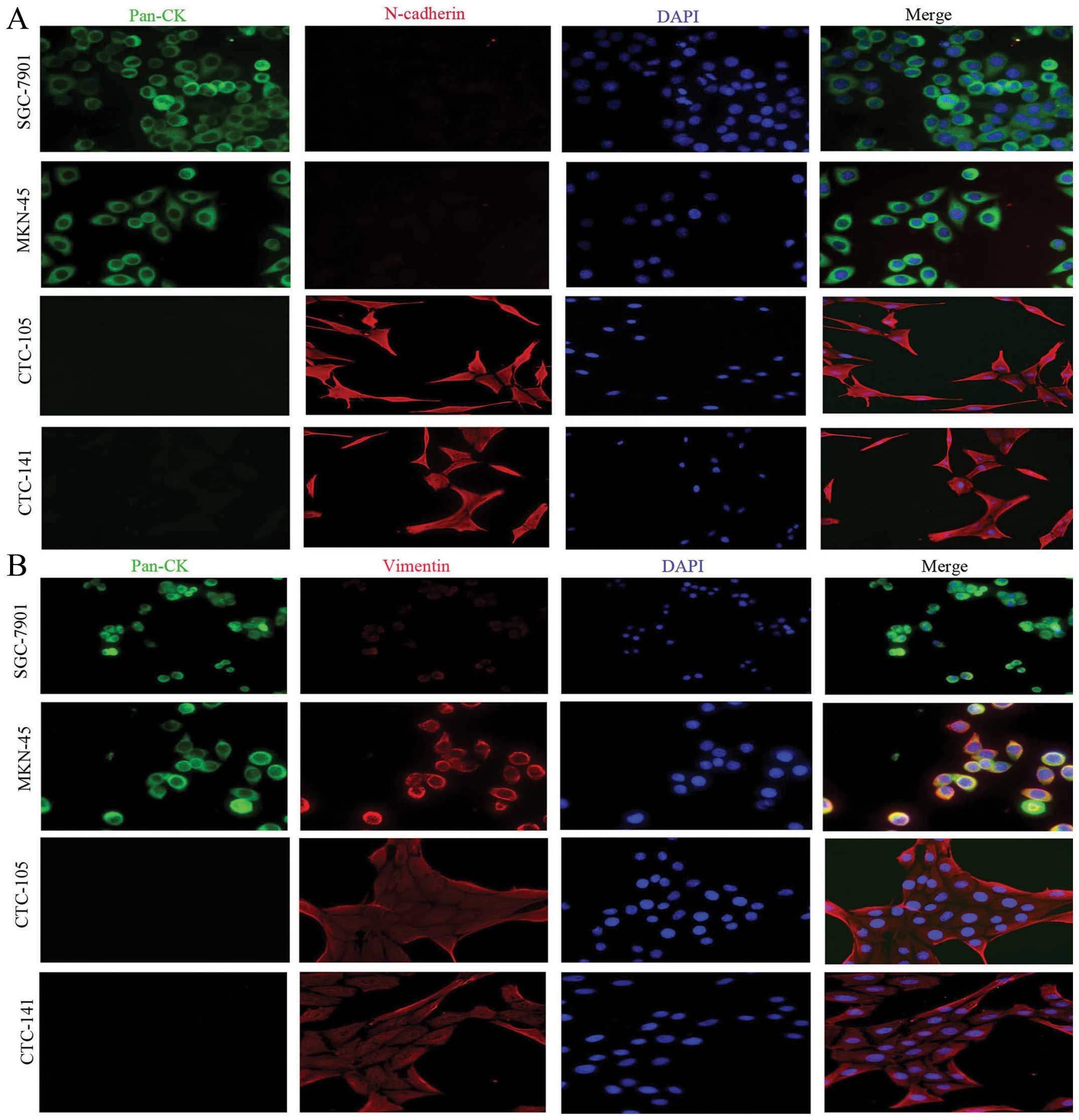
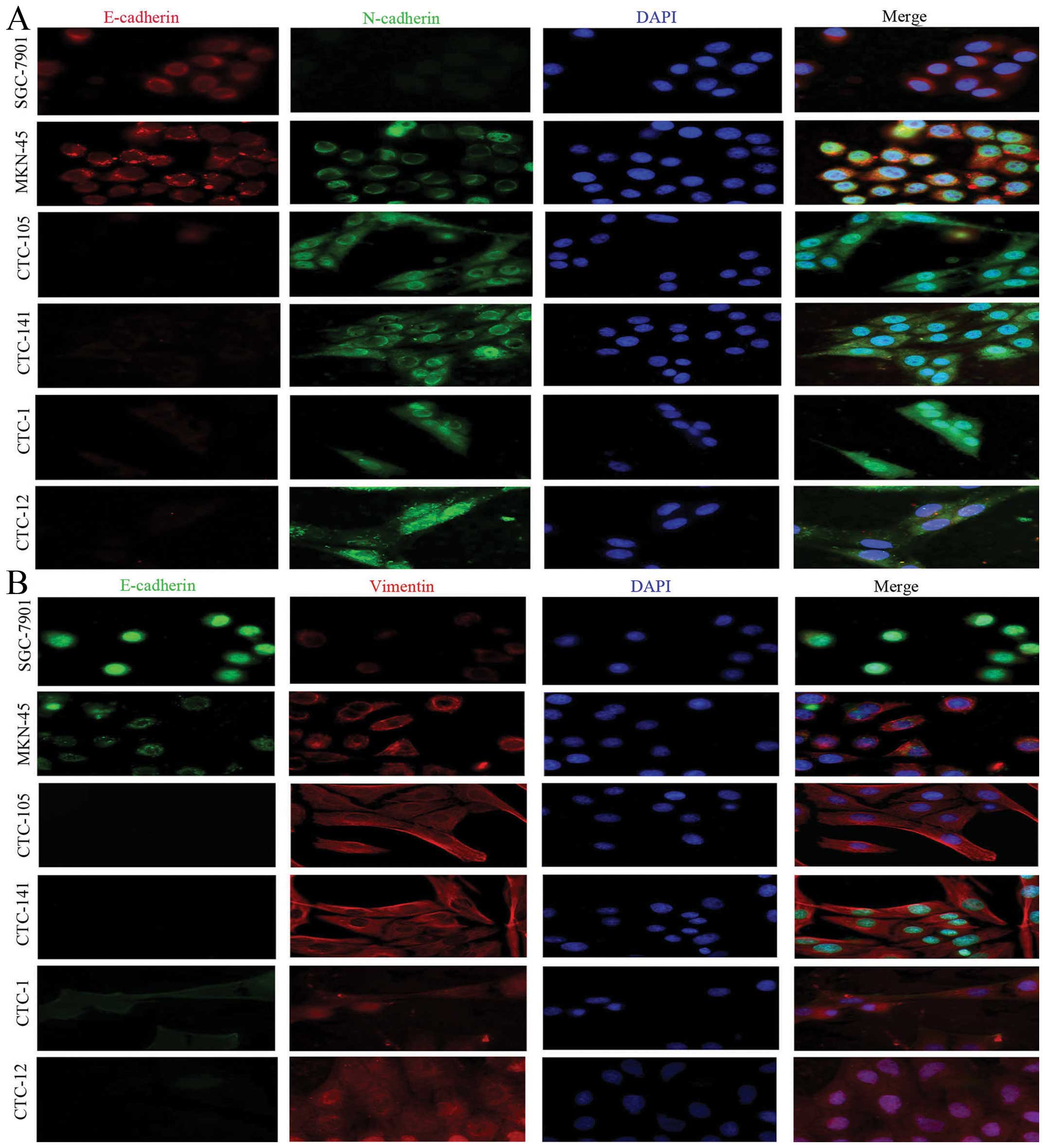
further studies of triple staining immunofluorescence suggested
that, compared with gastric cancer cell lines, CTCs highly
expressed the mesenchymal biomarkers vimentin and N-cadherin, but
lowly or weakly expressed epithelial biomarkers E-cadherin and
pan-CK (Figs. 2 and 3).
Expression of EMT related transcriptors
was reversely correlated with miR-200s and was positively
correlated with Akt kinases activation in gastric CTCs
To investigate the role of miR-200s, a family of
EMT-associated miRNAs, in human gastric CTCs, the relative
expression of miR-200s were examined by quantitative real-time PCR
assay. As expected, the relative expression of miR-200a, b and c
were all significantly decreased in human gastric circulating tumor
cells as compared with SGC-7901 and MKN-45 cells (P<0.05 for all
comparisons, Fig. 1D), which may
suggested that miR-200s were involved in the process of EMT. To
evaluate whether PI3K and Akt kinases signaling pathway is
activated to participate in the process of EMT in gastric CTCs
western blotting was performed to detect expression of both Akt and
p-Akt (s473). It was observed that expression of both Akt and p-Akt
(s473), especially p-Akt (s473), were activated in gastric CTCs
(Fig. 1E). Expression of
EMT-related transcription factor snail1, twist1, zeb1 was
significantly overexpressed in gastric CTCs, compared with gastric
cancer cell lines (P<0.05 for all comparation, Fig. 1F). Based on the above, expression
of EMT related transcriptors was reversely correlated with miR-200s
and was positively correlated with Akt kinase activation in gastric
CTCs.
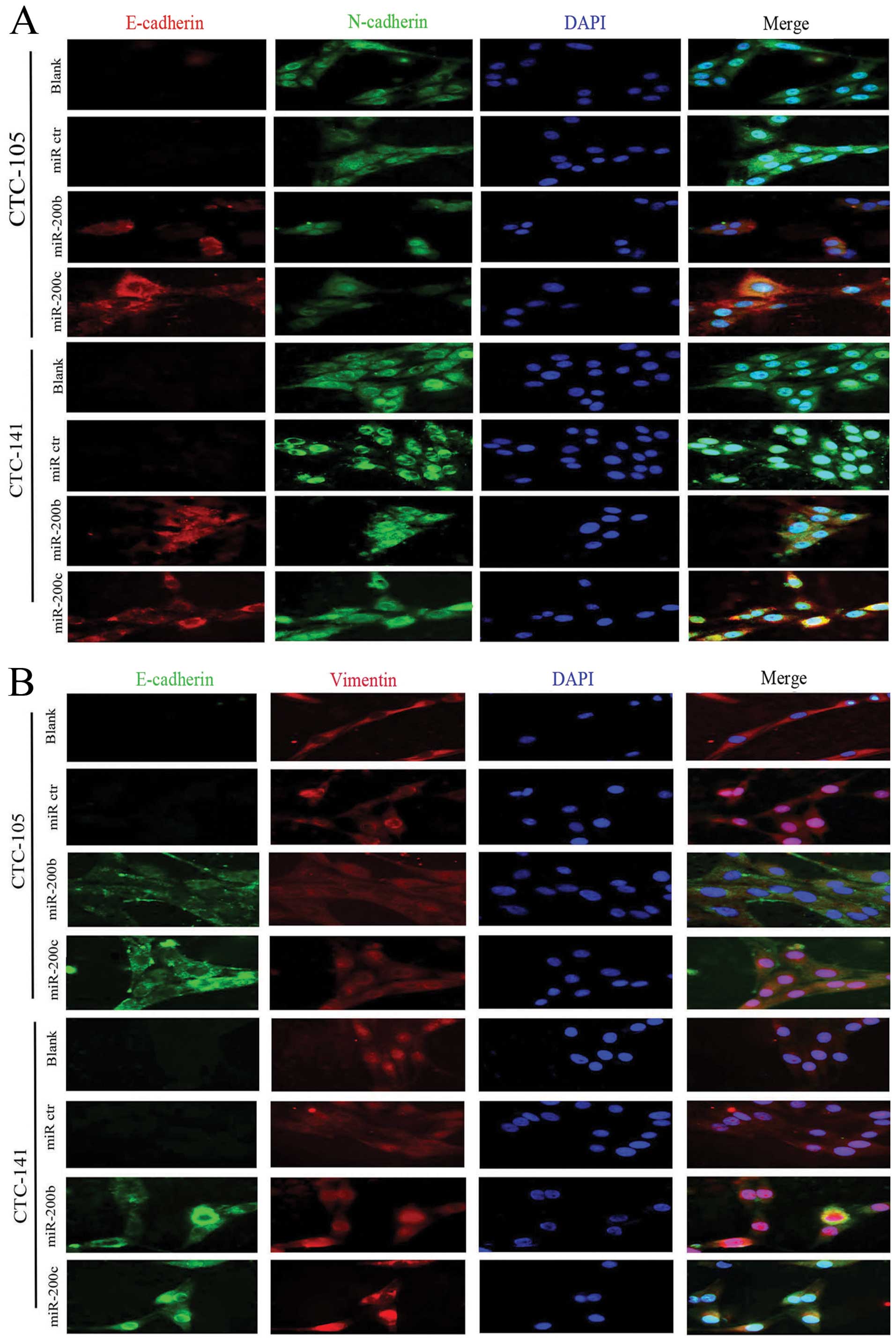
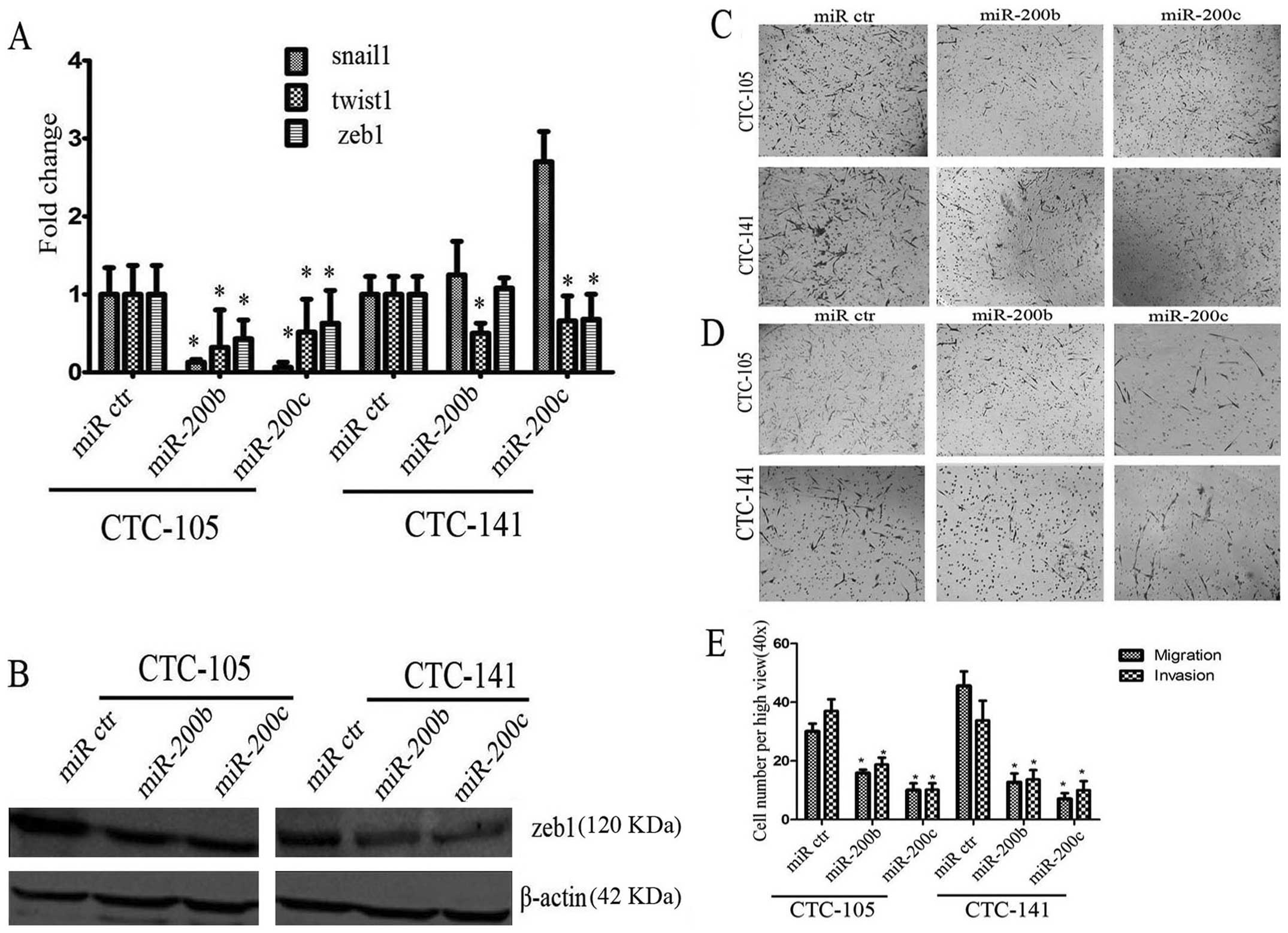
miR-200b and c promoted E-cadherin
expression and decreased twist1, zeb1 expression in gastric
CTCs
In order to investigate the impact of miR-200b and c
on human gastric CTCs, human hsa-miR-200b and c or negative control
miRNA were transfected into human gastric CTCs. After transfection,
triple staining immunofluorescence assays were performed. Ectopic
expression of miR-200b and c increased E-cadherin expression in
gastric CTCs (Fig. 4). In
addition, compared with negative control miRNA, ectopic expression
of miR-200b or c decreased mRNA expression of snail1, twist1, zeb1
by 7.6–15.6, 2–3 and 1.58–2.3 times, respectively, in CTC-105 cells
(P-values were all <0.05, Fig.
5A). mRNA expression of twist1, zeb1 in CTC-141 was
downregulated by 1.5–2 and 1.3–1.5 times, respectively, after
transfection of miR-200b or c (P-values were all <0.05, Fig. 5A), while snail1 expression tended
to increase, no significant difference was observed (P>0.05,
Fig. 5A). Consistent with the
results of real-time PCR, western blotting demonstrated that
ectopic expression of miR-200b and c decreased the expression of
zeb1 protein (Fig. 5B). Overall,
miR-200b and c promoted E-cadherin expression and decreased twist1,
zeb1 expression in gastric CTCs.
miR-200b and c inhibited migration and
invasion in gastric CTCs
miR-200b and c are known to play key roles in the
regulation of cell migration and invasion. To test whether the cell
migration and invasion potential of gastric CTCs transfected with
miR-200b or c were inhibited, transwell assay was performed. As
shown in Fig. 5C–E, compared with
negative control miRNA, ectopic expression of miR-200b or c
inhibited migration and invasion potential (P<0.05 for all
comparisons, Fig. 5E). Based on
the above, miR-200s play a negative role in invasion and metastasis
of human gastric CTCs. These results indicated that miR-200b and c
inhibited migration and invasion potential in gastric CTCs.
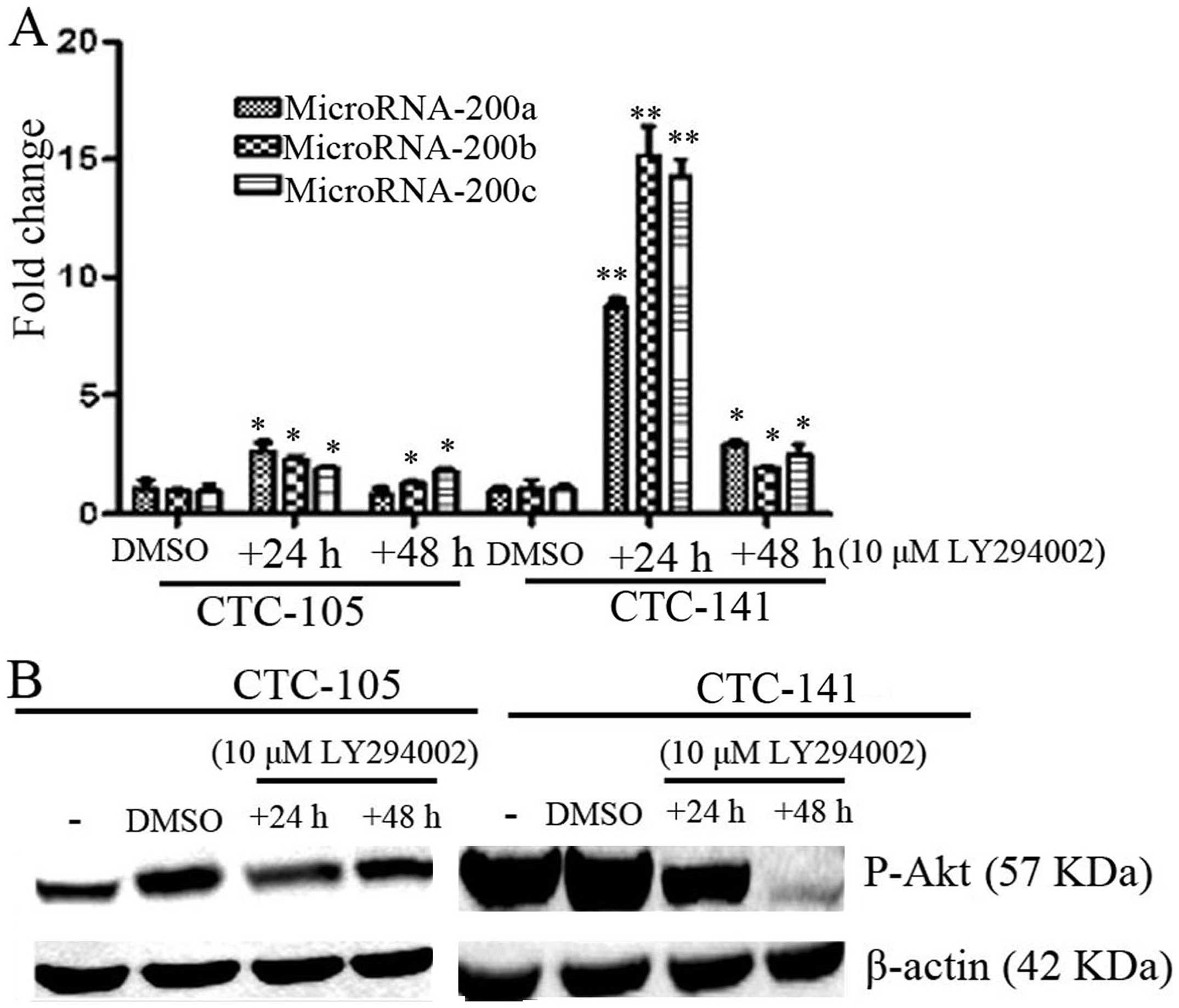
Inhibition of p-Akt increased expression
of MicroRNA-200s in gastric CTCs
Virtakoivu et al found that inhibited Akt2
could induce overexpression of miR-200s and then regulated invasion
and migration of prostate cancer cells (13). To detect the Akt kinase activation
on the expression of miR-200s, PI3K inhibitor 10 μM LY294002 was
added to CTCs and then expression of miR-200s were detected by
real-time PCR. As shown in Fig. 6,
the PI3K inhibitor LY294002 inhibited phosphorylation of AKt, and
increased the expression of miR-200a, b and c both in CTC-105 and
CTC-141 cells. This effect suggests that the function of miR-200s
in the gastric CTCs is likely, at least in part, regulated by p-AKt
signaling pathway.
Discussion
Epithelial-mesenchymal (EM) transition (EMT) is a
process where cells lose their epithelial properties and obtain
mesenchymal properties. EMT has the ability to enhance tumor cell
invasion and metastasis (7).
Usually in the process, suppressed expression of E-cadherin can
induce increased expression of N-cadherin, which is closely related
to tumor invasion. Except for invasion, EMT was also reported in
the process of drug resistance through multisignal transduction.
For example, Ren et al found that inhibition of ZEB1
reverses EMT and chemoresistance in docetaxel-resistant human lung
adenocarcinoma cell line (14),
Rosano et al found that activated endothelin A receptor
pathway enables cells to acquire EMT, thus contributing to
chemotherapy resistance to cisplatinum and taxol in ovarian cancer
cell lines (15).
In our study, CTCs in human gastric cancer patients
showed remarkable EMT process compared with human gastric cancer
cell lines SGC-7901 and MKN-45. In the process of dissemination to
peripheral blood, cells escaped the primary tumor and acquired
mesenchymal composition. N-cadherin, vimentin promoted cell
migration and invasion. The process of EMT occur in many tumors,
such as in breast cancer (16),
cervical cancer (17,18), colorectal cancer (19). EMT was regulated by many pathways
involving tumor invasion and metastasis. A study showed that
circulating breast tumor cells occurred in different stages of
breast cancer patients, and found that patients exhibited dynamic
EMT changes in CTCs (11). Others
found that breast CTCs co-expressed stem cellrelated markers and
EMT associated markers (20,21).
Interestingly, our results found that miR-200s were
downregulated by activated phosphor-Akt, and the signaling pathway
was involved in EMT, migration and invasion of human gastric CTCs.
The following signaling pathways involved in EMT process have been
reported: notch3-zeb-EMT mediated differentiation of esophageal
cancer cells (22), activation of
Akt-GSK3β-Snail-EMT pathway was involved in the phenomenon of
gefitinib resistance in lung cancer (23). TGF-β-p38 MAPK-EMT (24). Akt-HSF-1-Slug participated in EMT
in HER2-positive breast cancer (25). AKT-snail-EMT was involved in
migration of esophageal squamous cell carcinoma (26); β-catenin/tcf-zeb1 influenced EMT
thereby affecting tumor cell invasion (27). LPS-NF-KB-snail-EMT (28); miR-200a-Wnt/β-catenin-zeb-EMT
(29,30); activation of src induced EMT
(31). TNFα-AKT/GSK-3β-Snail
mediated EMT in colon cancer cells (32). Wnt3-Wnt/β-catenin signaling pathway
induced EMT-like phenotype and in turn affected the trastuzumab
resistance in HER2-positive breast cancer cells (33). mTOR complex 1 affects epithelial
type through the opposite regulation of ZEB1/ZEB2 and miR-200b and
miR-200c (34). Our study found
that, p-Akt-miR200szeb1/twist1 mediated EMT thus affected migration
and invasion of human gastric circulating tumor cells. Whether mTOR
was involved in the EMT process was not assessed. In conclusion,
combined with previous studies, multiple signaling pathways were
involved in EMT or EMT-related transcription factors by affecting
snail/slug/twist/zeb1/2, thereby affecting the biological behavior
in different cells, including cell differentiation, migration and
invasion and metastasis, and drug resistance.
Our study had several limitations. Four cases of
CTCs were detected, more cases should be involved in the study.
Another limitation is that the downstream molecular of p-Akt should
be detected in order to investigate which pathway was involved in
downregulation of miR-200s.
Acknowledgements
We greatly appreciate the financial support from the
National Basic Research Program of China (973 Program,
2011CB935800) (http://www.973.gov.cn/English/Index.aspx) and the
Program for Changjiang Scholars and Innovative Research Team in
University (PCSIRT, grant no. IRT1272) of China.
Abbreviations:
|
CTCs
|
circulating tumor cells
|
|
EMT
|
epithelial-mesenchymal transition
|
|
miR
|
microRNA
|
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v10, Cancer Incidence and Mortality
Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France:
International Agency for Research on Cancer; 2013, http://globocan.iarc.fr.
Accessed December 18, 2013
|
|
2
|
Bidard FC, Mathiot C, Delaloge S, et al:
Single circulating tumor cell detection and overall survival in
nonmetastatic breast cancer. Ann Oncol. 21:729–733. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cristofanilli M, Budd GT, Ellis MJ, et al:
Circulating tumor cells, disease progression, and survival in
metastatic breast cancer. N Engl J Med. 351:781–791. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayes DF, Cristofanilli M, Budd GT, et al:
Circulating tumor cells at each follow-up time point during therapy
of metastatic breast cancer patients predict progression-free and
overall survival. Clin Cancer Res. 12:4218–4224. 2006. View Article : Google Scholar
|
|
5
|
Pantel K and Alix-Panabières C:
Circulating tumour cells in cancer patients: challenges and
perspectives. Trends Mol Med. 16:398–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gheldof A and Berx G: Cadherins and
epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci.
116:317–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martinez-Alvarez C, Blanco MJ, Perez R, et
al: Snail family members and cell survival in physiological and
pathological cleft palates. Dev Biol. 265:207–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu W, Kamara H and Svoboda KK: The role of
twist during palate development. Dev Dyn. 237:2716–2725. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu M, Bardia A, Wittner BS, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Williams LV, Veliceasa D, Vinokour E and
Volpert OV: miR-200b inhibits prostate cancer EMT, growth and
metastasis. PLoS One. 8:e839912013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Virtakoivu R, Pellinen T, Rantala JK,
Perälä M and Ivaska J: Distinct roles of AKT isoforms in regulating
β1-integrin activity, migration, andinvasion in prostate cancer.
Mol Biol Cell. 23:3357–3369. 2012.
|
|
14
|
Ren J, Chen Y, Song H, Chen L and Wang R:
Inhibition of ZEB1 reverses EMT and chemoresistance in
docetaxel-resistant human lung adenocarcinoma cell line. J Cell
Biochem. 114:1395–1403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rosano L, Cianfrocca R, Spinella F, et al:
Acquisition of chemoresistance and EMT phenotype is linked with
activation of the endothelin A receptor pathway in ovarian
carcinoma cells. Clin Cancer Res. 17:2350–2360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao Q, Liu W, Cai J, Li M, Gao Y, Lin W
and Li Z: EphB2 promotes cervical cancer progression by inducing
epithelial-mesenchymal transition. Hum Pathol. 45:372–381. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mayer A, Hockel M, Schlischewsky N,
Schmidberger H, Horn LC and Vaupel P: Lacking hypoxia-mediated
downregulation of E-cadherin in cancers of the uterine cervix. Br J
Cancer. 108:402–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pichler M, Ress AL, Winter E, et al:
MiR-200a regulates epithelial to mesenchymal transition-related
gene expression and determines prognosis in colorectal cancer
patients. Br J Cancer. 110:1614–1621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raimondi C, Gradilone A, Naso G, et al:
Epithelial-mesenchymal transition and stemness features in
circulating tumor cells from breast cancer patients. Breast Cancer
Res Treat. 130:449–455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Armstrong AJ, Marengo MS, Oltean S, et al:
Circulating tumor cells from patients with advanced prostate and
breast cancer display both epithelial and mesenchymal markers. Mol
Cancer Res. 9:997–1007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohashi S, Natsuizaka M, Naganuma S, et al:
A NOTCH3-mediated squamous cell differentiation program limits
expansion of EMT-competent cells that express the ZEB transcription
factors. Cancer Res. 71:6836–6847. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maseki S, Ijichi K, Tanaka H, et al:
Acquisition of EMT phenotype in the gefitinib-resistant cells of a
head and neck squamous cell carcinoma cell line through
Akt/GSK-3β/snail signaling pathway. Br J Cancer. 106:1196–1204.
2012.PubMed/NCBI
|
|
24
|
Wei J, Li Z, Chen W, Ma C, Zhan F, Wu W
and Peng Y: AEG-1 participates in TGF-beta1-induced EMT through p38
MAPK activation. Cell Biol Int. 37:1016–1021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carpenter RL, Paw I, Dewhirst MW and Lo
HW: Akt phosphorylates and activates HSF-1 independent of heat
shock, leading to Slug overexpression and epithelial-mesenchymal
transition (EMT) of HER2-overexpressing breast cancer cells.
Oncogene. Jan 27–2014.(Epub ahead of print). View Article : Google Scholar
|
|
26
|
Okui G, Tobiume K, Rizqiawan A, et al: AKT
primes snail-induced EMT concomitantly with the collective
migration of squamous cell carcinoma cells. J Cell Biochem.
114:2039–2049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sanchez-Tillo E, de Barrios O, Siles L,
Cuatrecasas M, Castells A and Postigo A: beta-catenin/TCF4 complex
induces the epithelial-to-mesenchymal transition (EMT)-activator
ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci USA.
108:19204–19209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang T, Chen Z and Fang L: Curcumin
inhibits LPS-induced EMT through downregulation of NF-kappaB-Snail
signaling in breast cancer cells. Oncol Rep. 29:117–124.
2013.PubMed/NCBI
|
|
29
|
Liu J, Ruan B, You N, et al:
Downregulation of miR-200a induces EMT phenotypes and CSC-like
signatures through targeting the β-catenin pathway in hepatic oval
cells. PLoS One. 8:e794092013.PubMed/NCBI
|
|
30
|
Cong N, Du P, Zhang A, et al:
Downregulated microRNA-200a promotes EMT and tumor growth through
the wnt/beta-catenin pathway by targeting the E-cadherin repressors
ZEB1/ZEB2 in gastric adenocarcinoma. Oncol Rep. 29:1579–1587.
2013.PubMed/NCBI
|
|
31
|
Zhao Y, Li X, Sun X, Zhang Y and Ren H:
EMT phenotype is induced by increased Src kinase activity via
Src-mediated caspase-8 phosphorylation. Cell Physiol Biochem.
29:341–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Wang HS, Zhou BH, et al:
Epithelial-mesenchymal transit ion (EMT) induced by TNF-alpha
requires AKT/GSK-3beta-mediated stabilization of snail in
colorectal cancer. PLoS One. 8:e566642013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Y, Ginther C, Kim J, Mosher N, Chung S,
Slamon D and Vadgama JV: Expression of Wnt3 activates
Wnt/beta-catenin pathway and promotes EMT-like phenotype in
trastuzumab-resistant HER2-overexpressing breast cancer cells. Mol
Cancer Res. 10:1597–1606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mikaelian I, Malek M, Gadet R, et al:
Genetic and pharmacologic inhibition of mTORC1 promotes EMT by a
TGF-β-independent mechanism. Cancer Res. 73:6621–6631.
2013.PubMed/NCBI
|