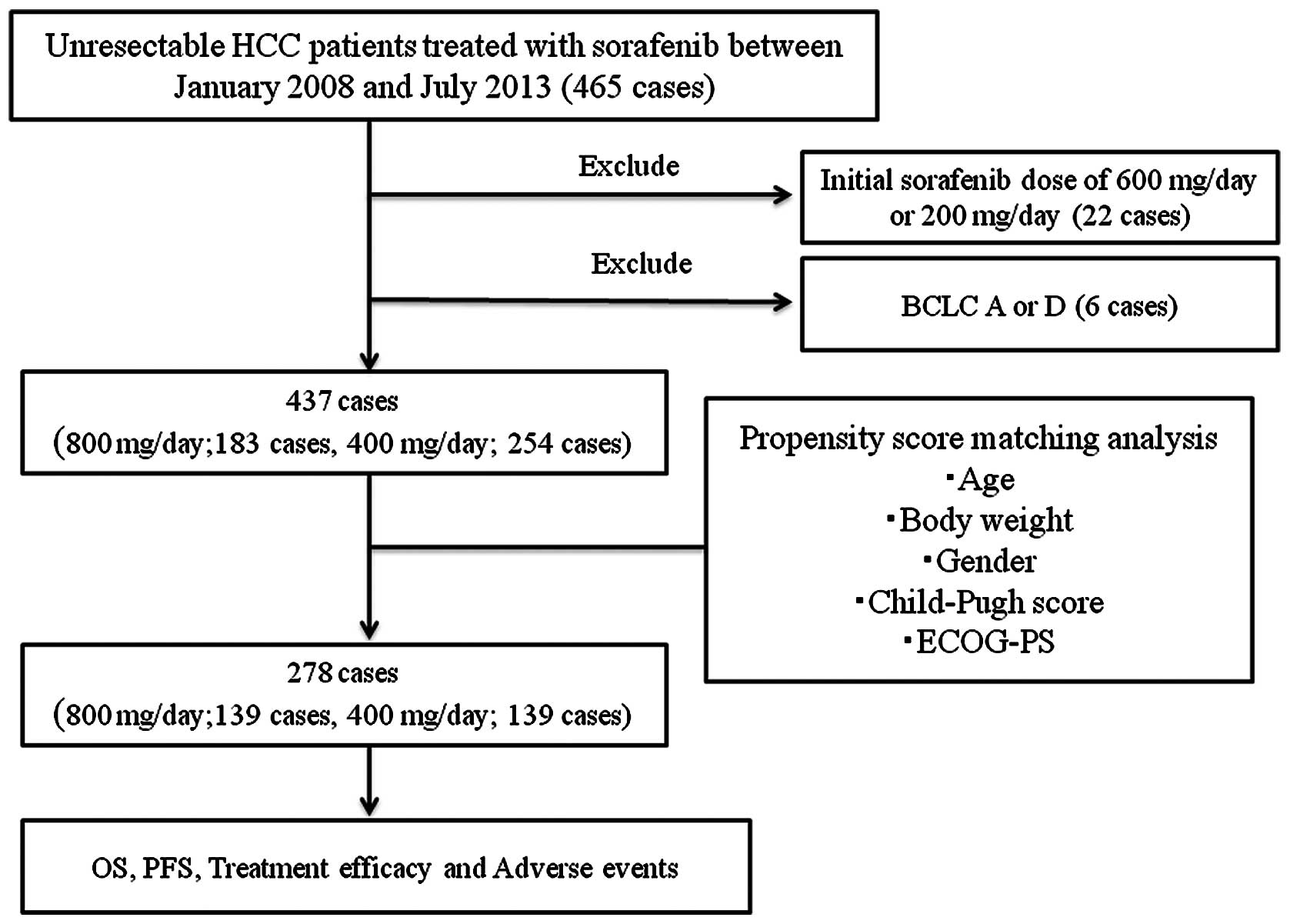
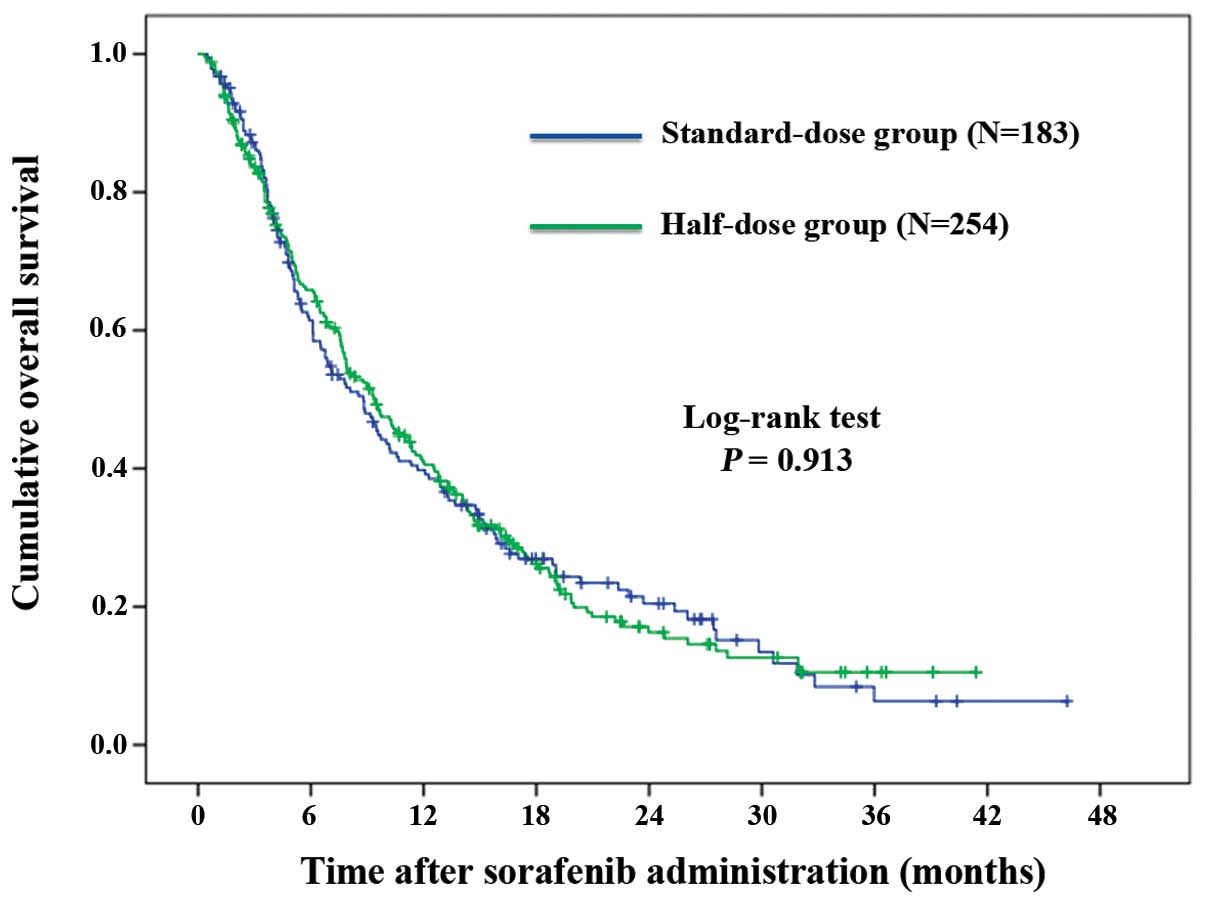
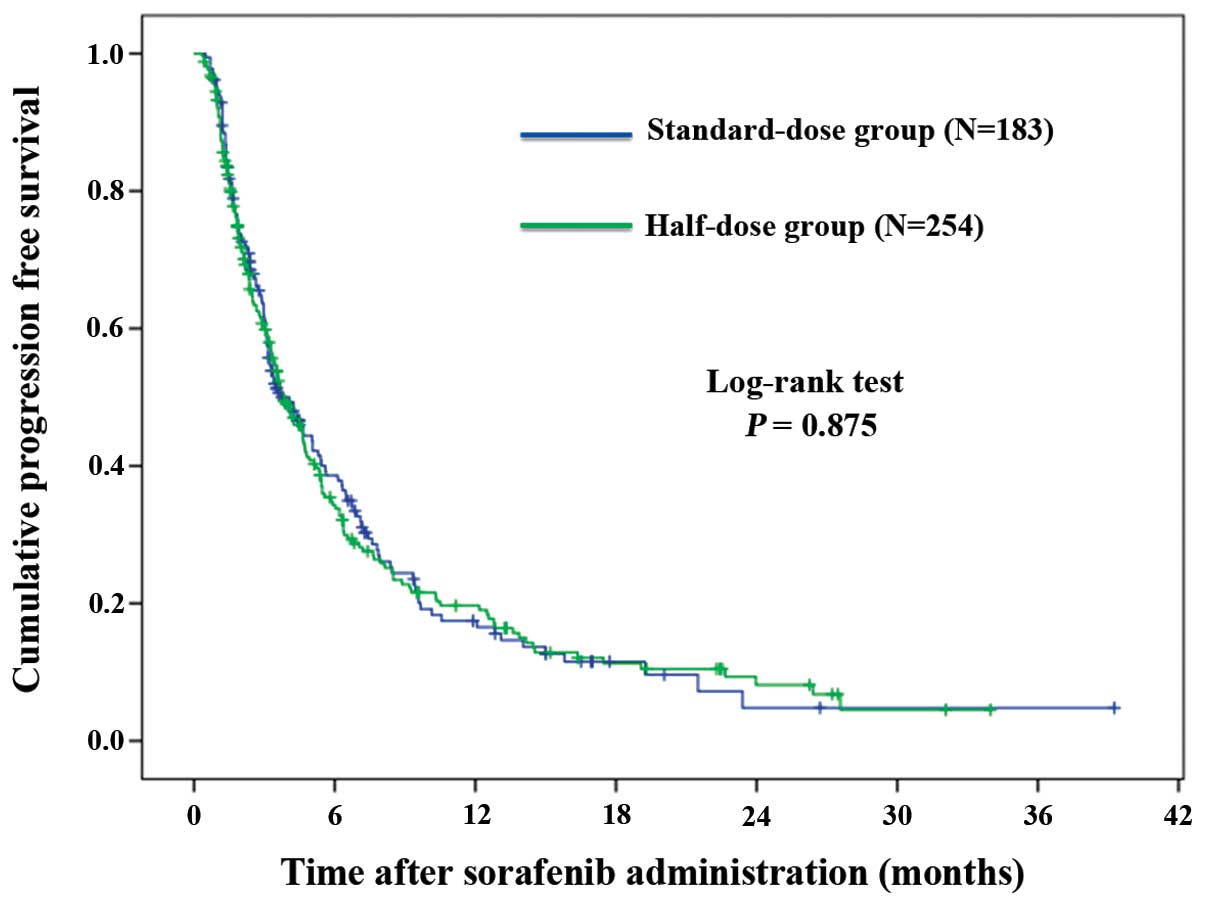
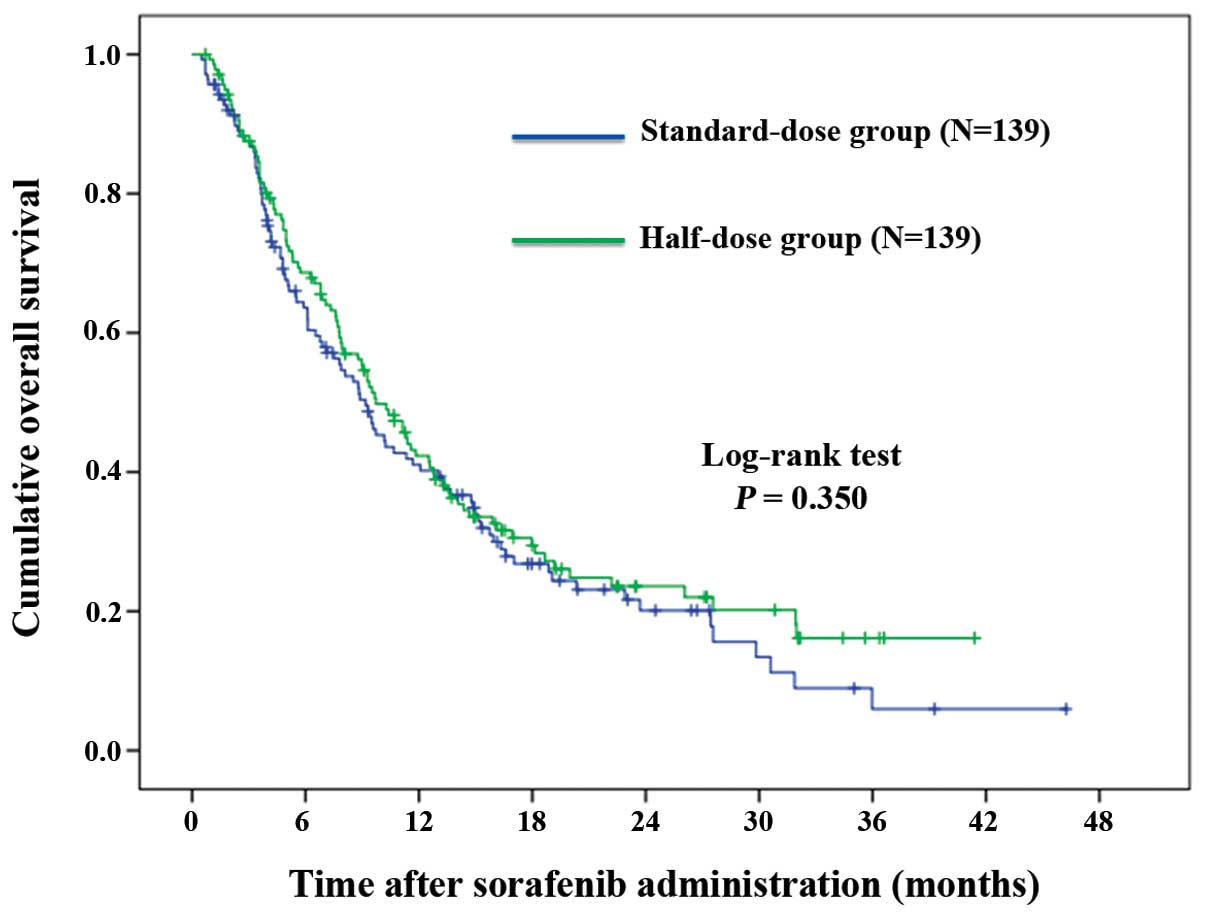
|
1
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lencioni R: Loco-regional treatment of
hepatocellular carcinoma. Hepatology. 52:762–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nishikawa H and Osaki Y: Non-B, non-C
hepatocellular carcinoma (Review). Int J Oncol. 43:1333–1342.
2013.PubMed/NCBI
|
|
5
|
Osaki Y and Nishikawa H: Treatment for
hepatocellular carcinoma in Japan over the last three decades: our
experience and literature review. Hepatol Res. Jun 26–2014.(Epub
ahead of print). View Article : Google Scholar
|
|
6
|
Nishikawa H, Arimoto A, Wakasa T, Kita R,
Kimura T and Osaki Y: Effect of transcatheter arterial
chemoembolization prior to surgical resection for hepatocellular
carcinoma. Int J Oncol. 42:151–160. 2013.PubMed/NCBI
|
|
7
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR,
Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici
M, Voliotis D and Bruix J; SHARP Investigators Study Group.
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J,
Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D and Guan Z:
Efficacy and safety of sorafenib in patients in the Asia-Pacific
region with advanced hepatocellular carcinoma: a phase III
randomised, double-blind, placebo-controlled trial. Lancet Oncol.
10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iavarone M, Cabibbo G, Piscaglia F,
Zavaglia C, Grieco A, Villa E, Cammà C and Colombo M; SOFIA
(SOraFenib Italian Assessment) study group. Field-practice study of
sorafenib therapy for hepatocellular carcinoma: a prospective
multicenter study in Italy. Hepatology. 54:2055–2063. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lencioni R, Kudo M, Ye SL, Bronowicki JP,
Chen XP, Dagher L, Furuse J, Geschwind JF, Ladrón de Guevara L,
Papandreou C, Sanyal AJ, Takayama T, Yoon SK, Nakajima K, Cihon F,
Heldner S and Marrero JA: First interim analysis of the GIDEON
(Global Investigation of therapeutic decisions in hepatocellular
carcinoma and of its treatment with sorafeNib) non-interventional
study. Int J Clin Pract. 66:675–683. 2012. View Article : Google Scholar
|
|
11
|
Lencioni R, Kudo M, Ye SL, Bronowicki JP,
Chen XP, Dagher L, Furuse J, Geschwind JF, de Guevara LL,
Papandreou C, Takayama T, Yoon SK, Nakajima K, Lehr R, Heldner S
and Sanyal AJ: GIDEON (Global Investigation of therapeutic
DEcisions in hepatocellular carcinoma and Of its treatment with
sorafeNib): second interim analysis. Int J Clin Pract. 68:609–617.
2014. View Article : Google Scholar
|
|
12
|
Di Marco V, De Vita F, Koskinas J, Semela
D, Toniutto P and Verslype C: Sorafenib: from literature to
clinical practice. Ann Oncol. 24(Suppl 2): ii30–ii37.
2013.PubMed/NCBI
|
|
13
|
Morimoto M, Numata K, Kondo M, Kobayashi
S, Ohkawa S, Hidaka H, Nakazawa T, Okuwaki Y, Okuse C, Matsunaga K,
Suzuki M, Morita S, Taguri M and Tanaka K: Field practice study of
half-dose sorafenib treatment on safety and efficacy for
hepatocellular carcinoma: A propensity score analysis. Hepatol Res.
May 6–2014.(Epub ahead of print). View Article : Google Scholar
|
|
14
|
Takeda H, Nishikawa H, Osaki Y, Tsuchiya
K, Joko K, Ogawa C, Taniguchi H, Orito E, Uchida Y and Izumi N;
Japanese Red Cross Liver Study Group. Clinical features associated
with radiological response to sorafenib in unresectable
hepatocellular carcinoma: a large multicenter study in Japan. Liver
Int. May 16–2014.(Epub ahead of print). View Article : Google Scholar
|
|
15
|
Nishikawa H, Takeda H, Tsuchiya K, Joko K,
Ogawa C, Taniguchi H, Orito E, Uchida Y, Osaki Y and Izumi N;
Japanese Red Cross Liver Study Group. Sorafenib therapy for BCLC
stage B/C hepatocellular carcinoma; clinical outcome and safety in
aged patients: a multicenter study in Japan. J Cancer. 5:499–509.
2014. View
Article : Google Scholar
|
|
16
|
Cheng AL, Amarapurkar D, Chao Y, Chen PJ,
Geschwind JF, Goh KL, Han KH, Kudo M, Lee HC, Lee RC, Lesmana LA,
Lim HY, Paik SW, Poon RT, Tan CK, Tanwandee T, Teng G and Park JW:
Re-evaluating transarterial chemoembolization for the treatment of
hepatocellular carcinoma: consensus recommendations and review by
an International Expert Panel. Liver Int. 34:174–183. 2014.
View Article : Google Scholar
|
|
17
|
European Association For The Study Of The
Liver; European Organisation For Research And Treatment Of Cancer.
EASL-EORTC clinical practice guidelines: management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012.PubMed/NCBI
|
|
18
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salvaggio G, Furlan A, Agnello F, Cabibbo
G, Marin D, Giannitrapani L, Genco C, Midiri M, Lagalla R and
Brancatelli G: Hepatocellular carcinoma enhancement on
contrast-enhanced CT and MR imaging: response assessment after
treatment with sorafenib: preliminary results. Radiol Med.
119:215–221. 2014. View Article : Google Scholar
|
|
20
|
Sacco R, Bargellini I, Ginanni B, Bertini
M, Faggioni L, Federici G, Romano A, Bertoni M, Metrangolo S,
Altomare E, Parisi G, Tumino E, Scaramuzzino A, Bresci G and
Bartolozzi C: Long-term results of sorafenib in advanced-stage
hepatocellular carcinoma: what can we learn from routine clinical
practice? Expert Rev Anticancer Ther. 12:869–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
D’Agostino RB Jr: Propensity score methods
for bias reduction in the comparison of a treatment to a
non-randomized control group. Stat Med. 17:2265–2281.
1998.PubMed/NCBI
|
|
22
|
Seung KB, Park DW, Kim YH, Lee SW, Lee CW,
Hong MK, Park SW, Yun SC, Gwon HC, Jeong MH, Jang Y, Kim HS, Kim
PJ, Seong IW, Park HS, Ahn T, Chae IH, Tahk SJ, Chung WS and Park
SJ: Stents versus coronary-artery bypass grafting for left main
coronary artery disease. N Engl J Med. 358:1781–1792. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ogasawara S, Kanai F, Obi S, Sato S,
Yamaguchi T, Azemoto R, Mizumoto H, Koushima Y, Morimoto N, Hirata
N, Toriyabe T, Shinozaki Y, Ooka Y, Mikata R, Chiba T, Okabe S,
Imazeki F, Yoshikawa M and Yokosuka O: Safety and tolerance of
sorafenib in Japanese patients with advanced hepatocellular
carcinoma. Hepatol Int. 5:850–856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marrero J, Venook A and Kudo M: Second
interim analysis of GIDEON (Global Investigation of therapeutic
DEcisions in unresectable hepatocellular carcinoma and Of its
treatment with sorafeNib): subgroup analysis by initial sorafenib
dose. Hepatology. 54:n21192011.
|
|
25
|
McCurry J: Japan battles with obesity.
Lancet. 369:451–452. 2007. View Article : Google Scholar
|
|
26
|
Examination Committee of Criteria for
‘Obesity Disease’ in Japan; Japan Society for the Study of
Obesity:. New criteria for ‘obesity disease’ in Japan. Circ J.
66:987–992. 2002.
|