Introduction
Gastric cancer is one of the most common cancers
worldwide, accounting for ~8% new cancers diagnosed each year
(1,2). The epidemiology studies indicate that
the male predominance of gastric cancer is a global phenomenon
(male:female ratio 2:1 or 3:1) (3). The predominance is also related to a
delay of 10–15 years in the appearance and onset of gastric cancer
in females compared with males (3,4).
From adolescence to menopause, women with multiple fertility have
relatively less frequent incidence of gastric cancer, while the
incidence of gastric cancer was high in nuns (5). Oral contraceptives and estrogen
replacement therapy reduced the incidence of gastric cancer in
women (1,6). The incidence of gastric cancer in the
prostate cancer patients treated with estrogen was lower than
non-treated patients (1,6). The incidence of gastric cancer
increased in women receiving oophorectomy and decreased in women
receiving estrogen replacement therapy (1,6).
Recent epidemiological survey results also indicated an association
between the estrogen level and histological type of gastric;
intestinal type gastric cancer occurred more often in middle-aged
males (1,6,7).
Animal experimental results also showed that in rats treated with
the chemical N-methyl-N′-nitro-of-N-nitrosoguanidine (MNNG), the
incidence of gastric cancer in male rats was higher than in female
(8). However, estrogen treatment
reduced the incidence of MNNG-induced gastric cancer in male rats
while oophorectomy increased the incidence of MNNG induced gastric
cancer in female rats. In addition, estrogen treatment reversed
precancer lesions of gastric mucosa induced by MNNG (8). Taken together, estrogen signaling is
involved in development of human gastric cancer, which is regulated
by endogenous estrogen level.
Estrogen signaling is mediated by estrogen
receptors, ER-α and ER-β. ER-α has three isoforms, namely, ER-α66
(the original estrogen receptor), ER-α46 and ER-α36 (9,10).
ER-α36 lacks the transcription activation domain AF-1 and AF-2 but
retains the DNA binding domain, receptor dimerization domain and
part of the ligand-binding domains (9). Previously, we reported that ER-α36 is
expressed in normal gastric and gastric cancer tissues, which was
correlated with TNM stage and metastasis of gastric cancer cells
(3,10,11).
However, the function and underlying mechanism of ER-α36-mediated
estrogen signaling in the growth of gastric cancer have not been
established. Here, we examined the expression patterns of ER-α36
and Cyclin D1 in human gastric cancer samples and studied effects
of different concentrations of estrogen on growth of various
gastric cancer cells.
Materials and methods
Cell culture
The human gastric cancer cell lines AGS, BGC823 and
SGC7901 and the normal gastric cell line GES-1 were obtained from
Chinese Academy of Medical Sciences Cell Center of Basic Medicine
(Beijing, China). Gastric cancer SGC7901 cells with different
levels of ER-α36 expression, SGC7901/High36 and SGC7901/Low36 cell
lines with high or low levels of ER-α36 expression, respectively,
were generated and characterized as described before (10). SGC7901, SGC7901/High36 and
SGC7901/Low36 cells were maintained in DMEM medium (Invitrogen,
USA) containing 10% fetal bovine serum (FBS, Invitrogen) at 37°C in
a 5% CO2 atmosphere. Before treated with indicated
concentrations of E2β or ethanol vehicle as a control, cells were
maintained for 3 days in phenol red-free DMEM plus 2.5%
dextran-charcoal stripped fetal calf serum. Following treatment for
7 days, the cells were trypsinized and counted with the Scepter™
2.0 handheld automated cell counter (Merck KGaA, Darmstadt,
Germany). Assays were performed in three dishes for each time-point
and all experiments were repeated three times.
Tumor sample and tissue microarray
Frozen tumor samples obtained from 40 gastric cancer
patients between 2009 and 2012 (Jiangda Pathology Institute), and
paraffin-embedded samples of gastric cancer obtained from 117
patients between 2006 and 2012 (Jiangda Pathology Institute) were
used for this study with the approval of the Institutional Review
Board of Jianghan University. Tumor tissues used for
immunohistochemistry (IHC) were fixed in 10% neutral formalin,
embedded in paraffin, processed and stained with hematoxylin and
eosin (H&E). Tissues for western blot analysis were snap-frozen
in liquid nitrogen and were kept at −150°C. The 117 samples for IHC
included 83 men and 34 women aged 30–59 years (mean age, 57.3
years) and the samples for western blot analysis were from 28 men
and 12 women aged 30–59 years (mean age, 56.2 years). None of the
patients had received any anticancer treatment prior to surgery.
Tumor size, histological differentiation, T stage and N stage were
evaluated according to the clinic pathological classification of
the World Health Organization (2010). Targeted tissue areas of 117
tumors were marked on H&E-stained sections. One tissue core,
1.0 mm in diameter and 3–4 mm in depth were removed from each block
using a manual microarray device (Beecher Instruments, Silver
Spring, MD, USA) with a total of 117 tissue cores inserted into the
recipient paraffin-block. The tissue microarray was sectioned at
4-micron thickness.
Western blot analysis
For western blot analysis, cells were washed with
cold PBS and lysed with the lysis buffer (50 mM Tris-HCl pH 8.0,
150 mM NaCl, 0.25 mM EDTA pH 8.0, 0.1% SDS, 1% Triton X-100, 50 mM
NaF) supplemented with protease and phosphatase inhibitors from
Sigma. Tumor tissues were dissected and homogenized in the lysis
buffer. The protein concentrations were determined with an Enhanced
BCA Protein Assay kit (Beyotime Institute of Biotechnology,
Shanghai, China). Cell lysates were mixed with loading buffer
(Beyotime Institute of Biotechnology), separated on 12% SDS-PAGE
gels and transferred to PVDF membranes (0.22 μm, Millipore,
Billerica, MA, USA). The membranes were probed with various primary
antibodies, appropriate secondary antibodies, and visualized with
enhanced chemiluminescence (ECL, Beyotime Institute of
Biotechnology) detection reagents (DNR Bio-Imaging Systems Ltd.,
Jerusalem, Israel). The densities of protein bands were assessed
with Totallab analysis software (Nonlinear Dynamics Technical, NC,
USA).
The anti-ER-α36 antibody was kindly provided by
Professor Zhaoyi Wang at Creighton University. Anti-Cyclin D1
antibody (SC-718) and anti-β-actin antibody (SC-47778) were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
RNA extraction and reverse transcription
PCR
Total RNA was extracted from the frozen tissues
using TRIzol reagent (Invitrogen, San Diego, CA, USA) according to
the manufacturer’s protocol. Total RNA was then reverse transcribed
into cDNA by reverse transcription-PCR kit (Shengong, Shanghai,
China). Following reverse transcription, PCR reaction was carried
out with 32 cycles using the following conditions: 94°C for 30 sec,
56°C for 30 sec and 72°C for 30 sec. Cyclin D1 primers and β-actin
primers were designed using Primer 5.0 (Primer Biosoft
International, Palo Alto, CA, USA) and were used simultaneously in
the same reaction. The following primers were used: Cyclin D1
forward primer 5′-ATGGAACACCAGCTCCTGTG-3′; Cyclin D1 reverse primer
5′-ACCTCCAGCATCCAGGTGGC-3′; β-actin forward primer
5′-ATGATGATATCGCCGCGCTC-3′; β-actin reverse primer
5′-GTACATGGCTGGGGTGTTGA-3′. PCR products (200 bp for Cyclin D1 and
395 bp for β-actin) were separated on a 1.5% agarose gel and
stained with Gelred (SBS Genetech Co., Beijing, China). The
densities of DNA bands were determined with analysis software
(Biostep Photoimpact, Beijing, China).
Statistical analysis
The statistical analysis was performed by SPSS 12.0
software. Results are shown as the mean ± SD in three replicate
samples and compared to the Student’s t-test and analysis of
variance (ANOVA). Differences were considered significant when
P<0.05. All experiments were repeated at least three times.
Results
Relationship between ER-α36 and Cyclin D1
expression and clinic pathological features in gastric
adenocarcinomas
The expression patterns of ER-α36 and Cyclin D1 were
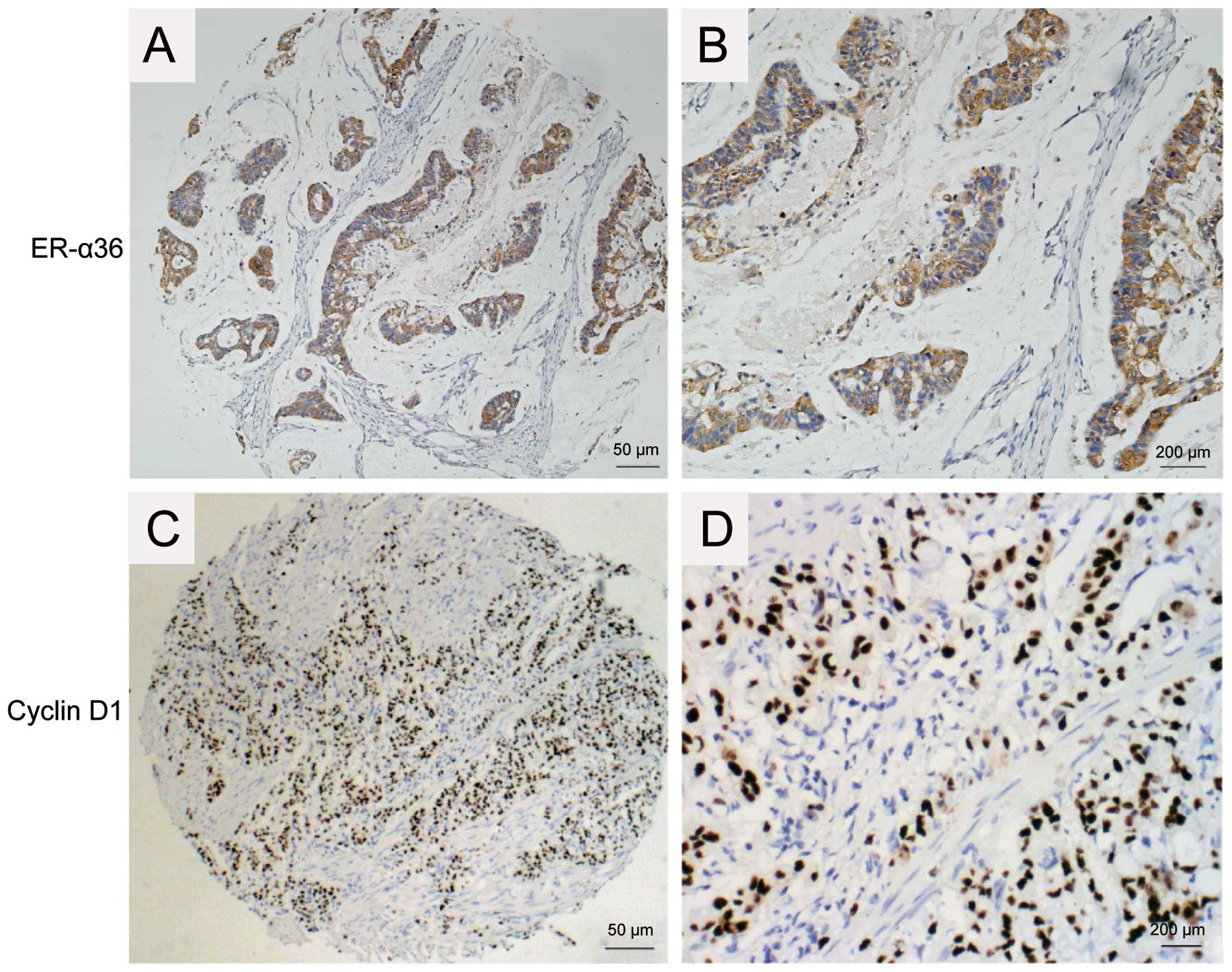
examined in 117 samples of gastric carcinoma with IHC. ER-α36
expression was detected predominantly in the cytomembrane and
cytoplasm of gastric carcinoma cells while Cyclin D1 was detected
predominantly in the nuclei of gastric carcinoma cells. Positive
expression of Cyclin D1 was observed in 81 of the 117 samples
(69.2%), and ER-α36 expression was detected in 101 of the 117 cases
(86.23%) (Table I and Fig. 1). A strong correlation was found
between Cyclin D1 and ER-α36 expression in IHC (P<0.01, Table I), suggesting that Cyclin D1 may be
one of the downstream effectors of ER-α36-mediated estrogen
signaling.
 | Table IRelationships of ER-α36 expression
with clinicopathological features and Cyclin D1 expression in
gastric carcinomas. |
Table I
Relationships of ER-α36 expression
with clinicopathological features and Cyclin D1 expression in
gastric carcinomas.
| ER-α36
expression | |
|---|
|
| |
|---|
| Factors | Negative | Positive | P-value |
|---|
| Age | | | 0.04 |
| ≤50 years | 7 | 18 | |
| >50 years | 9 | 83 | |
| Gender | | | 0.01 |
| Male | 8 | 75 | |
| Female | 8 | 26 | |
| Tumor size | | | 0.20 |
| ≤5 cm | 4 | 42 | |
| >5 cm | 12 | 59 | |
| Invasion to
serosa | | | 0.01 |
| Positive | 5 | 10 | |
| Negative | 11 | 91 | |
| Lymph node
metastasis | | | 0.71 |
| Positive | 3 | 27 | |
| negative | 13 | 74 | |
| Cyclin D1 | | | <0.01 |
| Positive | 2 | 79 | |
| Negative | 14 | 22 | |
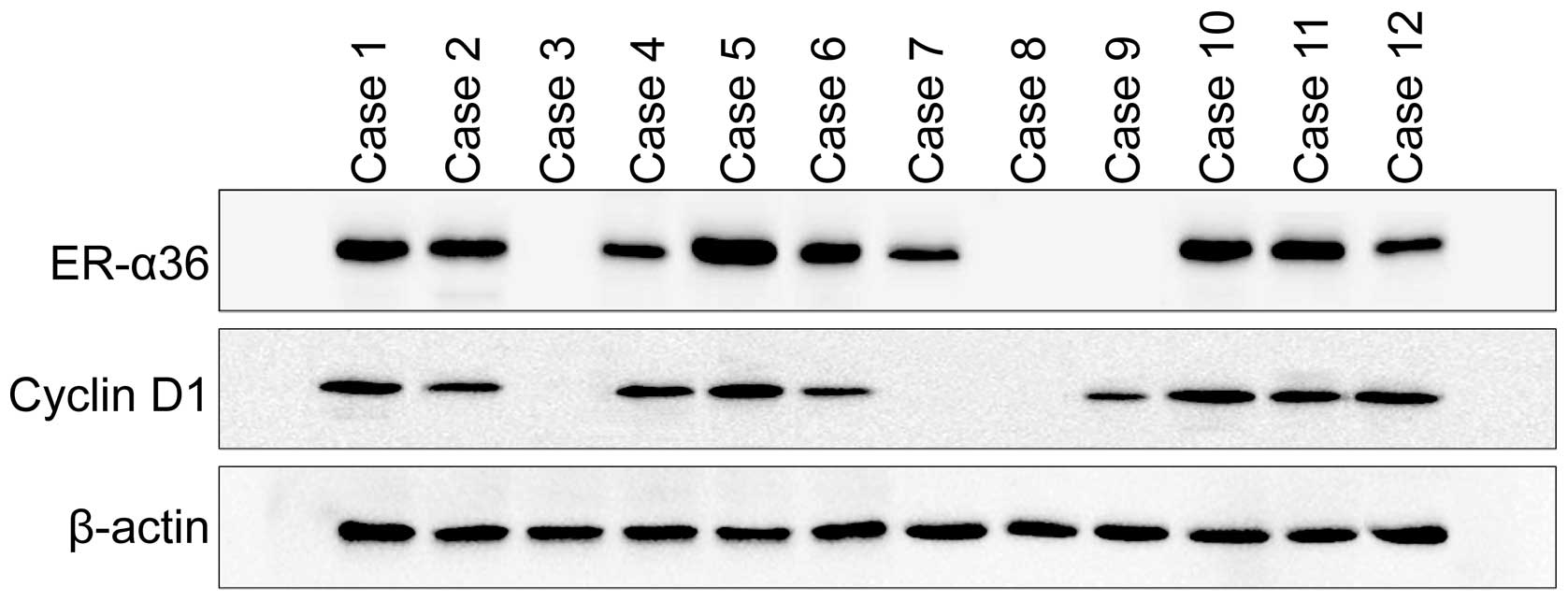
The expression patterns of ER-α36 and Cyclin D1 were
also examined in frozen gastric cancer tissues. ER-α36 and Cyclin
D1 expression were examined in 40 cases of frozen gastric cancer
tissues with western blot analysis and IHC (Fig. 2). Positive expression of ER-α36 was
detected in 32 of the 40 samples (80%) in western blot analysis and
31 of the 40 samples (77.5%) in immunohistochemistry. We also
observed positive expression of Cyclin D1 in 29 of the 40 samples
(72.5%) in western blot analysis and 27 of the 40 samples (77.5%)
in immunohistochemistry. A strong correlation was also found
between Cyclin D1 and ER-α36 expression in western blot analysis
(P<0.05).
The correlation between ER-α36 expression and other
clinicopathological features was also investigated. ER-α36
expression was correlated with the older patients (median age, 57.3
years old; range from 30 to 59 years old, P=0.04), gender
(male:female ratio 2.88:1; P=0.01), invasion to serosa (P=0.01),
but not with tumor size, histological differentiation and lymph
node metastasis (P>0.05; Table
I).
Estrogen stimulated growth of gastric
cancer cells
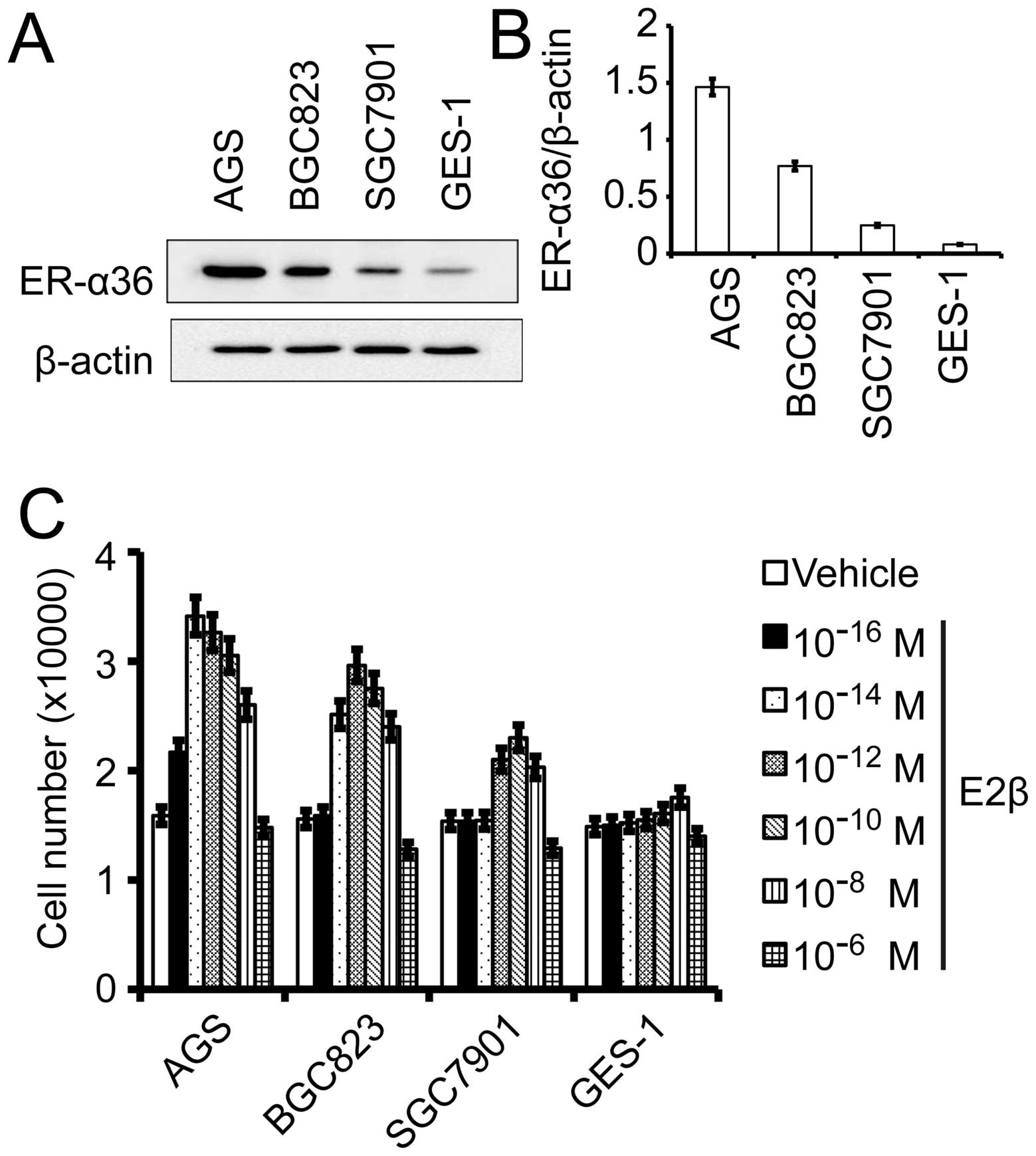
First, we examined ER-α36 expression in different
human gastric cancer cell lines and the normal gastric cell line
GES-1 using western blot analysis. As shown in Fig. 3A, ER-α36 expression was the lowest
in GES-1 cells and the highest in AGS cells with the second in
BGC823 cells and the third in SGC7901 cells. To determine the
effects of estrogen on growth of gastric cancer cells, gastric
cancer cells lines AGS, BGC823, SGC7901 and the normal gastric cell
line GES-1 were treated with different concentrations of
17β-estradiol (E2β) for 7 days and the cell number was determined.
All gastric cancer cells treated with E2β exhibited an increased
growth rate compared with cells treated with vehicle while normal
gastric GES-1 cells only slightly responded to E2β at
10−8 M of E2β. The dose-response curves of these cells
to E2β displayed a non-monotonic or biphasic pattern; increasing
concentrations of E2β that initially stimulated cell growth but
inhibited cell growth at higher concentrations (Fig. 3C). E2β at 10−16 M
started to weakly stimulate the growth of AGS cells that express
high levels of endogenous ER-α36 while BGC823 and SGC7901 cells
required 10−14 and 10−12 M, respectively. The
result suggested that ER-α36 expression is involved in the
sensitivity of gastric cancer cells to estrogen.
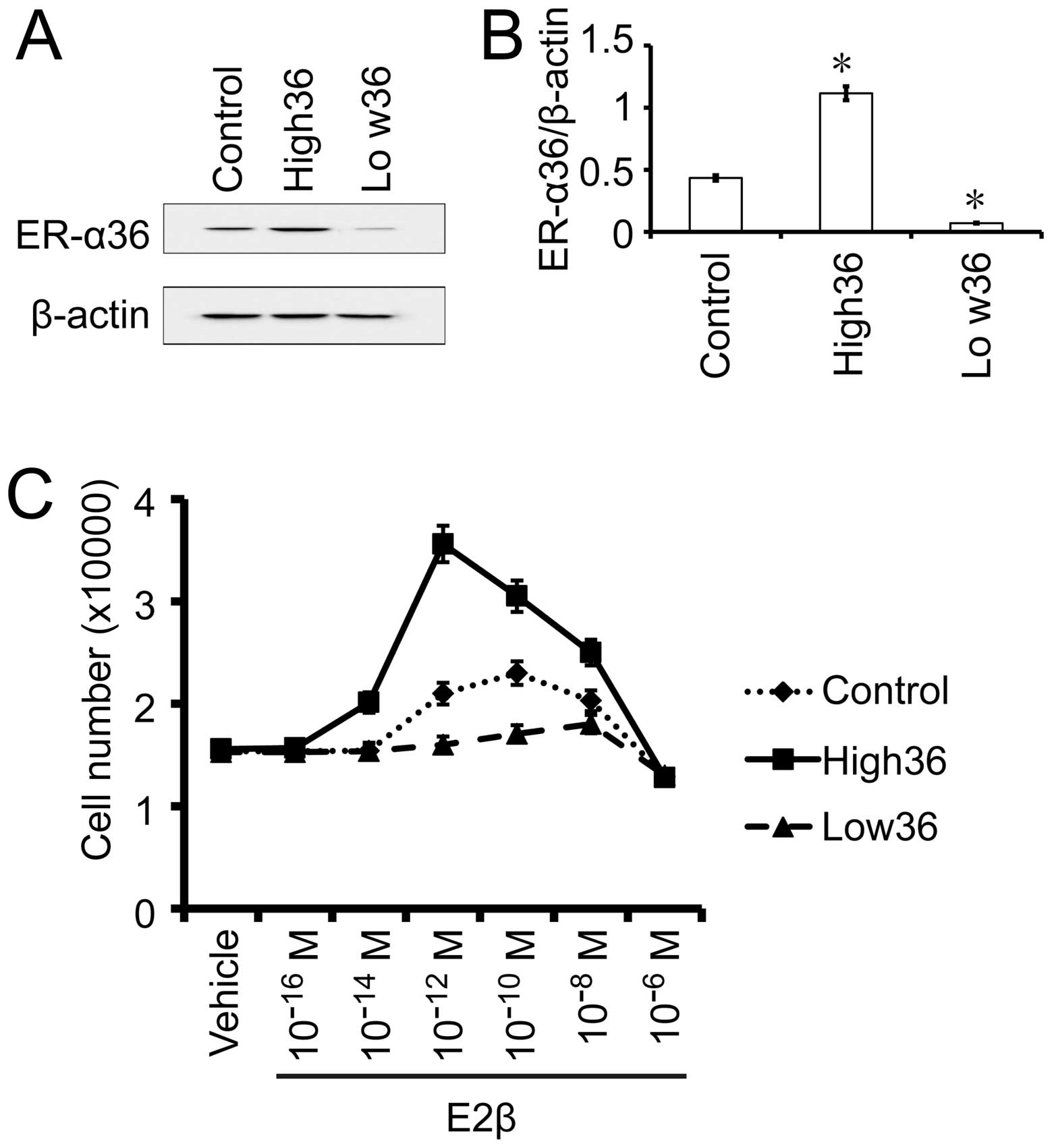
The effects of estrogen on gastric cancer
SGC7901 cells with different levels of ER-α36 expression
To confirm the influence of the levels of ER-α36
expression on the sensitivity of gastric cancer cells to estrogen,
we used SGC7901 cells with forced expression of recombinant ER-α36
(SGC7901/High36) and SGC7901 cells with knocked-down levels of
ER-α36 (SGC7901/Low36). The cells were treated with different
concentrations of E2β and cell growth was examined after 7 days. As
shown in Fig. 4, E2β at
10−10 M stimulated the most growth of the SGC7901/V
control cells while SGC7901/High36 cells responded the most to E2β
at 10−12 M. The growth of the SGC7901/Low36 cells that
express the lowest levels of ER-α36 started to be stimulated by
10−10 M of E2β, peaked at 10−8 M and
decreased when the E2β reached 10−6 M. Our results thus
indicated that the dose-response curves of gastric cancer cells to
E2β displayed a biphasic pattern and expression levels of ER-α36
shift the dose-response curve.
Correlation between estrogen expression
and Cyclin D1 expression in gastric cancer
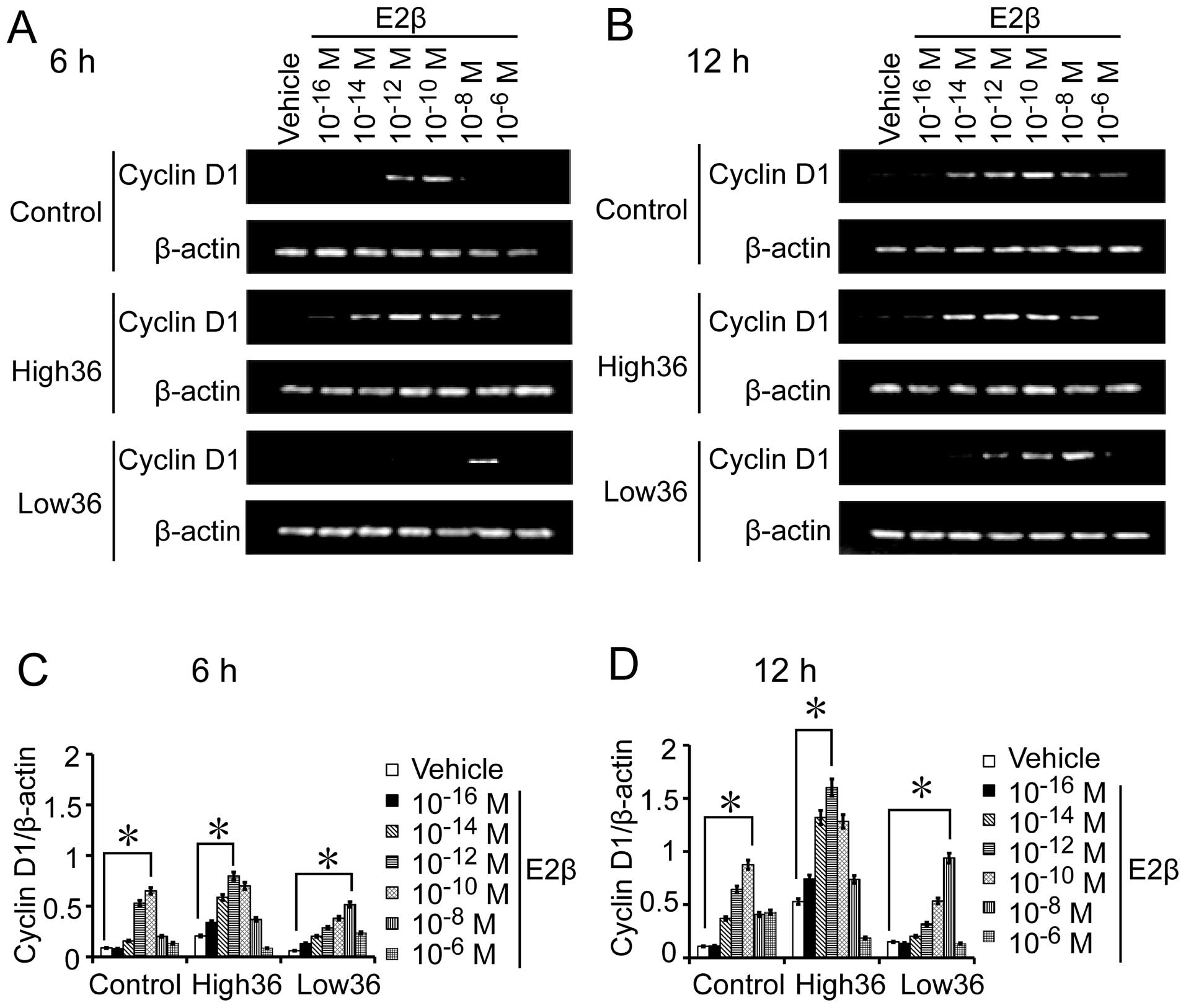
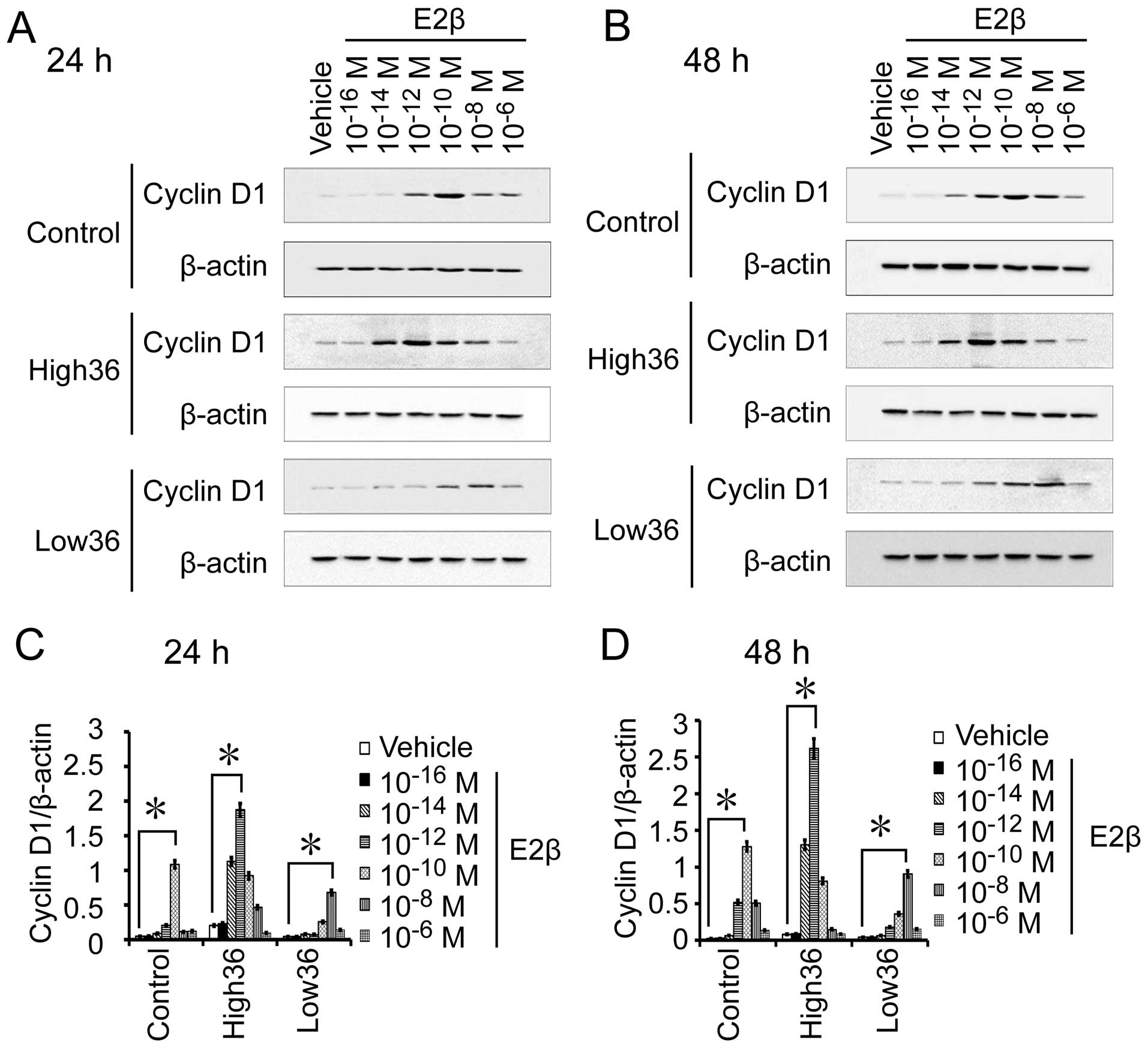
SGC7901 cells with different levels of ER-α36
expression. We also used different concentrations of estrogen to
treat SGC7901, SGC7901/High36 and SGC7901/Low36 cells for 6 and 12
h to examine Cyclin D1 mRNA expression, and for 24 and 48 h to
detect Cyclin D1 protein expression. As shown in Figs. 5 and 6, the 10−12 M of E2β-induced
Cyclin D1 expression in SGC7901 cells, which peaked at
10−10 M and decreased at 10−8 M. In
SGC7901/High36 cells, however, 10−14 M of E2β induced
Cyclin D1 expression, which peaked at 10−12 M and
decreased at 10−10 M (Figs.
5 and 6). The SGC7901/Low36
cells required higher concentrations of E2β (10−10 M) to
induce Cyclin D1 expression, which was decreased at 10−6
M (Figs. 5 and 6). Our results indicated that induction
of Cyclin D1 expression by estrogen in gastric cancer cells also
exhibited a biphasic pattern, which is influenced by expression
levels of ER-α36.
Discussion
Despite advances in various diagnostic tools and
therapy, the 5-year relative survival rate of gastric cancer is
still low (1,12–14).
Epidemiology studies have shown the male predominance of gastric
cancer (3). The predominance is
also shown by a delay of 10–15 years in the appearance and onset of
gastric cancer in females compared with males (3,4).
Accumulating evidence suggested that estrogen played a protective
role in the incidence of gastric cancer. However, the underlying
mechanisms of male dominance in gastric cancer have not been
established.
Our study showed that ER-α36 expression was
correlated well with male patients (P=0.01), invasion to serosa
(P=0.01) and Cyclin D1 expression (P<0.01) in gastric cancer
tissues. Our results thus suggested that ER-α36 may be involved in
observed male predominance in human gastric cancer. We also found
gastric cancer cells were stimulated by estrogen, which exhibited a
biphasic growth curve, and cells with high levels of ER-α36
expression are more sensitive to estrogen and required less
estrogen to stimulate cell growth compared to cells express lower
levels of ER-α36.
Cyclin D1 is an important regulatory factor for cell
cycle progression that is required to mediate the G1 to S
transition and cell cycle progression (15–17).
Cyclin D1 overexpression has been documented in several carcinomas,
including gastric cancer (18–21).
It has been reported that a gender difference in MNNG-induced rat
gastric carcinogenesis is involved in the gender difference of
Cyclin D1/cdk4 expression (22).
Here, we found that Cyclin D1 expression is induced by
ER-α36-mediated estrogen signaling in gastric cancer cells and is
also well correlated with ER-α36 expression in human gastric tumor
tissues. We found estrogen induction of Cyclin D1 expression also
exhibited a biphasic pattern; induces Cyclin D1 expression at low
concentrations and failed to do so at high concentrations,
consistent with growth curves of gastric cancer cells in response
to estrogen. Our results thus indicated that Cyclin D1 may be one
of the downstream effectors of ER-α36 mediated mitogenic estrogen
signaling in gastric cancer cells.
The findings that gastric cells with high levels of
ER-α36 require pM even fM range estrogen to stimulate cell growth
and estrogen at μM range to inhibit cell growth while cells with
low levels of ER-α36 respond to estrogen at μM range and fail to
respond to estrogen at nM range may provide a molecular explanation
to the male dominance in the incidence of human gastric cancer and
a mechanism for the protection role of estrogen in gastric
cancer.
Acknowledgements
This study was supported by National Natural Science
Foundation of China (nos. 81272754 and 30870981) and Science
Foundation of Health Office of Hubei Province (no. NX200727).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Brenner H, Rothenbacher D and Arndt V:
Epidemiology of stomach cancer. Methods Mol Biol. 472:467–477.
2009. View Article : Google Scholar
|
|
3
|
Fu Z, Deng H, Wang X, Yang X, Wang Z and
Liu L: Involvement of ER-alpha36 in the malignant growth of gastric
carcinoma cells is associated with GRP94 overexpression.
Histopathology. 63:325–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sipponen P and Correa P: Delayed rise in
incidence of gastric cancer in females results in unique sex ratio
(M/F) pattern: etiologic hypothesis. Gastric Cancer. 5:213–219.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chandanos E and Lagergren J: Oestrogen and
the enigmatic male predominance of gastric cancer. Eur J Cancer.
44:2397–2403. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
7
|
Parkin DM, Pisani P and Ferlay J: Global
cancer statistics. CA Cancer J Clin. 49:33–64. 1999. View Article : Google Scholar
|
|
8
|
Furukawa H, Iwanaga T, Koyama H and
Taniguchi H: Effect of sex hormones on carcinogenesis in the
stomachs of rats. Cancer Res. 42:5181–5182. 1982.PubMed/NCBI
|
|
9
|
Wang Z, Zhang X, Shen P, Loggie BW, Chang
Y and Deuel TF: A variant of estrogen receptor-{alpha},
hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent
membrane-initiated mitogenic signaling. Proc Natl Acad Sci USA.
103:9063–9068. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Wang L, Han R, et al:
Identification of the forkhead transcriptional factor 2 (FOXL2)
gene mutations in four Chinese families with blepharophimosis
syndrome. Mol Vis. 19:2298–2305. 2013.PubMed/NCBI
|
|
11
|
Deng H, Huang X, Fan J, et al: A variant
of estrogen receptor-alpha, ER-alpha36 is expressed in human
gastric cancer and is highly correlated with lymph node metastasis.
Oncol Rep. 24:171–176. 2010.PubMed/NCBI
|
|
12
|
D’Angelo A, Bluteau O, Garcia-Gonzalez MA,
et al: Hepatocyte nuclear factor 1alpha and beta control terminal
differentiation and cell fate commitment in the gut epithelium.
Development. 137:1573–1582. 2010.PubMed/NCBI
|
|
13
|
Yuasa Y: Control of gut differentiation
and intestinal-type gastric carcinogenesis. Nat Rev Cancer.
3:592–600. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wilkinson NW, Howe J, Gay G, Patel-Parekh
L, Scott-Conner C and Donohue J: Differences in the pattern of
presentation and treatment of proximal and distal gastric cancer:
results of the 2001 gastric patient care evaluation. Ann Surg
Oncol. 15:1644–1650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Q, Sakamoto K and Wagner KU: D-type
cyclins are important downstream effectors of cytokine signaling
that regulate the proliferation of normal and neoplastic mammary
epithelial cells. Mol Cell Endocrinol. 382:583–592. 2014.
View Article : Google Scholar
|
|
16
|
Glassford J, Rabin N, Lam EW and Yong KL:
Functional regulation of D-type cyclins by insulin-like growth
factor-I and serum in multiple myeloma cells. Br J Haematol.
139:243–254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sherr CJ: D-type cyclins. Trends Biochem
Sci. 20:187–190. 1995. View Article : Google Scholar
|
|
18
|
Sauter ER, Yeo UC, von Stemm A, et al:
Cyclin D1 is a candidate oncogene in cutaneous melanoma. Cancer
Res. 62:3200–3206. 2002.PubMed/NCBI
|
|
19
|
Udhayakumar G, Jayanthi V, Devaraj N and
Devaraj H: Interaction of MUC1 with beta-catenin modulates the Wnt
target gene cyclinD1 in H. pylori-induced gastric cancer. Mol
Carcinog. 46:807–817. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arber N, Gammon MD, Hibshoosh H, et al:
Overexpression of Cyclin D1 occurs in both squamous carcinomas and
adenocarcinomas of the esophagus and in adenocarcinomas of the
stomach. Hum Pathol. 30:1087–1092. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bar-Sela G, Hershkovitz D, Haim N,
Kaidar-Person O, Shulman K and Ben-Izhak O: The incidence and
prognostic value of HER2 overexpression and cyclin D1 expression in
patients with gastric or gastroesophageal junction adenocarcinoma
in Israel. Oncol Lett. 5:559–563. 2013.PubMed/NCBI
|
|
22
|
Motohashi M, Wakui S, Muto T, et al:
Cyclin D1/cdk4, estrogen receptors alpha and beta, in
N-methyl-N′-nitro-N-nitrosoguanidine-induced rat gastric
carcinogenesis: immunohistochemical study. J Toxicol Sci.
36:373–378. 2011.PubMed/NCBI
|