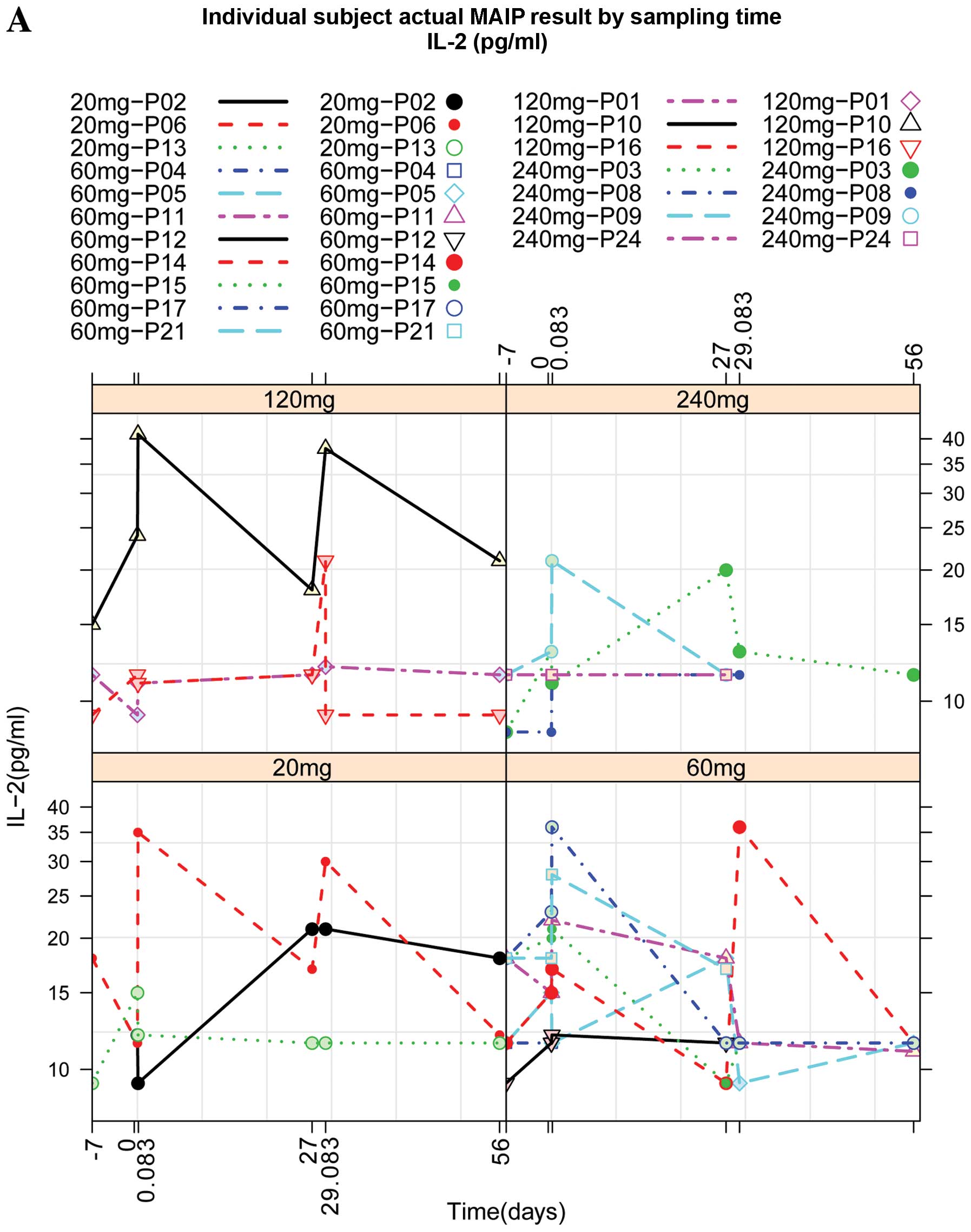
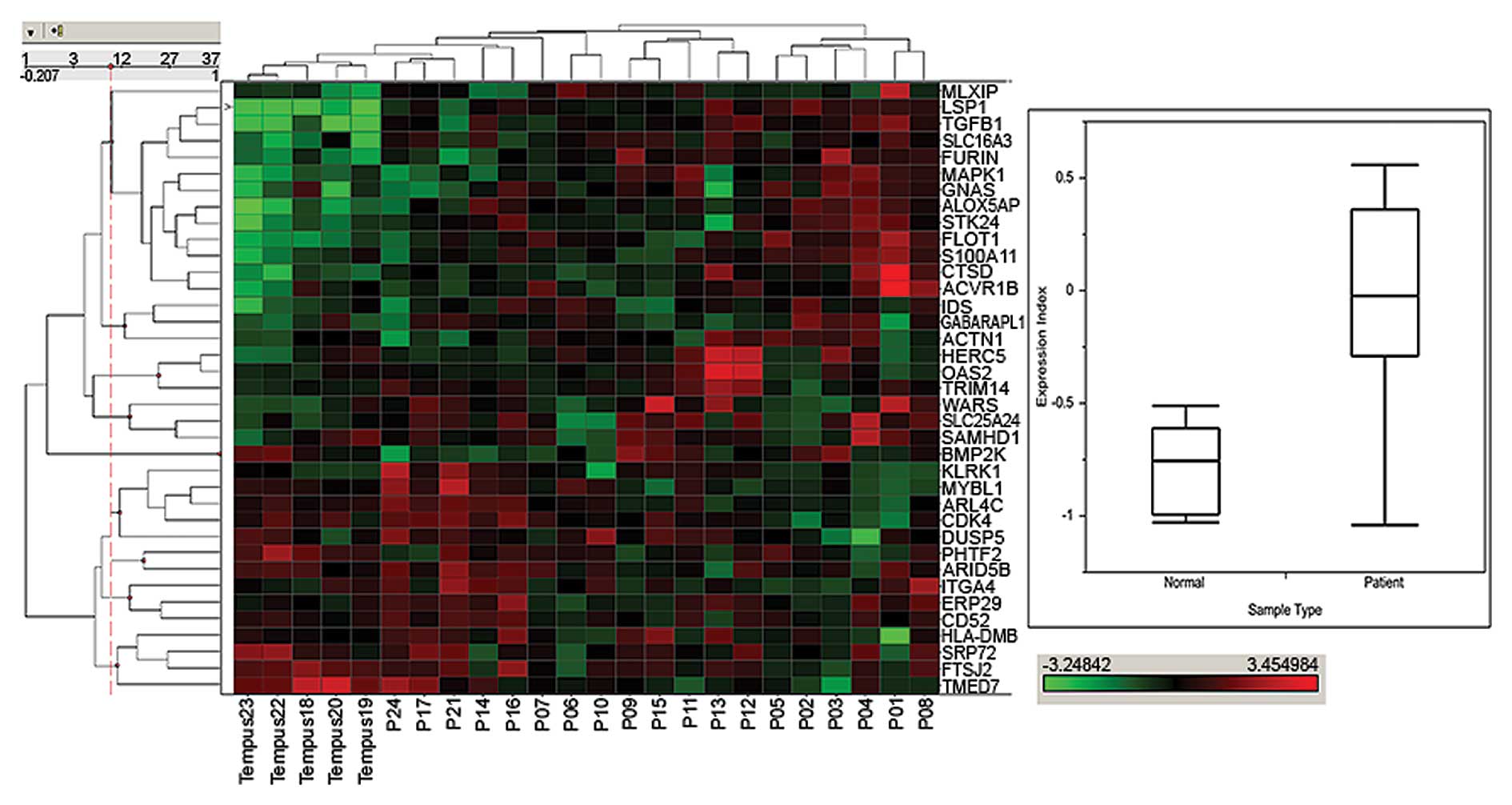
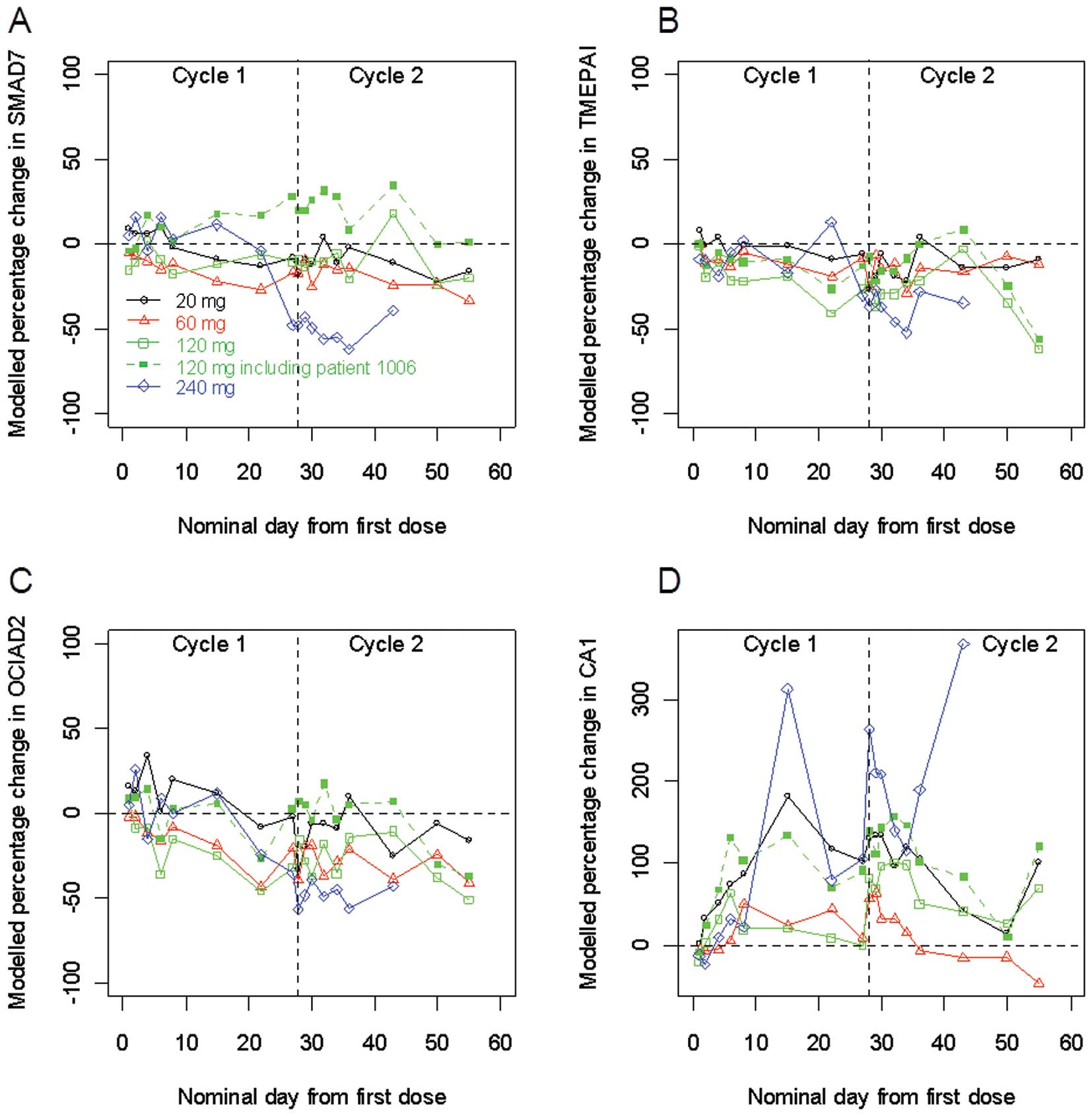
|
1
|
Massagué J: TGFβ signal transduction. Annu
Rev Biochem. 67:753–791. 1998.
|
|
2
|
Muraoka-Cook RS, Dumont N and Arteaga CL:
Dual role of transforming growth factor beta in mammary
tumorigenesis and metastatic progression. Clin Cancer Res.
11:937s–943s. 2005.PubMed/NCBI
|
|
3
|
Sun L: Tumour-suppressive and promoting
function of transforming growth factor beta. Front Biosci.
9:1925–1935. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Caestecker MP, Piek E and Robers AB:
Role of transforming growth factor-beta signaling in cancer. J Natl
Cancer Inst. 92:1388–1402. 2000.PubMed/NCBI
|
|
5
|
Park BJ, Park JI, Byun DS, Park JH and Chi
SG: Mitogenic conversion of transforming growth factor-beta1 effect
by oncogenic Ha-Ras-induced activation of the mitogen-activated
protein kinase-signaling pathway in human prostate cancer. Cancer
Res. 60:3031–3038. 2000.
|
|
6
|
Chen YG, Hata A, Lo RS, Wotton D, Shi Y,
Pavletich N and Massagué J: Determinants of specificity in TGF-beta
signal transduction. Genes Dev. 12:2144–2152. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siegel PM, Shu W, Cardiff RD, Muller WJ
and Massagué J: Transforming growth factor beta signaling impairs
neuinduced mammary tumorigenesis while promoting pulmonary
metastasis. Proc Natl Acad Sci USA. 100:8430–8435. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adler H, McCurdy M, Kattan M, Timme T,
Scardino P and Thompson T: Elevated levels of circulating
interleukin-6 and transforming growth factor-beta 1 in patients
with metastatic prostatic carcinoma. J Urol. 161:182–187. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shariat SF, Shaler M, Menesses-Diaz A, Kim
IY, Kattan MW, Wheeler TM and Slawin KM: Preoperative plasma levels
of transforming growth factor β-1 (TGF-β1) strongly predict
progression in patients undergoing radical prostatectomy. J Clin
Oncol. 19:2856–2864. 2001.
|
|
10
|
Shariat SF, Kattan MW, Traxel E, Andrews
B, Zhu K, Wheeler TM and Slawin KM: Association of pre- and
postoperative plasma levels of transforming growth factor beta (1)
and interleukin 6 and its soluble receptor with prostate cancer
progression. Clin Cancer Res. 10:1992–1999. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kattan M, Shariat SF, Andrews B, et al:
The addition of interleukin-6 soluble receptor and transforming
growth factor-beta1 improves a preoperative nomogram for predicting
biochemical progression in patients with clinically localized
prostate cancer. J Clin Oncol. 21:3573–3579. 2003. View Article : Google Scholar
|
|
12
|
Kakehi Y, Oka H, Mitsumori K, Itoh N,
Ogawa O and Yoshida O: Elevation of serum transforming growth
factor-β1 level in patients with metastatic prostate cancer. Urol
Oncol. 2:131–135. 1996.
|
|
13
|
Ivanovic V, Melman A, Davis-Joseph B,
Valcic M and Geliebter J: Elevated plasma levels of TGF-beta 1 in
patients with invasive prostate cancer. Nat Med. 1:282–284. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong F, Jirtle RL, Huang DH, Clough RW and
Anscher MS: Plasma transforming growth factor-beta1 level before
radiotherapy correlates with long term outcome of patients with
lung carcinoma. Cancer. 86:1712–1719. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kong FM, Anscher MS, Murase T, Abbott BD,
Iglehart JD and Jirtle RL: Elevated plasma transforming growth
factor-beta 1 levels in breast cancer patients decrease after
surgical removal of the tumor. Ann Surg. 222:155–162. 1995.
View Article : Google Scholar
|
|
16
|
Kong FM, Washington MK, Jirtle RL and
Anscher MS: Plasma transforming growth factor-beta 1 reflects
disease status in patients with lung cancer after radiotherapy: a
possible tumor marker. Lung Cancer. 16:47–59. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lahn M, Berry B, Kloeker S and Yingling J:
TGF-β receptor kinase inhibitors for the treatment of cancer. Smad
Signal Transduction: Smads in Proliferation, Differentiation and
Disease. Ten Dijke P and Heldin C-H: Springer-Verlad; New York, NY:
pp. 415–442. 2006
|
|
18
|
Shim KS, Kim KH, Han WS and Park EB:
Elevated serum levels of transforming growth factor-beta 1 in
patients with colorectal carcinoma: its association with tumor
progression and its significant decrease after curative surgery
resection. Cancer. 85:554–561. 1999. View Article : Google Scholar
|
|
19
|
Sheen-Chen SM, Chen HS, Sheen CW, Eng HL
and Chen WJ: Serum levels of transforming growth factor beta 1 in
patients with breast cancer. Arch Surg. 136:937–940. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsushima H, Ito N, Tamura S, et al:
Circulating transforming growth factor beta1 as a predictor of
liver metastasis after resection in colorectal cancer. Clin Cancer
Res. 7:1258–1262. 2001.PubMed/NCBI
|
|
21
|
Barthelemy-Brichant N, David JL, Bosquee
L, et al: Increased TGF-beta 1 plasma level in patients with lung
cancer: potential mechanisms. Eur J Clin Invest. 32:193–198. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiong B, Yuan HY, Hu MB, Zhang F, Wei ZZ,
Gong LL and Yang GL: Transforming growth factor-beta1 in invasion
and metastasis in colorectal cancer. World J Gastroenterol.
8:674–678. 2002.PubMed/NCBI
|
|
23
|
Hau P, Bogdahn U, Stauder G, Kunst M and
Schlingensiepen KH: TGF-β2 specific antisense oligonucleotide
(AP-12009) as continuous intratumoural treatment of recurrent
high-grade glioma patients. Arzneim-Forsch Drug Res.
53:4642003.
|
|
24
|
Dvorchik BH: The disposition (ADME) of
antisense oligonucleotides. Curr Opin Mol Ther. 2:253–257.
2000.PubMed/NCBI
|
|
25
|
Sawyer JS, Anderson BD, Beight DW, et al:
Synthesis and activity of new aryl- and heteroaryl-substituted
pyrazole inhibitors of the transforming growth factor-beta type I
receptor kinase domain. J Med Chem. 46:3953–3956. 2003. View Article : Google Scholar
|
|
26
|
Yingling JM, Blanchard KL and Sawyer JS:
Development of TGF-beta signaling inhibitors for cancer therapy.
Nat Rev Drug Discov. 3:1011–1022. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Robinson S, Pool R and Giffin R: Forum on
Drug Discovery, Development, and Translation: Emerging Safety
Science: Workshop Summary. National Academies Press; Washington,
DC: 2008
|
|
28
|
Lonning S, Mannick J and McPherson JM:
Antibody targeting of TGF-β in cancer patients. Curr Pharm
Biotechnol. 12:2176–2189. 2011.
|
|
29
|
Gatto B: Monoclonal antibodies in cancer
therapy. Curr Med Chem Anticancer Agents. 4:411–414. 2004.
View Article : Google Scholar
|
|
30
|
Muraoka RS, Dumont N, Ritter CA, et al:
Blockade of TGF-β inhibits mammary tumor cell viability, migration,
and metastases. J Clin Invest. 109:1551–1559. 2002.
|
|
31
|
Yang Y, Dukhanina O, Tang B, et al:
Lifetime exposure to a soluble TGF-β antagonist protects mice
against metastasis without adverse side effects. J Clin Invest.
109:1607–1615. 2002.PubMed/NCBI
|
|
32
|
Morris JC, Shapiro GI, Tan AR, et al:
Phase I study of GC1008: A human anti-transforming growth
factor-beta (TGFβ) monoclonal antibody (MAb) in patients with
advanced malignant melanoma (MM) or renal cell carcinoma (RCC). J
Clin Oncol. 26:90282008.PubMed/NCBI
|
|
33
|
Trachtman H, Fervenza FC, Gipson DS, et
al: A phase 1, single-dose study of fresolimumab, an anti-TGF-β
antibody, in treatment-resistant primary focal segmental
glomerulosclerosis. Kidney Int. 79:1236–1243. 2011.PubMed/NCBI
|
|
34
|
O’Brien PJ, Ramanathan R, Yingling JM, et
al: Analysis and variability of TGF-beta measurements in cancer
patients with skeletal metastases. Biologics. 2:563–569.
2008.PubMed/NCBI
|
|
35
|
Kadam S, Cleverly AL, Farmen M, et al: A
canonical transforming growth factor beta-dependent signaling
pathway is present in peripheral blood cells of cancer patients
with skeletal metastasis. J Mol Biomark Diagn. 4:1532005.
|
|
36
|
Zhaou L and Rocke DM: An expression index
for Affymetrix GeneChips based on the generalized logarithm.
Bioinformatics. 21:3983–3969. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang Y, Mariano JM, Angdisen J, Moody TW,
Diwan BA, Wakefield LM and Jakowlew SB: Enhanced tumorigenesis and
reduced transforming growth factor-β type II receptor in lung
tumors from mice with reduced gene dosage of transforming growth
factor-beta1. Mol Carcinog. 29:112–126. 2000.
|
|
38
|
Lobo ED, Hansen RJ and Balthsar JP:
Antibody pharmacokinetics and pharmacodynamics. J Pharmacol Sci.
93:2645–2668. 2004. View Article : Google Scholar
|
|
39
|
Classen S, Muth C, Debey-Pascher S, et al:
Application of T cell-based transcriptomics to identify three
candidate biomarkers for monitoring anti-TGF-βR therapy.
Pharmacogenet Genomics. 20:147–156. 2010.PubMed/NCBI
|
|
40
|
Baselga J, Rothenberg ML, Tabernero J, et
al: TGF-β signalling related markers in cancer patients with bone
metastasis. Biomarkers. 13:217–236. 2008.
|
|
41
|
Horstmann E, McCabe MS, Grochow L, et al:
Risks and benefits of phase 1 oncology trials, 1991 through 2002. N
Engl J Med. 352:895–904. 2005. View Article : Google Scholar : PubMed/NCBI
|