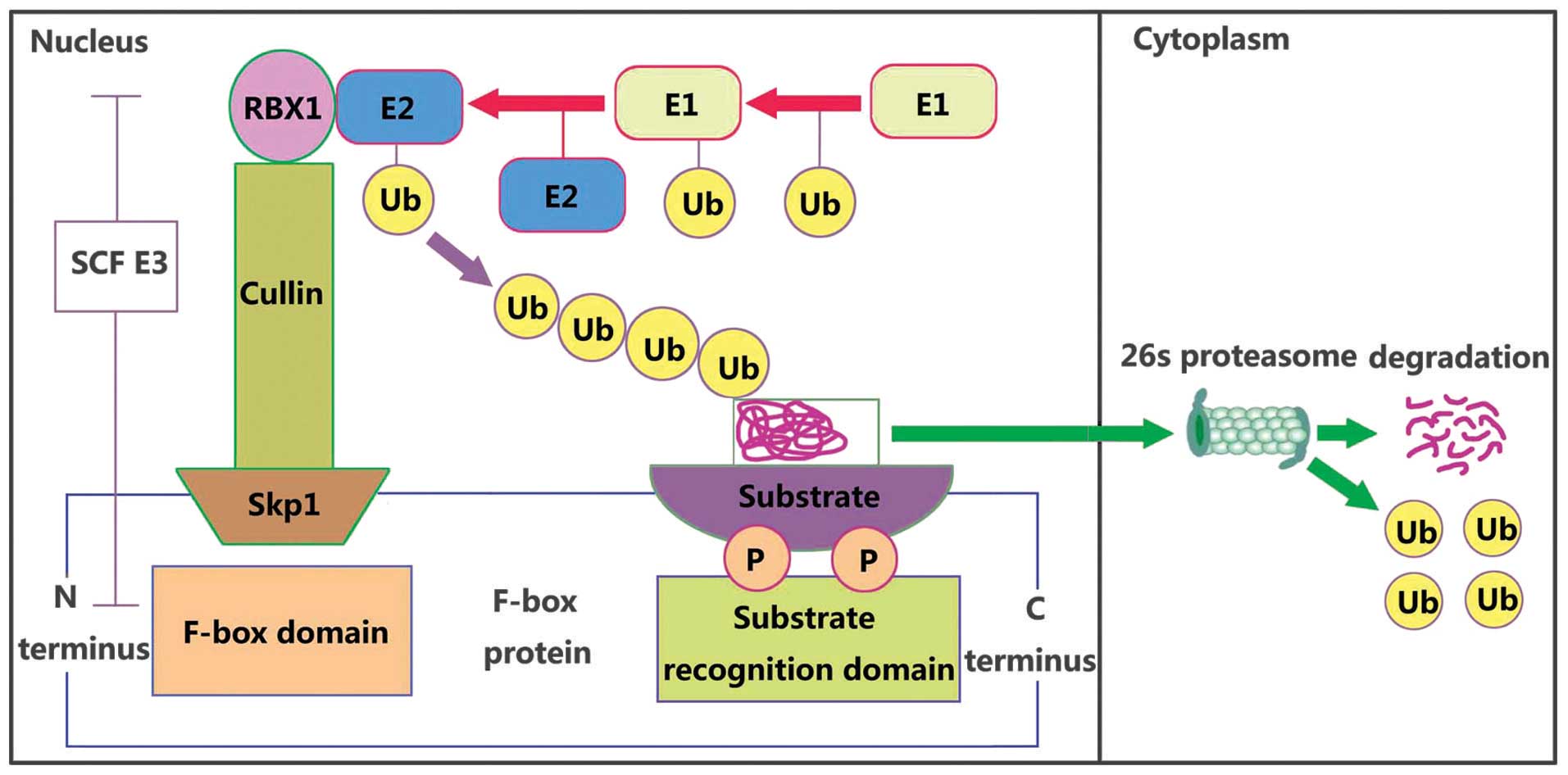
Protein ubiquitylation by the ubiquitin (Ub)
proteasome system (UPS) is a post-translational modification that
governs a broad array of basic cellular processes, and its
defective regulation is manifested in various human diseases
(1–3). UPS has a crucial role in maintaining
and regulating cellular homeostasis (4). The change of ubiquitination is
closely related to the occurrence of a wide variety of tumors. The
UPS exerts its functions mainly through the concerted efforts of a
group of enzymes (5–7) (Fig.
1): the E1 Ub-activating enzyme, E2 Ub-conjugating enzyme, and
E3 Ub ligase and 26S proteasome. Ub is activated in an
ATP-dependent manner by an Ub-activating enzyme (E1), and then
transferred to the active site cysteine of a conjugating enzyme
(E2) through a thioester bond. The E3 ligase facilitates the
attachment of Ub onto the substrate protein from the E2 enzyme.
Next, the Ub proteins are recognized and then degraded by 26S
proteasome to several small peptides. There are >1,000 putative
E3 Ub ligases belonging to two major families, the homologous to
E6-APC terminus (HECT) type and Ring/Cullin Ligase (RCL) type
(8,9). Among the E3 Ub ligase enzymes, the
RCL type of E3 ligases contain the largest number of family
members, among them, the Skp1-Cullin1-F-box (SCF) E3 ligase complex
has recently come to prominence (10–12).
The SCF-type E3 ligase complex consists of four units: Skp1, Rbx1
and Cullin1, and F-box protein (FBP), the latter of which being
responsible for the substrate targeting specificity of the complex
(13,14). FBPs are characterized by ~40 amino
acids. Because this kind of structure domain was originally found
in the cycle of F protein (FBXO1), it is named ‘F-box structure
domain’. Without taking into account the various isoforms that may
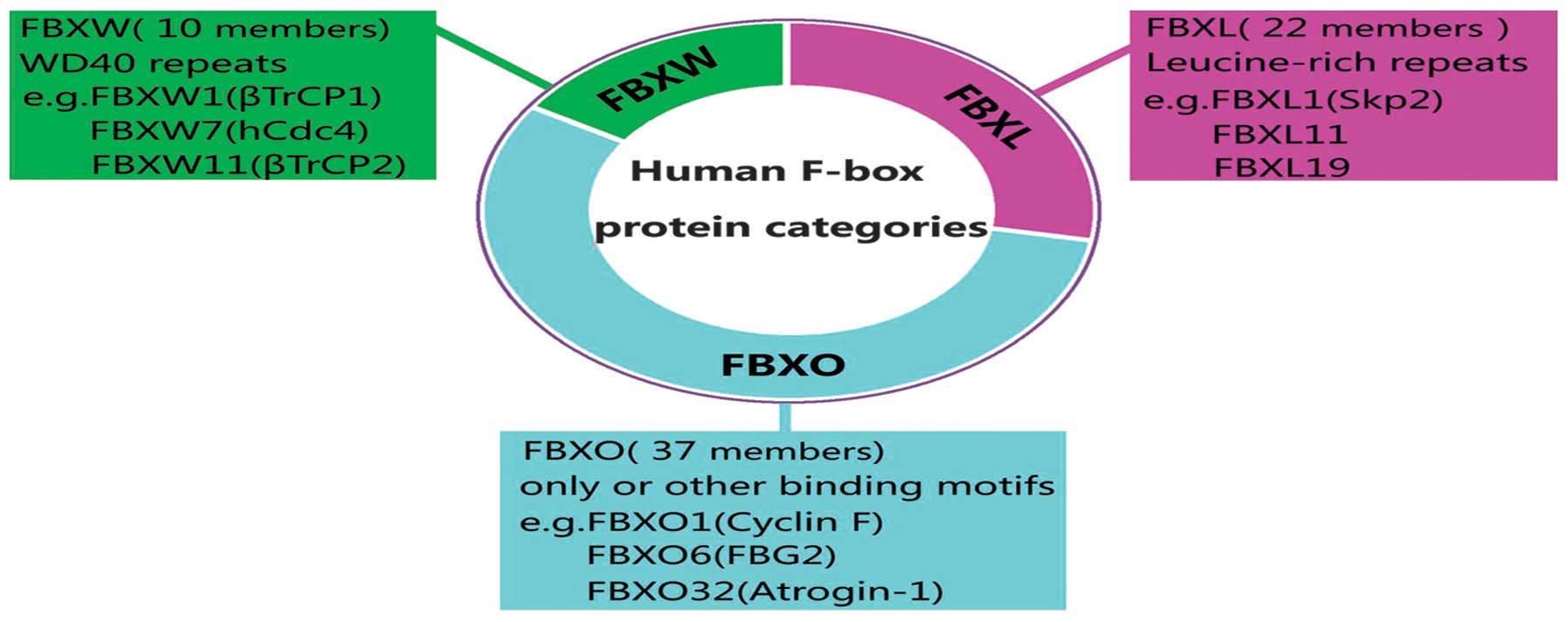
be produced, 69 human FBPs have been identified so far (10), but only few of them have been well
characterized. FBPs have been classified into three categories
according to their specific substrate recognition domains (Fig. 2) (15–17).
The FBXW subclass containing WD40 repeat domains is composed of 10
proteins. The FBXL family comprises of 22 proteins which are
leucine-rich repeat proteins. Other 37 F-box members containing
other domains such as zinc finger or ring finger constitute FBXO
family. FBPs are attractive candidates for drug discovery because
they play pivotal roles in various cancers.
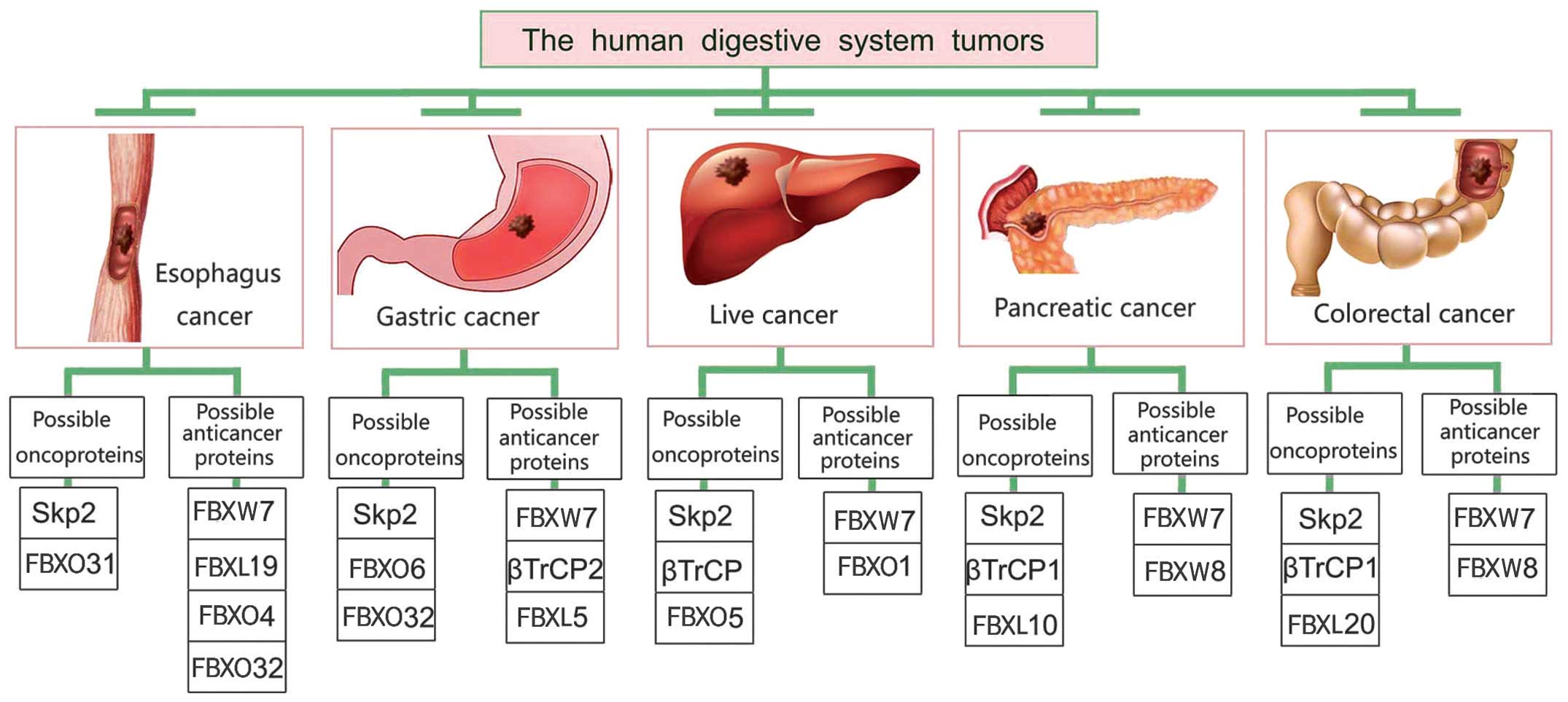
The common digestive system tumors are colorectal
cancer, gastric cancer, liver cancer, esophagus cancer and
pancreatic cancer (PC). According to the latest global cancer
statistics (Table I), colorectal
cancer is the third most common malignancy, while gastric cancer,
liver cancer and esophagus cancer are ranked the fourth, the fifth,
and the seventh respectively in all cancers. A total of 3,713,100
new cancer cases and 2,715,400 cancer deaths are responsible for
29.30% of worldwide total new cancer cases and 35.86% of deaths in
2008. There is a high necessity for accurate diagnosis of digestive
system tumors because of their poor prognosis due to
chemoresistance and a high recurrence rate. The main functions of
the digestive tract are the absorption, digestion and excretion.
The occurrence and development of digestive system tumors are
strongly associated with all sorts of stimulations and the
subsequently signal pathway activations caused by stimulations.
Studies have shown that FBP, one component of E3 ligase, can be
activated by the cell’s DNA damage caused by certain stimuli such
as heat and chemotherapy drugs (18,19).
Therefore, it is necessary and important to summarize the function
of FBPs in digestive system cancers.
The misregulated degradation of tumor suppressor
proteins or oncoproteins can drive tumorigenesis. Accordingly, FBPs
can function as oncoproteins when overexpressed (if their
substrates are tumor suppressors) or as tumor suppressors (if their
substrates are oncoproteins).
S-phase kinase-associated protein 2 (Skp2) is an
authenticated oncogenic protein (44). It was first discovered as an
element of CDK2/Cyclin A (45).
Then it was identified as a Skp1-binding protein to regulate cell
cycle progression (46). Skp2
drives cells from G1 to S phase through ubiquitylation and
degrading the p27 (47). p27, a
Cyclin-dependent kinase (CDK) inhibitor, is a negative regulator of
the cell cycle which is found decreased in cancers. So far, all
studied cases of cancer have indicated that high levels of Skp2
correlate with poor overall survival. The dysregulation of Skp2 and
p27 was found to be associated with tumor progression in human oral
(48), colon (49,50),
esophageal squamous cell carcinoma (ESCC) (51), gastric (52) and prostate cancer (53). Mouse models unequivocally confirmed
the role of the Skp2-p27 axis in tumorigenesis (54). Thus, Skp2 may serve as an
attractive target for the treatment of cancer.
β-transducin repeat-containing protein (βTrCP)
including βTrCP1 and βTrCP2 is overexpressed in multiple cancers,
such as colorectal cancer, pancreatic cancer, breast cancer and
melanoma, which supports an oncogenic function for these proteins
(55). However, in sharp contrast
to the tumor-promoting role of βTrCP described above, in gastric
cancer tumors, it has been shown to suppress tumor development
(56). Thus, βTrCP might have a
greater role as an oncogenic protein than as a tumor suppressor in
digestive system cancers (55).
Esophageal carcinoma is an age-related neoplasm with
a 5-year overall survival rate of <35% (57,58).
Esophageal cancer is one of the most frequently occurring
malignancies and the seventh leading cause of cancer-related deaths
in the world. ESCC is the most prevalent type of esophageal cancer
in China and the survival rate of ESCC patients is <10%
(59,60). Due to the changes in lifestyle such
as smoking and physical inactivity, the incidence of cancer becomes
increasingly high.
A total of 989,600 new stomach cancer cases and
738,000 deaths are estimated to have occurred in 2008, accounting
for 8% of the total cases and 10% of total deaths (83). The morbidity of gastric cancer is
the second most common, after lung cancer according to global
cancer statistics (83).
FBXO6, also named Fbg2, mainly targets checkpoint
kinase 1 (Chk1) for ubiquitination and degradation. Low expression
of FBXO6 causing Chk1 accumulation might increase tumor cell
resistance to chemotherapy drugs (95,96).
Chk1 is the main replication checkpoint for cellular sensitivity to
replicative stress. It has been proved to be overexpressed in
cancers (97). Intriguingly,
recent evidence questions the role of FBXO6 in gastric cancer.
Zhang et al (98) reported that
FBXO6 promotes the growth, proliferation and invasion of gastric
cancer cells as well as normal gastric cells. FBXO32 is also
involved in promoting tumorigenesis in gastric cancer cells
(99).
Acetaldehyde contributing to more aggressive
phenotypes in hepatocellular carcinoma cell line HEPG2 might result
from activating the expression of βTrCP (107). FBXW7, a universally
acknowledged tumor suppressor gene, decreased in hepatocellular
carcinoma tissues. FBXW7 was thought to be the strongest
independent risk factor for hepatocellular carcinoma recurrence or
prognostic marker (108). A
recent study shows that Yes-associated protein (YAP) may be a
potential target of FBXW7 in hepatocellular carcinoma (109). YAP is often overexpressed in
various types of human cancers (110). FBXW7 protein expression was
negatively correlated with c-Myc, Cyclin E and p53 in
hepatocellular carcinoma tissues (111). Recombinant human adenovirus-p53
can inhibit tumor cell growth with FBXW7 upregulation in murine
hepatocellular carcinoma model (112). This provides a new potential
therapy for HCC.
Notably, Cyclin F (FBXO1), is downregulated in liver
cancer, indicating poor survival and recurrence (113). FBXO5, named early mitotic
inhibitor-1 (Emi1), is highly expressed in 114 human hepatocellular
carcinoma samples. Emi1 increases hepatocellular carcinoma cell
proliferation by inhibiting the degradation of Skp2, thus reducing
the expression of p27 (114).
This result indicates possible crosstalk between individual FBPs.
FBXO31 functions as a tumor suppressor mainly through the
degradation of Cyclin D1 in liver cancer (77), which is consistent with the results
in breast cancer (79).
One study showed that Skp2 is also overexpressed in
both biliary tract carcinoma (BTC) cell lines and primary BTC
predicting poor prognosis. However, the levels of Skp2 in BTC and
p27 proteins were not correlated inversely with other tumors
(115). Also p27 can be degraded
by other means in BTC. Data from another study reported that the
expression of p27 and Skp2 are significantly inversely correlated
in 74 patients with ICCs (116).
Silencing of the Skp2 gene can on one hand slow down the
growth in a nude mouse tumor model, and on the other hand, inhibit
the proliferation, migration and invasiveness of gallbladder
carcinoma cell line GBC-SD by enhancing the expression of the p27
protein (117). Loss of FBXW7
expression is correlated with lymph node metastasis in ICC, which
tends to be an independent prognostic factor for both overall and
disease-free survival (32).
Pancreatic cancer (PC) is rare, with the incidence
rate 2.5% of all forms of cancers, while the mortality rate has
reached 96% (118). Because the
conventional treatments of PC have little effect on disease course,
the 5-year survival of PC is <5% (119,120). Most patients die within the first
year of diagnosis (121).
Therefore, better in-depth knowledge of the molecular mechanisms
might reveal new avenues for early diagnosis, and treatment of
patients.
The FBPs have rarely been studied in human PC. It
has been accepted by researchers that expression of Skp2 is high in
many advanced cancers. Consistent with a putative oncogenic role,
high expression level of Skp2 correlating with histological grade,
lymph node metastasis, lymphatic permeation and poor outcome has
been implicated in human pancreatic ductal carcinoma tissue
(122). Schüler et al
disclosed for Skp2 a novel function in pancreatic ductal
adenocarcinoma (PDAC) cells. Skp2 can resist TNF-related
apoptosis-inducing ligand (TRAIL)-induced apoptosis (123). Blocking the expression of βTRCP1
in PC cell line PancTu-1 can reduce nuclear factor-κB (NF-κB)
activation and chemoresistance (124). FBXW7 mutations were found in PC
(35). Genistein, a soy derived
isoflavone, exerts its antitumor activity partly through the
upregulation of FBXW7 and downregulation of miR-223 in PC cells
(125). Knockdown of FBXW8 can
inhibit cell proliferation of PC cells (126). FBXL10, a nucleolar protein that
represses transcription of ribosomal RNA genes (127), can promote leukemia mouse model
development (128), but its
expression is low in aggressive brain tumors (127). The expression of FBXL10 is high
in human PC tissues, and higher expression levels of FBXL10 are
correlated with disease grade and stage, as well as metastasis.
FBXL10 overexpression co-operated with KrasG12D, which promotes
PDAC formation in mouse models (129).
Colorectal cancer is the second most diagnosed
cancer in females and the third leading cause of cancer-related
death for females with an estimated 1.2 million new cases and
608,700 deaths in 2008 (3).
Colorectal cancer incidence rates are rapidly increasing in several
areas (130,131).
Earlier observation demonstrates that IκB and
β-catenin have a similar motif for the degradation via UPS pathway,
indicating that the ubiquitination of the two proteins is mediated
by the same E3 ligase (144).
IκB, inhibitor of NF-κB, functions as a tumor suppressor. β-catenin
is a downstream molecule of Wnt signaling pathways. β-catenin is an
oncoprotein that was found routinely activated in tumors and has
been correlated with poor prognosis and short survival (145,146). βTrCP targeting the degradation of
both β-catenin and IκB has been verified (147,148). Ougolkov et al (149) reported that 56% (25/45) of the
tumors had increased βTrCP1 mRNA aånd protein levels in colorectal
cancer compared to the normal colorectal tissues. Increased βTrCP1
levels were significantly associated with β-catenin activation.
This result indicated that βTrCP1 may act as an oncogene in
colorectal cancer.
During the last 10 years since the identification
and annotation of the FBP family, the continued identification and
characterization of novel substrates has greatly expanded our
knowledge. To date, 69 FBPs have been identified in humans.
However, only Skp2, FBXW7 and βTrCP are well recognized with their
matched downstream substrate in different cancers. The
identification of substrates for FBPs in different tissues remains
a major endeavor for researchers.
Above all, FBPs are important in the occurrence and
development of digestive system tumorigenesis, leading the high
level research into the pathogenesis of these tumors. We should
reveal further mechanism of the FBPs on the cellular and molecular
levels. Although a great number of FBPs have been identified in
digestive system tumors (Fig. 3),
this area of research and our current understanding of the FBP
family remains in its infancy. Plenty of questions remain to be
answered. Do the FBPs in a cell compete for binding to the Cullin
scaffold and consequently are unable to participate in
ubiquitination reactions in digestive system tumors? Will a certain
FBP function as a tumor suppressor or be oncogenic in different
stages of disease or different tissues of the same digestive system
tumor? Does intricate crosstalk exist among FBPs in digestive
system tumors? How does the FBP’s expression vary after primary
carcinomas metastasizing to lymph nodes in digestive system tumors?
Future study on FBP activity in these digestive system tumors will
be of great interest and the different biological characteristics
of a given FBP in different tissues will surely bring us new
insight. Bortezomib, a reversible inhibitor of the catalytic
activity of the 26S proteasome, has revealed effectiveness in the
treatment of mantle cell lymphoma and multiple myeloma (150,151). In addition, we believe inhibitors
targeting the FBPs are promising in the prevention and treatment of
digestive system tumors.
We are grateful to Liang Lv for graphic support, and
Dr Jirong Huo for English form revision of the review.
|
1
|
Genschik P, Sumara I and Lechner E: The
emerging family of CULLIN3-RING ubiquitin ligases (CRL3s): cellular
functions and disease implications. EMBO J. 32:2307–2320. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ciechanover A, Orian A and Schwartz AL:
Ubiquitin-mediated proteolysis: biological regulation via
destruction. Bioessays. 22:442–451. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smalle J and Vierstra RD: The ubiquitin
26S proteasome proteolytic pathway. Annu Rev Plant Biol.
55:555–590. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crusio KM, King B, Reavie LB and Aifantis
I: The ubiquitous nature of cancer: the role of the SCF(Fbw7)
complex in development and transformation. Oncogene. 29:4865–4873.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hershko A: The ubiquitin system for
protein degradation and some of its roles in the control of the
cell division cycle. Cell Death Differ. 12:1191–1197. 2005.
View Article : Google Scholar
|
|
6
|
Pickart CM and Rose IA: Functional
heterogeneity of ubiquitin carrier proteins. J Biol Chem.
260:1573–1581. 1985.PubMed/NCBI
|
|
7
|
Jadhav T and Wooten MW: Defining an
embedded code for protein ubiquitination. J Proteomics Bioinform.
2:3162009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakayama KI and Nakayama K: Ubiquitin
ligases: cell-cycle control and cancer. Nat Rev Cancer. 6:369–381.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jackson S and Xiong Y: CRL4s: the
CUL4-RING E3 ubiquitin ligases. Trends Biochem Sci. 34:562–570.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Z, Liu P, Inuzuka H and Wei W: Roles
of F-box proteins in cancer. Nat Rev Cancer. 14:233–247. 2014.
View Article : Google Scholar
|
|
11
|
Hicke L, Schubert HL and Hill CP:
Ubiquitin-binding domains. Nat Rev Mol Cell Biol. 6:610–621. 2005.
View Article : Google Scholar
|
|
12
|
Cardozo T and Pagano M: The SCF ubiquitin
ligase: insights into a molecular machine. Nat Rev Mol Cell Biol.
5:739–751. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nandi D, Tahiliani P, Kumar A and Chandu
D: The ubiquitin-proteasome system. J Biosci. 31:137–155. 2006.
View Article : Google Scholar
|
|
14
|
Zheng N, Schulman BA, Song L, et al:
Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin
ligase complex. Nature. 416:703–709. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cenciarelli C, Chiaur DS, Guardavaccaro D,
et al: Identification of a family of human F-box proteins. Curr
Biol. 9:1177–1179. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Winston JT, Koepp DM, Zhu C, et al: A
family of mammalian F-box proteins. Curr Biol. 9:1180–1182. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin J, Cardozo T, Lovering RC, et al:
Systematic analysis and nomenclature of mammalian F-box proteins.
Genes Dev. 18:2573–2580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J, Han L, Li B, et al: F-box only
protein 31 (FBXO31) negatively regulates p38 mitogen-activated
protein (MAPK) signaling by mediating lysine 48-linked
ubiquitination and degradation of MAP kinase kinase 6 (MKK6). J
Biol Chem. 289:21508–21518. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Santra MK, Wajapeyee N and Green MR: F-box
protein FBXO31 mediates cyclin D1 degradation to induce G1 arrest
after DNA damage. Nature. 459:722–725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koepp DM, Schaefer LK, Ye X, et al:
Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7
ubiquitin ligase. Science. 294:173–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Minella AC, Welcker M and Clurman BE: Ras
activity regulates cyclin E degradation by the Fbw7 pathway. Proc
Natl Acad Sci USA. 102:9649–9654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yada M, Hatakeyama S, Kamura T, et al:
Phosphorylation-dependent degradation of c-Myc is mediated by the
F-box protein Fbw7. EMBO J. 23:2116–2125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Welcker M, Orian A, Jin J, et al: The Fbw7
tumor suppressor regulates glycogen synthase kinase 3
phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad
Sci USA. 101:9085–9090. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei W, Jin J, Schlisio S, et al: The v-Jun
point mutation allows c-Jun to escape GSK3-dependent recognition
and destruction by the Fbw7 ubiquitin ligase. Cancer Cell. 8:25–33.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoeck JD, Jandke A, Blake SM, et al: Fbw7
controls neural stem cell differentiation and progenitor apoptosis
via Notch and c-Jun. Nat Neurosci. 13:1365–1372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tetzlaff MT, Yu W, Li M, et al: Defective
cardiovascular development and elevated cyclin E and Notch proteins
in mice lacking the Fbw7 F-box protein. Proc Natl Acad Sci USA.
101:3338–3345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan M, Zhao Y, Kim SJ, et al:
SAG/RBX2/ROC2 E3 ubiquitin ligase is essential for vascular and
neural development by targeting NF1 for degradation. Dev Cell.
21:1062–1076. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Akhoondi S, Sun D, von der Lehr N, et al:
FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer
Res. 67:9006–9012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyaki M, Yamaguchi T, Iijima T, et al:
Somatic mutations of the CDC4 (FBXW7) gene in hereditary
colorectal tumors. Oncology. 76:430–434. 2009.
|
|
30
|
Iwatsuki M, Mimori K, Ishii H, et al: Loss
of FBXW7, a cell cycle regulating gene, in colorectal cancer:
clinical significance. Int J Cancer. 126:1828–1837. 2010.PubMed/NCBI
|
|
31
|
Kemp Z, Rowan A, Chambers W, et al: CDC4
mutations occur in a subset of colorectal cancers but are not
predicted to cause loss of function and are not associated with
chromosomal instability. Cancer Res. 65:11361–11366. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Enkhbold C, Utsunomiya T, Morine Y, et al:
Loss of FBXW7 expression is associated with poor prognosis in
intrahepatic cholangiocarcinoma. Hepatol Res. Feb 19–2014.(Epub
ahead of print).
|
|
33
|
Lee JW, Soung YH, Kim HJ, et al:
Mutational analysis of the hCDC4 gene in gastric carcinomas.
Eur J Cancer. 42:2369–2373. 2006.
|
|
34
|
Sterian A, Kan T, Berki AT, et al:
Mutational and LOH analyses of the chromosome 4q region in
esophageal adenocarcinoma. Oncology. 70:168–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Calhoun ES, Jones JB, Ashfaq R, et al:
BRAF and FBXW7 (CDC4, FBW7, AGO, SEL10) mutations in distinct
subsets of pancreatic cancer: potential therapeutic targets. Am J
Pathol. 163:1255–1260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cassia R, Moreno-Bueno G,
Rodríguez-Perales S, et al: Cyclin E gene (CCNE)
amplification and hCDC4 mutations in endometrial carcinoma.
J Pathol. 201:589–595. 2003.
|
|
37
|
Woo LJ, Hwa SY, Young KS, et al: Somatic
mutation of hCDC4 gene is rare in lung adenocarcinomas. Acta
Oncol. 45:487–488. 2006.
|
|
38
|
Yan T, Wunder JS, Gokgoz N, et al: hCDC4
variation in osteo-sarcoma. Cancer Genet Cytogenet. 169:138–142.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kwak EL, Moberg KH, Wahrer DC, et al:
Infrequent mutations of Archipelago (hAGO, hCDC4,
Fbw7) in primary ovarian cancer. Gynecol Oncol. 98:124–128.
2005.
|
|
40
|
Inuzuka H, Fukushima H, Shaik S, et al:
Mcl-1 ubiquitination and destruction. Oncotarget. 2:239–244.
2011.
|
|
41
|
Inuzuka H, Shaik S, Onoyama I, et al:
SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for
ubiquitylation and destruction. Nature. 471:104–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wertz IE, Kusam S, Lam C, et al:
Sensitivity to antitubulin chemotherapeutics is regulated by MCL1
and FBW7. Nature. 471:110–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsunematsu R, Nakayama K, Oike Y, et al:
Mouse Fbw7/Sel-10/Cdc4 is required for notch degradation during
vascular development. J Biol Chem. 279:9417–9423. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gstaiger M, Jordan R, Lim M, et al: Skp2
is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci
USA. 98:5043–5048. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang H, Kobayashi R, Galaktionov K and
Beach D: p19Skp1 and p45Skp2 are essential elements of the cyclin
A-CDK2 S phase kinase. Cell. 82:915–925. 1995. View Article : Google Scholar
|
|
46
|
Bai C, Sen P, Hofmann K, et al: Skp1
connects cell cycle regulators to the ubiquitin proteolysis
machinery through a novel motif, the F-box. Cell. 86:263–274. 1996.
View Article : Google Scholar
|
|
47
|
Carrano AC, Eytan E, Hershko A and Pagano
M: Skp2 is required for ubiquitin-mediated degradation of the CDK
inhibitor p27. Nat Cell Biol. 1:193–199. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kudo Y, Kitajima S, Sato S, et al: High
expression of S-phase kinase-interacting protein 2, human F-box
protein, correlates with poor prognosis in oral squamous cell
carcinomas. Cancer Res. 61:7044–7047. 2001.PubMed/NCBI
|
|
49
|
Hershko D, Bornstein G, Ben-Izhak O, et
al: Inverse relation between levels of p27(Kip1) and of its
ubiquitin ligase subunit Skp2 in colorectal carcinomas. Cancer.
91:1745–1751. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mori M, Mimori K, Shiraishi T, et al: p27
expression and gastric carcinoma. Nat Med. 3:5931997. View Article : Google Scholar
|
|
51
|
Fukuchi M, Masuda N, Nakajima M, et al:
Inverse correlation between expression levels of p27 and the
ubiquitin ligase subunit Skp2 in early esophageal squamous cell
carcinoma. Anticancer Res. 24:777–783. 2004.
|
|
52
|
Masuda TA, Inoue H, Sonoda H, et al:
Clinical and biological significance of S-phase kinase-associated
protein 2 (Skp2) gene expression in gastric carcinoma: modulation
of malignant phenotype by Skp2 overexpression, possibly via p27
proteolysis. Cancer Res. 62:3819–3825. 2002.
|
|
53
|
Yang G, Ayala G, De Marzo A, et al:
Elevated Skp2 protein expression in human prostate cancer:
association with loss of the cyclin-dependent kinase inhibitor p27
and PTEN and with reduced recurrence-free survival. Clin Cancer
Res. 8:3419–3426. 2002.
|
|
54
|
Timmerbeul I, Garrett-Engele CM, Kossatz
U, et al: Testing the importance of p27 degradation by the SCFSkp2
pathway in murine models of lung and colon cancer. Proc Natl Acad
Sci USA. 103:14009–14014. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Frescas D and Pagano M: Deregulated
proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the
scales of cancer. Nat Rev Cancer. 8:438–449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kim CJ, Song JH, Cho YG, et al: Somatic
mutations of the beta-TrCP gene in gastric cancer. APMIS.
115:127–133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pisani P, Parkin DM and Ferlay J:
Estimates of the worldwide mortality from eighteen major cancers in
1985. Implications for prevention and projections of future burden.
Int J Cancer. 55:891–903. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sarbia M, Verreet P, Bittinger F, et al:
Basaloid squamous cell carcinoma of the esophagus: diagnosis and
prognosis. Cancer. 79:1871–1878. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Berger B and Belka C: Evidence-based
radiation oncology: oesophagus. Radiother Oncol. 92:276–290. 2009.
View Article : Google Scholar
|
|
60
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
61
|
Bai P, Xiao X, Zou J, et al: Expression of
p14 (ARF), p15 (INK4b), p16 (INK4a) and Skp2 increases during
esophageal squamous cell cancer progression. Exp Ther Med.
3:1026–1032. 2012.PubMed/NCBI
|
|
62
|
Wang XC, Tian LL, Tian J and Jiang XY:
Overexpression of SKP2 promotes the radiation resistance of
esophageal squamous cell carcinoma. Radiat Res. 177:52–58. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dong S, Zhao J, Wei J, et al: F-box
protein complex FBXL19 regulates TGFβ1-induced E-cadherin
down-regulation by mediating Rac3 ubiquitination and degradation.
Mol Cancer. 13:762014.PubMed/NCBI
|
|
64
|
Engers R, Ziegler S, Mueller M, et al:
Prognostic relevance of increased Rac GTPase expression in prostate
carcinomas. Endocr Relat Cancer. 14:245–256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Walker MP, Zhang M, Le TP, et al: RAC3 is
a pro-migratory co-activator of ERα. Oncogene. 30:1984–1994.
2011.PubMed/NCBI
|
|
66
|
van Roy F and Berx G: The cell-cell
adhesion molecule E-cadherin. Cell Mol Life Sci. 65:3756–3788.
2008.
|
|
67
|
Rodriguez FJ, Lewis-Tuffin LJ and
Anastasiadis PZ: E-cadherin’s dark side: possible role in tumor
progression. Biochim Biophys Acta. 1826:23–31. 2012.
|
|
68
|
Barbash O, Zamfirova P, Lin DI, et al:
Mutations in Fbx4 inhibit dimerization of the SCF(Fbx4) ligase and
contribute to cyclin D1 overexpression in human cancer. Cancer
Cell. 14:68–78. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Buckley MF, Sweeney KJ, Hamilton JA, et
al: Expression and amplification of cyclin genes in human breast
cancer. Oncogene. 8:2127–2133. 1993.PubMed/NCBI
|
|
70
|
Shinozaki H, Ozawa S, Ando N, et al:
Cyclin D1 amplification as a new predictive classification for
squamous cell carcinoma of the esophagus, adding gene information.
Clin Cancer Res. 2:1155–1161. 1996.PubMed/NCBI
|
|
71
|
Ikeguchi M, Sakatani T, Ueta T and Kaibara
N: Cyclin D1 expression and retinoblastoma gene protein (pRB)
expression in esophageal squamous cell carcinoma. J Cancer Res Clin
Oncol. 127:531–536. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Okabe H, Lee SH, Phuchareon J, et al: A
critical role for FBXW8 and MAPK in cyclin D1 degradation and
cancer cell proliferation. PLoS One. 1:e1282006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yu ZK, Gervais JL and Zhang H: Human CUL-1
associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1)
and cyclin D proteins. Proc Natl Acad Sci USA. 95:11324–11329.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kanie T, Onoyama I, Matsumoto A, et al:
Genetic reevaluation of the role of F-box proteins in cyclin D1
degradation. Mol Cell Biol. 32:590–605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Naganawa Y, Ishiguro H, Kuwabara Y, et al:
Decreased expression of FBXW7 is correlated with poor prognosis in
patients with esophageal squamous cell carcinoma. Exp Ther Med.
1:841–846. 2010.PubMed/NCBI
|
|
76
|
Kogo R, Mimori K, Tanaka F, et al: FBXO31
determines poor prognosis in esophageal squamous cell carcinoma.
Int J Oncol. 39:155–159. 2011.PubMed/NCBI
|
|
77
|
Huang HL, Zheng WL, Zhao R, et al: FBXO31
is down-regulated and may function as a tumor suppressor in
hepatocellular carcinoma. Oncol Rep. 24:715–720. 2010.PubMed/NCBI
|
|
78
|
Johansson P, Jeffery J, Al-Ejeh F, et al:
SCF-FBXO31 E3 ligase targets DNA replication factor Cdt1 for
proteolysis in the G2 phase of cell cycle to prevent
re-replication. J Biol Chem. 289:18514–18525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kumar R, Neilsen PM, Crawford J, et al:
FBXO31 is the chromosome 16q24.3 senescence gene, a
candidate breast tumor suppressor, and a component of an SCF
complex. Cancer Res. 65:11304–11313. 2005. View Article : Google Scholar
|
|
80
|
Dreissigacker U, Mueller MS, Unger M,
Siegert P, et al: Oncogenic K-Ras down-regulates Rac1 and RhoA
activity and enhances migration and invasion of pancreatic
carcinoma cells through activation of p38. Cell Signal.
18:1156–1168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tan J, Yang X, Zhuang L, et al:
Pharmacologic disruption of Polycomb-repressive complex 2-mediated
gene repression selectively induces apoptosis in cancer cells.
Genes Dev. 21:1050–1063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Guo W, Zhang M, Shen S, et al: Aberrant
methylation and decreased expression of the TGF-β/Smad target gene
FBXO32 in esophageal squamous cell carcinoma. Cancer.
120:2412–2413. 2014.
|
|
83
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
84
|
Ma XM, Liu Y, Guo JW, Liu JH and Zuo LF:
Relation of over-expression of S phase kinase-associated protein 2
with reduced expression of p27 and PTEN in human gastric carcinoma.
World J Gastroenterol. 11:6716–6721. 2005.PubMed/NCBI
|
|
85
|
Ma XM, Liu JH, Guo JW, et al: Correlation
of Skp2 expression in gastric carcinoma to expression of P27 and
PTEN. Ai Zheng. 25:56–61. 2006.(In Chinese).
|
|
86
|
Yang L, Kuang LG, Zheng HC, et al: PTEN
encoding product: a marker for tumorigenesis and progression of
gastric carcinoma. World J Gastroenterol. 9:35–39. 2003.PubMed/NCBI
|
|
87
|
Wei Z, Jiang X, Liu F, et al:
Downregulation of Skp2 inhibits the growth and metastasis of
gastric cancer cells in vitro and in vivo. Tumour Biol. 34:181–192.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cen G, Ding HH, Liu B and Wu WD: FBXL5
targets cortactin for ubiquitination-mediated destruction to
regulate gastric cancer cell migration. Tumour Biol. May
28–2014.(Epub ahead of print).
|
|
89
|
Saitoh T and Katoh M: Expression profiles
of βTRCP1 and βTRCP2, and mutation analysis of βTRCP2 in gastric
cancer. Int J Oncol. 18:959–964. 2001.
|
|
90
|
Milne AN, Leguit R, Corver WE, et al: Loss
of CDC4/FBXW7 in gastric carcinoma. Cell Oncol. 32:347–359.
2010.PubMed/NCBI
|
|
91
|
Yokobori T, Mimori K, Iwatsuki M, et al:
p53-altered FBXW7 expression determines poor prognosis in gastric
cancer cases. Cancer Res. 69:3788–3794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Martins CP, Brown-Swigart L and Evan GI:
Modeling the therapeutic efficacy of p53 restoration in tumors.
Cell. 127:1323–1334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ventura A, Kirsch DG, McLaughlin ME, et
al: Restoration of p53 function leads to tumour regression in vivo.
Nature. 445:661–665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Xue W, Zender L, Miething C, et al:
Senescence and tumour clearance is triggered by p53 restoration in
murine liver carcinomas. Nature. 445:656–660. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang YW, Brognard J, Coughlin C, et al:
The F box protein Fbx6 regulates Chk1 stability and cellular
sensitivity to replication stress. Mol Cell. 35:442–453. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Merry C, Fu K, Wang J, et al: Targeting
the checkpoint kinase Chk1 in cancer therapy. Cell Cycle.
9:279–283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Verlinden L, Vanden BI, Eelen G, et al:
The E2F-regulated gene Chk1 is highly expressed in
triple-negative estrogen receptor/progesterone receptor/HER-2
breast carcinomas. Cancer Res. 67:6574–6581. 2007.PubMed/NCBI
|
|
98
|
Zhang L, Hou Y, Wang M, et al: A study on
the functions of ubiquitin metabolic system related gene
FBG2 in gastric cancer cell line. J Exp Clin Cancer Res.
28:782009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lei KF, Liu BY, Wang YF, et al: SerpinB5
interacts with KHDRBS3 and FBXO32 in gastric cancer cells. Oncol
Rep. 26:1115–1120. 2011.PubMed/NCBI
|
|
100
|
Chen WQ, Zeng HM, Zheng RS, et al: Cancer
incidence and mortality in china, 2007. Chin J Cancer Res. 24:1–8.
2012. View Article : Google Scholar
|
|
101
|
Koga H, Harada M, Ohtsubo M, et al:
Troglitazone induces p27Kip1-associated cell-cycle arrest through
down-regulating Skp2 in human hepatoma cells. Hepatology.
37:1086–1096. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Liao YJ, Bai HY, Li ZH, et al: Longikaurin
A, a natural ent-kaurane, induces G2/M phase arrest via
downregulation of Skp2 and apoptosis induction through
ROS/JNK/c-Jun pathway in hepatocellular carcinoma cells. Cell Death
Dis. 5:e11372014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yan S, Yang X, Chen T, et al: The PPARγ
agonist Troglitazone induces autophagy, apoptosis and necroptosis
in bladder cancer cells. Cancer Gene Ther. 21:188–193. 2014.
|
|
104
|
Zou QF, Du JK, Zhang H, et al: Anti-tumour
activity of longikaurin A (LK-A), a novel natural diterpenoid, in
nasopharyngeal carcinoma. J Transl Med. 11:2002013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Xu H, Choe C, Shin SH, et al: Silencing of
KIF14 interferes with cell cycle progression and cytokinesis by
blocking the p27 (Kip1) ubiquitination pathway in hepatocellular
carcinoma. Exp Mol Med. 46:e972014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Thériault BL, Basavarajappa HD, Lim H, et
al: Transcriptional and epigenetic regulation of KIF14
overexpression in ovarian cancer. PLoS One. 9:e915402014.PubMed/NCBI
|
|
107
|
Hsiang CY, Wu SL, Chen JC, et al:
Acetaldehyde induces matrix metalloproteinase-9 gene expression via
nuclear factor-kappaB and activator protein 1 signaling pathways in
human hepato-cellular carcinoma cells: association with the
invasive potential. Toxicol Lett. 171:78–86. 2007. View Article : Google Scholar
|
|
108
|
Imura S, Tovuu LO, Utsunomiya T, et al:
The role of Fbxw7 expression in hepatocellular carcinoma and
adjacent non-tumor liver tissue. J Gastroenterol Hepatol. Apr
14–2014.(Epub ahead of print).
|
|
109
|
Tu K, Yang W, Li C, et al: Fbxw7 is an
independent prognostic marker and induces apoptosis and growth
arrest by regulating YAP abundance in hepatocellular carcinoma. Mol
Cancer. 13:1102014. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Steinhardt AA, Gayyed MF, Klein AP, et al:
Expression of Yes-associated protein in common solid tumors. Hum
Pathol. 39:1582–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Tu K, Zheng X, Zan X, et al: Evaluation of
Fbxw7 expression and its correlation with the expression of c-Myc,
cyclin E and p53 in human hepatocellular carcinoma. Hepatol Res.
42:904–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Tu K, Zheng X, Zhou Z, et al: Recombinant
human adenovirus-p53 injection induced apoptosis in hepatocellular
carcinoma cell lines mediated by p53-Fbxw7 pathway, which controls
c-Myc and Cyclin E. PLoS One. 8:e685742013. View Article : Google Scholar
|
|
113
|
Fu J, Qiu H, Cai M, et al: Low cyclin F
expression in hepatocellular carcinoma associates with poor
differentiation and unfavorable prognosis. Cancer Sci. 104:508–515.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhao Y, Tang Q, Ni R, et al: Early mitotic
inhibitor-1, an anaphase-promoting complex/cyclosome inhibitor, can
control tumor cell proliferation in hepatocellular carcinoma:
correlation with Skp2 stability and degradation of p27 (Kip1). Hum
Pathol. 44:365–373. 2013. View Article : Google Scholar
|
|
115
|
Sanada T, Yokoi S, Arii S, et al: Skp2
overexpression is a p27Kip1-independent predictor of poor prognosis
in patients with biliary tract cancers. Cancer Sci. 95:969–976.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Hashimoto N, Yachida S, Okano K, et al:
Immunohistochemically detected expression of p27(Kip1) and Skp2
predicts survival in patients with intrahepatic
cholangiocarcinomas. Ann Surg Oncol. 16:395–403. 2009. View Article : Google Scholar
|
|
117
|
Zhang B, Ji LH, Liu W, et al: Skp2-RNAi
suppresses proliferation and migration of gallbladder carcinoma
cells by enhancing p27 expression. World J Gastroenterol.
19:4917–4924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Ferlay J, Shin HR, Bray F, et al:
Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int
J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar
|
|
120
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar
|
|
121
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar
|
|
122
|
Einama T, Kagata Y, Tsuda H, et al:
High-level Skp2 expression in pancreatic ductal adenocarcinoma:
correlation with the extent of lymph node metastasis, higher
histological grade, and poorer patient outcome. Pancreas.
32:376–381. 2006. View Article : Google Scholar
|
|
123
|
Schüler S, Diersch S, Hamacher R, et al:
Skp2 confers resistance of pancreatic cancer cells towards
TRAIL-induced apoptosis. Int J Oncol. 38:219–225. 2011.PubMed/NCBI
|
|
124
|
Müerköster S, Arlt A, Sipos B, et al:
Increased expression of the E3-ubiquitin ligase receptor subunit
betaTRCP1 relates to constitutive nuclear factor-kappaB activation
and chemo-resistance in pancreatic carcinoma cells. Cancer Res.
65:1316–1324. 2005.
|
|
125
|
Ma J, Cheng L, Liu H, et al: Genistein
down-regulates miR-223 expression in pancreatic cancer cells. Curr
Drug Targets. 14:1150–1156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Wang H, Chen Y, Lin P, et al: The
CUL7/F-box and WD repeat domain containing 8 (CUL7/Fbxw8) ubiquitin
ligase promotes degradation of hematopoietic progenitor kinase 1. J
Biol Chem. 289:4009–4017. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Frescas D, Guardavaccaro D, Bassermann F,
et al: JHDM1B/ FBXL10 is a nucleolar protein that represses
transcription of ribosomal RNA genes. Nature. 450:309–313. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
He J, Nguyen AT and Zhang Y: KDM2b/JHDM1b,
an H3K36me2-specific demethylase, is required for initiation and
maintenance of acute myeloid leukemia. Blood. 117:3869–3880. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Tzatsos A, Paskaleva P, Ferrari F, et al:
KDM2B promotes pancreatic cancer via Polycomb-dependent and
-independent transcriptional programs. J Clin Invest. 123:727–739.
2013.PubMed/NCBI
|
|
130
|
Center MM, Jemal A and Ward E:
International trends in colorectal cancer incidence rates. Cancer
Epidemiol Biomarkers Prev. 18:1688–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Li JQ, Wu F, Mai T, et al: Correlation of
Skp2 with carcinogenesis, invasion, metastasis, and prognosis in
colorectal tumors. Int J Oncol. 25:87–95. 2004.PubMed/NCBI
|
|
133
|
Woenckhaus C, Maile S, Uffmann S, et al:
Expression of Skp2 and p27KIP1 in naevi and malignant melanoma of
the skin and its relation to clinical outcome. Histol Histopathol.
20:501–508. 2005.PubMed/NCBI
|
|
134
|
Shapira M, Ben-Izhak O, Linn S, et al: The
prognostic impact of the ubiquitin ligase subunits Skp2 and Cks1 in
colorectal carcinoma. Cancer. 103:1336–1346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Xu SY, Wang F, Wei G, et al: S-phase
kinase-associated protein 2 knockdown blocks colorectal cancer
growth via regulation of both p27 and p16 expression. Cancer Gene
Ther. 20:690–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Chen H, Mo X, Yu J, et al: Interference of
Skp2 effectively inhibits the development and metastasis of colon
carcinoma. Mol Med Rep. 10:1129–1135. 2014.PubMed/NCBI
|
|
137
|
Zhu J, Li K, Dong L and Chen Y: Role of
FBXL20 in human colorectal adenocarcinoma. Oncol Rep. 28:2290–2298.
2012.PubMed/NCBI
|
|
138
|
Zhu J, Deng S, Duan J, et al: FBXL20 acts
as an invasion inducer and mediates E-cadherin in colorectal
adenocarcinoma. Oncol Lett. 7:2185–2191. 2014.PubMed/NCBI
|
|
139
|
Babaei-Jadidi R, Li N, Saadeddin A, et al:
FBXW7 influences murine intestinal homeostasis and cancer,
targeting Notch, Jun, and DEK for degradation. J Exp Med.
208:295–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Rajagopalan H, Jallepalli PV, Rago C, et
al: Inactivation of hCDC4 can cause chromosomal instability.
Nature. 428:77–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Sancho R, Jandke A, Davis H, et al: F-box
and WD repeat domain-containing 7 regulates intestinal cell lineage
commitment and is a haploinsufficient tumor suppressor.
Gastroenterology. 139:929–941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Jahid S, Sun J, Edwards RA, et al: miR-23a
promotes the transition from indolent to invasive colorectal
cancer. Cancer Discov. 2:540–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Wang Y, Liu Y, Lu J, et al: Rapamycin
inhibits FBXW7 loss-induced epithelial-mesenchymal transition and
cancer stem cell-like characteristics in colorectal cancer cells.
Biochem Biophys Res Commun. 434:352–356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Aberle H, Bauer A, Stappert J, et al:
Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO
J. 16:3797–3804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zhang N, Wei P, Gong A, et al: FoxM1
promotes β-catenin nuclear localization and controls Wnt
target-gene expression and glioma tumorigenesis. Cancer Cell.
20:427–442. 2011.
|
|
146
|
Mokkapati S, Niopek K, Huang L, et al:
β-catenin activation in a novel liver progenitor cell type is
sufficient to cause hepatocellular carcinoma and hepatoblastoma.
Cancer Res. 74:4515–4525. 2014.
|
|
147
|
Shirane M, Hatakeyama S, Hattori K, et al:
Common pathway for the ubiquitination of IkappaBalpha, IkappaBbeta,
and IkappaBepsilon mediated by the F-box protein FWD1. J Biol Chem.
274:28169–28174. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Spiegelman VS, Slaga TJ, Pagano M, et al:
Wnt/beta-catenin signaling induces the expression and activity of
beta-TrCP ubiquitin ligase receptor. Mol Cell. 5:877–882. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Ougolkov A, Zhang B, Yamashita K, et al:
Associations among beta-TrCP, an E3 ubiquitin ligase receptor,
beta-catenin, and NF-kappaB in colorectal cancer. J Natl Cancer
Inst. 96:1161–1170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Alinari L, White VL, Earl CT, et al:
Combination bortezomib and rituximab treatment affects multiple
survival and death pathways to promote apoptosis in mantle cell
lymphoma. MAbs. 1:31–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Kane RC, Bross PF, Farrell AT and Pazdur
R: Velcade: U.S. FDA approval for the treatment of multiple myeloma
progressing on prior therapy. Oncologist. 8:508–513. 2003.
View Article : Google Scholar : PubMed/NCBI
|