Introduction
Oral squamous cell carcinoma (OSCC) is a common
malignancy worldwide, with a global estimate of 274,289 new cases
and 127,459 deaths in 2013 (1).
Although there are several types of oral cancer, >90% of all
diagnosed forms of oral cancer are squamous cell carcinomas
(2,3). OSCC is an aggressive epithelial
malignancy with a poor prognosis, despite advances in its diagnosis
and treatment (4). In addition,
despite multimodality approaches such as surgery, radiation, and
chemotherapy for oral cancer, the incidence of the disease has
continued to increase (5) showing
a low overall survival rate (6,7).
Therefore, the discovery and development of effective
chemotherapeutic agents for OSCC are expected to improve the
survival of OSCC patients.
Esculetin (6,7-dihydroxycoumarin) is a coumarin
derivative found in various plants used as folk medicines,
including Artemisia capillaries, Citrus limonia and
Euphorbia lathyris (8,9).
Esculetin is known to have pleiotropic biological activity and has
multiple beneficial effects, including anti-oxidant activity, the
inhibition of xanthine oxidase activity, platelet aggregation, the
growth inhibition of human leukemia cells and anticancer activity
(10–14). Esculetin induces the apoptosis of
oral cancer SAS cells (14) and
suppresses cancer cell proliferation (15). However, no study has reported on
the effect of esculetin on apoptosis in OSCC. The present study
examines esculetin the repression of a specific protein and cell
cycle progression and investigates whether esculetin can induce the
apoptotic cell death of oral cancer cells. Specific protein 1
(Sp1), which is expressed in all mammalian cells, is a family
factors specific protein Krὔppel-like factor of (KLF) (16). In addition, Sp1 is highly expressed
in various cancers such as breast carcinoma, thyroid cancer,
hepatocellular carcinoma, pancreatic, colorectal, gastric, cervical
and lung cancer (16–20). In this regard, to verify the
therapeutic potential of Sp1, the present study examined whether
esculetin regulated Sp1 target proteins can induce apoptosis by
suppressing the level of Sp1 in HN22 and HSC4 cells.
Materials and methods
Cell culture
OSCC cell lines HN22 and HSC4 were obtained from
Dankook University (Cheonan, Korea) and Hokkaido University
(Hokkaido, Japan), respectively. Cells were cultured in HyClone
Dulbecco’s modified Eagle’s medium (DMEM) containing 10%
heat-inactivated fetal bovine serum and 100 U/ml each of penicillin
and streptomycin at 37°C in humidified air with 5%
CO2.
Cell viability assay
The effect of esculetin on cell viability was
estimated using a
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)
(MTS) assay kit (Promega, Madison, WI, USA). Both HN22 and HSC4
cells were seeded onto a 96-well microtiter plate (HN22:
2×103 cells/well; HSC4: 3×103 cells/well) and
then treated with different doses of 2.5, 5, 10, 15 and 20 μg/ml
esculetin for 24 and 48 h. MTS solution was then added for 2 h at
37°C in 5% CO2. Absorbance at 490 nm was recorded using
the GloMax-Multi Microplate Multimode reader (Promega).
DAPI staining
The number of undergoing apoptotic cells by
esculetin was quantified using 4′-6-diamidino-2-phenylindole (DAPI)
staining. HN22 and HSC4 cells treated with esculetin, harvested by
trypsinization, and fixed in 100% methanol at room temperature for
20 min. The cells were spread on a slide, stained with DAPI (2
μg/ml), and subsequently monitored by the FluoView confocal laser
microscope (Fluoview FV10i; Olympus Corp., Tokyo, Japan).
Propidium iodide staining
After the esculetin treatment (5, 10 and 20 μg/ml)
for 48 h, detached HN22 and HSC4 cells were collected by
centrifugation and combined with adherent cells. The cells were
washed with cold PBS, fixed in 70% ice-cold ethanol overnight at
−20°C, and treated with 150 μg/ml RNase A and 20 μg/ml propidium
iodide (PI; Sigma-Aldrich, St. Louis, MO, USA). DNA content was
analyzed by flow cytometry using the MACSQuant Analyzer (Miltenyi
Biotec GmbH, Bergisch Gladbach, Germany).
Annexin V/7-AAD assay
Cells were seeded onto a 100-mm dish containing
5.2×105 cells/well for HN22 and 8.8×105
cells/well for HSC4 and treated with various concentrations of
esculetin for 48 h (5, 10 and 20 μg/ml). Both adherent and floating
cells were harvested and washed once with PBS. For the detection of
apoptosis, cells were incubated with Annexin V for 20 min at room
temperature in the dark. Apoptotic and necrotic cells were analyzed
by flow cytometry (Muse Cell Analyser; Merck Millipore, Billerica,
MA, USA) by using the Muse Annexin V and Dead Cell kit (MCH100105;
Merck Millipore). The experiment was conducted independently in
triplicate.
Reverse transcription-polymerase chain
reaction
Total RNA was extracted from cells by using the
TRIzol® reagent (Life Technologies, Carlsbad, CA, USA),
and 2.5 μg of RNA was used to synthesize cDNA by using the
HelixCript™ 1st Strand cDNA Synthesis kit (NanoHelix, Co., Ltd.,
Daejeon, Korea). cDNA was obtained by PCR using β-actin- and
Sp1-specific primers based on the method described below under the
following PCR conditions (35 cycles: 1 min at 95°C, 1 min at 56°C
and 1 min at 72°C). β-actin primers were forward 5′-GTG GGG CGC CCC
AGG CAC CA-3′ and reverse 5′-CTC CTT AAT GTC ACG CAC GAT TTC-3′,
and Sp1 primers were forward 5′-ATG CCT AAT ATT CAG TAT CAA GTA-3′
and reverse 5′-CCC TGA GGT GAC AGG CTG TGA-3′. PCR products were
analyzed by 1% agarose gel electrophoresis.
Immunocytochemistry
Cells were seeded over each sterilized glass
coverslip on 6-well tissue culture plates for 24 h and incubated
with esculetin for 48 h. The cells were then fixed and
permeabilized with Cytofix/Cytoperm solution for 30 min. For Sp1
expression, the cells were blocked with 1% BSA and then incubated
with a monoclonal Sp1 antibody at 4°C overnight. After the cells
were washed with PBST solution, the Sp1 and cleaved caspase-3
antibodies were reacted with the Jackson 488-conjugated anti-mouse
and Jackson 647-conjugated anti-rabbit secondary antibody at room
temperature for 1 h and then mounted with the Vectashield mounting
medium for fluorescence with DAPI (Vector Laboratories, Inc.,
Burlingame, CA, USA) onto the cells. These cells were visualized
using the FluoView confocal laser microscope.
Western blot analysis
Esculetin-treated HN22 and HSC4 cells were cultured
for 48 h and washed twice with cold PBS. The cells were then lysed
with the PRO-PREP™ protein extraction solution (iNtRON
Biotechnology, Gyeonggi-do, Korea) containing 1 μg/ml aprotinin, 1
μg/ml leupeptin and 1 mM PMSF. Extracted proteins were measured
using the Pierce® BCA protein assay kit (Thermo Fisher
Scientific, Rockford, IL, USA). Equal amounts of protein samples
were separated by 12 or 15% SDS-polyacrylamide gel electrophoresis
and then transferred to membranes that were blocked for 1 h at room
temperature with 5% non-fat dried milk in PBS containing 0.05%
Tween-20. The samples were then incubated overnight at 4°C with
specific antibodies. Protein bands were observed after treating
them with a horseradish peroxidase-conjugated secondary antibody
using the Pierce ECL western blotting substrate (Thermo Fisher
Scientific).
Statistical analysis
The results are presented as the means ± SD for at
least three independent experiments performed in triplicate. Data
were analyzed for statistical significance through a one-way
analysis of variance. P<0.05 was considered to indicate
significance.
Results
Esculetin suppressed the viability of
HN22 and HSC4 cells
The effects of esculetin on a OSCC cell line were
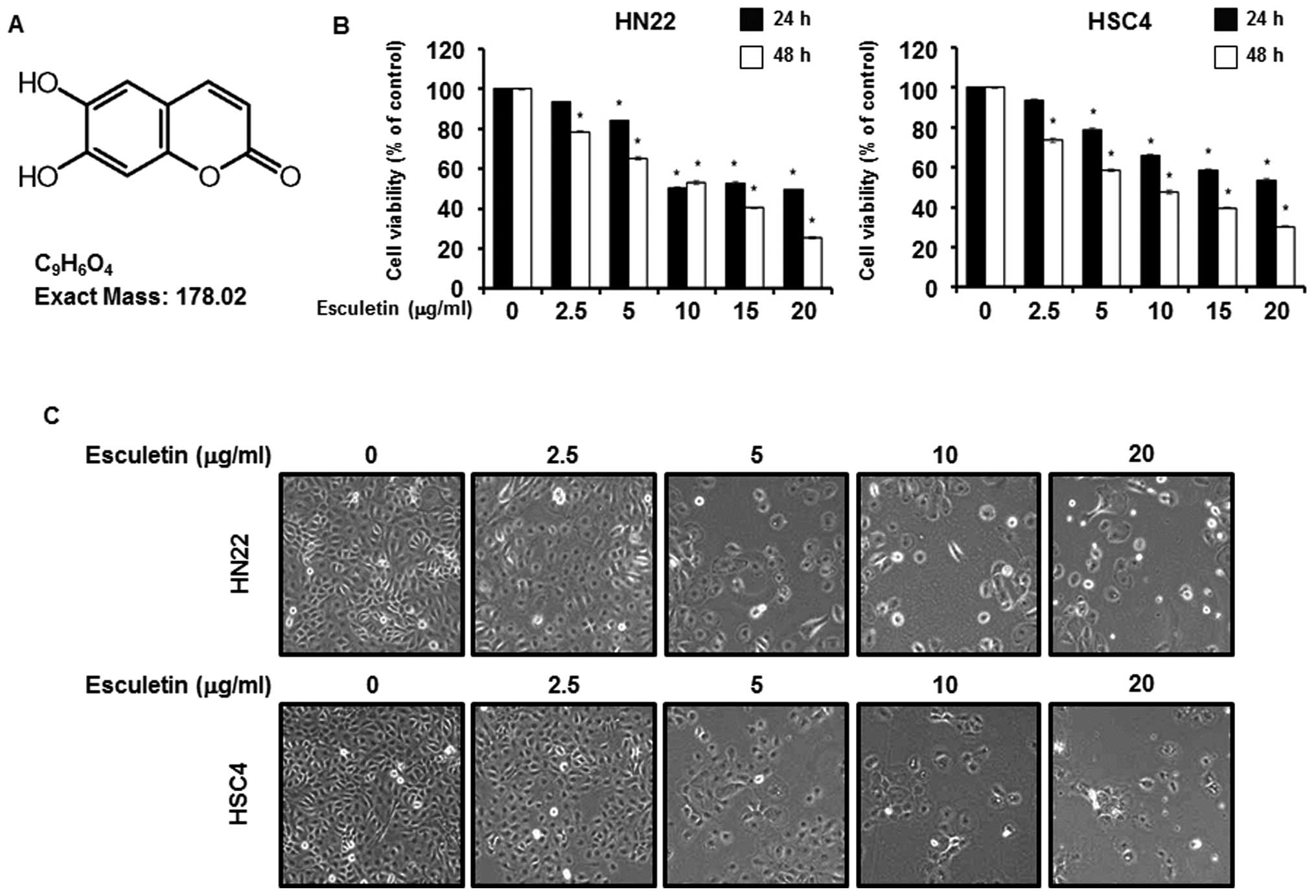
examined. Fig. 1A shows the
structure of esculetin. To establish the efficiency of esculetin as
an anticancer drug, the effects of esculetin were tested using an
MTS assay with HN22 and HSC4 cells. As shown in Fig. 1B, the MTS assay was conducted after
esculetin treatment at various concentrations (2.5, 5, 10, 15 and
20 μg/ml) for 24 and 48 h. To investigate morphologic changes, HN22
and HSC4 cells were treated with various concentrations (2.5, 5, 10
and 20 μg/ml) of esculetin for 48 h (Fig. 1C). These results indicate that
esculetin inhibited growth of human OSCC.
Esculetin induces apoptosis in G1 cell
cycle arrest of HN22 and HSC4 cells
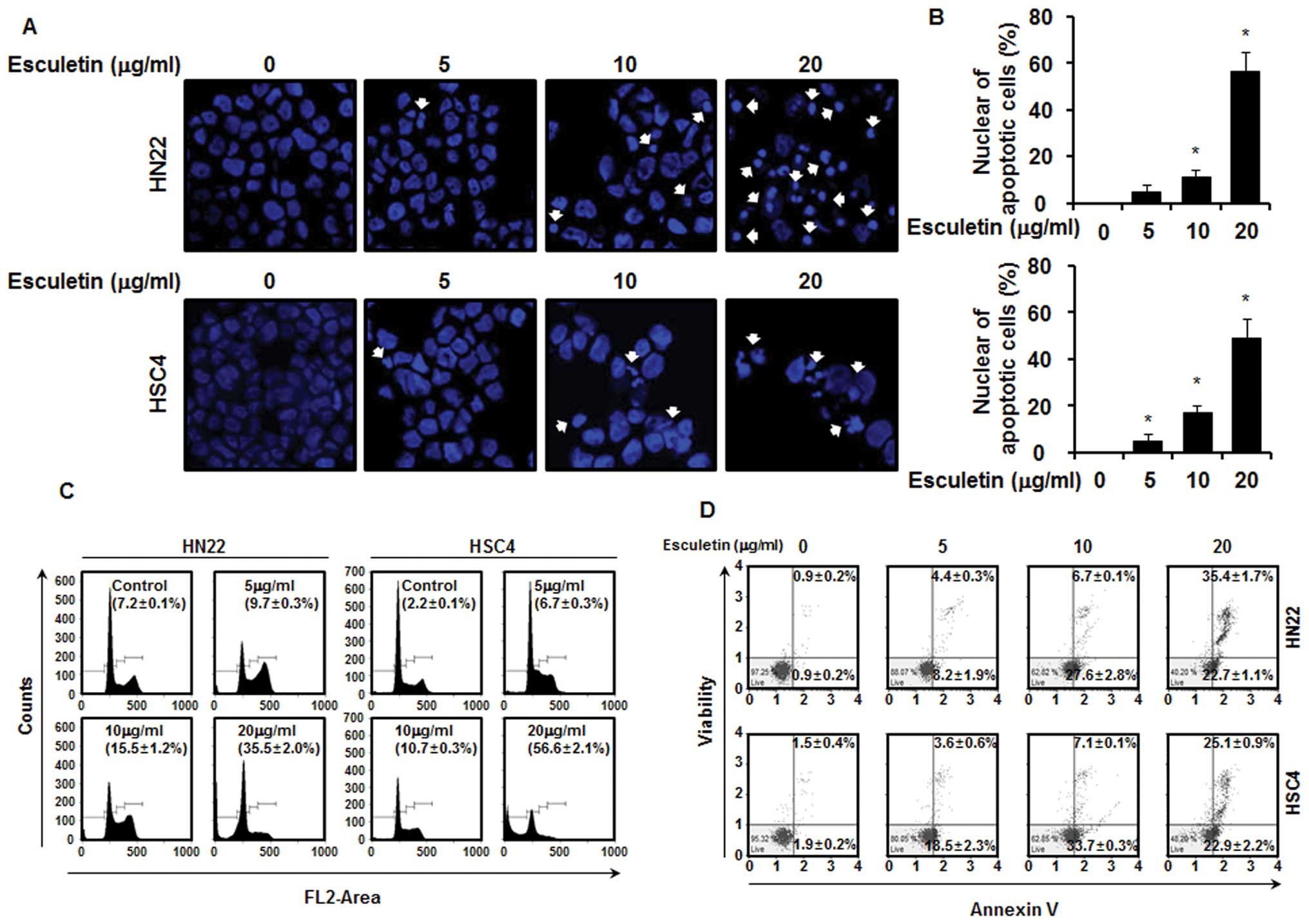
The effects of esculetin treatment on the apoptosis
of HN22 and HSC4 cells were determined by nuclear morphology based
on DAPI staining, which allowed the visualization of nuclear
shrinkage and fragmentation. The results indicate the presence of
nuclear condensation and perinuclear apoptotic bodies in HN22 and
HSC4 cells following esculetin treatment at concentrations of 5, 10
and 20 μg/ml for 48 h (Fig. 2A and
B). The cell cycle distribution was analyzed through the FACS
analysis. As shown in the graphs of Fig. 2C, there was significant increase in
the number of sub-G1 cells in HN22 cells: 9.7±0.3, 15.5±1.2 and
35.5±2.0% in the presence of 5, 10 and 20 μg/ml of esculetin,
respectively, in comparison to untreated control cells. An increase
in the number of sub-G1 cells was also observed in HSC4 cells:
6.7±0.3, 10.7±0.3 and 56.6±2.1% in the presence of 5, 10 and 20
μg/ml of esculetin, respectively, in comparison to untreated
control cells. Cells stained only with Annexin V were defined as
early apoptotic (lower right), and Annexin V and 7-AAD
double-stained cells were defined as late apoptotic (upper right).
As shown in Fig. 2D, esculetin
displayed marked effects to induce apoptosis of HN22 and HSC4 cells
in a dose-dependent manner. Treatment of the HN22 cells with 5, 10
and 20 μg/ml of esculetin for 48 h resulted in 8.2±1.9, 27.6±2.8
and 22.7±1.1% of early apoptotic cells (lower right) and 4.4±0.3,
6.7±0.1 and 35.4±1.7% of late apoptotic cells (upper right),
respectively. Similarly, treatment of HSC4 cells with esculetin
also led to 18.5±2.3, 33.7±0.3 and 22.9±2.2% of cells early
apoptotic cells (lower right) and 3.6±0.6, 7.1±0.1 and 25.1±0.9% of
late apoptotic cells (upper right) at the same three concentrations
as above, respectively.
Esculetin suppresses Sp1 expression and
is bound by Sp1 in HN22 and HSC4 cells
Sp1 has been found to play an important role in
tumor development and contribute to apoptotic cell death and cell
progression (21–24). To observe the level of Sp1
expression, both HN22 and HSC4 cells were treated with various
concentrations of esculetin at 5, 10 and 20 μg/ml for 48 h. As
shown in Fig. 3A, there was a
significant decrease in the level of Sp1 expression for both HN22
and HSC4 cells in a dose-dependent manner. To characterize the
apoptotic action of esculetin, the expression level of PARP was
determined by western blotting (Fig.
3B). In addition, the Sp1 mRNA was suppressed by esculetin in
both HN22 and HSC4 cells (Fig.
3C). Furthermore, immunocytochemical results show reduced
levels of Sp1-positive cells in a dose-dependent manner in HN22 and
HSC4 cells (Fig. 3D). These
results imply that the suppression of Sp1 by esculetin treatment
led to apoptotic cell death.
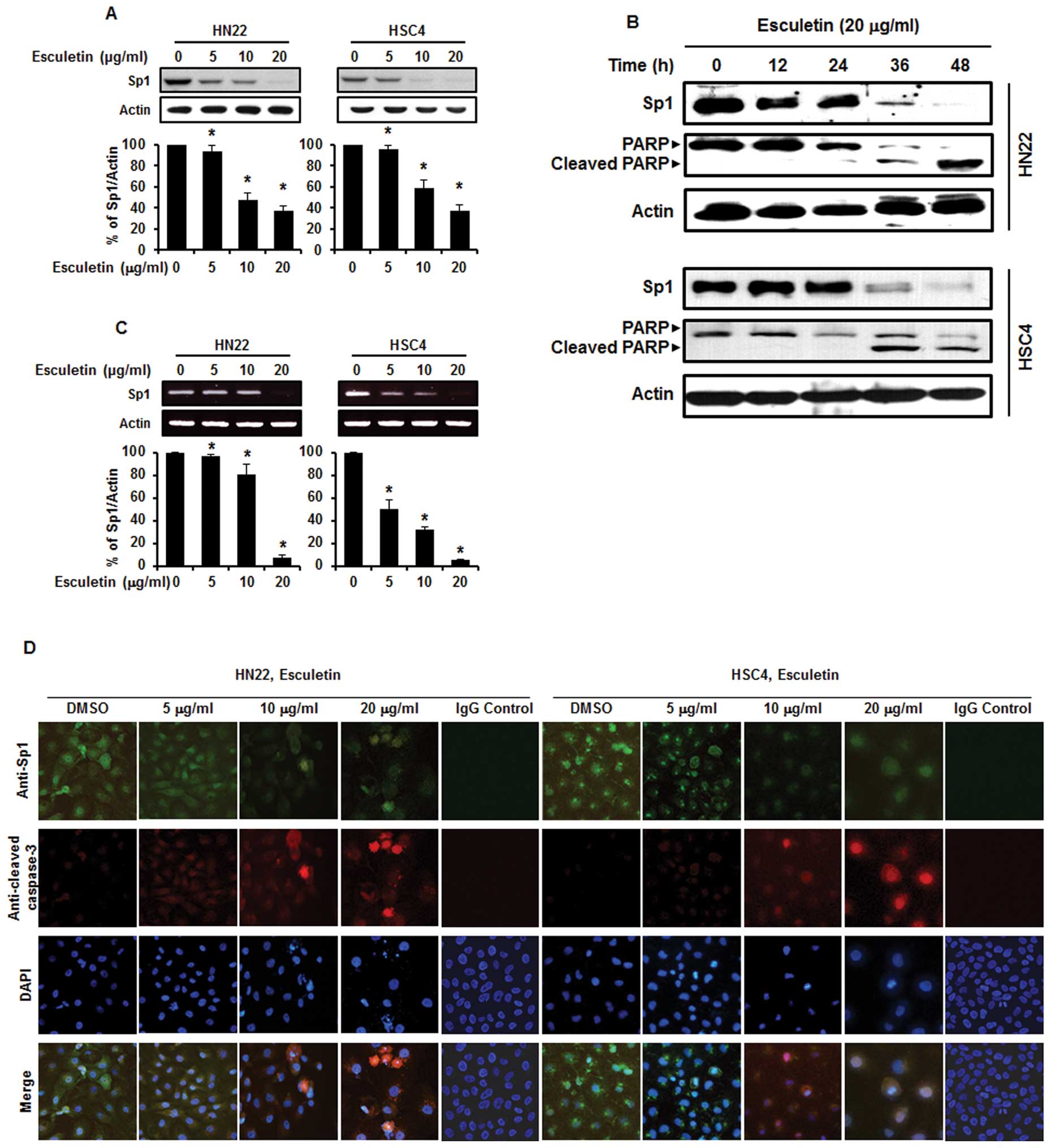 | Figure 3Esculetin suppressed Sp1 through
apoptosis in oral squamous cell carcinoma. (A) HN22 and HSC4 cells
were treated with 5, 10 and 20 μg/ml of esculetin for 48 h, and
whole-cell extracts were prepared, separated on SDS-PAGE, and
subjected to western blotting against Sp1 antibody. Actin was
employed as a loading control. The graphs indicate the ratio of Sp1
to actin expression. (B) Experiments to assess time-dependent
effects of esculetin on Sp1, PARP were conducted using HN22 and
HSC4 cells treated with 20 μg/ml esculetin for 0, 12, 24, 36 and 48
h. (C) Effects of esculetin (5, 10 and 20 μg/ml) for 48 h on the
Sp1 mRNA. (D) An immunofluorescence microscopy analysis was
conducted for HN22 and HSC4 cells treated with different
concentrations of esculetin (5, 10 and 20 μg/ml) for 48 h, and the
cells were immunostained with an anti-Sp1 antibody anti-cleaved
caspase-3. Then signals were detected with Jackson 488-conjugated
anti-mouse and Jackson 647-conjugated anti-rabbit secondary
antibody. DAPI was used for staining of the nucleus. |
Esculetin regulates the expression of
cell cycle arrest- and apoptosis-related molecules in HN22 and HSC4
cells
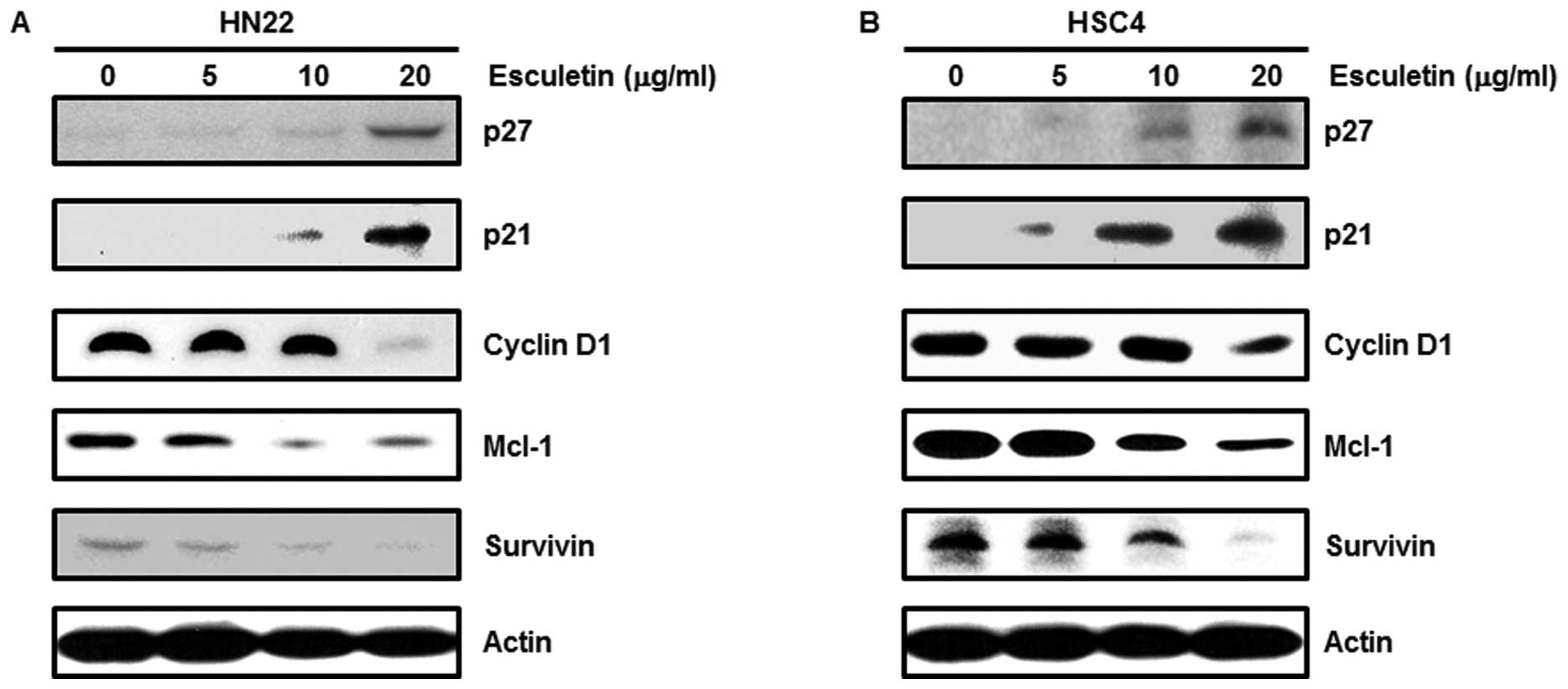
The treatment of HN22 and HSC4 cells with esculetin
regulated the expression level of several cell cycle arrest- and
apoptosis-related proteins. To clarify the relationship between
esculetin and Sp1-mediated apoptosis, a western blot analysis was
conducted. Cell cycle arrest-related proteins such as p27 and p21
increased, whereas proteins related to cell proliferation and
survival, including cyclin D1, Mcl-1 and survivin, showed
significant decreases by esculetin treatment in a dose-dependent
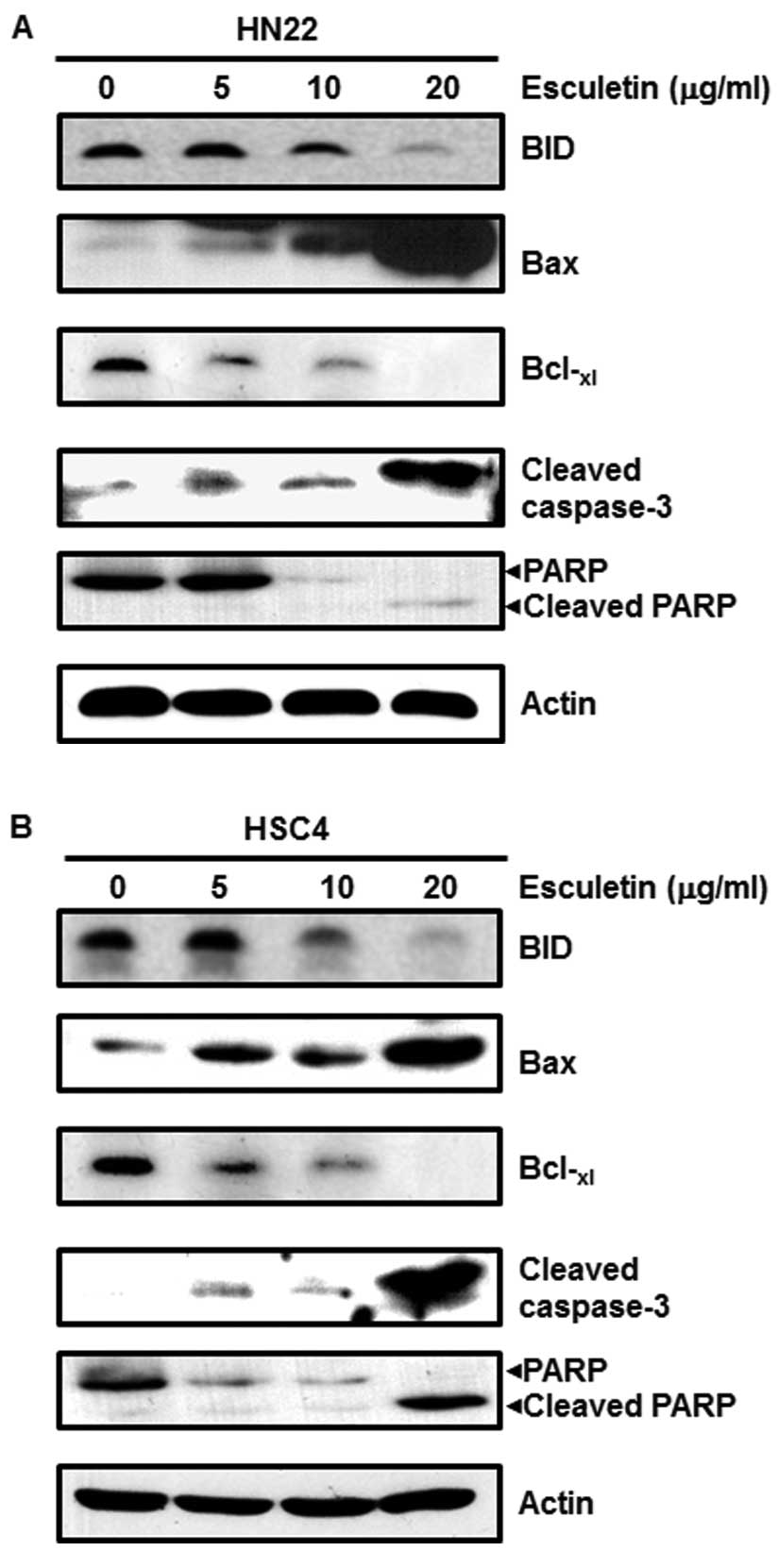
manner (Fig. 4). Apoptosis-related
proteins BID and PARP were decreased, and Bax, cleaved caspase-3
and cleaved PARP increased. The anti-apoptotic protein
Bcl-xl decreased by esculetin in a dose-dependent manner
(Fig. 5). These results show that
esculetin treatment of OSCC decreases Sp1, resulting in growth
arrest and apoptotic cell death.
Discussion
Esculetin (Fig. 1A)
is a naturally-occurring coumarin derivative showing
chemopreventive and chemotherapeutic activity against several types
of cancers (25). For example,
esculetin has been shown to have an anti-inflammatory effect in the
croton oil ear test (26), an
anti-proliferative effect on vascular smooth muscle cells (27), and an inhibitory effect on
N-methyl-N-nitrosourea-induced mammary carcinoma (28,29).
In addition, it is a scavenger of oxygen free radicals (10,30).
Esculetin induces the apoptosis of human oral cancer SAS cells
(14) and inhibits the
proliferation of cancer cells (15). However, the effects of esculetin on
apoptosis in HN22 and HSC4 cells have not been reported.
The present study investigated the molecular
mechanism of esculetin as a potential target of Sp1 for cancer
suppression by using OSCC cell lines. According to the results,
cell viability showed a significant decrease by esculetin treatment
in a dose- and time-dependent manner in both HN22 and HSC4 cells
(Fig. 1B). As shown in Fig. 1C, cell size decreased, and cells
became rounded. The results of the FACS analysis and DAPI staining
for both cell lines (Fig. 2A–C)
show that esculetin inhibited the proliferation of HN22 and HSC4
cells through cell cycle arrest at G0/G1 and induced apoptosis. In
addition, the Annexin V assay revealed that esculetin induced early
apoptosis (Fig. 2D). To determine
whether the level of Sp1 expression would be reduced by esculetin,
HN22 and HSC4 cells were treated with various concentrations (5, 10
and 20 μg/ml) of esculetin for 48 h and different durations (0, 12,
24, 36 and 48 h) at a single concentration of esculetin (20 μg/ml).
As shown in Fig. 3A and B,
esculetin treatment induced a significant decrease in the level of
Sp1 expression in HN22 and HSC4 cells in a dose- and time-dependent
manner. Further, the Sp1 mRNA was suppressed by esculetin in both
HN22 and HSC4 cells (Fig. 3C).
Immunocytochemistry results revealed a decreased level of Sp1 and
an increased level of cleaved caspase-3 in a dose-dependent manner
in HN22 and HSC4 cell lines (Fig.
3D). In addition, esculetin inhibited the transcriptional
activity and expression of Sp1 downstream proteins, including p27,
cyclin D1, Mcl-1 and survivin, in a dose-dependent manner (Fig. 4). Consistent with this result,
esculetin reduced the expression of BID, Bcl-xl and PARP
while increasing that of Bax, cleaved caspase-3 and cleaved PARP
(Fig. 5), implying that esculetin
regulated Sp1, ultimately producing apoptotic cell death.
Taken together, esculetin may be a promising
therapeutic agent in the treatment of oral cancer. The results
clearly suggest that Sp1 may play a potentially important role in
OSCC growth and that esculetin may be a potent anticancer drug
candidate that can suppress Sp1 express in various types of oral
cancer.
Acknowledgements
This study was supported by the Agenda Program
(PJ00932102) from Rural Development Administration, Republic of
Korea.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Hamada T, Wakamatsu T, Miyahara M, et al:
MUC4: a novel prognostic factor of oral squamous cell carcinoma.
Int J Cancer. 130:1768–1776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kruger M, Hansen T, Kasaj A and Moergel M:
The correlation between chronic periodontitis and oral cancer. Case
Rep Dent. 2013:2624102013.PubMed/NCBI
|
|
4
|
Forastiere A, Koch W, Trotti A and
Sidransky D: Head and neck cancer. N Engl J Med. 345:1890–1900.
2001. View Article : Google Scholar
|
|
5
|
Liang XH, Lewis J, Foote R, Smith D and
Kademani D: Prevalence and significance of human papillomavirus in
oral tongue cancer: the Mayo Clinic experience. J Oral Maxillofac
Surg. 66:1875–1880. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyn-geal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar
|
|
7
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
8
|
Chang WS, Lin CC, Chuang SC and Chiang HC:
Superoxide anion scavenging effect of coumarins. Am J Chin Med.
24:11–17. 1996. View Article : Google Scholar
|
|
9
|
Masamoto Y, Ando H, Murata Y, Shimoishi Y,
Tada M and Takahata K: Mushroom tyrosinase inhibitory activity of
esculetin isolated from seeds of Euphorbia lathyris L.
Biosci Biotechnol Biochem. 67:631–634. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin WL, Wang CJ, Tsai YY, Liu CL, Hwang JM
and Tseng TH: Inhibitory effect of esculetin on oxidative damage
induced by t-butyl hydroperoxide in rat liver. Arch Toxicol.
74:467–472. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Egan D, O’Kennedy R, Moran E, Cox D,
Prosser E and Thornes RD: The pharmacology, metabolism, analysis,
and applications of coumarin and coumarin-related compounds. Drug
Metab Rev. 22:503–529. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okada Y, Miyauchi N, Suzuki K, et al:
Search for naturally occurring substances to prevent the
complications of diabetes. II Inhibitory effect of coumarin and
flavonoid derivatives on bovine lens aldose reductase and rabbit
platelet aggregation. Chem Pharm Bull (Tokyo). 43:1385–1387. 1995.
View Article : Google Scholar
|
|
13
|
Wang CJ, Hsieh YJ, Chu CY, Lin YL and
Tseng TH: Inhibition of cell cycle progression in human leukemia
HL-60 cells by esculetin. Cancer Lett. 183:163–168. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kok SH, Yeh CC, Chen ML and Kuo MY:
Esculetin enhances TRAIL-induced apoptosis through DR5 upregulation
in human oral cancer SAS cells. Oral Oncol. 45:1067–1072. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Noguchi M, Kitagawa H, Miyazaki I and
Mizukami Y: Influence of esculetin on incidence, proliferation, and
cell kinetics of mammary carcinomas induced by
7,12-dimethylbenz[a]anthracene in rats on high- and low-fat diets.
Jpn J Cancer Res. 84:1010–1014. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davie JR, He S, Li L, et al: Nuclear
organization and chromatin dynamics-Sp1, Sp3 and histone
deacetylases. Adv Enzyme Regul. 48:189–208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chuang JY, Wu CH, Lai MD, Chang WC and
Hung JJ: Overexpression of Sp1 leads to p53-dependent apoptosis in
cancer cells. Int J Cancer. 125:2066–2076. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen L, Liu Q, Qin R, et al: Amplification
and functional characterization of MUC1 promoter and
gene-virotherapy via a targeting adenoviral vector expressing
hSSTR2 gene in MUC1-positive Panc-1 pancreatic cancer cells in
vitro. Int J Mol Med. 15:617–626. 2005.PubMed/NCBI
|
|
19
|
Kong LM, Liao CG, Fei F, Guo X, Xing JL
and Chen ZN: Transcription factor Sp1 regulates expression of
cancer-associated molecule CD147 in human lung cancer. Cancer Sci.
101:1463–1470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sankpal UT, Goodison S, Abdelrahim M and
Basha R: Targeting Sp1 transcription factors in prostate cancer
therapy. Med Chem. 7:518–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chae JI, Jeon YJ and Shim JH:
Downregulation of Sp1 is involved in honokiol-induced cell cycle
arrest and apoptosis in human malignant pleural mesothelioma cells.
Oncol Rep. 29:2318–2324. 2013.PubMed/NCBI
|
|
22
|
Kim DW, Ko SM, Jeon YJ, et al:
Anti-proliferative effect of honokiol in oral squamous cancer
through the regulation of specificity protein 1. Int J Oncol.
43:1103–1110. 2013.PubMed/NCBI
|
|
23
|
Chae JI, Cho JH, Lee KA, et al: Role of
transcription factor Sp1 in the quercetin-mediated inhibitory
effect on human malignant pleural mesothelioma. Int J Mol Med.
30:835–841. 2012.PubMed/NCBI
|
|
24
|
Jeon YJ, Ko SM, Cho JH, Chae JI and Shim
JH: The HDAC inhibitor, panobinostat, induces apoptosis by
suppressing the expresssion of specificity protein 1 in oral
squamous cell carcinoma. Int J Mol Med. 32:860–866. 2013.PubMed/NCBI
|
|
25
|
Kawase M, Sakagami H, Hashimoto K, Tani S,
Hauer H and Chatterjee SS: Structure-cytotoxic activity
relationships of simple hydroxylated coumarins. Anticancer Res.
23:3243–3246. 2003.PubMed/NCBI
|
|
26
|
Tubaro A, Del Negro P, Ragazzi E, Zampiron
S and Della Loggia R: Anti-inflammatory and peripheral analgesic
activity of esculetin in vivo. Pharmacol Res Commun. 20(Suppl 5):
83–85. 1988. View Article : Google Scholar
|
|
27
|
Huang HC, Lai MW, Wang HR, Chung YL, Hsieh
LM and Chen CC: Antiproliferative effect of esculetin on vascular
smooth muscle cells: possible roles of signal transduction
pathways. Eur J Pharmacol. 237:39–44. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsunaga K, Yoshimi N, Yamada Y, et al:
Inhibitory effects of nabumetone, a cyclooxygenase-2 inhibitor, and
esculetin, a lipoxygenase inhibitor, on
N-methyl-N-nitrosourea-induced mammary carcinogenesis in rats. Jpn
J Cancer Res. 89:496–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hecht SS, Kenney PM, Wang M, et al:
Evaluation of butylated hydroxyanisole, myo-inositol, curcumin,
esculetin, resveratrol and lycopene as inhibitors of benzo[a]pyrene
plus 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung
tumorigenesis in A/J mice. Cancer Lett. 137:123–130. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martin-Aragon S, Benedi JM and Villar AM:
Effects of the anti-oxidant (6,7-dihydroxycoumarin) esculetin on
the glutathione system and lipid peroxidation in mice. Gerontology.
44:21–25. 1998. View Article : Google Scholar : PubMed/NCBI
|