Introduction
Breast cancer is among the leading causes of cancer
mortality and accounts for more than 400,000 deaths annually
worldwide (1). The introduction of
new chemotherapeutic regimens in recent years has modestly improved
the survival rate of patients, but there are still major obstacles
that must be overcome before this disease can be successfully
treated (2,3). One of the major clinical issues is
the development of drug resistance, which accounts for a poor
response and reduces the overall survival rate in patients
(4,5). Among the drugs used for treating
breast cancer, the ones most commonly used belong to the taxane
group of agents, which kill tumor cells by targeting cellular
microtubules (6,7). Similar to other classes of agents,
however, a major drawback of taxane chemotherapy is that tumor
cells can develop resistance to these drugs (5,8–10).
Paclitaxel (PTX) is a prototype of
microtubule-stabilizing agents that disrupt cellular microtubule
function by stabilizing the microtubule assembly dynamics (6). Specifically, it suppresses the growth
and shortening dynamics of microtubules through its interaction
with the plus-end of the microtubule (11). As a result, it disrupts the normal
spindle architecture of the cell, which leads to chromosome
segregation defects, mitotic arrest and eventually apoptosis
(12,13). Earlier studies have demonstrated
that there is a close relationship between microtubule dynamics and
PTX resistance in cancer cells (14–20).
Microtubules associate with a specialized class of
proteins, known as the plus-end-binding proteins (+TIPs), that
localize to the growing plus-ends of microtubules and regulate
numerous plus-end-mediated processes. Recent studies have
emphasized the central role of end-binding (EB) family proteins
among the +TIPs (21–25). Mammals contain three EBs, EB1, EB2,
and EB3 with ~57–66% sequence identity and are encoded by separate
genes (26). EBs play critical
roles in the regulation of microtubule dynamics (27–30).
Recently, analysis using reconstituted microtubules showed that EBs
can modulate the dynamics of PTX-treated microtubules.
Specifically, EB3 has been shown to stimulate catastrophe events
and to rescue growth of PTX-treated microtubules, indicating that
it suppresses PTX’s ability to stabilize microtubule dynamics
(31). Among the EB family
proteins, EB1 was focused on previously as it is highly conserved
compared to other EB members and is ubiquitously expressed in all
cell types (26). High resolution
structural analysis showed that EB1 alters the structure of
microtubule plus-ends (32).
Consistent with its ability to modulate structure-function of
microtubules, EB1 plays essential roles in mitosis (33–36).
Recently, overexpression of EB1 has been shown to stimulate cell
growth in cultured human breast cancer cells, indicating that it
plays an important role in stimulation of cell proliferation
(37,38).
In addition to its microtubule regulatory roles, EB1
has been strongly linked to tumorigenesis. It binds to
adenomatous polyposis coli (APC), a major tumor suppressor
in colon. Mutations in the EB1-binding region of APC are commonly
found in colon cancer cells (39).
Recent proteomic and biochemical analyses have shown that there is
a high EB1 expression level in various human carcinomas and breast
cancer cells (38,40,41).
Consistent with a higher expression level in breast cancer cells,
EB1 has also been shown to be upregulated in breast cancer patients
(38). Specifically, its
expression level appears to be correlated with the
clinicopathological signature of the tumor malignancy in patients
(38). This study has also shown
that EB1 enhances breast tumor growth in nude mice (38).
Because of the close relationship between the
regulation of microtubule dynamics by EB1 and PTX, and the
correlation between EB1 expression and breast cancer, we speculated
that EB1 could regulate PTX-induced cytotoxicity and PTX-mediated
microtubule stabilization in breast cancer cells. In the present
study, we investigated the role of EB1 in regulating PTX-induced
cytotoxicity and microtubule stabilization in breast cancer cells.
Our results demonstrate that suppression of EB1 sensitizes cells to
PTX-induced cytotoxicity and apoptosis. Additional results revealed
details of the underlying mechanism associated with the regulation
of PTX sensitivity by EB1.
Materials and methods
Reagents and antibodies
PTX, vinblastine (VIN), cisplatin, propidium iodide
(PI), DAPI, GTP, PIPES, EGTA, reagents for the methylthiotetrazol
(MTT) assay and the Annexin V apoptosis detection kit were obtained
from Sigma (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium
(DMEM), Leibovitz (L-15) medium, RPMI-1640 medium, DMEM-F12 (1:1)
medium supplemented with HEPES and fetal bovine serum (FBS) were
purchased from HiMedia Laboratories (Mumbai, India). Rhodamine
labeled tubulin was obtained from Cytoskeleton, Inc. (Denver, CO,
USA), and Oregon green 488-labeled PTX (Ore-PTX) was obtained from
Invitrogen Life Technologies (Carlsbad, CA, USA). Monoclonal
antibodies against EB1, actin and BubR1 were obtained from BD
Biosciences (San Jose, CA, USA). Mouse monoclonal antibodies
against α-tubulin and p53 and the rabbit polyclonal anti-EB1 were
obtained from Sigma. Mouse monoclonal anti-Bax, rat monoclonal
anti-α-tubulin and rabbit polyclonal anti-Bcl2, anti-caspase-9 and
anti-PARP were obtained from Abcam (Cambridge, MA, USA). Rabbit
polyclonal p21 antibody was obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). FITC, TRITC and peroxidase
conjugated secondary antibodies were obtained from Jackson
ImmunoResearch (West Grove, PA, USA).
Cell culture, transfection with
ribonuclease III-prepared siRNA pools (esiRNA)
MCF-7, MDA MB-231 and T47D cells originally obtained
from ATCC (Manassas, VA, USA) were cultured in DMEM, L-15, and
RPMI-1640 media, respectively, which were supplemented with 10%
FBS, 2 mM L-glutamine, 1.5 mg/ml sodium bicarbonate, 100 μg/ml
penicillin and 100 μg/ml streptomycin. MCF-10 A cells were grown as
a monolayer culture in DMEM-F12 medium supplemented with 5% donor
horse serum (Invitrogen Life Technologies), 20 ng/ml epidermal
growth factor (EGF), 10 μg/ml insulin, 0.5 μg/ml hydrocortisone,
100 ng/ml cholera toxin (all from Sigma), 100 μg/ml penicillin and
100 μg/ml streptomycin (42).
To downregulate protein expression, we used
ribonuclease III-prepared small interfering RNA pools, which ensure
the efficient knockdown of proteins with minimum off-target effects
compared with single siRNAs (43,44).
esiRNA consists of enzymatically-prepared siRNA pools comprised of
a heterogeneous mixture of siRNAs which target the same mRNA
sequence of the gene (43). Cells
at ~50% confluence were transfected with either EB1 esiRNA (cat.
no. EHU045671; Sigma) against the 331–807 nucleotide region of EB1
(NM_012325.2) or luciferase control esiRNA (cat. no. EHUFLUC;
Sigma) using either oligofectamine (for MCF-7 cells) or
lipofectamine (for T47D and MDA MB-231 cells) as the vehicle.
Plasmids and proteins
pEGFP-EB1 was used as the template for PCR
amplification of the complete coding sequence of the human EB1
(NM_012325.2) gene. The amplified product was ligated into the
pET-28a (Novagen, Inc., Madison, WI, USA) expression vector and
transformed into BL21-(DE3) cells. The 6-His-tagged EB1 was
expressed under 1 mM IPTG and then purified through Nickel-NTA
(28). Tubulin was purified from
goat brains by repetitive cycles of polymerization and
depolymerization (45). Protein
concentrations were estimated by the Bradford method using BSA as
the standard (46).
Cell proliferation and apoptosis
assays
The effect of PTX on cell viability was measured by
MTT assay as previously described (47). The cells at ~60% confluence were
treated with gradients of PTX concentrations (1–200 nM), and
allowed to grow for the next 48 h prior to measuring cell viability
using MTT assay in the Multi-Mode Microplate Reader, SpectraMax 5
(Molecular Devices, Sunnyvale, CA, USA). The percentage of viable
cells as a function of drug concentration was plotted to determine
the drug concentration needed to inhibit cell viability by 50%,
half inhibitory concentrations (IC50). For determination
of IC50 values in the esiRNA-treated cells, the cells
after 24 h of transfection with esiRNA were treated with gradients
of PTX concentrations (5–200 nM) and then incubated for another 48
h prior to measuring cell viability using the MTT assay. For the
apoptosis assay, after 24 h of transfection with esiRNA, the cells
were treated with PTX (10 nM) for the next 48 h. Apoptosis was
measured using the Annexin V-FITC apoptosis assay kit followed by
flow cytometric analysis using the FACSAria III (BD Biosciences).
The quadrants of the raw sample data were determined by comparing
the data of unstained cells. Data were analyzed using FlowJo
software. Percentages of apoptosis were quantified from the sum of
first (Q1) and second (Q2) quadrant. Total cell death was
quantified from the sum of Q1, Q2 and the third quadrant (Q3).
Apoptosis was also detected using immunofluorescence-based imaging.
The cells were grown on coverslips, washed with PBS and then
treated with binding buffer containing Annexin V FITC and PI for 10
min (48). The relative levels of
Annexin V and PI staining were determined using confocal
microscopy.
Microtubule pull-down assay from cell
lysate
MCF-7 cells were lysed in BRB80 buffer, pH 6.8
supplemented with a 1X protease inhibitor cocktail and 0.1% Triton
X-100 and then centrifuged at 15,000 rpm for 15 min at 4°C.
Exogenously purified bovine tubulin (2 μM) and 1 mM GTP were then
added to the cell lysates, and the reaction mixture was polymerized
at 37°C for 15 min (49).
Microtubules were pelleted at 100,000 × g for 40 min at 35°C and
washed with warm BRB80 buffer. The pellet was treated with 4X SDS
sample buffer, and proteins were detected by western blot
analysis.
Western blot analysis
Protein samples and cell lysates were run on
SDS-PAGE, and the protein bands were transferred onto
polyvinylidene fluoride (PVDF) membranes. Target proteins were
detected by incubating the membranes in the appropriate primary
antibodies and then incubating them in the appropriate
HRP-conjugated secondary antibodies. Densitometric analyses of the
blots were performed using the Quantity One software (Bio-Rad,
Hercules, CA, USA).
Immunofluorescence microscopy and image
analysis
Cells fixed in either methanol at −20°C or 3.7%
paraformaldehyde were washed with PBS containing 2% bovine serum
albumin and 0.5% Triton X-100. The cells were then incubated in
primary antibody for 1 h and then incubated in secondary antibody
and DAPI for 45 and 1 min, respectively. Coverslips were mounted
using ProLong Gold (Invitrogen Life Technologies), and the images
(63X) were captured using a Leica SP5 laser confocal microscope.
The intensity measurements were taken using the system run software
provided by Leica. Briefly, intensity per pixel was quantified by
selecting regions of interest (ROI) of equal size in all the images
for comparison.
Microtubule polymerization and PTX
microtubule-binding assay
Reaction mixtures containing tubulin (15 μM) and the
desired concentration of EB1 were polymerized in BRB80 buffer (80
mM PIPES, 2 mM EGTA and 1 mM MgCl2), pH 6.8, containing
1 mM GTP and 0.1 μM PTX. Polymerization was monitored by measuring
the turbidity of the reaction mixtures at 360 nm. For the
quantitative estimation of microtubules, the reaction mixtures were
passed through a 15% glycerol cushion by centrifugation at 100,000
g after 30 min of polymerization. The pellets were resuspended in
cold buffer, and the proteins were run on 10% SDS-PAGE and then
subjected to Coomassie staining. For the quantitative analysis of
PTX binding to microtubules, tubulin (10 μM) was polymerized into
microtubules in vitro in BRB80 buffer containing 10%
glycerol and 1 mM GTP for 15 min at 37°C. Next, Ore-PTX (20 nM) was
added either to the control microtubules or the microtubules
pre-incubated with EB1 (20–200 nM) for 15 min. The reaction
mixtures were incubated for another 15 min prior to centrifugation
through a 15% sucrose cushion at 100,000 g. The microtubule pellets
were resuspended in ice-cold BRB80 buffer. The binding of Ore-PTX
to the microtubules was determined by measuring the fluorescence
intensity of Ore-PTX present in the microtubule solutions.
Fluorescence measurements were taken using a fluorescence
spectrophotometer (Horiba FluoroLog-3) with an excitation at 488 nm
and an emission in the 500–560 nm range. The percentage of Ore-PTX
binding on microtubules inhibited by EB1 was estimated by comparing
the fluorescence values at 525 nm of the EB1 containing
Ore-PTX-treated samples with the Ore-PTX only treated control
sample. All fluorescence measurements were taken using a 1-cm path
length cuvette. The pelleted microtubule solutions were also run
through 10% SDS-PAGE.
Statistical analysis
Results are presented as the mean ± standard error
(SE). Statistical significance was set at p<0.05. One-way
analysis of variance (ANOVA) was used for statistical analysis on
EB1 expression levels and IC50 values in different cell
lines. The comparison of differences between control and the
treated groups was performed using a two-tailed Student’s t-test.
The data were plotted using Origin 8.
Results
EB1 expression negatively correlates with
PTX sensitivity in breast cancer cell lines
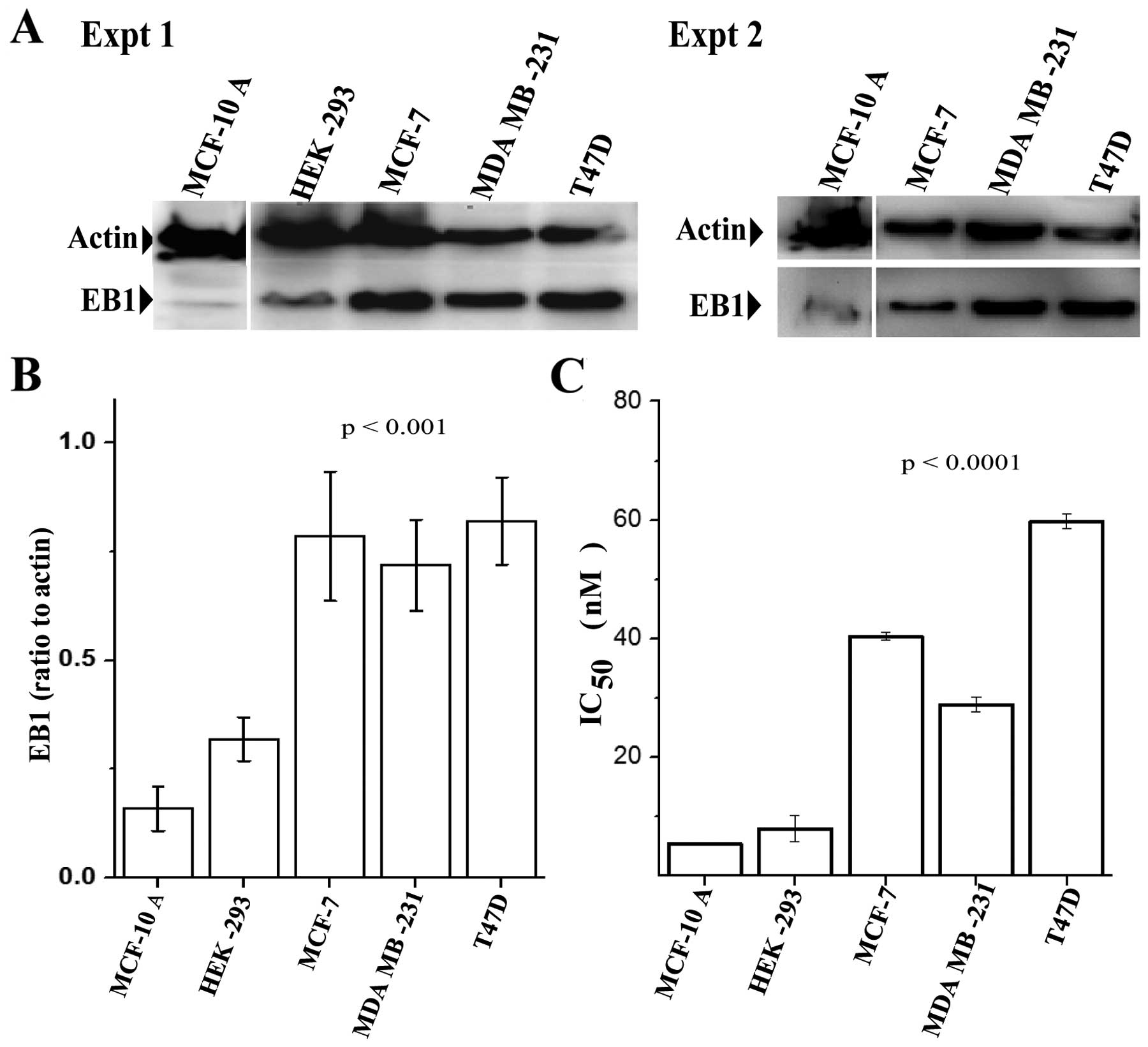
We examined the expression of EB1 by immunoblotting
in three breast cancer cell lines, the human breast adenocarcinoma
cells MCF-7, the metastatic human breast adenocarcinoma MDA MB-231
cells, and the human ductal breast epithelial tumor T47D cells. Two
non-tumorigenic cell lines, mammary MCF-10 A and the kidney
epithelial HEK-293 were used as control. A significantly high EB1
expression level was observed in all three breast cancer cell lines
compared with the normal MCF-10 A and HEK-293 cells (Fig. 1A and B). EB1 expressions in the
breast cancer cell lines were several folds higher as compared with
the MCF-10 A and HEK-293 cells. Statistical analyses were performed
based on the results of two independent western blot analysis
images, as described in Materials and methods. These results were
in good agreement with previously published results on EB1
expression in breast cancer cells (38). We then examined if the EB1
expression level correlates with the sensitivity of these cells to
PTX. To determine the sensitivity of these cells to PTX, we
performed in vitro cell viability assays. Cells treated with
gradients of PTX concentrations (1–200 nM) were analyzed for cell
viability by the MTT assay. The IC50 values of PTX,
which stand for the concentration of PTX needed to suppress cell
viability by 50%, in the cell lines were then determined.
Strikingly, we noted that MCF-7, MDA MB-231 and T47D cells had much
higher IC50 values than the control MCF-10 A cells
(Fig. 1C). Consistent with the
previously published data (50),
MCF-10 A cells exhibited a very low IC50 of ~6 nM.
However, the IC50 values for MCF-7, MDA MB-231 and T47D
cells were increased to 40, 28 and 62 nM, respectively with a range
of 5–10-fold increase compared to MCF-10 A. Thus, the expression
levels of EB1 correlated with the IC50 values of PTX.
Unlike PTX, we did not observe any significant correlation between
EB1 expression level in the cell lines and the cytotoxicity of VIN,
a microtubule destabilizing agent (not shown).
EB1 depletion increased PTX’s ability to
inhibit cell viability in breast cancer cells
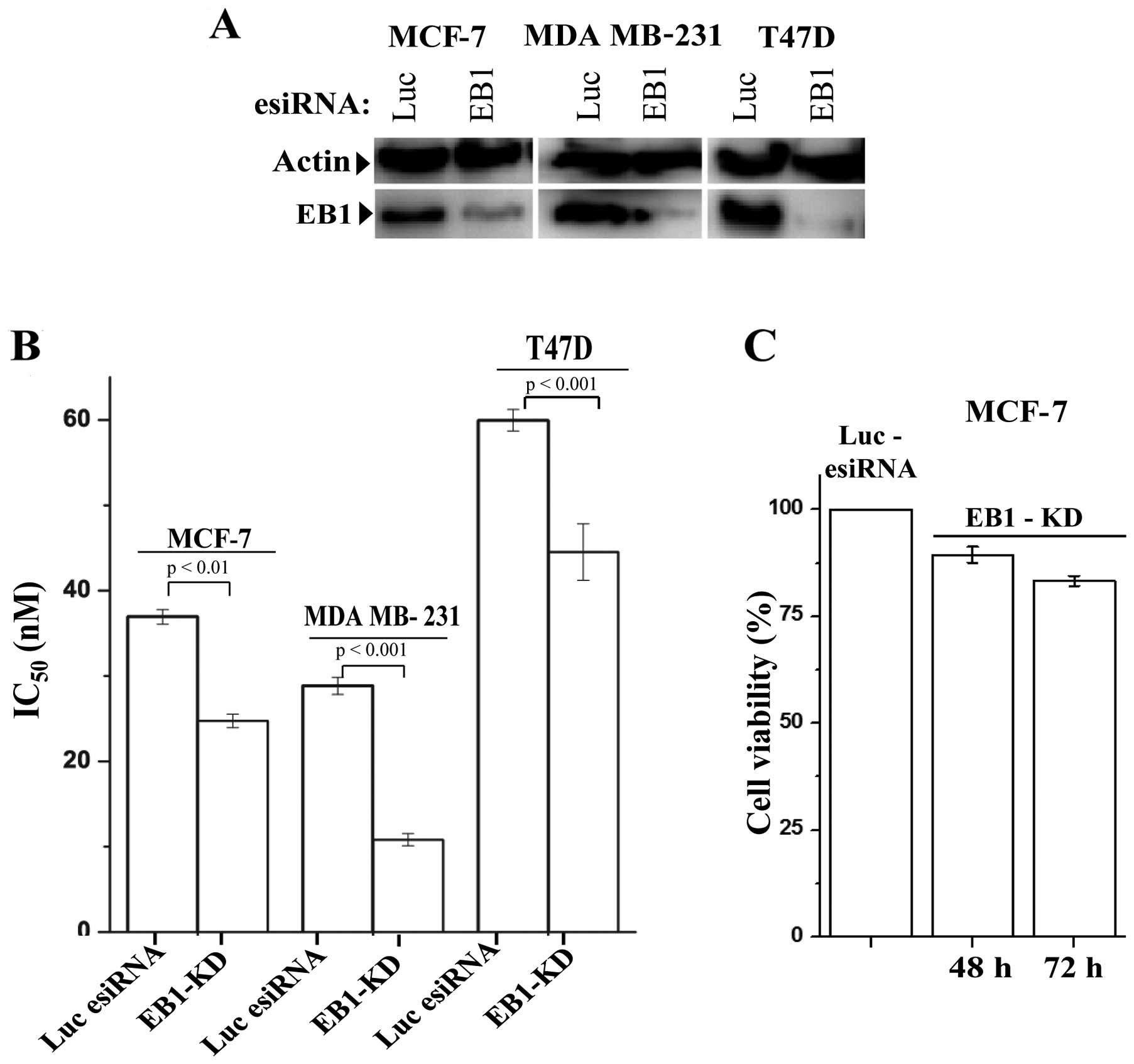
Next, we investigated the effect of EB1 depletion on
PTX-induced cytotoxicity in the breast cancer cells. For efficient
knockdown of EB1, we used ribonuclease III-prepared pool of siRNAs,
as described in Materials and methods. Cells after treatment with
EB1 esiRNA or control luciferase esiRNA for 24 h, were incubated
with gradients of PTX concentrations (5–200 nM) for another 48 h
prior to measuring cell viability. EB1 was effectively depleted
(90±6%) in all three breast cancer cell lines during this period
(Fig. 2A). The ability of PTX to
inhibit cell viability increased significantly in the EB1 knockdown
(EB1-KD) cells compared with the control cells. We observed that
the IC50 of PTX in all three breast cancer cell lines
were significantly reduced (Fig.
2B). The IC50 values for the MCF-7, MDA MB-231 and
T47D control cells were 38, 28 and 59 nM, respectively, and the
IC50 values for the EB1-KD MCF-7, MDA MB-231 and T47D
cells were 23, 11 and 43 nM, respectively. These results indicate a
40, 60 and 28% reduction in the IC50 in the EB1-KD
MCF-7, MDA MB-231 and T47D cells, respectively, compared with the
control cells. EB1-KD alone, in the absence of PTX, did not
significantly inhibit cell viability. After 72 h of EB1 depletion,
the percentage of viable cells was reduced only by 17% (Fig. 2C). Additionally, we also examined
the effect of EB1-KD on cytotoxic effects of VIN and the
microtubule unrelated drug cisplatin in MCF-7 cells. EB1-KD did not
have any effect on either VIN- or cisplatin-induced effects on cell
viability in MCF-7 cells (data not shown). We also found that
EB1-KD did not significantly enhance PTX sensitivity in the control
MCF-10 A and HEK-293 cells (data not shown), indicating that the
effect was specific to the breast cancer cells.
EB1 depletion sensitizes breast cancer
cells to PTX-induced apoptosis via activation of p53 and its
downstream apoptosis regulators
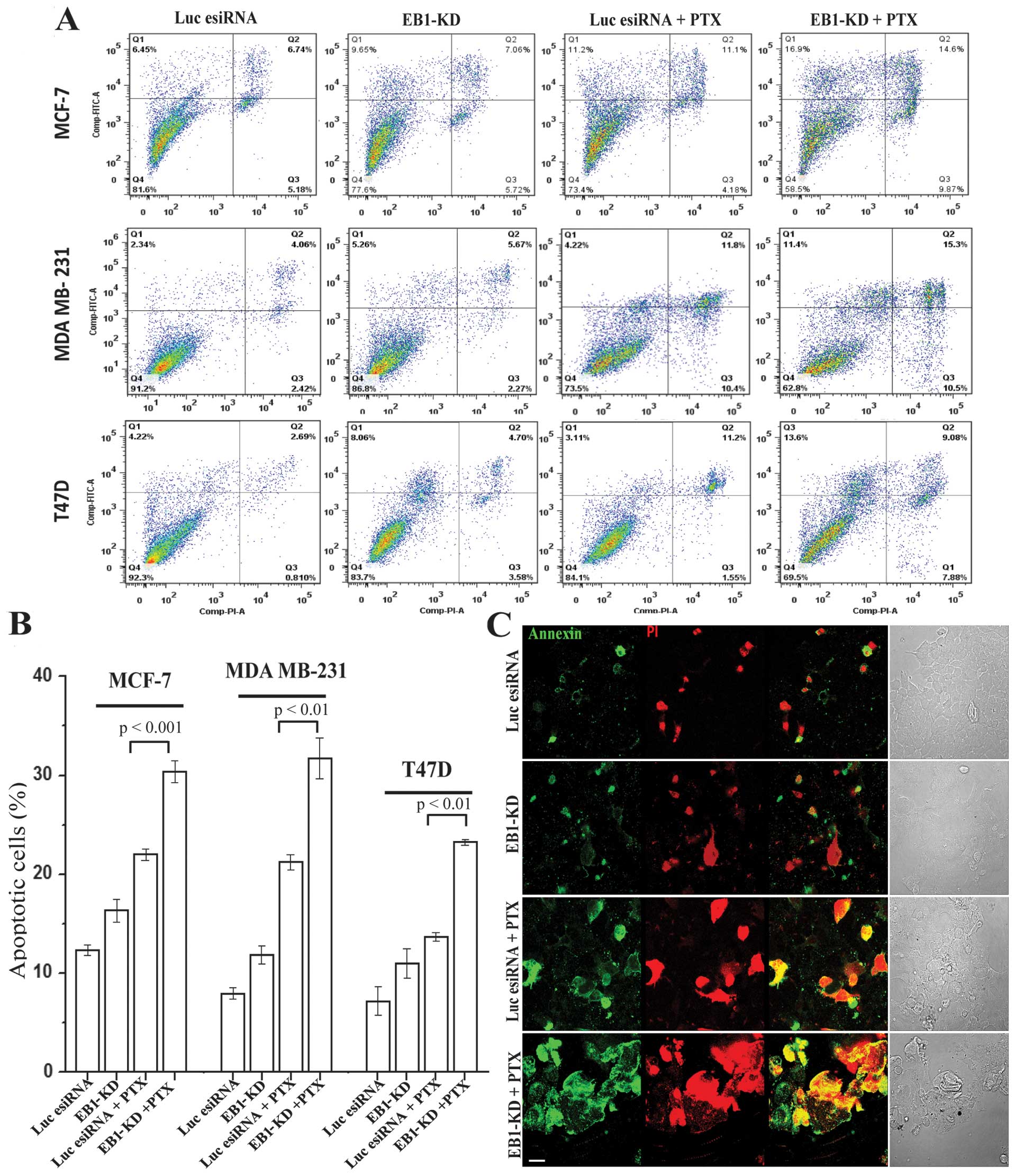
Next, we investigated the effect of EB1 depletion on
PTX-induced apoptosis in the breast cancer cell lines. After a 24-h
esiRNA transfection, the EB1-KD and control cells were treated with
a low dose of PTX (10 nM, ~1/4th of IC50 of PTX in
control MCF-7 cells) for the next 48 h prior to measuring apoptosis
using the Annexin V assay. In all three breast cancer cell lines,
there was a significant amount of apoptosis induced in the
EB1-depleted cells under PTX treatment (Fig. 3A). While the PTX treatment in the
control MCF-7, MDA MB-231 and T47D cells induced apoptosis in ~21,
18 and 14% cells, respectively, PTX treatment in the EB1-KD MCF-7,
MDA MB-231 and T47D cells induced apoptosis in ~30, 30 and 22%
cells, respectively (Fig. 3B).
These results were equivalent to a 1.4-, 1.6- and 1.5-fold increase
in apoptosis in the MCF-7, MDA MB-231 and T47D cells, respectively.
The total cell death increased from 26, 26 and 16% in the
PTX-treated control MCF-7, MDA MB-231 and T47D cells, respectively,
to 43, 37 and 31% in the PTX-treated EB1-KD MCF-7, MDA MB-231 and
T47D cells, respectively (Fig.
3A), as described in Materials and methods. Furthermore, EB1-KD
alone, in the absence of PTX, did not significantly induce
apoptosis in any of the three cell lines as compared with control
cells not treated with PTX (Fig. 3A
and B). These results were confirmed by the Annexin V and PI
immunofluorescence staining results (Fig. 3C).
To elucidate the molecular mechanism underlying
apoptosis induced in cells depleted of EB1 and treated with PTX, we
analyzed the expression patterns of various apoptosis-associated
proteins. The PTX-treated EB1-KD MCF-7 cells demonstrated a
time-dependent increase in the caspase-9 expression level and
poly(ADP-ribose) polymerase (PARP) cleavage compared with the
PTX-treated control cells (Fig.
4A). In addition, the p53 and Bax expression levels were
remarkably higher (Fig. 4A), and
the Bcl2 expression level was reduced in the PTX-treated EB1
deficient cells compared with the PTX-treated control cells
(Fig. 4B). The p21 expression
level, however, appeared similar under both of these conditions
(Fig. 4A). Furthermore, there was
no significant change in the expression levels of p53, Bax,
caspase-9 and PARP in the control EB1-KD cells in the absence of
PTX, but the level of p21 was slightly increased in these cells
(Fig. 4C).
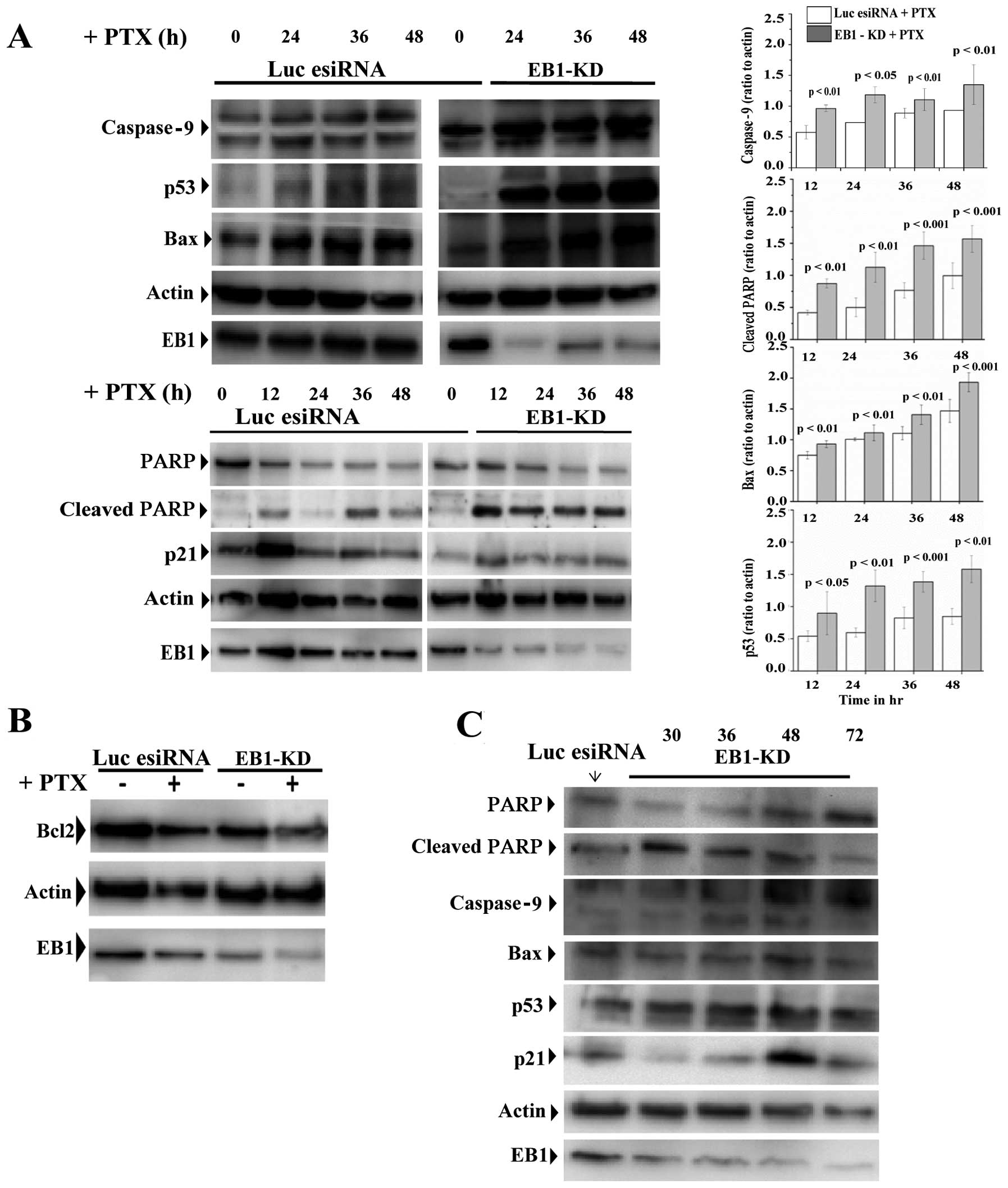 | Figure 4EB1 knockdown (EB1-KD) stimulates
activation of p53 and its downstream apoptosis regulators in the
paclitaxel (PTX)-treated MCF-7 cells. (A) Immunoblot of MCF-7 cell
extracts after luciferase or EB1 esiRNA (siRNA pools) (24 h)
treatment followed by the treatment with PTX (10 nM) during the
time periods as specified. The levels of cleaved poly(ADP-ribose)
polymerase (PARP), caspase-9, p53, Bax, and p21 in the luciferase
esiRNA-treated and EB1-KD cells are shown. β-actin was probed as
control. The bar graphs show the densitometric quantification of
caspase-9, Bax, cleaved PARP and p53 expression as compared to
actin upon PTX treatment during the specified time periods in
EB1-KD vs. control luciferase esiRNA-treated cells. Data [mean ±
standard error (SE)] presented are the mean
intensity/mm2 based on three independent experiments.
P-values were determined by Student’s t-test. (B) Immunoblot
showing the levels of Bcl2 in luciferase esiRNA and EB1-KD MCF-7
cells in the presence or absence of PTX (10 nM). (C) Immunoblot of
cleaved PARP, caspase-9, p53, Bax, and p21 in MCF-7 cell extracts
after EB1 esiRNA treatments for specified time periods. No PTX was
added to these cells. |
EB1 depletion induced nuclear
accumulation of p53 and increased p53 association with cellular
microtubules in PTX-treated MCF-7 cells
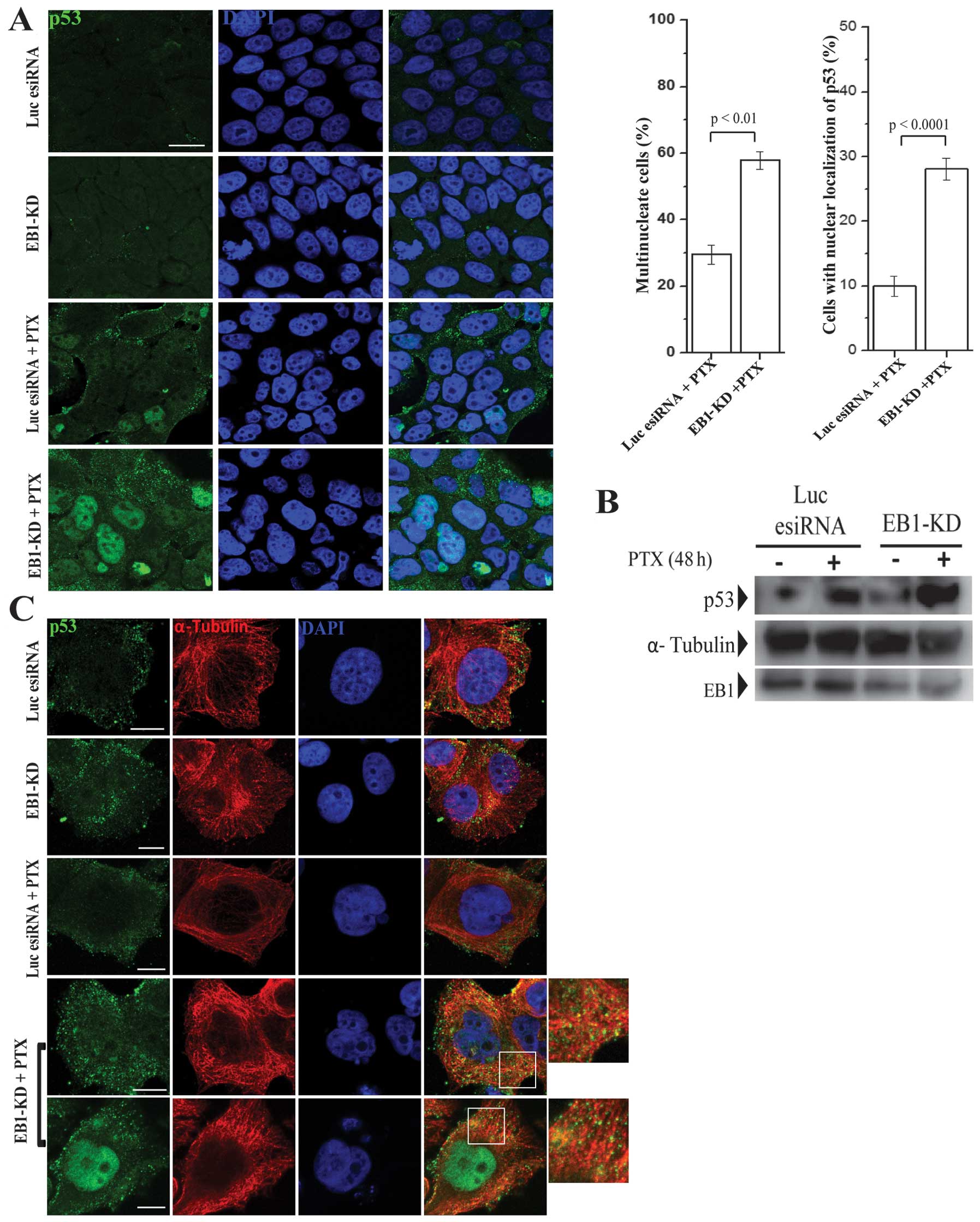
Next, we examined the cellular localization of p53
in EB1-KD vs. control cells. Consistent with the increase in p53
expression, the amount of p53 that accumulated in the nucleus was
significantly higher in the PTX-treated EB1-KD MCF-7 cells
(Fig. 5A). Furthermore, we also
observed an increase in the number of multinucleated cells in
PTX-treated EB1-KD cells. Approximately 56% interphase cells were
multinucleated, containing two or more nuclei (bar graph, Fig. 5A). Multinucleated cells were also
observed in the PTX-treated control cells; however, they were less
abundant (31%). The majority of the multinucleated cells in EB1-KD
condition demonstrated a higher accumulation of p53 in their
nuclei, whereas the PTX-treated control cells did not demonstrate a
higher nuclear accumulation of p53 (bar graph, Fig. 5A).
Next, we investigated whether the association
between p53 and the cellular microtubules was altered in the
PTX-treated EB1-KD cells. The cellular microtubules were assembled
in microtubule-stabilizing buffer and then centrifuged. Finally,
the amount of p53 associated with the microtubules was determined
using a biochemical analysis, as described in Materials and
methods. As expected and consistent with previous studies (51), the PTX-treated control cells
demonstrated a moderate increase in the amount of p53 associated
with microtubules compared with the cells not treated with PTX
(Fig. 5B). However, when compared
with PTX-treated EB1-KD cells, we observed a robust increase
(~3-fold) in the amount of p53 associated with the cellular
microtubules in the EB1-KD cells (Fig.
5B). Unlike the PTX-treated cells, the EB1-KD control cells did
not exhibit any noticeable increase in the amount of p53 associated
with the microtubules (Fig. 5B).
Consistent with the biochemical analysis results, the
immunofluorescence images also showed an increase in the amount of
p53 localized to the microtubules in the EB1-depleted PTX-treated
cells compared with the PTX-treated control and EB1-depleted
control cells (Fig. 5C). The
co-localization of p53 and the microtubules was evident from the
increase in co-staining (yellow) of α-tubulin and p53 on
microtubules in the merged images (enlarged images, Fig. 5C).
EB1 depletion induced formation of
multiple spindle foci in PTX-treated mitotic MCF-7 cells
We investigated the effect of EB1 depletion on the
effects of PTX in mitotic cells. First, we determined how the
EB1-KD expression affects the levels of the spindle assembly
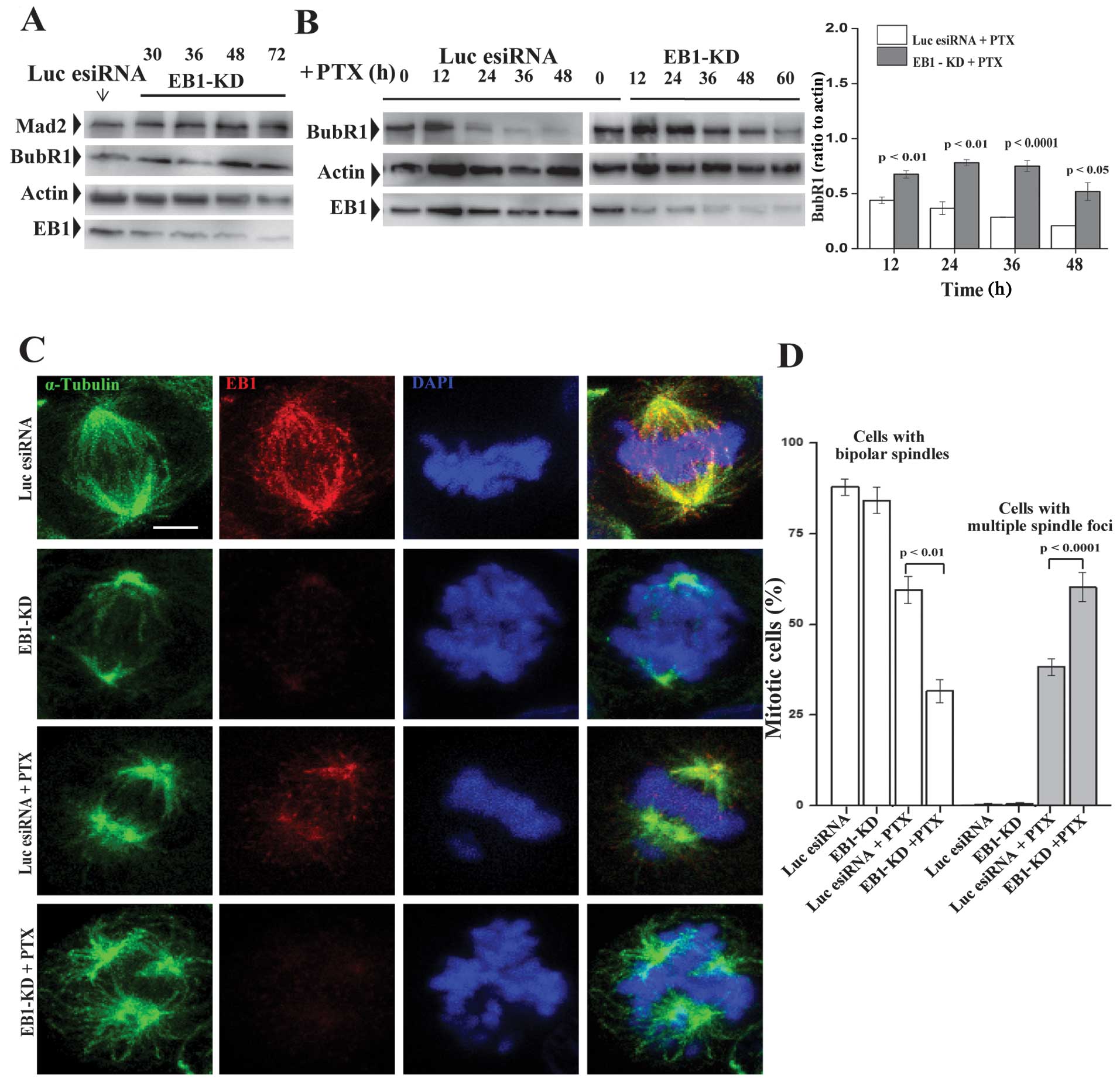
checkpoint (SAC) proteins in MCF-7 cells. There was a higher BubR1
level in the control EB1-KD cells, which was stable and persistent,
even after 72 h of esiRNA treatment (Fig. 6A). A similar change was observed in
the Mad2 expression level (Fig.
6A). In contrast, the EB1-KD and control cells demonstrated
time-dependent decreases in the BubR1 expression level, albeit at
different rates, when treated with PTX (Fig. 6B). While the BubR1 level quickly
decreased soon after 12 h of PTX treatment in the control cells,
the rate of BubR1 loss was relatively slower in the EB1-KD cells
(Fig. 6B).
Next, we examined the cellular phenotypes induced
under these different conditions. MCF-7 cells were immunostained to
visualize the chromosomes and spindle microtubules. EB1 depletion
alone induced chromosome congressional defects in the metaphase
cells (Fig. 6C). The chromosomes
appeared to be unorganized and poorly aligned along the metaphase
plate. Although the spindle poles appeared to be intact, their
positions along the spindle pole axis were disrupted. In the
majority of these cells, the poles were displaced from the axis,
which resulted in a loss of spindle symmetry. These findings are
consistent with previous findings in other cell lines (33,34).
We then investigated whether PTX treatment in the EB1-KD cells
intensifies the EB1 depletion-induced mitotic defects. PTX
treatment in the EB1-KD cells induced multiple spindle foci
formation with severe defects in the spindle organization and
chromosome congression (Fig. 6C).
The chromosomes appeared to be scattered around the spindle foci.
The bipolar arrangement of the spindle microtubules was lost. The
spindles appeared to be unusually long and curvy (Fig. 6C). About 60% mitotic cells had
multiple spindle foci in the EB1 deficient PTX-treated condition
(Fig. 6D). There was a strong
correlation between these defects and the level of EB1 depletion in
the cells. In contrast to the phenotypes observed in the
EB1-depleted cells, the PTX-treated control MCF-7 cells appeared to
be predominantly bipolar (Fig.
6C).
EB1 depletion potentiates PTX-induced
microtubule stabilization and stabilizes PTX localization in MCF-7
cells
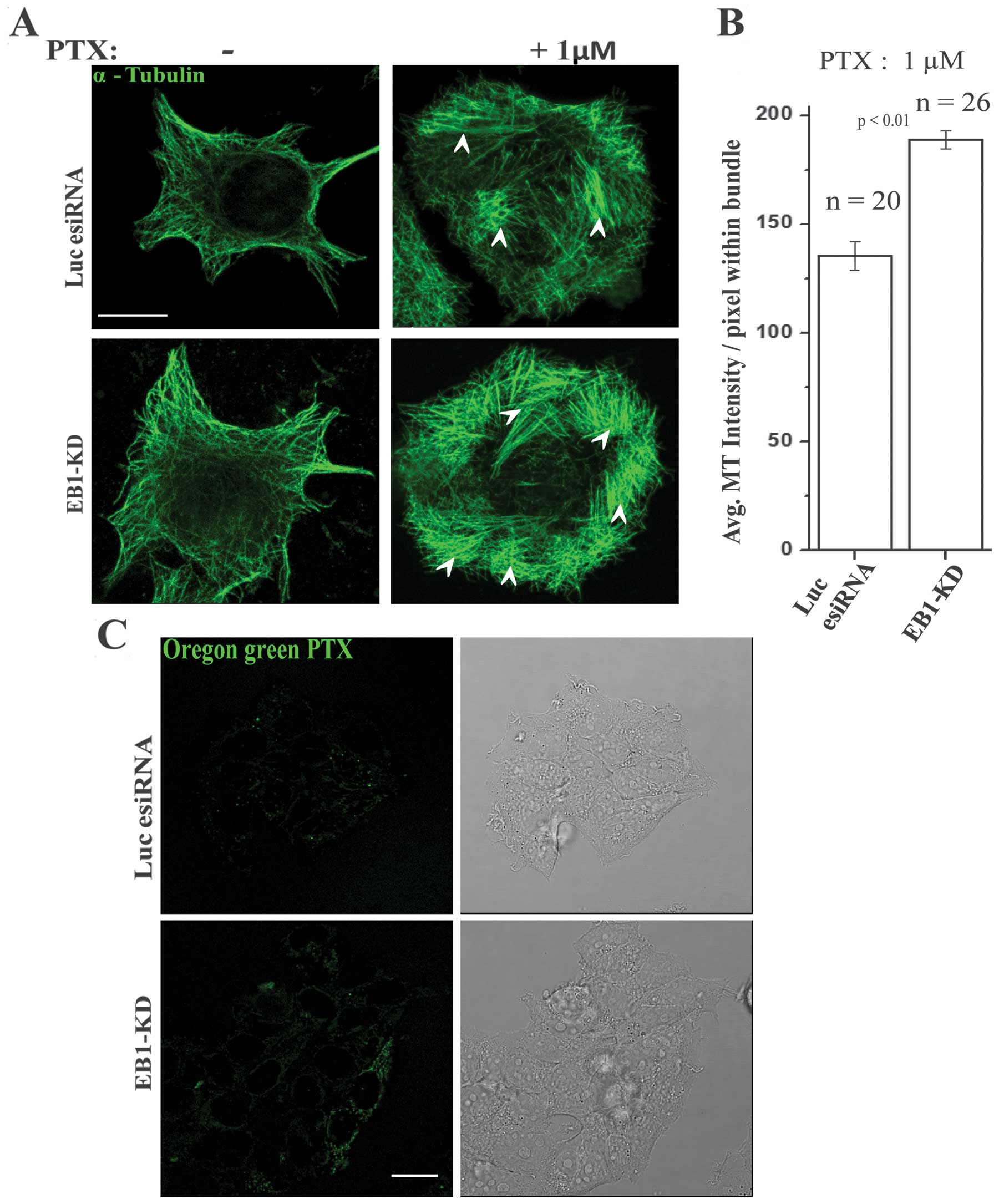
To investigate whether EB1-KD affects the ability of
PTX to induce microtubule stabilization in cells, we assessed the
stabilization of microtubules in PTX-treated control and EB1-KD
cells. We used microtubule bundling (lateral association of
microtubules) in an immunofluorescence assay to determine the
PTX-induced stabilization of microtubules because microtubule
bundle formation is the result of PTX-mediated microtubule polymer
stabilization and therefore, represents the cellular activity of
taxanes (15) (shown by arrows,
Fig. 7A). Consistent with the
previous studies (15), we
observed formation of microtubule bundles in MCF-7 cells upon PTX
treatment (Fig. 7A). However, the
relative size and the number density of the bundles were
significantly increased in the EB1-KD cells compared with the
PTX-treated control cells (Fig.
7A). Based on an analysis of a large population of cells, it
was found that the average microtubule intensity within the bundles
was ~40% higher in the PTX-treated EB1-KD cells compared with the
PTX-treated control cells (Fig.
7B). We also found that the average intensity of microtubules
that are not associated with the bundles was similar in the control
vs. the EB1-KD cells (not shown). To determine if this increase in
microtubule bundling is the result of an increase in PTX
localization in cells, we treated EB1-KD and control esiRNA-treated
cells with fluorescent Ore-PTX and then determined the
intracellular level of fluorescent PTX in the MCF-7 cells by live
imaging. The level of intracellular fluorescent PTX was visibly
higher in the PTX-treated EB1-KD cells compared with the
PTX-treated control cells (Fig.
7C).
EB1 inhibited PTX-induced microtubule
polymerization and PTX binding on microtubules
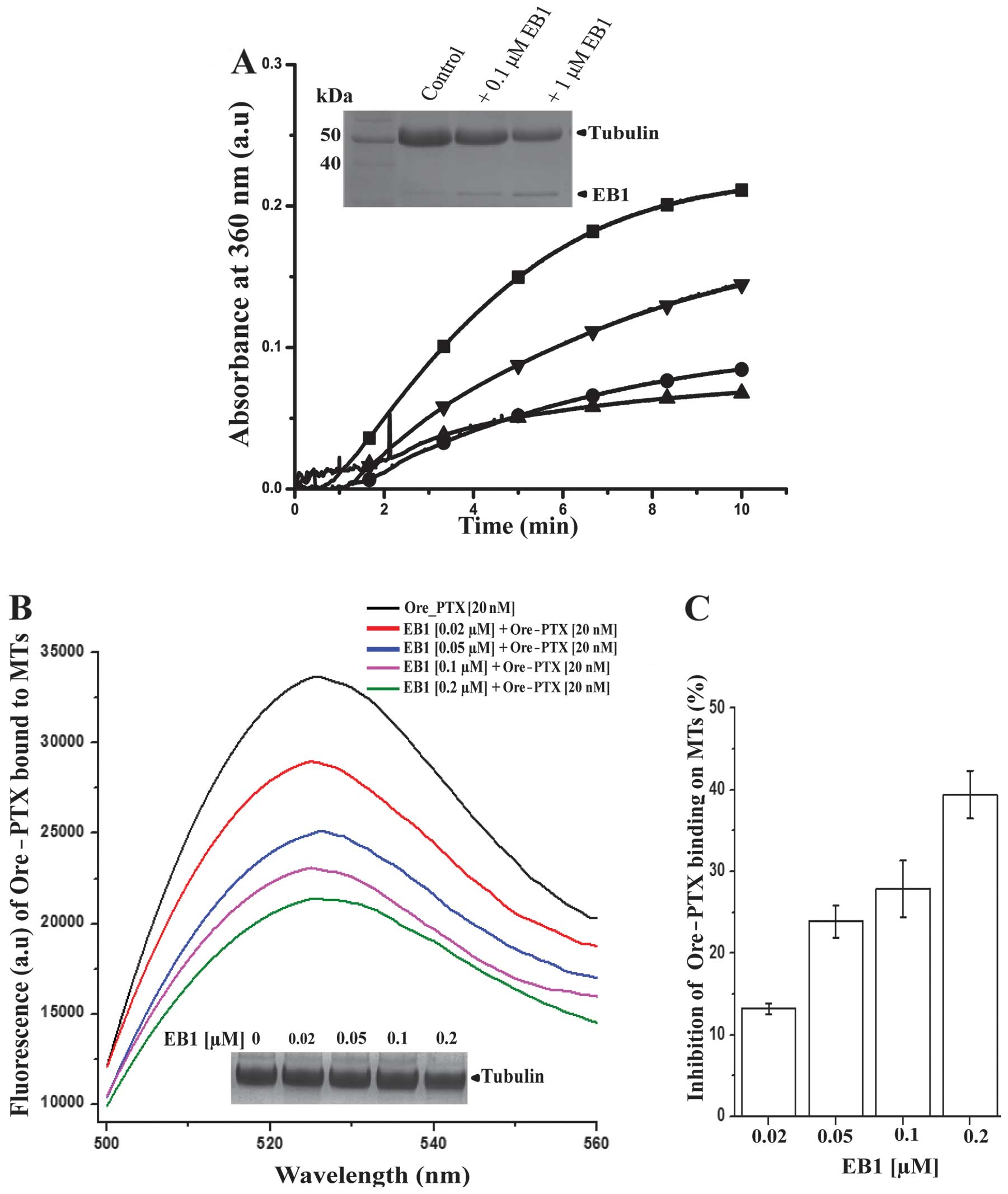
Next, we investigated whether EB1 regulates
PTX-induced microtubule polymerization. PTX-induced microtubule
polymerization was carried out in vitro by adding 0.1 μM PTX
to purified tubulin. The turbidity curve indicates that 0.1 μM PTX
was very efficient in inducing microtubule polymerization (square,
Fig. 8A). Polymerization was
attenuated when PTX was added to the pre-incubated mixture of
tubulin and EB1. It was reduced by ~40% with only 0.1 μM EB1 (1:1
molar ratio of EB1 and PTX) (lower triangle, Fig. 8A). A 2-fold molar excess of EB1
over PTX further suppressed the polymerization by ~60% (circle,
Fig. 8A). By using an unrelated
histidine-tagged protein, we also confirmed that the inhibition of
polymerization was not due to any non-specific effect of the 6-His
tag attached to EB1 (data not shown). The inhibition of
polymerization by EB1 was further confirmed by an SDS-PAGE analysis
of the PTX-assembled microtubules after separating the polymerized
proteins from the unpolymerized fractions through glycerol cushion
followed by centrifugation (inset, Fig. 8A), as described in Materials and
methods. The SDS-PAGE data also showed that the association between
EB1 and the microtubules increased in a concentration-dependent
manner (Fig. 8A). We also found
that EB1 did not inhibit microtubule polymerization when the
microtubules were polymerized from tubulin using DMSO as inducer
(not shown), indicating that the inhibitory effect of EB1 is very
much specific to the PTX-induced microtubule polymerization.
We then investigated whether EB1 affects PTX binding
on microtubules. For this, we performed a quantitative
fluorescence-based assay. The fluorescent Ore-PTX was allowed to
bind to pre-polymerized steady-state microtubules in vitro
in the absence or presence of increasing concentrations of EB1. The
amount of PTX bound to the microtubules was determined by measuring
the fluorescence intensity of the Ore-PTX associated with the
microtubules, as described in Materials and methods. The Ore-PTX
fluorescence in the microtubule solutions was reduced in the
presence of EB1 indicating that EB1 inhibited the association
between Ore-PTX and the microtubules in a dose-dependent manner
(Fig. 8B). At 100 nM EB1 which
corresponds to the molar ratio of PTX:EB1 as 1:5, the Ore-PTX
binding was inhibited by ~30%. The inhibition was further increased
to ~40% at 200 nM EB1 (Fig. 8B and
C). Increase of EB1 >200 nM did not show any additional
increase in the inhibition of PTX binding beyond 40%. We also
confirmed by SDS-PAGE analysis that EB1 did not significantly alter
the total microtubule polymer mass in the range of concentrations
(20–200 nM) used in the PTX-binding experiment (inset, Fig. 8B) (28). This result confirms that the
reduction of PTX binding to the microtubules in the presence of EB1
was not due to any decrease in the total microtubule polymer mass,
but rather it was due to inhibition of PTX binding by EB1 to the
microtubules. Altogether, these results indicate that EB1 inhibits
PTX-mediated microtubule polymerization by preventing PTX binding
to the microtubules.
Discussion
EB1 is a major +TIP and has previously been shown to
promote proliferation of breast cancer cells (22,38).
However, its effects on the sensitivity of breast cancer cells to
microtubule-targeted drugs have not been clearly understood. In the
present study, we demonstrate that EB1 plays an important role in
regulating the sensitivity of breast cancer cells to
microtubule-stabilizing drug, PTX. We show that PTX sensitivity
correlates negatively with the EB1 expression level in breast
cancer cell lines (Fig. 1). The
EB1-KD increases PTX sensitivity in breast cancer cells by
stimulating PTX-induced apoptosis and inhibition of cell viability
(Figs. 2–4). Our findings support a mechanism in
which EB1 downregulates PTX sensitivity by impairing PTX-induced
stabilization of microtubule polymerization and inhibiting PTX
binding to the microtubules (Figs.
6–8). During the preparation
of this manuscript, we found a related report published by Luo
et al (52), in which they
investigated the role of EB1 on PTX sensitivity in breast cancer
cells. However, it did not provide a clear conclusion due to a
number of reasons. First, EB1 depletion was not significant in the
study. Furthermore, GFP-fused EB1 was used to examine the effect of
EB1 overexpression on PTX sensitivity. Fusion of GFP either to the
N- or the C-terminus of EB1 has previously been shown to inhibit
EB1’s ability to bind to the microtubules and to other +TIPs
(53). The results of Luo et
al (52) also could not
clearly address the effect of EB1 on PTX-induced microtubule
polymerization as the EB1 protein used in their assay was tagged
with glutathione S-transferase (GST). Since GST is a bulky molecule
and it itself exerts an effect on microtubule polymerization
(52), the results could not
clearly describe the exclusive role of EB1 in PTX-induced
microtubule polymerization. In this study, we used esiRNAs, which
ensured >90% suppression of EB1 expression in all the breast
cancer cell lines used in this study (Fig. 2A). In the biochemical assays, we
used purified recombinant human EB1, which clearly describes the
exclusive effect of EB1 on PTX-induced microtubule polymerization
(Fig. 8).
It has been previously reported that an increase in
the nuclear translocation of p53 in cancer cells occurs in response
to treatment with microtubule-targeting drugs, including PTX
(51,56). It is generally accepted that the
drug-induced stabilization of microtubules is the primary mechanism
responsible for the increase in the p53 association with
microtubules and its nuclear export (51,54,55).
Consistently, we found that the association between p53 and
cellular microtubules increased in response to PTX treatment in the
control cells (51) (Fig. 5B and C). However, the association
between p53 and cellular microtubules was further increased in the
PTX-treated EB1-KD cells (Fig. 5B and
C). The increased p53-microtubule association in PTX-treated
EB1-KD cells could be the result of an increase in the
stabilization of microtubules in the PTX-treated EB1-KD cells
compared with the PTX-treated control cells. In support of this
mechanism, we showed that microtubule bundling was greater in the
PTX-treated EB1 deficient interphase cells compared with the
PTX-treated control interphase cells (Fig. 7A). Consistently, we also observed
an increase in the abundance of multiple spindle foci with
abnormally long spindles in the PTX-treated EB1-KD mitotic cells
(Fig. 6D). We also found that the
level of intracellular PTX was higher in the EB1 deficient cells,
which suggests that the association between PTX and the cellular
microtubules is increased in the absence of EB1 (Fig. 7B). Altogether, these results
support a mechanism in which EB1 inhibits the
microtubule-stabilizing function of PTX. The results also support
the previously proposed hypothesis that microtubule stabilization
and p53 activation are closely linked (51,56).
EB1 mediates several key interactions that are
essential for stabilizing spindle-chromosome attachment and
activation of the SAC. For example, it has been shown that the
disruption of its interaction with the plus-end protein TIP150
activates the SAC in HeLa cells (35). Consistently, we found that the
EB1-KD resulted in a prolonged and stable accumulation of BubR1 and
Mad2 in the control cells (Fig.
6A). It suggests that the EB1-deficient control cells undergo a
stable mitotic arrest. On the contrary, EB1-deficient cells treated
with PTX exhibited a time-dependent decrease in the BubR1 level,
which indicates that the SAC became less effective or was
suppressed by PTX treatment (Fig.
6B). It has been shown previously that mitotic cells with weak
SAC activation may undergo apoptosis through either of these
mechanisms: apoptosis may be triggered at the mitotically arrested
stage, or the cells may exit mitosis as viable cells but then die
at a multinucleated G1-stage (57). Our findings that PTX treatment
increased apoptosis (Fig. 4A) and
induced multinucleation with increased nuclear accumulation of p53
in the EB1-KD cells (Fig. 5A),
suggest that apoptosis was triggered at the multinucleated stage
after exiting mitosis rather than at the mitosis. We also found
that the loss of BubR1 occurred relatively more rapidly in the
PTX-treated control cells than in the PTX-treated EB1-KD cells
(Fig. 6B). This indicates that the
SAC activation was relatively weaker and transient in the
PTX-treated control cells than the PTX-treated EB1-KD cells. As the
PTX-treated control cells did not exhibit significant apoptosis
like the PTX-treated EB1-KD cells (Fig. 3), a likely possibility is that
these cells proceeded to the next round of division. In support of
this possibility, we found that PTX-treated control cells displayed
higher cell viability (higher IC50 of PTX) as compared
with the PTX-treated EB1-KD cells (Fig. 2).
Recent high resolution electron microscopy analysis
of interaction between the Schizosaccharomyces pombe EB1
homolog Mal3p and microtubules showed that EB1 binds between the
microtubule protofilaments at and near the plus-ends (58,59).
Combination of in vitro reconstitution and the sub-pixel
level fluorescence analysis with human recombinant EB1 further
revealed that EB1 binding accelerates the conformational maturation
of microtubules at the polymerizing ends (32). Because EB1 is involved in
conformational maturation of the ends, it may affect the assembly
of protofilaments during PTX-mediated microtubule polymerization.
Consistent with this mechanism, we found that PTX-induced
microtubule polymerization was inhibited by EB1 (Fig. 8). Although EB1 effectively
inhibited microtubule polymerization induced by PTX, it did not
inhibit DMSO-induced microtubule polymerization (not shown), or it
did not depolymerize the pre-polymerized steady-state microtubules
in vitro (Fig. 8B)
(28). These findings suggest that
EB1 specifically inhibits the action of PTX on the microtubules,
presumably by preventing PTX to bind to the tubulin subunits on the
microtubules and to induce the assembly of the protofilaments.
How could EB1 being a +TIP inhibit the anti-mitotic
action of PTX, which is known to bind all along the microtubule
protofilament? It has been demonstrated previously that PTX exerts
its anti-mitotic action in cancer cells at a very
sub-stoichiometric level, the concentrations at which it does not
significantly affect microtubule polymer mass, but suppresses
microtubule dynamics very efficiently (11,12).
This suggested that the anti-mitotic action of PTX is predominantly
attributed to its binding at the microtubule plus-ends rather than
its binding along the microtubule surface (6). Thus, the increased accumulation of
EB1 on plus-ends of microtubules could specifically inhibit binding
of PTX to the ends, but PTX binding along the surface of
microtubule may not be affected under such condition. Consistent
with this possibility, we observed that EB1 inhibited binding of
PTX on the microtubules to a maximum extent of 40%, and the
remaining 60% PTX was still bound to the microtubules (Fig. 8B). The inhibitory effect of EB1 on
PTX-induced microtubule polymerization is also in good support with
the effects of the EB proteins on the dynamics of PTX-treated
microtubules shown by Mohan et al (31) in which they showed that the overall
dynamicity of PTX-treated microtubules is increased in the presence
of EB proteins in vitro. Specifically, the EB proteins
increased the frequencies of growth (rescue) and shortening
(catastrophe) of the PTX-treated microtubules (31). Similar to these findings, study by
Pagano et al, showed that at low PTX concentration,
microtubule dynamics is increased instead of suppressed in the
presence of EB protein, EB3 (60).
In summary, we show from our cellular and
biochemical results that inhibition of EB1 expression sensitizes
breast cancer cells to PTX-induced cytotoxicity and apoptosis. The
ability of EB1 to inhibit PTX-induced microtubule polymerization
and PTX binding to the microtubules suggests that EB1 confers
resistance against the microtubule-stabilizing activity of PTX. The
results support the hypothesis that EB1 downregulates PTX
sensitivity through a mechanism in which it inhibits PTX binding to
the microtubules and suppresses PTX-induced stabilization of
microtubule polymerization.
Acknowledgements
We thank S. Murty Srinivasula,
IISER-Thiruvananthapuram for critical comments and suggestions, and
for providing cell lines. We also thank Stephen Doxsey, UMass
Medical (Worcester, MA, USA) for providing pGFP-EB1 plasmid. This
study was supported by a CSIR-Govt. of India research grant to
T.M.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographical regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
2
|
Mamounas EP, Bryant J, Lembersky B,
Fehrenbacher L, Sedlacek SM, Fisher B, Wickerham DL, Yothers G,
Soran A and Wolmark N: Paclitaxel after doxorubicin and
cyclophosphamide as adjuvant chemotherapy for node-positive breast
cancer: results from NSABP B-28. J Clin Oncol. 23:3686–3696. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin M, Seguí MA, Antón A, Ruiz A, Ramos
M, Adrover E, Aranda I, Rodríguez-Lescure A, Grosse R, Calvo L, et
al: GEICAM 9805 Investigators: Adjuvant docetaxel for high-risk,
node-negative breast cancer. N Engl J Med. 363:2200–2210. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Henderson IC, Berry DA, Demetri GD,
Cirrincione CT, Goldstein LJ, Martino S, Ingle JN, Cooper MR, Hayes
DF, Tkaczuk KH, et al: Improved outcomes from adding sequential
Paclitaxel but not from escalating Doxorubicin dose in an adjuvant
chemotherapy regimen for patients with node-positive primary breast
cancer. J Clin Oncol. 21:976–983. 2003. View Article : Google Scholar
|
|
5
|
McGrogan BT, Gilmartin B, Carney DN and
McCann A: Taxanes, microtubules and chemoresistant breast cancer.
Biochim Biophys Acta. 1785:96–132. 2008.PubMed/NCBI
|
|
6
|
Jordan MA and Wilson L: Microtubules as a
target for anticancer drugs. Nat Rev Cancer. 4:253–265. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Manfredi JJ, Parness J and Horwitz SB:
Taxol binds to cellular microtubules. J Cell Biol. 94:688–696.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giannakakou P, Gussio R, Nogales E,
Downing KH, Zaharevitz D, Bollbuck B, Poy G, Sackett D, Nicolaou KC
and Fojo T: A common pharmacophore for epothilone and taxanes:
molecular basis for drug resistance conferred by tubulin mutations
in human cancer cells. Proc Natl Acad Sci USA. 97:2904–2909. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Orr GA, Verdier-Pinard P, McDaid H and
Horwitz SB: Mechanisms of Taxol resistance related to microtubules.
Oncogene. 22:7280–7295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kavallaris M, Kuo DY, Burkhart CA, Regl
DL, Norris MD, Haber M and Horwitz SB: Taxol-resistant epithelial
ovarian tumors are associated with altered expression of specific
beta-tubulin isotypes. J Clin Invest. 100:1282–1293. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Derry WB, Wilson L and Jordan MA:
Substoichiometric binding of taxol suppresses microtubule dynamics.
Biochemistry. 34:2203–2211. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jordan MA, Toso RJ, Thrower D and Wilson
L: Mechanism of mitotic block and inhibition of cell proliferation
by taxol at low concentrations. Proc Natl Acad Sci USA.
90:9552–9556. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blagosklonny MV, Robey R, Sheikh MS and
Fojo T: Paclitaxel-induced FasL-independent apoptosis and slow
(non-apoptotic) cell death. Cancer Biol Ther. 1:113–117. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ganguly A, Yang H, Pedroza M, Bhattacharya
R and Cabral F: Mitotic centromere-associated kinesin (MCAK)
mediates paclitaxel resistance. J Biol Chem. 286:36378–36384. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sung M and Giannakakou P: BRCA1 regulates
microtubule dynamics and taxanes-induced apoptotic cell signaling.
Oncogene. 33:1418–1428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Liu B, Zhang C, Peng G, Liu M, Li
D, Gu F, Chen Q, Dong JT, Fu L and Zhou J: Parkin regulates
paclitaxel sensitivity in breast cancer via a microtubule-dependent
mechanism. J Pathol. 218:76–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alli E, Bash-Babula J, Yang JM and Hait
WN: Effect of stathmin on the sensitivity to antimicrotubule drugs
in human breast cancer. Cancer Res. 62:6864–6869. 2002.PubMed/NCBI
|
|
18
|
Sun X, Li D, Yang Y, Ren Y, Li J, Wang Z,
Dong B, Liu M and Zhou J: Microtubule-binding protein CLIP-170 is a
mediator of paclitaxel sensitivity. J Pathol. 226:666–673. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu Q and Luduena RF: Removal of beta III
isotype enhances taxol induced microtubule assembly. Cell Struct
Funct. 18:173–182. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kamath K, Wilson L, Cabral F and Jordan
MA: Beta-tubulin induces paclitaxel resistance in association with
reduced effects on microtubule dynamic instability. J Biol Chem.
280:12902–12907. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akhmanova A and Steinmetz MO: Tracking the
ends: a dynamic protein network controls the fate of microtubule
tips. Nat Rev Mol Cell Biol. 9:309–322. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Honnappa S, Okhrimenko O, Jaussi R,
Jawhari H, Jelesarov I, Winkler FK and Steinmetz MO: Key
interaction modes of dynamic +TIP networks. Mol Cell. 23:663–671.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Komarova Y, Lansbergen G, Galjart N,
Grosveld F, Borisy GG and Akhmanova A: EB1 and EB3 control CLIP
dissociation from the ends of growing microtubules. Mol Biol Cell.
16:5334–5345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Askham JM, Vaughan KT, Goodson HV and
Morrison EE: Evidence that an interaction between EB1 and
p150(Glued) is required for the formation and maintenance of a
radial microtubule array anchored at the centrosome. Mol Biol Cell.
13:3627–3645. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dixit R, Barnett B, Lazarus JE, Tokito M,
Goldman YE and Holzbaur EL: Microtubule plus-end tracking by
CLIP-170 requires EB1. Proc Natl Acad Sci USA. 106:492–497. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su LK and Qi Y: Characterization of human
MAPRE genes and their proteins. Genomics. 71:142–149. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tirnauer JS, Grego S, Salmon ED and
Mitchison TJ: EB1-microtubule interactions in Xenopus egg extracts:
role of EB1 in microtubule stabilization and mechanisms of
targeting to microtubules. Mol Biol Cell. 13:3614–3626. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Manna T, Honnappa S, Steinmetz MO and
Wilson L: Suppression of microtubule dynamic instability by the
+TIP protein EB1 and its modulation by the CAP-Gly domain of
p150glued. Biochemistry. 47:779–786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vitre B, Coquelle FM, Heichette C, Garnier
C, Chrétien D and Arnal I: EB1 regulates microtubule dynamics and
tubulin sheet closure in vitro. Nat Cell Biol. 10:415–421. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Komarova Y, De Groot CO, Grigoriev I,
Gouveia SM, Munteanu EL, Schober JM, Honnappa S, Buey RM,
Hoogenraad CC, Dogterom M, et al: Mammalian end binding proteins
control persistent microtubule growth. J Cell Biol. 184:691–706.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mohan R, Katrukha EA, Doodhi H, Smal I,
Meijering E, Kapitein LC, Steinmetz MO and Akhmanova A: End-binding
proteins sensitize microtubules to the action of
microtubule-targeting agents. Proc Natl Acad Sci USA.
110:8900–8905. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maurer SP, Cade NI, Bohner G, Gustafsson
N, Boutant E and Surrey T: EB1 accelerates two conformational
transitions important for microtubule maturation and dynamics. Curr
Biol. 24:372–384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Draviam VM, Shapiro I, Aldridge B and
Sorger PK: Misorientation and reduced stretching of aligned sister
kinetochores promote chromosome missegregation in EB1- or
APC-depleted cells. EMBO J. 25:2814–2827. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brüning-Richardson A, Langford KJ, Ruane
P, Lee T, Askham JM and Morrison EE: EB1 is required for spindle
symmetry in mammalian mitosis. PLoS One. 6:e288842011.PubMed/NCBI
|
|
35
|
Ward T, Wang M, Liu X, Wang Z, Xia P, Chu
Y, Wang X, Liu L, Jiang K, Yu H, et al: Regulation of a dynamic
interaction between two microtubule-binding proteins, EB1 and
TIP150, by the mitotic p300/CBP-associated factor (PCAF)
orchestrates kinetochore microtubule plasticity and chromosome
stability during mitosis. J Biol Chem. 288:15771–15785. 2013.
View Article : Google Scholar
|
|
36
|
Green RA, Wollman R and Kaplan KB: APC and
EB1 function together in mitosis to regulate spindle dynamics and
chromosome alignment. Mol Biol Cell. 16:4609–4622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun L, Gao J, Dong X, Liu M, Li D, Shi X,
Dong J, Lu X, Liu C and Zhou J: EB1 promotes Aurora-B kinase
activity through blocking its inactivation by protein phosphatase
2A. Proc Natl Acad Sci USA. 105:7153–7158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dong X, Liu F, Sun L, Liu M, Li D, Su D,
Zhu Z, Dong JT, Fu L and Zhou J: Oncogenic function of microtubule
end-binding protein 1 in breast cancer. J Pathol. 220:361–369.
2010.PubMed/NCBI
|
|
39
|
Jaïs P, Sabourin JC, Bombled J, Rougier P,
Lasser P, Duvillard P, Bénard J and Bressac-de Paillerets B:
Absence of somatic alterations of the EB1 gene adenomatous
polyposis coli-associated protein in human sporadic colorectal
cancers. Br J Cancer. 78:1356–1360. 1998.PubMed/NCBI
|
|
40
|
Nishigaki R, Osaki M, Hiratsuka M, Toda T,
Murakami K, Jeang KT, Ito H, Inoue T and Oshimura M: Proteomic
identification of differentially-expressed genes in human gastric
carcinomas. Proteomics. 5:3205–3213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Zhou X, Zhu H, Liu S, Zhou C,
Zhang G, Xue L, Lu N, Quan L, Bai J, et al: Overexpression of EB1
in human esophageal squamous cell carcinoma (ESCC) may promote
cellular growth by activating beta-catenin/TCF pathway. Oncogene.
24:6637–6645. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Soule HD, Maloney TM, Wolman SR, Peterson
WD Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF and Brooks
SC: Isolation and characterization of a spontaneously immortalized
human breast epithelial cell line, MCF-10. Cancer Res.
50:6075–6086. 1990.PubMed/NCBI
|
|
43
|
Kittler R, Heninger AK, Franke K,
Habermann B and Buchholz F: Production of endoribonuclease-prepared
short interfering RNAs for gene silencing in mammalian cells. Nat
Methods. 2:779–784. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Calegari F, Haubensak W, Yang D, Huttner
WB and Buchholz F: Tissue-specific RNA interference in
post-implantation mouse embryos with endoribonuclease-prepared
short interfering RNA. Proc Natl Acad Sci USA. 99:14236–14240.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Manna T, Thrower DA, Honnappa S, Steinmetz
MO and Wilson L: Regulation of microtubule dynamic instability in
vitro by differentially phosphorylated stathmin. J Biol Chem.
284:15640–15649. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang L, Lau YK, Xia W, Hortobagyi GN and
Hung MC: Tyrosine kinase inhibitor emodin suppresses growth of
HER-2/neu-overexpressing breast cancer cells in athylic mice and
sensitizes these cells to the inhibitory effects of paclitaxel.
Clin Cancer Res. 5:343–353. 1999.
|
|
48
|
Gireesh KK, Rashid A, Chakraborti S, Panda
D and Manna T: CIL-102 binds to tubulin at colchicine binding site
and triggers apoptosis in MCF-7 cells by inducing monopolar and
multinucleated cells. Biochem Pharmacol. 84:633–645. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Young A, Dictenberg JB, Purohit A, Tuft R
and Doxsey SJ: Cytoplasmic dynein-mediated assembly of pericentrin
and gamma tubulin onto centrosomes. Mol Biol Cell. 11:2047–2056.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ciardiello F, Caputo R, Pomatico G, De
Laurentiis M, De Placido S, Bianco AR and Tortora G: Resistance to
taxanes is induced by c-erbB-2 overexpression in human MCF-10A
mammary epithelial cells and is blocked by combined treatment with
an antisense oligonucleotide targeting type I protein kinase A. Int
J Cancer. 85:710–715. 2000. View Article : Google Scholar
|
|
51
|
Giannakakou P, Sackett DL, Ward Y, Webster
KR, Blagosklonny MV and Fojo T: p53 is associated with cellular
microtubules and is transported to the nucleus by dynein. Nat Cell
Biol. 2:709–717. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Luo Y, Li D, Ran J, Yan B, Chen J, Dong X,
Liu Z, Liu R, Zhou J and Liu M: End-binding protein 1 stimulates
paclitaxel sensitivity in breast cancer by promoting its actions
toward microtubule assembly and stability. Protein Cell. 5:469–479.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Skube SB, Chaverri JM and Goodson HV:
Effect of GFP tags on the localization of EB1 and EB1 fragments in
vivo. Cytoskeleton (Hoboken). 67:1–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Giannakakou P, Nakano M, Nicolaou KC,
O’Brate A, Yu J, Blagosklonny MV, Greber UF and Fojo T: Enhanced
microtubule-dependent trafficking and p53 nuclear accumulation by
suppression of microtubule dynamics. Proc Natl Acad Sci USA.
99:10855–10860. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rathinasamy K and Panda D: Kinetic
stabilization of microtubule dynamic instability by benomyl
increases the nuclear transport of p53. Biochem Pharmacol.
76:1669–1680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Carney BK, Caruso Silva V and Cassimeris
L: The microtubule cytoskeleton is required for a G2 cell cycle
delay in cancer cells lacking stathmin and p53. Cytoskeleton
(Hoboken). 69:278–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tao W, South VJ, Zhang Y, Davide JP,
Farrell L, Kohl NE, Sepp-Lorenzino L and Lobell RB: Induction of
apoptosis by an inhibitor of the mitotic kinesin KSP requires both
activation of the spindle assembly checkpoint and mitotic slippage.
Cancer Cell. 8:49–59. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Maurer SP, Fourniol FJ, Bohner G, Moores
CA and Surrey T: EBs recognize a nucleotide dependent structural
cap at growing microtubule ends. Cell. 149:371–382. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sandblad L, Busch KE, Tittmann P, Gross H,
Brunner D and Hoenger A: The Schizosaccharomyces pombe EB1
homolog Mal3p binds and stabilizes microtubule lattice seam. Cell.
127:1415–1424. 2006. View Article : Google Scholar
|
|
60
|
Pagano A, Honoré S, Mohan R, Berges R,
Akhmanova A and Braguer D: Epothilone B inhibits migration of
glioblastoma cells by inducing microtubule catastrophes and
affecting EB1 accumulation at microtubule plus ends. Biochem
Pharmacol. 84:432–443. 2012. View Article : Google Scholar : PubMed/NCBI
|