1. Discovery of BTG2 in TOB/BTG gene
family
The TOB/BTG genes belong to the anti-proliferative
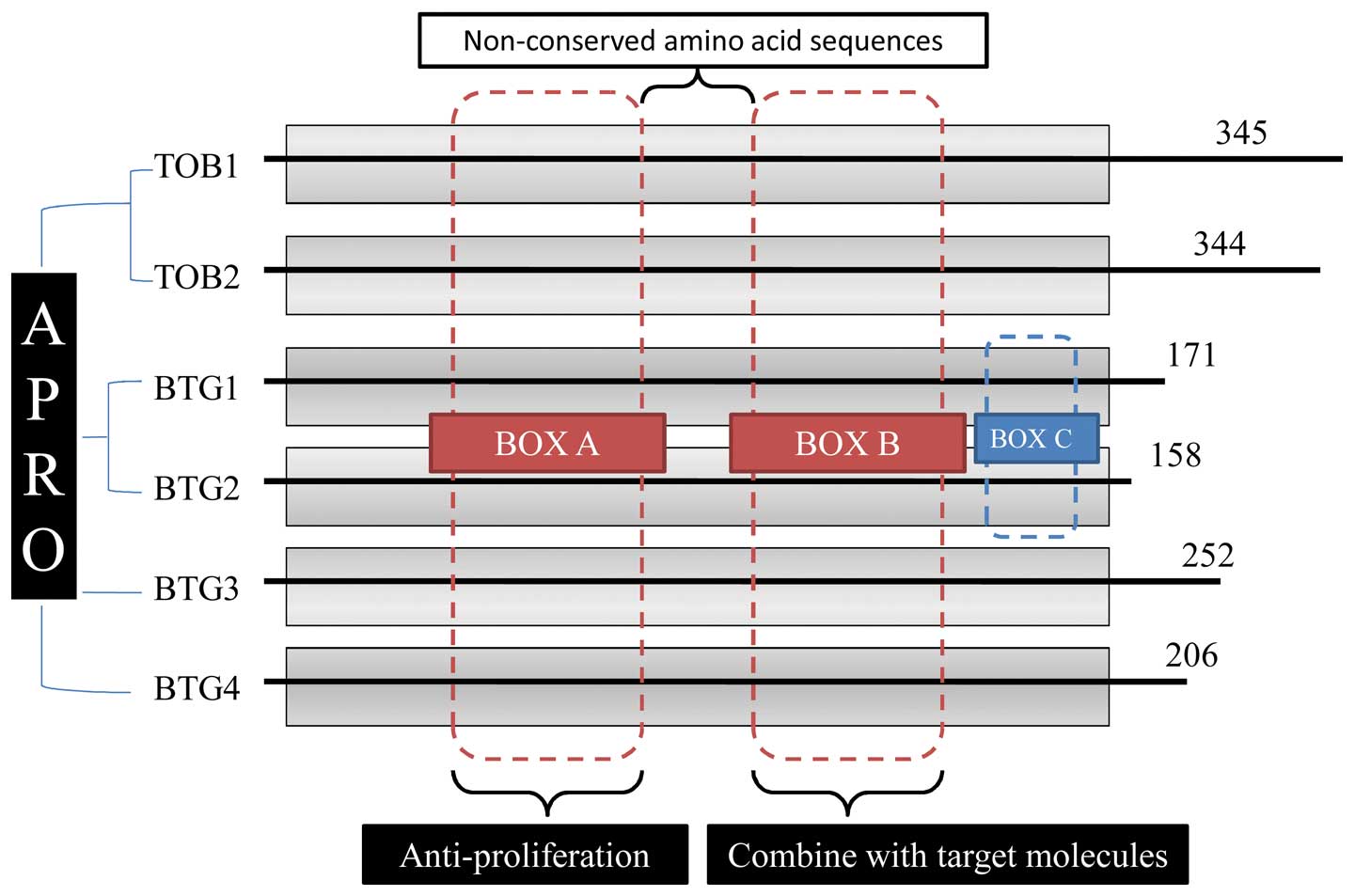
gene family that includes six different genes in vertebrates: TOB1,
TOB2, BTG1 BTG2/TIS21/PC3, BTG3 and BTG4 (Fig. 1). The conserved domain of BTG
N-terminal contains two regions, named box A and box B, which show
a high level of homology to the other domains (1–5). Box
A has a major effect on cell proliferation, while box B plays a
role in combination with many target molecules. Compared with other
family members, BTG1 and BTG2 have an additional region named box C
(Fig. 1) (6). Both box A and box B have a
relationship with CCR4-associated factor 1 (Caf1), which attributes
to the ribonuclease D (RNase D) family of deadenylases and belongs
to the CCR4-Not deadenylase complex (7).
The BTG domain has an effect on a protein-protein
interactions, and the interaction exists between BTG1/2 and a
homeobox transcription factor Hoxb9 (5). In myoblasts, BTG1 has an effect on
the activity of several transcription factors (8). BTG3 can affect E2F1, an important
transcription factor in cell cycle progression especially for the
S-phase. Moreover, the binding between DNA and the E2F1-DP1
transcription factor complex can be inhibited by BTG3, which has a
negative effect on the inhibition of S-phase progression (9).
To our best knowledge, BTG2 is the first identified
gene in the BTG/TOB family (10),
which is located on band 2, region 3 of the long arm of chromosome
1 and encodes 158 amino acids (11). In 1991, the PC3 gene was discovered
as an immediate early gene induced by nerve growth factor (NGF) in
rat PC12 cell line during neuronal differentiation (12), which has the characteristics of
anti-proliferation molecules. Fletcher et al (13) reported that tetradecanoyl phorbol
acetate (TPA), a tumor-promoting agent, induced mouse NIH3T3 cell
line to produce TIS21 that has the same gene sequence as PC3. In
1996, Rouault et al (14)
cloned and localized the human BTG2 gene, and reported that its
expression is regulated by a p53-dependent mechanism and its
function is related to cell cycle regulation and cellular reaction
to DNA damage. TIS21, PC3, and BGT2 are the homologous genes in
mice, rats, and humans, respectively.
2. Biological functions of BTG2
BTG2 is expressed in various organs and tissues,
such as the spleen, thymus, lungs, stomach and large intestine,
with high expression levels being reported in the lung, intestines,
pancreas and prostate (15). It
has been demonstrated that BTG2 can arrest cells at G1/S and G2/M
transition, increase apoptosis (14), promote retinoic acid-induced
differentiation in hematopoietic cells (16) and inhibit expansion of thymocytes
(17). Moreover, BTG2 also plays
important roles in cell differentiation, proliferation, DNA damage
repair and apoptosis.
Differentiation
It has been demonstrated that BTG2 gene is involved
in the development and differentiation of nerve cells and
hematopoietic cells (18). It is
overexpressed in the G0/G1 phase of the cell cycle in
neuroepithelial cells (18),
suggesting that it plays a role in the induction of nerve growth
and differentiation. Overexpression of BTG2 is involved in the
differentiation process of HL-60 cells after treatment with
12-O-tetra-decanoylphorbol-13-acetate (TPA) or retinoic acid (RA)
(19). Most notably, BTG2 gene
expression occurs earlier than p21 expression, indicating BTG2
plays an important role in the differentiation processes of
hematopoietic cells (19).
Anti-proliferation
BTG2 plays an important role in regulation of cell
proliferation, through regulating the cell cycle in some solid
tumors. Zhang et al (20)
reported that BTG2 inhibited the proliferation and invasion of
gastric cancer cells. Ma and Ni (21) demonstrated that BTG2 overexpression
can suppress the cell growth and promote apoptosis in pancreatic
cancer cells. Moreover, BTG2 overexpression inhibits the expression
of cyclin D1, MMP-1, and MMP-2, and suppresses the proliferation of
lung cancer cells (22). These
findings support that BTG2 acts as an anti-proliferative gene in
carcinogenesis. However, Wagener et al (23) showed that BTG2 promoted the
migration of bladder cancer cells and BTG2 overexpression is
associated with poor survival in patients with bladder cancer.
Thus, the roles of BTG2 in oncogenesis may be cancer
type-dependent.
DNA damage repair
Our previous studies demonstrated that p53, the most
investigated tumor suppressor gene, can upregulate BTG2 expression,
and downregulate cyclins D1 and E during hepatocarcinogenesis
(1,24). When DNA is damaged, p53 is
upregulated, which will induce DNA repair and cause the cell cycle
arrest at G1/S-phase. If damage cannot be repaired, p53 will induce
programmed cell death or apoptosis. It has been demonstrated that
BTG2 exerts its antitumor effect through p53-dependent Ras signal
transduction pathway (3). Thus,
the BTG2 gene expression is significantly increased when DNA is
damaged. Various DNA-damaging agents, such as, ionizing radiation,
some chemical substances and ultraviolet may induce the BTG2
expression via p53 and cause the cell cycle arrest at G1 phase
through inhibiting cyclin D1, which may promote DNA damage repair
(25,26). Recently, Choi et al
(27) reported that TIS21/BTG2/PC3
plays a role in the DNA damage response. In their study,
TIS21/BTG2/PC3 accelerates the repair of DNA double strand breaks
(DSBs) according to the increased activity of the methylation of
Mre 11 and protein arginine methyltransferase 1 (PRMT1) (27). However, the detailed mechanisms for
the involvement of BTG2/TIS21/ PC3 in DNA damage repair are not
fully understood and need further investigation.
Apoptosis
Several studies demonstrated that BTG2 can promote
or induce cell apoptosis (21,28).
Zhang et al (20) reported
that BTG2 not only restrains the biological activities of gastric
cancer cells, such as cell growth and proliferation, cell migration
and invasion, but also promotes tumor cell apoptosis. Zhang et
al (28) found that BTG2
induces cell apoptosis and suppresses cell invasion of human
triple-negative breast cancer MDA-MB-231 cells. Hong et al
(29) report that the treatment of
U937 cells with BTG2 inhibits the expression of cell division cycle
2 kinase (cyclin B1-CDC2) and causes cell apoptosis. However, it is
reported that high level of PC3 gene in PC12 cells can inhibit cell
apoptosis (30). Thus, the effects
of BTG2 on cell apoptosis may be cell type-dependent and the
precise mechanisms need further investigation.
3. Regulation of BTG2 gene
BTG2 is considered an early growth response gene. In
our previous study, we established an improved diethylnitrosamine
(DEN)-induced primary hepatocellular carcinoma (HCC) rat model and
evaluated the expression of BTG2, p53, cyclins D1 and E in HCC
(1). We found that expression of
the BTG2 and p53 was detected at the early stage of DEN treatment,
and peaked at 5 weeks and then declined gradually. The expression
of cyclins D1 and E was increased significantly during
hepatocarcinogenesis (1).
Actually, the expression of BTG2 appears to be time-dependent in
the cell cycle, but the regulatory mechanism of BTG2 is
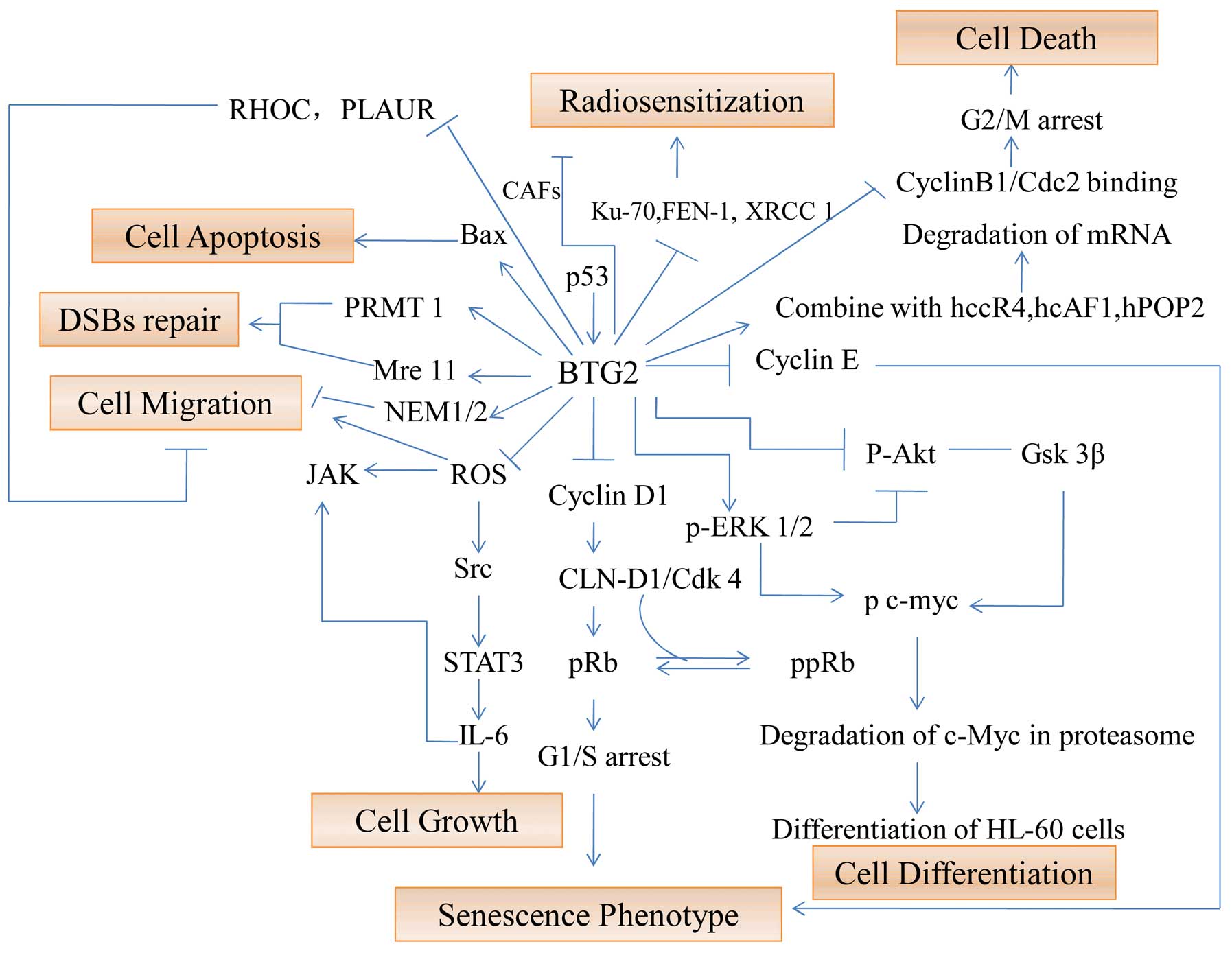
complicated. BTG2 regulates cell growth, death, migration,
apoptosis, and radiosensitization through several important
signaling pathways (Fig. 2).
The classical pathway of BTG2 on
senescence phenotype
As aforementioned, following DNA damage, BTG2
expression is stimulated by p53 and the expression of cyclin D1 is
downregulated, inhibiting G1/S transition via the pRB pathway. This
pathway is a classical pathway and the activation of BTG2 is
p53-dependent. BTG2 expression is also upregulated by oxidative
stress via reactive oxygen species-protein kinase C-NFκB pathway,
which is independent of p53 status (31). Additionally, BTG2 suppresses the
expression of cyclin E and inhibits G1/S transition in 293 cells in
the absence of p53, Rb and cyclin D1 (32).
The pathway of BTG2 on cell
migration
The low expression of BTG2 is found in many human
cancer tissues and its loss is related to cell migration and
invasion. Lim et al (33)
reported that BTG2 has a negative effect on cancer cell metastasis
by inhibiting Src-FAK (focal adhesion kinase) signaling through
downregulation of reactive oxygen species (ROS) generation. Wagener
et al (23) also reported
that endogenous BTG2 expression contributes to the migratory
potential of bladder cancer cells and the high levels of BTG2 in
bladder cancers are correlated with poor clinical prognosis in
patients with bladder cancer.
The pathway of BTG2 on cell growth
A recent study demonstrated that cisplatin
attenuates prostate cancer cell proliferation in part by
upregulation of BTG2 through the p53-dependent pathway or
p53-independent NF-κB pathway (34). BTG2 negatively regulates JAK2/STAT3
signaling through the regulation of ROS generation indirectly
(35). Therefore, it is likely
that BTG2 exerts its antitumor activity via the downregulation of
cytokine secretion.
The pathway of BTG2 on cell
differentiation
PC3/Tis21 knockout mice display impaired terminal
differentiation of hippocampal granule neurons and defective
contextual memory (36). Moreover,
BTG2 regulates the differentiation of HL-60 cells mainly through
activation of Erk1/2 and inhibition of Akt, resulting in the
downregulation of c-Myc (37).
The pathway of BTG2 on cell death
BTG2 negatively regulates cyclin D1 and decreases
the expression of FoxM1 cell cycle regulatory factor through
inhibiting the binding of cyclin B1/Cdks (38). Ryu et al (39) found that BTG2 is upregulated by
PKC-δ in U937 cells, and induces G2/M arrest and cell death through
inhibiting cyclin B1-Cdc2 binding. BTG2 stimulates PRMT1 (protein
arginine methyltransferase 1), promoting DNA repair of DSBs and Mre
11 methylation (27,40). In addition, BTG2 upregulates Bax
gene to promote apoptosis (41).
The pathway of BTG2 on
radiosensitization
It has been demonstrated that BTG2 is one of the
important radiation response genes (25). A recent study showed that BTG2
improves the radiosensitivity of breast cancer cells by affecting
cell cycle distribution, enhancing radiation-induced apoptosis, and
inhibiting DNA repair-related protein expression (42). Moreover, Chu et al (26) reported that cancer-associated
fibroblasts (CAFs) can reduce the levels of GADD45 and BTG2, both
are major radiation-induced genes. These data suggest that BTG2 may
be one of the most important response genes during
radiotherapy.
In summary, BTG2 plays an important role in cancer
development and progression (Fig.
2). However, like other genes, the regulatory mechanisms of
BTG2 in cancer are complex and need further investigation.
4. Relationship between miRNAs and BTG2
It is well documented the miRNAs inhibit mRNA
translation and reduce mRNA stability by binding to the
3′-untranslated region (3′UTR) of the target mRNAs (43–45).
Nearly 30% of human genes are regulated by miRNAs (46). A miRNA has multiple target genes,
while a target gene can be regulated by multiple miRNAs. It has
been found that miRNAs are associated with tumor formation and
progression through regulation of oncogenes or tumor suppressor
genes (46).
BTG2 and miR-21
miRNA-21 (miR-21) is one of the important miRNAs and
is widely distributed in human tissues and cells. The
overexpression of miRNA-21 has been observed in a variety of
carcinomas, such as prostate, gastric, colon breast and lung
cancers; miRNA-21 promotes cell growth and proliferation and
inhibits cell apoptosis (47–53).
Recent studies demonstrated that BTG2 is a new target gene of
miR-21 in prostate cancer cells (49), laryngeal cancer cells (52) and melanoma cells (53). Liu et al (52) uncovered that miR-21 promotes the
proliferation of laryngeal cancer cells through downregulation of
BTG2. Sun et al (54)
reported that miR-21 is overexpressed and negatively regulates BTG2
expression in lung cancer cells, promoting lung cancer cells
growth, progression and invasion. Coppola et al (49) demonstrated that the loss of BTG2
and upregulation of miR-21 contribute to the epithelial-mesenchymal
transition in prostate cancer cells. Thus, miR-21 plays an
important role in regulating BTG2 gene during carcinogenesis.
BTG2 and other miRNAs
Several studies have shown that BTG2 is regulated by
other miRNAs in different cancers. A recent study demonstrated that
miR-21, miR-23A and miR-27A cooperatively regulate the expression
of tumor suppressor genes, including PDCD4, BTG2 and NEDD4L
(55). Jalava et al
(56) reported that
androgen-mediated miR-32 is overexpressed in castration-resistant
prostate cancer (CRPC), resulting in a reduced expression of BTG2.
Alvarez-Saavedra et al (57) report that BTG2 and Paip2a are
direct targets of miR-132 in the mouse suprachiasmatic nucleus.
Interestingly, our previous study also observed that overexpression
of miR-18 in hepatocellular cancer cells negatively regulates the
BTG2 expression (58).
5. Relationship between BTG2 and cancer
Several studies have suggested that BTG2 can be a
potential biomarker for prognosis in cancer patients (23,28,59,60).
BTG2 is closely associated with other tumor suppressor genes, such
as RB, p53 and p73 (1–3,14);
BTG2 expression is downregulated in laryngeal carcinoma (52). In patients with pancreatic cancer,
the mRNA expression of BTG2 is obviously suppressed in tumor
tissues compare with the surrounding non-cancerous tissues
(21). Struckmann et al
(61) reported that the BTG2
expression is reduced in clear cell renal cell carcinomas. Zhang
et al (20) demonstrated
that BTG2 inhibits cell invasion and proliferation of the gastric
adenocarcinoma cells SGC7901 and MKN45. Takahashi et al
(59) revealed that low expression
of BTG2 is related to tumor size, grade, metastasis, recurrence and
poor survival in patients with breast carcinoma. Our study also
found that BTG2 is involved in the differentiation of cancer cells
and associated with the clinical pathology (24). The low level of BTG2 expression is
associated with poor differentiation of cancer cells. Moreover, Hu
et al (42) found that BTG2
overexpression in human breast cancer cell line MCF-7 increases
cell sensitivity to ionizing radiation; increased apoptosis is
observed alongside decreased expression of cyclins D1 and B1, Ku70,
FEN-1, and XRCC1 protein as well as increased BAX protein
expression. Furthermore, Quy et al (35) observed that BTG2 plays a role in
cancer microenvironment by downregulating IL-6 expression in human
dermal fibroblasts through inhibiting STAT3 activation. In summary,
BTG2 acts as a tumor suppressor in most solid tumors. However,
upregulation of BTG2 promotes cell migration and is related to poor
survival in bladder cancer. Future studies should explore the
underlying mechanisms (Table
I).
 | Table IThe roles of BTG2 in different
cancers. |
Table I
The roles of BTG2 in different
cancers.
| Cancer type | Major findings |
|---|
| Laryngeal
carcinoma | BTG2 is a pan-cell
cycle regulator and tumor suppressor (51) |
| Lung cancer | BTG2 inhibits cell
proliferation and invasion (22,44,47,54) |
| Prostate
cancer | BTG2 loss leads to
acquisition of epithelial-mesenchymal transition (EMT) (49) |
| Gastric cancer | BTG2 inhibits cell
invasion and proliferation (20) |
| Renal cell
carcinoma | Impaired BTG2
expression is found in clear cell renal carcinoma (61) |
| Breast cancer | Downregulation of
BTG2 is associated with poor survival in patients with breast
carcinoma; upregulation of BTG2 increases the radiosensitivity of
breast cancer cells (42,50) |
| Pancreatic
cancer | BTG2 inhibits cell
growth and induces apoptosis (21) |
| Bladder cancer | BTG2 promotes cell
migration and BTG2 overexpression is associated with poor survival
in patients with bladder cancer (23) |
6. Concluding remark
In this review, we summarized evidence supporting
that the BTG2 gene has anti-proliferative properties in most human
cancers. BTG2 mediates the expression of several genes that decide
the fate of cells through activation of some downstream factors in
different pathways. It is also a radiosensitizer in breast cancer
cells, through inducing cell cycle arrest, promoting
radiation-induced apoptosis, and modulating the expression of DNA
repair-related proteins. However, the underlying mechanisms of the
BTG2 tumor suppressing activities are not fully understood. There
are many issues to be addressed in future investigations, including
the regulation of BTG2-associated signal pathways, identification
of BTG2 target molecules, interactions between miRNAs and BTG2
expression and function of the different roles of BTG2 in different
cancers, and the potential of BTG2 as a diagnostic and prognostic
marker in cancer patients.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81272498, 30973457 and
30901764).
References
|
1
|
Zhang Z, Chen C, Wang G, Yang Z, San J,
Zheng J, Li Q, Luo X, Hu Q, Li Z and Wang D: Aberrant expression of
the p53-inducible antiproliferative gene BTG2 in hepatocellular
carcinoma is associated with overexpression of the cell
cycle-related proteins. Cell Biochem Biophys. 61:83–91. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Horvilleur E, Bauer M, Goldschneider D,
Mergui X, de la Motte A, Benard J, Douc-Rasy S and Cappellen D:
p73alpha isoforms drive opposite transcriptional and
post-transcriptional regulation of MYCN expression in neuroblastoma
cells. Nucleic Acids Res. 36:4222–4232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boiko AD, Porteous S, Razorenova OV,
Krivokrysenko VI, Williams BR and Gudkov AV: A systematic search
for downstream mediators of tumor suppressor function of p53
reveals a major role of BTG2 in suppression of Ras-induced
transformation. Genes Dev. 20:236–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horiuchi M, Takeuchi K, Noda N, Muroya N,
Suzuki T, Nakamura T, Kawamura-Tsuzuku J, Takahasi K, Yamamoto T
and Inagaki F: Structural basis for the antiproliferative activity
of the Tob-hCaf1 complex. J Biol Chem. 284:13244–13255. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winkler GS: The mammalian
anti-proliferative BTG/Tob protein family. J Cell Physiol.
222:66–72. 2010. View Article : Google Scholar
|
|
6
|
Yang X, Morita M, Wang H, Suzuki T, Yang
W, Luo Y, Zhao C, Yu Y, Bartlam M, Yamamoto T and Rao Z: Crystal
structures of human BTG2 and mouse TIS21 involved in suppression of
CAF1 deadenylase activity. Nucleic Acids Res. 36:6872–6881. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duriez C, Moyret-Lalle C, Falette N,
El-Ghissassi F and Puisieux A: BTG2, its family and its tutor. Bull
Cancer. 91:E242–E253. 2004.PubMed/NCBI
|
|
8
|
Busson M, Carazo A, Seyer P, Grandemange
S, Casas F, Pessemesse L, Rouault JP, Wrutniak-Cabello C and
Cabello G: Coactivation of nuclear receptors and myogenic factors
induces the major BTG1 influence on muscle differentiation.
Oncogene. 24:1698–1710. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ou YH, Chung PH, Hsu FF, Sun TP, Chang WY
and Shieh SY: The candidate tumor suppressor BTG3 is a
transcriptional target of p53 that inhibits E2F1. EMBO J.
26:3968–3980. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buanne P, Corrente G, Micheli L, Palena A,
Lavia P, Spadafora C, Lakshmana MK, Rinaldi A, Banfi S, Quarto M,
Bulfone A and Tirone F: Cloning of PC3B, a novel member of the
PC3/BTG/ TOB family of growth inhibitory genes, highly expressed in
the olfactory epithelium. Genomics. 68:253–263. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim IK: TIS21 (/BTG2/PC3) as a link
between ageing and cancer: cell cycle regulator and endogenous cell
death molecule. J Cancer Res Clin Oncol. 132:417–426. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bradbury A, Possenti R, Shooter EM and
Tirone F: Molecular cloning of PC3, a putatively secreted protein
whose mRNA is induced by nerve growth factor and depolarization.
Proc Natl Acad Sci USA. 88:3353–3357. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fletcher BS, Lim RW, Varnum BC, Kujubu DA,
Koski RA and Herschman HR: Structure and expression of TIS21, a
primary response gene induced by growth factors and tumor
promoters. J Biol Chem. 266:14511–14518. 1991.PubMed/NCBI
|
|
14
|
Rouault JP, Falette N, Guehenneux F,
Guillot C, Rimokh R, Wang Q, Berthet C, Moyret-Lalle C, Savatier P,
Pain B, Shaw P, Berger R, Samarut J, Magaud JP, Ozturk M, Samarut C
and Puisieux A: Identification of BTG2, an antiproliferative
p53-dependent component of the DNA damage cellular response
pathway. Nat Genet. 14:482–486. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Melamed J, Kernizan S and Walden PD:
Expression of B-cell translocation gene 2 protein in normal human
tissues. Tissue Cell. 34:28–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Passeri D, Marcucci A, Rizzo G, Billi M,
Panigada M, Leonardi L, Tirone F and Grignani F: Btg2 enhances
retinoic acid-induced differentiation by modulating histone H4
methylation and acetylation. Mol Cell Biol. 26:5023–5032. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Konrad MA and Zuniga-Pflucker JC: The
BTG/TOB family protein TIS21 regulates stage-specific proliferation
of developing thymocytes. Eur J Immunol. 35:3030–3042. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsuda S, Rouault J, Magaud J and Berthet
C: In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS
Lett. 497:67–72. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cho BO, Jeong YW, Kim SH, Park K, Lee JH,
Kweon GR and Park JC: Up-regulation of the BTG2 gene in TPA- or
RA-treated HL-60 cell lines. Oncol Rep. 19:633–637. 2008.PubMed/NCBI
|
|
20
|
Zhang L, Huang H, Wu K, Wang M and Wu B:
Impact of BTG2 expression on proliferation and invasion of gastric
cancer cells in vitro. Mol Biol Rep. 37:2579–2586. 2010. View Article : Google Scholar
|
|
21
|
Ma XM and Ni KL: Expression of
antiproliferation gene BTG2 in human pancreatic cancer and its
antiproliferation effect. World J Tumor. 9:20–24. 2010.(In
Chinese).
|
|
22
|
Wei S, Hao C, Li X, Zhao H, Chen J and
Zhou Q: Effects of BTG2 on proliferation inhibition and
anti-invasion in human lung cancer cells. Tumour Biol.
33:1223–1230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wagener N, Bulkescher J, Macher-Goeppinger
S, Karapanagiotou-Schenkel I, Hatiboglu G, Abdel-Rahim M,
Abol-Enein H, Ghoneim MA, Bastian PJ, Muller SC, Haferkamp A,
Hohenfellner M, Hoppe-Seyler F and Hoppe-Seyler K: Endogenous BTG2
expression stimulates migration of bladder cancer cells and
correlates with poor clinical prognosis for bladder cancer
patients. Br J Cancer. 108:973–982. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang ZM, Wang G, Yang ZX, Shan JL, Chen
C, Jin F, Xu W, Li Q, Luo XZ, Wang D and Li ZP: The expression of
B-cell translocation gene 2 in diethylnitrosamine-induced primary
hepatocellular carcinoma rat model. Chin J Hepatol. 17:107–111.
2009.(In Chinese).
|
|
25
|
Kis E, Szatmári T, Keszei M, Farkas R,
Esik O, Lumniczky K, Falus A and Sáfrány G: Microarray analysis of
radiation response genes in primary human fibroblasts. Int J Radiat
Oncol Biol Phys. 66:1506–1514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chu TY, Yang JT, Huang TH and Liu HW:
Crosstalk with cancer-associated fibroblasts increases the growth
and radiation survival of cervical cancer cells. Radiat Res.
181:540–547. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi KS, Kim JY, Lim SK, Choi YW, Kim YH,
Kang SY, Park TJ and Lim IK: TIS21(/BTG2/PC3) accelerates the
repair of DNA double strand breaks by enhancing Mre11 methylation
and blocking damage signal transfer to the Chk2(T68)-p53(S20)
pathway. DNA Repair (Amst). 11:965–975. 2012. View Article : Google Scholar
|
|
28
|
Zhang YJ, Wei L, Liu M, Li J, Zheng YQ,
Gao Y and Li XR: BTG2 inhibits the proliferation, invasion, and
apoptosis of MDA-MB-231 triple-negative breast cancer cells. Tumour
Biol. 34:1605–1613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hong JW, Ryu MS and Lim IK:
Phosphorylation of serine 147 of tis21/BTG2/pc3 by p-Erk1/2 induces
Pin-1 binding in cytoplasm and cell death. J Biol Chem.
280:21256–21263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Farioli-Vecchioli S, Tanori M, Micheli L,
Mancuso M, Leonardi L, Saran A, Ciotti MT, Ferretti E, Gulino A,
Pazzaglia S and Tirone F: Inhibition of medulloblastoma
tumorigenesis by the antiproliferative and pro-differentiative gene
PC3. FASEB J. 21:2215–22125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Imran M and Lim IK: Regulation of
Btg2(/TIS21/PC3) expression via reactive oxygen species-protein
kinase C-NFκB pathway under stress conditions. Cell Signal.
25:2400–2412. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lim IK, Lee MS, Ryu MS, Park TJ, Fujiki H,
Eguchi H and Paik WK: Induction of growth inhibition of 293 cells
by downregulation of the cyclin E and cyclin-dependent kinase 4
proteins due to overexpression of TIS21. Mol Carcinog. 23:25–35.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lim SK, Choi YW, Lim IK and Park TJ: BTG2
suppresses cancer cell migration through inhibition of Src-FAK
signaling by downregulation of reactive oxygen species generation
in mitochondria. Clin Exp Metastasis. 29:901–913. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chiang KC, Tsui KH, Chung LC, Yeh CN, Feng
TH, Chen WT, Chang PL, Chiang HY and Juang HH: Cisplatin modulates
B-cell translocation gene 2 to attenuate cell proliferation of
prostate carcinoma cells in both p53-dependent and p53-independent
pathways. Sci Rep. 4:55112014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Quy LN, Choi YW, Kim YH, Chwae YJ, Park TJ
and Lim IK: TIS21(/BTG2/PC3) inhibits interleukin-6 expression via
downregulation of STAT3 pathway. Cell Signal. 25:2391–2399. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Farioli-Vecchioli S, Saraulli D, Costanzi
M, Leonardi L, Cinà I, Micheli L, Nutini M, Longone P, Oh SP,
Cestari V and Tirone F: Impaired terminal differentiation of
hippocampal granule neurons and defective contextual memory in
PC3/Tis21 knockout mice. PLoS One. 4:e83392009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Imran M, Park TJ and Lim IK:
TIS21/BTG2/PC3 enhances downregulation of c-Myc during
differentiation of HL-60 cells by activating Erk1/2 and inhibiting
Akt in response to all-transretinoic acid. Eur J Cancer.
48:2474–2485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park TJ, Kim JY, Oh SP, Kang SY, Kim BW,
Wang HJ, Song KY, Kim HC and Lim IK: TIS21 negatively regulates
hepatocarcinogenesis by disruption of cyclin B1-Forkhead box M1
regulation loop. Hepatology. 47:1533–1543. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ryu MS, Lee MS, Hong JW, Hahn TR, Moon E
and Lim IK: TIS21/BTG2/PC3 is expressed through PKC-delta pathway
and inhibits binding of cyclin B1-Cdc2 and its activity,
independent of p53 expression. Exp Cell Res. 299:159–170. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Miyata S, Mori Y and Tohyama M: PRMT1 and
Btg2 regulates neurite outgrowth of Neuro2a cells. Neurosci Lett.
445:162–165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Del Puerto HL, Martins AS, Moro L, Milsted
A, Alves F, Braz GF and Vasconcelos AC: Caspase-3/-8/-9, Bax and
Bcl-2 expression in the cerebellum, lymph nodes and leukocytes of
dogs naturally infected with canine distemper virus. Genet Mol Res.
9:151–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hu X, Xing L, Jiao Y, Xu J, Wang X, Han A
and Yu J: BTG2 overexpression increases the radiosensitivity of
breast cancer cells in vitro and in vivo. Oncol Res. 20:457–465.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Taenzer A, Alix-Panabieres C, Wikman H and
Pantel K: Circulating tumor-derived biomarkers in lung cancer. J
Thorac Dis. 4:448–449. 2012.PubMed/NCBI
|
|
45
|
Plaisance-Bonstaff K and Renne R: Viral
miRNAs. Methods Mol Biol. 721:43–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kato M and Slack FJ: MicroRNAs: small
molecules with big roles - C. elegans to human cancer. Biol Cell.
100:71–81. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu J, Liu X, Cui F, Chen G, Guan Y and He
J: The efficacy of the inhalation of an aerosolized Group A
streptococcal preparation in the treatment of lung cancer. Chin J
Cancer Res. 24:346–352. 2012.(In Chinese). View Article : Google Scholar
|
|
48
|
Pass HI: Biomarkers and prognostic factors
for mesothelioma. Ann Cardiothorac Surg. 1:449–456. 2012.
|
|
49
|
Coppola V, Musumeci M, Patrizii M,
Cannistraci A, Addario A, Maugeri-Sacca M, Biffoni M,
Francescangeli F, Cordenonsi M, Piccolo S, Memeo L, Pagliuca A,
Muto G, Zeuner A, De Maria R and Bonci D: BTG2 loss and miR-21
upregulation contribute to prostate cell transformation by inducing
luminal markers expression and epithelial-mesenchymal transition.
Oncogene. 32:1843–1853. 2013. View Article : Google Scholar
|
|
50
|
Niu J, Shi Y, Tan G, Yang CH, Fan M,
Pfeffer LM and Wu ZH: DNA damage induces NF-kappaB-dependent
microRNA-21 up-regulation and promotes breast cancer cell invasion.
J Biol Chem. 287:21783–21795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY and
Yan M: microRNA-21 promotes tumor proliferation and invasion in
gastric cancer by targeting PTEN. Oncol Rep. 27:1019–1026.
2012.PubMed/NCBI
|
|
52
|
Liu M, Wu H, Liu T, Li Y, Wang F, Wan H,
Li X and Tang H: Regulation of the cell cycle gene, BTG2, by miR-21
in human laryngeal carcinoma. Cell Res. 19:828–837. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang CH, Yue J, Pfeffer SR, Handorf CR and
Pfeffer LM: MicroRNA miR-21 regulates the metastatic behavior of
B16 melanoma cells. J Biol Chem. 286:39172–39178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sun Q, Hang M, Guo X, Shao W and Zeng G:
Expression and significance of miRNA-21 and BTG2 in lung cancer.
Tumour Biol. 34:4017–4026. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Frampton AE, Castellano L, Colombo T,
Giovannetti E, Krell J, Jacob J, Pellegrino L, Roca-Alonso L, Funel
N, Gall TM, De Giorgio A, Pinho FG, Fulci V, Britton DJ, Ahmad R,
Habib NA, Coombes RC, Harding V, Knösel T, Stebbing J and Jiao LR:
MicroRNAs cooperatively inhibit a network of tumor suppressor genes
to promote pancreatic tumor growth and progression.
Gastroenterology. 146:268–277.e18. 2014. View Article : Google Scholar
|
|
56
|
Jalava SE, Urbanucci A, Latonen L,
Waltering KK, Sahu B, Jänne OA, Seppälä J, Lähdesmäki H, Tammela TL
and Visakorpi T: Androgen-regulated miR-32 targets BTG2 and is
overexpressed in castration-resistant prostate cancer. Oncogene.
31:4460–4471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Alvarez-Saavedra M, Antoun G, Yanagiya A,
Oliva-Hernandez R, Cornejo-Palma D, Perez-Iratxeta C, Sonenberg N
and Cheng HY: miRNA-132 orchestrates chromatin remodeling and
translational control of the circadian clock. Hum Mol Genet.
20:731–751. 2011. View Article : Google Scholar :
|
|
58
|
Li Q, Wang G and Zhang ZM: The
relationship between microRNA-18 and BTG2 in the carcinogenesis of
hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi. 17:42–45.
2009.(In Chinese). PubMed/NCBI
|
|
59
|
Takahashi F, Chiba N, Tajima K, Hayashida
T, Shimada T, Takahashi M, Moriyama H, Brachtel E, Edelman EJ,
Ramaswamy S and Maheswaran S: Breast tumor progression induced by
loss of BTG2 expression is inhibited by targeted therapy with the
ErbB/HER inhibitor lapatinib. Oncogene. 30:3084–3095. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Choi JA and Lim IK: TIS21/BTG2 inhibits
invadopodia formation by downregulating reactive oxygen species
level in MDA-MB-231 cells. J Cancer Res Clin Oncol. 139:1657–1665.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Struckmann K, Schraml P, Simon R,
Elmenhorst K, Mirlacher M, Kononen J and Moch H: Impaired
expression of the cell cycle regulator BTG2 is common in clear cell
renal cell carcinoma. Cancer Res. 64:1632–1638. 2004. View Article : Google Scholar : PubMed/NCBI
|