Introduction
Radiotherapy (RT) is commonly used for the treatment
of non-small cell lung cancer (NSCLC) but tumor control and
survival outcomes remain poor for affected patients due to RT
resistance (1,2). Combination therapies involving
radiosensitizing drugs and RT are therefore currently recommended
for NSCLC cases (3).
Cis-diamminedichloroplatinum(II) (cisplatin, CDDP) is a
well-known radiosensitizing agent and is administered as part of a
primary intervention, particularly for advanced NSCLC treatment
regimens (4). However, the use of
CDDP is often limited as it is severely nephrotoxic (5). CDDP metabolites also induce
nephrotoxicity through a biotransformation pathway (6). Hence, the development of alternatives
to CDDP is of great interest.
Liposome (LP) is well characterized as a classical
carrier for drug delivery system (DDS) (7). LP can alter both the pharmacokinetics
and the biodistribution of drugs by affecting the size, surface
charge and membrane lipid packing (8). A size-controlled LP can efficiently
deliver a drug to the site of a tumor through an enhanced
permeability and retention (EPR) effect (passive targeting) and
protect the drug from metabolic processes that may clear it from
the body prematurely (9). LP is
insufficient however to actively target a specific site or sustain
the long-term circulation in the bloodstream because of its
elimination through the reticuloendothelial system (RES).
Epidermal growth factor receptor (EGFR) is
frequently targeted as an anticancer therapy strategy as its
overexpression has been identified in many types of human cancer
including NSCLC (10–12). Furthermore, EGFR overexpression
plays a major role in reducing the radiosensitivity of NSCLC cells
(13,14). Recently, an active targeting
approach has emerged involving the display of a tumor-specific
ligand or antibody on an LP (15,16).
In our current study, we conjugated an EGFR antibody to an
liposomal CDDP (LP-CDDP) and evaluated its ability to enhance the
efficacy of targeted RT without the adverse nephrotoxic effects of
CDDP.
Materials and methods
Preparation of CDDP-incorporated
immunoliposome conjugated with EGFR antibodies (EGFR:LP-CDDP)
CDDP was purchased from Sigma (St. Louis, MO, USA).
Monoclonal anti-EGFR antibodies were prepared from the hybridoma
line HB-8509 (ATCC, Manassas, VA, USA). LP-CDDP was prepared as
previously described (17).
Briefly, dipalmitoylphosphatidylcholine, cholesterol, ganglioside,
diacetyl phosphate and dipalmitoylphosphatidylethanolamine
(35:40:15:5:5 as the molar ratio; Katayama Chemical Industries Co.,
Ltd., Osaka, Japan) were dissolved in methanol/chloroform (1:1 v/v)
solution. The lipid film was produced by evaporating and drying
under vacuum. It was dissolved in 10 mM
N-tris(hydroxymethyl)methyl-3-amino-propanesulfonic acid (TAPS)
buffer (pH 8.4), followed by sonication to obtain small unilamellar
vesicles. The LP encapsulated CDDP (17). The lipid concentration was measured
as total cholesterol in the 0.5% Triton X-100 (Sigma-Aldrich Korea,
Ltd., Gyeonggi, Korea), using a Determiner TC 555 kit. Total lipid
concentration was calculated by multiplying 2.5 by cholesterol
concentration. Anti-EGFR antibodies were displayed on the LP
surface using 3,3′-dithiobis(sulfo-succinimidylpropionate) (DTSSP)
(Pierce Biotechnology, Inc., Rockford, IL, USA). Tris was then
added to a final concentration of 132 mg/ml to terminate the
reaction (18). To quantify the
number of EGFR antibodies on the LP-CDDP, western blotting was
used. Briefly, the samples were boiled in sample buffer and
separated using 4–15% gradient SDS-PAGE. The resolved proteins were
then transferred onto a polyvinylidene difluoride (PVDF) membrane
(Millipore, Bedford, MA, USA) which was blocked in a 5% skim milk
solution (Becton-Dickinson & Co., Sparks, MD, USA) in
Tris-buffered saline with Tween-20 (TBST) (50 mM Tris-HCl pH 7.4,
150 mM NaCl, 0.1% Tween-20) for 1 h. The filter was then incubated
with peroxidase-conjugated donkey anti-mouse IgG (Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 2 h.
Immunoreactive protein was visualized using ECL Western Blotting
Detection Reagents (GE Healthcare, Buckinghamshire, UK).
Physicochemical characterization
The sizes and ζ-potentials of the LP-CDDP and
EGFR:LP-CDDP were measured at 25°C by dynamic light scattering
(DLS) using a Zetasizer Nano ZS device (Malvern Instruments Ltd.,
Worcestershire, UK). The polydispersity index (PDI) is a width
parameter for the ζ-average as an intensity mean. The compounds
were adsorbed onto a carbon-coated grid, negatively stained using
2% (w/v) phosphotungstric acid (pH 7.0), and subjected to
transmission electron microscopy (TEM) (JEM-1011; JEOL, Tokyo,
Japan).
Measurement of in vitro anticancer
effects
Human epidermoid carcinoma A431 cells (ATCC no.
CRL-1555), human lung carcinoma A549 cells (ATCC no. CCL-185) and
human colon carcinoma RKO cells (ATCC no. CRL-2577) were obtained
from ATCC and maintained in Dulbecco’s modified Eagle’s medium,
Ham’s F12K medium and Eagle’s minimum essential medium,
respectively, supplemented with 10% fetal bovine serum (all from
Invitrogen Life Technologies, Grand Island, NY, USA). These cell
lines were selected as they have been shown to express EGFR at
different levels; A431 and A549 cells show high EGFR expression and
RKO cells demonstrate low expression of this receptor (10). Cells were seeded in a 96-well
culture plate, grown overnight, and treated with CDDP, LP-CDDP or
EGFR:LP-CDDP. After incubation for 24 h, cell viability was assayed
using Cell Counting kit-8 (Dojindo, Kumamoto, Japan) in accordance
with the manufacturer’s instructions. For clonogenic assays, A549
cells were seeded onto 6-well plates at a density of 100–1,000
cells/well depending on the intended doses of CDDP and ionizing
radiation (IR) (CL/1800; Varian Medical Systems, Inc., Palo Alto,
CA, USA). After CDDP treatment, cells were irradiated at 0–10 Gy
and added fresh media in the next day, then incubated for 12 days
to allow colony formation. The emerging colonies containing >50
cells were counted. The plating efficiency was defined using the
non-irradiated cells as: plating efficiency (PE) = (mean colonies
counted)/(cells plated). The survival fraction was calculated as:
survival fraction (SF) = (mean colonies counted)/[(cells plated) ×
PE], as previously described (19).
In vivo tumor growth delay
All animal experiments were performed in accordance
with the protocols of the Institutional Animal Care and Use
Committee of the Asan Institute for Life Sciences (Seoul, Korea)
(2010-12-180). To generate a xenograft tumor model, A549 cells
(1×106 cells) were subcutaneously injected into the
right hind legs of Balb/c nude mice. When the tumors grew to a size
of ~200 mm3, the mice were randomly divided into eight
experimental groups (n=12) and injected intravenously with 10 mg/kg
(CDDP dose equivalent) of CDDP, LP-CDDP or EGFR:LP-CDDP. At 2 h
after these injections, the tumors were irradiated with 5 Gy using
a 6 MV photon beam linear accelerator (CL/1800; Varian Medical
Systems, Inc.). The tumor size and body weights of the mice were
then measured every week using caliper (Mitutoyo, Kanagawa, Japan).
The tumor volume (V) was calculated as: V (mm3) =
[(largest length) × (shortest length)2]/2. To evaluate
the efficacy of each treatment, change of tumor growth was compared
between treated group and control group (T/C). The T/C (%) on the
final date of this experiment was calculated as: T/C (%) = [(change
in tumor growth for treated group)/(change in tumor growth for
control group)] ×100. On the final day of the experiment, the
kidneys were collected and weighed.
Evaluation of nephrotoxicity
To evaluate kidney function, the blood urea nitrogen
(BUN) and creatinine levels were measured in 5-week-old Balb/c mice
following a single injection of CDDP, LP-CDDP or EGFR:LP-CDDP (10
mg/kg CDDP dose equivalent) (n=6). At 3 days after this treatment,
the BUN levels were determined using the modified Berthelot
reaction of Bio-Quant, Inc. (San Diego, CA, USA). Creatinine was
measured using Creatinine Colorimetric Detection kit (Enzo Life
Sciences, Inc., Farmingdale, NY, USA). To assess nephrotoxicity,
Balb/c nude mice bearing an A549-derived tumor were treated with
CDDP, LP-CDDP or EGFR:LP-CDDP (10 mg/kg CDDP dose equivalent) (n=6)
and sacrificed 30 days later. The kidney, lungs and liver were
harvested and fixed in 4% paraformaldehyde and the tissues were
embedded in paraffin and sliced at a 5 μm thickness. The resulting
sections were stained with hematoxylin and eosin and observed under
a microscope (DP71; Olympus, Tokyo, Japan).
Statistical analysis
Statistical analysis of the group differences in
these assays were performed by one-way ANOVA, Tukey’s test. The
values are the mean ± standard deviation. The value of P<0.05
was considered to be statistically significant.
Results
Characterization of EGFR:LP-CDDP
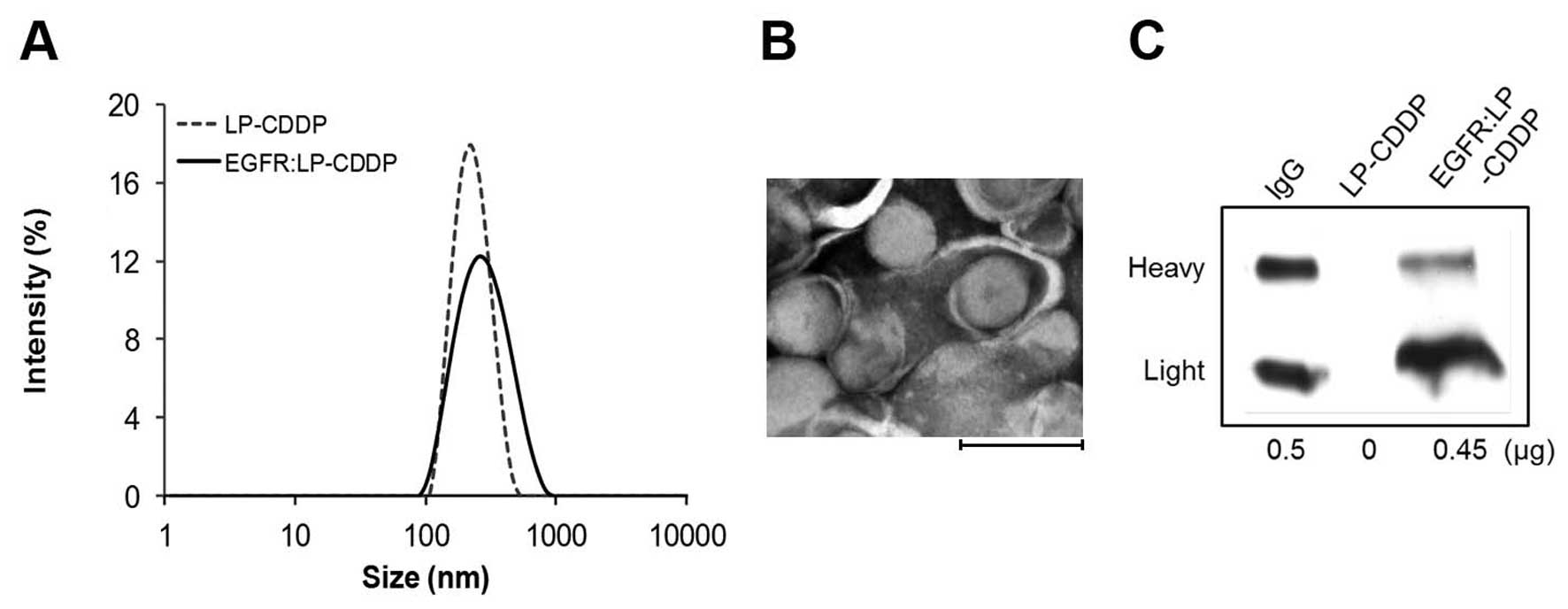
The size distribution of LP-CDDP and EGFR:LP-CDDP,
measured by the DLS method, is shown in Fig. 1A and was measured at 212.4 nm (PDI,
0.100) and 247.9 nm (PDI, 0.148), respectively (Table I). A TEM image of EGFR:LP-CDDP
revealed a size and its spherical shape that was consistent with
these values (Fig. 1B). The level
of CDDP incorporated into EGFR:LP was 3.2 mg CDDP/14.2 mg lipid/ml
and the loading efficiency was 22.5%. The amount of EGFR Ab
displayed on the LP was calculated by measuring the density of the
bands detected by western blotting (Fig. 1C). A total of 20 μl of LP-CDDP
(containing 280 μg of lipid) contained 0.45 μg EGFR Ab.
 | Table IAnalytical information. |
Table I
Analytical information.
| Size (nm) | ζ potential (mV) |
|---|
| LP-CDDP | 212.4 | −64 |
| EGFR:LP-CDDP | 247.9 | −60 |
In vitro anticancer effects of
EGFR:LP-CDDP
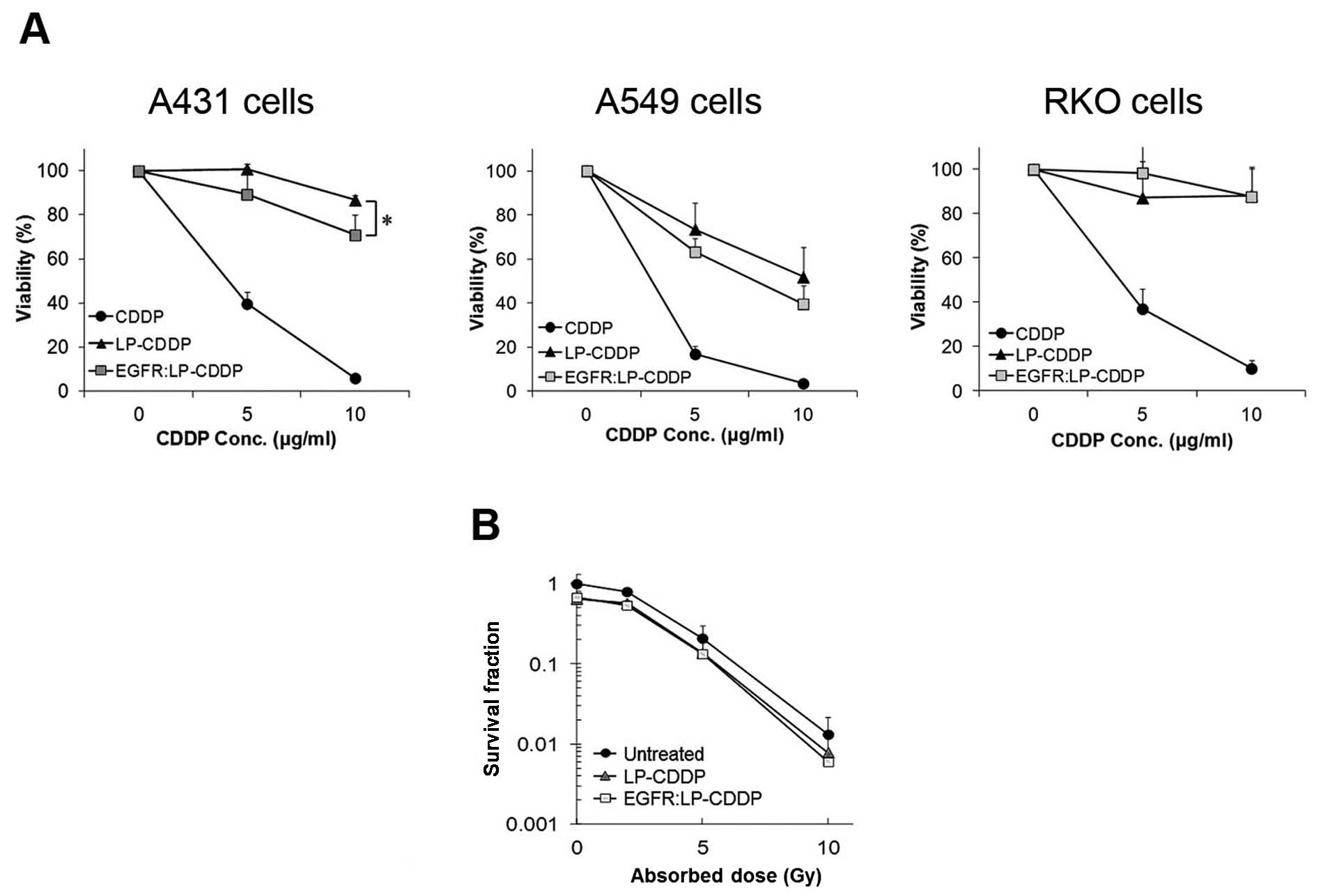
The targeting ability of EGFR:LP-CDDP in
EGFR-expressing cancer cells was evaluated in A431 and A549 cells
that express EGFR at a high level. Both lines showed a lower
viability following treatment with EGFR:LP-CDDP compared with
LP-CDDP. Specially, cytotoxicity of EGFR:LP-CDDP was higher than
that of LP-CDDP at 10 μg/ml against A431 and A549 cells. In
contrast, the viability of RKO cells which express a rare variant
of EGFR did not differ between EGFR:LP-CDDP and LP-CDDP treatments
(Fig. 2A). These results indicated
that EGFR:LP-CDDP can target EGFR-expressing cancer cells leading
to enhanced cytotoxicity. To further investigate their
chemoradiotherapeutic effects, A549 cells were treated with LP-CDDP
or EGFR:LP-CDDP (5 μg/ml) and irradiated at 0, 2, 5, or 10 Gy after
2 h. The survival fractions were calculated using a colony
formation assay as described in Materials and methods as 1, 0.79,
0.20, and 0.01 at the radiation doses of 0, 2, 5, and 10 Gy,
respectively (Fig. 2B). The cells
treated with a combination of LP-CDDP with IR showed survival
fractions of 0.6, 0.57, 0.14, and 0.008 at 0, 2, 5, and 10 Gy. In
the cells exposed to a combination of EGFR:LP-CDDP with IR, these
values were 0.6, 0.54, 0.13, and 0.006 at 0, 2, 5, and 10 Gy. These
data revealed that both LP-CDDP and EGFR:LP-CDDP enhance
radiosensitivity but that EGFR:LP-CDDP was slightly more
potent.
Enhanced in vivo chemoradiotherapeutic
efficacy and reduced toxicity of EGFR:LP-CDDP
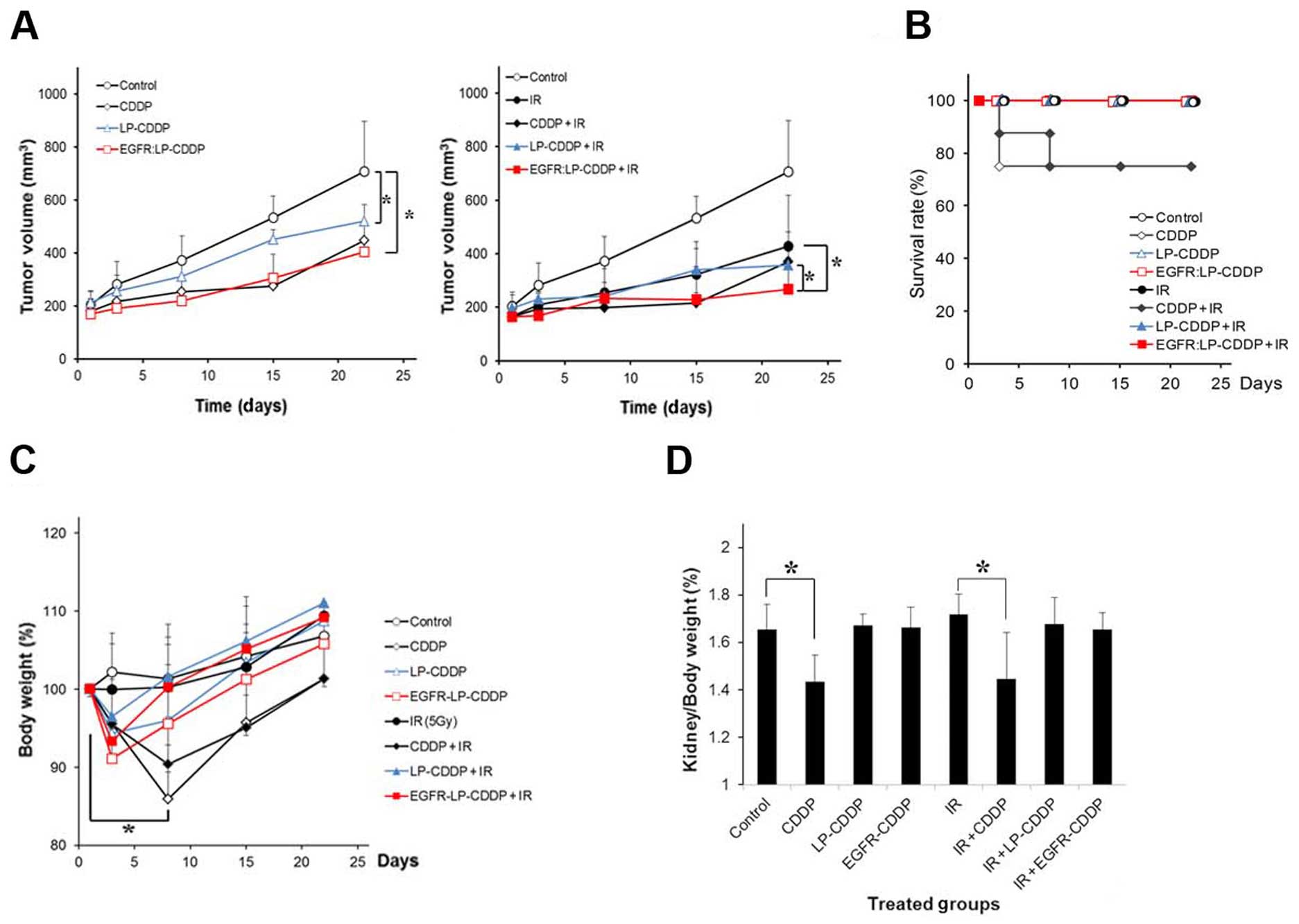
The chemoradiotherapeutic efficacy of LP and EGFR:LP
containing CDDP was compared with that of free CDDP in mice bearing
A549-derived tumors in the right hind leg. The mice were
intravenously injected with CDDP, LP-CDDP, or EGFR:LP-CDDP at 10
mg/kg (CDDP concentration equivalent). After 2 h, the tumors were
irradiated at 5 Gy. As shown in Fig.
3A, the tumor growth in the CDDP-, LP-CDDP-, or
EGFR:LP-CDDP-treated animals was delayed compared with that of the
control. In combination therapies of IR and drugs (Fig. 3A, right panel), CDDP, LP-CDDP, and
EGFR:LP-CDDP all enhanced the radiotherapeutic efficacy of the
treatment. At day 22, the T/C (%) values of the control-, CDDP-,
LP-CDDP-, or EGFR:LP-CDDP-treated mice were 100%, 53.1±4.9%,
61.3±7.3%, and 46.8±4.3%, respectively. Values of 51.8±7.4%,
40.7±4.9%, 32.2±8.4%, and 20.7±4.2% were obtained after IR, CDDP
with IR, LP-CDDP with IR, and EGFR:LP-CDDP with IR, respectively.
These results reveal a higher chemotherapeutic and
chemoradiotherapeutic efficacy of EGFR:LP-CDDP among the compounds
tested.
During the experiments, 25% of the mice treated with
CDDP or with a combination of CDDP and IR died within 1 week
(Fig. 3B and Table II). The body weights of the
surviving animals in the groups treated with CDDP or with a
combination of CDDP and IR fell to 85% of normal levels at the
beginning of the therapy and then slowly recovered (Fig. 3C). These results indicate that
although free CDDP has anticancer effects, it is severely toxic.
The other treatment groups showed equivalent survival rate and body
weight profiles. On the final day of the experimental period, the
kidney weights of the mice in each treatment group were compared.
As shown in Fig. 3D, only two
treatments (CDDP and CDDP with IR) caused a significant loss in
kidney weight. These results suggest that the LP formulation
prevented CDDP-induced damage.
 | Table IIKaplan-Meier analysis. |
Table II
Kaplan-Meier analysis.
| Survival rate
(%) |
|---|
|
|
|---|
| Groups | (days) 1 | 3 | 8 | 15 | 22 |
|---|
| Control | 100 | 100 | 100 | 100 | 100 |
| CDDP | 100 | 75 | 75 | 75 | 75 |
| LP-CDDP | 100 | 100 | 100 | 100 | 100 |
| EGFR:LP-CDDP | 100 | 100 | 100 | 100 | 100 |
| IR | 100 | 100 | 100 | 100 | 100 |
| CDDP + IR | 100 | 87.5 | 75 | 75 | 75 |
| LP-CDDP + IR | 100 | 100 | 100 | 100 | 100 |
| EGFR:LP-CDDP +
IR | 100 | 100 | 100 | 100 | 100 |
Pathological changes and
nephrotoxicity
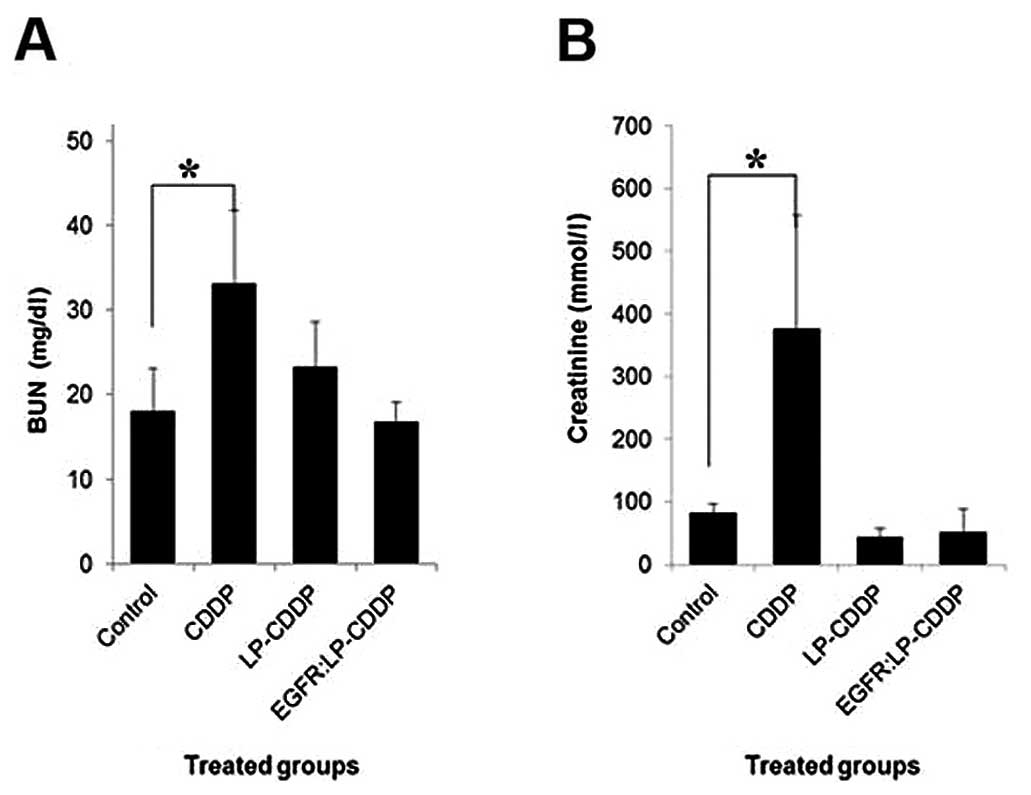
To examine and compare the effects of LP-CDDP and
EGFR:LP-CDDP on renal function, the kidney injury markers BUN and
creatinine were assayed in the tumor mice treated with CDDP,
LP-CDDP and EGFR:LP-CDDP (Fig. 4).
At 3 days after these treatments (10 mg/kg CDDP dose equivalent),
the BUN values in the control-, CDDP-, LP-CDDP- and
EGFR:LP-CDDP-treated animals were measured at 6.4±1.7, 11.8±3.1,
8.3±1.8, and 6.0±0.8 mmol/l, respectively (Fig. 4A). In the case of creatinine, the
values for the control, CDDP, LP-CDDP and EGFR:LP-CDDP animals were
81.6±15.4, 375.9±180, 4.35±14.2, and 50.9±36.9 mmol/l, respectively
(Fig. 4B). Only CDDP caused
significant nephrotoxicity as expected, whilst LP-CDDP and
EGFR:LP-CDDP did not show any evidence of such toxic effects.
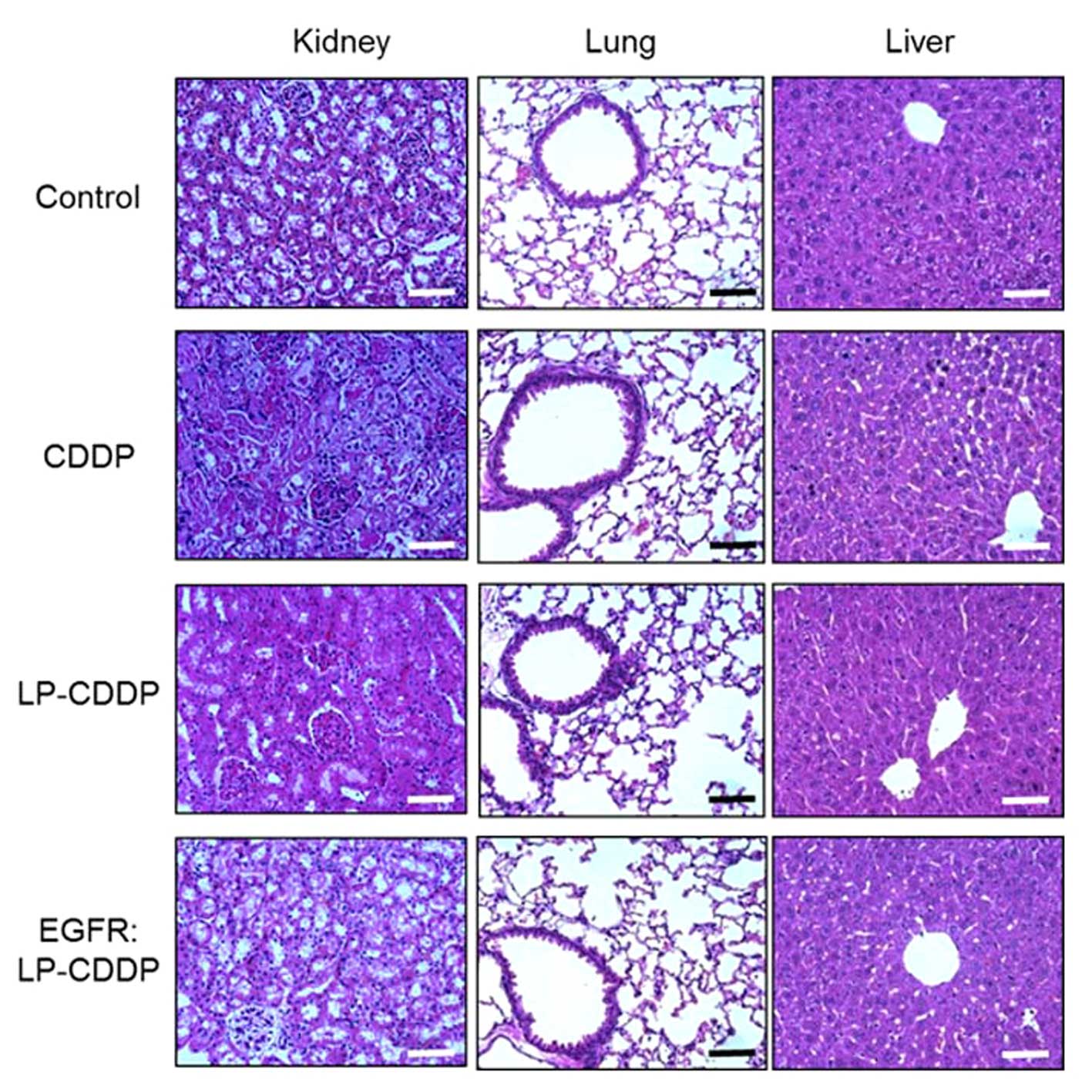
The mice treated with CDDP, LP-CDDP or EGFR:LP- CDDP
were then histopathologically evaluated. Consistently, acute
cortical tubular degeneration and regeneration was observed in the
animals treated with CDDP (Fig.
5). On the other hand, the kidneys of the mice treated with
LP-CDDP and EGFR:LP-CDDP did not show any toxic damage (Fig. 5). There were no pathological
changes observed in the lungs or liver in any group. These results
indicated that encapsulation of CDDP using LP eliminates the
nephrotoxic properties of this compound.
Discussion
A combination of chemotherapy and RT is regarded as
the standard treatment regimen for various cancers including lung,
head and neck, and cervical cancers. For chemoradiotherapy
interventions to treat NSCLC, CDDP is frequently used (3). However, although CDDP has remarkable
radiosensitization effects, its nephrotoxic properties severely
limit its clinical application. To reduce this toxicity of CDDP,
encapsulation has been attempted using gelatin nanoparticles,
polymeric micelles, carbon nanohorns and LP (20–22).
These carriers containing CDDP are a promising new class of
radiosensitizers. However, a CDDP-incorporated LP has not been
studied previously in this context. In our present study, we have
developed the EGFR:LP-CDDP compound and we provide compelling
evidence that it enhances theradiotherapeutic efficacy of a
combination IR regimen without causing nephrotoxicity in
vivo. In particular, it is meaningful for further tailored
chemoradiotherapy strategies that EGFR:LP-CDDP targets
radioresistant cells expressing a high level of EGFR.
The conjugation of LP-CDDP to EGFR antibodies via a
crosslinker produced a strong interaction (Fig. 1) which contributed to the effective
targeting ability of the resulting EGFR:LP-CDDP both in
vitro and in vivo. The surface charge of EGFR:LP-CDDP is
also sufficiently negative to avoid the binding of non-specific
blood proteins (Table I). The
cytotoxicity of EGFR:LP-CDDP or LP-CDDP was found to be weaker than
that of CDDP in an in vitro assay, suggesting that these
compounds might be slowly taken up by cells (Fig. 2A) (23). However, the cellular selectivity of
EGFR:LP-CDDP was observed to be dependent on the EGFR expression
levels. The radiosensitizing effects of EGFR:LP-CDDP and LP-CDDP
were compared using a clonogenic assay. Although few differences
were found, we speculate this was due to an insufficient time for
CDDP uptake into the cells.
Whilst the anticancer effects of EGFR:LP-CDDP were
weak in vitro, these effects were found to be significant
in vivo (Figs. 3–5). In terms of tumor growth, the
EGFR:LP-CDDP-treated mice appeared to show a delay in comparison
with the LP-CDDP- or CDDP-injected animals. This suggested that
EGFR:LP-CDDP had successfully targeted the tumor. Moreover, the
combination of EGFR:LP-CDDP and IR enhanced tumor growth delay in
the model mice compared with separate EGFR:LP-CDDP or IR therapies,
indicating that EGFR:LP-CDDP effectively radiosensitizes tumor
cells. The free CDDP-treated group seemed to show a greater delay
in tumor growth than the LP-CDDP-treated animals since several mice
in this group died. Additionally, body weight loss was significant
in the free CDDP-treated group. Although the EGFR:LP-CDDP- or
LP-CDDP-treated groups showed slight body weight loss at 3 days
after the injections, these weights quickly recovered. This
indicated that the encapsulation of CDDP by LP reduces its
toxicity. The kidney weights were also significantly reduced in the
mice treated with CDDP or with a combination of CDDP and IR
(Fig. 3D). Nephrotoxicity is a
well-known side effect of CDDP and several markers of this
complication have been reported including urea, creatinine, and a
fractional excretion of sodium (24,25).
Creatinine and BUN levels are easily measurable via blood tests.
The normal value of creatinine is <1.3 mg/dl and of BUN is
<23 mg/dl (24). Both of these
values were increased in mice treated with CDDP, but not in the
other treatment groups. CDDP-induced nephrotoxicity was also
observed by histopathological analysis (Fig. 5). Renal tubular degeneration and
regeneration were observed only in the mice treated with CDDP. In
the renal cortical tubules of these mice, tubular dilation, cell
necrosis, and sloughing of cells were also evident. In addition,
nephrotoxicity was similarly observed in the animals treated with a
combination of CDDP and IR (data not shown). Overall, our results
indicate that EGFR:LP-CDDP in combination with IR enhances the
radiotherapeutic efficacy through the active targeting of
EGFR-expressing NSCLC cells and that CDDP-induced nephrotoxicity is
eliminated by LP encapsulation.
In conclusion, EGFR:LP-CDDP is an effective targeted
radiosensitizer in EGFR-overexpressing NSCLC cells. This maximizes
the chemoradiotherapeutic efficacy of combination regimens in NSCLC
cells by neutralizing both the toxicity of CDDP and the IR
resistance of the cells.
Acknowledgements
This research was supported by grants from the
Korean Health Technology R&D Project through the Korea Health
Industry Development Institute (KHIDI) (Seoul, Korea), funded by
the Ministry for Health and Welfare, Seoul, Republic of Korea
(HI06C0868, HI10C2014 and HI14C1090), and the National Research
Foundation of Korea (NRF) (Seoul, Korea) grant funded by the Korea
government (MEST) (NRF-2012R1A2A2A01014671 and
NRF-2013R1A1A2011346).
References
|
1
|
Ghafoori P, Marks LB, Vujaskovic Z and
Kelsey CR: Radiation-induced lung injury. Assessment, management,
and prevention. Oncology (Williston Park). 22:37–47; discussion
52–33. 2008.
|
|
2
|
Graves EE, Maity A and Le QT: The tumor
microenvironment in non-small-cell lung cancer. Semin Radiat Oncol.
20:156–163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baas P, Belderbos JS and van den Heuvel M:
Chemoradiation therapy in nonsmall cell lung cancer. Curr Opin
Oncol. 23:140–149. 2011. View Article : Google Scholar
|
|
4
|
Giaccone G: Twenty-five years of treating
advanced NSCLC: what have we achieved? Ann Oncol. 4(Suppl 15):
iv81–iv83. 2004.
|
|
5
|
Yao X, Panichpisal K, Kurtzman N and
Nugent K: Cisplatin nephrotoxicity: a review. Am J Med Sci.
334:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wainford RD, Weaver RJ, Stewart KN, Brown
P and Hawksworth GM: Cisplatin nephrotoxicity is mediated by gamma
glutamyltranspeptidase, not via a C-S lyase governed
biotransformation pathway. Toxicology. 249:184–193. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang M and Thanou M: Targeting
nanoparticles to cancer. Pharmacol Res. 62:90–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hwang KJ, Padki MM, Chow DD, Essien HE,
Lai JY and Beaumier PL: Uptake of small liposomes by
non-reticuloendothelial tissues. Biochim Biophys Acta. 901:88–96.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Drummond DC, Meyer O, Hong K, Kirpotin DB
and Papahadjopoulos D: Optimizing liposomes for delivery of
chemotherapeutic agents to solid tumors. Pharmacol Rev. 51:691–743.
1999.PubMed/NCBI
|
|
10
|
Jeong SY, Park SJ, Yoon SM, et al:
Systemic delivery and preclinical evaluation of Au nanoparticle
containing beta-lapachone for radiosensitization. J Control
Release. 139:239–245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van der Meel R, Oliveira S, Altintas I, et
al: Tumor-targeted nanobullets: anti-EGFR nanobody-liposomes loaded
with anti-IGF-1R kinase inhibitor for cancer treatment. J Control
Release. 159:281–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song S, Liu D, Peng J, et al: Peptide
ligand-mediated liposome distribution and targeting to EGFR
expressing tumor in vivo. Int J Pharm. 363:155–161. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang K, Ang KK, Milas L, Hunter N and Fan
Z: The epidermal growth factor receptor mediates radioresistance.
Int J Radiat Oncol Biol Phys. 57:246–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baumann M, Krause M, Dikomey E, et al:
EGFR-targeted anti-cancer drugs in radiotherapy: preclinical
evaluation of mechanisms. Radiother Oncol. 83:238–248. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ansell SM, Harasym TO, Tardi PG,
Buchkowsky SS, Bally MB and Cullis PR: Antibody conjugation methods
for active targeting of liposomes. Methods Mol Med. 25:51–68.
2000.PubMed/NCBI
|
|
16
|
Kolhatkar R, Lote A and Khambati H: Active
tumor targeting of nanomaterials using folic acid, transferrin and
integrin receptors. Curr Drug Discov Technol. 8:197–206. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirai M, Minematsu H, Hiramatsu Y, et al:
Novel and simple loading procedure of cisplatin into liposomes and
targeting tumor endothelial cells. Int J Pharm. 391:274–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirai M, Minematsu H, Kondo N, Oie K,
Igarashi K and Yamazaki N: Accumulation of liposome with Sialyl
Lewis X to inflammation and tumor region: application to in vivo
bio-imaging. Biochem Biophys Res Commun. 353:553–558. 2007.
View Article : Google Scholar
|
|
19
|
Jung J, Kim EJ, Chung HK, Park HJ, Jeong
SY and Choi EK: c-Myc down-regulation is involved in proteasome
inhibitor-mediated enhancement of radiotherapeutic efficacy in
non-small cell lung cancer. Int J Oncol. 40:385–390. 2012.
|
|
20
|
Tseng CL, Su WY, Yen KC, Yang KC and Lin
FH: The use of biotinylated-EGF-modified gelatin nanoparticle
carrier to enhance cisplatin accumulation in cancerous lungs via
inhalation. Biomaterials. 30:3476–3485. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishiyama N, Okazaki S, Cabral H, et al:
Novel cisplatin-incorporated polymeric micelles can eradicate solid
tumors in mice. Cancer Res. 63:8977–8983. 2003.PubMed/NCBI
|
|
22
|
Ajima K, Murakami T, Mizoguchi Y, et al:
Enhancement of in vivo anticancer effects of cisplatin by
incorporation inside single-wall carbon nanohorns. ACS Nano.
2:2057–2064. 2008. View Article : Google Scholar
|
|
23
|
Castelo-Branco PA, Rubinger MM, de Alves
LC, et al: Synthesis and antifungal activity of aromatic
bis-gamma-lactones analogous to avenaciolide. Chem Biodivers.
4:2745–2754. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yaklin KM: Acute kidney injury: an
overview of pathophysiology and treatments. Nephrol Nurs J.
38:13–19. 2011.PubMed/NCBI
|
|
25
|
Dirkes S: Acute kidney injury: not just
acute renal failure anymore? Crit Care Nurse. 31:37–50. 2011.
View Article : Google Scholar : PubMed/NCBI
|