Introduction
Indoleamine 2,3-dioxygenase (IDO) is an enzyme that
catalyzes the essential amino acid tryptophan and leads to the
starvation of tryptophan and the accumulation of tryptophan
metabolites such as kynurenine. Therefore, it induces apoptosis or
cell cycle arrest of activated T cells and the differentiation of
new regulatory T cells, which results in immunologic suppression
(1–3). This control may be essential for
physiological acquired immunological tolerance. For example, IDO
expressed by the placenta prevents a maternal allo-reaction against
the fetus (4,5) and enables the maintenance of
gestation as shown by the fact that the IDO inhibitor,
1-methyl-tryptophan (1-MT), induced the immune-mediated rejection
of allogeneic concepti (6). IDO is
also expressed in some tumor cells, and it plays a pivotal role in
the escape from immunological attack by inducing immunological
anergy in such tumors (7,8). 1-MT could potentiate antitumor
immunity in these IDO-expressing tumors, and could induce tumor
regression in animal models (9).
Based on this result, several clinical trials evaluating the use of
1-MT against solid tumors are currently ongoing. However, the role
of IDO in lymphoid tumors such as lymphoma or lymphocytic leukemia
has not been fully revealed.
Experimental autoimmune encephalomyelitis (EAE) is a
well-known animal model of multiple sclerosis and is mediated by
autoimmune TH1 cells (10). Altered peptide ligands (APLs) that
had modified the strength of T-cell receptor signaling had induced
self-tolerance to autoantigens. Platten et al (10) showed that the IDO expression was
induced by APLs and that the administration of
N-[3′,4′-dimethoxycinnamoyl] anthranilic acid (tranilast), a
synthetic derivative of the tryptophan metabolite anthranilic acid,
could reverse paralysis in mice with EAE. IDO has also been
reported to be associated with acute graft-versus-host disease
(GVHD) in murine bone marrow transplantation. IDO expression is
induced at the site of GVHD. Moreover, exogenous kynurenines can
reduce GVHD lethality (11,12).
These reports indicated that the tryptophan metabolites as well as
the forced IDO expression may have some effect on the fate of
activated lymphocytes.
It is possible that an analysis of the expression
and effect of IDO in lymphoid malignancies will provide a clue
regarding their treatment. In the present study, we found that the
lymphoid malignant cells were susceptible to cytotoxicity by IDO
and that tranilast also induced growth suppression of these
cells.
Materials and methods
Ethics statement
After written informed consent was obtained in
compliance with the Declaration of Helsinki, samples (peripheral
blood, bone marrow, lymph node, ascites and pleural effusion) were
collected from the patients. Approval was obtained from the ethics
committee of the Tokyo Medical and Dental University. All animal
studies were approved by the Animal Subjects Committee of the Tokyo
Medical and Dental University and were performed in accordance with
the institutional guidelines.
Cells and reagents
Splenocytes were obtained from 8-week-old female
BALB/c mice using a RBC lysis buffer [0.155 M NH4Cl,
0.01 M NH4HCO3 and 0.1 mM EDTA (pH 7.5)].
Peripheral blood mononuclear cells (PBMCs) from patients were
isolated through density-gradient centrifugation from freshly
collected blood samples using SEPARATE-L (Muto Pure Chemicals,
Tokyo, Japan). Cells were preserved with CELLBANKER (Nippon Zenyaku
Kogyo Co., Ltd., Fukushima, Japan) at −80°C. Human and murine cell
lines were obtained from the American Type Culture Collection
(ATCC, Manassas, VA, USA) and cultured in RPMI-1640 medium or
Dulbecco’s modified Eagle’s medium (DMEM) medium with 10% fetal
calf serum (FCS). Tranilast was provided by Kissei Pharmaceutical
(Nagano, Japan). IFNγ, concanavalin A (ConA), L-kynurenine, 3-HAA,
Kynurenic acid, fludarabine, α-naphthoflavone, and
2,3,7,8-Tetrachlorodibenzodioxin (TCDD) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). IL-2 was purchased from R&D
Systems (Minneapolis, MN, USA). CD19 Pan B Dynabeads and human
T-activated CD3/CD28 Dynabeads were purchased from Invitrogen Life
Technologies (Grand Island, NY, USA). 4-Hydroperoxy
Cyclophosphamide were purchased from Wako Pure Chemical Industries
(Osaka, Japan). JNK inhibitor SP600125 was purchased from Enzo Life
Sciences (Farmingdale, NY, USA).
Real time (RT)-PCR
Samples from patients with B-cell lymphoma were
used. RNA was isolated using TRIzol (Gibco-BRL, Gaithersburg, MD,
USA). Reverse transcription using an oligo dT primer was performed
with a SuperScript II RT kit (Invitrogen-Life Technologies, Grand
Island, NY, USA). The synthesized cDNA was amplified with primers
specific for human IDO (forward, 5′-CCTGACTTATGAGAA
CATGGACGT-3′ and reverse, 5′-ATACACCAGACCGTCTG ATAGCTG-3′); or
mouse IDO (forward, 5′-TTCGAAAGGTG CTGCCCCGC-3′ and reverse,
5′-GCCCTTGTCGCAGTCCC CAC-3′); or human GAPDH (forward,
5′-CTGACTTCAAC AGCGACACC-3′ and reverse, 5′-TCCTCTTGTGCTCTTGC
TGG-3′); or mouse GAPDH (forward, 5′-TGCGACTTCAAC
AGCAACTC-3′ and reverse, 5′-CTTGCTCAGTGTCCTTGC TG-3′). Quantitative
RT-PCR was performed with the LightCycler FastStart DNA Master Plus
SYBR-Green I kit using LightCycler software version 3.5 (Roche
Applied Science, Indianapolis, IN, USA).
Cloning of mouse IDO (mIDO)
The murine Ido-1 cDNA containing the full
length open reading frame was amplified with the specific primers
(forward, 5′-GGAGTAGA CAGCAATGGCAC-3′ and reverse, 5′-GAGCTTGCTA
CACTAAGGCC-3′) using the RNA from splenocytes as a template. The
purified product of 1250 bp was cloned into the pGEM-T Easy Vector
system (Invitrogen-Life Technologies). After sequencing, it was
subcloned into the expression vector, pcDNA3 (Invitrogen-Life
Technologies) (pcDNA3-mIDO).
Transfection
Chinese hamster ovary (CHO) cells were transfected
with pcDNA3-mIDO using Lipofectamine LTX (Invitrogen-Life
Technologies) and selected with 1 mg/ml geneticin (G418) (Roche
Applied Science). The stable transfected CHO cells were subcloned,
and the clones with high levels of mIDO protein expression were
selected. Furthermore, pcDNA3-mIDO was transfected to the murine B
lymphoma cell lines A20 and M12 by electroporation. RNA and protein
were extracted from the transfected cells after selection with
G418. A plasmid containing the dominant negative form of c-Jun
N-terminal kinase (JNK) was obtained from the Addgene repository
[13761; pcDNA3 Flag Jnk2a2 (apf)] (13). The coding region (DN-JNK2) was
subcloned into retroviral vector pQCXIN (Clontech: Palo Alto, CA,
USA). A20 cells were transduced with pQCXIN-DN-JNK2 and selected
with G418.
Conditioned medium
We cultured CHO cells transfected with the
mIDO gene or control vector in a minimum essential medium
(αMEM) medium. On the following day, the αMEM in the dish was
changed to RPMI-1640 medium and the CHO cells were cultured for 5
days at 37°C and in 5% CO2. The collected supernatant
from the dish was adjusted to a pH of 7.0 with 1 N NaOH and then 2
mM L-glutamine (Invitrogen-Life Technologies) was added. This
medium was sterilized by a filter.
Measurement of cell viability and viable
cell number
In the present study, the determination of cell
viability was based on an analysis of mitochondrial transmembrane
potential using 3,3′-dehexyloxacarbocyamine iodine (DiOC6)
(Molecular Probes, Eugene, OR, USA) and the cell membrane
permeability to propidium iodide (PI) (Molecular Probes) by flow
cytometry with a FACSCalibur™ flow cytometer (Becton-Dickinson,
Franklin Lakes, NJ, USA) as previously described (14). The fluorescence-activated cell
sorting (FACS) data were analyzed with FlowJo software version
6.3.3 (Tree Star, Inc., Ashland, OR, USA). The DiOC6-positive and
PI-negative cells were considered to be viable cells. The viable
cell number was calculated as the cell number that was acquired
during 30 sec multiplied by the percentage of viable cells.
Immunoblot analysis
Cells were lysed in a lysis buffer containing 1%
Triton X-100, 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM
sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 10
mg/ml each of aprotinin and leupeptin (Sigma-Aldrich, St. Louis,
MO, USA). The cell lysates were separated using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and
electroblotted to polyvinylidene difluoride membranes. Membranes
were probed with primary antibodies and probed with HRP that was
conjugated anti-mouse or rabbit IgG (GE Healthcare Life Sciences,
Piscataway, NJ, USA) as the second antibody. Antibodies against IDO
(Oriental Yeast Co., Ltd., Tokyo, Japan), SAPK/JNK,
phospho-Thr183/Tyr185-SAPK/JNK, AKT, phospho-S473-AKT, p38,
phospho-Thr180/Tyr182-p38 (Cell Signaling Technology, Danvers, MA,
USA) and β-actin (Sigma-Aldrich) were used.
Mice
Eight-week-old female BALB/c mice were purchased
from CLEA Japan (Tokyo, Japan) and were housed under specific
pathogen-free conditions. After intraperitoneal injection of
anesthesia using 2,2,2-tribromoethanol (Avertin; Sigma-Aldrich),
the bilateral hind flanks of each mouse were transplanted with
2×105 of A20, which was a B lymphoma cell line derived
from the BALB/c mice. These cells generated palpable tumors at the
site of injection in 100% of the injected mice. Seven days after
the inoculation, tranilast that was suspended in 0.5% carboxymethyl
cellulose (CMC) solution or CMC solution alone had been
administered by oral gavages with a disposable flexible tube twice
a day for 3 weeks. The tumor progression was monitored biweekly,
and the tumor volumes were calculated with the following formula:
Volume = (width)2 × length/2.
Statistical analysis
The significance of the differences between groups
was determined by the Mann-Whitney U test, the Student’s t-test, or
the Student’s paired t-test using GraphPad Prism software (La
Jolla, CA, USA).
Results
IDO expression in human B-lymphoid
malignancies
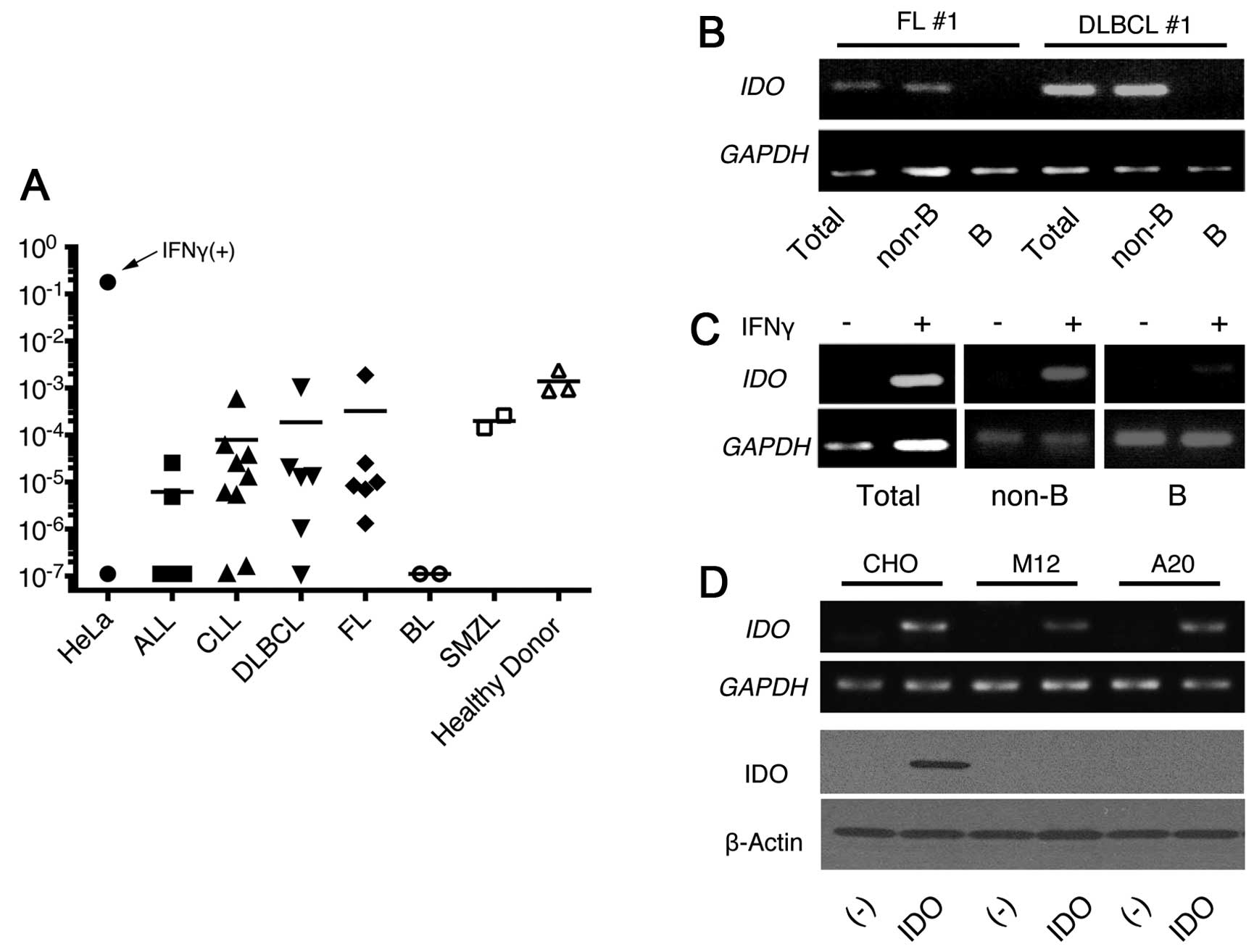
First, we examined the IDO expression in 30
samples from the patients with B-cell malignancies by Rq-PCR using
specific primers for IDO gene. We set HeLa cells that were
treated with 10 ng/ml IFNγ and untreated HeLa cells as the positive
and negative controls, respectively. The expression of IDO
from 22 samples was detected as 10-fold higher than those of the
negative controls although all samples were less than one tenth of
the positive controls. The results were comparable to the
expression of PBMCs from three healthy donors. The expression was
heterogeneous in the samples and had no clear correlation with the
malignancy types (Fig. 1A) except
for Burkitt’s lymphoma (BL), which had no detectable expression. We
further evaluated the IDO expression in the samples that had
a relatively high expression after the separation of the B cells
and non-B cells. The IDO expression was mainly detected in
non-B cell populations in five examined samples (Fig. 1B). We then examined IDO
induction after IFNγ treatment (10 ng/ml) using the PBMCs from CLL
patients because this cytokine strongly induced IDO in dendritic
cells. IDO was clearly upregulated after the treatment in
PBMCs from patients with CLL. However, the findings demonstrated
that this induction was associated with the non-B cells, but not
with the CLL-B cells after the separation of B cells and non-B
cells (Fig. 1C).
Establishment of cell lines transfected
with the murine IDO gene
Next, we cloned the murine IDO cDNA from the
splenocytes of the BALB/c mice in the pcDNA3 vector containing the
G418-resistant gene, and transfected it into CHO cells. Multiple
clones were selected by G418 that had high cytoplasmic IDO protein
as well as IDO mRNA expression confirmed by immunoblot and
RT-PCR (Fig. 1D, CHO-IDO). We next
introduced the IDO gene into the murine B-cell lines A20 and
M12 to examine the effect of IDO on the development of
lymphoid malignancies. Compared with the control vector without the
insert, the IDO-containing vector produced an almost equal
number of G418-resistant clones. Although comparable amounts of
IDO mRNA to CHO-IDO could be detected in these cells by
RT-PCR, IDO protein was not detected, which suggested
post-transcriptional downregulation of IDO in these lymphoid cell
lines (Fig. 1D). Although some
proteins have been regulated by their stability
post-translationally, the proteasome inhibitors could not induce
the IDO expression in these transfectants (data not shown).
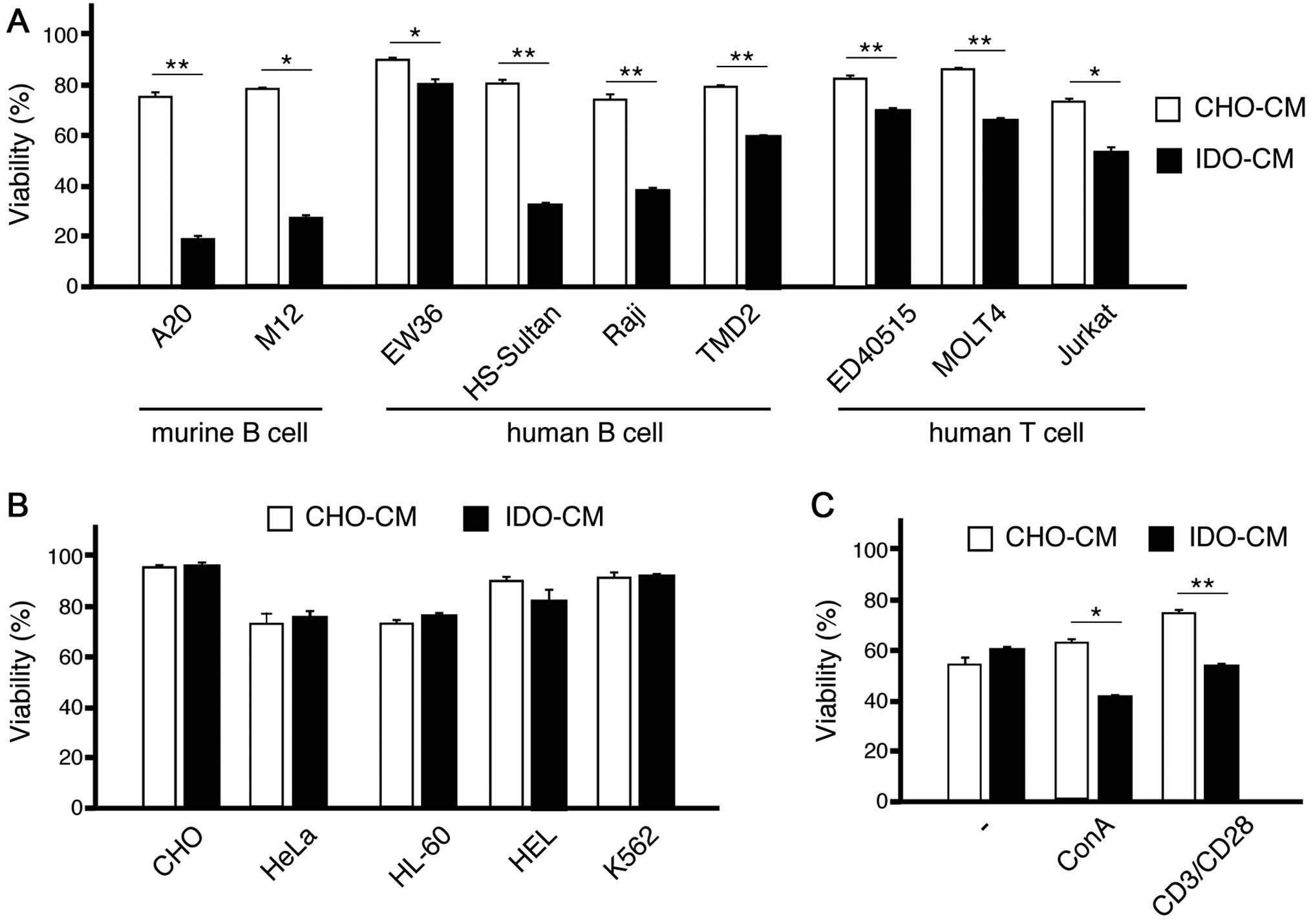
Conditioned medium with CHO-IDO is
cytotoxic to lymphoid cell lines
This downmodulation allowed us to consider the
possibility that IDO may be toxic to these lymphoid cell lines. To
examine this possibility, we created the conditioned medium with
CHO (CHO-CM) and CHO-IDO (IDO-CM), and cultured these murine
lymphoid cell lines in this media. The viability of both A20 and
M12 was markedly decreased in IDO-CM compared with those in CHO-CM
(Fig. 2A).
We then examined the viability of various cell lines
cultured in CHO-CM or IDO-CM. All lymphoid cell lines examined,
including both T-cell and B-cell lines, showed less viability in
IDO-CM than in CHO-CM (Fig. 2A).
In contrast, the viability of non-lymphoid cell lines in IDO-CM was
not changed significantly in comparison with those in CHO-CM
(Fig. 2B).
When T cells were cultured with only 30 U/ml IL-2,
these cells showed similar viability in IDO-CM than in CHO-CM.
However, when T cells were cultured with 10 ng/ml concanavalin A
(ConA) or human T-activated CD3/CD28 Dynabeads, IDO-CM decreased
their viability (Fig. 2C)
indicating that signals from antigenic stimuli may induce
susceptibility to IDO toxicity.
Collectively, the cytotoxic effect of IDO may be
specific to activated lymphocytes and lymphoid lines.
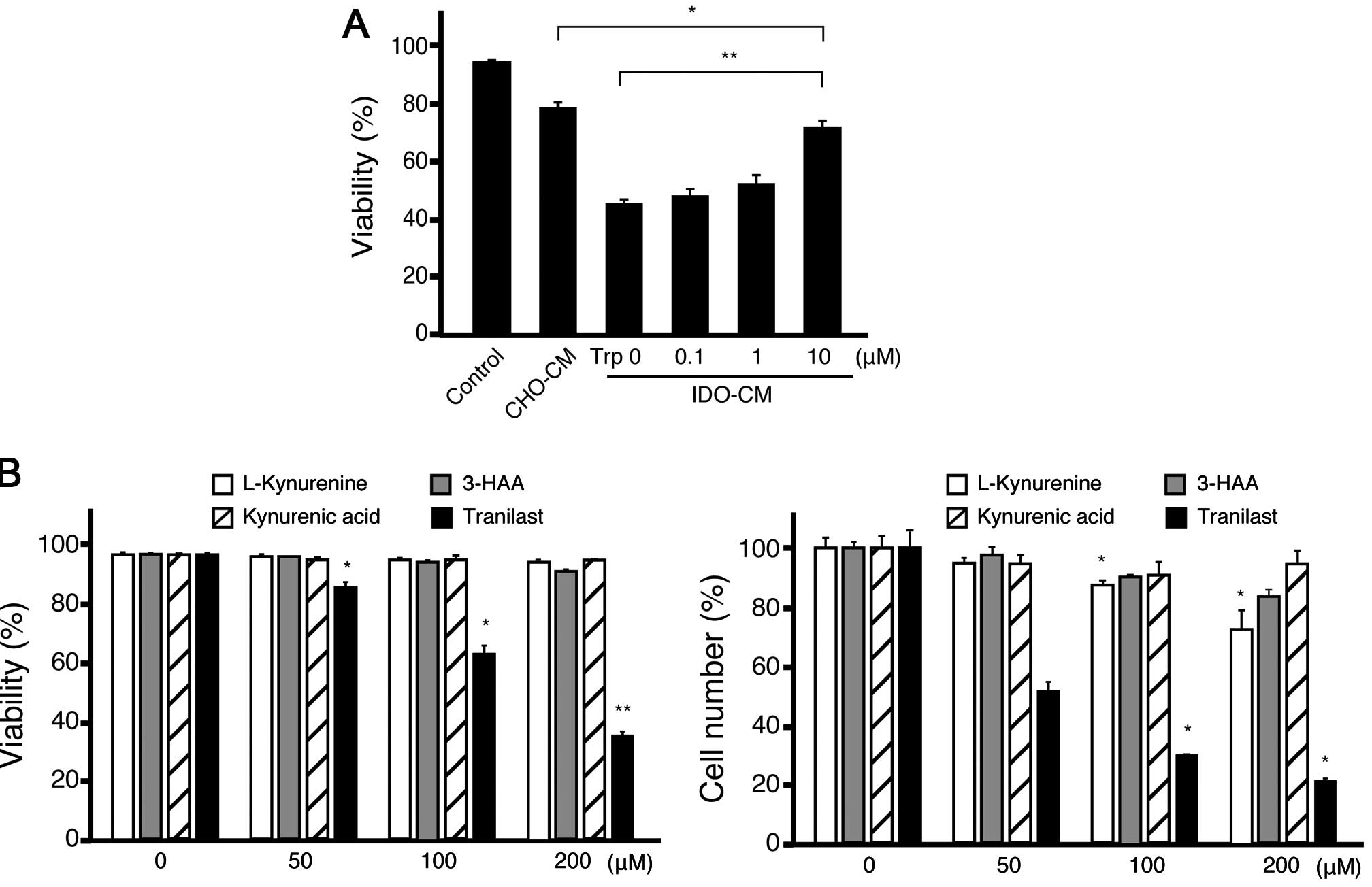
Tryptophan metabolites inhibit the
proliferation of lymphoid cell lines
Both the starvation of tryptophan and the production
of tryptophan metabolites induced by IDO have been reported to be
responsible for IDO immune suppression (2). Actually the concentration of
trypatophan in IDO-CM was undetectable (data not shown). Therefore,
we cultured IDO-sensitive lymphoid lines with added IDO-CM and an
excess amount of tryptophan. The addition of tryptophan restored
the viability in IDO-CM, though the restoration was not complete
(Fig. 3A); this indicated that
tryptophan metabolites as well as the starvation of tryptophan
affected the lymphoid lines.
We then examined the effects of the various
tryptophan metabolites on the viability of IDO-sensitive lymphoid
line A20 (Fig. 3B). After
treatment with L-Kynurenine or 3-HAA, the viable number of A20
cells was decreased although the viability had not changed. These
results indicated that these molecules could suppress proliferation
although they were not cytotoxic. Kynurenic acid could not change
proliferation. Compared with these molecules, tranilast markedly
decreased the viability of A20 cells. Tranilast not only induced
cell death, but also attenuated the proliferation of A20 cells, as
estimated by carboxyfluorescein succinimidyl ester (CFSE) dilution
assay (data not shown). This effect appears to be independent of
the cell cycle phase because the treated cells remained unchanged
by the cell cycle analysis (data not shown). When tranilast was
washed out from the medium after 1 day of treatment, A20 cells
proliferated as much as the untreated cells, indicating that the
effect of tranilast was reversible at this time-point. However, the
A20 cells did not proliferate after 2 days of tranilast treatment
(data not shown). We then examined the effect of tranilast in
combination with chemotherapeutic reagents. Tranilast showed
additive cytotoxicity with active metabolites of
4-hydroperoxycyclophosphamide and fludarabine (data not shown).
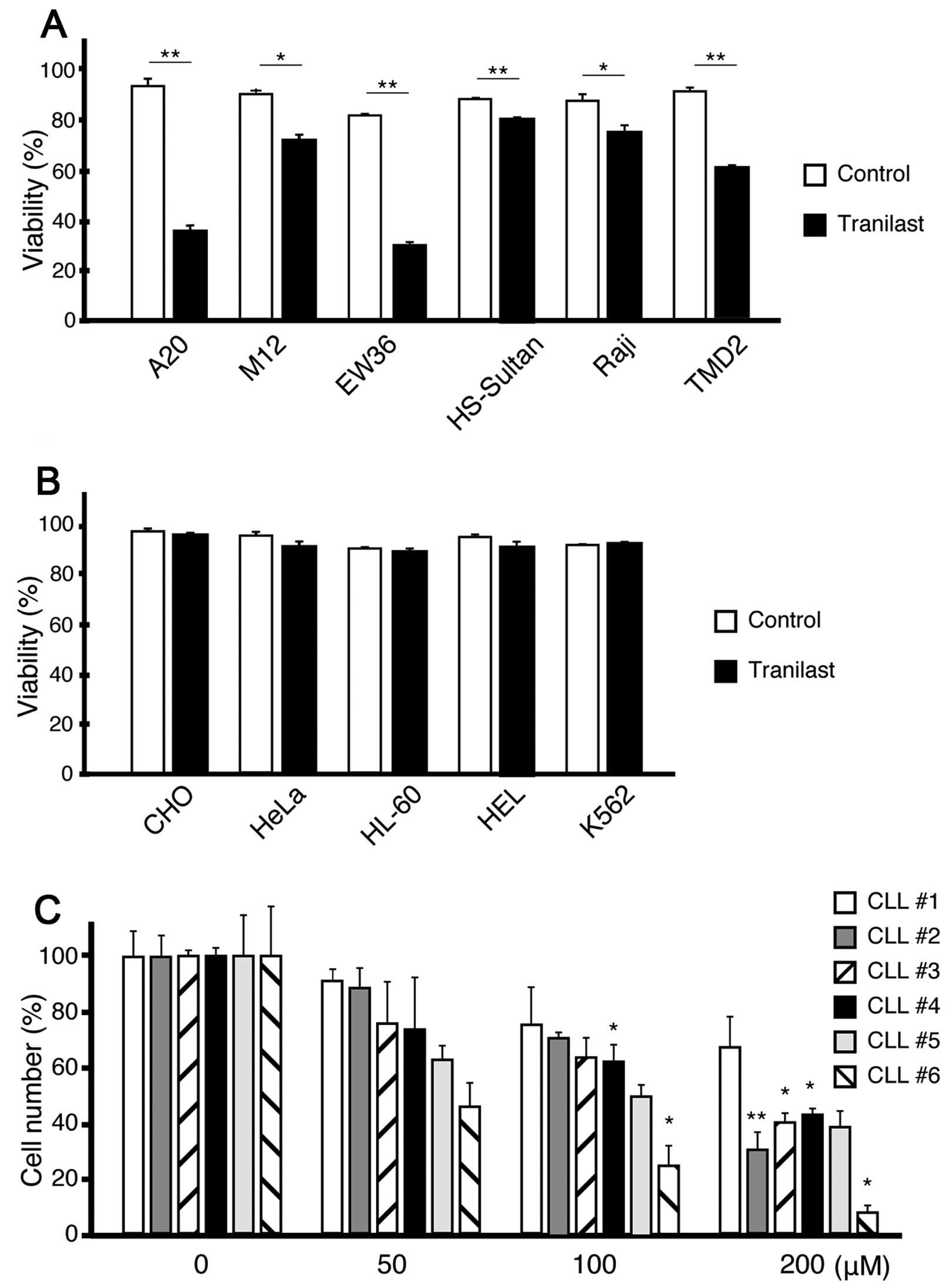
Tranilast induces cell death of lymphoid
malignant cells
We examined the effect of tranilast treatment on
various cell lines. Similar to IDO-CM, tranilast induced cell death
in all lymphoid lines (Fig. 4A)
but not in the myeloid or epithelial cell lines (Fig. 4B) examined; however, the effects of
IDO-CM and tranilast were not totally equivalent. Tranilast induced
more cell death of EW36 than IDO-CM, and conversely, IDO-CM was
more toxic than tranilast for HS-Sultan. We also analyzed the
effect of tranilast against the primary samples from six patients
with CLL. Tranilast dose-dependently augmented the spontaneous
apoptosis of CLL cells (Fig.
4C).
Tranilast induces the phosphorylation of
JNK
To reveal the molecular mechanism of cytotoxicity
induced by tranilast, we checked the activation of some kinases in
A20, TMD2 (15), and Raji cells by
immunoblot after tranilast treatment. The phosphorylation of 42 kD
of extracellular signal-regulated kinase (Erk-2) was upregulated
transiently after the addition of tranilast in these lines
(Fig. 5A; and data not shown). In
addition, JNK activation was induced in A20 cells 12 h after
tranilast treatment (Fig. 5A).
Although JNK activation was not obvious in the human B cell lines,
TMD2 and Raji, after the addition of tranilast alone, tranilast
clearly augmented the phosphorylation of JNK after B-cell receptor
signaling using anti-IgM treatment (data not shown). Because the
JNK activation could promote not only cell survival but also cell
death depending on the cell type, we examined the effect of JNK
inhibition on tranilast treatment. The addition of JNK inhibitor
SP600125 augmented the cytotoxicity of tranilast only slightly
(Fig. 5B). We also induced the
dominant negative form of JNK2 into some A20 cells. Tranilast
induced the cell death of these cells as compared to that of
wild-type A20 cells, indicating that JNK was not required for the
cytotoxicity of tranilast (Fig.
5C).
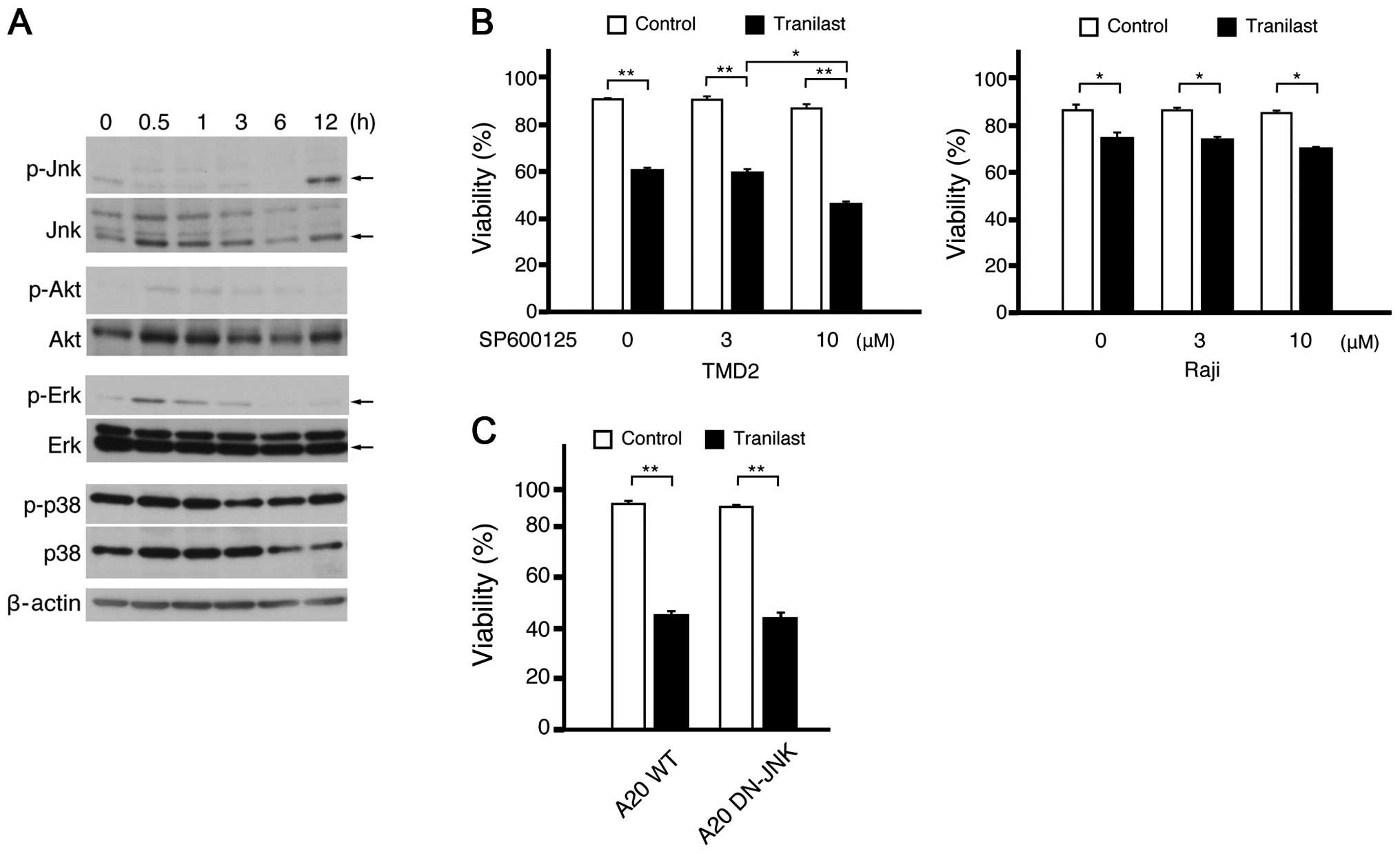 | Figure 5c-Jun N-terminal kinase (JNK)
activation induced by tranilast. (A) A20 cells were treated with
200 μM of tranilast and harvested at the indicated time. The whole
cell lysate was subjected to immunoblot using antibodies against
the indicated anti-phosphorylated proteins (p-JNK, p-Akt, p-Erk and
p-p38). The same membranes were reprobed with anti-total proteins
(JNK, Akt, Erk and p38). The major band of p-JNK was the 46 kD form
of JNK, and the major band of p-Erk was the 42 kD form of Erk
(Erk-2) as determined by the comparison with bands of total JNK or
Erk (arrows). (B) The human B cell lines, TMD2 and Raji, were
treated with or without 200 μM of tranilast in addition to the
indicated concentration of the JNK inhibitor, SP600125. After 2
days of culture, the viabilities were analyzed and shown as the
means with SD of triplicates. (C) A20 was transfected with
pQCXIN-dominant negative form of JNK2 and selected with G418.
Enhanced 54 kD of JNK and diminished auto-phosphorylation of 46 kD
of JNK were verified by immunoblot analysis (data not shown). The
viabilities were analyzed after 2 days of culture with 200 μM of
tranilast. The astersks indicate significant differences in
compared with untreated cells (*P<0.01,
**P<0.001). |
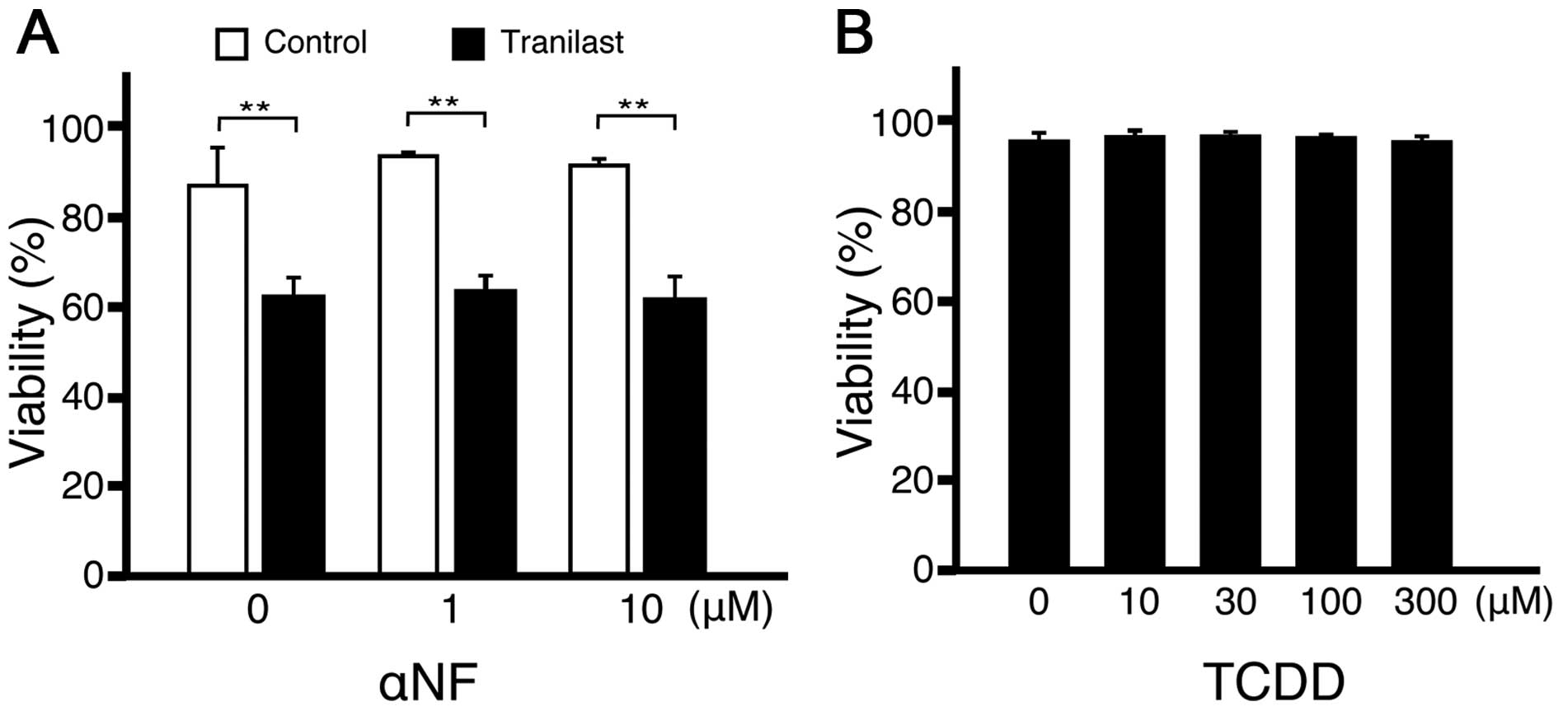
The effect of tranilast is independent of
aryl hydrocarbon receptor (AhR)
Kynurenines can act as endogenous AhR ligand
(16,17). Furthermore, tranilast could act as
a ligand for AhR (18). To examine
if the cytotoxic effect of tranilast on lymphoid cells was mediated
by AhR, the AhR antagonist, α-naphthoflavone (α-NF), was added to
the culture with tranilast. The addition of α-NF did not change the
effect of tranilast (Fig. 6A). In
addition, the potent agonist of AhR,
2,3,7,8-tetrachlorodibenzodioxin (TCDD), was not as cytotoxic to
A20 cells as tranilast (Fig. 6B).
Collectively, the cytotoxic effect of tranilast appeared to be
independent of AhR.
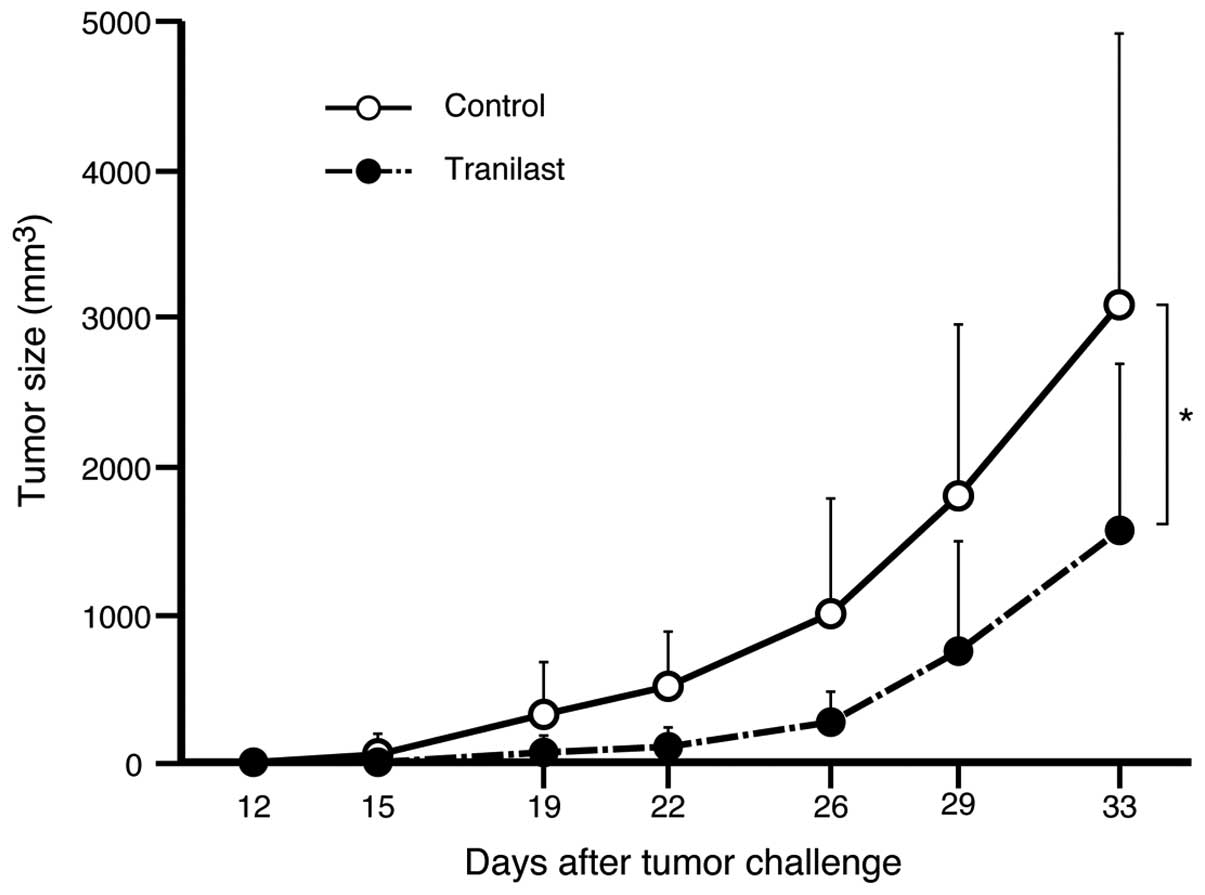
Tranilast attenuates the growth of
lymphoid tumor in vivo
To examine the effect of tranilast in vivo,
we inoculated A20 into BALB/c mice subcutaneously. After 7 days of
inoculation, treatment with oral administration of 100 mg/kg of
tranilast suspended in CMC solution or CMC solution alone was
started. The treatment was applied twice daily for 3 weeks. The
mice that underwent tranilast treatment had slower tumor
development compared with the mice that were dosed with CMC
solution alone. The tumor mass at 5 weeks after the challenge was
significantly smaller in mice treated with tranilast compared with
the control mice (P=0.02, Fig.
7).
Discussion
In the present study, we showed that conditioned
medium with IDO-expressing cells and a tryptophan metabolite,
tranilast, both decreased proliferation and induced cell death of
activated lymphocytes and specifically lymphoid tumor cells. In
agreement with our results, it has been reported that mesenchymal
stem cells treated with IFNγ inhibited the proliferation of normal
and follicular lymphoma B cells in an IDO-dependent manner
(19). Because tryptophan is an
essential amino acid, its starvation and the presence of
metabolites could influence all tissues including the placenta
(4) or dendritic cells (6), which contain a significant amount of
IDO. The specificity of cytotoxicity induced by IDO against
activated lymphoid cells may explain the machinery for the
protection of IDO-expressing cells themselves. In agreement with
this result, we could not get an increased exogenous expression of
IDO protein in murine lymphoid cell lines; however, we detected
IDO mRNA in these transfected cells. In the same way, it has
been reported that IDO protein has weak or absent enzymatic
activity in human B cells, although it can be induced by CD40L and
IFNγ together or by a TLR agonist (20). We analyzed IDO expression in
lymphoid tumor samples by RT-PCR (Fig.
1A). The expression in tumors was not increased compared with
those in PBMCs from healthy donors. Conversely, the IDO
expression in most tumor samples were weak and in some barely
detectable, particularly in BL. Because the percentage of malignant
B cells in BL were almost 100% (data not shown), the decrease of
IDO in malignant tissues may represent the decrease of
IDO-expressing non-B cells. In agreement of this, the IDO
expression was mainly derived from non-malignant bystander cells
from the samples examined. A report has described that the
functional expression of IDO could be detected in a portion of
diffuse large B-cell lymphoma (DLBCL) cases, and this expression
was correlated with worse prognosis (21). Although we did not have any
IDO-expressing DLBCL cases, these IDO-expressing lymphoma cells may
have the same protective properties as non-lymphoid cells against
IDO toxicity. It was also possible that this property could result
in resistance to chemotherapeutic reagents and contribute to the
poor prognosis and escape from immunological surveillance.
As tranilast was the most effective tryptophan
metabolite for EAE as reported by Platten et al (10), it was also the most potent
cytotoxic reagent for lymphoid tumor cells among the tryptophan
metabolites examined. Tranilast has an inhibitory effect on various
cancer cells including breast, prostate cancer and glioma (22). In our analysis, lymphoid cell lines
were more susceptible to the cytotoxicity of tranilast than the
non-lymphoid cells examined. As kynurenines can act as endogenous
AhR agonists and suppress immune response, tranilast is also an AhR
agonist. Its effect on breast cancer stem cells is AhR dependent
(18). The effect of tranilast for
lymphoid tumor was not inhibited by AhR antagonist α-NF indicating
that the effect was independent of AhR in the present study. To
date, the mechanisms of specific cytotoxicity to lymphoid tumor
cells induced by tranilast have not been elucidated. Tranilast
induced Erk-2 activation transiently and JNK activation after 1 day
of treatment in A20 cells. In contrast, it was reported that
tranilast suppressed LPS-induced Erk-2 activation in microglial
cells (23) and IL-1β-induced JNK
activation of mesangial cells (24). These opposite effects of tranilast
on MAP kinase signaling may explain the specific suppression
against lymphoid cells. JNK can mediate cell survival and cell
death dependent on cell type (25,26).
But the fact that tranilast could not be inhibited by the inhibitor
or the dominant negative form of JNK indicated that JNK was not
essential for the effect of tranilast. Because JNK was activated by
cellular stress such as UV, tranilast may also induce similar
stress in lymphoid malignant cells.
Tranilast has been widely used as oral anti-allergic
reagent in Japan and South Korea, and its safety is well
recognized. Because infectious complications have not been reported
as the side-effect of tranilast, this reagent should not induce
profound immunosuppression. Adjuvant use of tranilast that
potentiates the effect of chemotherapy is warranted.
Acknowledgements
The authors would like to thank Ms. Kaori Okada, Ms.
Sakie Endo, Ms. Ayumi Kiyokawa and Ms. Shihomi Endo for skillful
technical assistance and Dr Toshie Suzuki and Dr Hideki Kudo for
helpful discussions. The authors also thank Kissei Pharmaceutical
Corporation for providing the tranilast, and Dr Roger Davis for
providing the Jnk2a2 plasmid.
References
|
1
|
Fallarino F, Grohmann U, Vacca C, et al: T
cell apoptosis by tryptophan catabolism. Cell Death Differ.
9:1069–1077. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fallarino F, Grohmann U, You S, et al: The
combined effects of tryptophan starvation and tryptophan
catabolites down-regulate T cell receptor zeta-chain and induce a
regulatory phenotype in naive T cells. J Immunol. 176:6752–6761.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frumento G, Rotondo R, Tonetti M, Damonte
G, Benatti U and Ferrara GB: Tryptophan-derived catabolites are
responsible for inhibition of T and natural killer cell
proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med.
196:459–468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Munn DH, Zhou M, Attwood JT, et al:
Prevention of allogeneic fetal rejection by tryptophan catabolism.
Science. 281:1191–1193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mellor AL, Sivakumar J, Chandler P, et al:
Prevention of T cell-driven complement activation and inflammation
by tryptophan catabolism during pregnancy. Nat Immunol. 2:64–68.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mellor AL and Munn DH: IDO expression by
dendritic cells: tolerance and tryptophan catabolism. Nat Rev
Immunol. 4:762–774. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inaba T, Ino K, Kajiyama H, et al: Role of
the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the
progression of ovarian carcinoma. Gynecol Oncol. 115:185–192. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ino K, Yamamoto E, Shibata K, et al:
Inverse correlation between tumoral indoleamine 2,3-dioxygenase
expression and tumor-infiltrating lymphocytes in endometrial
cancer: its association with disease progression and survival. Clin
Cancer Res. 14:2310–2317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sharma MD, Baban B, Chandler P, et al:
Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes
directly activate mature Tregs via indoleamine 2,3-dioxygenase. J
Clin Invest. 117:2570–2582. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Platten M, Ho PP, Youssef S, et al:
Treatment of autoimmune neuroinflammation with a synthetic
tryptophan metabolite. Science. 310:850–855. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jasperson LK, Bucher C,
Panoskaltsis-Mortari A, et al: Indoleamine 2,3-dioxygenase is a
critical regulator of acute graft-versus-host disease lethality.
Blood. 111:3257–3265. 2008. View Article : Google Scholar
|
|
12
|
Jasperson LK, Bucher C,
Panoskaltsis-Mortari A, Mellor AL, Munn DH and Blazar BR: Inducing
the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase
(IDO), for suppression of graft-versus-host disease (GVHD)
lethality. Blood. 114:5062–5070. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gupta S, Barrett T, Whitmarsh AJ, et al:
Selective interaction of JNK protein kinase isoforms with
transcription factors. EMBO J. 15:2760–2770. 1996.PubMed/NCBI
|
|
14
|
Hu D and Kipps T: Reduction in
mitochondrial membrane potential is an early event in
Fas-independent CTL-mediated apoptosis. Cell Immunol. 195:43–52.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tohda S, Nara N, Murohashi I and Aoki N:
Establishment of an interleukin-3-dependent leukemic cell line from
a patient with chronic lymphocytic leukemia in the acute phase.
Blood. 78:1789–1794. 1991.PubMed/NCBI
|
|
16
|
Opitz CA, Litzenburger UM, Sahm F, et al:
An endogenous tumour-promoting ligand of the human aryl hydrocarbon
receptor. Nature. 478:197–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nguyen NT, Kimura A, Nakahama T, et al:
Aryl hydrocarbon receptor negatively regulates dendritic cell
immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad
Sci USA. 107:19961–19966. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Prud’homme GJ, Glinka Y, Toulina A, Ace O,
Subramaniam V and Jothy S: Breast cancer stem-like cells are
inhibited by a non-toxic aryl hydrocarbon receptor agonist. PLoS
One. 5:e138312010. View Article : Google Scholar
|
|
19
|
Maby-El Hajjami H, Amé-Thomas P, Pangault
C, et al: Functional alteration of the lymphoma stromal cell niche
by the cytokine context: role of indoleamine-2,3 dioxygenase.
Cancer Res. 69:3228–3237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Godin-Ethier J, Hanafi LA, Duvignaud JB,
Leclerc D and Lapointe R: IDO expression by human B lymphocytes in
response to T lymphocyte stimuli and TLR engagement is biologically
inactive. Mol Immunol. 49:253–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ninomiya S, Hara T, Tsurumi H, et al:
Indoleamine 2,3-dioxy-genase in tumor tissue indicates prognosis in
patients with diffuse large B-cell lymphoma treated with R-CHOP.
Ann Hematol. 90:409–416. 2011. View Article : Google Scholar
|
|
22
|
Rogosnitzky M, Danks R and Kardash E:
Therapeutic potential of tranilast, an anti-allergy drug, in
proliferative disorders. Anticancer Res. 32:2471–2478.
2012.PubMed/NCBI
|
|
23
|
Platten M, Eitel K, Wischhusen J, Dichgans
J and Weller M: Involvement of protein kinase Cdelta and
extracellular signal-regulated kinase-2 in the suppression of
microglial inducible nitric oxide synthase expression by
N-[3,4-dimethoxycinnamoyl]-anthranilic acid (tranilast). Biochem
Pharmacol. 66:1263–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chikaraishi A, Hirahashi J, Takase O, et
al: Tranilast inhibits interleukin-1beta-induced monocyte
chemoattractant protein-1 expression in rat mesangial cells. Eur J
Pharmacol. 427:151–158. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Johnson GL and Nakamura K: The c-jun
kinase/stress-activated pathway: regulation, function and role in
human disease. Biochim Biophys Acta. 1773:1341–1348. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Behrens A, Sibilia M and Wagner EF:
Amino-terminal phosphorylation of c-Jun regulates stress-induced
apoptosis and cellular proliferation. Nat Genet. 21:326–329. 1999.
View Article : Google Scholar : PubMed/NCBI
|