Introduction
Prostate cancer is the second most frequently
diagnosed cancer in men (914,000 new cases, 13.8% of the total),
the fifth most common cancer across both genders, and the sixth
leading cause of death from cancer in men in 2008 (1). Hormone ablation therapy is a
first-line treatment for prostate cancer; however, the duration of
remission is limited and androgen-independent regrowth is observed
in most patients (2).
Additionally, most chemotherapeutic agents provide only a marginal
benefit of survival or quality of life. Therefore, the development
of early diagnostic imaging agents and effective therapeutics for
prostate cancer is important for prolongation of survival time and
enhancement of patient quality of life.
Somatostatin and bombesin/gastrin-releasing peptide
(BBN/GRP) receptors are highly expressed in prostate cancers and
can be selectively targeted by cytotoxic peptide conjugates.
Peptide analogues are an exciting potential treatment for
androgen-independent prostate cancer (3). BBN is a 14-amino-acid neuropeptide,
first isolated from frog skin (4,5), and
it has high affinity for the gastrin-releasing peptide receptor
(GRPR). BBN and its mammalian counterpart, GRP, have similar
biological properties and share nearly identical C-terminal amino
acid sequences. In prostate cancer, GRPR expression is closely
associated with neoplastic transformation (6), cell migration (7,8),
proliferation (6,9), and invasion capacity (10,11).
GRPR is an important target for radiolabeled BBN
analogues that function as diagnostics or radionuclide therapies
for GRPR-positive tumors (12).
Various BBN analogues have been labeled with radiometals and
suitable chelators and used for positron emission tomography (PET)
imaging in GRPR-positive tumors (13–15).
99mTc-labeled BBN analogues have been synthesized with
diaminedithiol (DADT) and the dithiadiphosphine framework
(P2S2-COOH) as chelators. These analogues
were evaluated in normal mice (16,17)
and PC3 tumor-bearing mice, which represented a model of prostate
cancer (18,19). Bifunctional chelators and linkers
are necessary for specific uptake into the tumors and to reduce
uptake of radiometals into normal tissue. Rogers et al
labeled 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid
(DOTA)-Aoc is 8-aminooctanoic acid (Aoc)-BBN(7-14)
with 64Cu and then applied this radiotracer to a PC3
xenograft model (20). The tumor
was well-visualized; however, sustained blood concentrations and
persistent liver and kidney retention limited the potential
clinical application of this tracer.
Peptides as targeting molecules have unique
characteristics in vivo including fast washout and excretion
via the kidney because of their hydrophilic nature. To modulate the
pharmacokinetic characteristics of radiolabeled peptide in
vivo, researchers have tried to optimize to lower uptake in
non-target organ and higher in target organ by modification of
chelator and linker. Parry et al reported that shorter
aliphatic linkers (from C4 to C12) between the DOTA and BBN
resulted in lower liver uptake with simultaneous higher uptake into
GRPR-positive breast tumors (21).
Another group applied 1,4,7-triazacyclononane-1,4-diacetic acid
(NO2A) and various aliphatic linkers as a
1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) derivative
chelator and a pharmacokinetic modifier to BBN for 64Cu
labeling to improve tumor uptake and pharmacokinetic properties,
respectively. They reported that shorter and more hydrophilic
linker produced superior small animal PET images, with minimal
accumulation in collateral tissue but the longer and more
lipophilic linker resulted in high accumulation of radioactivity in
liver and abdominal region (22).
Parry et al introduced short peptides as a linker between
DOTA and BBN to reduce retention of the tracer in the liver by
activating the hepatobiliary pathway (23). Glycosylating the peptides is an
alternative strategy to improve pharmacokinetic properties
(24,25). Arg-Gly-Asp (RGD) peptide conjugates
([123 or 125I]Gluco-RGD (25) and [18F] Galacto-RGD)
(24,26,27),
which can be used to target new vascularization in tumors, showed
very similar kinetics in the kidney. In addition, RGD peptide
conjugates displayed clearly reduced activity in the liver as well
as increased uptake and retention in the tumor compared with the
unmodified parent peptides. 64Cu is widely used because
it is easy to produce with a medical cyclotron (28). 64Cu can be used in PET
to evaluate the kinetics of protein or peptide interactions with
target cells, and has a suitable positron energy (17.8%,
Eβ+max=656 keV), and a β-emitter (39.6%,
Eβ−max=573 keV) with a relatively long
half-life (12.7 h) (29).
In this study, we evaluated 64Cu-labeled
1,4,7-triazacy-clononane, 1-glutaric acid-4,7 acetic acid
(NODAGA)-BBN and 64Cu-labeled NODAGA-galacto-BBN in an
in vitro receptor-binding assay and for in vivo tumor
targeting by visualizing PC3 prostate xenograft tumors with small
animal PET.
Materials and methods
Materials
All reagents were purchased from commercial sources
and used as received without further purification.
NODAGA-BBN(7-14)NH2 and
NODAGA-galacto-BBN(7-14) NH2 were obtained from
AnyGen Co., Ltd. (Jeollanam-do, Korea).
[125I]Tyr4-BBN(1-14)
was obtained from PerkinElmer, Inc. (Branford, CT, USA). Ham’s
F-12K medium was purchased from WelGENE, Inc. (Daegu, Korea).
Bovine serum albumin (BSA), CaCl2, glacial acetic acid,
NaCl, NaOH, MnCl2, penicillin, phosphate-buffered saline
(PBS), sodium acetate, streptomycin, and
tris(hydroxymethyl)aminomethane (Tris) were purchased from the
Sigma-Aldrich Corp. (St. Louis, MO, USA). Matrigel (BD Matrigel
Basement Membrane Matrix) was purchased from BD Biosciences (San
Jose, CA, USA). Instant Thin Layer Chromatography-Silica Gel
(ITLC-SG) was purchased from Agilent Technologies, Inc. (Santa
Clara, CA, USA). Antibiotics and antimycotics, including
amphotericin B, streptomycin, and penicillin, were purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA). The PC3 human
prostate cancer cell line was obtained from the American Type
Culture Collection (Manassas, VA, USA).
High-performance liquid chromatography
and mass spectrometry
The purity of NODAGA-BBN(7-14)NH
2 and NODAGA-galacto-BBN(7-14)NH
2 was analyzed using high-performance liquid
chromatography (HPLC) (Shimadzu HPLC 10AVP system) with a C18
analytical column (5 μm, 3.0×150 mm). The elution conditions were
as follows: a mixture of an aqueous solution of solvent A [0.1%
trifluoro-acetic acid (TFA) in acetonitrile] and B (0.1% TFA in
water) with a 30-min linear gradient from 5 to 65% at a flow rate
of 1 ml/min. Matrix-assisted laser desorption/ionization (MALDI)
and MALDI time-of-flight (MALDI-TOF) experiments were performed on
a Kratos Shimadzu Axima-CFR.
Synthesis
Solid-phase peptide synthesis (SPPS) was performed
on a PIT-Symphony Peptide Synthesizer using the 9-f
luorenylmethoxycarbonyl (Fmoc) methodology to produce the
BBN(7-14)NH2 peptide (22). Conjugation of NODAGA to the
BBN(7-14)NH2 peptide resulted in
NODAGA-BBN(7-14)NH2 via an active ester. A
solution of NODAGA (286 μmol) in dimethylformamide (DMF) (1 ml) and
N,N-diisopropylethylamine (DIEA) (2 M, 800 μl) was added on the
resin with stirring at room temperature (RT). The stirring was
continued for 12 h, after which the resin was washed three times
with DMF. Fmoc deprotection was performed in 20% piperidine;
subsequently, the resin was washed three times with DMF. The
NODAGA-BBN(7-14)NH2 was isolated by
HPLC.
The preparation of NODAGA-galacto-BBN(7-14)NH2 was essentially similar
to the preparation of NODAGA-BBN(7-14)
NH2 described above. Galacturonic acid was conjugated
via an active ester onto the amine of glutamine (Q) on the
BBN(7-14)NH2. To a mixture of
galacturonic acid (10 μmol) in DMF (140 μl), hydroxybenzotriazole
(HOBt) (2 M, 40 μl) and 1,3-diisopropylcarbodiimide (DIC) (2 M, 40
μl) were added on the resin with stirring at RT. The stirring was
continued for 12 h, after which the resin was washed three times
with DMF. Fmoc deprotection was performed in 20% piperidine, and
the resin was washed three times with DMF. A solution of NODAGA
(286 μmol) in DMF (1 ml) and DIEA (2 M, 800 μl) was added to the
resin with stirring at RT for 12 h. NODAGA-galacto-BBN(7-14)NH2 was isolated by HPLC.
HPLC analysis and mass spectroscopy were used to confirm the
identity of the product.
Preparation of
[64Cu]NODAGA-BBN and [64Cu]NODAGA-
galacto-BBN
64Cu was produced at the Korea Institute
of Radiological and Medical Sciences (KIRAMS) (Seoul, Korea) with
50 MeV of cyclotron irradiation using methods reported previously
(30). The solvent was evaporated
for 30 min under a stream of argon at 110°C. To prepare the
[64Cu]NODAGA-BBN and
[64Cu]NODAGA-galacto-BBN, a 64Cu solution
(74–185 MBq) was added to a solution of NODAGA-BBN and
NODAGA-galacto-BBN (24 μg/1 ml 0.2 M sodium acetate buffer, pH 4.0)
in a glass vial. The resulting mixture was shaken at 70°C for 20
min. Subsequently, 64Cu incorporation was determined by
radio-thin layer chromatography (radio-TLC).
Cell culture and cell binding assay
The human prostate cancer PC3 cell line cultured in
Ham’s F-12K medium containing 20% (v/v) FBS and 1% (v/v)
penicillin/streptomycin solution. Cultures were maintained in a
37°C incubator with 5% CO2 in air, and the medium was
changed every 3 days.
Cell binding assays for [64Cu]NODAGA-BBN
or [64Cu] NODAGA-galacto-BBN on PC3 cells were performed
as described previously (13). PC3
cells (2×106/100 μl) were resuspended in binding buffer
(Ham’s F-12K medium containing 20 mM Tris, pH 7.4, 150 mM NaCl, 2
mM CaCl2, 1 mM MnCl2, and 0.1% BSA). For the
assay, equal volumes of non-radioactive ligands (NODAGA-BBN or
NODAGA-galacto-BBN) and radioactive ligand (3.7 MBq
[125I]Tyr4-BBN; PerkinElmer, Inc., Waltham,
MA, USA) were added. The GRPR ligands were added at concentrations
10−4–10−13 M. The tubes were incubated for 60
min at RT. Then, the reaction medium was removed and the cells were
washed three times with cold PBS. The cells were harvested and the
bound radioactivity was counted with a gamma counter (1480 Wizard
3″ Automatic Gamma Counter; PerkinElmer, Inc.). Data were analyzed
with GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA)
to determine the half maximal inhibitory concentration
(IC50) value. Experiments were performed in
triplicate.
Partition coefficient determination
The octanol/water partition coefficient of
[64Cu]NODAGA-BBN and [64Cu]
NODAGA-galacto-BBN was determined using the following protocol.
Approximately 3.7 MBq of [64Cu]NODAGA-BBN or
[64Cu]NODAGA-galacto-BBN in 3 ml of PBS (pH 7.4) was
added to 4 ml of octanol. The mixture was then vigorously stirred
for 5 min and centrifuged (3,000 rpm, 5 min). The activity of both
the PBS and octanol phases was measured in the gamma counter, and
logP values were calculated (n=3).
Internalization studies and in vitro
stability of [64Cu] NODAGA-BBN and
[64Cu]NODAGA-galacto-BBN in human serum
Internalization studies for
[64Cu]NODAGA-BBN or [64Cu]NODAGA-galacto-BBN
on PC3 cells were performed as described previously (18,19).
One million PC3 cells per tube were prepared for internalization
studies. The cells were washed with PBS and then incubated with
[64Cu]NODAGA-BBN or [64Cu]NODAGA-galacto-BBN
(0.0001 MBq/tube) for 2 h at 4°C. To remove unbound radioactivity,
the cells (n=3) were washed twice with ice-cold PBS and incubated
with prewarmed culture medium at 37°C for 0, 5, 15, 30, 45, 60, 90,
and 120 min to allow internalization.
To remove cell surface-bound radiotracer, the cells
were washed twice with acid (50 mM glycine-HCl/100 mM NaCl, pH
2.8). The acid solution was collected, and radioactivity was
measured with the gamma counter. The results were measured as
surface-bound activity. The cells were treated with 1 M NaOH, and
the resulting lysate was then measured with the gamma counter. The
results are expressed as the percentage of internalized activity
relative to the total activity (surface-bound activity +
internalized activity). The results are expressed as the mean ±
standard deviation (SD).
The stability of compounds
[64Cu]NODAGA-BBN and [64Cu]NODAGA-galacto-BBN
was assessed with a radio-TLC scanner and instant TLC (ITLC) paper.
Either [64Cu]NODAGA-BBN or
[64Cu]NODAGA-galacto-BBN (185 MBq/50 μl) was incubated
at 37°C in 1 ml of human serum and mouse serum for different time
intervals (1, 4 and 24 h). Free 64Cu was used as a
reference. The mobile phase consisted of 20 mM sodium acetate and
50 mM EDTA (pH 5.0). ITLC was performed using a radio-TLC scanner
(Aloka, Tokyo, Japan).
Pharmacokinetic study of
[64Cu]NODAGA-BBN and [64Cu]
NODAGA-galacto-BBN
The care, maintenance, and treatment of animals in
these studies followed protocols approved by the Institutional
Animal Care and Use Committee (IACUC) of the KIRAMS (IACUC no.
KIRAMS 2013-80). Female nude mice (BALB/cSlc-nu/nu, 6-weeks old)
were purchased from Japan SLC, Inc. (Shizuoka, Japan). Animals were
maintained in a temperature-controlled chamber at 22±3°C with a
12-h light/dark cycle. Relative humidity was maintained at 55±20%.
Sterilized rodent diet and purified tap water were supplied ad
libitum. Animals were acclimatized to the condition described
above for a week prior to use for the study.
Nude mice received [64Cu]NODAGA-BBN or
[64Cu] NODAGA-galacto-BBN via their tail veins at 7.4
MBq/head dose(n=6each). Blood samples were collected from
retro-orbital plexus via the sodium-heparinized capillary tube (75
mm; Paul Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany)
at 0, 0.0833, 0.167, 0.25, 0.5, 1, 2, 4, 6, 8 and 24 h after
administration. Dosing solution (20 μl) and blood samples (50 μl)
were transferred to polyethylene tubes. Each radioactivity of them
was counted by a scintillation counter. The radioactivity
concentration of blood sample was expressed as the percentage of
injected dose per a milliliter of blood (% ID/ml).
Pharmacokinetic parameters were estimated from the
blood radioactivity concentration vs. time data by
non-compartmental method using the non-linear least-squares
regression program, WinNonlin ver. 2.0 (Pharsight Corp., Cary, NC,
USA).
Biodistribution studies
Male athymic nude (Nu/Nu) BALB/c mice (Nara Biotech
Co., Ltd., Seoul, Korea) were injected subcutaneously with
1×107 PC3 human prostate cancer cells suspended in 100
μl of a Matrigel and PBS mixture (Matrigel:PBS = 1:1) at 6 weeks of
age in the flank of the left thigh. The mice were subjected to
biodistribution studies and PET imaging when the tumor reached
0.7–0.9 cm in diameter (18–21 days after implantation).
The PC3 tumor-bearing nude mice (n=3 for each group)
were injected with ~1.48 MBq of [64Cu]NODAGA-BBN or
[64Cu]NODAGA-galacto-BBN via the tail vein and
sacrificed at 1 or 3 h after injection. The tumor and normal
tissues of interest were removed and weighed, and their
radioactivity levels were measured using a gamma counter. The
uptake of radioactivity in the tumor and normal tissues was
expressed as percent of injected dose per gram (% ID/g).
Small animal PET studies
Whole-body PET images were obtained using a
dedicated small animal PET/CT scanner (Inveon™; Siemens Preclinical
Solutions, Malvern, PA, USA). Animals were anesthetized with 1.5%
isoflurane (Foran; Choongwae Pharma Co., Seoul, Korea) and injected
with 11.1–18.5 MBq (100 μl) of [64Cu]NODAGA-BBN or
[64Cu] NODAGA-galacto-BBN via the caudal vein, and
30-min static scans were acquired at 1, 3, 24, 48 and 72 h after
injection. In vivo GRPR-blocking experiment performed to
confirm specific tumor-targeting efficiency. To block GRPR, 15
mg/kg of NODAGA-BBN or NODAGA-galacto-BBN intravenously injected 30
min ahead of [64Cu]NODAGA-BBN or [64Cu]
NODAGA-galacto-BBN injection, then PET images were acquired at 1 h
after injection. PET images were reconstructed using an ordered
subset expectation maximization (OSEM) 2D algorithm with four
iterations. Regions of interest (ROIs) were drawn on the
reconstructed images using Inveon Research Workplace (IRW),
provided by Siemens Preclinical Solutions.
Statistical analysis
Data were analyzed with GraphPad Prism statistical
software (GraphPad Software, Inc.). Student’s two-tailed t-test was
used to determine statistical significance at the 95% confidence
level, with P<0.05 considered significantly different.
Results
Chemistry and radiochemistry
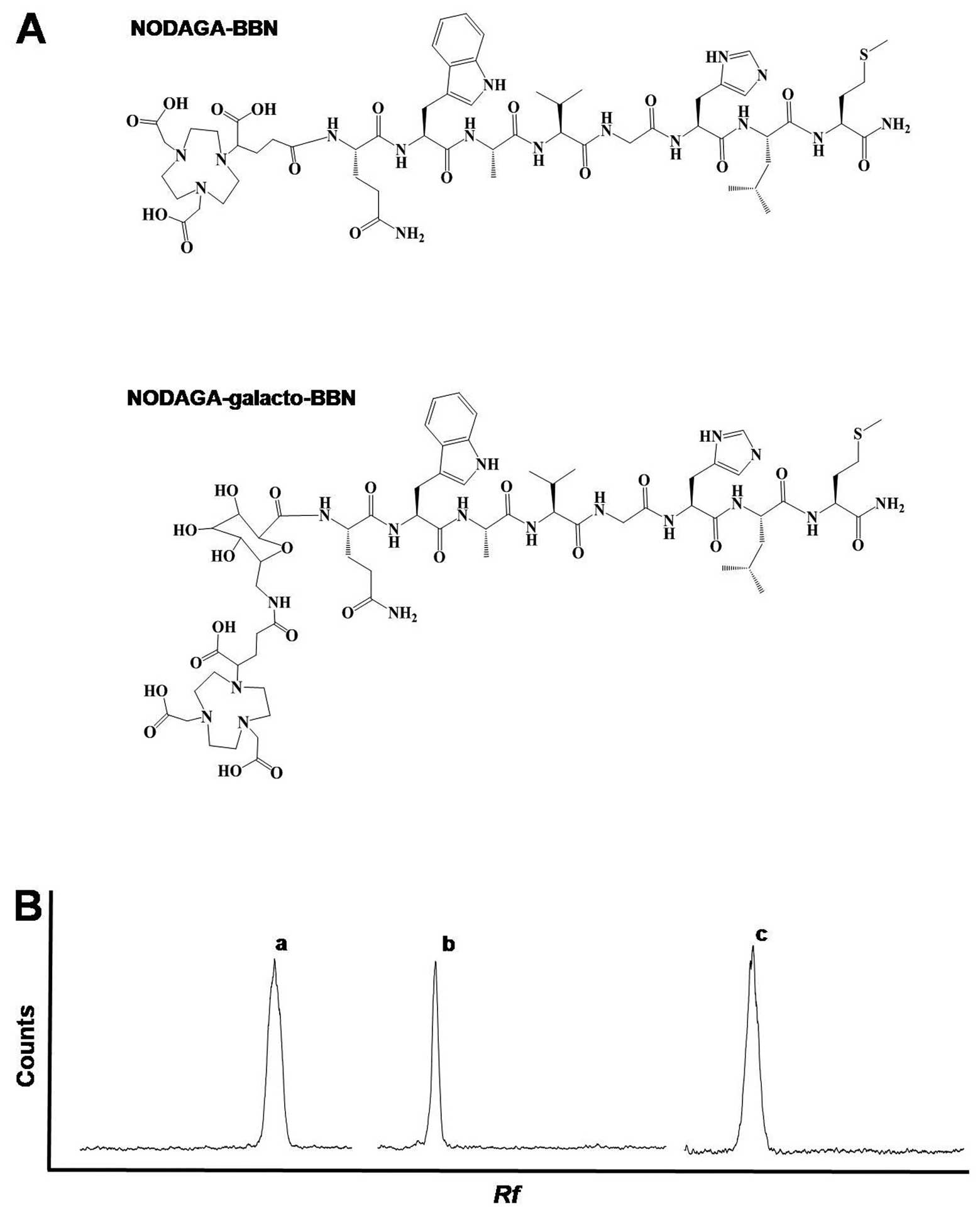
The NODAGA-BBN(7-14)
NH2 conjugate was obtained with a 97.2% yield and a
19.2-min retention time (Rt) on analytical HPLC. The MALDI-TOF mass
of NODAGA-BBN(7-14)NH2 was m/z=1298.3
(calculated for
C58H88N16O16S1,
1297.5) (Table I).
 | Table IThe MALDI-TOF mass of NODAGA. |
Table I
The MALDI-TOF mass of NODAGA.
| NODAGA-BBN |
NODAGA-galacto-BBN |
|---|
| Chemical
formula |
C58H88N16O16S1 |
C65H99N17O21S1 |
| Calculated MW | 1297.5 | 1486.6 |
| MALDI-TOF mass | 1298.3 | 1487.2 |
| LogP | −3.09±0.03 | −3.29±0.08 |
| Radiolabeling | 100±0.00 | 100±0.00 |
| efficiency (%) | | |
The NODAGA-galacto-BBN(7-14)NH2 conjugate was obtained
in 95.2% yield with a 19.8-min Rt on analytical HPLC. The MALDI-TOF
mass of NODAGA-galacto-BBN(7-14) NH
2 was m/z=1487.2 (calculated for
C65H99N17O21S1,
1486.6) (Table I). NODAGA-BBN and
NODAGA-galacto-BBN were labeled with 64Cu at 70°C for 20
min. The final products of the NODAGA-BBN and NODAGA-galacto-BBN
complexes are shown in Fig. 1A.
The radiolabeling yields of NODAGA-BBN and NODAGA-galacto-BBN were
>99% (Table I and Fig. 1B).
Pharmacokinetic study of
[64Cu]NODAGA-BBN and
[64Cu]NODAGA-galacto-BBN
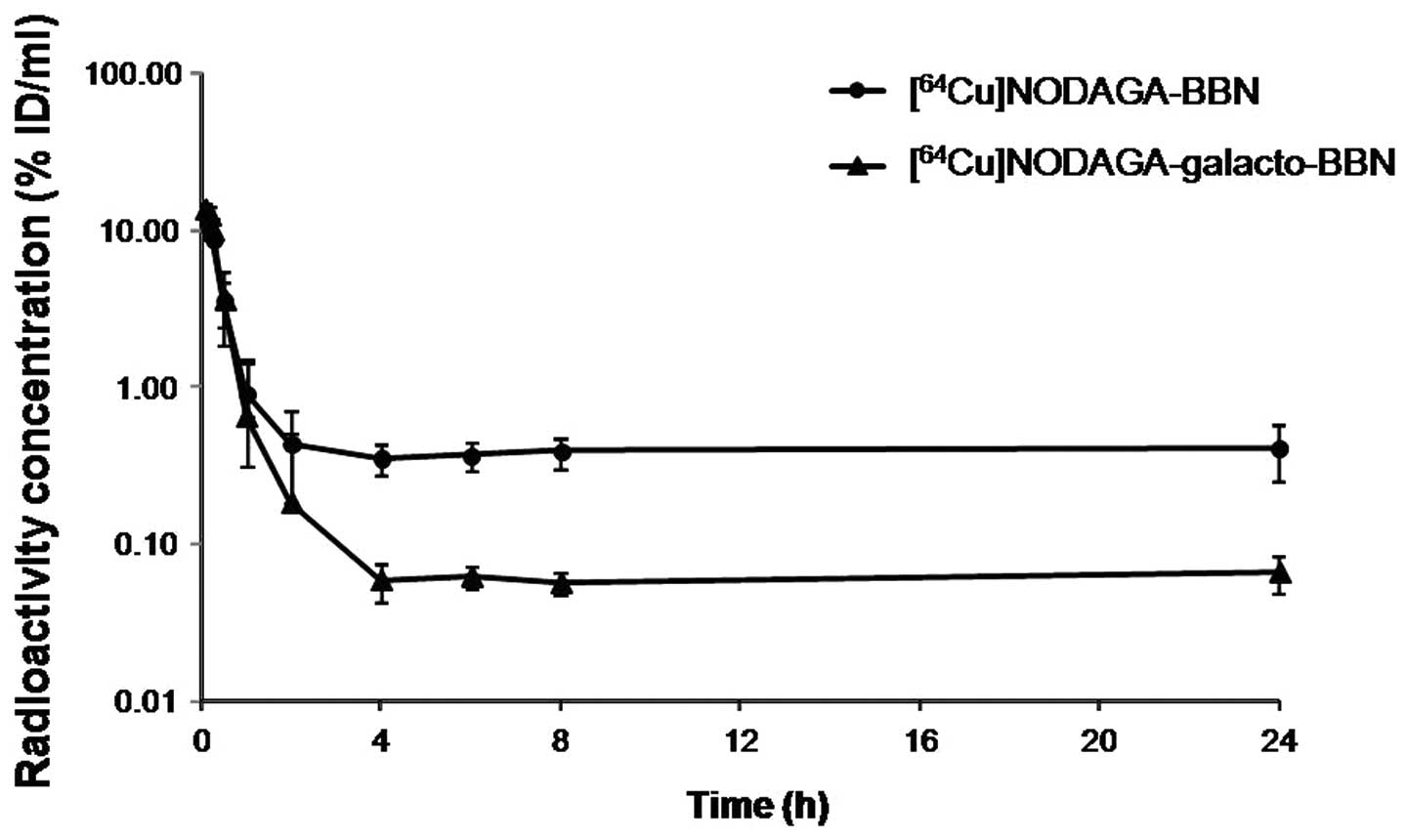
The blood radioactivity concentration-time profiles
following single intra-venous (i.v.) bolus injection of
[64Cu]NODAGA-BBN or [64Cu] NODAGA-galacto-BBN
in nude mice are shown in Fig. 2.
In both, the blood radioactivity concentrations decreased
biexponentially and reached the steady state at 4 h after
administration. At the steady state, the blood radioactivity level
of [64Cu]NODAGA-BBN was ~6 times higher than that of
[64Cu]NODAGA-galacto-BBN. Table II summarizes the blood
pharmacokinetic parameters obtained by non-compartmental analysis.
The blood concentration extrapolated to time zero (C0)
was 15.8±1.74 and 16.2±1.48% ID/ml, the terminal elimination
half-life (t1/2,z) was 70.7±43.71 and 29.2±20.61 h, and
the area under blood concentration-time curve (AUC0–24
h) was 14.6±3.00 and 7.44±2.02% ID·h/ml, respectively, after
i.v. injection of [64Cu]NODAGA-BBN or
[64Cu]NODAGA-galacto-BBN. The volume of distribution at
terminal phase (Vz) was 195±75.04 and 395±171.95 ml, the
systemic clearance (Cl) was 2.22±0.96 and 10.3±2.89 ml/h, and the
mean retention time (MRT) was 7.87±0.87 and 2.70±0.24 h,
respectively, after i.v. injection of [64Cu]NODAGA-BBN
or [64Cu]NODAGA-galacto-BBN.
 | Table IINon-compartmental pharmacokinetic
parameters of radioactivity in blood obtained after i.v. injection
of [64Cu] NODAGA-BBN or
[64Cu]NODAGA-galacto-BBN (injected dose: 7.4 MBq/head)
in nude mice (n=6 each). |
Table II
Non-compartmental pharmacokinetic
parameters of radioactivity in blood obtained after i.v. injection
of [64Cu] NODAGA-BBN or
[64Cu]NODAGA-galacto-BBN (injected dose: 7.4 MBq/head)
in nude mice (n=6 each).
| Parameter |
[64Cu]NODAGA-BBN |
[64Cu]NODAGA- galacto-BBN |
|---|
| C0 (%
ID/ml) | 15.8±1.74 | 16.2±1.48 |
| t1/2,z
(h) | 70.7±43.71 | 29.2±20.61 |
| AUC0–24
h | 14.6±3.00 | 7.44±2.02 |
| (% ID·h/ml) |
| Vz
(ml) | 195±75.04 | 395±171.95 |
| Cl (ml/h) | 2.22±0.96 | 10.3±2.89 |
| MRTlast
(h) | 7.87±0.87 | 2.70±0.24 |
In vitro characterization
The stability of[64Cu]NODAGA-BBN and
[64Cu]NODAGA-galacto-BBN, in terms of the percentage of
the intact conjugate, was measured in human or mouse serum. No
de-radiometallation or major degradation of the BBN tracer was
detected after incubation with human and mouse sera at 37°C for 1 h
as monitored by radio-TLC.
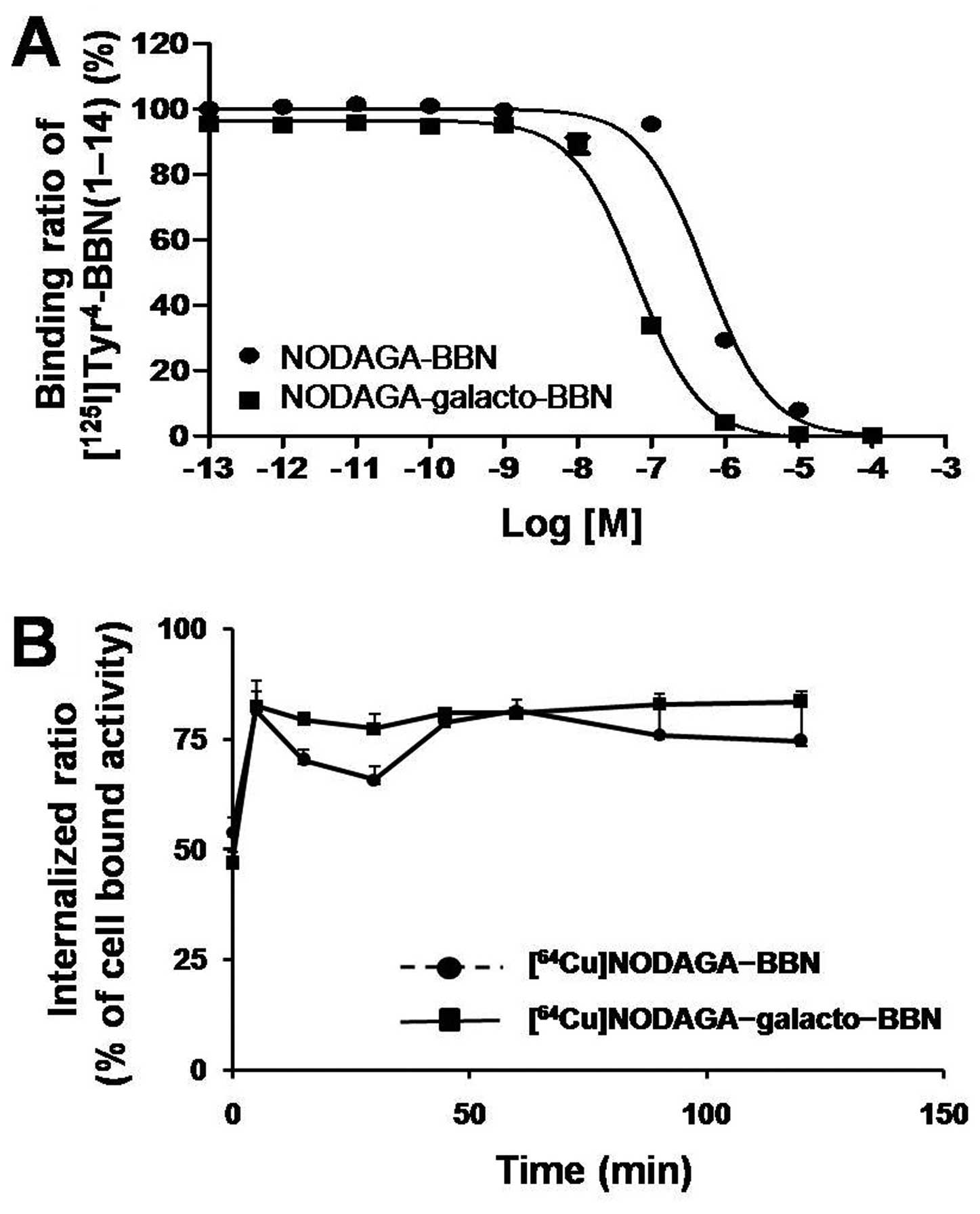
Cell binding assays with NODAGA-BBN and NODAGA-
galacto-BBN showed that both compounds inhibited the binding of
[125I]Tyr4-BBN to PC3 cells in a
concentration-dependent manner. As shown in Fig. 3A, the calculated IC50
value of NODAGA-BBN was 547±38.9 nM, whereas a somewhat higher
affinity (64.1±6.34 nM) was observed for NODAGA-galacto-BBN.
Both [64Cu]NODAGA-BBN and
[64Cu]NODAGA-galacto- BBN showed similar logP values of
−3.09±0.03 and −3.29±0.08, respectively, indicating that the
complexes were highly hydrophilic and that adding the galactose
moiety made the peptide even more hydrophilic.
The internalization studies presented in Fig. 3B showed that
[64Cu]NODAGA-BBN and [64Cu]NODAGA-galacto-BBN
are rapidly taken up (within 5 min) into PC3 cells. The
intracellular uptake of [64Cu]NODAGA-BBN and
[64Cu] NODAGA-galacto-BBN reached a plateau at 15 min;
75–80% of cell-bound activity was attributed to the internalization
of the peptides in the cells.
Biodistribution studies
The in vivo uptake of [64Cu]
NODAGA-BBN and [64Cu] NODAGA-galacto-BBN intoseveral
organs, including a GRPR-expressing PC3 prostate tumor, was
measured at 1 and 3 h after i.v. injection (Fig. 4). Rapid blood clearance was
observed for both [64Cu]NODAGA-BBN and
[64Cu]NODAGA-galacto-BBN, with <0.5% ID/g remaining.
Liver uptake of the [64Cu]NODAGA-galacto-BBN-injected
group was relatively low (1 h, 1.13±0.19% ID/g; 3 h, 0.82±0.35%
ID/g) (1 h, P<0.05; 3 h, P<0.01), compared with the
[64Cu]NODAGA-BBN-injected group (1 h, 2.69±0.60% ID/g; 3
h, 2.47±0.23% ID/g). However, the tumor uptake of [64Cu]
NODAGA-galacto-BBN was significantly greater (P<0.01) than that
of [64Cu]NODAGA-BBN; the tumor uptake at 1 and 3 h for
[64Cu]NODAGA-galacto-BBN was 3.26±0.53 and 1.96±1.16%
ID/g, respectively, whereas the tumor uptake at 1 and 3 h for
[64Cu]-NODAGA-BBN was 1.62±0.23 and 1.18±0.18% ID/g,
respectively. There was a significant difference in uptake pattern
between [64Cu]NODAGA-BBN and
[64Cu]NODAGA-galacto-BBN in other organs including the
blood, heart, lungs, and intestines (Fig. 4A and B). At 1 and 3 h, the ratio of
tumor to non-target (i.e., liver, kidney, and blood) for
[64Cu]NODAGA-galacto-BBN was higher than that of
[64Cu]NODAGA-BBN (Fig. 4C
and D). Therefore, [64Cu] NODAGA-galacto-BBN has a
higher targeting efficiency than [64Cu]NODAGA-BBN.
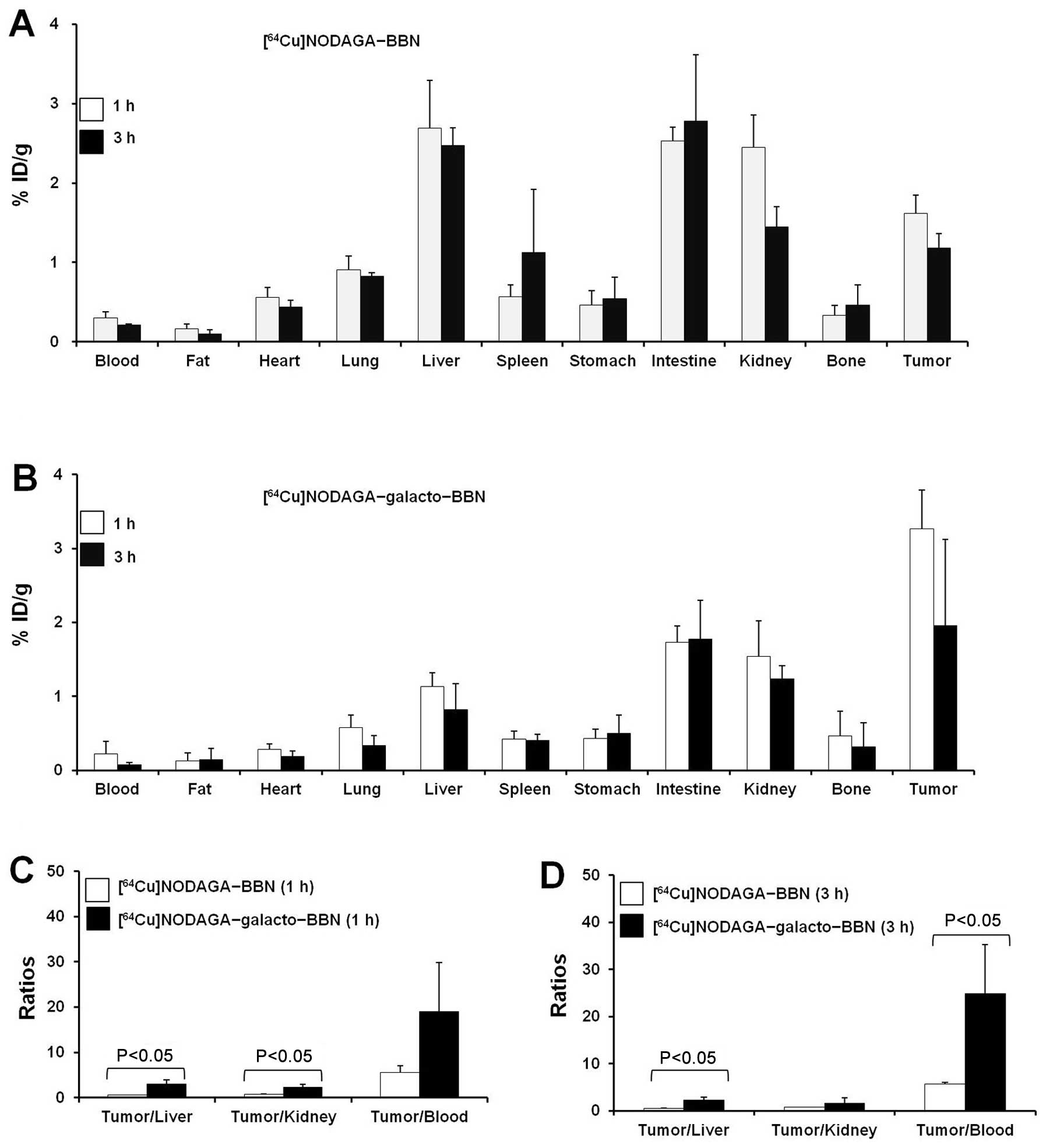 | Figure 4(A and B) Biodistribution and (C and
D) calculated tumor-to-normal tissue uptake ratios for
[64Cu]1,4,7-triazacyclononane, 1-glutaric acid-4,7
acetic acid (NODAGA)-BBN and [64Cu]NODAGA-galacto-BBN in
PC3 tumor-bearing mice. (A and B) Biodistribution data of (A)
[64Cu]NODAGA-BBN (n=3) and (B)
[64Cu]NODAGA-galacto-BBN (n=3), 1 h (open bar) and 3 h
(closed bar) after BBN probe injection. The data are presented as
percent of injected dose per gram (% ID/g) [mean ± standard
deviation (SD)]. (C and D) Tumor-to-normal tissue (liver, kidney,
and blood) uptake ratios for [64Cu]NODAGA-BBN (open bar,
n=3) and [64Cu]NODAGA-galacto-BBN (closed bar, n=3) at
(C) 1 h and (D) 3 h after tracer injection. Data are presented as
the mean ± SD, P<0.05 ([64Cu] NODAGA-BBN vs.
[64Cu]NODAGA-galacto-BBN). |
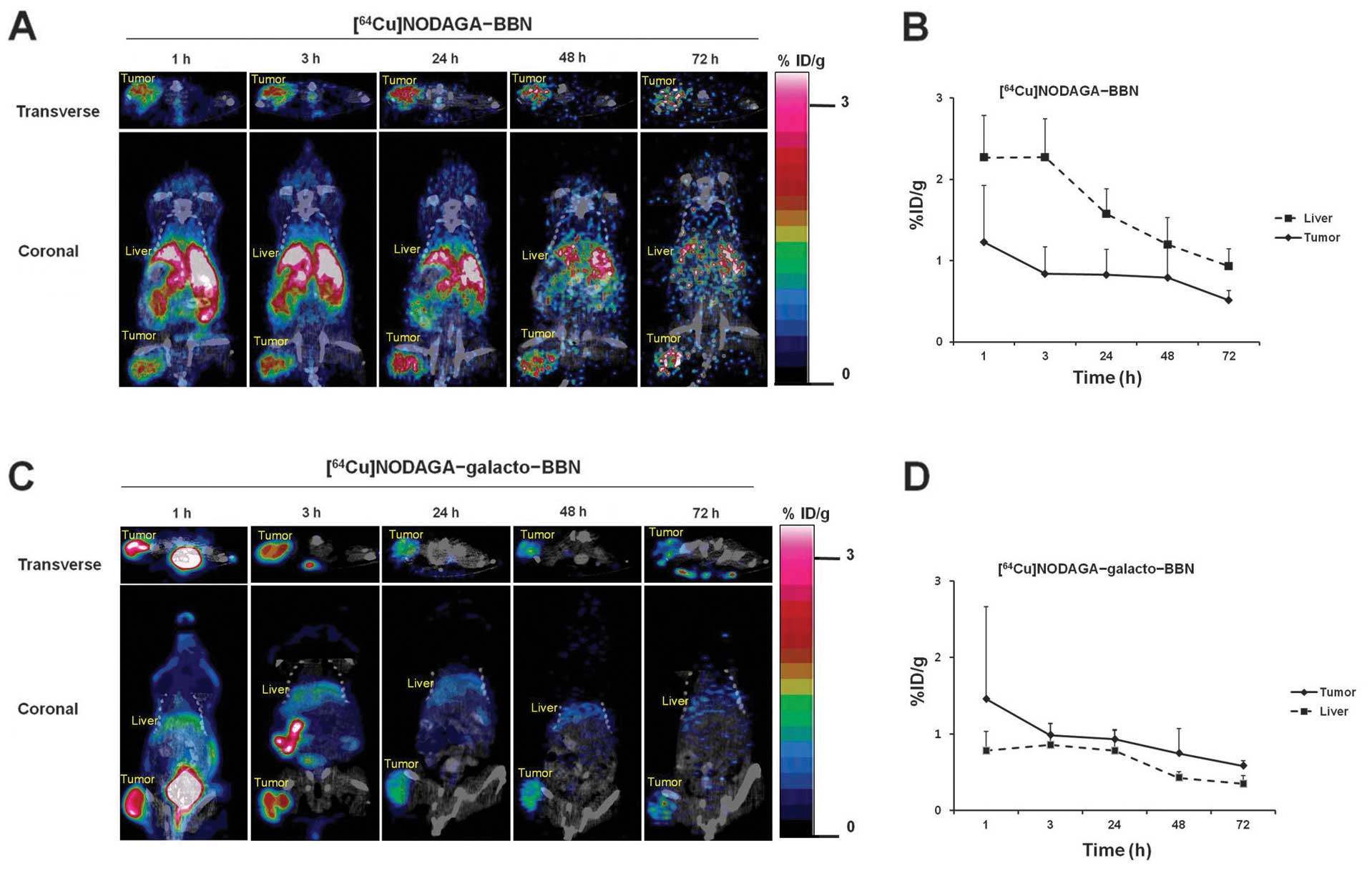
Small animal PET imaging studies
To evaluate the in vivo kinetics of
[64Cu]NODAGA-BBN and [64Cu]NODAGA-
galacto-BBN, small animal PET imaging studies were performed in PC3
prostate cancer-bearing mice. Fig.
5 shows a single slice of the transverse and coronal small
animal PET images of a representative mouse at 1, 3, 24, 48 and 72
h after injection of [64Cu]NODAGA-BBN or
[64Cu]NODAGA-galacto-BBN. [64Cu]NODAGA-BBN
and [64Cu]NODAGA-galacto-BBN exhibited high tumor uptake
at 1 h; the location of tumor was clearly visible for 72 h
(Fig. 5A and C). Although
differences of tumor uptake between two probes were not
statistically significant by quantitative ROI analysis (P>0.05,
from 1 to 72 h), liver uptake of
[64Cu]NODAGA-galacto-BBN was significantly lower than
that of [64Cu]NODAGA-BBN throughout the scanning periods
(P<0.05, from 1 to 72 h). Tumor and liver uptake of
[64Cu] NODAGA-BBN and
[64Cu]NODAGA-galacto-BBN longitudinally decreased >72
h (Fig. 5B and D). Tumor uptake of
[64Cu] NODAGA-BBN or [64Cu]NODAGA-galacto-BBN
was barely detectable upon administration of 15 mg/kg NODAGA-BBN or
NODAGA-galacto-BBN as a blocking agent 30 min before tracer
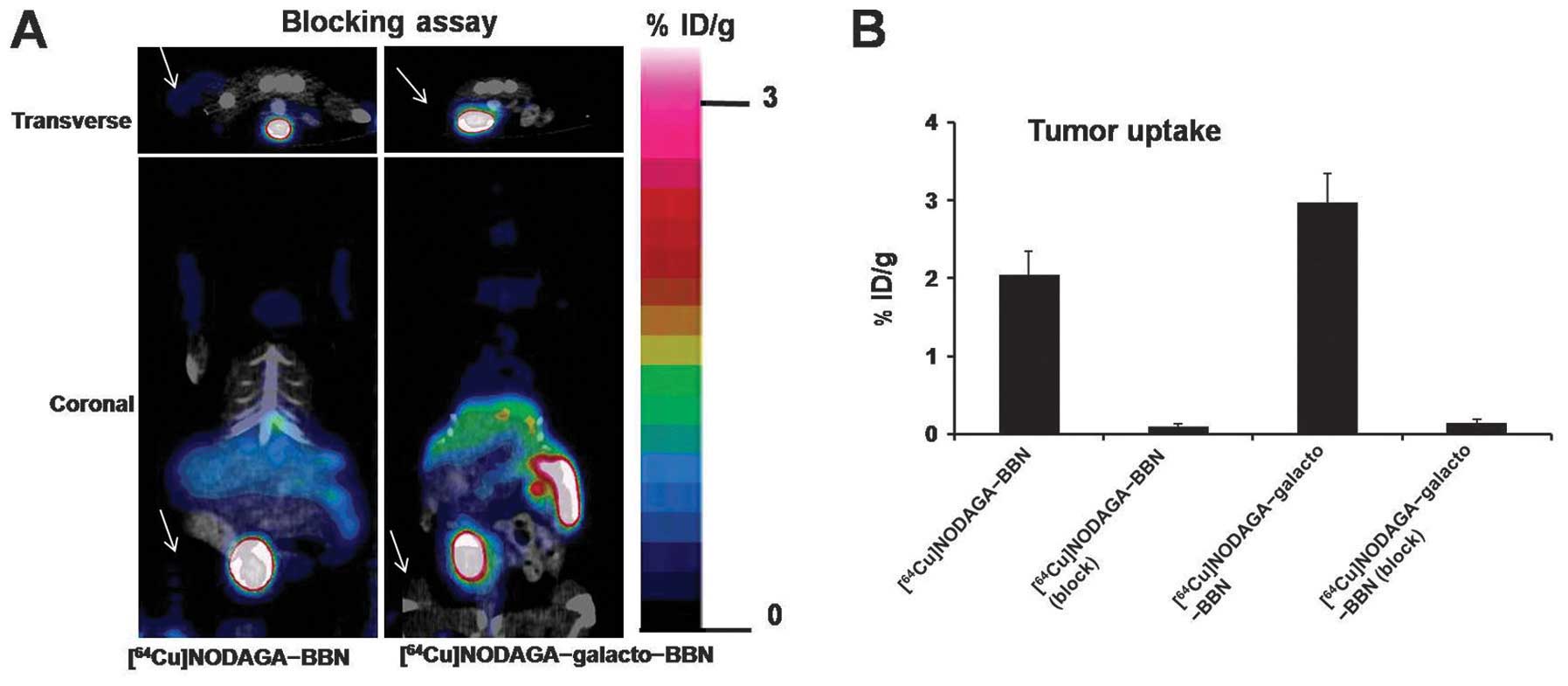
injection (Fig. 6A). Tumor uptake
was decreased in the group treated with the blocking agent
([64Cu]NODAGA-BBN (block): 0.09±0.03% ID/g and
[64Cu]NODAGA-galacto-BBN (block): 0.14±0.05% ID/g)
compared with the group that did not receive the blocking agent
([64Cu]NODAGA-BBN: 1.23±0.70% ID/g and
[64Cu]NODAGA-galacto-BBN: 1.46±1.20% ID/g) (Fig. 6B).
Discussion
Here, for the first time, we evaluated BBN
analogues radiolabeled with 64Cu that contained a
galactose moiety as a linker between the BBN peptide and NODAGA
chelator. These analogues were designed to provide relatively low
liver and high prostate tumor uptake. In order to investigate the
effects of galactose moiety in BBN in systemic circulation, blood
radioactivity concentration-time profiles of
[64Cu]NODAGA-BBN or [64Cu]NODAGA-galacto-BBN
were determined. The blood radioactivity level of
[64Cu]NODAGA-BBN was ~6 times higher than that of
[64Cu]NODAGA-galacto-BBN at steady state as shown in
Fig. 2 and Table II. This result suggests that
[64Cu]NODAGA-galacto-BBN is faster excreted from blood
circulation compared to that of [64Cu]NODAGA-BBN. In the
PET imaging and biodistribution analysis, [64Cu]
NODAGA-galacto-BBN was more favorable in vivo tumor
retention and pharmacokinetic properties than [64Cu]
NODAGA-BBN (Figs. 4 and 5). In the biodistribution studies, the
tumor uptake for [64Cu]NODAGA-galacto-BBN was higher
than the tumor uptake for [64Cu]NODAGA-BBN at 1 and 3 h
(3.26±0.53 vs. 1.62±0.23% at 1 h and 1.96±1.16 vs. 1.18±0.18% at 3
h, respectively). Moreover, the liver uptake for [64Cu]
NODAGA-galacto-BBN was lower than the liver uptake for
[64Cu]NODAGA-BBN from 1 to 72 h according to PET imaging
(Fig. 5). In the biodistribution
studies, liver uptake of [64Cu]NODAGA-galacto-BBN was
significantly lower than that of [64Cu]NODAGA-BBN at 1
and 3 h (1.13±0.19 vs. 2.69±0.69 at 1 h and 0.82±0.35 vs.
2.47±0.23% at 3 h, respectively) (Fig.
4). The tumor/blood ratios for
[64Cu]NODAGA-galacto-BBN are higher at 1 h (19.05±10.82)
and 3 h (24.89±10.36) to any previously reported results in
[64Cu]NO2A-(AMBA)-BBN (2.6 at 24 h) (22), [64Cu]NOTA-Bn-SCN-Aoc-BBN
(18.029 at 4 h) (31), and
[64Cu]DOTA-amino acid linker-BBN (~4–8) (23). The favorable tumor/blood ratio of
[64Cu]NODAGA-galacto-BBN might be due to its fast
clearance from systemic circulation as shown in Fig. 2. These pharmacokinetic properties
might be caused by use of NODAGA and galactose as a chelator and a
linker, respectively. i) NODAGA as a chelator; The group of Rogers
hypothesized that a six-coordinate 64Cu-labeled
NOTA-Bn-SCN-Aoc-BBN would have greater in vivo stability
than five-coordinate 64Cu-labeled NO2A-Aoc-BBN and
tumor/blood ratio of six-coordinate complex at 4 h after injection
was found to be 18 (31). NODAGA
is a chelator that can form with 64Cu to six-coordinated
state. However, the formation of six-coordinated structure of
NODAGA with 64Cu in [64Cu] NODAGA-BBN or
[64Cu]NODAGA-galacto-BBN in vivo state must be
determined in further study. ii) Galactose as a linker; the
improved tumor/blood ratios of [64Cu]NODAGA-galacto-BBN
might be a role of galactose as a linker. Generally, purpose of
linker insertion in radiometal-labeled peptide analogues could be
the enhancement of in vivo stability or targeting
efficiency. The use of galactose as a linker could reduce the
lipophilicity of the conjugate, reduce liver accumulation and
result in fast excretion of radioactivity.
Radiolabeling NODAGA-BBN with 64Cu is
simple. Previously, Liu etaldeveloped a PET probe using
NODAGA-RM1 and NODAGA-AMBA as a BBN analogue with 64Cu;
this strategy provided a radiochemical yield of >85% (32). In the current study,
[64Cu]NODAGA-BBN and [64Cu]NODAGA-galacto-BBN
were obtained with higher radiochemical yields (>99%) after
incubation at 70°C for 20 min (Table
I). After labeling with 64Cu, NODAGA-BBN and
NODAGA-galacto-BBN were very stable in both human and mouse serum
at 37°C for 1 h, demonstrating no de-radiometallation.
Mansi et al produced a new conjugate, RM2,
with the chelator DOTA coupled to
D-Phe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2 via cationic
spacer 4-amino-1-carboxymethyl- piperidine for labeling with and
presented [111In]RM2 and [68Ga] RM2 as ideal
candidates for single photon emission computed tomography (SPECT)
and PET agents, respectively (33). [68Ga] DOTA-BBN,
BAY86-7548 has been investigated for safety, metabolism,
pharmacokinetics, biodistribution and radiation dosimetry in five
healthy men (34), and applied to
prostate cancer patients for detecting intraprostatic prostate
cancer (35).
Peptides used as targeting molecules have been
labeled with 64Cu using various suitable chelators such
as DOTA, NOTA, and NO2A including NODAGA (13,14,32,36).
Improved in vivo kinetics is important for
64Cu-based PET imaging and therapy. Clearly, the
targeting efficiency of [64Cu] NODAGA-galacto-BBN to PC3
tumor might be not superior to previously reported data (22,23,32).
However, this study demonstrates that use of galactose and NODAGA
in complex could induce the favorable pharmacokinetic
characteristic such as rapid clearance from systemic circulation.
Given its serum stability in vitro and favorable in
vivo tumor-targeting properties (Figs. 3 and 4), [64Cu]NODAGA-galacto-BBN
warrants further investigation for clinical translation.
Additionally, 67Cu (t1/2=61 h) has favorable
therapeutic β energy (100%, Eβ-max=577 keV,
Eγ=195 keV), and nearly equivalent to 64Cu in
coordination chemistry. Moreover, the in vivo kinetics of
67Cu-labeled molecules can be predicted with
64Cu-labeled compounds (37). Thus, 67Cu-labeled BBN
analogues may be utilized for radiotherapy of GRPR-expressing
tumors. In conclusion, NODAGA-BBN and NODAGA-galacto-BBN have been
successfully prepared and radiolabeled with 64Cu. The
radiolabeling procedures were simple and straightforward. Both
[64Cu]NODAGA-BBN and [64Cu]NODAGA-galacto-BBN
stable against in vitro de-radiometallation and showed
excellent in vivo tumor-targeting properties. In further
studies, we will perform precise evaluation and characterization of
these PET probes in vitro and in vivo.
Acknowledgements
This study was supported by the Nuclear R&D
(grant code: NRF-2012M2A2A7013480 and NRF-2012M2A2A7013722)
Programs of NRF, funded by MEST.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View Article : Google Scholar
|
|
3
|
Stangelberger A, Schally AV and Djavan B:
New treatment approaches for prostate cancer based on peptide
analogues. Eur Urol. 53:890–900. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fischer R, Hill RM and Warshay D: Effects
of psychodysleptic drug psilocybin on visual perception. Changes in
brightness preference. Experientia. 25:166–169. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagalla SR, Barry BJ, Falick AM, Gibson
BW, Taylor JE, Dong JZ and Spindel ER: There are three distinct
forms of bombesin. Identification of [Leu13]bombesin,
[Phe13]bombesin, and [Ser3, Arg10, Phe13]bombesin in the frog
Bombina orientalis. J Biol Chem. 271:7731–7737. 1996.PubMed/NCBI
|
|
6
|
Albrecht M, Doroszewicz J, Gillen S, Gomes
I, Wilhelm B, Stief T and Aumüller G: Proliferation of prostate
cancer cells and activity of neutral endopeptidase is regulated by
bombesin and IL-1beta with IL-1beta acting as a modulator of
cellular differentiation. Prostate. 58:82–94. 2004. View Article : Google Scholar
|
|
7
|
Aprikian AG, Tremblay L, Han K and
Chevalier S: Bombesin stimulates the motility of human
prostate-carcinoma cells through tyrosine phosphorylation of focal
adhesion kinase and of integrin-associated proteins. Int J Cancer.
72:498–504. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagakawa O, Ogasawara M, Fujii H, Murakami
K, Murata J, Fuse H and Saiki I: Effect of prostatic neuropeptides
on invasion and migration of PC-3 prostate cancer cells. Cancer
Lett. 133:27–33. 1998. View Article : Google Scholar
|
|
9
|
Bologna M, Festuccia C, Muzi P, Biordi L
and Ciomei M: Bombesin stimulates growth of human prostatic cancer
cells in vitro. Cancer. 63:1714–1720. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishimaru H, Kageyama Y, Hayashi T, Nemoto
T, Eishi Y and Kihara K: Expression of matrix metalloproteinase-9
and bombesin/gastrin-releasing peptide in human prostate cancers
and their lymph node metastases. Acta Oncol. 41:289–296. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Festuccia C, Angelucci A, Gravina G,
Eleuterio E, Vicentini C and Bologna M: Bombesin-dependent
pro-MMP-9 activation in prostatic cancer cells requires beta1
integrin engagement. Exp Cell Res. 280:1–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Markwalder R and Reubi JC:
Gastrin-releasing peptide receptors in the human prostate: relation
to neoplastic transformation. Cancer Res. 59:1152–1159.
1999.PubMed/NCBI
|
|
13
|
Fournier P, Dumulon-Perreault V,
Ait-Mohand S, Langlois R, Bénard F, Lecomte R and Guérin B:
Comparative study of
64Cu/NOTA-[D-Tyr6,βAla11,Thi13,Nle14]BBN(6–14) monomer
and dimers for prostate cancer PET imaging. EJNMMI Res. 2:82012.
View Article : Google Scholar
|
|
14
|
Zhang H, Abiraj K, Thorek DL, Waser B,
Smith-Jones PM, Honer M, Reubi JC and Maecke HR: Evolution of
bombesin conjugates for targeted PET imaging of tumors. PLoS One.
7:e440462012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Varasteh Z, Aberg O, Velikyan I, Lindeberg
G, Sörensen J, Larhed M, Antoni G, Sandström M, Tolmachev V and
Orlova A: In vitro and in vivo evaluation of a
18F-labeled high affinity NOTA conjugated bombesin
antagonist as a PET ligand for GRPR-targeted tumor imaging. PLoS
One. 8:e819322013. View Article : Google Scholar
|
|
16
|
Baidoo KE, Lin KS, Zhan Y, Finley P,
Scheffel U and Wagner HN Jr: Design, synthesis, and initial
evaluation of high-affinity technetium bombesin analogues.
Bioconjug Chem. 9:218–225. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karra SR, Schibli R, Gali H, Katti KV,
Hoffman TJ, Higginbotham C, Sieckman GL and Volkert WA:
99mTc-labeling and in vivo studies of a bombesin
analogue with a novel water-soluble dithiadiphosphine-based
bifunctional chelating agent. Bioconjug Chem. 10:254–260. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
La Bella R, Garcia-Garayoa E, Langer M,
Bläuenstein P, Beck-Sickinger AG and Schubiger PA: In vitro and in
vivo evaluation of a 99mTc(I)-labeled bombesin analogue
for imaging of gastrin releasing peptide receptor-positive tumors.
Nucl Med Biol. 29:553–560. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
La Bella R, Garcia-Garayoa E, Bahler M,
Bläuenstein P, Schibli R, Conrath P, Tourwé D and Schubiger PA: A
99mTc(I)-postlabeled high affinity bombesin analogue as
a potential tumor imaging agent. Bioconjug Chem. 13:599–604. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rogers BE, Bigott HM, McCarthy DW, Della
Manna D, Kim J, Sharp TL and Welch MJ: MicroPET imaging of a
gastrin-releasing peptide receptor-positive tumor in a mouse model
of human prostate cancer using a 64Cu-labeled bombesin
analogue. Bioconjug Chem. 14:756–763. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Parry JJ, Andrews R and Rogers BE:
MicroPET imaging of breast cancer using radiolabeled bombesin
analogs targeting the gastrin-releasing peptide receptor. Breast
Cancer Res Treat. 101:175–183. 2007. View Article : Google Scholar
|
|
22
|
Lane SR, Nanda P, Rold TL, Sieckman GL,
Figueroa SD, Hoffman TJ, Jurisson SS and Smith CJ: Optimization,
biological evaluation and microPET imaging of copper-64-labeled
bombesin agonists, [64Cu-NO2A-(X)-BBN(7-14)NH2], in a
prostate tumor xenografted mouse model. Nucl Med Biol. 37:751–761.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parry JJ, Kelly TS, Andrews R and Rogers
BE: In vitro and in vivo evaluation of 64Cu-labeled
DOTA-linker-bombesin(7–14) analogues containing different amino
acid linker moieties. Bioconjug Chem. 18:1110–1117. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haubner R, Wester HJ, Weber WA, Mang C,
Ziegler SI, Goodman SL, Senekowitsch-Schmidtke R, Kessler H and
Schwaiger M: Noninvasive imaging of alpha(v)beta3 integrin
expression using 18F-labeled RGD-containing glycopeptide
and positron emission tomography. Cancer Res. 61:1781–1785.
2001.PubMed/NCBI
|
|
25
|
Haubner R, Wester HJ, Burkhart F,
Senekowitsch-Schmidtke R, Weber W, Goodman SL, Kessler H and
Schwaiger M: Glycosylated RGD-containing peptides: tracer for tumor
targeting and angiogenesis imaging with improved biokinetics. J
Nucl Med. 42:326–336. 2001.PubMed/NCBI
|
|
26
|
Haubner R, Kuhnast B, Mang C, Weber WA,
Kessler H, Wester HJ and Schwaiger M: [18F]Galacto-RGD:
synthesis, radiolabeling, metabolic stability, and radiation dose
estimates. Bioconjug Chem. 15:61–69. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haubner R, Weber WA, Beer AJ, Vabuliene E,
Reim D, Sarbia M, Becker KF, Goebel M, Hein R, Wester HJ, Kessler H
and Schwaiger M: Noninvasive visualization of the activated
alphavbeta3 integrin in cancer patients by positron emission
tomography and [18F]Galacto-RGD. PLoS Med. 2:e702005.
View Article : Google Scholar
|
|
28
|
Wu AM, Yazaki PJ, Tsai Sw, Nguyen K,
Anderson AL, McCarthy DW, Welch MJ, Shively JE, Williams LE,
Raubitschek AA, Wong JY, Toyokuni T, Phelps ME and Gambhir SS:
High-resolution microPET imaging of carcinoembryonic
antigen-positive xenografts by using a copper-64-labeled engineered
antibody fragment. Proc Natl Acad Sci USA. 97:8495–8500. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Williams HA, Robinson S, Julyan P, Zweit J
and Hastings D: A comparison of PET imaging characteristics of
various copper radioisotopes. Eur J Nucl Med Mol Imaging.
32:473–480. 2005. View Article : Google Scholar
|
|
30
|
Kim JY, Park H, Lee JC, Kim KM, Lee KC, Ha
HJ, Choi TH, An GI and Cheon GJ: A simple Cu-64 production and its
application of Cu-64 ATSM. Appl Radiat Isot. 67:1190–1194. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Craft JM, De Silva RA, Lears KA, Andrews
R, Liang K, Achilefu S and Rogers BE: In vitro and in vivo
evaluation of a 64Cu-labeled NOTA-Bn-SCN-Aoc-bombesin
analogue in gastrin-releasing peptide receptor expressing prostate
cancer. Nucl Med Biol. 39:609–616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y, Hu X, Liu H, Bu L, Ma X, Cheng K,
Li J, Tian M, Zhang H and Cheng Z: A comparative study of
radiolabeled bombesin analogs for the PET imaging of prostate
cancer. J Nucl Med. 54:2132–2138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mansi R, Wang X, Forrer F, Waser B,
Cescato R, Graham K, Borkowski S, Reubi JC and Maecke HR:
Development of a potent DOTA-conjugated bombesin antagonist for
targeting GRPr-positive tumours. Eur J Nucl Med Mol Imaging.
38:97–107. 2011. View Article : Google Scholar
|
|
34
|
Roivainen A, Kähkönen E, Luoto P,
Borkowski S, Hofmann B, Jambor I, Lehtiö K, Rantala T, Rottmann A,
Sipilä H, Sparks R, Suilamo S, Tolvanen T, Valencia R and Minn H:
Plasma pharmacokinetics, whole-body distribution, metabolism, and
radiation dosimetry of 68Ga bombesin antagonist BAY
86-7548 in healthy men. J Nucl Med. 54:867–872. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kähkönen E, Jambor I, Kemppainen J, Lehtiö
K, Grönroos TJ, Kuisma A, Luoto P, Sipilä HJ, Tolvanen T, Alanen K,
Silén J, Kallajoki M, Roivainen A, Schäfer N, Schibli R, Dragic M,
Johayem A, Valencia R, Borkowski S and Minn H: In vivo imaging of
prostate cancer using [68Ga]-labeled bombesin analog
BAY86-7548. Clin Cancer Res. 19:5434–5443. 2013. View Article : Google Scholar
|
|
36
|
Jackson AB, Nanda PK, Rold TL, Sieckman
GL, Szczodroski AF, Hoffman TJ, Chen X and Smith CJ:
64Cu-NO2A-RGD-Glu-6-Ahx-BBN(7-14)NH2: a heterodimeric
targeting vector for positron emission tomography imaging of
prostate cancer. Nucl Med Biol. 39:377–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park JA and Kim JY: Recent advances in
radiopharmaceutical application of matched-pair radiometals. Curr
Top Med Chem. 13:458–469. 2013. View Article : Google Scholar : PubMed/NCBI
|