Introduction
Although the incidence rate for gastric cancer has
steadily declined in past decades, gastric cancer is the second
leading cause of cancer-related deaths, with one of the highest
mortality rates in the world (1,2).
Therefore, identifying the molecular changes that occur in gastric
carcinogenesis would contribute to an improved understanding of the
pathogenesis of this disease, and would offer the potential for
subsequent advances in prevention, earlier diagnosis and perhaps
better intervention strategies.
Cancer progression, such as tumor invasion and
metastasis results from the accumulation of alterations in genes
involved in cell proliferation, mitogenesis, invasiveness and
angiogenesis as well as in the inhibition of apoptosis (3–5). In
particular, it has been widely accepted that tumor cell
proliferation and inhibition of apoptosis are the most crucial
steps in tumor invasion and metastasis (6,7).
The Bcl-2 (B-cell lymphoma-2) family of proteins,
which consists of anti-apoptotic and pro-apoptotic members, is a
critical regulator for the mitochondrial pathway of apoptosis
through controlling the integrity of the outer mitochondrial
membrane (8). Bcl-2 family members
can be divided into three groups, based on their conserved homology
domains, named the Bcl-2 homology (BH) domains. The first group is
anti-apoptotic proteins, which have all four BH domains (BH1-BH4)
including Bcl-2, Bcl-xL, myeloid cell leukemia-1 (Mcl-1), Bcl-w and
A1. The second group is pro-apoptotic proteins, which contain three
conserved domains (BH1–BH3) including Bax, Bak and Bok. The third
group is the BH3-only pro-apoptotic proteins including Bid, Bim,
Puma, Noxa, Bad, Bmf, Hrk and Bik (8).
Anti-apoptotic Bcl-2 family members promote cell
survival by inhibiting pro-apoptotic proteins, directly binding and
blocking activation of caspases by cytochrome C and preserving the
integrity of the mitochondrial outer membrane against apoptotic
stimuli. Anti-apoptotic Bcl-2 family members are known to be
expressed at high levels in variable human cancers, and allow cells
to evade apoptotic signals and attain a neoplastic state (9–11).
Mcl-1 is a potent anti-apoptotic Bcl-2 protein and
exerts its anti-apoptotic function by heterodimerizing with other
Bcl-2 family members and preventing the permeabilization of the
mitochondrial outer membrane (12). It is overexpressed in many human
cancers and can confer resistance to apoptotic signaling and
treatment (13,14). In addition, the expression of Mcl-1
is associated with tumor progression and adverse patient outcome in
many human cancers including gastric cancer (15–21).
Recently, several molecules that specifically inhibit Mcl-1 have
been discovered, providing a potential role for Mcl-1 as a
therapeutic target in cancer (22–25).
The aims of current study were to evaluate whether
Mcl-1 affects the survival or death of gastric cancer cells, and to
investigate the prognostic value of its expression in gastric
cancer.
Materials and methods
Patients and tissue samples
For this study, formalin-fixed, paraffin-embedded
tissue blocks were obtained from 139 randomly chosen patients who
had undergone surgery for gastric cancer at the Chonnam National
University Hwasun Hospital (Jeonnam, Korea) between January 1997
and December 1998. Patients who had received preoperative
chemotherapy or irradiation before surgery were excluded from this
study. The clinicopathologic parameters at the time of surgery were
reviewed through the medical records. Tumor staging was conducted
in accordance with the American Joint Committee on Cancer (AJCC)
staging system (26). Survival was
measured from the time of surgery until follow-up on December 31,
2012. This study was approved by the Institutional Review Board of
Chonnam National University Hwasun Hospital.
Cell culture and siRNA transfection
Human gastric cancer cell lines, SNU638 and TMK1
were obtained from the American Type Culture Collection Line Inc.
Cell lines were cultured in RPMI-1640 (Hyclon, Loan, UT, USA)
supplemented with 10% fetal bovine serum and antibiotics. Mcl-1 and
scramble siRNA were purchased from Bioneer (Daejeon, Korea) and
Qiagen (Germantown, MD, USA), respectively. Mcl-1 construction was
subcloned into a pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA).
The transfection of the specific gene was performed with
Lipofectamine™ RNAiMAX (Invitrogen) and Fugene 6 (Promega, Madison,
WI, USA) according to the manufacturer’s recommendations,
respectively. In addition, gene-transfected cells were selectively
treated with pharmacological caspase inhibitor, Z-VAD-FMK (5 μM/ml,
MBL, Woburn, MA, USA) and 5-Fluorouracil (5-FU) (10 μg/ml,
Choong-Wae, Chung-Nam, Korea).
Western blotting
Total cell proteins were prepared with
RIPA® reagent (Thermo Scientific, Rockford, IL, USA).
Cytosolic and mitochondrial proteins were isolated with the
Mitochondria Isolation kit (Thermo Scientific) according to the
manufacturer’s recommendations. The protein was subjected to
SDS-polyacrylamide electrophoresis and electrotransferred onto a
PVDF membrane. Blots were visualized by the luminescent image
analyzer LAS-4000 (Fujifilm, Tokyo, Japan) with an enhanced
chemiluminescence detection system HRP substrate. The following
antibodies were used; antibodies against Mcl-1, cleaved caspase-3,
-7, cleaved poly (ADP-ribose) polymerase (PARP), second
mitochondria-derived activator of caspases/direct inhibitors of
apoptosis protein (IAP) binding protein with low PI (Smac/DIABLO),
Omi/high-temperature requirement protein A2 (Omi/HtrA2), CoxIV,
cyclin D1, cyclin D3, phospho-cell division cycle gene 2
(phospho-cdc2), cyclin-dependent kinase 4 (CDK4), CDK6, p21, p27,
phospho-extracellular signal-regulated kinase1/2 (phospho-ERK1/2),
phospho-glycogen synthase kinase-3β (phopho-GSK3β), phospho-AKT,
Janus kinase 2 (JAK2), phospho-JAK2, Signal transducers and
activators of transcription-3 (STAT3), and phopho-STAT3. They were
purchased from Cell Signaling Technology (Danvers, MA, USA).
Antibodies against β-tubulin and GAPDH were used and purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell proliferation assay
Proliferation of transfected cells was measured with
the EZ-CyTox Cell Viability Assay kit (Daeil Lab Inc., Seoul,
Korea), which contains WST-1. EZ-CyTox cell viability reagent was
applied at 37°C and allowed to develop. Cell viability was measured
at 490 nm absorbance using a microplate reader (Infinite M200,
Tecan, Austria GmbH, Austria). All experiments were conducted at
least in triplicate.
Flow cytometric analysis
For the apoptosis assay, attached cells were
collected and stained in APC Annexin V and 7-amino-actinomycin D
(7-AAD) (BD Biosciences, San Diego, CA, USA). For cell cycle
analysis, cells were incubated in ribonuclease A (Sigma-Aldrich,
St. Louis, MO, USA) and propidium iodide (PI) (Sigma-Aldrich). The
apoptotic cells and cell cycle phase were analyzed on the BD Cell
Quest® version 3.3 (Becton Dickinson, San Jose, CA, USA)
and WinMDI version 2.9 (The Scripps Research Institute, San Diego,
CA, USA), respectively.
Immunohistochemical analysis
Immunostaining was performed using a Dako Real™
Envision HRP/DAB detection system (Dako Cytomation, Glostrup,
Denmark). After antigen retrieval, the endogenous peroxidase
activity was blocked with a peroxidase-blocking solution and
incubated with polyclonal rabbit anti-human Mcl-1, Ki-67
(Dakopatts, Glostrup, Denmark) in primary diluent solution
(Invitrogen) overnight at 4°C. After rinsing in TBST, tissues were
stained using 3,3-diaminobenzidine (DAB) liquid, counterstained
with Mayer hematoxylin (Sigma-Aldrich). Stained tissues were viewed
and photographed using a light microscope (Olympus, Tokyo,
Japan).
Evaluation of Mcl-1 expression
Immunoreactivity of Mcl-1 expression was evaluated
independently on the basis of the intensity and extent of staining
by two observers without knowledge of the clinicopathological data.
The staining intensity was graded on a scale of four: 0, no
staining of cancer cells; 1, weak staining; 2, moderate staining;
3, strong staining. The staining extent was also graded on a scale
of four: 0, none; 1, <10%; 2, 10–50%; 3, >50%. The intensity
rating was multiplied by the extent rating to obtain a mean overall
score. The mean overall score of 4.0 was chosen as the cut-off
point for the positive status of Mcl-1 expression.
Assessment of tumor cell proliferation
and apoptosis
The proliferative ability of tumor cells was
presented with the Ki-67 labeling index (KI). The KI was determined
by the number of Ki-67-positive nuclei per 1000 tumor cell nuclei.
For the detection and quantification of apoptosis, the DeadEnd™
Colorimetric terminal deoxynucleotidyl transferase dUTP nick end
labeling (TUNEL) system (Promega) was used, following the
instructions of the manufacturer. TUNEL-positive, darkly stained
nuclei or nuclear fragments with a cytoplasmic halo were considered
as positive apoptotic cells. The apoptotic index (AI) was expressed
as a number of TUNEL positive nuclei including apoptotic body among
1000 tumor cell nuclei.
Statistical analysis
Statistical analysis was performed with the
Statistical Package for the Social Sciences (SPSS/PC+ 15.0,
Chicago, IL, USA). The expression of Mcl-1 with relation to various
clinicopathological parameters was assessed with the χ2
test and Fisher’s exact test. The survival rates of patients was
estimated with the Kaplan-Meier method and analyzed using a
log-rank test. The relationship between Mcl-1 expression and KI or
AI was evaluated by the Student’s t-test. In the in vitro
experiments, statistical significance was determined by a Student’s
t-test. Differences were considered significant when p≤0.05.
Results
Impact of Mcl-1 on apoptosis in gastric
cancer cells
To explore the effect of Mcl-1 on the survival or
death of gastric cancer cells, Mcl-1 siRNA or pcDNA3.1-Mcl-1 were
used to silence or overexpress Mcl-1 expression in gastric cancer
cell lines, SNU638 and TMK1, respectively. Mcl-1 expression in all
tested cells, showed a specific decrease by transfection with Mcl-1
siRNA and a specific increase by transfection with pcDNA3.1-Mcl-1
(data not shown). To study the effect of Mcl-1 on apoptosis, we
performed flow cytometric analyses. The cell apoptotic rate induced
by transfection of Mcl-1 siRNA significantly increased, compared
with that induced by transfection of the scramble siRNA (20.8 and
9.2 vs. 27.2 and 19.4%) in the SNU638 and TMK1 cells, and these
increases were blocked by treatment with the pharmacological
caspase inhibitor Z-VAD-FMK (Fig.
1A). 5-FU is well known to induce apoptosis and affect the cell
cycle of cancer cells. Overexpression of Mcl-1 by transfection of
pcDNA3.1-Mcl-1 inhibited the apoptosis of the SNU638 and TMK1 cells
in response to 5-FU (Fig. 1B).
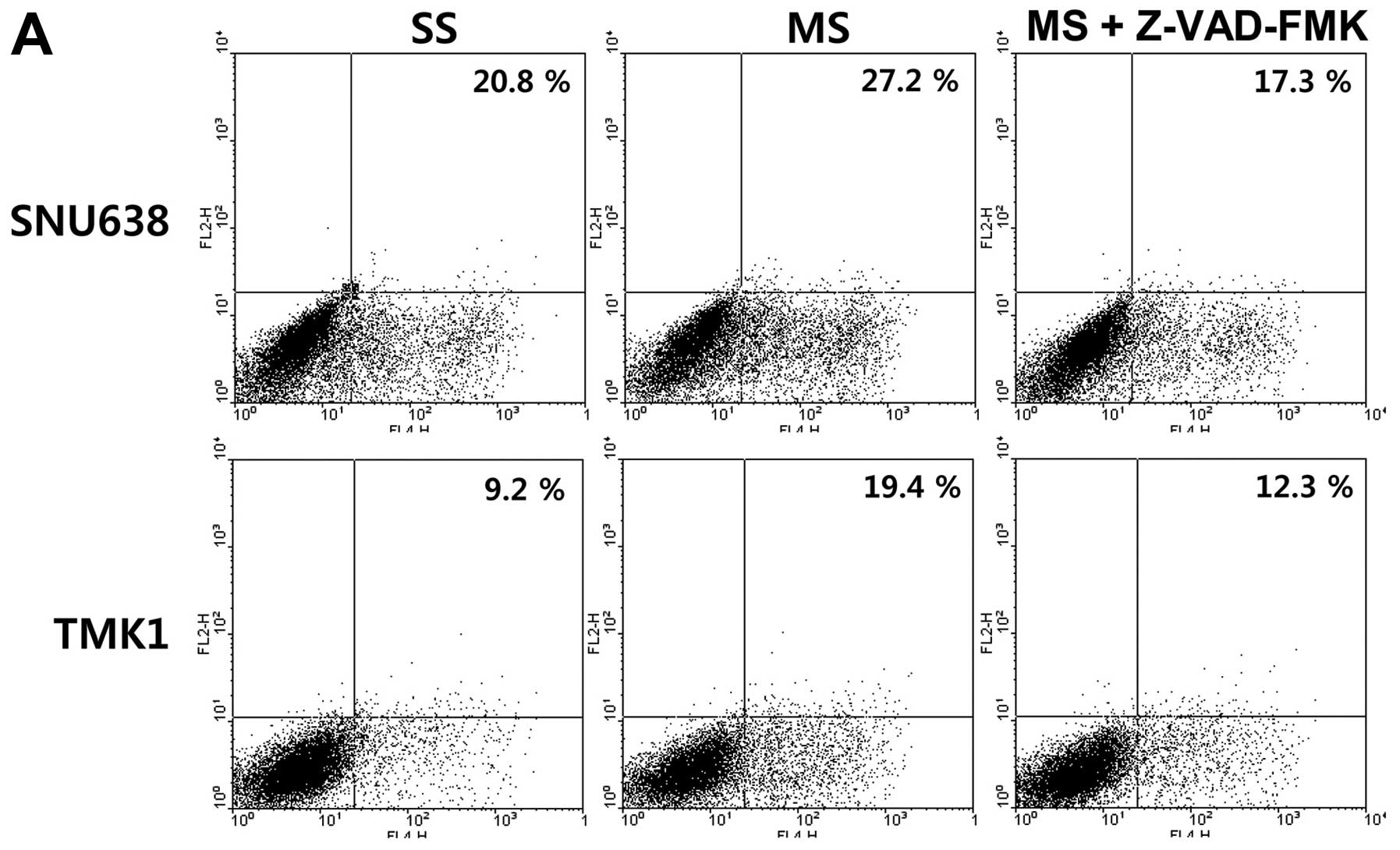 | Figure 1The impact of Mcl-1 on apoptosis in
gastric cancer cells. (A) The cell apoptotic rate induced by the
transfection of Mcl-1 siRNA significantly increased, compared with
that induced by the transfection of the scramble siRNA (20.8 and
9.2 vs. 27.2 and 19.4%) in the SNU638 and TMK1 cells, and the
increases were blocked by treatment with a pharmacological caspase
inhibitor, Z-VAD-FMK. (B) Overexpression of Mcl-1 by transfection
of pcDNA3.1-Mcl-1 inhibited the apoptosis of SNU638 and TMK1 cells
in response to 5-FU. (C) The cleaved caspase-3, -7, and PARP
expression levels were upregulated in the SNU638 and TMK1 cells
after Mcl-1 knockdown and downregulated after the overexpression of
Mcl-1. (D) The release of Smac/DIABLO and Omi/HtrA2 into the
cytoplasm was induced by Mcl-1 knockdown and inhibited by the
overexpression of Mcl-1. PARP, poly (ADP-ribose) polymerase;
Smac/DIABLO, second mitochondria-derived activator of
caspases/direct IAP binding protein with low pI; Omi/HtrA2,
Omi/high-temperature requirement protein A2; SS, scramble siRNA;
MS, Mcl-1 siRNA; EV, empty-pcDNA3.1; MV, pcDNA3.1-Mcl-1; 5-FU,
5-fluorouracil. |
To determine the activation of caspases during
knockdown and overexpression of Mcl-1, we further investigated
caspase-specific activities. The cleaved caspase-3, -7, and PARP
expression levels were upregulated in the SNU638 and TMK1 cells
after Mcl-1 knockdown and downregulated after overexpression of
Mcl-1 (Fig. 1C). The release of
Smac/DIABLO and HtrA2/Omi from the mitochondrial membrane to the
cytoplasm triggers activation of the caspase cascade and ultimately
induces apoptosis. To analyze the release of Smac/DIABLO and
Omi/HtrA2 to the cytoplasm due to Mcl-1 expression, we examined the
expression of SmacC/DIABLO and Omi/HtrA2 in the cytoplasmic and
mitochondrial proteins using western blots. The release of
Smac/DIABLO and Omi/HtrA2 into the cytoplasm was induced by Mcl-1
knockdown and inhibited by the overexpression of Mcl-1 (Fig. 1D).
Impact of Mcl-1 on cell cycle
distribution in gastric cancer cells
To detect whether Mcl-1 could change cell cycle
distribution, we performed flow cytometric analyses. Mcl-1
knockdown resulted in cell cycle arrest in the sub G1 phase of
SNU638 and TMK1 cells. Cell cycle arrest in the sub-G1 phase
induced by 5-FU treatment was inhibited by the overexpression of
Mcl-1 (Fig. 2A). Next, we
evaluated the effects of Mcl-1 on various cell cycle regulatory
proteins in gastric cancer cells. As shown in Fig. 2B, the cyclin D1, P-cdc2, CDK4 and
CDK6 protein levels were significantly decreased by Mcl-1
knockdown, and increased by the overexpression of Mcl-1 in SNU638
and TMK1 cells. However, p21 and p27 protein levels remained
unchanged by knockdown and overexpression of Mcl-1.
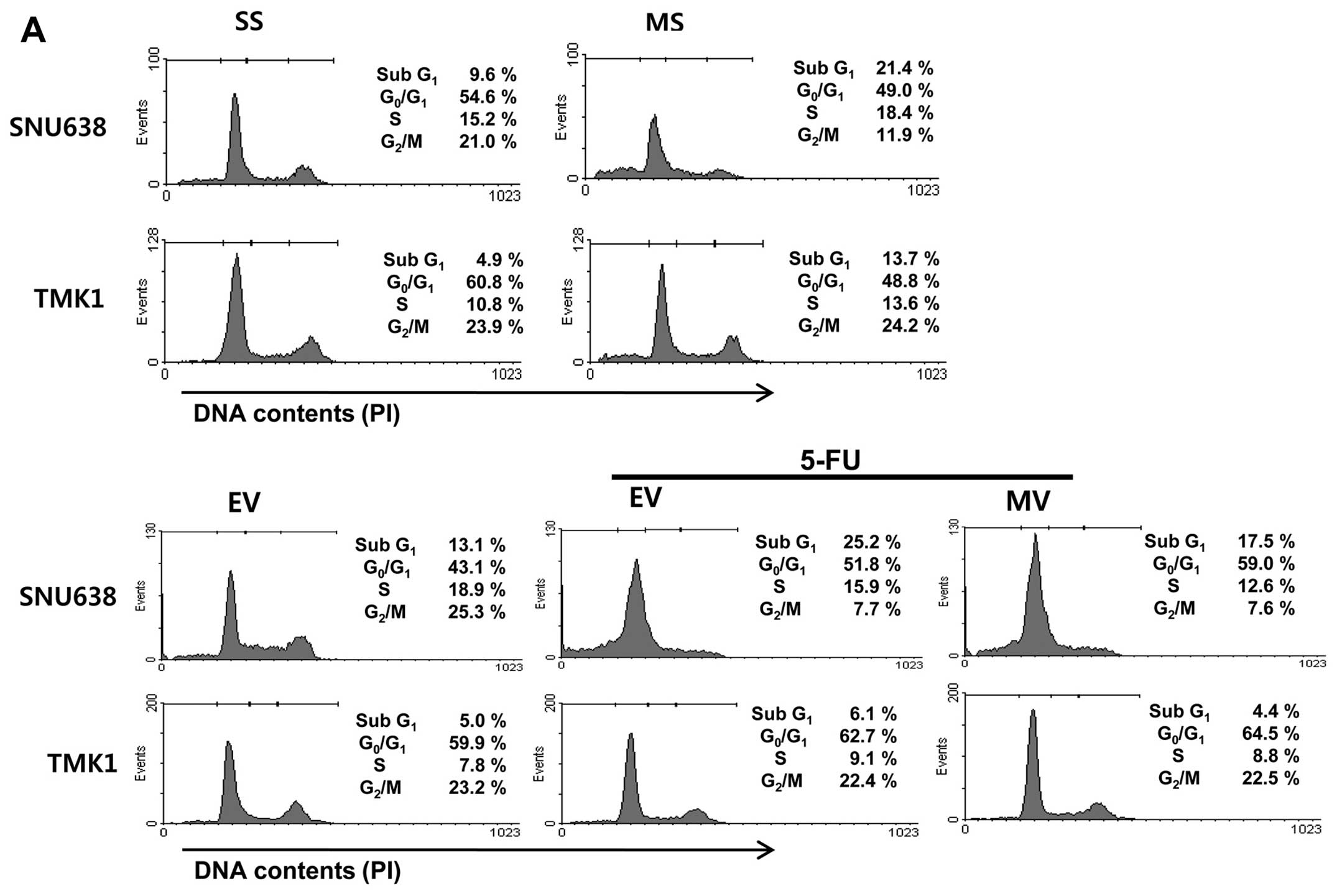 | Figure 2The impact of Mcl-1 on cell cycle
distribution in gastric cancer cells. (A) Mcl-1 knockdown resulted
in cell cycle arrest in the sub G1 phase of SNU638 and TMK1 cells.
The cell cycle arrest in the sub-G1 phase induced by 5-FU treatment
was inhibited by the overexpression of Mcl-1. (B) The cyclin D1,
P-cdc2, CDK4 and CDK6 protein levels were significantly decreased
by Mcl-1 knockdown, and increased by the overexpression of Mcl-1 in
SNU638 and TMK1 cells. However, p21 and p27 protein levels remained
unchanged by knockdown and overexpression of Mcl-1. SS, scramble
siRNA; MS, Mcl-1 siRNA; EV, empty-pcDNA3.1; MV, pcDNA3.1-Mcl-1;
5-FU, 5-fluorouracil; cdc2, cell division cycle gene 2; CDK,
cyclin-dependent kinase. |
Impact of Mcl-1 on the proliferation of
gastric cancer cells
The proliferating cells, as determined by
absorbance, were significantly increased in the
pcDNA3.1-Mcl-1-transfected SNU638 cells compared to the
empty-pcDNA3.1-transfected cells at 3 and 4 days (p=0.045 and
0.049, respectively), but there was no significant difference in
the TMK1 cells. Moreover, in all tested cells, there was no
significant difference in cell proliferation between Mcl-1 siRNA
and scramble siRNA transfected cells (Fig. 3).
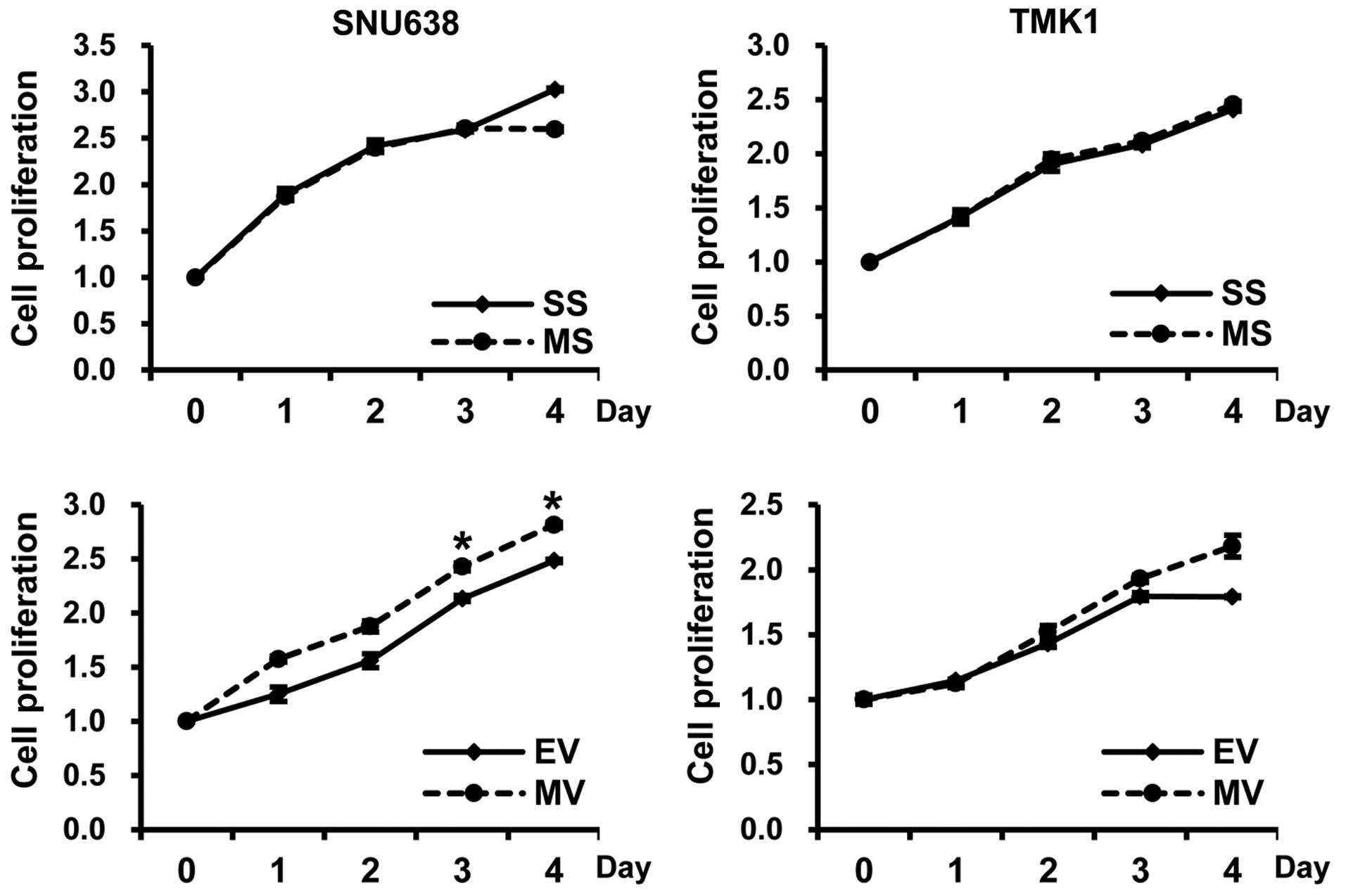 | Figure 3The impact of Mcl-1 on the
proliferation of gastric cancer cells. The proliferating cells, as
determined by absorbance, were significantly increased in the
pcDNA3.1-Mcl-1-transfected SNU638 cells compared to the
empty-pcDNA3.1-transfected cells at 3 and 4 days (mean ± SE, n=3;
*p<0.05), but there was no significant difference in
TMK1 cells. In addition, in all tested cells, there was no
significant difference regarding cell proliferation between Mcl-1
siRNA and scramble siRNA transfected cells. SS, scramble siRNA; MS,
Mcl-1 siRNA; EV, empty-pcDNA3.1; MV, pcDNA3.1-Mcl-1. |
Impact of Mcl-1 on intracellular
signaling pathways involved in the apoptosis and cell cycle
distribution of gastric cancer cells
To explore the potential mechanisms involved in
apoptosis and cell cycle distribution, we studied the effect of
Mcl-1 on the stimulation of intracellular signaling pathways
leading to apoptosis and cell cycle distribution in SNU638 and TMK1
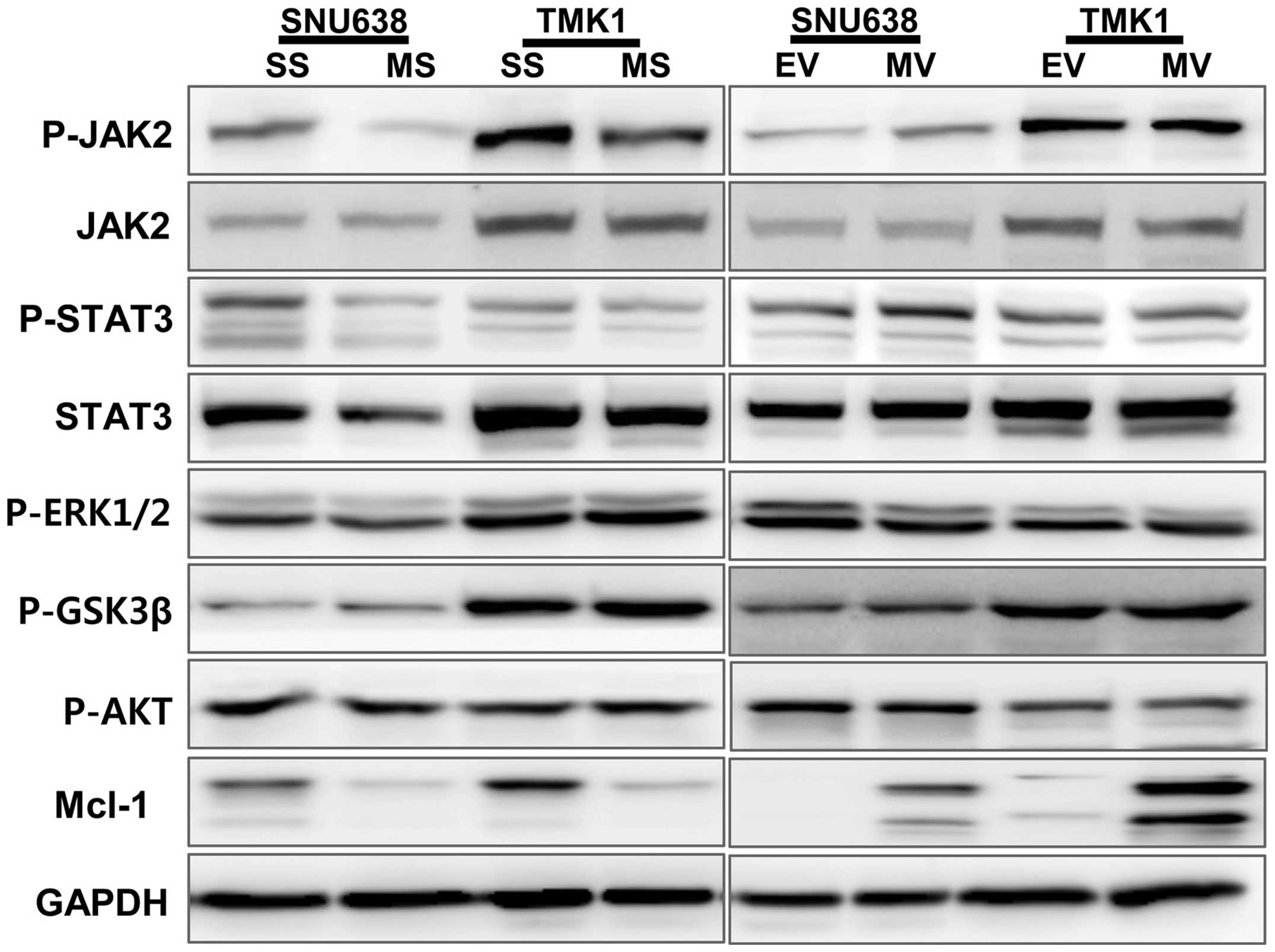
cells. The phosphorylation levels of JAK2 and STAT3 were
downregulated by Mcl-1 knockdown SNU638 and TMK1 cells, and the
overexpression of Mcl-1 upregulated the phosphorylation of JAK2 and
STAT3 in SNU638 cells (Fig. 4).
The phosphorylation levels of ERK1/2, GSK3β and AKT were not
changed by knockdown and overexpression of Mcl-1.
Correlations between the expression of
Mcl-1 and clinicopathological features in gastric cancers
To study the prognostic role of Mcl-1 in human
gastric cancer progression, we investigated the expression of the
Mcl-1 protein immunohistochemically in formalin-fixed,
paraffin-embedded tissue blocks obtained from 139 gastric cancer
patients with clinicopathological data. Survival and the
correlation between immunostaining of Mcl-1 and clinicopathological
parameters was analyzed. The immunostaining of Mcl-1 protein did
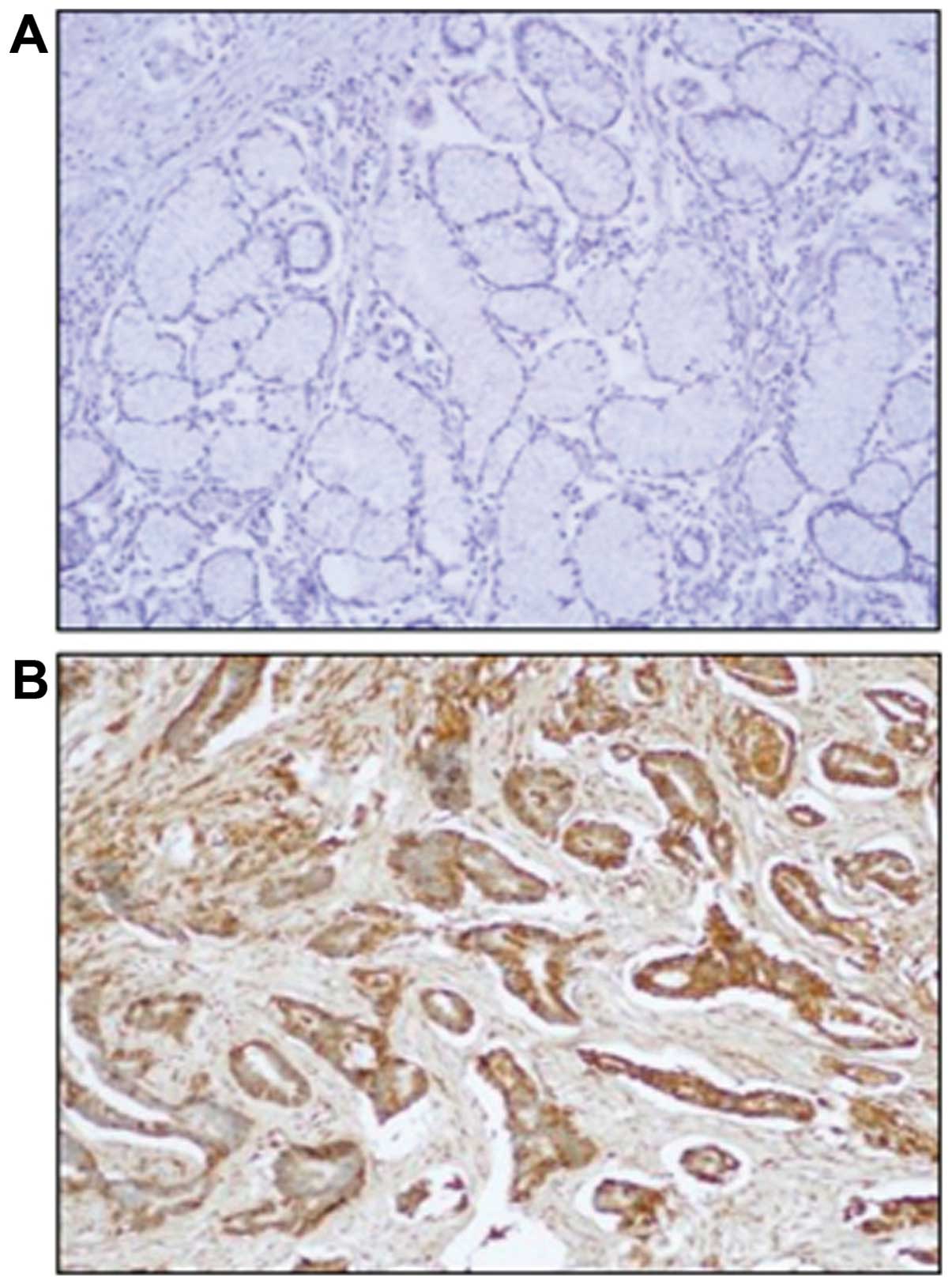
not or weakly stained the normal gastric mucosa (Fig. 5A). The immunostaining of Mcl-1
protein was predominantly identified in the cytoplasm of cancer
cells and was not detectable in the tumor stroma (Fig. 5A). The percentage of positive tumor
cells and the staining intensity for each sample were recorded. For
the 139 patient samples evaluated, positive Mcl-1 expression was
observed in 41.0% of the gastric cancer tissues (57/139) (Table I). Immunostaining of Mcl-1 was
significantly associated with age, tumor size, stage, depth of
invasion and lymph node metastasis (p=0.001, 0.013, 0.003, 0.022
and 0.007, respectively) (Table
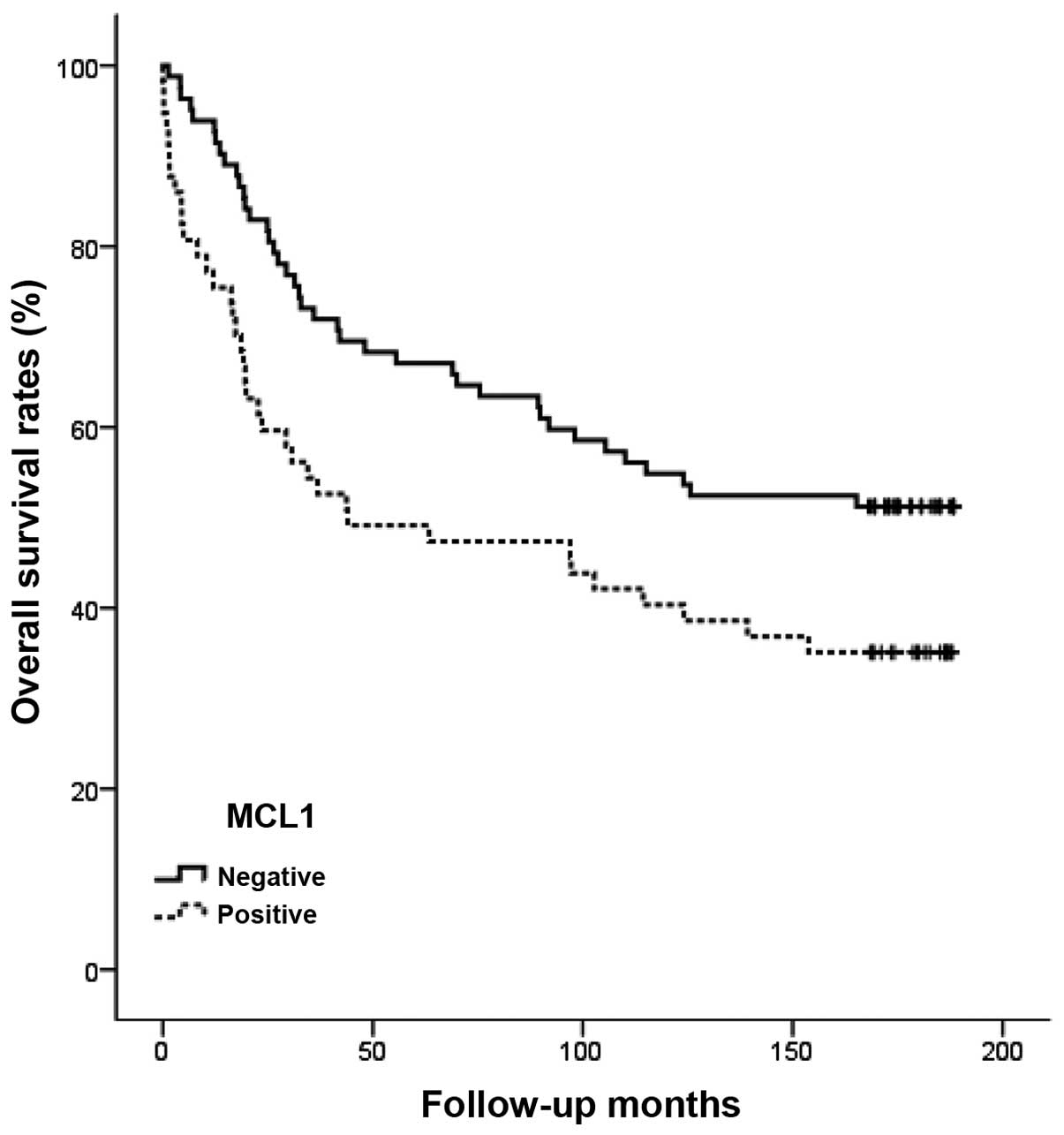
I). Moreover, overall survival for patients with positive Mcl-1
immunostaining was significantly shorter than for the negative
patients (p=0.020) (Fig. 6).
 | Table ICorrelation between Mcl-1 expression
and the clinicopathological parameters of gastric cancer. |
Table I
Correlation between Mcl-1 expression
and the clinicopathological parameters of gastric cancer.
| | Mcl-1 | |
|---|
| |
| |
|---|
| Characteristics | Total (n=139) | Neg. (n=82) | Pos. (n=57) | p-value |
|---|
| Age (years) | | | | 0.001 |
| <57 | 57 | 43 | 14 | |
| ≥57 | 82 | 39 | 43 | |
| Gender | | | | 0.366 |
| Male | 94 | 53 | 41 | |
| Female | 45 | 29 | 16 | |
| Tumor size
(cm) | | | | 0.013 |
| <4.0 | 83 | 56 | 27 | |
| ≥4.0 | 56 | 26 | 30 | |
| Stage | | | | 0.003 |
| I | 66 | 46 | 20 | |
| II | 19 | 14 | 5 | |
| III | 34 | 16 | 18 | |
| IV | 20 | 6 | 14 | |
| Lauren
classification | | | | 0.054 |
| Intestinal | 84 | 44 | 40 | |
| Diffuse | 54 | 38 | 16 | |
| Mixed | 1 | 0 | 1 | |
| Histologic
type | | | | 0.190 |
| Well
differentiated | 39 | 19 | 20 | |
| Moderately
differentiated | 13 | 6 | 7 | |
| Poorly
differentiated | 81 | 54 | 27 | |
| Signet ring
cell | 6 | 3 | 3 | |
| Depth of invasion
(T) | | | | 0.022 |
| T1 | 59 | 41 | 18 | |
| T2 | 15 | 10 | 5 | |
| T3 | 53 | 28 | 25 | |
| T4 | 12 | 3 | 9 | |
| Lymph node
metastasis (N) | | | | 0.007 |
| N0 | 75 | 52 | 23 | |
| N1–3 | 64 | 30 | 34 | |
| Distant metastasis
(M) | | | | NA |
| M0 | 139 | 82 | 57 | |
| M1 | 0 | 0 | 0 | |
Correlation between the expression of
Mcl-1 and tumor cell proliferation or apoptosis in gastric
cancers
The KI for the 139 tumors ranged from 14.8 to 86.5,
with a mean KI of 49.8±17.8. The mean KI value of Mcl-1 positive
tumors was 56.4±17.2, and significantly lower than the KI of Mcl-1
negative tumors (p=0.018) (Table
II). The AI for 139 tumors ranged from 0 to 6.3 with a mean AI
of 1.8±1.4. There was no significant difference between Mcl-1
expression and AI (p=0.528) (Table
II).
 | Table IICorrelation between Mcl-1 expression
and tumor cell proliferation or apoptosis in gastric cancers. |
Table II
Correlation between Mcl-1 expression
and tumor cell proliferation or apoptosis in gastric cancers.
| | Mcl-1
expression | |
|---|
| |
| |
|---|
| Indices | Total (n=139) | Neg. (n=82) | Pos. (n=57) | p-value |
|---|
| KI (mean ± SD) | 49.8±17.8 | 38.8±13.0 | 56.4±17.2 | 0.018 |
| AI (mean ± SD) | 1.8±1.4 | 1.7±1.7 | 1.9±1.4 | 0.528 |
Discussion
Apoptosis is an essential mechanism for
physiological and pathological cell death. The balance of cell
growth and apoptosis determines normal tissue homeostasis.
Inhibition of apoptosis plays a significant role in cancer
promotion and resistance to chemotherapy (6,7).
Several inhibitors of apoptosis proteins (IAPs) have been
identified, including the Bcl-2, p53 and p73 families (8–11).
Mcl-1 has been identified as a member of anti-apoptotic Bcl-2
family proteins and is frequently expressed in various cancer
tissues (12–14). Our study showed that Mcl-1
knockdown induced apoptosis, while the overexpression of Mcl-1
inhibited apoptosis in gastric cancer cells, suggesting a role of
Mcl-1 in the alteration of the invasive and oncogenic phenotypes of
gastric cancer cells.
Generally, there are two main pathways in apoptosis:
the mitochondrial pathway, which involves the release of cytochrome
C from the mitochondria into the cytoplasm, and the cell surface
pathway, which is stimulated by cell surface death receptors such
as Fas and TNFR. Both pathways share the activation of caspases,
which are considered to be crucial effectors of the cell death
machinery (6,7). In our study, the cleaved caspase-3,
-7, and PARP expression levels were upregulated in gastric cancer
cells after Mcl-1 knockdown and downregulated after the
overexpression of Mcl-1.
Mcl-1 inhibits the progression of apoptosis by
interacting with pro-apoptotic proteins such as Bak and Bax on the
mitochondrial membrane preventing such molecules from dimerizing to
form pro-apoptotic pores, and the subsequent release of cytochrome
C into the cytoplasm (12–14). Recently, it has been found that
Smac/DIABLO and Omi/HtrA2 bind IAPs and promote apoptosis. The
apoptotic activity of Smac/DIABLO and Omi/HtrA2 is regulated by the
release of Smac/DIABLO and Omi/HtrA2 from the mitochondrial
membrane to the cytoplasm, following apoptotic stimuli (27–29).
In our study, the release of Smac/DIABLO and Omi/HtrA2 into the
cytoplasm was induced by Mcl-1 knockdown and inhibited by the
overexpression of Mcl-1. Therefore, the anti-apoptotic mechanism of
Mcl-1 is mediated by the direct inhibition of caspase activity and
is negatively regulated by the endogenous IAPs antagonist
Smac/DIABLO and Omi/HtrA2 in gastric cancer cells.
The cell cycle is the cascade of events that promote
a growing cell to duplicate all its components and ultimately split
into two daughter cells. The cell cycle is governed by CDK, and the
activity is regulated by the positive regulators, including the
cyclins and CDK binding proteins, by negative regulators, including
the CDKIs, and by phosphorylation and dephosphorylation events
(30,31). In addition, Cdc2 is one of the most
important regulated kinases of cell cycle. Its activity is
regulated positively by cyclin B1 and negatively by CKI (32). Our study showed that knockdown of
Mcl-1 induced cell cycle arrest by decreasing cyclin D1, cdc2, and
CDK 4 and 6 in gastric cancer cells. In contrast, overexpression of
Mcl-1 inhibited cell cycle arrest. However, CDKIs, such as p21 and
p27, remained unchanged by the knockdown and overexpression of
Mcl-1. Alterations in genes involved in the regulation of cell
cycle progression are frequent events in human cancers (30–32).
Therefore, Mcl-1 may contribute to gastric cancer progression via
cell cycle regulation by controlling cyclin, cdc2 and CDK
expression.
Cell proliferation and apoptosis is a tightly
regulated process under the control of multiple intracellular
signaling pathways including AKT, ERK1/2, GSK3β, JAK2 and STAT3
(33–36). In our study, the phosphorylation
levels of JAK2 and STAT3 were significantly blocked by the
knockdown of Mcl-1. Also, the phosphorylation levels of JAK2 and
STAT3 were increased by overexpression of Mcl-1. The results
suggest that Mcl-1 might mainly regulate gastric cancer cell growth
through the JAK2 and STAT3 signaling pathways.
Previously, expression of Mcl-1 has been reported to
be highly expressed in various human cancer types and has also been
associated with tumor progression and adverse clinical outcomes
(15–21). In our study, Mcl-1 expression was
significantly increased in gastric cancer tissues compared to
normal gastric mucosa tissues, and associated with age, tumor size,
stage, depth of invasion, lymph node metastasis and poor survival.
This result is in agreement with the results of previous studies
(18–20). Previously, silencing the Mcl-1 gene
using antisense oligonulceotides produced a significant increase in
apoptosis and decrease in cell growth, and in combination with
chemotherapy displayed synergistic antitumor activity in gastric
cancer cells (37,38). Therefore, Mcl-1 may be considered
as a potential therapeutic target in the treatment of colorectal
cancer.
Tumor volume is balanced by tumor cell proliferation
and apoptosis, and while both activities often increase in tandem,
proliferative activity is generally linked to tumor progression
(3–7). Therefore, we evaluated the
correlation between Mcl-1 expression and tumor cell proliferation
or apoptosis in gastric cancer tissues. In our study, the mean KI
value of Mcl-1-positive tumors was significantly lower than that of
Mcl-1-negative tumors. However, there was no significant difference
between Mcl-1 expression and AI.
Taken together, we found that Mcl-1 plays an
important role in human gastric cancer progression by modulating
tumor cell proliferation and apoptosis and may be used as a
molecular marker for the prediction of clinical outcomes in gastric
cancer.
Acknowledgements
This work was supported by research funds from the
Research Institute of Clinical Medicine, Chonnam National
University Hospital in 2013 (CRI 13025-1), Republic of Korea.
References
|
1
|
Guggenheim DE and Shah MA: Gastric cancer
epidemiology and risk factors. J Surg Oncol. 107:230–236. 2013.
View Article : Google Scholar
|
|
2
|
Krejs GJ: Gastric cancer: Epidemiology and
risk factors. Dig Dis. 28:600–603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Allan AL, Vantyghem SA, Tuck AB and
Chambers AF: Tumor dormancy and cancer stem cells: Implications for
the biology and treatment of breast cancer metastasis. Breast Dis.
26:87–98. 2007.PubMed/NCBI
|
|
4
|
Brábek J, Mierke CT, Rösel D, Veselý P and
Fabry B: The role of the tissue microenvironment in the regulation
of cancer cell motility and invasion. Cell Commun Signal. 8:222010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kiechle FLand Zhang X: Apoptosis:
biochemical aspects and clinical implications. Clin Chim Acta.
326:27–45. 2002. View Article : Google Scholar
|
|
7
|
Schultz DR and Harrington WJ Jr:
Apoptosis: Programmed cell death at a molecular level. Semin
Arthritis Rheum. 32:345–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Llambi F and Green DR: Apoptosis and
oncogenesis: Give and take in the BCL-2 family. Curr Opin Genet
Dev. 21:12–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weyhenmeyer B, Murphy AC, Prehn JH and
Murphy BM: Targeting the anti-apoptotic Bcl-2 family members for
the treatment of cancer. Exp Oncol. 34:192–199. 2012.PubMed/NCBI
|
|
11
|
Davids MS and Letai A: Targeting the
B-cell lymphoma/leukemia 2 family in cancer. J Clin Oncol.
30:3127–3135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomas LW, Lam C and Edwards SW: Mcl-1;
the molecular regulation of protein function. FEBS Lett.
584:2981–2989. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akgul C: Mcl-1 is a potential therapeutic
target in multiple types of cancer. Cell Mol Life Sci.
66:1326–1336. 2009. View Article : Google Scholar
|
|
14
|
Quinn BA, Dash R, Azab B, et al: Targeting
Mcl-1 for the therapy of cancer. Expert Opin Investig Drugs.
20:1397–1411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang T, Zhao C, Luo L, Zhao H, Cheng J
and Xu F: The expression of Mcl-1 in human cervical cancer and its
clinical significance. Med Oncol. 29:1985–1991. 2012. View Article : Google Scholar
|
|
16
|
Luo L, Zhang T, Liu H, Lv T, Yuan D, Yao
Y, Lv Y and Song Y: MiR-101 and Mcl-1 in non-small-cell lung
cancer: Expression profile and clinical significance. Med Oncol.
29:1681–1686. 2012. View Article : Google Scholar
|
|
17
|
Henderson-Jackson EB, Helm J, Ghayouri M,
Hakam A, Nasir A, Leon M, Bui M, Yeatman T and Coppola D:
Correlation between Mcl-1 and pAKT protein expression in colorectal
cancer. Int J Clin Exp Pathol. 3:768–774. 2010.PubMed/NCBI
|
|
18
|
Likui W, Qun L, Wanqing Z, Haifeng S,
Fangqiu L and Xiaojun L: Prognostic role of myeloid cell leukemia-1
protein (Mcl-1) expression in human gastric cancer. J Surg Oncol.
100:396–400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maeta Y, Tsujitani S, Matsumoto S, et al:
Expression of Mcl-1 and p53 proteins predicts the survival of
patients with T3 gastric carcinoma. Gastric Cancer. 7:78–84. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsujitani S, Saito H, Wakatsuki T,
Ikeguchi M, Shirabe K, Morita M, Kakeji Y, Yano T and Maehara Y:
Relationship between expression of apoptosis-related proteins and
the efficacy of postoperative chemotherapy in patients with T3
gastric cancer. Surg Today. 42:225–232. 2012. View Article : Google Scholar
|
|
21
|
Akagi H, Higuchi H, Sumimoto H, et al:
Suppression of myeloid cell leukemia-1 (Mcl-1) enhances
chemotherapy-associated apoptosis in gastric cancer cells. Gastric
Cancer. 16:100–110. 2013. View Article : Google Scholar
|
|
22
|
Mandelin AM II and Pope RM: Myeloid cell
leukemia-1 as a therapeutic target. Expert Opin Ther Targets.
11:363–373. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Perciavalle RM and Opferman JT: Delving
deeper: MCL-1’s contributions to normal and cancer biology. Trends
Cell Biol. 23:22–29. 2013. View Article : Google Scholar
|
|
24
|
Hartman ML and Czyz M: Anti-apoptotic
proteins on guard of melanoma cell survival. Cancer Lett.
331:24–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bose P and Grant S: Mcl-1 as a therapeutic
target in acute myelogenous leukemia (AML). Leuk Res Rep. 2:12–14.
2013.PubMed/NCBI
|
|
26
|
Frederick L, Greene D and Irvin D: AJCC
cancer staging manual. 6th edition. Springer-Verlag; New York: pp.
17–57. 2002
|
|
27
|
Obexer P and Ausserlechner MJ: X-linked
inhibitor of apoptosis protein - a critical death resistance
regulator and therapeutic target for personalized cancer therapy.
Front Oncol. 4:1972014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Almagro MC and Vucic D: The inhibitor
of apoptosis (IAP) proteins are critical regulators of signaling
pathways and targets for anti-cancer therapy. Exp Oncol.
34:200–211. 2012.PubMed/NCBI
|
|
29
|
Kilbride SM and Prehn JH: Central roles of
apoptotic proteins in mitochondrial function. Oncogene.
32:2703–2711. 2013. View Article : Google Scholar
|
|
30
|
Graña X and Reddy EP: Cell cycle control
in mammalian cells: Role of cyclins, cyclin dependent kinases
(CDKs), growth suppressor genes and cyclin-dependent kinase
inhibitors (CKIs). Oncogene. 11:211–219. 1995.PubMed/NCBI
|
|
31
|
Soták M, Sumová A and Pácha J: Cross-talk
between the circadian clock and the cell cycle in cancer. Ann Med.
46:221–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fisher D, Krasinska L, Coudreuse D and
Novák B: Phosphorylation network dynamics in the control of cell
cycle transitions. J Cell Sci. 125:4703–4711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu HG, Ai YW, Yu LL, et al:
Phosphoinositide 3-kinase/Akt pathway plays an important role in
chemoresistance of gastric cancer cells against etoposide and
doxorubicin induced cell death. Int J Cancer. 122:433–443. 2008.
View Article : Google Scholar
|
|
34
|
Choi IJ, Kim JS, Kim JM, Jung HC and Song
IS: Effect of inhibition of extracellular signal-regulated kinase 1
and 2 pathway on apoptosis and bcl-2 expression in Helicobacter
pylori-infected AGS cells. Infect Immun. 71:830–837. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McCubrey JA, Steelman LS, Bertrand FE, et
al: GSK-3 as potential target for therapeutic intervention in
cancer. Oncotarget. 5:2881–2911. 2014.PubMed/NCBI
|
|
36
|
Amoyel M, Anderson AM and Bach EA:
JAK/STAT pathway dysregulation in tumors: A Drosophila perspective.
Semin Cell Dev Biol. 28:96–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wacheck V, Cejka D, Sieghart W, Losert D,
Strommer S, Crevenna R, Monia BP and Selzer E: Mcl-1 is a relevant
molecular target for antisense oligonucleotide strategies in
gastric cancer cells. Cancer Biol Ther. 5:1348–1354. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zangemeister-Wittke U and Huwiler A:
Antisense targeting of Mcl-1 has therapeutic potential in gastric
cancer. Cancer Biol Ther. 5:1355–1356. 2006. View Article : Google Scholar : PubMed/NCBI
|