Introduction
Hepatocellular carcinoma (HCC) is one of the most
frequent malignant tumors worldwide and ranks as the second most
common cause of cancer-related death in China (1–3). In
recent years, in line with the rising morbidity of hepatitis C
virus infection, the incidence of HCC is increasing in many western
countries including the United States (4–6). At
present, due to the high recurrence rate and early metastasis, the
prognosis of the HCC patients remains very poor (7,8).
Hence, understanding the pathogenesis of HCC and exploring
prognostic markers and therapeutic targets is imperative for the
treatment of HCC.
Activated Cdc42 associated kinase (ACK1, also known
as TNK2) was originally considered as a Cdc42-interacting protein,
and was suggested to be a Cdc42 effector (9,10).
In addition to interaction with Cdc42, other ACK1 interacting
partners include clathrin, ubiquitin, Grb2 and Nedd4-2 E3 ligase
(11,12). As a ubiquitously expressed
non-receptor tyrosine kinase, ACK1 has emerged as an important
transducer of variety of extracellular signals (13). Importantly, ACK1 gene amplification
can cause ACK1 phosphorylation (p-ACK1) and auto-activation, which
results in the activation of ACK1 signal transduction (14,15).
Activated ACK1 senses extracellular signals through interacting
with activated receptor-tyrosine kinases including AKT, EGFR, HER2
and MERTK (15,16). Such interactions result in
ACK1-activated signal transduction through the activation of
multiple downstream effectors including androgen receptor (AR)
(17). Through these ACK1
activated signaling networks, ACK1 participates in cell survival,
invasion, migration and tumorigenesis (18).
WW domain-containing oxidoreductase (WWOX) is a
newly found tumor suppressor protein (19). Extensive research indicates that
WWOX is downregulated in various tumor types including HCC
(19–22). Notably, WWOX is a target of ACK1
signaling in prostate cancer and ACK1 could negatively regulate
WWOX protein expression and promote WWOX degradation (23).
The AKT signaling pathway, is critical in promoting
cell proliferation, invasion and migration, and is frequently
dysregulated in multiply tumor types (24). Furthermore, it has been reported
that AKT signaling plays a key role in facilitating MMP2 and MMP9
expression, which are important factors in the progression of HCC
(25). Noteworthy, while AKT
signaling pathway is understood to a broad extent in several
cancers, new study evidence has emerged demonstrating that AKT
signaling activation can occur in PI3K-independent pattern
(26). Several studies have
already identified a novel mechanism of ACK1 mediated AKT
activation, which leads to AKT signaling translocation to the cell
plasma membrane and subsequent molecule activation (23,26).
However, the mechanism of ACK1-mediated AKT activation in HCC is
not clear.
Several studies have implicated ACK1 amplification
and high-expression in carcinogenesis of multiple tissue types such
as lung, breast and prostate (27–29).
Overexpression level of ACK1 correlates with tumor invasion and
migration in renal and pancreatic cancers (30,31).
Importantly, ACK1 activation- induced robust AKT signaling
activation has been found in various tumor types (23). However, the role and mechanisms
involved in ACK1 are still unknown in human HCC.
In this study, we demonstrate that ACK1 is an
independent prognostic marker for predicting both the overall and
disease- free survival of patients with HCC. ACK1 can negatively
regulate WWOX expression in HCC cells. Knockdown of ACK1 resulted
in upregulation of WWOX and inactivation of AKT signaling. In this
study, we also found that knockdown of ACK1 resulted in the
downregulation of MMP2 and MMP9 in HCC. Notably, our results
indicate that ACK1 is a candidate oncogene in HCC because it plays
an important role in hepatocarcinogenesis.
Materials and methods
Cell culture and clinical samples
The immortalized human liver cell line L02 was
obtained from the American Type Culture Collection (Manassas, VA,
USA). HCC cell lines SMMC-7721, Huh-7, HepG2, BEL-7402 and MHCC-97H
were purchased from the Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). All the cell lines were
maintained in Dulbecco’s modified Eagle’s medium (Gibco, USA)
containing 10% fetal bovine serum (Gibco, USA) and cultured in a
humidified incubator containing 5% CO2 at 37°C.
A total of 150 pairs of HCC and corresponding
adjacent non-tumorous liver samples (>2.0 cm from the resection
margin) were obtained from the patients who underwent hepatectomy
between January 2005 and June 2009 at First Affiliated Hospital of
Gannan Medical University. The tumor stages were classified
according to the new 7th edition (TNM-7) of the American Joint
Committee on Cancer (AJCC)/International Union Against Cancer
(UICC) TNM system (32). The
demographic features and clinicopathologic data of these patients
are shown in Table I. Our study
enrolled 118 males and 32 females with a median age of 52 years.
All samples were collected immediately after hepatectomy,
snap-frozen in liquid nitrogen, and stored at −80°C until use. All
recruited patients provided written informed consent before
surgical resection, and all the protocols were approved by the
Ethics Committee of First Affiliated Hospital of Gannan Medical
University according to the 1975 Declaration of Helsinki.
 | Table ICorrelations between ACK1 expression
and clinicopathologic features in HCC. |
Table I
Correlations between ACK1 expression
and clinicopathologic features in HCC.
| | ACK1 protein | |
|---|
| |
| |
|---|
|
Characteristics | n | High | Low | P-value |
|---|
| Gender |
| Female | 32 | 20 | 12 | 0.162 |
| Male | 118 | 55 | 63 | |
| Age (year) |
| ≤45 | 42 | 17 | 25 | 0.203 |
| >45 | 108 | 58 | 50 | |
| HBsAg
statusa |
| Negative | 32 | 13 | 19 | 0.319 |
| Positive | 118 | 62 | 56 | |
| Cirrhosis |
| No | 48 | 22 | 26 | 0.600 |
| Yes | 102 | 53 | 49 | |
| AFP (μg/l)b |
| ≤400 | 67 | 29 | 38 | 0.189 |
| >400 | 83 | 46 | 37 | |
| Tumor size |
| ≤5 cm | 55 | 26 | 29 | 0.735 |
| >5 cm | 95 | 49 | 46 | |
| Tumor number |
| Single | 118 | 51 | 67 | 0.002c |
| Multiple | 32 | 24 | 8 | |
| Tumor capsule |
| Complete | 35 | 13 | 22 | 0.122 |
| Incomplete | 115 | 62 | 53 | |
| Vascular
invasion |
| No | 128 | 57 | 71 | 0.002c |
| Yes | 22 | 18 | 4 | |
| Edmondson
grade |
| I/II | 91 | 37 | 54 | 0.007c |
| III/IV | 59 | 38 | 21 | |
| TNM stage |
| I/II | 96 | 38 | 58 | 0.001c |
| III/IV | 54 | 37 | 17 | |
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
Total RNA was isolated from cell lines using TRIzol
reagent (Invitrogen, USA). The RNA samples were reverse transcribed
into cDNA with a RevertAid Premium First Strand cDNA Synthesis kit
(Fermentas, Canada). qRT-PCR was done in an ABI 7500 system using
the SYBR® Premix Ex Taq™ II (Takara, Japan). The
following primers were used: ACK1 sense primer:
5′-AGAGCCTGAAGACACGCACC-3′; antisense primer:
5′-GGATCTGACTGCCGTTGAGG-3′. β-actin sense primer:
5′-GGGAAATCGTGCGTGACAT-3′; antisense primer:
5′-CTGGAAGGTGGACAGCGAG-3′. Three experimental replicates were
performed.
Western immunoblotting
The primary rabbit anti-ACK1 polyclonal antibody
(ab135672), primary rabbit anti-p-ACK1 polyclonal antibody
(ab74091) and primary rabbit anti-WWOX polyclonal antibody
(ab189410) were purchased from Abcam (Cambridge, UK). The primary
rabbit anti-Ki-67 polyclonal antibody (sc-15402), rabbit anti-AKT
polyclonal antibody (sc-8312, which can be used to detect total
proteins of AKT1, AKT2 and AKT3), rabbit anti-p-AKT polyclonal
antibody (sc-293095, which can be used to detect phosphorylated
AKT1, AKT2 and AKT3), rabbit anti-MMP2 polyclonal antibody
(sc-10736), rabbit anti-MMP9 polyclonal antibody (sc-10737) and
rabbit anti-β-actin polyclonal antibody (sc-130656) were purchased
from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The secondary
goat anti-rabbit antibody (sc-2004) was also obtained from Santa
Cruz Biotechnology. The blots were examined with the secondary
antibody conjugated with HRP and reactions were visualized using
the HyGLO HRP detection kit from Diagenode Inc. (Denville, NJ,
USA).
Immunohistochemical staining
Immunohistochemical staining was performed on
paraformaldehyde-fixed paraffin sections. The ACK1, WWOX and p-ACK1
primary antibodies were used in the immunohistochemistry assays.
Immunohistochemical staining was performed as previous reported
(25). Immunostaining intensity
was evaluated as four grades: 0, negative; 1, weak; 2, moderate; 3,
strong. The percentage of positive cells was categorized as grades:
0, 0%; 1, 1–10%; 2, 11–50%; 3, 51–80%; and 4, >80%. The
immunostaining intensity and average percentage of positive cells
were evaluated for ten independent high magnification fields. By
multiplying the staining intensity and the percentage of positive
cells, the final weighed expression score was obtained (0–12).
RNAi transfection
The ACK1 shRNA and scrambled shRNA vector pRS were
purchased from OriGene Technologies Inc. (Rockville, MD, USA). ACK1
unique 29mer shRNA constructs in pRS Vector was transfected into
MHCC-97H cells using TurboFectin Transfection Reagent purchased
from OriGene Technologies Inc. as ACK1-shRNA MHCC-97H cells. The
non-effective 29-mer scrambled shRNA cassette in pRS Vector was
transfected into MHCC-97H cells as the control cells.
Cell proliferation and viability
assays
For the HCC cell proliferation assay, tumor cells
were seeded into 96-well plates at 5×103 cells per well
for 24 h and performed using a Cell Proliferation ELISA, BrdU
(5-bromodeoxyuridine) (chemiluminescent) (Roche, Indianapolis, IN,
USA). The cell viability of HCC cells was determined using 3-(4,
5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)
assay. HCC cells were seeded in a 96-well plate. The absorbance of
the samples was measured at 24, 48 and 72 h.
Cell apoptotic assays
An Annexin V-FLUOS Staining kit (Roche) was used to
determine the level of cell apoptosis, according to the
manufacturer’s protocols. The caspase 3/7 activity assay was
performed using an Apo-ONE® Homogeneous Caspase-3/7
Assay (Promega, WI, USA), as described in a previous study
(24).
Statistical analysis
Statistical analysis was performed using the SPSS
16.0 statistical software package (SPSS Inc., Chicago, IL, USA). A
two-tailed Student’s t-test, a Kaplan–Meier plot, a log-rank test,
a Spearman correlation coefficient analysis, a Chi-square test or
Fisher’s exact test was used to evaluate statistical significance.
Independent prognostic factors were assessed by the Cox
proportional hazards stepwise regression model. Data are shown as
the mean ± SEM. P-values were two-sided, and a P-value of <0.05
was considered to indicate statistical significance.
Results
Expression level of ACK1 in cell
lines
We first detected the expression level of ACK1 in
L02, SMMC-7721, Huh-7, HepG2, BEL-7402 and MHCC-97H cell lines
using qRT-PCR and immunoblotting. We found that the expression
level of ACK1 mRNA in immortalized nontumorigenic human hepatocyte
cell line L02 was significantly lower than that in HCC cell lines
including SMMC-7721, Huh-7, HepG2, BEL-7402 and MHCC-97H
(P<0.01, respectively, Fig.
1A). Furthermore, MHCC-97H expressed the highest expression
level of ACK1 mRNA in HCC cell lines among the five HCC cell lines
(Fig. 1A). Our results of
immunoblotting assay also verified these findings (Fig. 1B). Thereby, MHCC-97H cells were
used in ACK1 knockdown experiment.
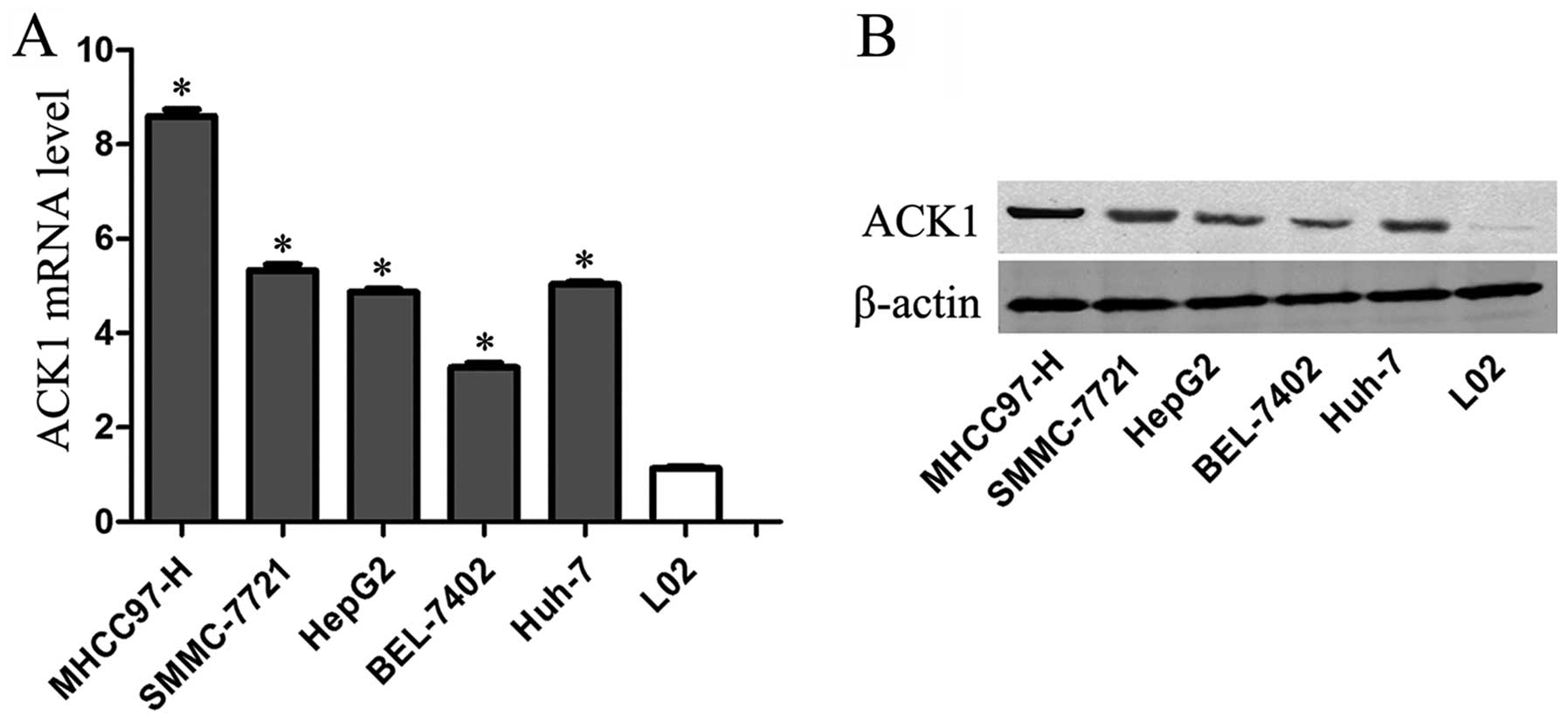 | Figure 1The expression of ACK1 in cell lines.
(A) ACK1 mRNA expression in the immortalized normal human liver
cell line L-02 and five hepatoma cell lines (SMMC-7721, BEL-7402,
Huh-7, HepG2 and MHCC-97H) using qRT-PCR (n=3,
*P<0.01, vs. L-02 group, respectively). (B) ACK1
protein expression in the immortalized normal human liver cell line
L-02 and five hepatoma cell lines (SMMC-7721, BEL-7402, Huh-7,
HepG2 and MHCC-97H) using immunoblotting (n=3, P<0.01). |
Clinical significance of ACK1 expression
in HCC samples
To explore the clinical significance of ACK1 in HCC,
we examined the expression level of ACK1 in 150 pairs of HCC and
matched tumor-adjacent liver tissues using qRT-PCR, immunoblotting
and immunohistochemical staining, and found that the expression
level of ACK1 was significantly higher in the HCC tissues than that
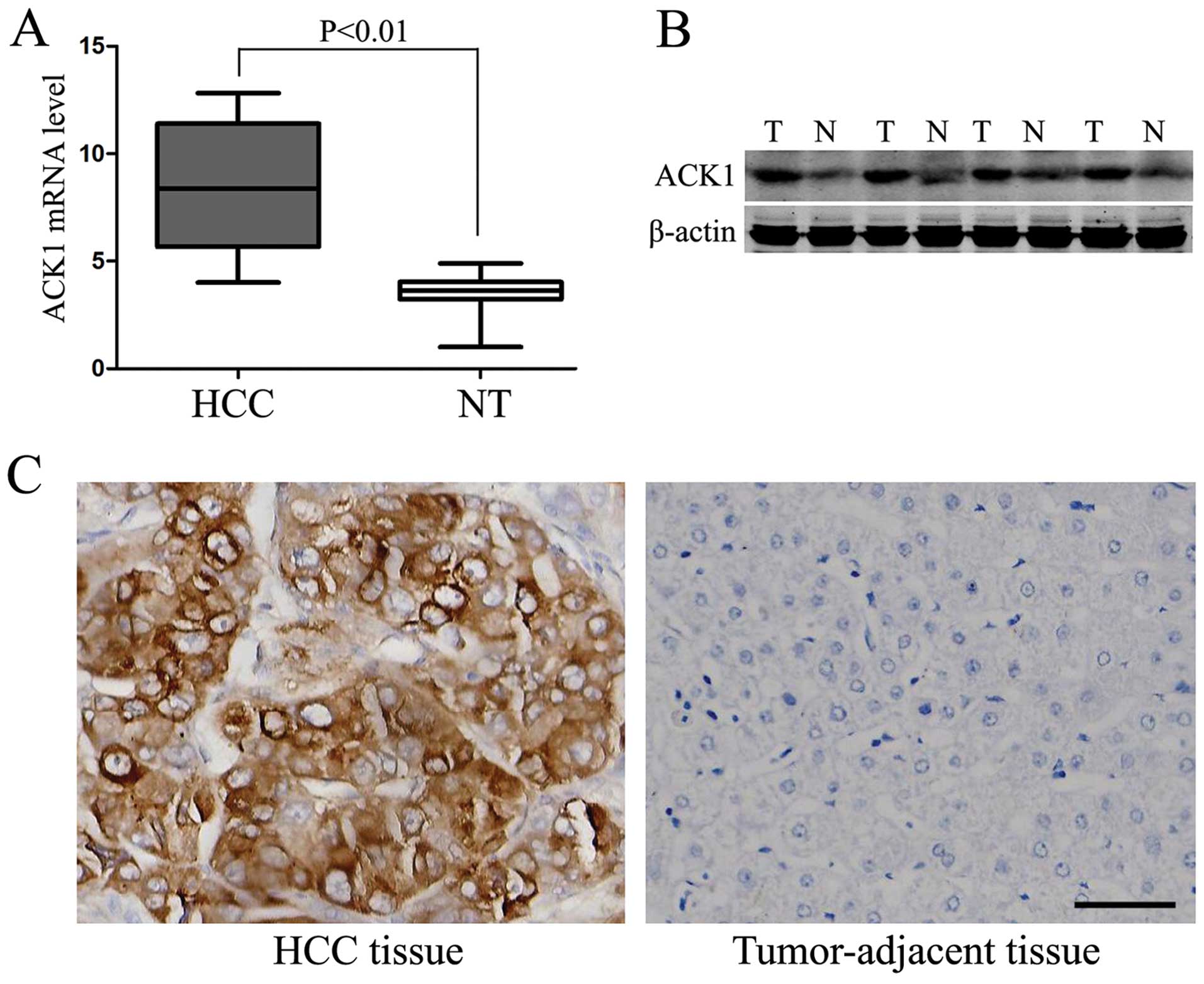
in the noncancerous tissues (P<0.01, respectively, Fig. 2A–C). To further investigate the
clinical role of ACK1 in HCC, we determined the correlations of the
ACK1 protein expression with clinicopathological characteristics,
including patient gender, age, HBsAg, AFP level, tumor size,
cirrhosis, capsule formation, Edmondson-Steiner grade, tumor
number, vascular invasion and TNM stage. The median expression
level of ACK1 protein was used as the cutoff point to divide into
low-expressing and high-expressing groups. In this study, the
expression level of ACK1 was evidently correlated with the
Edmondson-Steiner grade, tumor number, vascular invasion and TNM
stage (P<0.05, respectively). However, no significant
correlation was found between the expression of TPX2 and patients
gender, age, HBsAg, AFP level, tumor size, cirrhosis and capsule
formation (P>0.05, respectively). These results are listed in
Table I.
ACK1 expression is an independent
prognostic factor for HCC
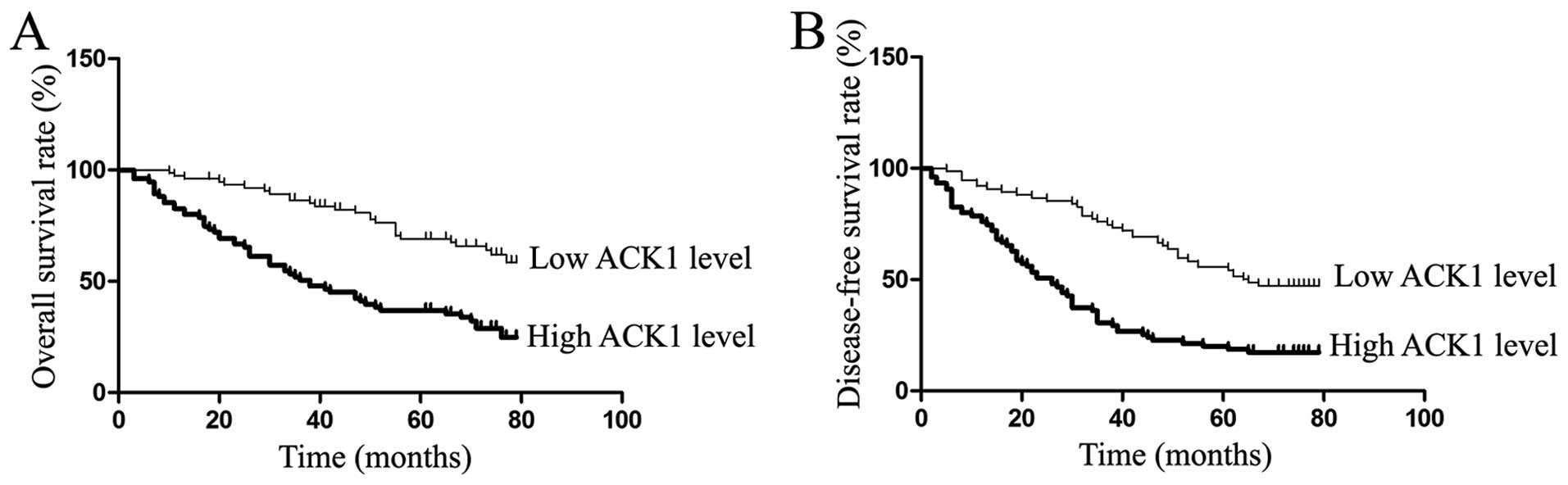
In our study, the median expression level of ACK1
protein was used as the cutoff point to divide into low-expressing
and high-expressing groups for HCC patients’ survival. The HCC
patients with high ACK1 expression had evidently reduced overall
survival and disease-free survival. The 5-year overall survival
rate of the low ACK1 expressing group was 69.0%, which was
evidently higher than that of the high-expressing group (36.9%,
P<0.01) (Fig. 3A). The 5-year
disease-free survival rate of the low ACK1 expressing group was
55.6%, which was significantly higher than that of the
high-expressing group (20.0%, P<0.01) (Fig. 3B). The univariate analysis showed
that hepatitis B surface antigen (HBsAg) status, tumor number,
vascular invasion, Edmonson-Steiner classification, TNM stage and
ACK1 expression were the prognosis factors for HCC patients
(Table II). In a multivariate
analysis model, the expression level of ACK1 was evidently
associated with overall survival (HR 2.523; 95% CI, 1.496–4.255;
P<0.01) and disease-free survival (HR 2.318; 95% CI,
1.479–3.634; P<0.01) (Table
III). Our results indicated that the ACK1 expression level was
an independent prognosis factor in HCC patients.
 | Table IIUnivariate prognostic analysis of
overall survival and disease-free survival in HCC patients. |
Table II
Univariate prognostic analysis of
overall survival and disease-free survival in HCC patients.
| | Overall survival
rate (%) | Disease-free
survival rate (%) |
|---|
| |
|
|
|---|
| Variable | n | 3-year | 5-year | P-value | 3-year | 5-year | P-value |
|---|
| Gender |
| Female | 32 | 84.2 | 67.7 | 0.165 | 59.4 | 46.9 | 0.165 |
| Male | 118 | 64.2 | 48.9 | | 51.7 | 35.3 | |
| Age (year) |
| ≤45 | 42 | 80.8 | 56.3 | 0.577 | 64.3 | 40.8 | 0.742 |
| >45 | 108 | 63.7 | 51.7 | | 49.1 | 36.8 | |
| HBsAg
statusa |
| Negative | 32 | 81.3 | 78.0 | 0.026c | 75.0 | 56.3 | 0.012c |
| Positive | 118 | 64.9 | 45.8 | | 47.5 | 32.8 | |
| Cirrhosis |
| No | 48 | 68.7 | 64.2 | 0.143 | 62.5 | 45.3 | 0.078 |
| Yes | 102 | 68.3 | 47.7 | | 49.0 | 34.3 | |
| AFP (μg/l)b |
| ≤400 | 67 | 65.3 | 57.3 | 0.289 | 55.2 | 44.5 | 0.350 |
| >400 | 83 | 71.0 | 49.2 | | 51.8 | 32.5 | |
| Tumor size |
| ≤5 cm | 55 | 68.5 | 54.8 | 0.951 | 49.1 | 37.8 | 0.568 |
| >5 cm | 95 | 68.4 | 51.7 | | 55.8 | 37.9 | |
| Tumor number |
| Single | 118 | 73.4 | 58.8 | <0.001c | 59.3 | 44.7 | <0.001c |
| Multiple | 32 | 50.0 | 31.3 | | 31.3 | 12.5 | |
| Tumor capsule |
| Complete | 35 | 73.7 | 60.8 | 0.310 | 57.1 | 40.0 | 0.904 |
| Incomplete | 115 | 66.9 | 50.5 | | 52.2 | 37.1 | |
| Vascular
invasion |
| No | 128 | 68.4 | 55.1 | 0.036c | 55.5 | 41.2 | 0.018c |
| Yes | 22 | 68.2 | 39.2 | | 40.9 | 18.2 | |
| Edmondson
grade |
| I/II | 91 | 76.8 | 57.6 | 0.020c | 67.0 | 48.0 | <0.001c |
| III/IV | 59 | 55.6 | 45.1 | | 32.2 | 22.0 | |
| TNM stage |
| I/II | 96 | 71.4 | 61.4 | 0.001c | 60.4 | 46.6 | 0.001c |
| III/IV | 54 | 63.0 | 37.7 | | 40.7 | 22.2 | |
| ACK1 protein
level |
| Low | 75 | 86.5 | 69.0 | <0.001c | 76.0 | 55.6 | <0.001c |
| High | 75 | 50.6 | 36.9 | | 30.7 | 20.0 | |
 | Table IIIMultivariate analysis of factors
contributing to overall survival and disease-free survival in HCC
patients. |
Table III
Multivariate analysis of factors
contributing to overall survival and disease-free survival in HCC
patients.
| Overall survival
rate | Disease-free
survival rate |
|---|
|
|
|
|---|
| Variable | HR (95% CI)b | P-value | HR (95% CI)b | P-value |
|---|
| HBsAg
statusa | 2.512
(1.301–4.849) | 0.006c | 2.410
(1.354–4.290) | 0.003c |
| Tumor number | 2.596
(1.264–5.333) | 0.009c | 2.422
(1.268–4.628) | 0.007c |
| Vascular
invasion | 0.654
(0.333–1.286) | 0.219 | 0.739
(0.395–1.384) | 0.345 |
| TNM stage | 1.142
(0.560–2.329) | 0.715 | 1.039
(0.564–1.917) | 0.902 |
| Edmondson
grade | 1.029
(0.630–1.680) | 0.910 | 1.505
(0.983–2.305) | 0.060 |
| ACK1 level | 2.523
(1.496–4.255) | 0.001c | 2.318
(1.479–3.634) | <0.001c |
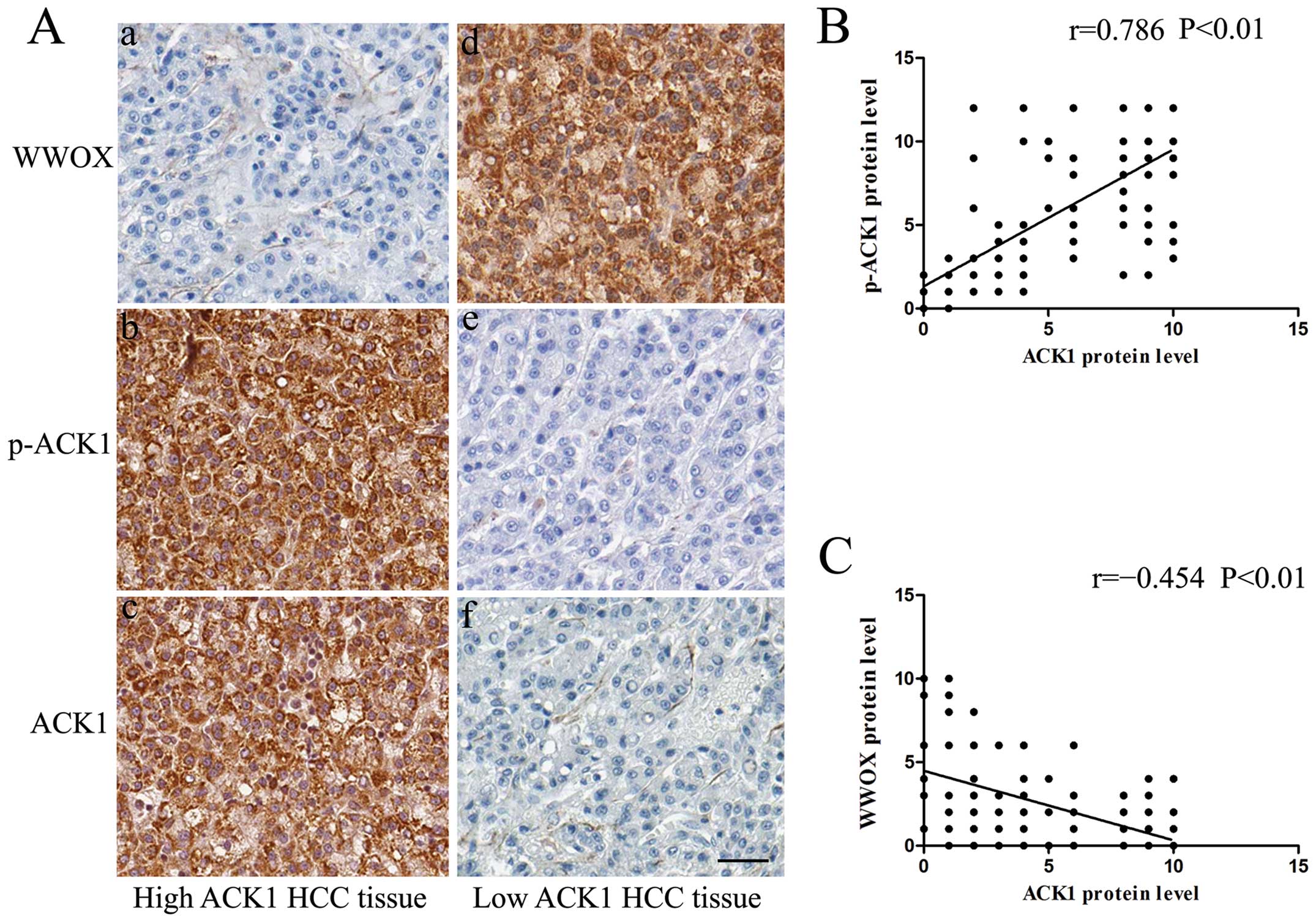
ACK1 is positively associated with p-ACK1
and negatively associated with WWOX expression in HCC samples
To explore whether ACK1 amplification is associated
with ACK1 activation, we detected the phosphorylated ACK1
expression (phosphorylated at Tyr284, p-ACK1) using
immunohistochemical staining, which was previously shown to be
associated with ACK1 activation in many studies. As we expected,
there was indeed an evident positive correlation between the
expression level of ACK1 and p-ACK1 (r=0.786, P<0.01, Spearman’s
correlation test) (Fig. 4A and B).
Our results indicate that ACK1 amplification is related to the
increase in activated p-ACK1.
Since WWOX is downregulated in HCC and is an
interacting protein of ACK1, we examined the correlation between
ACK1 and WWOX in 150 pairs of HCC and matched tumor-adjacent liver
tissues using immunohistochemical staining. IHC scores were used
for semiquantitative analysis of ACK1 and WWOX expression. In this
study, there was indeed an evident negative correlation between the
expression level of ACK1 and WWOX (r= -0.454, P<0.01, Spearman’s
correlation test) (Fig. 4A and
C).
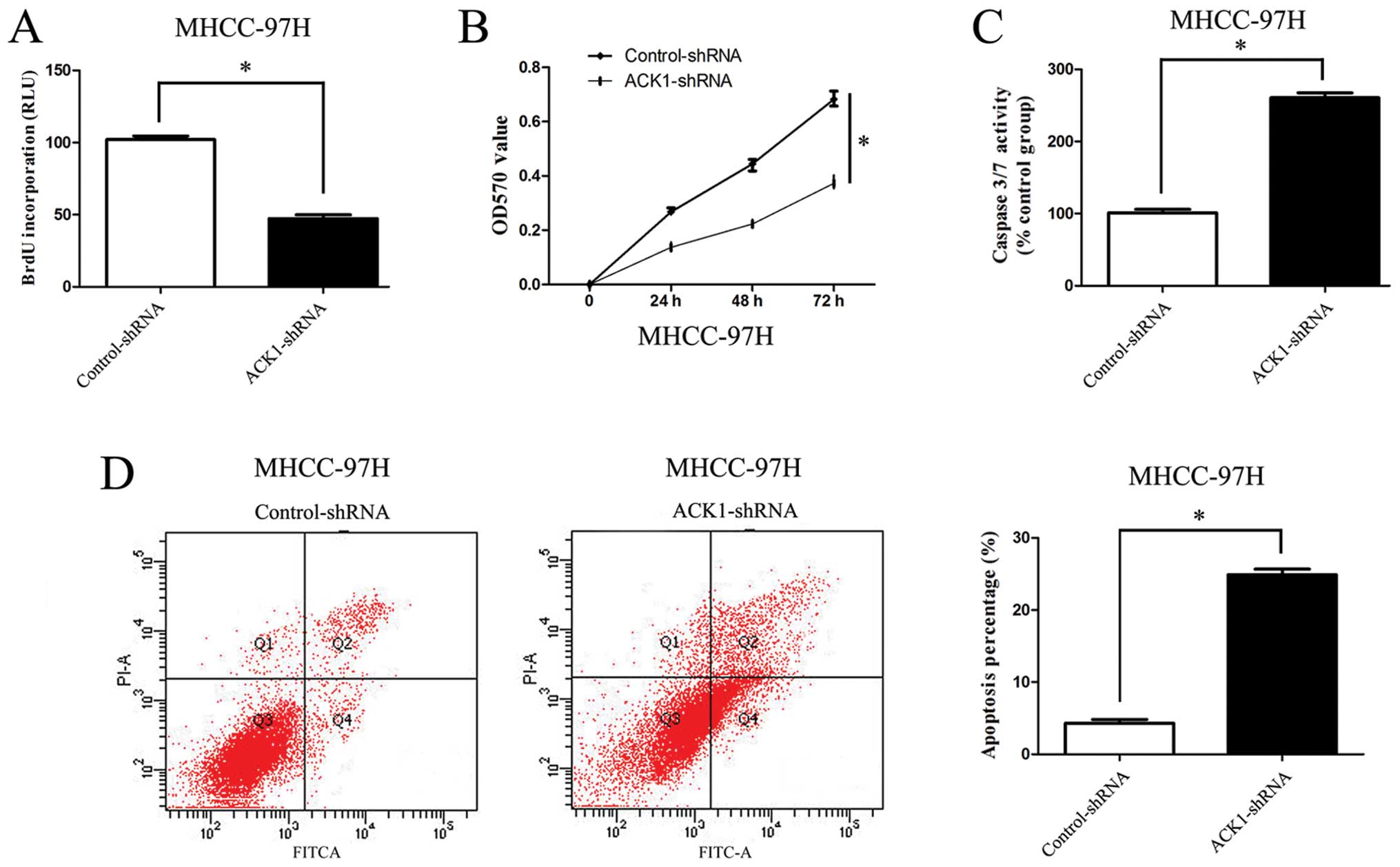
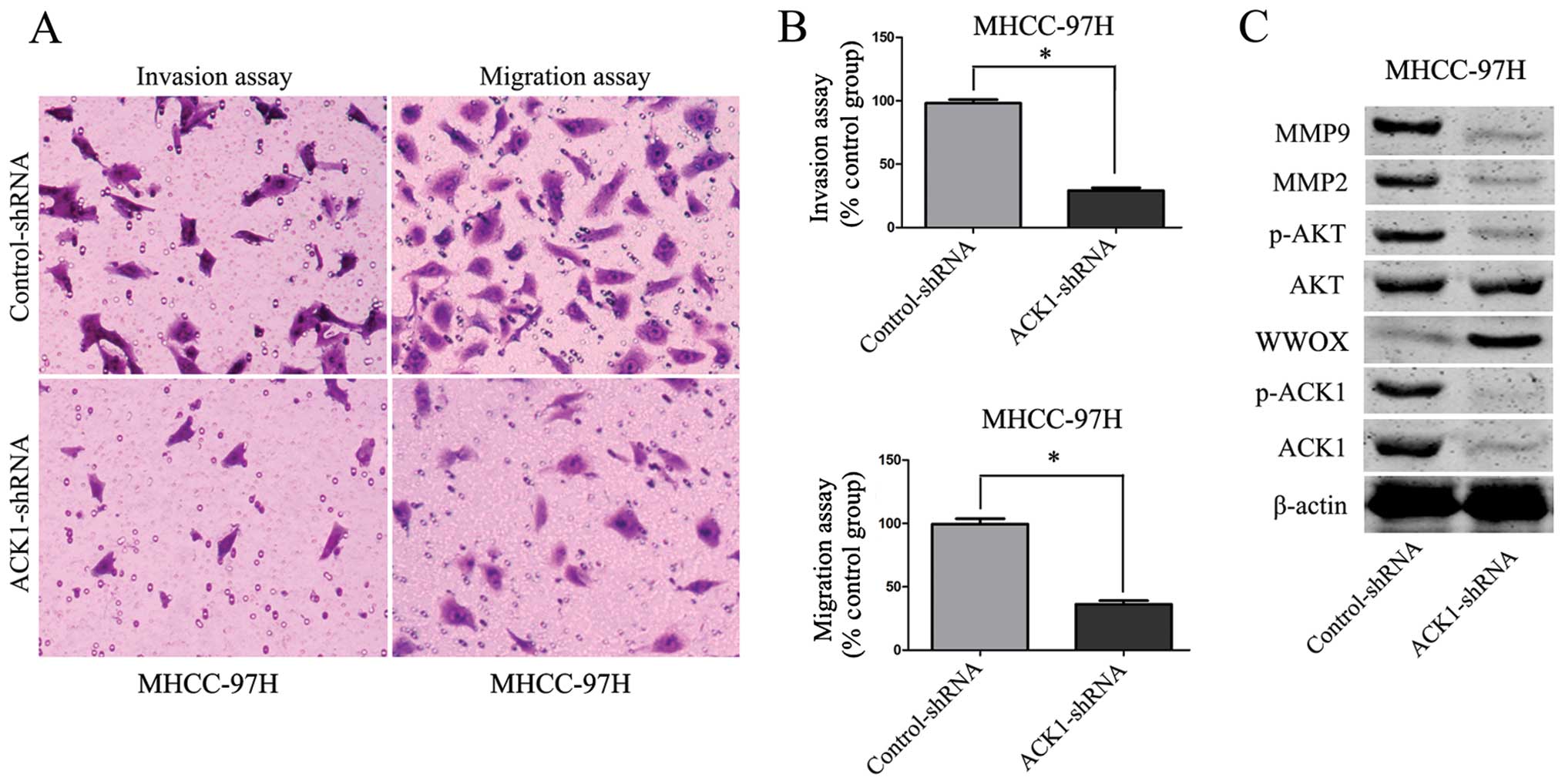
Knockdown of ACK1 promotes MHCC-97H cell
apoptosis and represses invasion, migration and proliferation
To further identify the effect of ACK1 on cell
apoptosis, invasion, migration and proliferation of HCC, we
downregulated the expression of ACK1 by ACK1-shRNA vectors in
MHCC-97H cells. As compared with the control group, silencing ACK1
was found to inhibit MHCC-97H cell proliferation and viability by
BrdU and MTT assays (P<0.01, respectively, Fig. 5A and B). On the other hand, our
study found that the activity of caspase 3/7 in MHCC-97H cells was
significantly increased after knockdown of ACK1 (P<0.01,
respectively, Fig. 5C). Flow
cytometry assay revealed that the percentage of apoptosis in HCC
cells in ACK1-shRNA group was increased (P<0.01, respectively,
Fig. 5D). Our study also found
that knockdown of ACK1 expression significantly reduced the number
of migrated and invaded MHCC-97H cells (P<0.01, respectively,
Fig. 6A and B).
Knockdown of ACK1 is associated with
upregulation of WWOX and inactivation of AKT signaling in MHCC-97H
cells
To identify the underlying mechanism by which ACK1
regulates tumor cell apoptosis, invasion, migration and
proliferation in HCC, we investigated the effect of ACK1 on p-ACK1,
WWOX and AKT signaling. Knockdown of ACK1 evidently increased the
level of WWOX and attenuated the phosphorylation of ACK1 and AKT in
MHCC-97H cells (P<0.01, Fig.
6C). Furthermore, to better understand the downstream molecules
involved in ACK1 induced-AKT signaling activation, we identified
whether ACK1 affected MMP2 and MMP9 expression in HCC cells. As
expected, we found that knockdown of ACK1 expression markedly
decreased the MMP2 and MMP9 expression in MHCC-97H cells
(P<0.01, Fig. 6C).
Discussion
Due to the high frequency of tumor recurrence, the
prognosis of patients with HCC remains very poor (2). Many biomarkers have been associated
to the progression of HCC, but most of them turned out not to be of
clinical utility (6). Thus, it is
urgent to explore effective biomarkers to improve the treatment
efficacy of HCC. ACK1, an intracellular tyrosine kinase, is
considered to be implicated in regulating multiple aspects of tumor
progression (13,16). Several studies have indicated that
ACK1 overexpression is commonly observed in human cancers including
lung and prostate cancer (27,33).
Furthermore, it has been demonstrated that ACK1 can promote tumor
growth via regulating pro-survival molecules such as AKT and AR in
various cancers (26,31,33).
The role of ACK1 have been investigated in multiple
tumor types (31,33). However, the ACK1 expression and its
related molecule mechanisms in HCC remained unknown. In this study,
we initially examined the expression of ACK1 in an immortalized
nontumorigenic hepatocyte cell line L02 and five HCC cell lines,
such as SMMC-7721, Huh-7, HepG2, BEL-7402 and MHCC-97H. We found
that ACK1 expression in immortalized nontumorigenic hepatocyte cell
line L02 was lower than that in HCC cell lines. We further
investigated ACK1 expression in 150 HCC patients using qRT-PCR,
immunohistochemical staining and western blotting, and our results
found that the expression level of ACK1 was evidently higher in HCC
compared with matched normal tumor adjacent liver tissues.
Moreover, ACK1 expression level was significantly correlated with
several clinicopathological parameters including Edmondson-Steiner
grade, tumor number, vascular invasion and TNM stage. Notably, our
results also indicates that high-expression level of ACK1 was
evidently correlated with shorter overall survival and disease-free
survival, which is an assumed biomarker for HCC negative prognosis.
Additionally, multivariate Cox repression analysis further
confirmed that ACK1 is an independent factor for HCC patients. Our
results revealed that the expression level of ACK1 is a critical
factor for prognosis determination and ACK1 may promote tumor
progression in HCC.
In this study, we detected the phosphorylated ACK1
expression (p-ACK1), which was previously shown to be associated
with ACK1 activation. As expected, our results indicate that ACK1
amplification is related to the increase in activated p-ACK1. Our
data are consistent with previous studies on gastric cancer and
breast cancer, suggesting the ACK1 gene amplification can cause
ACK1 phosphorylation and auto-activation (15,16).
It has been demonstrated that ACK1 is responsible for the
regulation of several important molecule proteins, which are
involved in tumor cell apoptosis, invasion, migration and
proliferation, such as WWOX, AKT and AR (21,23).
In our study, ACK1 expression was inversely associated with WWOX
expression in HCC samples. The in vitro experiments
presented in this study also indicate that ACK1 is an important
regulatory factor in HCC cell apoptosis, invasion, migration and
proliferation. Our in vitro studies also revealed that
knockdown of ACK1 by shRNA in MHCC-97H, a cell line with high
expression level of the target gene, consistently showed
degradation of the p-AKT, MMP2 and MMP9 expression. Moreover, our
study confirmed that ACK1 mediates the degradation of WWOX and
activates AKT signaling in HCC.
As a recently discovered tumor suppressor, the
expression level of WWOX have been shown to be downregulated in
various tumor types including HCC (19,20).
WWOX protein plays an inhibitory role in Wnt/β-catenin signaling
pathway, which is an important signaling for tumor progression
(19). High frequency of loss of
WWOX is observed in breast, prostate and ovarian cancer (20–22).
Additionally, it has been reported that WWOX was identified to be
an ACK1 interacting protein and Ack1 was shown to be prerequisite
for WWOX protein degradation (23). In this study, we found that ACK1
downregulated WWOX expression, which is consistent with a previous
study (23).
Previously, activated AKT signaling has been
considered to facilitate tumor cell proliferation, invasion and
migration (24). Multiple studies
found that AKT signaling pathway is aberrantly hyperactivated in
HCC, which results in the promotion of tumor progression (6,24).
Furthermore, it has been reported that AKT signaling plays a key
role in facilitating MMP2 and MMP9 expression (6). As two critical members of the MMPs
family, MMP2 and MMP9 are important factors in the invasion and
migration of HCC (34). In this
study, we found that knockdown of ACK1 expression resulted in
inactivation of AKT signaling and downregulation the expression of
MMP2 and MMP9 in HCC cells. Our data indicate that ACK1 promotes
cell invasion and migration through AKT signaling activation and
MMP2/9 upregulation and ACK1 may be an important alternate AKT
activator in HCC.
In summary, we found that ACK1 expression is
upregulated in HCC tissues as compared with non cancerous liver
tissues and that overexpression level of ACK1 is correlated with
clinicopathological features of poor prognosis in HCC.
Additionally, we identified that ACK1 expression is an independent
factor for predicting the overall survival and disease-free
survival of HCC patients. ACK1 was positively associated with
p-ACK1 and was negatively associated with WWOX expression. In
vitro studies showed that knockdown of ACK1 promoted HCC cells
apoptosis and repressed HCC cell invasion, migration and
proliferation. Furthermore, knockdown of ACK1 resulted in
upregulation of WWOX and inactivation of AKT signaling. In this
study, we also found that knockdown of ACK1 resulted in the
downregulation of MMP2 and MMP9 in HCC. Notably, our results
indicate that ACK1 is a candidate oncogene in HCC because it plays
an important role in hepatocarcinogenesis. This investigation
revealed a potential target of ACK1 and supplied us with a new
insight into the degradation of WWOX and activation of AKT
signaling in HCC. ACK1 may potentially act as a prognostic
biomarker, and may also be a therapeutic target in HCC.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (30600156), the Natural Science
Foundation of Jiangxi Province (2012ZBAB205001), the Science and
Technology Projects Foundation of Jiangxi Province (20112BBG70037)
and the Projects Foundation of Jiangxi Province Education
Department (GJJ14681). The authors would like to thank all the
patients who participated in this study.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen WQ, Zeng HM, Zheng RS, Zhang SW and
He J: Cancer incidence and mortality in China, 2007. Chin J Cancer
Res. 24:1–8. 2012. View Article : Google Scholar
|
|
3
|
Nishikawa H, Osaki Y, Endo M, et al:
Japanese Red Cross Liver Study Group: Comparison of standard-dose
and half-dose sorafenib therapy on clinical outcome in patients
with unresectable hepatocellular carcinoma in field practice: A
propensity score matching analysis. Int J Oncol. 45:2295–2302.
2014.PubMed/NCBI
|
|
4
|
Venook AP, Papandreou C, Furuse J and de
Guevara LL: The incidence and epidemiology of hepatocellular
carcinoma: A global and regional perspective. Oncologist. 15(Suppl
4): 5–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alazawi W, Cunningham M, Dearden J and
Foster GR: Systematic review: Outcome of compensated cirrhosis due
to chronic hepatitis C infection. Aliment Pharmacol Ther.
32:344–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Q, Tu K, Zhang H, Zheng X, Yao Y and
Liu Q: TPX2 as a novel prognostic biomarker for hepatocellular
carcinoma. Hepatol Res. Sep 29–2014.(Epub ahead of print).
View Article : Google Scholar
|
|
7
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases. Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han S, Han L, Yao Y, Sun H, Zan X and Liu
Q: Activated hepatic stellate cells promote hepatocellular
carcinoma cell migration and invasion via the activation of
FAK-MMP9 signaling. Oncol Rep. 31:641–648. 2014.
|
|
9
|
Nur-E-Kamal MS, Kamal JM, Qureshi MM and
Maruta H: The CDC42-specific inhibitor derived from ACK-1 blocks
v-Ha-Ras-induced transformation. Oncogene. 18:7787–7793. 1999.
View Article : Google Scholar
|
|
10
|
Prieto-Echagüe V, Gucwa A, Craddock BP,
Brown DA and Miller WT: Cancer-associated mutations activate the
nonreceptor tyrosine kinase Ack1. J Biol Chem. 285:10605–10615.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chan W, Tian R, Lee YF, Sit ST, Lim L and
Manser E: Down-regulation of active ACK1 is mediated by association
with the E3 ubiquitin ligase Nedd4–2. J Biol Chem. 284:8185–8194.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grøvdal LM, Johannessen LE, Rødland MS,
Madshus IH and Stang E: Dysregulation of Ack1 inhibits
down-regulation of the EGF receptor. Exp Cell Res. 314:1292–1300.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kelley LC and Weed SA: Cortactin is a
substrate of activated Cdc42-associated kinase 1 (ACK1) during
ligand-induced epidermal growth factor receptor downregulation.
PLoS One. 7:e443632012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gajiwala KS, Maegley K, Ferre R, He YA and
Yu X: Ack1: Activation and regulation by allostery. PLoS One.
8:e539942013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shinmura K, Kiyose S, Nagura K, Igarashi
H, Inoue Y, Nakamura S, Maeda M, Baba M, Konno H and Sugimura H:
TNK2 gene amplification is a novel predictor of a poor prognosis in
patients with gastric cancer. J Surg Oncol. 109:189–197. 2014.
View Article : Google Scholar
|
|
16
|
Howlin J, Rosenkvist J and Andersson T:
TNK2 preserves epidermal growth factor receptor expression on the
cell surface and enhances migration and invasion of human breast
cancer cells. Breast Cancer Res. 10:R362008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mahajan K, Coppola D, Challa S, et al:
Ack1 mediated AKT/PKB tyrosine 176 phosphorylation regulates its
activation. PLoS One. 5:e96462010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mahajan K and Mahajan NP: ACK1 tyrosine
kinase: Targeted inhibition to block cancer cell proliferation.
Cancer Lett. 338:185–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li YP, Wu CC, Chen WT, Huang YC and Chai
CY: The expression and significance of WWOX and β-catenin in
hepatocellular carcinoma. APMIS. 121:120–126. 2013. View Article : Google Scholar
|
|
20
|
Salah Z, Aqeilan R and Huebner K: WWOX
gene and gene product: Tumor suppression through specific protein
interactions. Future Oncol. 6:249–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang J and Zhang W: WWOX tumor suppressor
gene. Histol Histopathol. 23:877–882. 2008.PubMed/NCBI
|
|
22
|
Lewandowska U, Zelazowski M, Seta K,
Byczewska M, Pluciennik E and Bednarek AK: WWOX, the tumour
suppressor gene affected in multiple cancers. J Physiol Pharmacol.
60(Suppl 1): 47–56. 2009.PubMed/NCBI
|
|
23
|
Mahajan K and Mahajan NP: Shepherding AKT
and androgen receptor by Ack1 tyrosine kinase. J Cell Physiol.
224:327–333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng X, Gai X, Ding F, Lu Z, Tu K, Yao Y
and Liu Q: Histone acetyltransferase PCAF up-regulated cell
apoptosis in hepatocellular carcinoma via acetylating histone H4
and inactivating AKT signaling. Mol Cancer. 12:962013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Q, Yang P, Tu K, Zhang H, Zheng X, Yao
Y and Liu Q: TPX2 knockdown suppressed hepatocellular carcinoma
cell invasion via inactivating AKT signaling and inhibiting MMP2
and MMP9 expression. Chin J Cancer Res. 26:410–417. 2014.PubMed/NCBI
|
|
26
|
Mahajan K and Mahajan NP: PI3K-independent
AKT activation in cancers: a treasure trove for novel therapeutics.
J Cell Physiol. 227:3178–3184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan DS, Haaland B, Gan JM, et al:
Bosutinib inhibits migration and invasion via ACK1 in KRAS mutant
non-small cell lung cancer. Mol Cancer. 13:132014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van der Horst EH, Degenhardt YY, Strelow
A, et al: Metastatic properties and genomic amplification of the
tyrosine kinase gene ACK1. Proc Natl Acad Sci USA. 102:15901–15906.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mahajan K, Coppola D, Rawal B, Chen YA,
Lawrence HR, Engelman RW, Lawrence NJ and Mahajan NP: Ack1-mediated
androgen receptor phosphorylation modulates radiation resistance in
castration-resistant prostate cancer. J Biol Chem. 287:22112–22122.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chua BT, Lim SJ, Tham SC, Poh WJ and
Ullrich A: Somatic mutation in the ACK1 ubiquitin association
domain enhances oncogenic signaling through EGFR regulation in
renal cancer derived cells. Mol Oncol. 4:323–334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mahajan K, Coppola D, Chen YA, Zhu W,
Lawrence HR, Lawrence NJ and Mahajan NP: Ack1 tyrosine kinase
activation correlates with pancreatic cancer progression. Am J
Pathol. 180:1386–1393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng CH, Lee CF, Wu TH, Chan KM, Chou HS,
Wu TJ, Yu MC, Chen TC, Lee WC and Chen MF: Evaluation of the new
AJCC staging system for resectable hepatocellular carcinoma. World
J Surg Oncol. 9:1142011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mahajan K, Challa S, Coppola D, Lawrence
H, Luo Y, Gevariya H, Zhu W, Chen YA, Lawrence NJ and Mahajan NP:
Effect of Ack1 tyrosine kinase inhibitor on ligand-independent
androgen receptor activity. Prostate. 70:1274–1285. 2010.PubMed/NCBI
|
|
34
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|