Introduction
Regulatory T cells (Tregs) are key players in
maintaining immune homeostasis and tolerance. The forkhead
transcription factor Foxp3 is essential for differentiation and
activation of Tregs (1), and used
to be regarded as specific transcription factor of Tregs (2,3). In
2007, Hinz et al (4) first
reported that pancreatic cancer cells expressed Foxp3. Subsequent
studies reported that breast cancer cells expressed Foxp3, and that
Foxp3 positivity was associated with poor prognosis (5). However, other studies reported that
Foxp3 acts as a tumor suppressor in breast cancer and prostate
cancer (6–8). Thus, the role of Foxp3 expression in
cancer cells (referred as ‘cancer cell-derived Foxp3’ in this
report) remains incompletely understood, especially regarding
molecular mechanisms.
At the molecular level, FOXP3 binds to multiple
transcription factors, such as NFAT, NF-κB, STAT3, AML1/Runx1 to
regulate T cells function (9–12).
It also modulates gene expression through epigenetic mechanisms,
such as chromatin remodeling and histone deacetylation (13,14).
Zheng et al (15) first
performed a genome-wide analysis of Foxp3 in mouse Tregs and found
that Foxp3 acts as both a transcriptional activator and repressor
in Tregs. Recently, Rudra et al (16) reported that Foxp3 binds to 361
proteins in Tregs and is involved in the transcriptional regulation
of most of these proteins. The above demonstrate a complex nature
of the interaction of Foxp3 with its target genes. However, less is
known about the role of Foxp3 in the transcriptional regulation in
cancer cells. In particular, it is unknown whether Foxp3 regulates
transcription in cancer cells as it does in Tregs.
Our previous study revealed the expression of Foxp3
in tongue squamous cell carcinoma (TSCC) cells, and showed that the
expression of cancer cell-derived Foxp3 was positively associated
with the pathologic differentiation and T stage, and inversely
associated with overall survival of TSCC patients (17). To achieve further knowledge on
these influences, and how cancer cell-derived Foxp3 can regulate
TSCC, the present study was performed, using genome-wide analysis
of Foxp3 target genes in TSCC cells with a combination of chromatin
immunoprecipitation array profiling (ChIP-on-chip assay) and
expression profiling (whole-genome microarray assay). We also
compared Foxp3 biding sites in TSCC cells with the known binding
sites in human Tregs to show the differences in transcriptional
regulation profile. This study revealed the relationship between
direct and indirect targets genes of Foxp3 in TSCC cells and
provide molecular basis of cancer cell-derived Foxp3 function.
Materials and methods
Cell cultures
Three human TSCC cell lines (CAL 27, SCC-9, and
SCC-5) were purchased from American Type Culture Collection (ATCC).
CAL 27 cells were maintained in DMEM (Gibco, Grand Island, NY, USA)
that contained 10% fetal bovine serum (FBS) (Gibco). SCC-9 cells
and SCC-5 cells were maintained in DMEM/F-12 (Gibco) that contained
10% FBS.
Cytoimmunofluorescence staining
CAL 27, SCC-9, and SCC-5 cells were seeded into
48-well plates for routine culturing. After washing in PBS, cells
were fixed in 4% formaldehyde for 20 min at room temperature,
treated with 1% Triton, and then blocked in 5% bovine serum albumin
(BSA) at room temperature for 50 min. The cells were then incubated
with goat anti-human Foxp3 antibody (10 μg/ml, R&D Systems,
Minneapolis, MN, USA) at 4°C overnight and Northern Lights
anti-goat IgG-NL557 (1:200, R&D Systems) at room temperature in
the dark for 1 h. After nuclear staining with 5 μg/ml DAPI for 1
min, cells were observed under an inverted microscope (Axio
observer Z1, Zeiss). Negative control was performed by replacing
the primary antibody with PBS.
ChIP-on-chip and bioinformatics
analysis
SCC-9 cells were seeded into 6-well plates and
cultured for 48 h. After washing in PBS twice, 2 ml of fresh medium
and 54 μl of 37% formaldehyde were added to each well, followed by
incubation at room temperature for 10 min. Then, 200 μl of glycine
was added, followed by incubation for 5 min at room temperature.
The medium was removed and cells were washed twice with pre-chilled
5 mM EDTA. Then, 200 ml of PBS with 1% PMSF was added to each well,
and the cells were harvested. The ChIP-on-chip assay (Shanghai
Kangcheng Biotech Co., Ltd., Shanghai, China) was performed with
goat polyclonal antibody against FOXP3-ChIP Grade (Abcam, Hong
Kong, China) and NimbleGen HG18 3×720K RefSeq promoter microarray
(Roche, Mannheim, Germany). Gene ontology (GO) and pathway analysis
were performed with the cooperation with Shanghai Kangcheng Biotech
Co., Ltd. The binding sites of FOXP3 in the genome of human Tregs
were obtained from the ChIP-on-chip results of Sadlon et al
(18).
Gene silencing with siRNA
SCC-9 cells were routinely cultured. When the
confluence reached 30–50% (24 h), cells were harvested and treated
with 50 nM siRNA (Guangzhou RiboBio Co., Ltd., Guangzhou, China)
and an equal amount of Lipofectamine 2000 (Invitrogen, Carlsbad,
CA, USA), followed by incubation for 5 h. At 24 and 48 h after
transfection, total RNA and protein were extracted for real-time
PCR and western blot assays to assess the effect of Foxp3 on
interference. The siRNA sequences were described earlier (4,17).
Western blot assays
As described in our earlier study (17), cells were harvested in RIPA buffer
(Beyotime, Shanghai, China), and protein concentration was
determined by Bradford assay (Bio-Rad Laboratories, Shanghai,
China). Equal amounts of total protein were subjected to 10%
SDS-PAGE and then transferred onto PVDF membranes (Millipore,
Billerica, MA, USA). The membrane was blocked in Tris-buffered
saline (TBST) containing 5% BSA for 2 h at room temperature, and
then incubated with 1.0 μg/ml anti-human Foxp3 antibody (R&D
Systems) at 4°C overnight. After washing in TBST, the membrane was
incubated in horseradish peroxidase-conjugated anti-goat IgG
(1:10,000; Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) for
1 h. Each sample was probed with an anti-GAPDH antibody (1:1,000;
Santa Cruz) as a loading control.
RNA extraction, reverse transcription and
real-time PCR
Total RNA was extracted with the High Pure RNA
Isolation kit (Roche) according to the manufacturer’s instructions.
RNA (1 μg of each sample) was then used for reverse transcription
into cDNA with the Transcriptor First Strand cDNA Synthesis kit
(Roche) according to the manufacturer’s instructions. The mRNA
expression of Foxp3 was examined by real-time PCR using
Light-Cycler 480 SYBR Green I Master (Roche, Mannheim, Germany) and
the thermal cycling conditions and primers were the same as
described in our earlier study or selected from PrimerBank
(17,19). PCRs were conducted in triplicate
for each sample. GAPDH was used as internal reference and the
2−ΔΔCt method was used to determine gene expression.
Human genome-wide expression
profiling
After silencing with siRNA for 48 h, RNA in SCC-9
cells was extracted, and the Human Genome U133 plus 2.0 array
(Affymetrix, USA) was used for the whole genome array assay.
Microarray hybridization was carried out at CapitalBio Corp.
(Beijing, China). Cluster analysis was performed with Cluster 3.0
software. Data analysis was performed using Significance Analysis
of Microarray software (SAM 3.0, Stanford University, USA;
http://www-stat.stanford.edu).
Statistical analysis
Statistical analysis was carried out using SPSS 17.0
statistical software package. Quantitative data analysis employed
Student’s t-test to compare two groups and one-way analysis of
variance (ANOVA) to compare multiple groups. A P-value <0.05 was
considered statistically significant.
Results
Translocation of Foxp3 into nuclei of
TSCC cells
Nuclear translocation is essential for transcription
factor function, so at first we used immunofluorescent staining to
make clear the subcellular distribution of Foxp3 in TSCC cells.
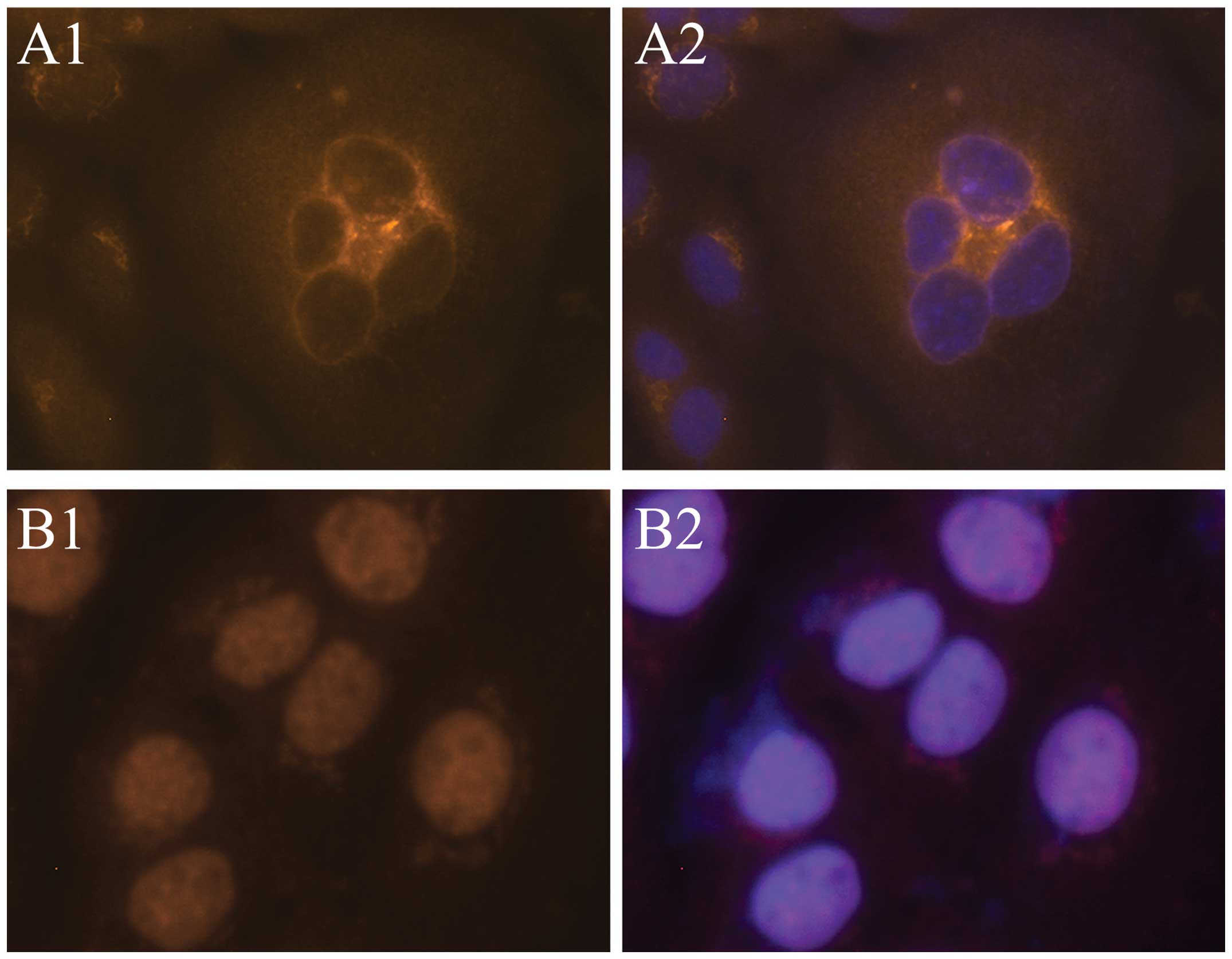
After culture of SCC-9 cells for 24 h, the immunofluorescence
showed that Foxp3 was present throughout the cells, with the
greatest concentration at the nuclear membrane, which appears as a
round fluorescent body (Fig. 1A).
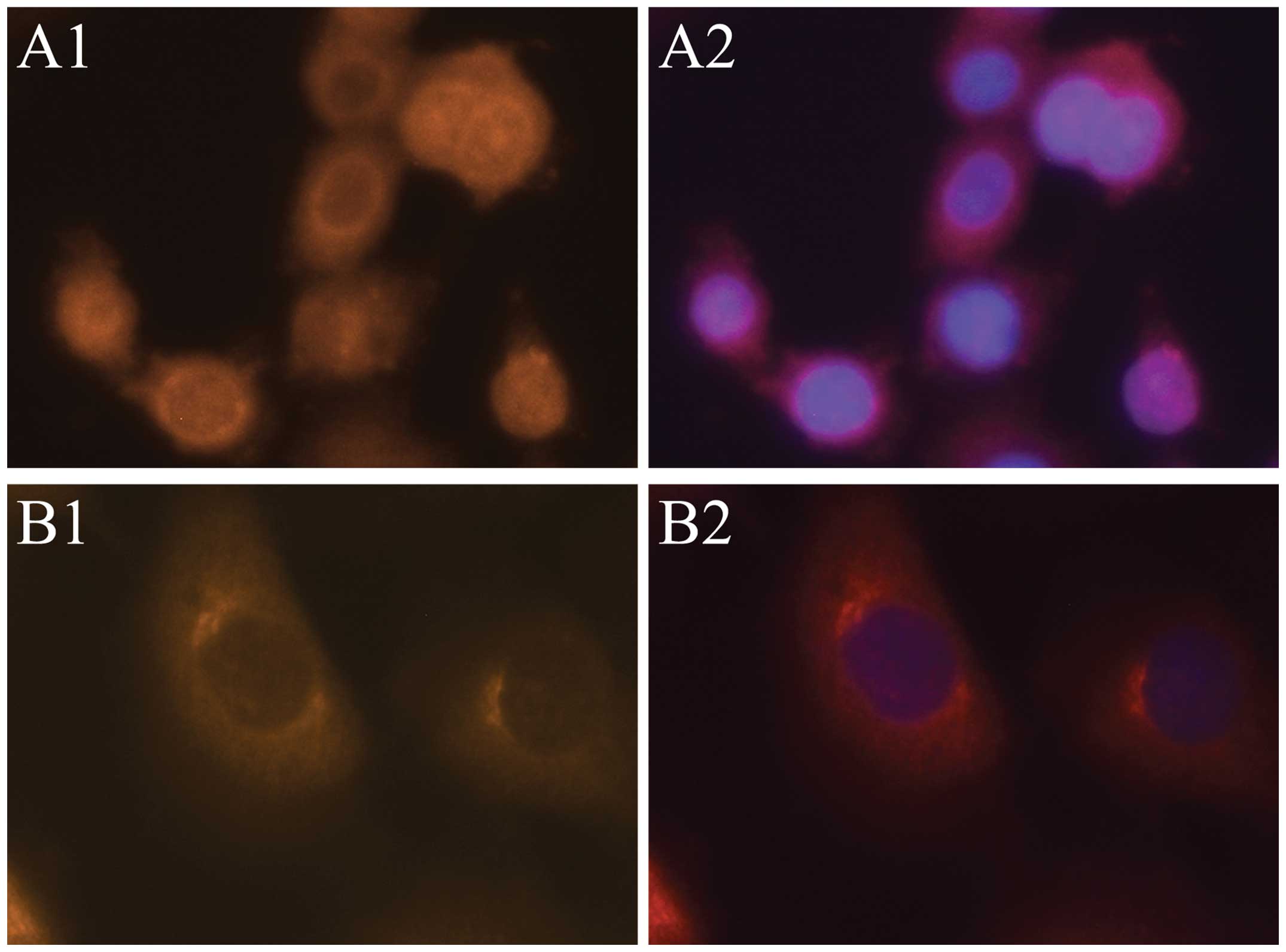
This expression pattern could also be observed in SCC-15 and CAL 27
cells (Fig. 2). After 48 h, Foxp3
was mainly expressed within the nucleus, and the round body shape
of fluorescent staining at the nuclear membrane disappeared
(Fig. 1B), indicating that Foxp3
was transported into nucleus gradually.
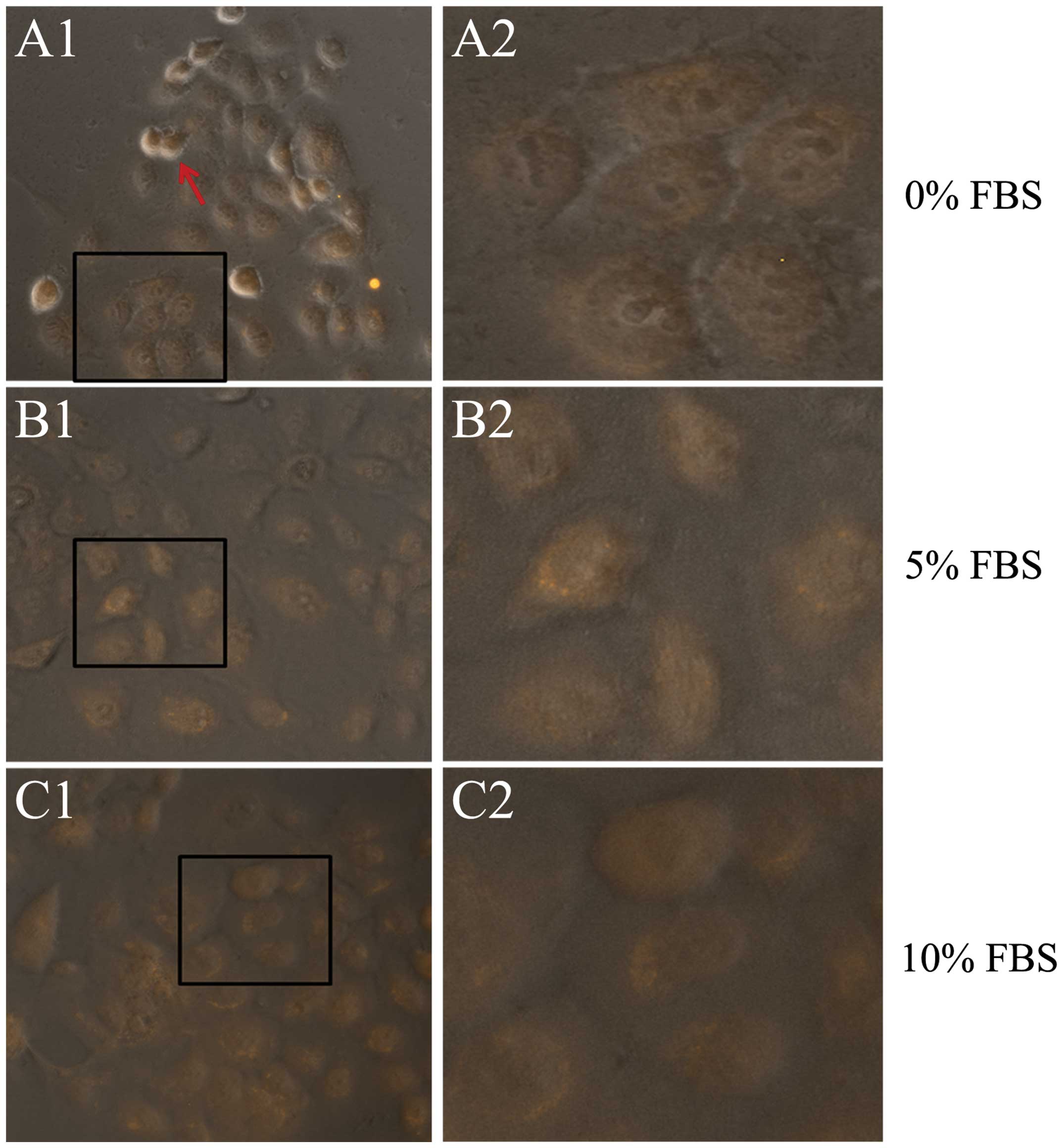
Next, SCC-9 cells were cultured in DMEM-F12 that
contained 0, 5 and 10% FBS for 24 h and then underwent Foxp3
immunofluorescent staining. It can be observed that cells in the 0%
FBS group had a poor growth, and some cells became round and
suspended. However, there was Foxp3 expression in the nuclei of
cells in all three groups (Fig.
3). This culture assay shows that stimulation from other cell
types are not needed in the nucleus translocation of Foxp3 in TSCC
cells.
Foxp3 binding sites and functional
annotation in the genome of TSCC cells
The ChIP-on-chip assay identified 4140 binding sites
of Foxp3 in the genome of SCC-9 cells [false discovery rate (FDR)
<0.05]; after accounting for identical genes, there were 3573
Foxp3-binding genes. Among all genes with FDR values <0.005,
there were 25 transcriptional factors: POU3F1, HEY1, TEAD1, POU4F2,
VEZF1, KCNIP4, KLF12, E2F1, REST, FOXO4, NR4A1, HOXB8, POU2F1,
HOXD9, HIC1, ZBTB16, TCF7, KLF11, IGFBP7, NFIC, PKNOX1, TWIST1,
ING1, MEF2A and LITAF.
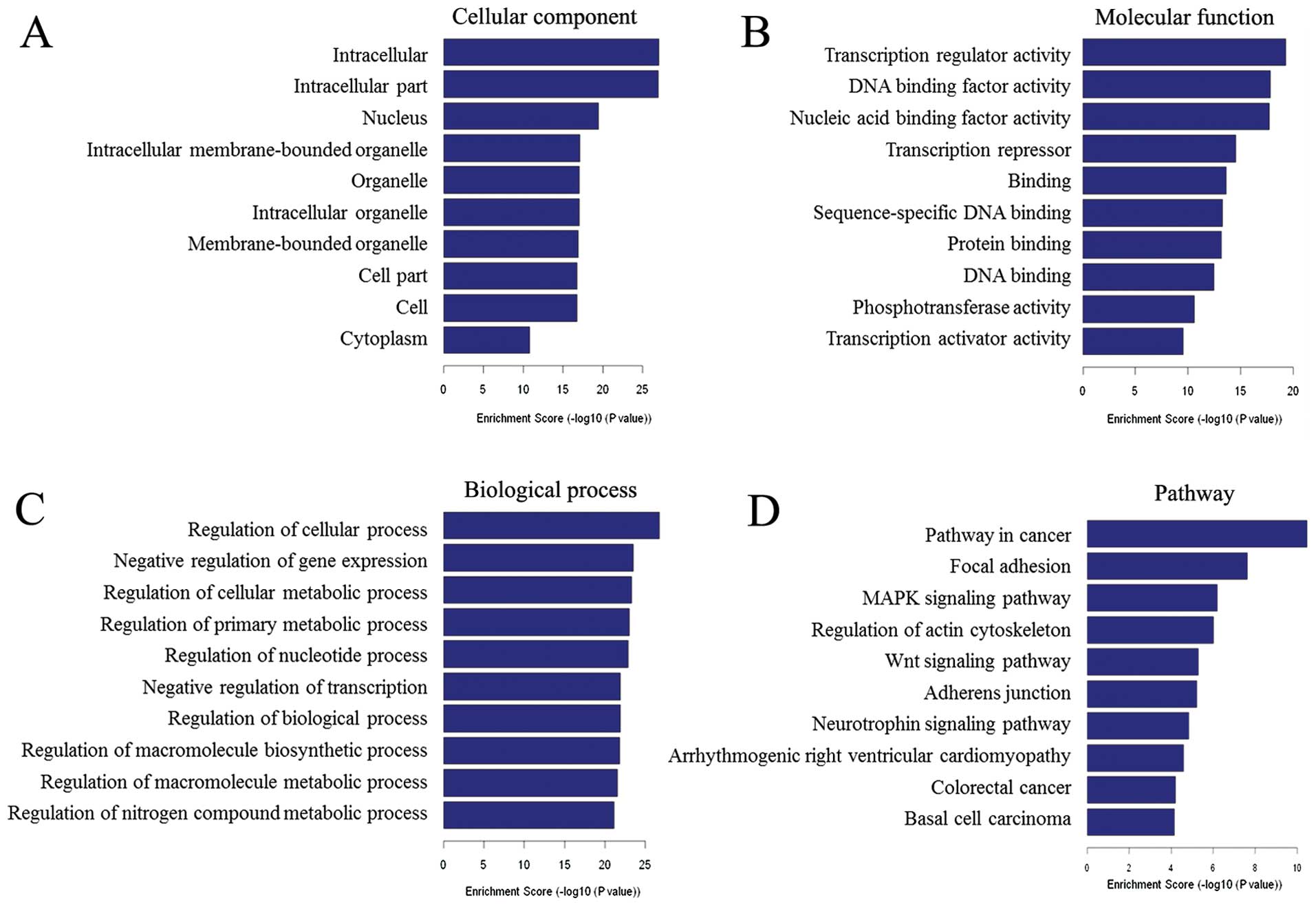
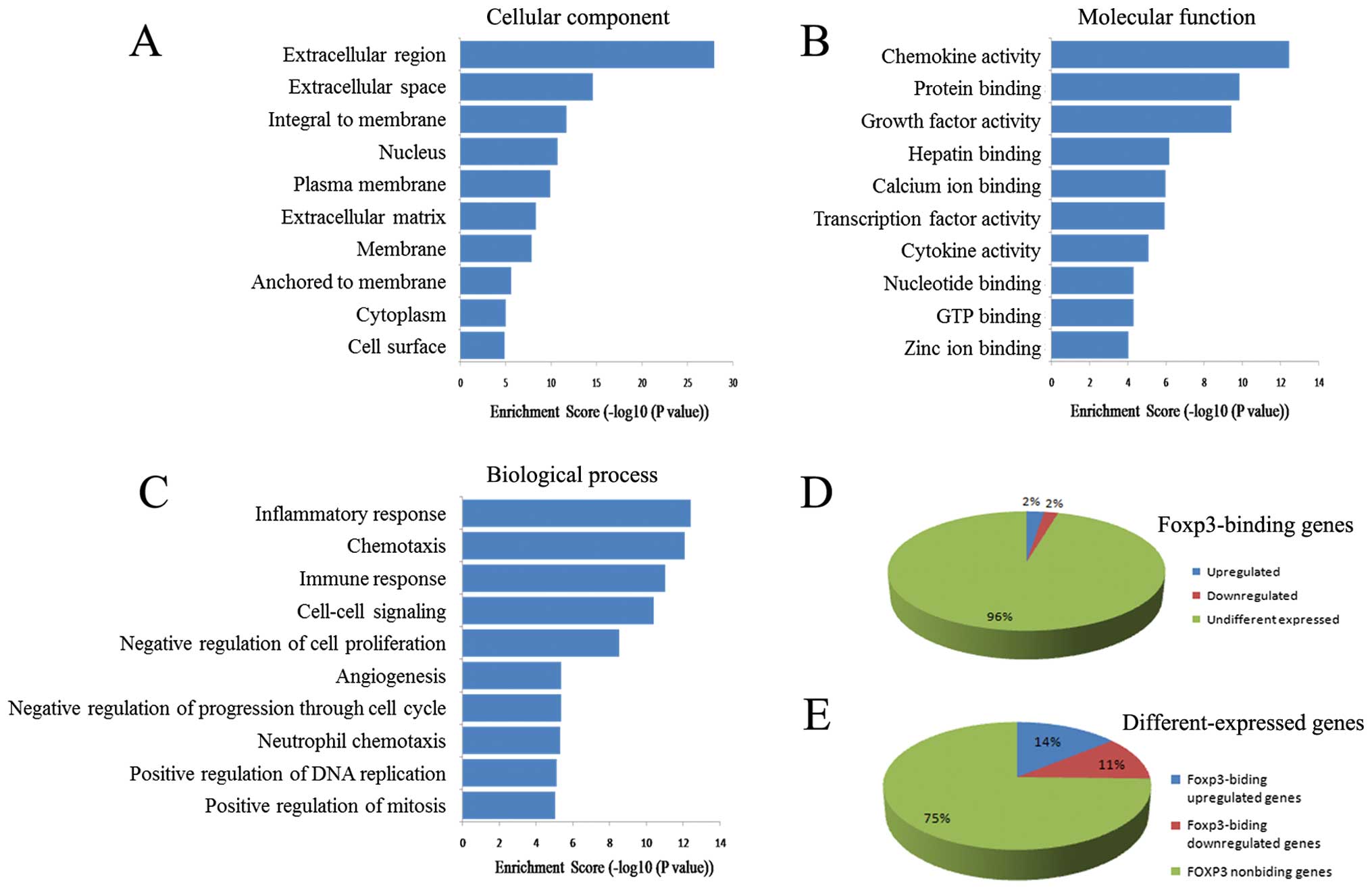
To reveal general functional features of the
molecular program implemented by Foxp3 in TSCC cell, we conducted
GO analysis of Foxp3-binding genes. Results showed that the
proteins encoded by Foxp3-binding genes mainly located in
intracellular parts of TSCC cells (top 10 GO terms - cellular
component, Fig. 4A), functioned in
transcriptional regulation and biological macromolecules biding
(top 10 GO terms - molecular function, Fig. 4B). Biological analysis showed that
the proteins encoded by Foxp3-binding genes were involved in many
general regulations (top 10 GO terms - biological process, Fig. 4C), and in the top 324 GO terms with
P-values <0.001, 131 terms (40.43%) were associated with
transcriptional regulation, both upregulation and downregulation.
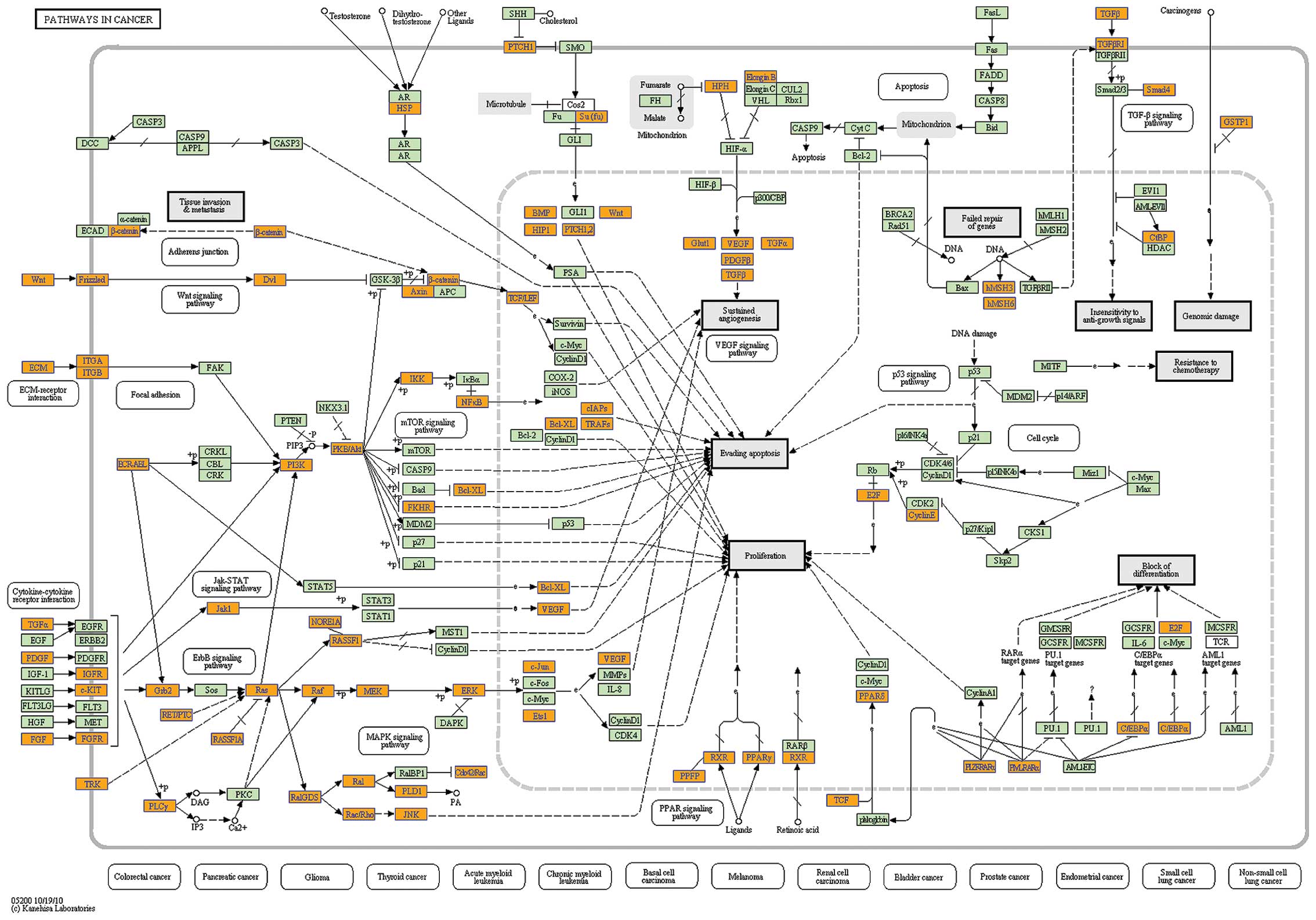
Analysis of our data with the KEGG database indicated that 9 of the
top 10 pathways were associated with cancer (Figs. 4D and 5).
Comparison of Foxp3-binding genes in
human TSCC cells and Tregs
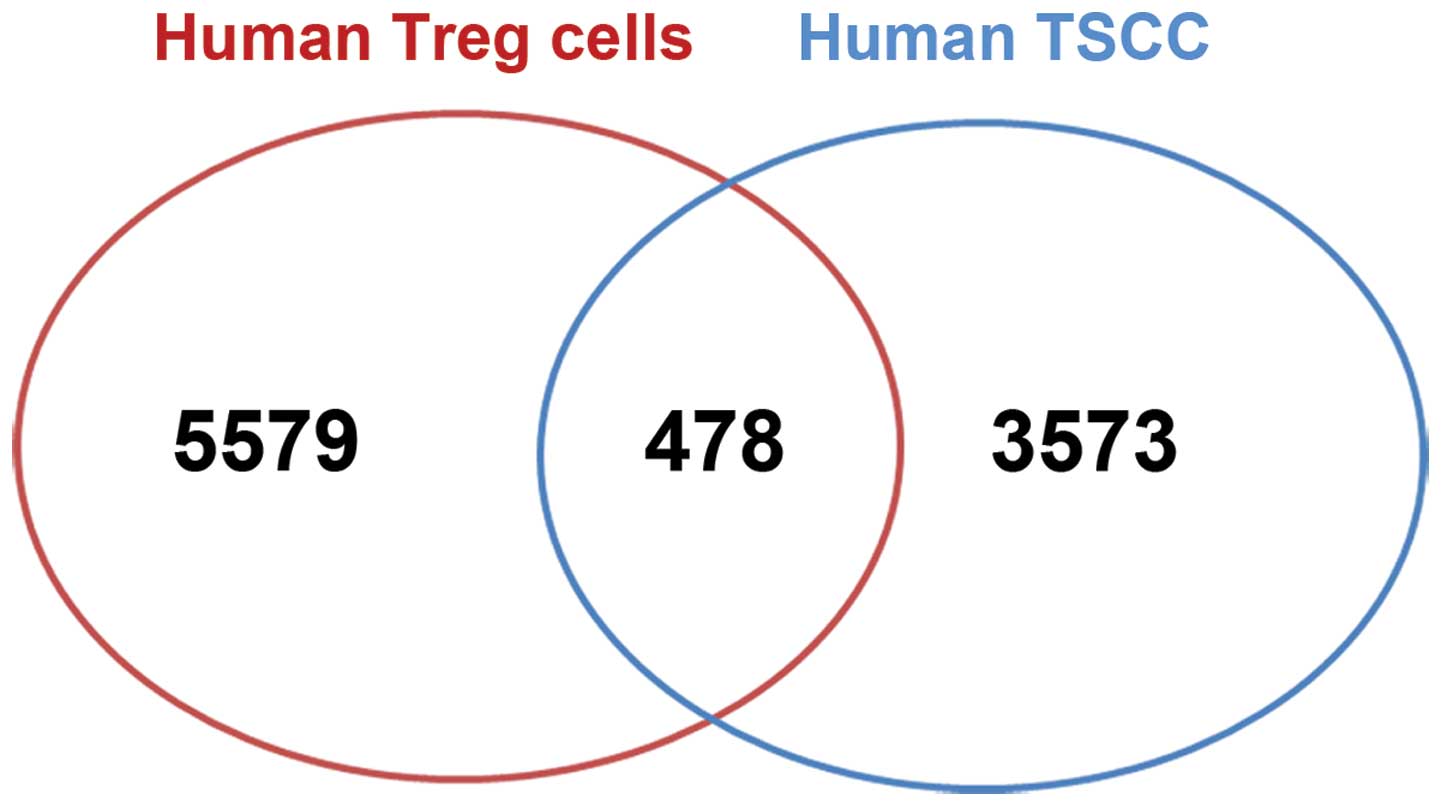
We compared the Foxp3-binding genes in human TSCC
cells with the known Foxp3 binding sites in human Tregs (18). Previous ChIP-on-chip data showed
that 5,579 genes were bound by Foxp3 in Tregs (18). Comparison results showed that 478
Foxp3-binding genes in our ChIP-on-chip data set were also
Foxp3-binding genes in human Tregs. This overlap corresponds to
12.28% of the Foxp3-binding genes in human TSCC cells and 8.75% of
the Foxp3-binding genes in Tregs (Fig.
6).
GO analysis of these 478 overlapped genes showed
similar results with the Foxp3-binding genes in human TSCC cells.
The encoded proteins were mainly localized on the cell membrane and
in the intracellular parts, and functioned in the regulation of
transcription and binding to nucleic acids or proteins, including
NF-κB (P=4.22×10−8), but rarely involved in T
cell-specific biological processes.
Previous analysis indicated that Foxp3-binding genes
in Tregs take part in 86 pathways, most of which are associated
with the differentiation, activation, and death of T cells under
normal and pathological conditions. However, pathway analysis of
overlapped genes also showed similar results with the Foxp3-binding
genes in human TSCC cells, and 7 of the top 10 pathways were
involved in cancer-related pathways.
Effect of Foxp3 on gene expression in
human TSCC cells
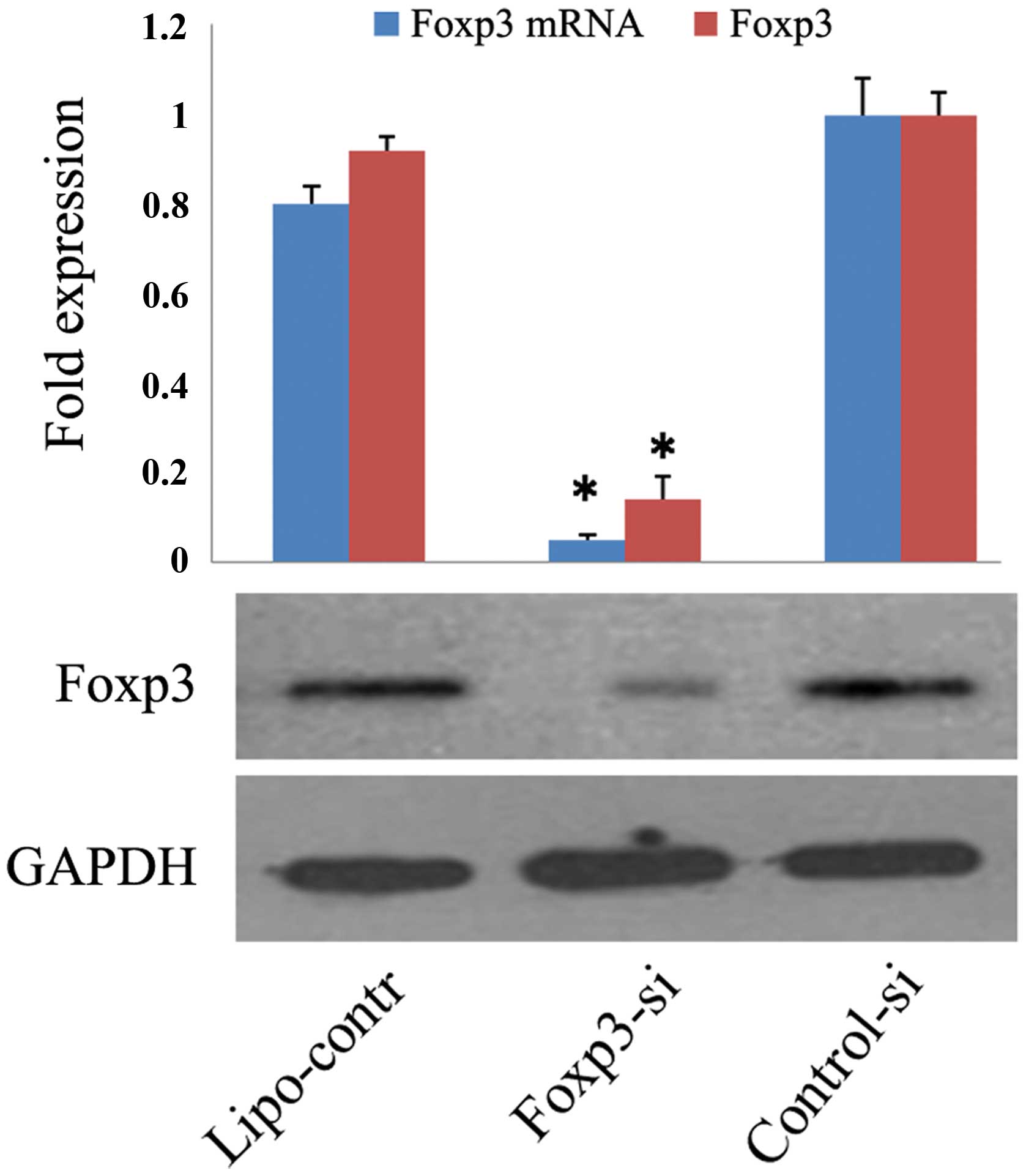
Next, we used siRNA to downregulate Foxp3 expression
in TSCC cells. In these experiments, SCC-9 cells were transfected
with Foxp3 siRNA (Foxp3-si group), control-siRNA (control-si
group), or Lipofectamine 2000 (lipo-control group). At 48 h after
transfection of SCC-9 cells, Foxp3 expression was downregulated by
85% in the Foxp3-si group relative to the control-si group
(P=0.001). There was no marked difference between control-si and
lipo-control groups (Fig. 7).
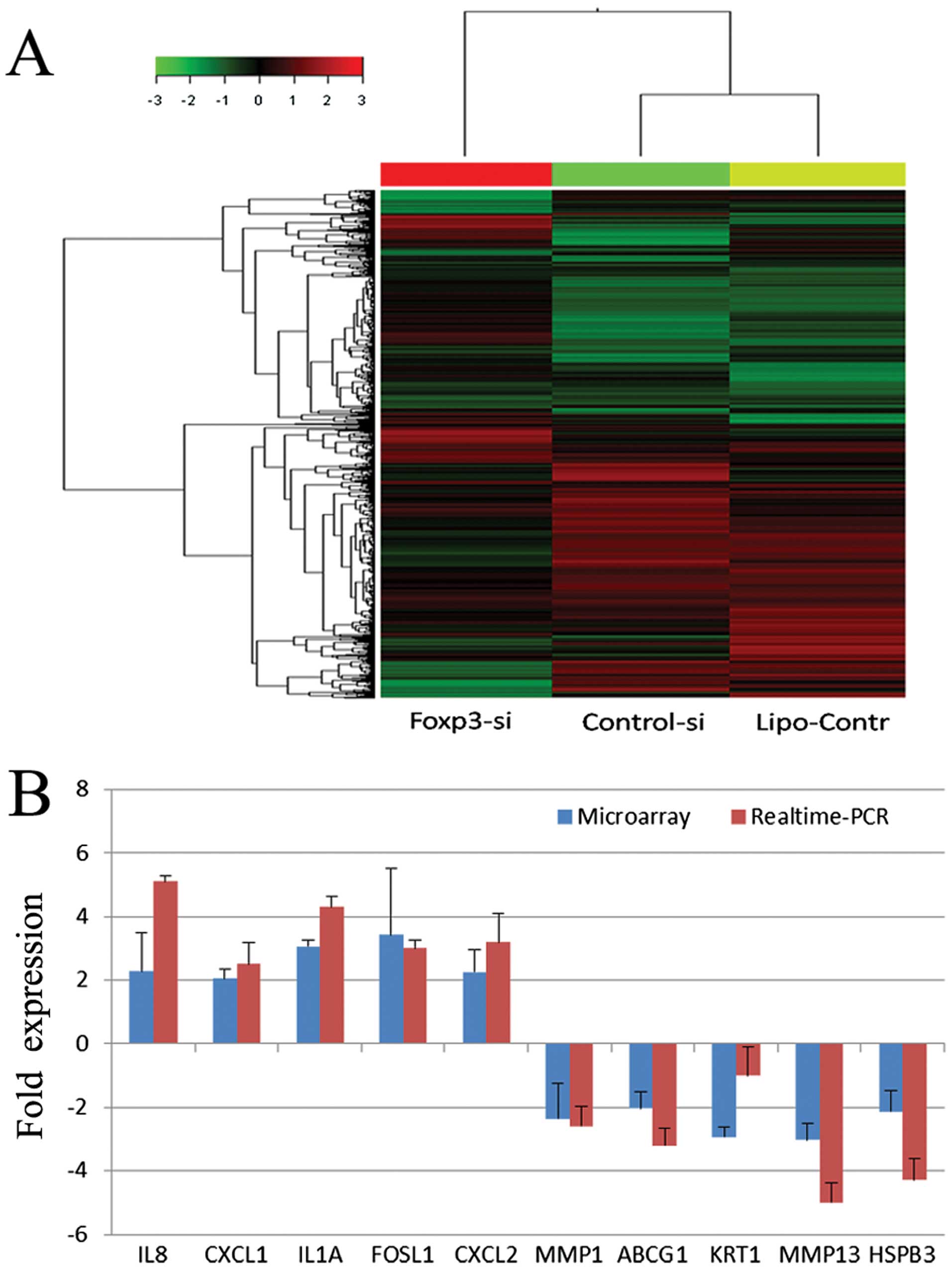
Then human whole-genome microarray assay showed that
there was no significant difference in the gene expression profiles
of the control-si group and the lipo-control group, and that the
Foxp3-si group was significantly different from the other two
groups (Fig. 8A). When the cut-off
ratio was set at 2-fold change in expression, 30 genes were
upregulated and 36 genes were downregulated in the Foxp3-si group.
When the cut-off ratio was set at 1.5-fold change in expression,
269 genes were upregulated and 330 genes were downregulated in the
Foxp3-si group. Real-time PCR of 10 randomly selected
different-expressed genes was performed to validate the microarray
results, as shown in Fig. 8B.
We further performed bioinformatics analysis on
these differently expressed genes. Cellular component analysis
showed that the proteins encoded by differently expressed genes
were mainly distributed in the extracellular parts and the cell
membrane (Fig. 9A). Molecular
function analysis showed that they were closely related to the
cytokine network in that they influenced chemokine activity, growth
factor activity, and cytokine activity (Fig. 9B). Analysis of the biological
processes showed that the top 10 terms were mainly associated with
the regulation of the microenvironment and immunity, such as
inflammatory responses, chemotaxis, immune responses, cell-cell
signaling, angiogenesis, and neutrophil chemotaxis (Fig. 9C).
Pathway analysis also showed that the proteins
encoded by differently expressed genes mainly took part in pathways
associated with cytokines and inflammatory reactions, such as
cytokine-cytokine receptor interactions, adhesion and diapedesis of
lymphocytes, adhesion and diapedesis of granulocytes, molecules
involved in local acute inflammatory responses, cytokines and
cytokine networks, and inflammatory responses.
Direct regulation of gene transcription
by Foxp3 in TSCC cells
The ChIP-on-chip and human genome-wide expression
profiling assay showed significant difference between the
differently expressed genes (after downregulation of Foxp3
expression) and Foxp3-binding genes in TSCC cells. Thus, we tried
to reveal the correlation between the data set of ChIP-on-chip and
profiling assay to identify genes that are directly regulated by
Foxp3. After cross-referencing the data set of Foxp3-binding genes
and differently expressed genes, results show that 152 genes
(associated genes) were identical in the ChIP-on-chip and
expression profiling, with 85 genes being upregulated and 67 genes
being downregulated. These associated genes accounted for 4.25%
(152/3573) of the Foxp3-binding genes (Fig. 9D) and 25.38% (152/599) of the
differently expressed genes (Fig.
9E).
When these associated genes were further analyzed,
results showed that the top GO term in cell component was the
nucleus. Molecular functions focused on nucleic acid and protein
biding, and had little association with the regulation of
cytokines. Analysis of biological processes showed that these genes
were similar to Foxp3-binding genes in TSCC cells, and that they
are extensively involved in different biological processes.
Notably, these genes were not specific for the regulation of
cytokines, immune responses, inflammatory reactions, and the
cellular microenvironment.
Pathway analysis by use of the KEGG database showed
that pathways with P-values <0.001 included adherens junction
(P=4.88×10−4), ECM-receptor interaction
(P=5.83×10−4), small cell lung cancer
(P=6.25×10−4), focal adhesion (P=6.77×10−4),
and nitrogen metabolism (P=9.80×10−4). These results are
also similar to those of FOXP3-binding genes in TSCC cells.
Discussion
In the present study, we performed a genome-wide
analysis of Foxp3 target genes in TSCC cells using a combination of
ChIP-on-chip and whole-genome microarray assays. We identified
direct and indirect target genes of cancer cell-derived Foxp3 for
the first time. Our data suggest that cancer cell-derived Foxp3
directly regulates the transcription of genes that affect certain
internal biological processes of TSCC cells, and indirectly
influences the extracellular inflammatory micro-environment.
Foxp3 in Tregs is a well-known inducible
transcriptional factor. In Tregs, Foxp3 mainly localize in the
cytoplasm or adjacent to the nucleus when cells are unstimulated.
Upon stimulation with the anti-CD3 or anti-CD28 antibody, Foxp3
undergoes T cell receptor (TCR)-mediated post-transcriptional
modification, and within 1 h is translocated into the nucleus
(13). However, additional factors
may also be needed in Foxp3 translocation after TCR stimulation
(20). Moreover, TCR is a specific
receptor on the surface of T cells rather than other cell types.
Therefore, it is necessary and still difficult to elucidate the
mechanism of Foxp3 translocation in tumor cells. Our results showed
that Foxp3 expressed in TSCC cells can enter the nucleus, even in
the absence of serum. This suggests that cancer cell-derived Foxp3
entry into the nucleus is independent of exogenous stimuli. We
speculate that non-microenvironment dependent signal peptide may
exist in cancer cell-derived Foxp3, and that factors expressed by
TSCC cells themselves may promote Foxp3 translocation into the
nucleus, even that TSCC cells can secret factors into
microenvironment to induce Foxp3 translocation in Tregs, which can
be an exquisite ‘cross-talk’ between tumor cells and lymphocytes.
Elucidation of the specific mechanism requires further
investigation.
The capability of nucleus translocation of Foxp3 in
these cell lines created basic conditions for genome study. We
initially speculated that cancer cell-derived Foxp3 may directly
regulate the transcription of some extracellular factors, such as
cytokines and chemokines, similarly to Foxp3 in Tregs. However,
when we used the ChIP-on-chip assay to identify Foxp3 binding sites
in the genome of TSCC cells, bioinformatic analysis indicated that
proteins encoded by these genes are mainly localized within TSCC
cells, and many of these genes are involved in cancer related
biological processes. In particular, analysis of molecular function
showed that these proteins may bind to multiple proteins, including
other transcriptional factors, and this may lead to co-regulation
of related genes or alter the levels of free transcriptional
factors and thereby affect transcription. Therefore, cancer
cell-derived Foxp3 appears to regulate gene transcription through
multiple patterns, such as direct regulation, regulation of other
transcriptional factors, and regulation of proteins that bind to
other transcriptional factors. In the study of Tregs, Rudra et
al (16) also showed that,
Foxp3 directly binds to genes and regulates the expression of
proteins that bind to and regulate Foxp3 itself. This is the first
report presenting the DNA binding profile of cancer cell-derived
Foxp3. Pathway analysis further showed that the proteins encoded by
Foxp3-binding genes are associated with cancer-related
pathways.
Sadlon et al (18) performed ChIP-on-chip studies of
Foxp3 in human Tregs in 2010 and the genes they identified were
distributed in 86 pathways, most of which were associated with the
functions and life activities of T cells. In this study, 11 of the
pathways that we identified in TSCC cells were also present in Treg
cells; four pathways with high enrichment were closely related to
cancer. We also compared the Foxp3-binding genes in TSCC cells with
those in Treg cells. The results showed that only 478 genes (13.38%
of Foxp3-binding genes in TSCC cells) were in both TSCC cells and
Tregs. These findings suggest that there are significant
differences in the genes regulated by Foxp3 in Tregs and TSCC
cells, and that cancer cell-derived Foxp3 has distinct biological
functions.
We performed genome-wide expression profiling in
TSCC cells after downregulation of Foxp3 by RNAi. The results
confirmed that cancer cell-derived Foxp3 affected gene expression
in TSCC cells. Analysis showed that the proteins encoded by these
differently expressed genes were mainly distributed in the
extracellular domain and cell membrane, and functioned to influence
the extracellular microenvironment and inflammation, such as
components of the extracellular matrix, intercellular signal
transduction, activities of chemokines, growth factors and
cytokines (including IL-8 signaling, down-regulation of IFN-γ and
upregulation of IL-6). As cytokine network in tumor
microenvironment affect Tregs proliferation and function (21,22),
this part of findings are consistent with the hypothesis that
cancer cell-derived Foxp3 regulates the microenvironment through
its association with Tregs, thereby influencing the tumor immune
response. Still, this hypothesis requires confirmation by further
studies.
Notably, the proteins encoded by Foxp3-binding genes
were mainly localized within TSCC cells. Therefore, the differently
expressed genes and Foxp3-binding genes were significantly
different in profile (only 25.38% overlap), protein distribution
and function, which indicated that genes eventually influenced by
cancer cell-derived Foxp3 are significantly different from those
directly regulated by cancer cell-derived Foxp3. This suggests that
cancer cell-derived Foxp3 indirectly affect the inflammatory
microenvironment of TSCC.
However, the 25.38% overlap (associated genes)
indicated that cancer cell-derived Foxp3 could also directly
regulate gene transcription in TSCC cells. The results of
bioinformatic analysis associated genes are similar to those of
Foxp3-binding genes in TSCC cells and are widely involved in a
variety of cellular processes, rather than the regulation of
microenvironment and inflammation. These findings indicate that
cancer cell-derived Foxp3 may directly regulate gene transcription
and influence a fraction of biological processes in TSCC cells, and
indirectly regulate gene transcription to affect the extra-cellular
inflammatory microenvironment. Further studies such as
dual-luciferase reporter gene assay and microenvironment
co-cultured model could be used to confirm the mechanism of
regulating specific genes by cancer cell-derived Foxp3.
In conclusion, we have, for the first time,
identified direct and indirect target genes of cancer cell-derived
Foxp3 in TSCC cells. Cancer cell-derived Foxp3 directly regulate
the transcription of genes that affect certain internal biological
processes of TSCC cells, and indirectly influence the extracellular
microenvironment.
Acknowledgements
This study was supported by grants from National
Natural Science Foundation of China (nos. 81172566, 81372884 and
81302367), Specialized Research Fund for the Doctoral Program of
Higher Education of China (no. 20130171120125), Natural Science
Foundation of Guangdong Province (no. S2013040015004).
References
|
1
|
Marson A, Kretschmer K, Frampton GM,
Jacobsen ES, Polansky JK, MacIsaac KD, Levine SS, Fraenkel E, von
Boehmer H and Young RA: Foxp3 occupancy and regulation of key
target genes during T-cell stimulation. Nature. 445:931–935. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gavin MA, Rasmussen JP, Fontenot JD, Vasta
V, Manganiello VC, Beavo JA and Rudensky AY: Foxp3-dependent
programme of regulatory T-cell differentiation. Nature.
445:771–775. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakaguchi S: Immunology: Conditional
stability of T cells. Nature. 468:41–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hinz S, Pagerols-Raluy L, Oberg HH,
Ammerpohl O, Grüssel S, Sipos B, Grützmann R, Pilarsky C,
Ungefroren H, Saeger HD, et al: Foxp3 expression in pancreatic
carcinoma cells as a novel mechanism of immune evasion in cancer.
Cancer Res. 67:8344–8350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Merlo A, Casalini P, Carcangiu ML,
Malventano C, Triulzi T, Mènard S, Tagliabue E and Balsari A: FOXP3
expression and overall survival in breast cancer. J Clin Oncol.
27:1746–1752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zuo T, Wang L, Morrison C, Chang X, Zhang
H, Li W, Liu Y, Wang Y, Liu X, Chan MW, et al: FOXP3 is an X-linked
breast cancer suppressor gene and an important repressor of the
HER-2/ErbB2 oncogene. Cell. 129:1275–1286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ladoire S, Arnould L, Mignot G, Coudert B,
Rébé C, Chalmin F, Vincent J, Bruchard M, Chauffert B, Martin F, et
al: Presence of Foxp3 expression in tumor cells predicts better
survival in HER2-overexpressing breast cancer patients treated with
neoadjuvant chemotherapy. Breast Cancer Res Treat. 125:65–72. 2011.
View Article : Google Scholar
|
|
8
|
Wang L, Liu R, Li W, Chen C, Katoh H, Chen
GY, McNally B, Lin L, Zhou P, Zuo T, et al: Somatic single hits
inactivate the X-linked tumor suppressor FOXP3 in the prostate.
Cancer Cell. 16:336–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Y, Borde M, Heissmeyer V, Feuerer M,
Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, et al:
FOXP3 controls regulatory T cell function through cooperation with
NFAT. Cell. 126:375–387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bettelli E, Dastrange M and Oukka M: Foxp3
interacts with nuclear factor of activated T cells and NF-kappa B
to repress cytokine gene expression and effector functions of T
helper cells. Proc Natl Acad Sci USA. 102:5138–5143. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chaudhry A, Rudra D, Treuting P, Samstein
RM, Liang Y, Kas A and Rudensky AY: CD4+ regulatory T
cells control TH17 responses in a Stat3-dependent manner. Science.
326:986–991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ono M, Yaguchi H, Ohkura N, Kitabayashi I,
Nagamura Y, Nomura T, Miyachi Y, Tsukada T and Sakaguchi S: Foxp3
controls regulatory T-cell function by interacting with AML1/Runx1.
Nature. 446:685–689. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen C, Rowell EA, Thomas RM, Hancock WW
and Wells AD: Transcriptional regulation by Foxp3 is associated
with direct promoter occupancy and modulation of histone
acetylation. J Biol Chem. 281:36828–36834. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li B, Samanta A, Song X, Iacono KT, Bembas
K, Tao R, Basu S, Riley JL, Hancock WW, Shen Y, et al: FOXP3
interactions with histone acetyltransferase and class II histone
deacetylases are required for repression. Proc Natl Acad Sci USA.
104:4571–4576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng Y, Josefowicz SZ, Kas A, Chu TT,
Gavin MA and Rudensky AY: Genome-wide analysis of Foxp3 target
genes in developing and mature regulatory T cells. Nature.
445:936–940. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rudra D, deRoos P, Chaudhry A, Niec RE,
Arvey A, Samstein RM, Leslie C, Shaffer SA, Goodlett DR and
Rudensky AY: Transcription factor Foxp3 and its protein partners
form a complex regulatory network. Nat Immunol. 13:1010–1019. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang YJ, Liu HC, Su YX, Zhang TH, Chu M,
Liang LZ and Liao GQ: Foxp3 expressed by tongue squamous cell
carcinoma cells correlates with clinicopathologic features and
overall survival in tongue squamous cell carcinoma patients. Oral
Oncol. 47:566–570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sadlon TJ, Wilkinson BG, Pederson S, Brown
CY, Bresatz S, Gargett T, Melville EL, Peng K, D’Andrea RJ, Glonek
GG, et al: Genome-wide identification of human FOXP3 target genes
in natural regulatory T cells. J Immunol. 185:1071–1081. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X and Seed B: A PCR primer bank for
quantitative gene expression analysis. Nucleic Acids Res.
31:e1542003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hancock WW and Ozkaynak E: Three distinct
domains contribute to nuclear transport of murine Foxp3. PLoS One.
4:e78902009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Medzhitov R: Inflammation 2010: New
adventures of an old flame. Cell. 140:771–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cantini G, Pisati F, Mastropietro A,
Frattini V, Iwakura Y, Finocchiaro G and Pellegatta S: A critical
role for regulatory T cells in driving cytokine profiles of Th17
cells and their modulation of glioma microenvironment. Cancer
Immunol Immunother. 60:1739–1750. 2011. View Article : Google Scholar : PubMed/NCBI
|