Introduction
Gastrointestinal cancers account for a large portion
of cancer-related death worldwide, including Korea. Among them,
gastric cancer is still one of the most common leading causes of
death, and if it is diagnosed in advanced or metastatic stage, the
overall prognosis is still poor even though there has been rapid
progress for therapeutic modalities of gastric cancer, including
surgery or chemotherapy. Thus, there is a need to find novel
therapeutic agents effective against malignant gastric disease,
especially for advanced or metastatic cancer.
Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) is
a quinonoid constituent extracted from the roots of the medicinal
plant Plumbago zeylanica L (1). The roots of Plumbago zeylanica
have been used for various treatment aims in Oriental medicine
fields. One promising effect of plumbagin is that it shows
antitumor potential against various types of cancer. For example,
an in vivo study demonstrated that plumbagin significantly
inhibited the growth of azoxymethane-induced intestinal tumors in
rats (2), and several in
vitro studies reported that plumbagin showed anti-carcinogenic
effects including cell proliferation or invasion or induced cell
cycle arrest or apoptosis in breast cancer (3), melanoma (4), non-small lung cancer (5) or prostate cancer cells (6).
Several pivotal studies have clearly demonstrated
the molecular mechanisms of antitumor effects of plumbagin in
various types of cancer cells. Hafeez et al reported that
plumbagin inhibited constitutive expression of epidermal growth
factor receptor (EGFR), phosphorylation and DNA binding activity of
signal transducer and activator of transcription 3 (STAT3) and
nuclear factor-κB (NF-κB) in pancreas cancer cells (7). Manu et al showed that
plumbagin has a potential blocking activity of CXC chemokine
receptor 4 (CXCR4) and potential for inhibition of invasion and
migration in breast and gastric cancer cells (8). A recent in vitro study
investigated the underlying mechanism of plumbagin in gastric
cancer cells, and demonstrated that plumbagin inhibited NF-κB p65
nuclear translocation and phosphorylation of p65, IκBα and IκBα
kinase (IKKα), and downregulated NF-κB-related gene products, such
as inhibitor of apoptosis 1 (IAP1), X-linked inhibitor of apoptosis
(XIAP), B-cell lymphoma-2 (Bcl-2), Bcl-xL and vascular endothelial
growth factor (VEGF) (9). Another
well-designed in vitro study showed that plumbagin
suppresses STAT3 activation pathway through induction of
SH2-containing protein tyrosine phosphatase 1 (SHP1), a
non-receptor type protein tyrosine phosphatase (PTPase), in
multiple myeloma cells (10).
However, impact of plumbagin on STAT3 signaling pathway in gastric
carcinogenesis has not been reported yet.
Previously, in vitro, we observed that SHP1
expression was markedly reduced or negative in various gastric
cancer cell lines, which was mainly caused by epigenetic silencing
mechanism, and exogenous introduction of SHP1 plasmid significantly
downregulated Janus kinase 2 (JAK2)/STAT3 pathway and their target
genes (unpublished data). From this background, we aimed in this
in vitro study to demonstrate the ability of plumbagin to
induce SHP1 expression and suppress JAK2/STAT3 signaling pathway in
gastric cancer cells.
Materials and methods
Reagents and cell line
Plumbagin (purity >97%) was purchased from
Sigma-Aldrich (St. Louis, MO, USA) and, dissolved in dimethyl
sulfoxide (DMSO) to a concentration of 100 mmol/l and stored at
−20°C, and diluted to indicated concentration immediately before
use. Recombinant human interleukin-6 (IL-6) and broad-acting PTPase
inhibitor sodium pervanadate was purchased from Sigma-Aldrich. A
rabbit polyclonal IgG antibody against human SHP1 (sc-287) and
β-actin (sc-47778) was purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Mouse monoclonal IgG antibodies against
human STAT3 (no. 9139) and phospho-STAT3 (Tyr 705, no. 4113), and
rabbit polyclonal antibodies against human JAK2 (no. 3230) and
phospho-JAK2 (Tyr 1007/1008, no. 3771) were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA).
The human gastric cancer cell line (MKN-28) was
obtained from Korean Cell Line Bank (Seoul National University,
Seoul, Korea), and cultured in RPMI supplemented with 10%
heat-inactivated FBS and penicillin/streptomycin (1.0%) (all from
Gibco, Carlsbad, CA, USA). Cells were incubated in a humidified
atmosphere of 5% CO2 at 37°C.
Western blot analysis
Total 80–100 μg of cytoplasmic proteins were
extracted using CelLytic M (C2978; Sigma-Aldrich) with Complete
Mini (pretease inhibitor cocktail; Roche Diagnostics GmbH,
Mannheim, Germany). Primary antibodies were diluted at 1:1,000 in
the blocking buffer (Tris-buffered saline with Tween-20; Biosesang,
Gyeonggi, Korea) containing 5% non-fat skim milk (Difco;
Becton-Dickinson and Co., Sparks, MD, USA). Probed membranes were
incubated for 12 h at 4°C. The membranes were incubated with goat
anti-mouse or anti-rabbit IgG as a secondary antibody for 1 h at
room temperature. The protein bands were detected by exposing
membrane to enhanced chemiluminescence (Perkin-Elmer, Wathman, MA,
USA) for 1 min.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from whole cells using
TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA) method,
and subsequently complementary DNA (cDNA) was produced by using
High Capacity cDNA Reverse Transcription kit (Applied Biosystems,
Foster City, CA, USA) and treatment with 1 unit of DNase (Promega
Corp., Fitchburg, WI, USA). We performed RT-PCR by modifying a
previously described method. In brief, 20 ng of prepared cDNA was
used to make 25 μl of PCR product using EconoTaq® Plus
Green Master Mix (Lucigen Corp., Middleton, WI, USA). PCR was done
under the following conditions: initial denaturation at 94°C (2
min), followed by 30–40 cycles of denaturation at 94°C (15 sec),
annealing at 55°C (15 sec), extension at 72°C (15 sec) and final
extension at 72°C (10 min). The oligonucleotide sequences are
summarized in Table I.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as
a housekeeping gene for each sample. PCR products (5 μl) were
loaded on a 2% agarose gel, and positive bands were obtained by
staining with ethidium bromide (Amresco LLC, Solon, OH, USA).
 | Table ICharacteristics of primers for
RT-PCR. |
Table I
Characteristics of primers for
RT-PCR.
| Gene | Primers
(5′→3′) | GenBank accession
no. | Size (bp) | Reference |
|---|
| SHP1 | F :
ACTGAGCTGCATCTGAG
R : CCACTGACGAGAGC | NM_080549.2 | 240 | (31) |
| Cyclin
D1 | F :
TTCGATGATTGGAATAGC
R : TGTGAGCTGCTCATTGAG | XM_006718653.1 | 150 | (44) |
| VEGF-1 | F :
GGAGTGTGTGCACGAGAGTCAC
R : GGTCGACTGAGAGCT | NM_001287044.1 | 343 | (45) |
|
Survivin | F :
TTCTGCACATCTGAGTCG
R : TGTCGAGAGCTCAGT | NM_001012271.1 | 391 | (46) |
| MMP-9 | F :
TTGACAGCGACAGAGTG
R : GCATTCACGTCGTCCTTAT | NM_004994.2 | 179 | (47) |
| GAPDH | F :
GGTCTCTCTGACTCACA
R : AGCATTCGTTGTCATAC | NM_002046 | 116 | (48) |
Water soluble tetrazolium salt-1 (WST-1)
cell proliferation assay
To quantify the inhibitory effect of plumbagin on
cellular proliferation, we used a commercial WST-1 assay kit
(EZ-Cytox; DoGen, Seoul, Korea) as manufacturer’s instructions
(11). Briefly, 1×104
MKN-28 cells/well were cultured in a 96-well plate at 37°C for 24
h, and treated with plumbagin at 20 or 40 μM for 3, 6 and 9 h. We
also cultured untreated cells for the same time period as a
control. After treatment, 10 μl of WST was added in each well for 4
h, and absorbance at 450 nm was measured by an ELISA reader (Epoch;
BioTek Instruments, Inc., Seoul, Korea). All the experiments were
performed in triplicate.
Wound healing assay
After treated with plumbagin at 20 or 40 μM for 6 h,
cells were equally seeded on a 6-well plate chamber, and after
attachment, a monolayer wound was made using 200 μl pipette tip.
The media were changed to remove floating debris, and the vertical
distance between the sides of the wound was measured at 24 and 48 h
after wound injury using software (12). All the experiments were performed
in triplicate.
Matrigel invasion assay
Following treatment with plumbagin at 20 or 40 μM
for 6 h, 4×104 cells/well were placed into the 24-well
Matrigel Invasion Chambers (BD Biosciences, Franklin Lakes, NJ,
USA) in 2% FBS medium, and in the lower wells 10% FBS was added.
After 24 h of incubation, filter membranes were stained with
crystal violet, and the number of positive invading cells which
penetrated through membrane pore was counted under ×20
magnification in at least five randomly selected separate
areas.
Annexin V assay
To compare the different percentage of apoptotic
cells by plumbagin treatment, we used an Annexin V-FITC assay kit
(Anse Technologies Co., Ltd., Seoul, Korea) according to the
manufacturer’s instructions. Briefly, 1×106 MKN-28
cells/well were treated with 20 or 40 μM of plumbagin for 6 h,
washed and resuspended in binding buffer, followed by staining with
an Annexin V-FITC and propidium iodide (PI) solution for 10 and 30
min, respectively. After staining, samples were analyzed using
FACSCalibur Flow Cytometer (Becton-Dickinson and Co., San Jose, CA,
USA).
Statistical analysis
The SPSS ver. 19.0 (SPSS, Inc., Chicago, IL, USA)
was used for all analyses. Continuous data are presented as mean ±
standard deviation. A Student’s t-test was performed for continuous
data, and p<0.05 was considered as statistically
significant.
Results
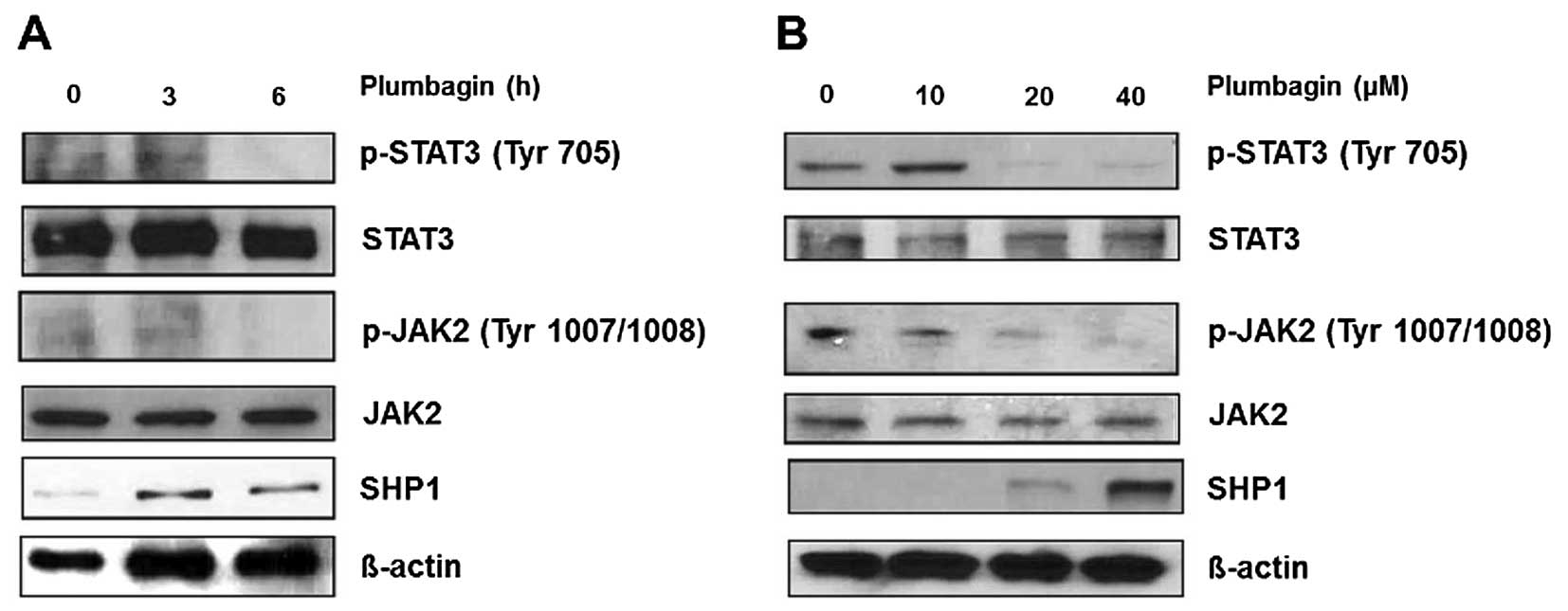
Plumbagin inhibits phosphorylation of
constitutive STAT3 in MKN-28 cells
First, we investigated the effect of plumbagin to
modulate the activity of JAK2/STAT3 signaling in the gastric cancer
cells. MKN-28 cells were treated with different concentration (10,
20 and 40 μM) of plumbagin for 6 h, and western blot analysis was
performed to measure phosphorylation level of JAK2 at tyrosine
1007/1008 and STAT3 at tyrosine 705. Plumbagin significantly
inhibited phosphorylation of JAK2 and STAT3 from 20 μM, and in
contrast, total JAK2 and STAT3 showed similar level regardless of
plumbagin treatment, which supports that plumbagin negatively
modulates JAK2/STAT3 activity mainly via dephosphorylation rather
than protein degradation. Because SHP1 is one of non-transmembrane
PTPase which negatively modulate JAK2/STAT3 signaling in epithelial
cells (13), we also observed
induction of SHP1 expression by plumbagin treatment starting at 20
μM (Fig. 1A). Our data suggest
that plumbagin might dephosphorylate and downregulate JAK2/STAT3
activity by induction of SHP1 expression in MKN-28 cells. We also
observed the time-course to inhibit phosphorylation of JAK2/STAT3
and to induce SHP1 by treatment with 40 μM of plumbagin for
indicated time points. Phosphorylation of JAK2/STAT3 was
ameliorated at 6 h, whereas SHP1 expression appeared at 3 h, which
suggests that a time-lag might exist between induction of SHP1 and
downregulation of JAK2/STAT3 activity in MKN-28 cells (Fig. 1B).
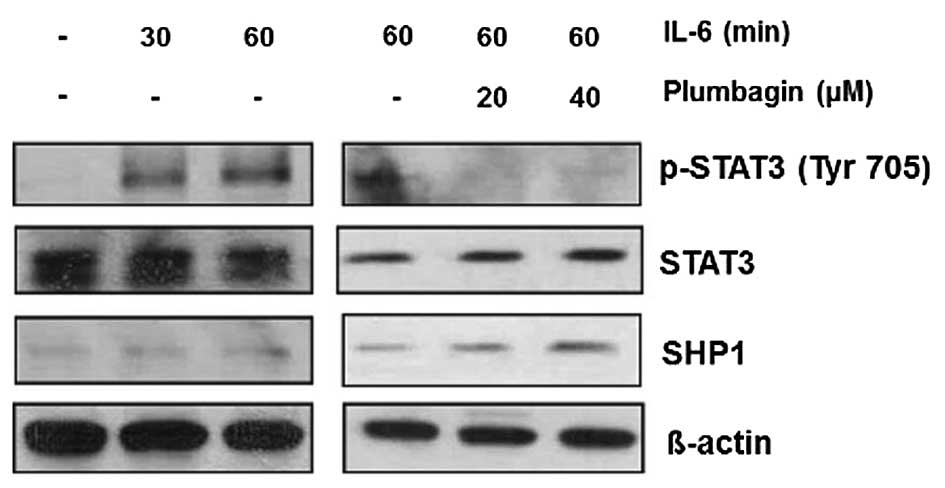
Plumbagin inhibits IL-6-induced STAT3
phosphorylation in MKN-28 cells
Previous in vitro studies demonstrated that
stimulation with human recombinant IL-6 upregulates phosphorylated
STAT3 level to promote invasive activity in gastric cancer cells
(14,15). Thus, we investigated whether
plumbagin could modulate IL-6-induced phosphorylation of STAT3 in
gastric cancer cells. MKN-28 cells were stimulated with 50 ng/ml of
IL-6 at indicated time points (30 and 60 min), and we observed that
phosphorylated STAT3 was significantly upregulated from 30 min,
whereas SHP1 expression continued to be weakly positive. However,
treatment with 20 and 40 μM of plumbagin for 6 h, phosphorylated
STAT3 ameliorated even in 60 min stimulation with 50 ng/ml of IL-6,
and SHP1 expression was restored during stimulation (Fig. 2). These findings suggest that
plumbagin can also suppress IL-6-induced STAT3 phosphorylation as
well as constitutive STAT3 phosphorylation, and SHP1 might play a
critical role in this process.
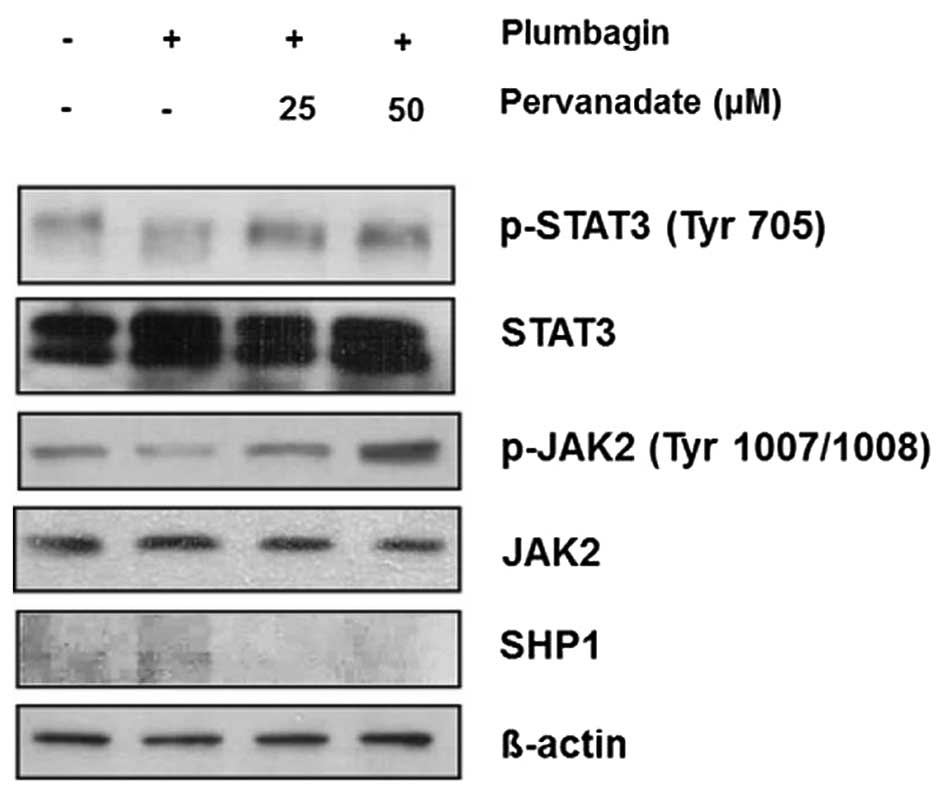
Inhibition of SHP1 restores
phosphorylation of JAK2 and STAT3 in MKN-28 cells
Because we observed that SHP1 was implicated in the
dephosphorylation and inactivation of JAK2/STAT3 signaling, for the
next step, we validated this mechanism by using PTPase inhibitor
sodium pervanadate (10,16,17).
Treatment with indicated concentration of pervanadate and 40 μM of
plumbagin for 6 h, and western blot analysis showed that plumbagin
downregulated phosphorylation of JAK2 and STAT3, this effect was
restored by adding 25 and 50 μM of pervanadate, whereas SHP1
expression showed the opposite pattern, it was restored by
treatment with plumbagin but ameliorated by combination with
pervanadate (Fig. 3). Taken
together, these findings also support our suggestion that SHP1
might be closely related to the mechanism of plumbagin-induced
inhibition of JAK2/STAT3 activity in MKN-28 cells.
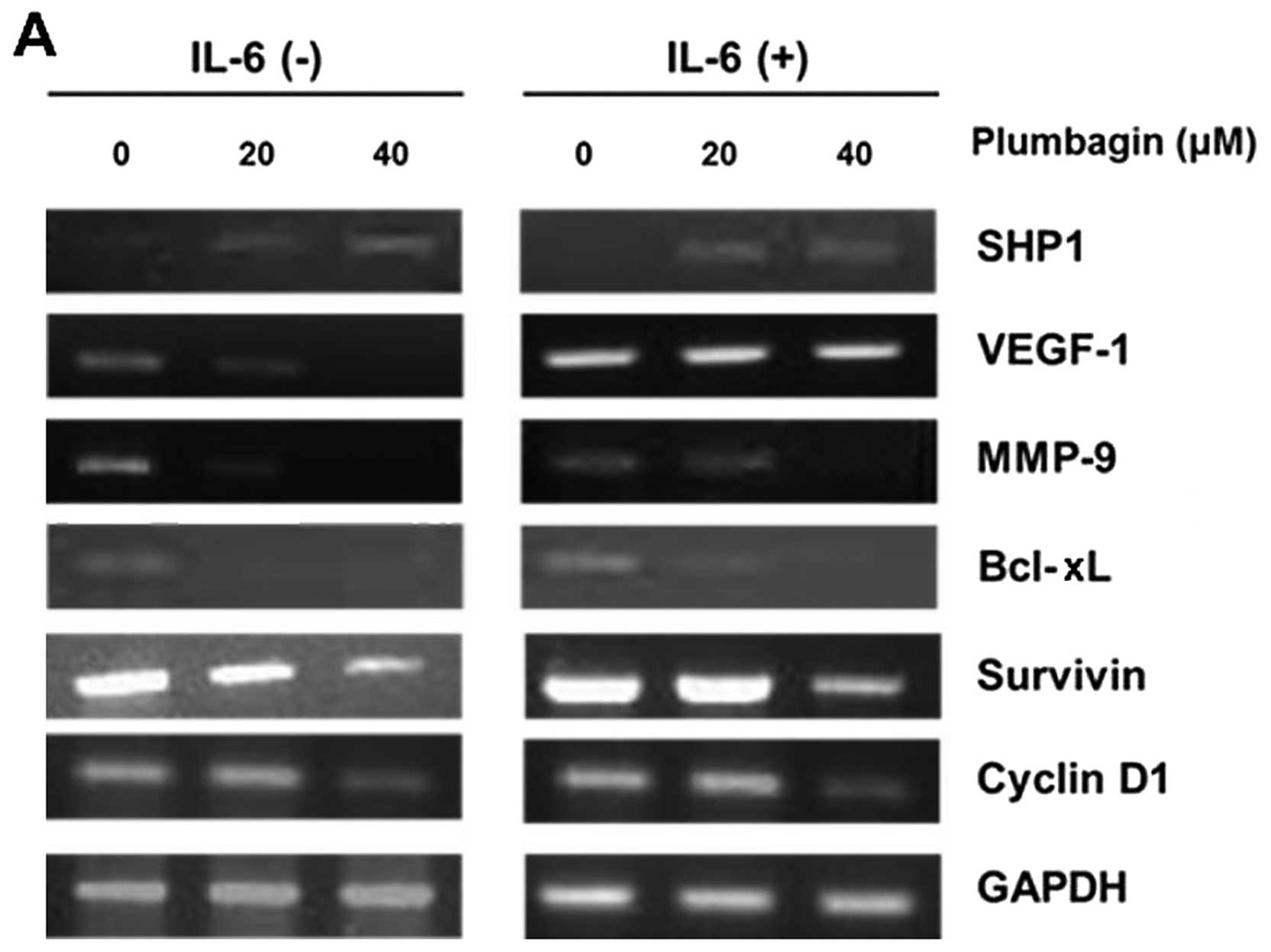
Plumbagin downregulates STAT3-associated
target genes in MKN-28 cells
In various human malignancies including gastric
cancer, STAT3 is commonly activated and acts as a pivotal
transcription factor to upregulate multiple target genes involving
proliferation, invasion/metastasis and anti-apoptosis, such as
VEGF-1, matrix metalloproteinase-9 (MMP-9), Bcl-xL, survivin or
cyclin D1 (18). To investigate
the effect of plumbagin in regulating target gene expression
related to STAT3 pathway in gastric cancer cells, treatment with 20
or 40 μM of plumbagin was performed for 6 h with or without
stimulation of IL-6, and by RT-PCR. Plumbagin restored SHP1
expression in both constitutive and IL-6-stimulated conditions, and
reduced gene expression of VEGF-1, MMP-9, Bcl-xL, survivin and
cyclin D1, which were maximally reduced by 40 μM concentration
(Fig. 4). These findings suggest
that plumbagin modulates mRNA expression of STAT3-related target
genes via restoration of SHP1 expression in MKN-28 cells.
Plumbagin inhibits cell proliferation,
migration and invasion, and induces apoptosis in MKN-28 cells
To determine functional effects of plumbagin for
STAT3-related cellular proliferation, migration, invasion and
apoptosis in gastric cancer cells, MKN-28 cells were treated with
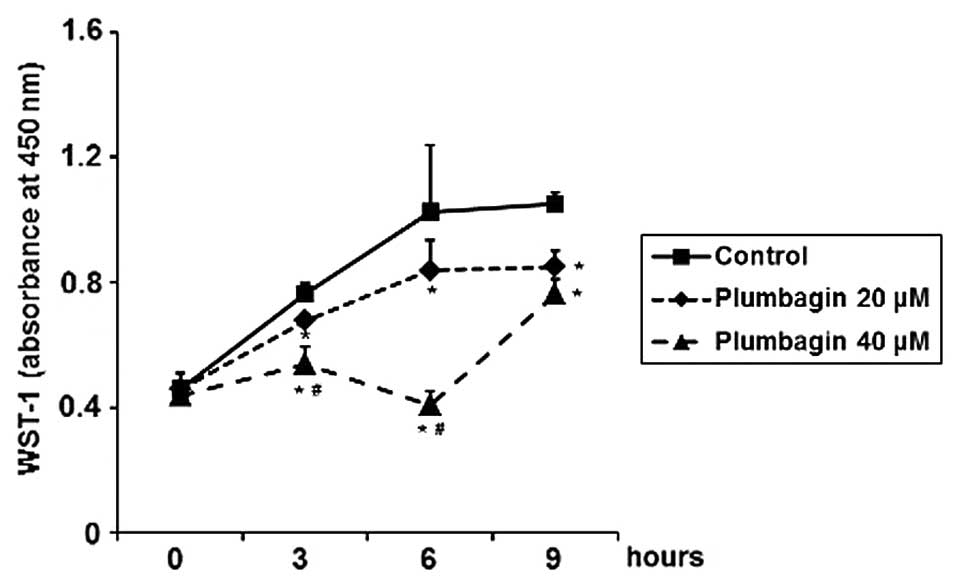
20 or 40 μM of plumbagin. By performing WST-1 cell proliferation
assay, we observed that plumbagin significantly inhibited cell
proliferation in a time- and dose-dependent manner, with maximal
inhibitory effect at 40 μM in 6 h treatment (Fig. 5). After treatment with plumbagin
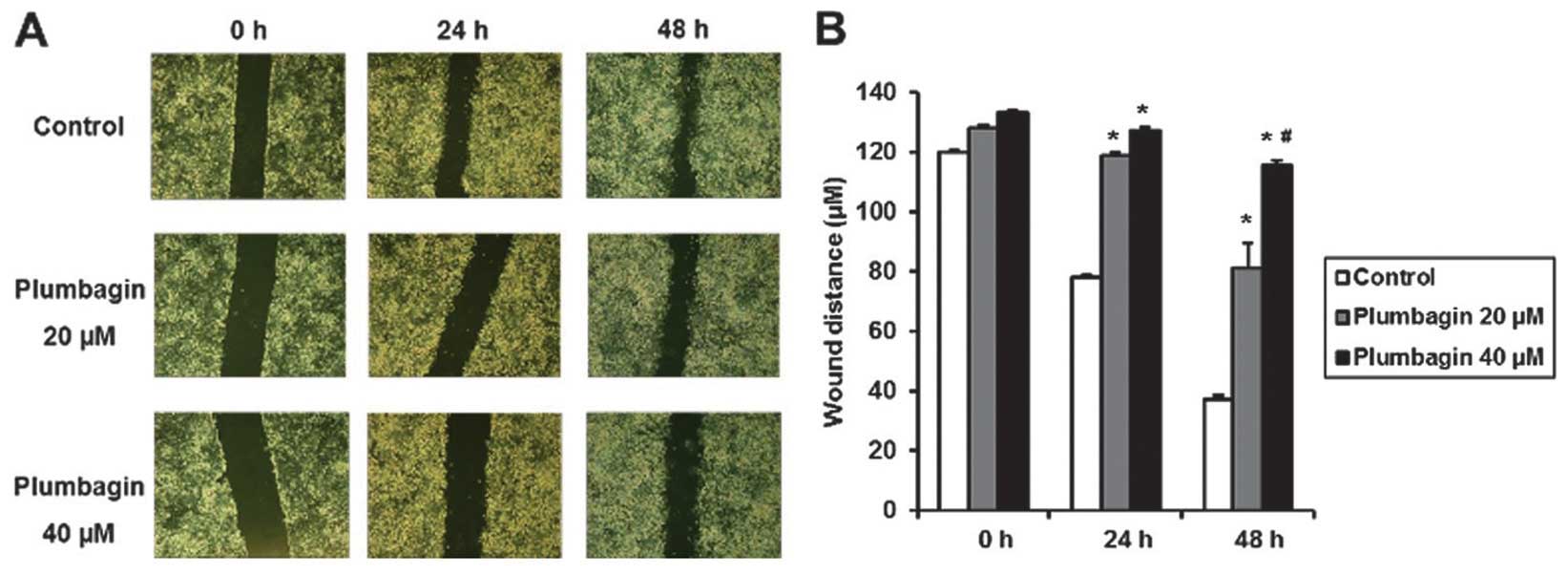
for 6 h, we made wound injury in a 6-well plate using a pipette
tip, and we observed that plumbagin significantly inhibited wound
closure at 24 and 48 h after injury and this inhibitory effect was
more prominent at 40 than 20 μM of plumbagin (Fig. 6). We treated MKN-28 cells with 20
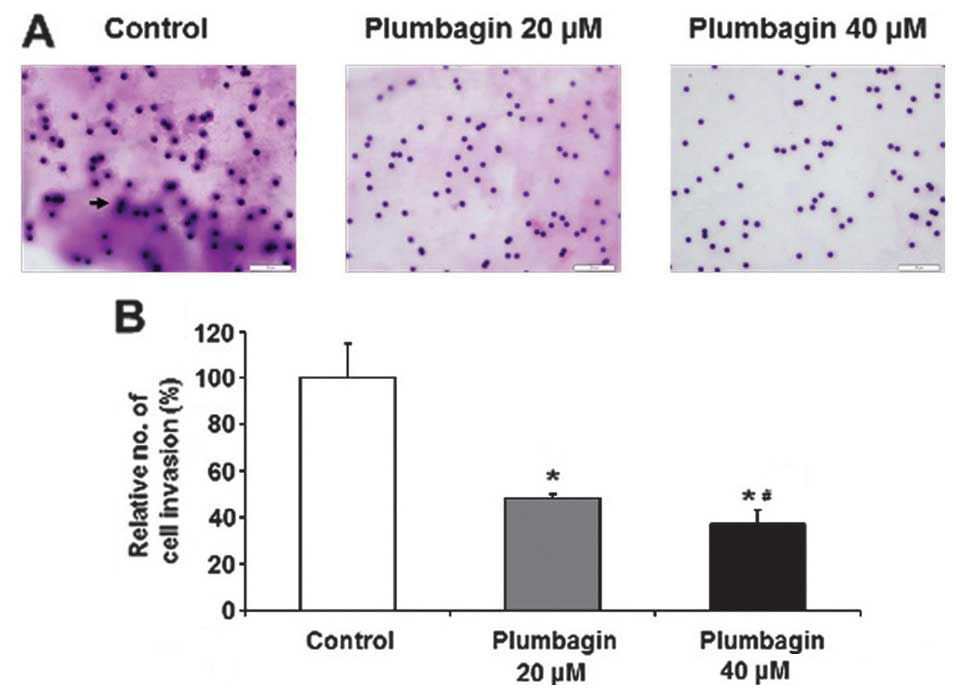
or 40 μM of plumbagin for 6 h, and cultured for 24 h in Transwell
plate with a pore membrane, and then fixed and stained the cells by
crystal violet. We also observed that plumbagin significantly
reduced the relative number of invading cells, and 40 μM of
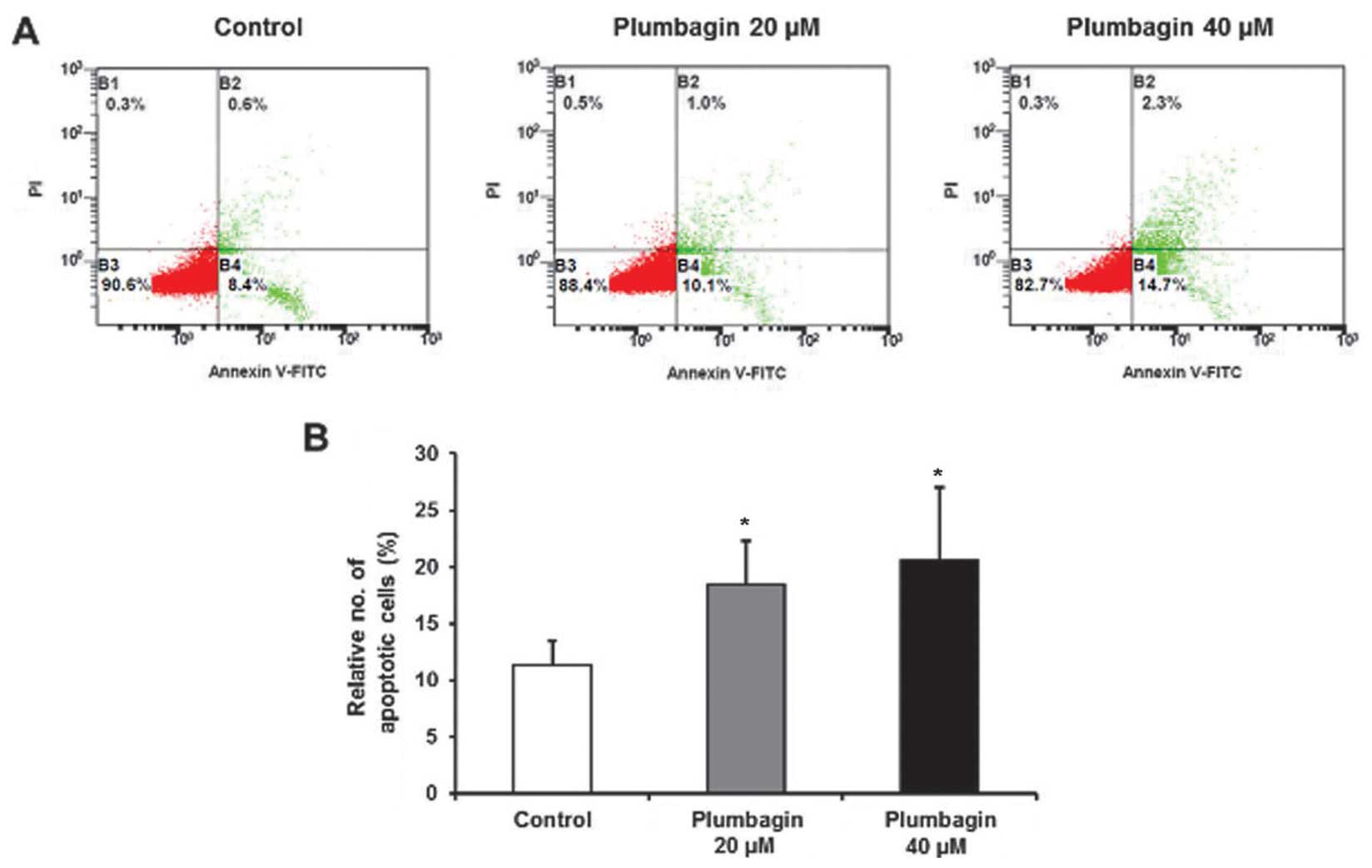
plumbagin was more effective than 20 μM (Fig. 7). Finally, we investigated
pro-apoptotic effect of plumbagin by Annexin V assay, and relative
number of apoptotic cells was significantly increased by 40 μM of
plumbagin, rather than 20 μM (Fig.
8). Taken together, these findings suggest that plumbagin
significantly inhibits cell proliferation, migration and invasion,
and induces cellular apoptosis in MKN-28 cells, and these
functional effects are dose-dependent.
Discussion
In this in vitro study, we showed that
plumbagin restored SHP1 expression to downregulated JAK2/STAT3
activity and their target genes, and consequentially, led to
anti-proliferative, migratory, invasive and pro-apoptotic effects
in gastric carcinoma cells. To our knowledge, our study firstly
demonstrates that plumbagin inhibits STAT3 pathway through
induction of SHP1 activity in stomach cancer cells. Little has been
reported about anti-cancer role of plumbagin in gastric cancer, and
only few studies focused on its inhibitory effects on NF-κB or
CXCR4 pathway (8,9), or cytoxic effect through generation
of reactive oxygen species (ROS) (19). Also, recent studies reported
several candidate molecules to downregulate JAK2/STAT3 activity and
exhibit antitumor effect in gastric cancer cells (20–23).
However, none of them showed molecular link between SHP1 and
JAK2/STAT3 pathway in gastric cancer cells.
SHP1 is non-receptor-type PTPase, which is encoded
by PTPN6 gene located on human chromosome 12p13 (24), and it has been reported as a
negative regulator of JAK2/STAT3 activity by dephosphorylation of
JAK2 and STAT3 to act as a PTPase (25,26).
Previous studies demonstrated that SHP1 is inactivated by aberrant
methylation of CpG island promoter in various hematopoietic
malignancies, and their functional roles have been extensively
investigated in hematopoietic cancer cells (27–30).
However, only few studies reported CpG island promoter
hypermethylation in epithelial cells such as colon cancer cells
(13,31), and little is known about reduced
gene expression or promoter hypermethylation of SHP1 in gastric
cancer cells except that several studies briefly reported the
methylation rate of CpG island promoter of SHP1 in gastric
carcinoma tissues (32,33). In colon cancer cells, SHP1
expression was mainly regulated by DNA methylation and upregulated
by DNA methyltransferase inhibitors such as 5-aza-2′-deoxycytidine
(5-aza-dC). Furthermore, increased SHP1 expression by transfection
with SHP1 plasmid vector or treatment with demethylating agent such
as 5-aza-dC substantially decreased p-JAK2/p-STAT3 level (13). We observed previously that SHP1
expression was epigenetically regulated and closely related with
STAT3 activity in gastric cancer cells (unpublished data). Thus, as
the next step, we searched for a candidate molecule which can
induce SHP1 expression in gastric cancer cells, and demonstrated
that plumbagin might be a potential inducer of SHP1 to inhibit
JAK2/STAT3 pathway.
Previous pivotal studies extensively investigated
the crucial role of STAT3 for initiation and progression of gastric
cancer. Persistent infection of CagA-positive Helicobacter
pylori (H. pylori) strain activates constitutive STAT3
via chronic JAK2 activity, which in turn promotes target gene
transcription associated with proliferation, invasion, metastasis
and angiogenesis. In terms of gastric epithelial cells, IL-6 family
ligands such as IL-6 and IL-11 is associated with chronic
inflammation by CagA-positive H. pylori and development of
gastric cancer (18,34). The suppressors of cytokine
signaling (SOCS) family proteins such as SOCS-1 or -3 have been
reported as important regulators of JAK2/STAT3 pathway by negative
feedback mechanism (35,36). Numerous PTPases have been presented
as promising targets to inhibit STAT3 signaling in various kinds of
cancer, including SHP1 (37), SHP2
(38), PTP-1D (39) and PTEN (40). However, little is known about the
expression level and roles of PTPase in gastric tumorigenesis, and
their inhibitory action on STAT3 signaling is still controversial.
Several previous studies have focused on the effect of SHP2, and an
in vitro study demonstrated that phosphorylated CagA
preferentially activates SHP2/extracellular signal-regulated kinase
(ERK) pathway and induces cell growth inhibition (41), whereas immunohistochemical studies
using human stomach tissues showed that SHP2 expression was
significantly enhanced in H. pylori-infected gastric cancer
(42,43). Previously, we observed that
exogenous expression of SHP1 in gastric cancer cells significantly
inhibited cellular proliferation, migration and invasion
(unpublished data), however, this phenomenon should be further
validated in immunohistochemical studies using human gastric
carcinoma tissues. This study might further support the hypothesis
that SHP1 negatively regulates STAT3 activity because induction of
SHP1 by plumbagin inhibited JAK2/STAT3 signaling and inhibition of
SHP1 reversed the plumbagin effects on JAK2/STAT3 pathway.
In conclusion, our study suggests that plumbagin
might have promising anti-cancer potential via upregulation of SHP1
expression and inhibition of JAK2/STAT3 pathway in gastric cancer
cells, and SHP1 might be an alternative target to regulate STAT3
activity in gastric carcinogenesis. Further preclinical studies
concerning the effects of plumbagin in STAT3 overexpressing gastric
cancer should be performed, and also other promising agents which
can upregulate SHP1 expression in gastric cancer cells need to be
investigated.
Acknowledgements
This research was financially supported by grants
from Korea University (Seoul, Korea) (grant no. R1211041) and
Pacific Pharma Corp. (Seoul, Korea) (grant no. Q1307141).
References
|
1
|
Sandur SK, Ichikawa H, Sethi G, Ahn KS and
Aggarwal BB: Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone)
suppresses NF-kappaB activation and NF-kappaB-regulated gene
products through modulation of p65 and IkappaBalpha kinase
activation, leading to potentiation of apoptosis induced by
cytokine and chemotherapeutic agents. J Biol Chem. 281:17023–17033.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sugie S, Okamoto K, Rahman KM, Tanaka T,
Kawai K, Yamahara J and Mori H: Inhibitory effects of plumbagin and
juglone on azoxymethane-induced intestinal carcinogenesis in rats.
Cancer Lett. 127:177–183. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuo PL, Hsu YL and Cho CY: Plumbagin
induces G2-M arrest and autophagy by inhibiting the AKT/mammalian
target of rapamycin pathway in breast cancer cells. Mol Cancer
Ther. 5:3209–3221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsu YL, Cho CY, Kuo PL, Huang YT and Lin
CC: Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) induces
apoptosis and cell cycle arrest in A549 cells through p53
accumulation via c-Jun NH2-terminal kinase-mediated phosphorylation
at serine 15 in vitro and in vivo. J Pharmacol Exp Ther.
318:484–494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang CC, Chiang YM, Sung SC, Hsu YL, Chang
JK and Kuo PL: Plumbagin induces cell cycle arrest and apoptosis
through reactive oxygen species/c-Jun N-terminal kinase pathways in
human melanoma A375.S2 cells. Cancer Lett. 259:82–98. 2008.
View Article : Google Scholar
|
|
6
|
Powolny AA and Singh SV: Plumbagin-induced
apoptosis in human prostate cancer cells is associated with
modulation of cellular redox status and generation of reactive
oxygen species. Pharm Res. 25:2171–2180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hafeez BB, Jamal MS, Fischer JW, Mustafa A
and Verma AK: Plumbagin, a plant derived natural agent inhibits the
growth of pancreatic cancer cells in in vitro and in vivo via
targeting EGFR, Stat3 and NF-κB signaling pathways. Int J Cancer.
131:2175–2186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Manu KA, Shanmugam MK, Rajendran P, Li F,
Ramachandran L, Hay HS, Kannaiyan R, Swamy SN, Vali S, Kapoor S, et
al: Plumbagin inhibits invasion and migration of breast and gastric
cancer cells by downregulating the expression of chemokine receptor
CXCR4. Mol Cancer. 10:1072011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Shen L, Lu FR, Qin Y, Chen R, Li J,
Li Y, Zhan HZ and He YQ: Plumbagin inhibits cell growth and
potentiates apoptosis in human gastric cancer cells in vitro
through the NF-κB signaling pathway. Acta Pharmacol Sin.
33:242–249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sandur SK, Pandey MK, Sung B and Aggarwal
BB: 5-hydroxy-2-methyl-1,4-naphthoquinone, a vitamin K3 analogue,
suppresses STAT3 activation pathway through induction of protein
tyrosine phosphatase, SHP-1: Potential role in chemosensitization.
Mol Cancer Res. 8:107–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang Z, Lee H, Lee E, Kang SK, Nam JM and
Lee M: Responsive nematic gels from the self-assembly of aqueous
nanofibres. Nat Commun. 2:4592011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dallol A, Agathanggelou A, Tommasi S,
Pfeifer GP, Maher ER and Latif F: Involvement of the RASSF1A tumor
suppressor gene in controlling cell migration. Cancer Res.
65:7653–7659. 2005.PubMed/NCBI
|
|
13
|
Xiong H, Chen ZF, Liang QC, Du W, Chen HM,
Su WY, Chen GQ, Han ZG and Fang JY: Inhibition of DNA
methyl-transferase induces G2 cell cycle arrest and apoptosis in
human colorectal cancer cells via inhibition of JAK2/STAT3/STAT5
signalling. J Cell Mol Med. 13:3668–3679. 2009. View Article : Google Scholar
|
|
14
|
Lin MT, Lin BR, Chang CC, Chu CY, Su HJ,
Chen ST, Jeng YM and Kuo ML: IL-6 induces AGS gastric cancer cell
invasion via activation of the c-Src/RhoA/ROCK signaling pathway.
Int J Cancer. 120:2600–2608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu BH, Chen HY, Zhan WH, Wang CY, Cai SR,
Wang Z, Zhang CH and He YL: (-)-Epigallocatechin-3-gallate inhibits
VEGF expression induced by IL-6 via Stat3 in gastric cancer. World
J Gastroenterol. 17:2315–2325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rhee YH, Jeong SJ, Lee HJ, Lee HJ, Koh W,
Jung JH, Kim SH and Sung-Hoon K: Inhibition of STAT3 signaling and
induction of SHP1 mediate antiangiogenic and antitumor activities
of ergosterol peroxide in U266 multiple myeloma cells. BMC Cancer.
12:282012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JH, Chiang SY, Nam D, Chung WS, Lee J,
Na YS, Sethi G and Ahn KS: Capillarisin inhibits constitutive and
inducible STAT3 activation through induction of SHP-1 and SHP-2
tyrosine phosphatases. Cancer Lett. 345:140–148. 2014. View Article : Google Scholar
|
|
18
|
Jackson CB and Giraud AS: STAT3 as a
prognostic marker in human gastric cancer. J Gastroenterol Hepatol.
24:505–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JA, Lee EK, Park SJ, Kim ND, Hyun DH,
Lee CG, Lee JH, Yang KM, Heo K and Son TG: Novel anti-cancer role
of naphthazarin in human gastric cancer cells. Int J Oncol.
40:157–162. 2012.
|
|
20
|
Chen J, Wang J, Lin L, He L, Wu Y, Zhang
L, Yi Z, Chen Y, Pang X and Liu M: Inhibition of STAT3 signaling
pathway by nitidine chloride suppressed the angiogenesis and growth
of human gastric cancer. Mol Cancer Ther. 11:277–287. 2012.
View Article : Google Scholar
|
|
21
|
Jia Y, Liu D, Xiao D, Ma X, Han S, Zheng
Y, Sun S, Zhang M, Gao H, Cui X, et al: Expression of AFP and STAT3
is involved in arsenic trioxide-induced apoptosis and inhibition of
proliferation in AFP-producing gastric cancer cells. PLoS One.
8:e547742013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim MJ, Nam HJ, Kim HP, Han SW, Im SA, Kim
TY, Oh DY and Bang YJ: OPB-31121, a novel small molecular
inhibitor, disrupts the JAK2/STAT3 pathway and exhibits an
antitumor activity in gastric cancer cells. Cancer Lett.
335:145–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katsha A, Arras J, Soutto M, Belkhiri A
and El-Rifai W: AURKA regulates JAK2-STAT3 activity in human
gastric and esophageal cancers. Mol Oncol. 8:1419–1428. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
López-Ruiz P, Rodriguez-Ubreva J, Cariaga
AE, Cortes MA and Colás B: SHP-1 in cell-cycle regulation.
Anticancer Agents Med Chem. 11:89–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Somani AK and Siminovitch KA:
Roles of the SHP-1 tyrosine phosphatase in the negative regulation
of cell signalling. Semin Immunol. 12:361–378. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu C, Sun M, Liu L and Zhou GW: The
function of the protein tyrosine phosphatase SHP-1 in cancer. Gene.
306:1–12. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han Y, Amin HM, Franko B, Frantz C, Shi X
and Lai R: Loss of SHP1 enhances JAK3/STAT3 signaling and decreases
proteosome degradation of JAK3 and NPM-ALK in ALK+ anaplastic
large-cell lymphoma. Blood. 108:2796–2803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oka T, Ouchida M, Koyama M, Ogama Y,
Takada S, Nakatani Y, Tanaka T, Yoshino T, Hayashi K, Ohara N, et
al: Gene silencing of the tyrosine phosphatase SHP1 gene by
aberrant methylation in leukemias/lymphomas. Cancer Res.
62:6390–6394. 2002.PubMed/NCBI
|
|
29
|
Koyama M, Oka T, Ouchida M, Nakatani Y,
Nishiuchi R, Yoshino T, Hayashi K, Akagi T and Seino Y: Activated
proliferation of B-cell lymphomas/leukemias with the SHP1 gene
silencing by aberrant CpG methylation. Lab Invest. 83:1849–1858.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chim CS, Fung TK, Cheung WC, Liang R and
Kwong YL: SOCS1 and SHP1 hypermethylation in multiple myeloma:
Implications for epigenetic activation of the Jak/STAT pathway.
Blood. 103:4630–4635. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ruchusatsawat K, Wongpiyabovorn J,
Shuangshoti S, Hirankarn N and Mutirangura A: SHP-1 promoter 2
methylation in normal epithelial tissues and demethylation in
psoriasis. J Mol Med (Berl). 84:175–182. 2006. View Article : Google Scholar
|
|
32
|
Bernal C, Aguayo F, Villarroel C, Vargas
M, Díaz I, Ossandon FJ, Santibáñez E, Palma M, Aravena E,
Barrientos C, et al: Reprimo as a potential biomarker for early
detection in gastric cancer. Clin Cancer Res. 14:6264–6269. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ksiaa F, Ziadi S, Amara K, Korbi S and
Trimeche M: Biological significance of promoter hypermethylation of
tumor-related genes in patients with gastric carcinoma. Clin Chim
Acta. 404:128–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Giraud AS, Menheniott TR and Judd LM:
Targeting STAT3 in gastric cancer. Expert Opin Ther Targets.
16:889–901. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Souma Y, Nishida T, Serada S, Iwahori K,
Takahashi T, Fujimoto M, Ripley B, Nakajima K, Miyazaki Y, Mori M,
et al: Antiproliferative effect of SOCS-1 through the suppression
of STAT3 and p38 MAPK activation in gastric cancer cells. Int J
Cancer. 131:1287–1296. 2012. View Article : Google Scholar
|
|
36
|
Inagaki-Ohara K, Mayuzumi H, Kato S,
Minokoshi Y, Otsubo T, Kawamura YI, Dohi T, Matsuzaki G and
Yoshimura A: Enhancement of leptin receptor signaling by SOCS3
deficiency induces development of gastric tumors in mice. Oncogene.
33:74–84. 2014. View Article : Google Scholar
|
|
37
|
Tenev T, Böhmer SA, Kaufmann R, Frese S,
Bittorf T, Beckers T and Böhmer FD: Perinuclear localization of the
protein-tyrosine phosphatase SHP-1 and inhibition of epidermal
growth factor-stimulated STAT1/3 activation in A431 cells. Eur J
Cell Biol. 79:261–271. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim H and Baumann H: Dual signaling role
of the protein tyrosine phosphatase SHP-2 in regulating expression
of acute-phase plasma proteins by interleukin-6 cytokine receptors
in hepatic cells. Mol Cell Biol. 19:5326–5338. 1999.PubMed/NCBI
|
|
39
|
Gunaje JJ and Bhat GJ: Involvement of
tyrosine phosphatase PTP1D in the inhibition of
interleukin-6-induced Stat3 signaling by alpha-thrombin. Biochem
Biophys Res Commun. 288:252–257. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Irie-Sasaki J, Sasaki T, Matsumoto W,
Opavsky A, Cheng M, Welstead G, Griffiths E, Krawczyk C, Richardson
CD, Aitken K, et al: CD45 is a JAK phosphatase and negatively
regulates cytokine receptor signalling. Nature. 409:349–354. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee IO, Kim JH, Choi YJ, Pillinger MH, Kim
SY, Blaser MJ and Lee YC: Helicobacter pylori CagA phosphorylation
status determines the gp130-activated SHP2/ERK and JAK/STAT signal
transduction pathways in gastric epithelial cells. J Biol Chem.
285:16042–16050. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang J, Jin MS, Kong F, Wang YP, Jia ZF,
Cao DH, Ma HX, Suo J and Cao XY: Increased expression of tyrosine
phosphatase SHP-2 in Helicobacter pylori-infected gastric cancer.
World J Gastroenterol. 19:575–580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim JS, Shin OR, Kim HK, Cho YS, An CH,
Lim KW and Kim SS: Overexpression of protein phosphatase
non-receptor type 11 (PTPN11) in gastric carcinomas. Dig Dis Sci.
55:1565–1569. 2010. View Article : Google Scholar
|
|
44
|
Jiménez DJ, Montaña JS, Alvarez D and
Baena S: A novel cold active esterase derived from Colombian high
Andean forest soil metagenome. World J Microbiol Biotechnol.
28:361–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gu X, Cun Y, Li M, Qing Y, Jin F, Zhong Z,
Dai N, Qian C, Sui J and Wang D: Human apurinic/apyrimidinic
endonuclease siRNA inhibits the angiogenesis induced by X-ray
irradiation in lung cancer cells. Int J Med Sci. 10:870–882. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
McTavish N, Copeland LA, Saville MK,
Perkins ND and Spruce BA: Proenkephalin assists stress-activated
apoptosis through transcriptional repression of NF-kappaB- and
p53-regulated gene targets. Cell Death Differ. 14:1700–1710. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Takei Y, Kadomatsu K, Yuzawa Y, Matsuo S
and Muramatsu T: A small interfering RNA targeting vascular
endothelial growth factor as cancer therapeutics. Cancer Res.
64:3365–3370. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rivera MN, Kim WJ, Wells J, Stone A,
Burger A, Coffman EJ, Zhang J and Haber DA: The tumor suppressor
WTX shuttles to the nucleus and modulates WT1 activity. Proc Natl
Acad Sci USA. 106:8338–8343. 2009. View Article : Google Scholar : PubMed/NCBI
|