Introduction
Cholangiocarcinoma (CC) is a malignant tumor that
arises from the ductal epithelium of the biliary tree (1). CC accounts for ~3% of all
gastrointestinal malignancies; however, it represents the second
most common hepatic malignancy after hepatocellular carcinoma (HCC)
(2). CC is currently classified as
intrahepatic CC and extrahepatic CC, depending on the carcinogenic
site. Extrahepatic CCs are further divided into hilar (Klatskin
tumor) or distal tumors and the incidence of this cancer has been
increasing in recent years (3).
Surgical resection remains the only potentially curative
therapeutic option, however, as a result of the early invasion and
metastasis, more than half of patients present with unresectable
disease at diagnosis (4). Although
many patients may receive extensive surgical resection and adjuvant
chemotherapy to improve chance of cure, the therapeutic effect and
postoperative prognosis are still unsatisfactory, with a 5-year
survival rate of 30–42% for hilar CC, and 18–54% for distal CC
(5,6). Thus, discovery of new relevant
biomarker to increase specificity or sensitivity for early
diagnosis and to improve the prognosis of extrahepatic CC is
important and urgently needed.
Fork-head factors are transcription factors that
share an evolutionarily conserved DNA-binding domain, termed the
‘fork-head’ or ‘winged-helix’ domain (7). Most members of the Fork-head Box
(FOX) family have been reported to be widely distributed across
several organs and tissues in very different species from yeast to
humans and play extraordinarily diverse roles that are critical to
the organism (8). Nowadays,
mounting evidence suggests that FOX family members are important
for a wide spectrum of biological processes, including metabolism,
development, differentiation, proliferation, apoptosis, migration,
invasion, and longevity (9).
Deregulation of FOX family genes leads to congenital, diabetes
mellitus, or carcinogenesis (10).
Given their important role in the expression of
numerous genes that affect cell proliferation, differentiation and
survival, FOX family members may represent direct targets, and
indirect effectors of therapeutic intervention. In addition, FOX
family members have been shown to be upregulated or downregulated
in many cancers. For example, Foxp1 transcription factor may
function as either an oncogene or as a tumor suppressor depending
on the cell types (11–14). FOXA1 gene is amplified and
overexpressed in esophageal and lung cancer (15). Furthermore, FOXJ1 was remarkably
upregulated in human HCC specimens (16). Increased expression of FOXM1
protein was found in variety of human tumors, including HCC
(17), intrahepatic CC (18).
Foxj2 is a novel forkhead factor, belonging to the
FOX family, with a dual DNA binding specificity (19). Some studies have shown that FoxJ2
is a transcription factor of this family that shows a rather broad
expression pattern, both in the adult as well as during embryonic
development, but the levels of expression vary between organs and,
in each organ, not all cells types express the factor (20). Recent study has reported that
upregulation of FOXJ2 might inhibit of cell migration and invasion
of breast cancer (21). However,
the role of FOXJ2 in EHCC has not been explored thus far. In this
study, we surveyed the expression of FOXJ2 in human patient
samples. To explore its associated molecular mechanisms in
extrahepatic CC cells, we examined the effect of targeted
overexpression of FOXJ2 gene on cell proliferation, migration and
invasion in vitro. These studies will be useful in
identifying potential candidates for targeted therapeutic
intervention of extrahepatic CC.
Materials and methods
Patients and clinical samples
We included a total of 63 paraffin-embedded
extrahepatic CC and matched paracancer normal bile duct tissue
samples from patients who underwent surgical treatment at
Affiliated Hospital of Nantong University during the period from
January 2005 to January 2009. The patients or their legal guardian
provided written informed consent to the surgical procedures and
gave permission to use resected tissue specimens for research
purposes. The patients with preoperative history of radiotherapy,
chemotherapy, and positive surgical margins were excluded.
Furthermore, a diagnosis of extrahepatic CC was confirmed
pathologically by two independent experienced pathologists. All
specimens excluding the pathological diagnosis of pancreatic ductal
carcinoma and periampullary carcinoma confirmed pathological
diagnosis and were classified according to the World Health
Organization (WHO) criteria. The tumor stage was performed
according to the 7th Union for International Cancer Control
(UICC)-TNM Staging. The follow-up data of the extrahepatic CC
patients in this study are available and complete. Overall
survival, which was defined as the time from the operation to the
time of patient death or the last follow-up, was used as a measure
of prognosis. Postoperative follow-up occurred at our outpatient
department and included clinical and laboratory examinations every
3 months for the first 2 years, every 6 months during the third to
fifth years until patient death.
Cell culture and transfection
The human extrahepatic CC cell line QBC939 were
purchased from a cell bank at the Chinese Academy of Sciences and
grown in RPMI-1640 medium (Hyclone, Logan, UT, USA) supplemented
with 10% fetal calf serum (Gibco, Grand Island, NY, USA). All cell
lines were cultured at 37°C in a humidified atmosphere of 5%
CO2. Transfection reagent Lipofectamine 2000 was
purchased from Invitrogen (St. Louis, MO, USA). For overexpression
of FOXJ2, the full-length FOXJ2 cDNA was amplified and cloned into
the pEGFP-N-3 expression vector (GeneChem, Shanghai, China). QBC939
cells were then transfected with a negative control vector or a
FOXJ2 expressing plasmid using Lipofectamine 2000 according to the
manufacturer’s instructions.
Real-time quantitative PCR
Total RNA was extracted from tissues lysate using a
TRIzol kit (Invitrogen, Carlsbad, CA, USA), and cDNA was
subsequently synthesized from total RNA using an Omniscript RT kit
(Qiagen, Valencia, CA, USA) according to the manufacturer’s
instructions. For detecting the mRNA level of FOXJ2, quantitative
real-time RT-PCR was conducted on the Mastercycler ep realplex
(Eppendorf 2S, Hamburg, Germany). A 25-μl reaction mixture
contained 1 μl of cDNA from samples, 12.5 μl of 2× Fast
EvaGreen™qPCR Master Mix, 1 μl primers (10 mM), and 10.5 μl of
RNase/DNase-free water. PCR procedures: incubation at 96°C for 2
min, 40 cycles at 96°C for 15 sec and 60°C for 1 min. The Ct value
was defined as the cycle number at which the fluorescence intensity
reached a certain threshold where amplification of each target gene
was within the linear region of the reaction amplification curves.
Relative expression level for each target gene was normalized by
the Ct value of GAPDH (internal control) using the
2-ΔΔCt relative quantification method. The sequences of
the primers for FOXJ2 were: FOXJ2 forward:
5′-TATGGTAGGGCATGAGGACAAC-3′; FOXJ2 reverse:
5′-GCAAACAATTAAAGGAGGACAAAC-3′. The glyceraldehyde-3′ phosphate
dehydrogenase (GAPDH) gene served as an internal control.
Western blot analysis
Since January 2013, fresh surgical specimens after
surgical removal were collected and immediately frozen in liquid
nitrogen until used for western blot analysis. The extrahepatic CC
samples, including tumor and para-carcinoma normal tissues, as well
as cell lines, were lysed in RIPA lysis buffer, and the lysates
were harvested by centrifugation (12,000 rpm) at 4°C for 30 min.
Approximately 50-mg protein samples were then separated by
electrophoresis in a 12% sodium dodecyl sulfate polyacrylamide gel
and transferred onto a polyvinylidene fluoride membrane. After
blocking the non-specific binding sites for 60 min with 5% non-fat
milk, the membranes were incubated overnight at 4°C with a goat
polyclonal antibody against FOXJ2 (Santa Cruz Biotechnology, USA at
a 1:1,000 dilution). The membranes were then washed three times
with TBST (Tris-buffered saline with Tween-20) for 10 min and
probed with the horseradish peroxidase (HRP)-conjugated donkey
anti-goat IgG antibody (Immunology Consultants Laboratory, USA, at
a 1:2,000 dilution) at 37°C for 1 h. After three washes, the
membranes were developed by an enhanced chemiluminescence system
(Cell Signaling Technology, Danvers, MA, USA). The band intensity
was measured by densitometry using Quantity One software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The protein levels were
normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Immunohistochemistry (IHC)
The paraffin-embedded sections were deparaffinized
with dimethylbenzene and rehydrated with raded alcohol solutions.
After three washes in phosphate-buffered saline (PBS), the slides
were boiled in antigen retrieval buffer containing 0.01 M sodium
citrate-hydrochloric acid (pH 6.0) for 15 min in a microwave oven.
After rinsing with PBS, the tissue sections were incubated with
goat polyclonal anti-human FOXJ2 antibody (1:100, Santa Cruz
Biotechnology, Inc., USA) and E-cadherin (diluted 1:1,000, Santa
Cruz Biotechnology, Inc.), and then rinsed in 3% peroxidase
quenching solution (Invitrogen) to block endogenous peroxidase. The
sections were then incubated with a donkey anti-goat second
antibody conjugated horseradish peroxidase (1:5,000; Abcam,
Cambridge, UK) at 4°C overnight. After washing in PBS, the
visualization signal was developed with 3, 3′-diaminobenzidine
(DAB) solution, and all of the slides were counterstained with
hematoxylin. As negative controls, adjacent sections were processed
as described above except that they were incubated overnight at 4°C
in blocking solution without the primary antibody. The IHC results
were scored by two experienced pathologists, who were blinded to
clinical data. The total FOXJ2 immunostaining score was calculated
as the sum of the percentage of positively stained tumor cells and
the staining intensity and ranged from 0 to 9. Briefly, the
percentage of positive staining was scored as 0 (0–9%, negative), 1
(10–25%, sporadic), 2 (26–50%, focal) or 3 (51–100%, diffuse), and
the intensity was scored as 0 (no staining), 1 (weak staining), 2
(moderate staining) or 3 (strong staining). The expression level of
FOXJ2 was defined as follows: ‘−’ (negative, score of 0), ‘+’
(weakly positive, score of 1–3), ‘++’ (positive, score of 4–6),
‘+++’ (strongly positive, score of 7–9). We defined strong FOXJ2
expression as a total score of >3, and weak FOXJ2 expression as
a total score of ≤3. As described elsewhere (22), we evaluated the intensity of
E-cadherin staining on tumor cells, based on the staining of the
control normal bile duct epithelium. E-cadherin was considered
positive (high expression), if the staining intensity on tumor
cells was the same as in normal bile duct epithelial cells. When
the intensity of E-cadherin staining on tumor cells was weaker than
the normal cells, E-cadherin was considered negative (low
expression).
Cell proliferation assay
The tetrazolium-based cell viability (MTT) assay was
performed to test cell proliferation. Cells transfected with the
FOXJ2 plasmid or empty vector were seeded in a 96-well plate at
1×103 cells/well containing 200 μl DMEM supplemented
with 10% FBS. After 1, 2 and 3 days of incubation, 100 μl of
sterile MTT dye (0.5 mg/ml, Sigma) was added to each well and
cultured for another 4 h. The supernatant was discarded and then
150 μl of dimethyl sulphoxide (DMSO) (Sigma, St. Louis, MO, USA)
was added to each well, the spectrophotometric absorbance was
measured for each sample at 490 nm, all the experiments were
performed in triplicate and repeated 3 times, and the average was
calculated.
Cell invasion and migration assays
The cell invasion capacity was determined using
transwell chambers (Corning, Corning, NY, USA). The membrane
filters were coated with Matrigel. Briefly, cells
(1×105/well) were suspended in 100 μl serum-free medium
and then added to the upper chamber of the inserts, RPMI-1640
medium (Gibco) containing 10% FBS (500 μl) was added to the lower
chamber as the chemotactic factor. After 48 h of incubation, the
cells that had invaded through the Matrigel were visualized using
0.1% crystal violet staining. The numbers of migrated cells were
calculated by counting five different views under the microscope.
Independent experiments were repeated three times.
In addition, we examined migration using QBC939
cells that were transfected with either pEGFP-N-3-FOXJ2 or control
vector. Transfected cells were cultured on 6-well plates. The
confluent monolayers were scraped in a line across the slides with
a sterile 20-μl plastic pipette tip and incubated in serum-free
medium for 48 h. Plates were then imaged at 0, 12, 24 and 48 h with
Olympus IX71 fluorescence microscope with a TH4-200 camera.
Quantification was blinded, and performed by creating a
longitudinal axis over the area of minimal density that
corresponded to the site of wound formation. The average baseline
wound area was centered over the axis and all the cells presented
in that area were assumed to have migrated there. These cells were
counted for data analysis.
Statistical analysis
All quantified data represented an average of at
least triplicate samples. SPSS 17.0 (SPSS Inc, Chicago, IL, USA)
was used for statistical analysis. Data are expressed as mean ±
SEM. The significance of the differences between values was
determined using Student’s t-test. χ2 test or Fischer’s
were used to identify differences between categorical variables.
Survival curves for the patients were calculated using the
Kaplan-Meier method and analyzed using the log-rank test.
Prognostic factors were examined by univariate and multivariate
analyses using a Cox proportional hazards model. Values of
P<0.05 were considered to indicate statistically significant
results in all cases.
Results
Downregulated expression of FOXJ2 gene in
extrahepatic CC and adjacent non-cancer normal bile duct
tissues
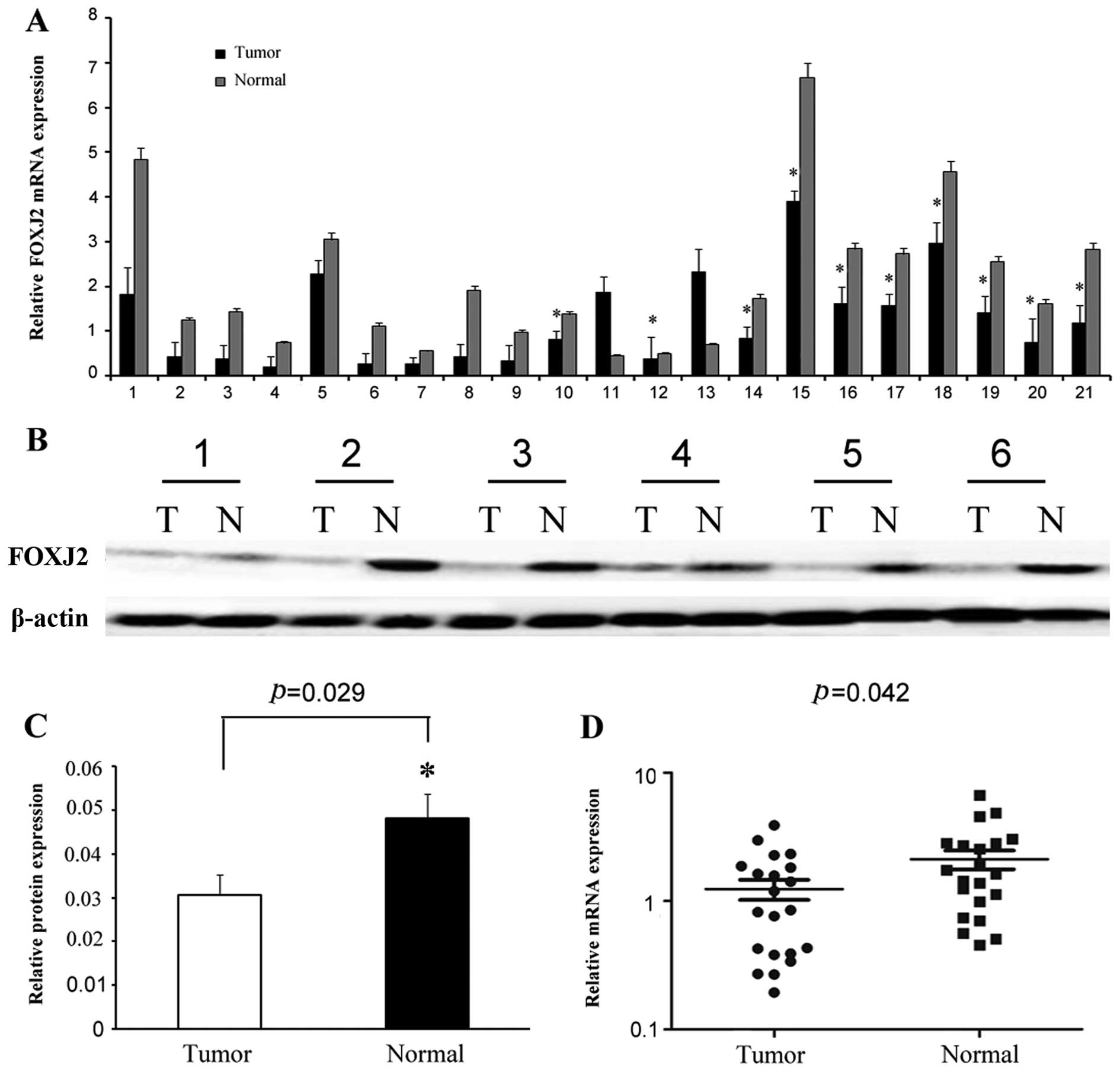
In order to assess the role of FOXJ2 in extrahepatic
CC, we performed real-time PCR to measure the expression of FOXJ2
mRNA in 21 freshly collected extrahepatic CC tissues and
corresponding paracancer normal bile duct tissues. FOXJ2 protein
was found to be markedly downregulated in 19 cases of extrahepatic
CC compared with corresponding adjacent non-cancer normal bile duct
tissues by western blotting (P=0.029, Fig. 1B and C). In the 21 extrahepatic CC
specimens, 9 cases had lymph node metastasis, and the other 12
cases were without metastasis. We also found that the mRNA level of
FOXJ2 in metastatic extrahepatic CCs was significantly decreased
compared with that in the extrahepatic CCs without lymph node
metastasis (*P<0.05, Fig. 1A). Compared with match paracancer
normal tissues, extrahepatic CC tissues exhibited lower expression
levels of FOXJ2 mRNA (P=0.042, Fig.
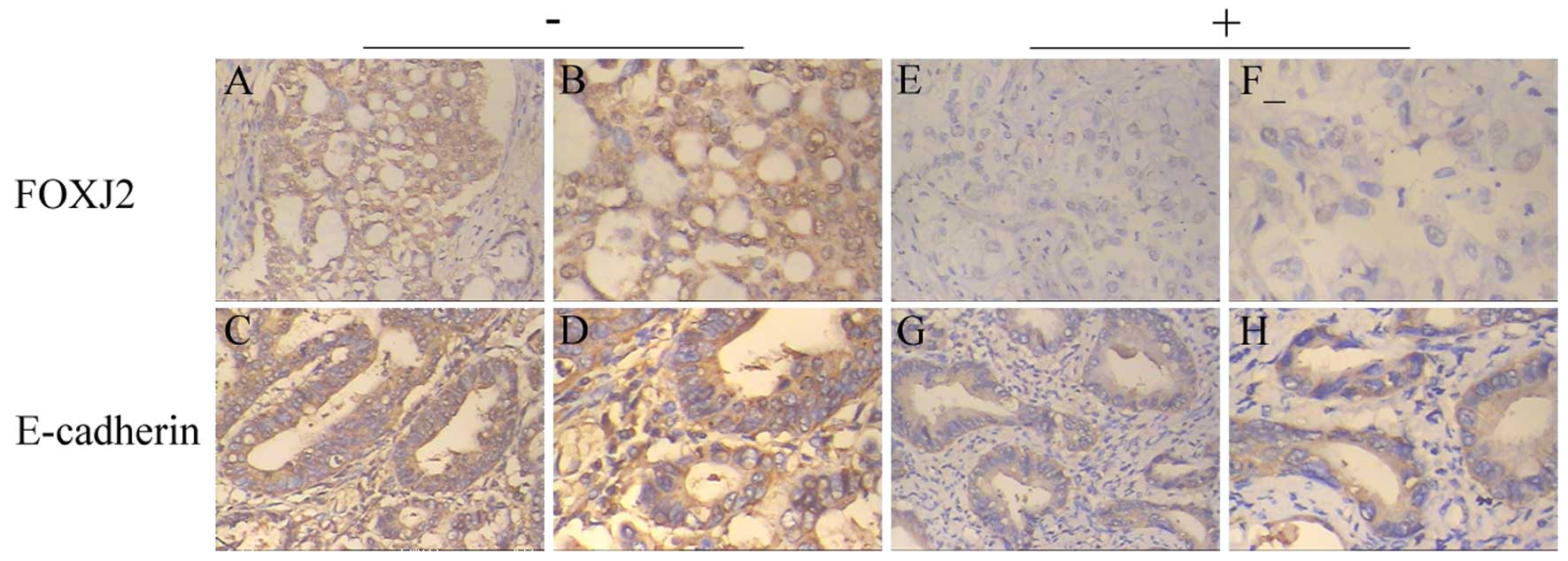
1D). We also measured the expression levels and subcellular
localization of FOXJ2 in 63 paraffin-embedded extrahepatic CC
samples by immunohistochemistry (Fig.
2). FOXJ2 protein showed low expression in 66.7% (42/63) of
extrahepatic CC samples, while high expression in 33.3% (21/63) of
extrahepatic CC samples, with staining mainly observed in the
nucleus of the tumor cells.
The correlations between the expression
of FOXJ2 and various clinicopathological characteristics
The relationship between clinicopathologic
characteristics and FOXJ2 expression levels in individuals with
extrahepatic CC were assessed by the χ2 analysis. We
found no significant association between FOXJ2 expression levels
and the patient age, sex, location, tumor size, differentiation,
perineural invasion or tumor stage in the 63 extrahepatic CC cases.
However, we observed that the expression level of FOXJ2 was
positively correlated with lymphatic invasion (P=0.045), venous
invasion (P=0.031), lymph node metastasis (P=0.012), TNM stage
(P=0.006) and E-cadherin expression (P=0.011) in extrahepatic CC
patients (Table I).
 | Table ICorrelation between FOXJ2 expression
and the clinicopathological characteristics of extrahepatic CC
patients. |
Table I
Correlation between FOXJ2 expression
and the clinicopathological characteristics of extrahepatic CC
patients.
| FOXJ2
expression |
|---|
|
|
|---|
| Factor | Cases (n=63) | Low (n=42) | High (n=21) | P-valuea | χ2 |
|---|
| Age | | | | 0.589 | 0.292 |
| ≤60 | 27 | 19 | 8 | | |
| >60 | 36 | 23 | 13 | | |
| Gender | | | | 0.202 | 1.625 |
| Male | 38 | 23 | 15 | | |
| Female | 25 | 19 | 6 | | |
| Location | | | | 0.475 | 0.511 |
| Hilar | 34 | 24 | 10 | | |
| Distal | 29 | 18 | 11 | | |
| Tumor size | | | | 0.280 | 1.167 |
| ≤2 cm | 27 | 16 | 11 | | |
| >2 cm | 36 | 26 | 10 | | |
|
Differentiation | | | | 0.143 | 3.886 |
| Well | 12 | 6 | 6 | | |
| Moderate | 33 | 21 | 12 | | |
| Poor | 18 | 15 | 3 | | |
| Lymphatic
invasion | | | | 0.045b | 4.012 |
| − | 25 | 13 | 12 | | |
| + | 38 | 29 | 9 | | |
| Venous
invasion | | | | 0.031b | 4.667 |
| − | 27 | 14 | 13 | | |
| + | 36 | 28 | 8 | | |
| Perineural
invasion | | | | 0.205 | 1.604 |
| − | 26 | 15 | 11 | | |
| + | 37 | 27 | 10 | | |
| Tumor stage | | | | 0.209 | 1.575 |
| T1–2 | 35 | 21 | 14 | | |
| T3–4 | 28 | 21 | 7 | | |
| Lymph node
metastasis | | | | 0.012b | 6.262 |
| − | 34 | 18 | 16 | | |
| + | 29 | 16 | 5 | | |
| TNM stage
(UICC) | | | | 0.006b | 7.572 |
| I–II | 39 | 21 | 18 | | |
| III–IV | 24 | 21 | 3 | | |
| E-cadherin
expression | | | | 0.011b | 6.499 |
| Low | 38 | 30 | 8 | | |
| High | 25 | 12 | 13 | | |
Expression of FOXJ2 and clinical
outcome
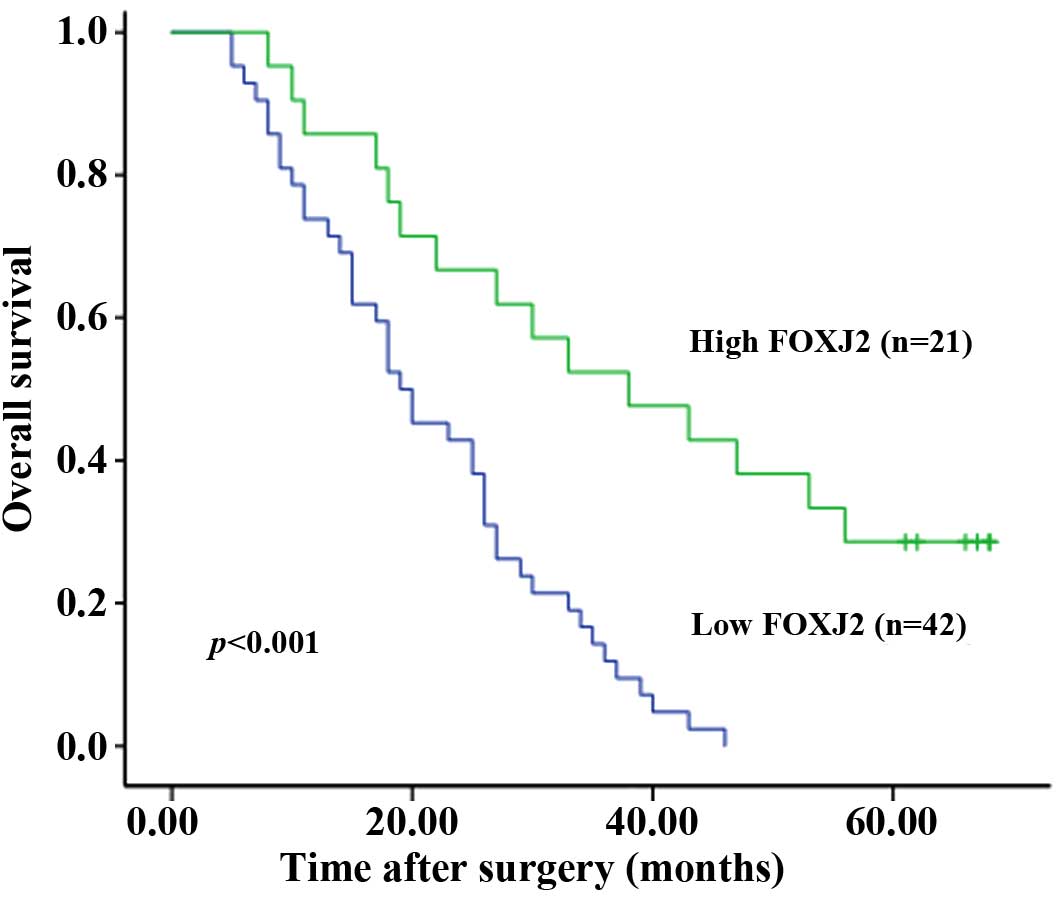
The overall survival of patients with low FOXJ2
expression was significantly poorer than that of FOXJ2-high
patients (P<0.001, log-rank test (Fig. 3). Univariate Cox regression
analyses showed that FOXJ2 expression was a prognostic factor for
poor survival (P<0.001), differentiation, lymphatic invasion,
venous invasion, tumor stage and lymph node metastasis were also
prognostic factors in the univariate analysis (Table II). Furthermore, a multivariate
Cox regression analysis confirmed FOXJ2 expression (P=0.012), tumor
stage and lymph node metastasis as independent predictors of the
overall survival of extrahepatic CC patients (Table II).
 | Table IIUnivariate and multivariate analysis
of prognostic factors using Cox proportional hazards model. |
Table II
Univariate and multivariate analysis
of prognostic factors using Cox proportional hazards model.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age
(≤60/>60) | 0.628 | 0.368–1.070 | 0.087 | - | - | - |
| Sex (M/F) | 1.241 | 0.731–2.108 | 0.423 | - | - | - |
| Location
(hilar/distal) | 0.884 | 0.524–1.491 | 0.664 | - | - | - |
| Tumor size (≤2
cm/>2 cm) | 1.514 | 0.888–2.579 | 0.127 | - | - | - |
| Differentiation
(well/mod/poor) | 2.136 | 1.040–4.390 | 0.039a | 1.631 | 0.782–3.402 | 0.192 |
| Lymphatic invasion
(−/+) | 2.448 | 1.395–4.295 | 0.002a | 1.621 | 0.909–2.893 | 0.102 |
| Venous invasion
(−/+) | 2.339 | 1.332–4.106 | 0.003a | 1.545 | 0.876–2.752 | 0.140 |
| Perineural invasion
(−/+) | 1.653 | 0.915–2.669 | 0.102 | - | - | - |
| Tumor stage
(T1–2/3–4) | 2.778 | 1.615–4.779 | <0.001a | 2.008 | 1.149–3.509 | 0.014a |
| Lymph node
metastasis (−/+) | 3.121 | 1.807–5.393 | <0.001a | 2.161 | 1.226–3.809 | 0.008a |
| FOXJ2 expression
(low/high) | 0.277 | 0.141–0.545 | <0.001a | 2.393 | 1.215–4.712 | 0.012a |
Overexpression of FOXJ2 inhibits
extrahepatic CC cell invasion, migration and proliferation in
vitro
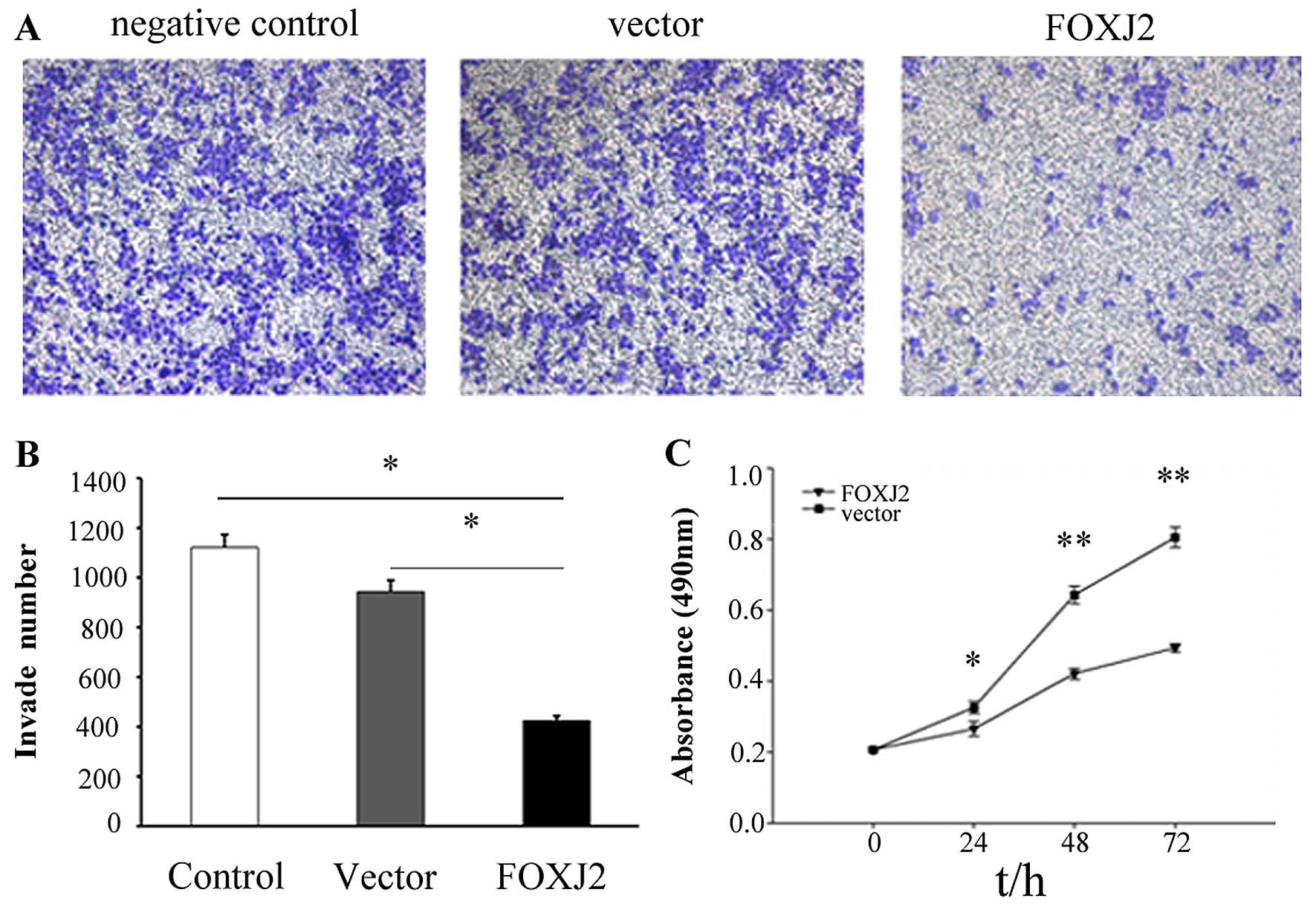
Because our above results indicated that FOXJ2
expression was reduced in extrahepatic CC and FOXJ2 might act as a
tumor suppressor, we next explored the function of FOXJ2 in
extrahepatic CC development. To evaluate the effects of FOXJ2 on
cell invasion, the FOXJ2 overexpressing vector and the empty vector
were respectively transfected into QBC939 cells. The cells were
seeded in the chamber and their invasion ability was determined 48
h later. The results revealed that overexpression of FOXJ2 was
associated with a significant reduction of invasion compared to
control empty vector extrahepatic CC cells (Fig. 5A and B, P=0.039), which showed that
FOXJ2 significantly repressed the invasion of extrahepatic CC
cells.
The above results were further confirmed by the
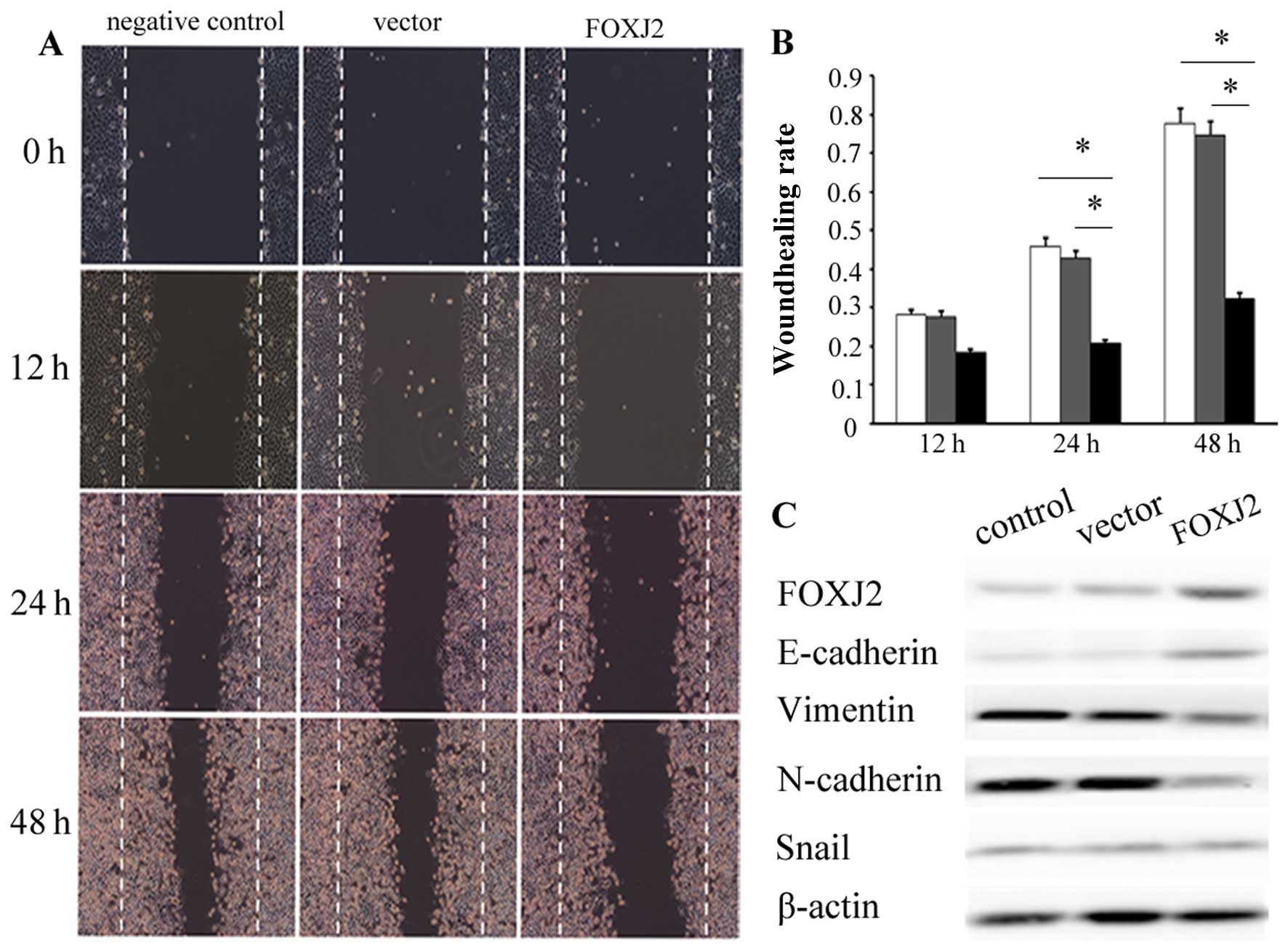
wound healing assay. The overexpression of FOXJ2 significantly
inhibited the migration of QBC939 cells at 48 h after transfection
(Fig. 4A and B, P<0.05).
Moreover, the cell growth by MTT assay revealed that cell growth
rate in FOXJ2-transfected extrahepatic CC cells were significantly
lower than empty vector-transfected extrahepatic CC cells (Fig. 5C, P<0.05). These data showed
that the overexpression of FOXJ2 inhibited extrahepatic CC cell
invasion, migration and proliferation in vitro.
To reveal how FOXJ2 regulates cell invasion and
migration in QBC939 cells, we decided to study EMT because the
cells acquire migrating potential and may invade the surrounding
stroma and enter circulating blood (23). To determine whether EMT is involved
in the effects of FOXJ2 on cell invasion and migration, change in
expression level of EMT-related genes was measured by using western
blotting, including E-cadherin, vimentin, N-cadherin and snail
(Fig. 4C). The results imply that
the changed expression of FOXJ2 was accompanied by the upregulation
of epithelial marker E-cadherin and downregulation of mesenchymal
marker vimentin and N-cadherin, and the protein expression level of
snail, a transcriptional regulator, was not significantly
changed.
Discussion
Extrahepatic CC is an aggressive malignancy with
dismal prognosis and characterized by early invasion, metastasis
and postoperative recurrence. Therefore, understanding the main
molecular mechanisms of this malignancy is the key for the
development of novel and effective therapeutic strategies for
extrahepatic CC. This is the first report on the
clinicopathological significance of FOXJ2 expression in patients
with extrahepatic CC. We found that the expression of FOXJ2 was
significantly reduced at both mRNA and protein levels in
extrahepatic CCs compared with paired paracancer normal bile duct
tissues. We also found that extrahepatic CC patients with low
expression of FOXJ2 showed shorter postoperative survival than high
FOXJ2 expression patients. Therefore, it is proposed that
downregulation of FOXJ2 may contribute to extrahepatic CC
initiation and progression.
Lymph node metastasis frequently occurs in patients
with extrahepatic CC. Recent studies have reported rates for lymph
node metastasis of 24–47% for hilar CC, and 25–63% for distal CC
(5, 6, 24–31).
In the present study, lymph node involvement was found in 42.8% of
all patients. Our results showed that both the level of FOXJ2 mRNA
and immunostaining rates in the extrahepatic CCs with lymph node
metastasis were significantly decreased compared with those in
extrahepatic CCs without lymph node metastasis. In addition, we
showed that the low level of FOXJ2 expression has a propensity to
be associated with lymphatic invasion, and lymph node metastasis in
extrahepatic CC patients. We also found that the expression level
of FOXJ2 was positively correlated with of E-cadherin. According to
previous reports, lymph node metastasis and E-cadherin may serve as
independent prognostic factors for extrahepatic CC (26,28,30,32–34).
By combining with the results of univariate and multivariate
analyses, these data suggested that FOXJ2 may be a new prognostic
marker for extrahepatic CC patients after surgical resection.
Epithelial-mesenchymal transition (EMT) is essential
for phenotypic transition during embryogenesis and wound healing,
and could also be reactivated during the malignant progression of
numerous cancers (35). During
EMT, tumor cells are expected to lose their epithelial phenotype
and gradually acquire a mesenchymal phenotype. E-cadherin is a
transmembrane glycoprotein which serves as the prime mediator of
epithelial adhesion and also plays a critical role in suppression
of tumor progression (35). Some
research has shown that expression of EMT-related proteins is
closely associated with tumor progression and a poor prognosis in
extrahepatic CC (34,36), suggesting that the EMT process may
act as an important molecular event during the progression and
metastasis of extrahepatic CC.
Immunostaining of individual EMT markers, such as
E-cadherin, has shown that high expression of FOXJ2 is correlated
with high E-cadherin, while low expression of FOXJ2 is correlated
with low E-cadherin in the same tissue. Western blotting showed
that exogenous FOXJ2 overexpression resulted in the increase of
epithelial markers E-cadherin, and decrease of mesenchymal marker
vimentin and N-cadherin, whereas FOXJ2 overexpression had no effect
on Snail expression. Thus, the above data indicate that the FOXJ2
protein is able to bind to E-cadherin promoters and transactivate
their transcription, which suggest that FoxJ2 is involved in the
regulation of cell adhesion events (37).
FOX family genes are implicated in carcinogenesis
through gene amplification, retroviral integration, chromosomal
translocation, and transcriptional regulation (38). FOXJ2 belongs to the human Fox
family and was able to activate transcription (19). Furthermore, FOXJ2 appeared to be
involved in positively regulating the progression of the cell cycle
or contributing to tumorigenesis (39). Currently, several FOX subfamilies
such as FOXA, FOXC, FOXM, FOXO, FOXP have been shown to play an
important role in tumorigenesis and the progression of certain
cancers (9). Besides, previous
evidence suggests that FOXJ2 might actively participate in the
metastatic process. In this study, we identified and functionally
characterized FOXJ2 as an important role in extrahepatic CC
progression. Whereas, our data, obtained by modulating FOXJ2
expression, establish a role for FOXJ2 in modulating the biological
properties of extrahepatic CC cells, including proliferation,
migration and aggressiveness in vitro. Overexpression of
FOXJ2 resulted in suppression of cell migration and invasion, which
suggests a potential role for FOXJ2 in the regulation of tumor cell
migration, invasion, in line with previous studies (21). Considering cell proliferation
function of FOXJ2 identified in spinal cord injury, FOXJ2
expression was increased predominantly in astrocytes, which highly
expressed proliferating cell nuclear antigen, a marker for
proliferating cells (40), we
suggest that exogenous FOXJ2 resulted in arrest of extrahepatic CC
cell proliferation in this study.
Thus, based on previous studies and the present
study, we suggest that overexpression of FOXJ2 contributed to
extrahepatic CC initiation and progression through promoting the
migration and invasion of extrahepatic CC cells, and possibly
functions as a tumor suppressor gene, which is consist with in an
earlier study (21). Therefore,
the data in this study suggest reasons to believe that FOXJ2 could
be a new therapeutic target for improving the treatment efficiency
of extrahepatic cholangiocarcinoma.
In conclusion, we provide compelling evidence that
overexpression of FOXJ2 leads to suppressed cell growth, migration
and invasion in extrahepatic CC cells. FOXJ2 expression may be a
therapeutic target, or useful to guide therapy of extrahepatic CC
patients. However, the complex molecular mechanisms of FOXJ2
contributing to extrahepatic CC require further investigation.
Acknowledgements
This study was supported by Medical innovation team
and talents of Jiangsu province (LJ201134).
References
|
1
|
Augustine MM and Fong Y: Epidemiology and
risk factors of biliary tract and primary liver tumors. Surg Oncol
Clin North Am. 23:171–188. 2014. View Article : Google Scholar
|
|
2
|
Shaib Y and El-Serag HB: The epidemiology
of cholangiocarcinoma. Semin Liver Dis. 24:115–125. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van der Gaag NA, Kloek JJ, de Bakker JK,
Musters B, Geskus RB, Busch OR, Bosma A, Gouma DJ and van Gulik TM:
Survival analysis and prognostic nomogram for patients undergoing
resection of extrahepatic cholangiocarcinoma. Ann Oncol.
23:2642–2649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaira K, Sunose Y, Ohshima Y, Ishioka NS,
Arakawa K, Ogawa T, Sunaga N, Shimizu K, Tominaga H, Oriuchi N, et
al: Clinical significance of L-type amino acid transporter 1
expression as a prognostic marker and potential of new targeting
therapy in biliary tract cancer. BMC Cancer. 13:4822013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirano S, Kondo S, Tanaka E, Shichinohe T,
Tsuchikawa T, Kato K, Matsumoto J and Kawasaki R: Outcome of
surgical treatment of hilar cholangiocarcinoma: A special reference
to postoperative morbidity and mortality. J Hepatobiliary Pancreat
Sci. 17:455–462. 2010. View Article : Google Scholar
|
|
6
|
Sakamoto Y, Kosuge T, Shimada K, Sano T,
Ojima H, Yamamoto J, Yamasaki S, Takayama T and Makuuchi M:
Prognostic factors of surgical resection in middle and distal bile
duct cancer: An analysis of 55 patients concerning the significance
of ductal and radial margins. Surgery. 137:396–402. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaufmann E and Knöchel W: Five years on
the wings of fork head. Mech Dev. 57:3–20. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lehmann OJ, Sowden JC, Carlsson P, Jordan
T and Bhattacharya SS: Fox’s in development and disease. Trends
Genet. 19:339–344. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Myatt SS and Lam EW: The emerging roles of
forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 7:847–859.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benayoun BA, Caburet S and Veitia RA:
Forkhead transcription factors: Key players in health and disease.
Trends Genet. 27:224–232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Banham AH, Connors JM, Brown PJ, Cordell
JL, Ott G, Sreenivasan G, Farinha P, Horsman DE and Gascoyne RD:
Expression of the foxp1 transcription factor is strongly associated
with inferior survival in patients with diffuse large B-cell
lymphoma. Clin Cancer Res. 11:1065–1072. 2005.PubMed/NCBI
|
|
12
|
Schuster MB and Porse BT: C/EBPalpha: A
tumour suppressor in multiple tissues? Biochim Biophys Acta.
1766:88–103. 2006.PubMed/NCBI
|
|
13
|
Barrans SL, Fenton JA, Banham A, Owen RG
and Jack AS: Strong expression of FOXP1 identifies a distinct
subset of diffuse large B-cell lymphoma (DLBCL) patients with poor
outcome. Blood. 104:2933–2935. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Banham AH, Beasley N, Campo E, Fernandez
PL, Fidler C, Gatter K, Jones M, Mason DY, Prime JE, Trougouboff P,
et al: The FOXP1 winged helix transcription factor is a novel
candidate tumor suppressor gene on chromosome 3p. Cancer Res.
61:8820–8829. 2001.PubMed/NCBI
|
|
15
|
Lin L, Miller CT, Contreras JI, Prescott
MS, Dagenais SL, Wu R, Yee J, Orringer MB, Misek DE, Hanash SM, et
al: The hepatocyte nuclear factor 3 alpha gene, HNF3alpha (FOXA1),
on chromosome band 14q13 is amplified and overexpressed in
esophageal and lung adenocarcinomas. Cancer Res. 62:5273–5279.
2002.PubMed/NCBI
|
|
16
|
Chen HW, Huang XD, Li HC, He S, Ni RZ,
Chen CH, Peng C, Wu G, Wang GH, Wang YY, et al: Expression of FOXJ1
in hepatocellular carcinoma: Correlation with patients’ prognosis
and tumor cell proliferation. Mol Carcinog. 52:647–659. 2013.
View Article : Google Scholar
|
|
17
|
Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z,
Roskams T, Durnez A, Demetris AJ and Thorgeirsson SS:
Classification and prediction of survival in hepatocellular
carcinoma by gene expression profiling. Hepatology. 40:667–676.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Obama K, Ura K, Li M, Katagiri T, Tsunoda
T, Nomura A, Satoh S, Nakamura Y and Furukawa Y: Genome-wide
analysis of gene expression in human intrahepatic
cholangiocarcinoma. Hepatology. 41:1339–1348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pérez-Sánchez C, Arias-de-la-Fuente C,
Gómez-Ferrería MA, Granadino B and Rey-Campos J: FHX.L and FHX.S,
two isoforms of the human fork-head factor FHX (FOXJ2) with
differential activity. J Mol Biol. 301:795–806. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pérez-Sánchez C, Gómez-Ferrería MA, de La
Fuente CA, Granadino B, Velasco G, Esteban-Gamboa A and Rey-Campos
J: FHX, a novel fork head factor with a dual DNA binding
specificity. J Biol Chem. 275:12909–12916. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Yang S, Ni Q, He S, Zhao Y, Yuan
Q, Li C, Chen H, Zhang L, Zou L, et al: Overexpression of forkhead
box J2 can decrease the migration of breast cancer cells. J Cell
Biochem. 113:2729–2737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shiozaki H, Tahara H, Oka H, Miyata M,
Kobayashi K, Tamura S, Iihara K, Doki Y, Hirano S, Takeichi M, et
al: Expression of immunoreactive E-cadherin adhesion molecules in
human cancers. Am J Pathol. 139:17–23. 1991.PubMed/NCBI
|
|
23
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murakami Y, Uemura K, Sudo T, Hayashidani
Y, Hashimoto Y, Nakamura H, Nakashima A and Sueda T:
Gemcitabine-based adjuvant chemotherapy improves survival after
aggressive surgery for hilar cholangiocarcinoma. J Gastrointest
Surg. 13:1470–1479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Igami T, Nishio H, Ebata T, Yokoyama Y,
Sugawara G, Nimura Y and Nagino M: Surgical treatment of hilar
cholangiocarcinoma in the ‘new era’: The Nagoya University
experience. J Hepatobiliary Pancreat Sci. 17:449–454. 2010.
View Article : Google Scholar
|
|
26
|
Lee SG, Song GW, Hwang S, Ha TY, Moon DB,
Jung DH, Kim KH, Ahn CS, Kim MH, Lee SK, et al: Surgical treatment
of hilar cholangiocarcinoma in the new era: The Asan experience. J
Hepatobiliary Pancreat Sci. 17:476–489. 2010. View Article : Google Scholar
|
|
27
|
Unno M, Katayose Y, Rikiyama T, Yoshida H,
Yamamoto K, Morikawa T, Hayashi H, Motoi F and Egawa S: Major
hepatectomy for perihilar cholangiocarcinoma. J Hepatobiliary
Pancreat Sci. 17:463–469. 2010. View Article : Google Scholar
|
|
28
|
Murakami Y, Uemura K, Hayashidani Y, Sudo
T, Hashimoto Y, Ohge H and Sueda T: Prognostic significance of
lymph node metastasis and surgical margin status for distal
cholangiocarcinoma. J Surg Oncol. 95:207–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ebata T, Nagino M, Nishio H, Igami T,
Yokoyama Y and Nimura Y: Pancreatic and duodenal invasion in distal
bile duct cancer: Paradox in the tumor classification of the
American Joint Committee on Cancer. World J Surg. 31:2008–2015.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Woo SM, Ryu JK, Lee SH, Yoo JW, Park JK,
Kim YT, Jang JY, Kim SW, Kang GH and Yoon YB: Recurrence and
prognostic factors of ampullary carcinoma after radical resection:
Comparison with distal extrahepatic cholangiocarcinoma. Ann Surg
Oncol. 14:3195–3201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hong SM, Pawlik TM, Cho H, Aggarwal B,
Goggins M, Hruban RH and Anders RA: Depth of tumor invasion better
predicts prognosis than the current American Joint Committee on
Cancer T classification for distal bile duct carcinoma. Surgery.
146:250–257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Murakami Y, Uemura K, Sudo T, Hashimoto Y,
Nakashima A, Kondo N, Sakabe R, Ohge H and Sueda T: Prognostic
factors after surgical resection for intrahepatic, hilar, and
distal cholangiocarcinoma. Ann Surg Oncol. 18:651–658. 2011.
View Article : Google Scholar
|
|
33
|
Li Q, Wang JM, Liu C, Xiao BL, Lu JX and
Zou SQ: Correlation of aPKC-iota and E-cadherin expression with
invasion and prognosis of cholangiocarcinoma. Hepatobiliary
Pancreat Dis Int. 7:70–75. 2008.PubMed/NCBI
|
|
34
|
Nitta T, Mitsuhashi T, Hatanaka Y,
Miyamoto M, Oba K, Tsuchikawa T, Suzuki Y, Hatanaka KC, Hirano S
and Matsuno Y: Prognostic significance of epithelial-mesenchymal
transition-related markers in extrahepatic cholangiocarcinoma:
Comprehensive immunohistochemical study using a tissue microarray.
Br J Cancer. 111:1363–1372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang KJ, Wang DS, Zhang SY, Jiao XL, Li
CW, Wang XS, Yu QC and Cui HN: The E-cadherin repressor slug and
progression of human extrahepatic hilar cholangiocarcinoma. J Exp
Clin Cancer Res. 29:882010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martín-de-Lara F, Sánchez-Aparicio P,
Arias de la Fuente C and Rey-Campos J: Biological effects of FoxJ2
over-expression. Transgenic Res. 17:1131–1141. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Katoh M and Katoh M: Human FOX gene family
(Review). Int J Oncol. 25:1495–1500. 2004.PubMed/NCBI
|
|
39
|
Kehn K, Berro R, Alhaj A, Bottazzi ME, Yeh
WI, Klase Z, Van Duyne R, Fu S and Kashanchi F: Functional
consequences of cyclin D1/BRCA1 interaction in breast cancer cells.
Oncogene. 26:5060–5069. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen X, Cao X, Tao G, Cao Z, Wang S, Zhou
F, Xie W, Zhao P, Zhang Z and Cui Z: Foxj2 expression in rat spinal
cord after injury and its role in inflammation. J Mol Neurosci.
47:158–165. 2012. View Article : Google Scholar : PubMed/NCBI
|