Introduction
Breast cancer is the most frequently diagnosed
cancer and leading cause of cancer deaths among women in the world.
Currently, breast cancer is the most common cancer among women in
China; new cases account for 12.2% and the mortality rate is 9.6%
of all breast cancer patients worldwide (1). In 2013, a total of 232,340 cases of
invasive breast cancer and 39,620 breast cancer deaths were
reported among US women (2).
Although the mortality has dropped over the past decades, distant
metastasis is still a main cause of death among breast cancer.
By supplying nutrients and providing the vascular
route for haematogenous metastasis, vascular-dependent diseases
such as breast cancer can be affected by angiogenesis (3–5).
Since angiogenesis plays a pivotal role in breast cancer
development, and seriously effects cancer cell invasion and
metastasis, inhibition of tumor angiogenesis is considered as an
attractive and effective strategy for the therapy of breast cancer
(6). Angiogenesis is a complex
process, which is regulated by different molecular pathways
(7).
Metadherin (MTDH), as a novel multifunctional
oncogene, originally identified in 2002 (8). In our previous studies, we have found
that MTDH improves the invasiveness of breast cancer cells by
inducing epithelial to mesenchymal transition, is involved in
inflammation-induced tumor progression, modulates TRAIL-resistance
in breast cancer cells through caspase-8 downregulation and Bcl-2
upregulation, mediates estrogen-independent growth and tamoxifen
resistance through PTEN downregulation and that >40% tumors
overexpress MTDH, which correlates with metastasis and
poor-prognosis of breast cancer (9–13).
MTDH is frequently overexpressed in tumor tissues and its
expression level is associated with the progression and worse
prognosis of malignant tumor such as hepatocellular carcinoma, lung
cancer, bladder cancer, laryngeal squamous cell carcinoma and
breast cancer (14–19) Therefore, knockdown of MTDH can
significantly inhibit prostate cancer progression, sensitize
endometrial cancer cells to cell death induction by TRAIL and
sensitize breast cancer cells to AZD6244 (20–22).
In this study, we explored the inhibition of angiogenesis through
knockdown of MTDH in breast cancer and the potential of MTDH as a
therapeutic target for anti-angiogenesis.
Materials and methods
Reagents
Antibodies against ERK1/2, p-ERK, PTEN, MMP-2 and
VEGF were purchased from Cell Signaling Technology (Beverly, MA,
USA). Anti-MTDH rabbit antibody was obtained from Invitrogen
(Carlsbad, CA, USA). Anti-CD31 antibody was obtained from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). The other reagents were
obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Breast cancer cell line MDA-MB-231 and human
umbilical vein endothelial cells (HUVECs) were purchased from
American Type Culture Collection. They were cultured in Dulbecco’s
modified Eagle’s medium supplemented with 10% fetal bovine serum
(FBS) at 37°C and under 5% CO2 incubator.
Plasmid construction and
transfection
The MTDH knockdown plasmids were constructed as
previously described (23). In
brief, the 19-nt sequence 5′-ATGAACCAGAATCAGTCAGC-3′ was used to
construct MTDH shRNA. The 60-nt oligonucleotides were annealed and
inserted into the pSUPER.retro.pure (OligoEngine, Seattle, WA,
USA). According to the manufacturer’s protocol, the MDA-MB-231
cells were transfected with Lipofectamine 2000 (Invitrogen). The
shRNA interference vector was applied to establish the
MDA-MB-231-prpM cell line and the empty vector was used to
establish the MDA-MB-231-prpn cell line. Cells were selected with
0.5 μg/ml puromycin to generate stable cell lines. miR-21-mimics
and the corresponding negative control (NC) (Gene Pharma, Shanghai,
China) were used for upregulation of miR-21. Transiently
transfected cells were harvested at 48 h for mRNA and at 72 h for
protein analysis.
Quantitative real-time PCR
Total RNA was isolated using TRIzol by the
manufacturer’s protocol (Takara, Dalian, China). Total RNA was
reverse transcribed to cDNA by applying Prime Script RT reagent kit
(Takara). Quantitative RT-PCR was performed with the SYBR green
detection (Takara) in Applied Biosystems StepOne Plus Real-Time PCR
System (Applied Biosystems, Carlsbad, CA, USA). The level of GAPDH
was used as the endogenous control for detection of mRNA expression
analysis and U6 was used for microRNAs. The 2−ΔΔCt
method was employed for data analysis.
Western blot analysis
Cells were harvested and lysed in ice-cold RIPA
buffer (1X PBS, 1% NP40, 0.1% SDS, 5 mM EDTA, 0.5% sodium
deoxycholate and 1 mM sodium orthovanadate) with protease and
phosphatase inhibitors. The proteins were quantified using the BCA
Protein Assay kit (Merck, Darmstadt, Germany). Same amount of
protein was separated by 10% SDS-PAGE gel and then transferred onto
PVDF membrane (Millipore, Bedford, MA, USA). After blocking with 5%
non-fat dry milk, the PVDF membrane was first incubated overnight
at 4°C with primary antibodies, rinsed and incubated with the
secondary antibodies. The corresponding signals were detected with
enhanced chemiluminescence (ECL).
Tube formation assay
Tumor cell conditioned medium (TCM) without FBS was
obtained as described (24). The
96-well plates were covered with 50 μl Matrigel and placed at 37°C
for 30 min to polymerize. The HUVECs were suspended using different
TCM and 100 μl of HUVECs were added to each well. Then HUVECs were
incubated for 7 h. Tube-like structures were photographed with an
Olympus digital camera and macroscopic quantities were recorded by
counting at least 10 microscopic fields.
Mouse aortic ring assay
Mouse aortas were dissected from BALB/c mice and cut
into ~1-mm long sections as previously described (25). A 48-well plate was first covered
with 100 μl of Matrigel and incubated for 30 min at 37°C. The
aortic rings were put into the wells and then covered with an
overlay of 100 μl Matrigel, followed by addition of 200 μl of TCM.
The cultures were kept at 37°C in a humidified environment for a
week and the result of the fields covered by sprouting from the
aortic rings was examined with an Olympus microscope at appropriate
magnification.
Immunohistochemistry (IHC)
Seventy-seven breast cancer tissue samples were
obtained from the Department of Pathology of Qilu Hospital of
Shandong University from 2011 to 2014. To quantify the microvessel
density (MVD), the SP-9000 Histostain™-Plus kits (Zhongshan
Goldenbridge Biotechnology Co.) were used to detect CD31 expression
following standard steps as previously described (26,27).
MicroRNA array analysis
Total RNA was isolated using TRIzol by the
manufacturer’s protocol. A microarray with 873 miRNA probes was
designed in accordance with Sanger miRbase release 12.0. RNA
labeling and hybridization were performed as previously described
(28). After hybridization,
microarrays were investigated by the LuxScan 10K Microarray Scanner
(CapitalBio, Beijing, China), and the images were analyzed by
GenePix Pro 6.0 software (Axon Instruments, Foster City, CA, USA).
The data are available in the Gene Expression Omnibus (GEO).
Statistical analysis
Statistical software SPSS 18.0 was used. The data
are shown as mean ± SD. The difference in statistics was analyzed
through the Student’s t-test and regarded as statistically
significant for P-values <0.05.
Results
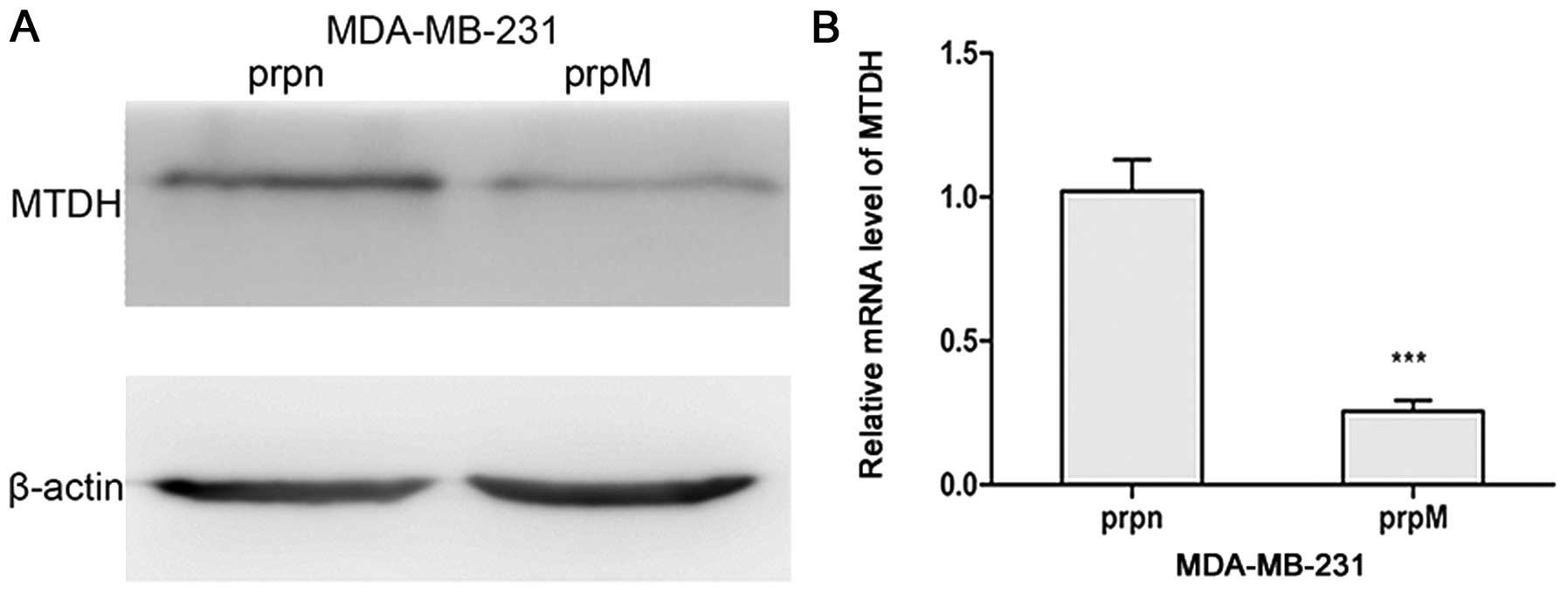
Establishment of the MTDH knockdown cell
line
Due to the high level expression of MTDH in
MDA-MB-231 cells, we first designed the short hairpin RNA and then
transfected the plasmid into MDA-MB-231 cells to establish the MTDH
knockdown cell line. The stable cell line was selected by adding
puromycin to DMEM. MTDH expression levels were detected by qRT-PCR
and western blot analysis. As shown in Fig. 1, MTDH expression levels were
obviously lower in MDA-MB-231-prpM cells than that in control
MDA-MB-231-prpn cells.
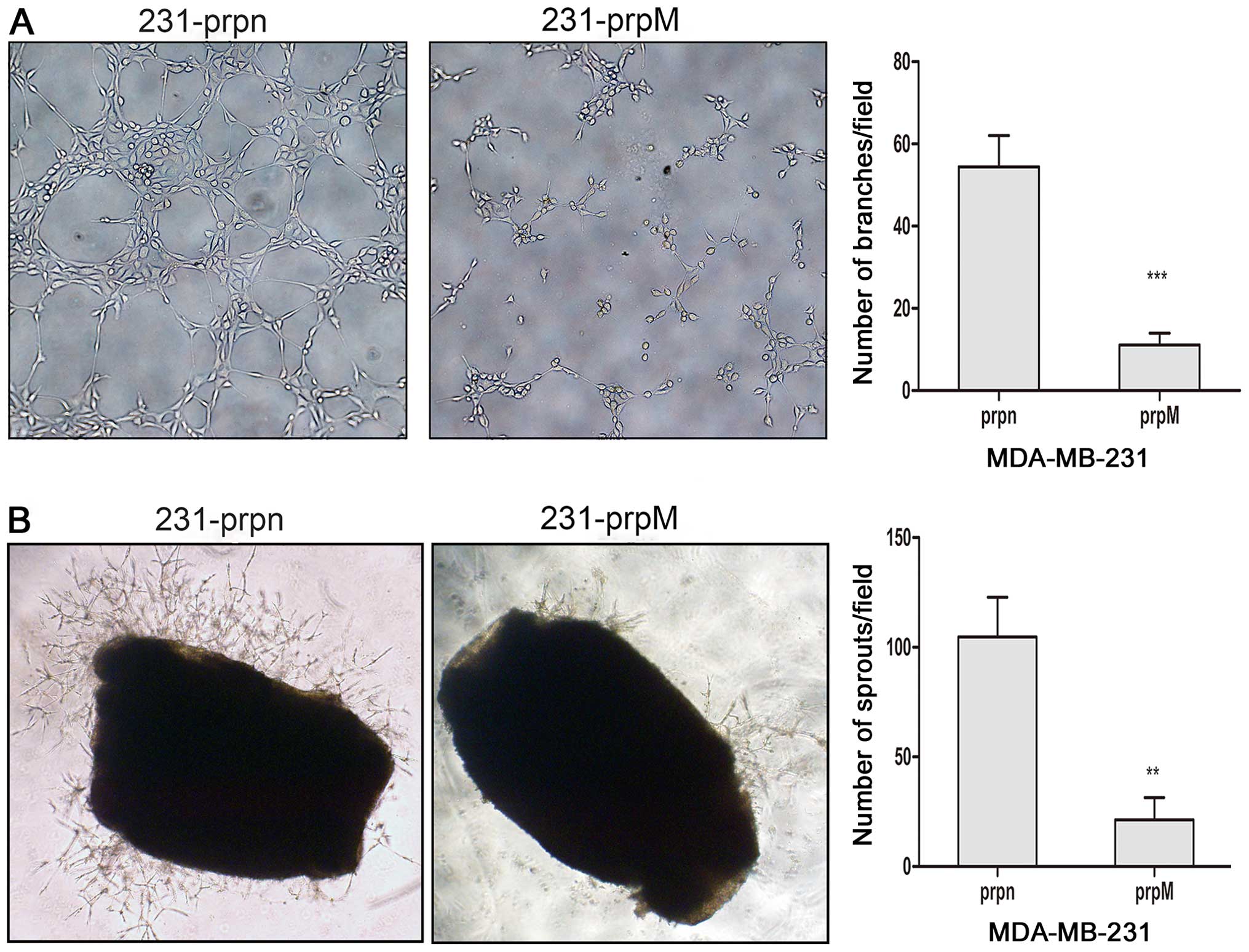
Knockdown of MTDH inhibits angiogenesis
in vitro
To confirm whether the knockdown of MTDH could
inhibit angiogenesis of breast cancer cells, a tube formation assay
was performed as an important indicator of endothelial function in
angiogenesis. HUVECs cultured on Matrigel rapidly align and finally
form tube-like structures. Since the tube formation can be affected
by different medium components, TCM obtained from different breast
cells was added to HUVECs cultured on Matrigel and the tube-like
structures were quantitatively investigated. Compared to the
control MDA-MB-231-prpn cells, HUVECs cultured with TCM from
MDA-MB-231-prpM cells caused an obvious decrease in tube formation,
as shown in Fig. 2A.
Knockdown of MTDH inhibits angiogenesis
ex vivo
To investigate the inhibition of angiogenesis ex
vivo, we detected the sprouting of vessels from mouse aortic
rings. Mouse aortas cultured in Matrigel were treated with TCM from
MDA-MB-231-prpM cells and the control group. The effect on
angiogenesis of MTDH was demonstrated through comparing the fields
covered by sprouting from the aortic rings. As shown in Fig. 2B, the knockdown of MTDH
significantly inhibited the formation of microvessel structures
around the mouse aortic rings.
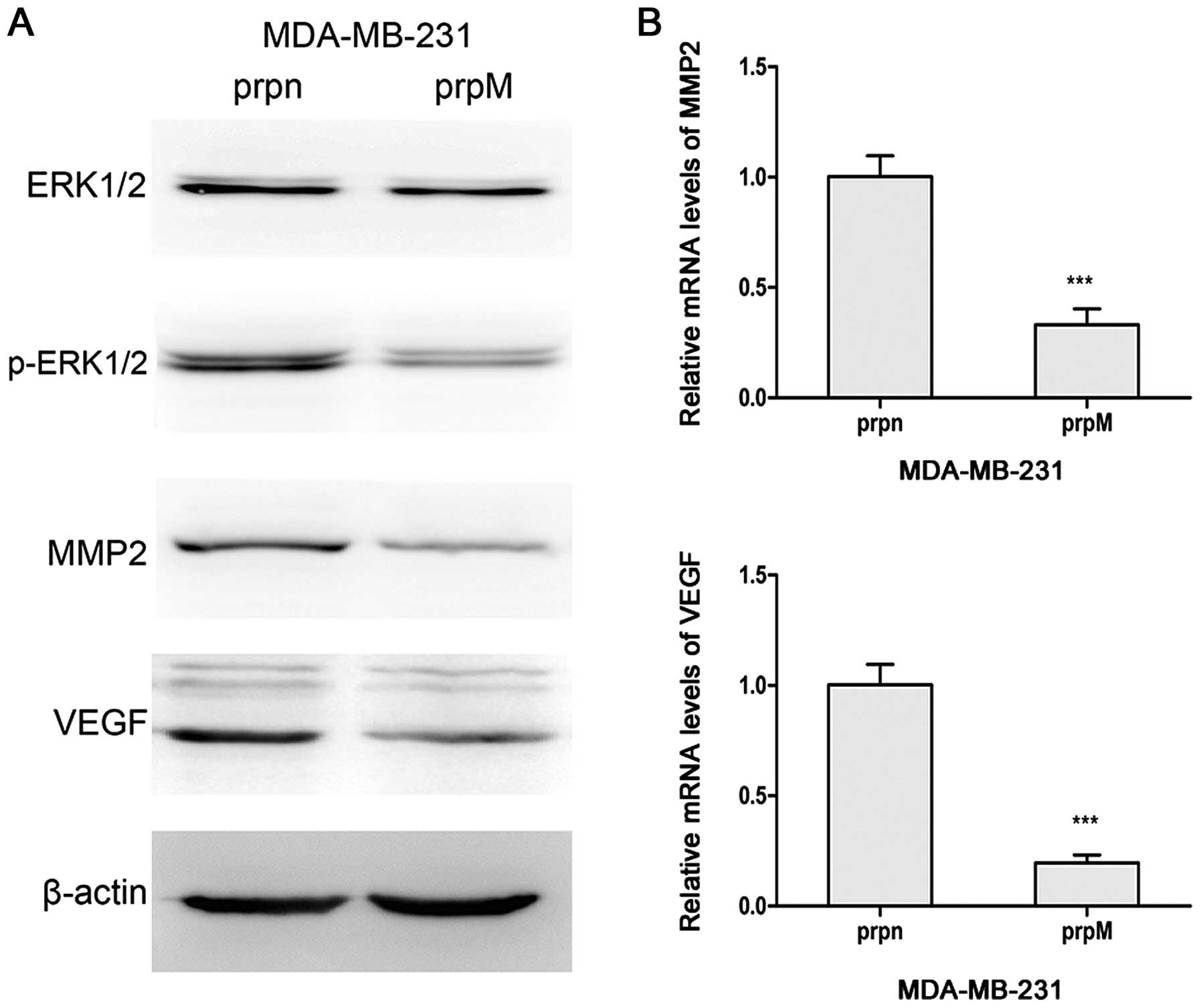
The knockdown of MTDH downregulates the
expression of p-ERK1/2 and decreases the levels of VEGF and
MMP2
ERK1/2 molecule was widely regarded as a signal
pathway activator of angiogenesis (29,30).
Therefore, the protein levels of ERK1/2 and p-ERK1/2 were monitored
in our test to explore a potential mechanism of action. As shown in
Fig. 3, the knockdown of MTDH
downregulated the expression of p-ERK1/2 in western blot analysis.
Because p-ERK1/2 could regulate angiogenesis through MMP-2 and VEGF
(31), we measured the mRNA levels
and protein levels of MMP-2 and VEGF in MTDH knockdown cells. The
levels of the two markers were significantly reduced in
MDA-MB-231-prpM cells.
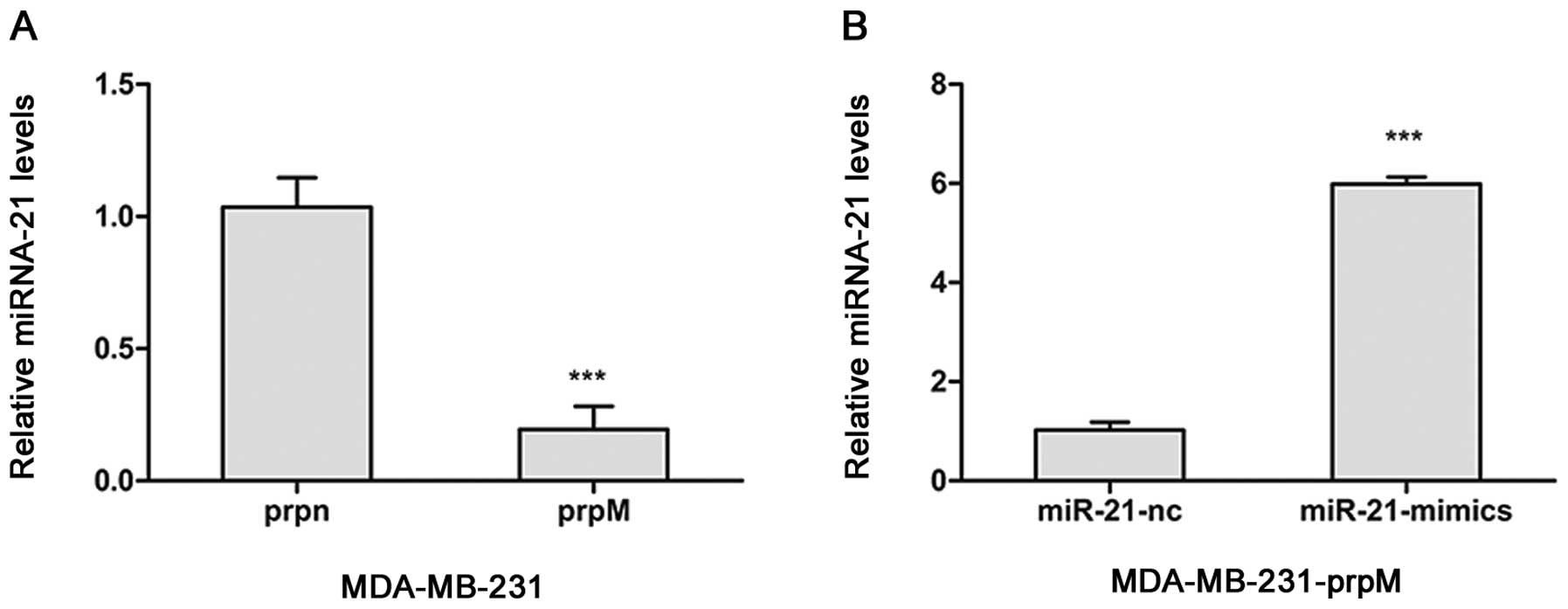
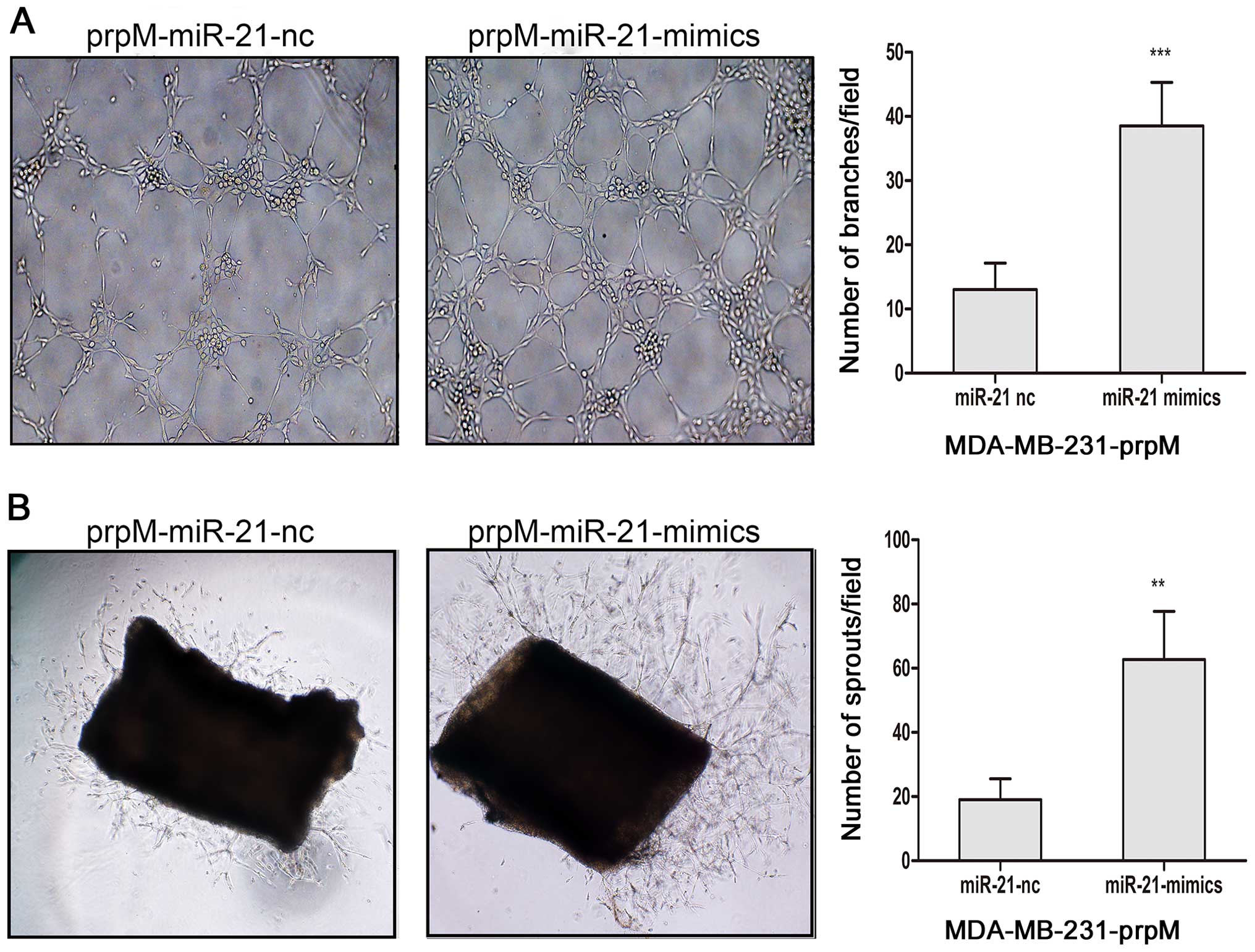
miR-21-mimics increase miR-21-inducing
ERK1/2, MMP2 and VEGF expression, and promote tumor
angiogenesis
To investigate the influence of MTDH knockdown on
the inhibition of angiogenesis, by regulating levels of miRNAs, the
miRNA arrays was adopted to detect the changes of miRNAs after MTDH
knockdown. From the miRNA array data, we found MTDH regulated miRNA
expression in MDA-MB-231-prpM cell (data not shown). Among them,
miR-21 level was significantly decreased. To investigate whether
miR-21 was involved in the inhibition of angiogenesis, we
transected miR-21-mimics with Lipofectamine 2000 into
MDA-MB-231-prpM to upregulate the level of miR-21. As shown in
Fig. 4, the level of miR-21
obviously increased. Then, we applied tube formation assay and
mouse aortic ring assay to explore the role of miR-21 in
angiogenesis. The results showed that upregulated expression of
miR-21 could partially reverse the inhibition of angiogenesis in
MDA-MB-231-prpM cells (Fig. 5).
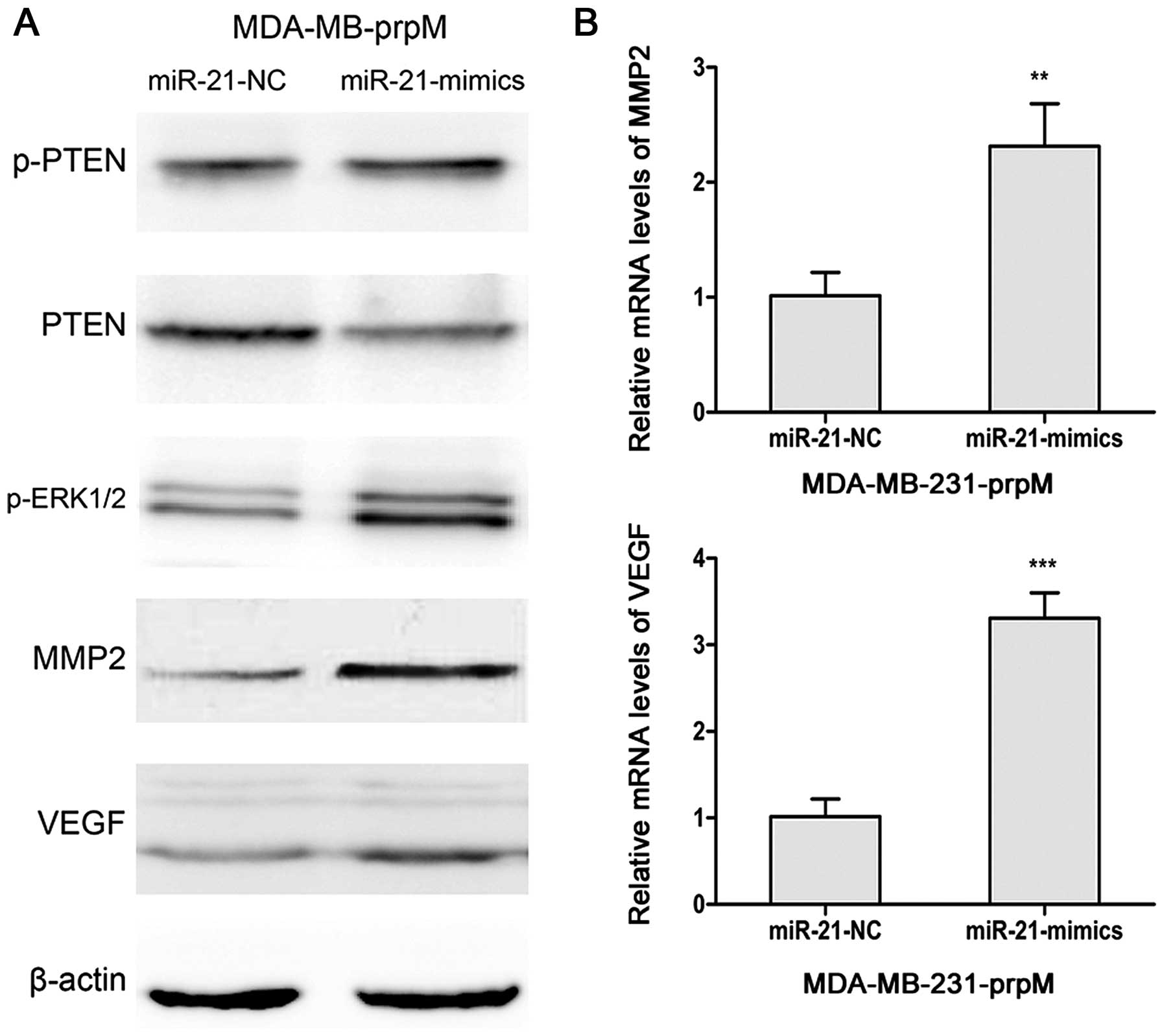
Previous studies indicated that miR-21 regulated expression of PTEN
(32,33). Therefore, we detected PTEN and
p-PTEN in miR-21 mimic-transfected MDA-MB-231-prpM cells. As shown
in Fig. 6, upregulated expression
of miR-21 increased the protein level of p-ERK1/2 via suppressing
PTEN, and then increased the levels of MMP2 and VEGF.
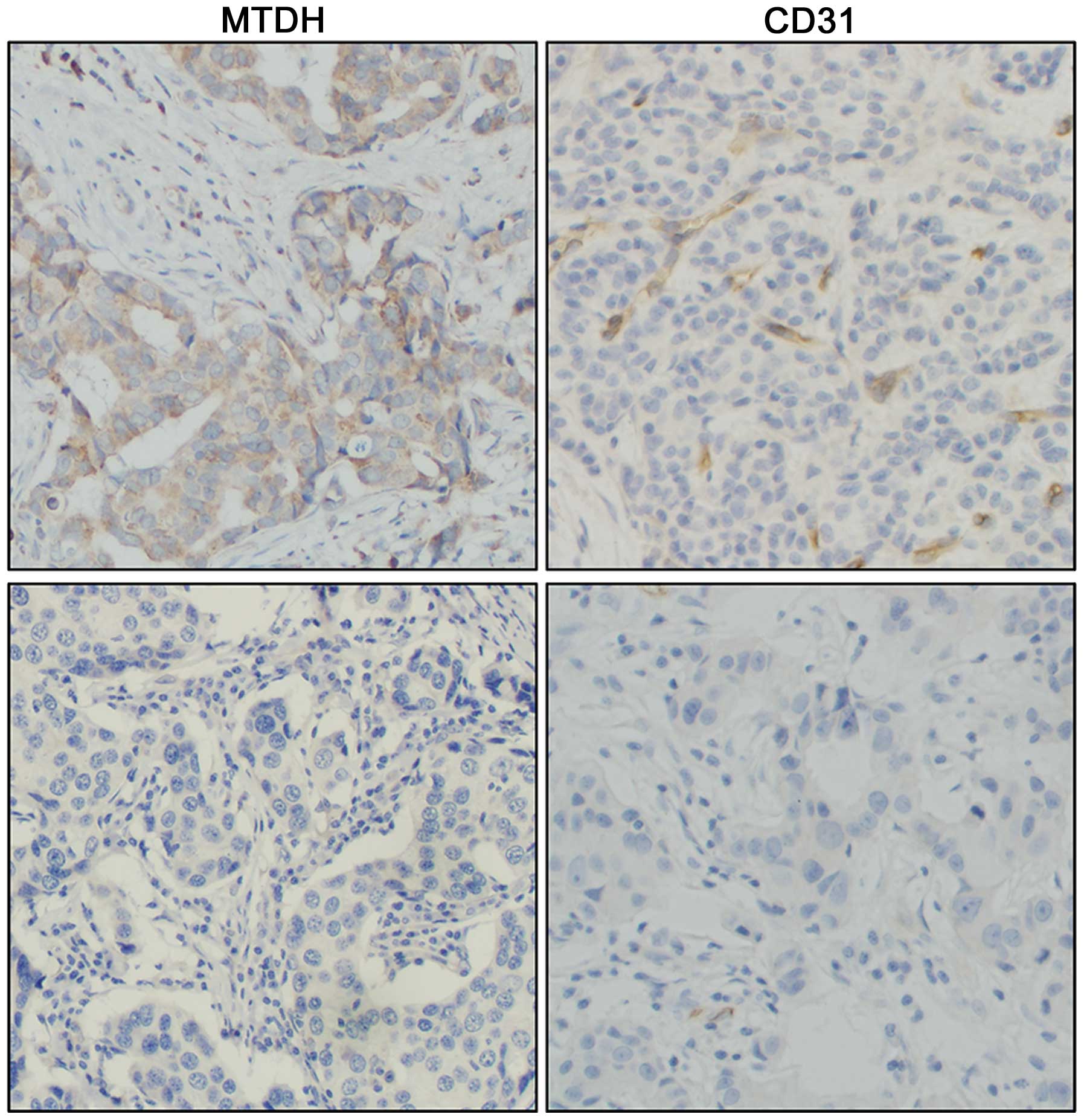
Lower MVD is linked with low expression
of MTDH
To confirm the relationship between MTDH and
angiogenesis in breast cancer tissue, we stained 77 breast cancer
tissue samples with the MTDH and CD31 antibody. As shown in
Fig. 7, 43 cases were
CD31-positive in the 61 MTDH-positive cases in total. Of these, 6
cases were CD31-positive in the total 16 MTDH-negative staining
cases. Therefore, compared to the MTDH overexpressing tissues, the
result showed that low expression of MTDH was linked with lower
MVD. The data were evaluated with the χ2 test
(P=0.032).
Discussion
Angiogenesis is mediated by multiple molecules
including vascular endothelial growth factor (VEGF), epiderma1
growth factor (EGF), epidermal growth factor receptor (EGFR) and
matrix metalloproteinases (MMPs).
Due to the increased secretion of pro-angiogenic
factors, malignant cells become more angiogenic originating from
the activation of the oncogene or inactivation of the tumor
suppressor gene. For example, in breast cancer, P53 mutations
promote angiogenesis by upregulation of EGFR (34); activated RhoA promotes VEGF
expression and angiogenesis by decrease of P53 stability (35); 53BP1 inhibits angiogenesis by
decrease of MMP2 and MMP9 (36).
Previous studies demonstrated that MTDH as an oncogene promotes
invasion and metastasis of malignant cells. In triple-negative
breast cancer, it was reported that MTDH correlates with
angiogenesis and worse clinical outcomes through
immunohistochemical staining of 125 specimens. However, the
mechanism was not clarified (37).
To explore the function of the knockdown of MTDH to
modulate angiogenesis in breast cancer, the expression of MTDH was
manipulated with RNA interference in MDA-MB-231 cells. Our results
showed that the knockdown of MTDH was able to suppress tube
formation of HUVECs and sprouting of the mouse aortic rings. To
further investigate the potential molecular mechanism of the
knockdown of MTDH in inhibition of angiogenesis, we focused on the
ERK1/2 signaling pathway, which is essential in cell proliferation,
differentiation, apoptosis and angiogenesis (29,38,39).
The knockdown of MTDH downregulated the level of p-ERK1/2 in
MDA-MB-231-prpM cells, and then decreased the levels of MMP2 and
VEGF. These results demonstrated that the knockdown of MTDH is an
effective method of anti-angiogenesis in breast cancer and p-ERK1/2
signaling is an essential event in this process.
MicroRNAs (miRNAs), 20–25 nucleotides
non-protein-coding RNAs, have been proven to be involve in
regulation of gene expression (40). The function of miRNAs have been
confirmed not only in many biological processes but also in various
pathological situations including cancer (41). Angiogenesis is a key process in
cancer development, and the process can also be regulated by many
miRNAs such as miR-29b in hepatocellular carcinoma, miR-18a in
gastric cancer, miR-497 in ovarian cancer, miR-1246 in colorectal
cancer cells, and miR-21 in prostate cancer through different
molecular pathways (24,33,42–44).
As one of the best-evaluated miRNA, miR-21 has been reported as an
oncogene (45). Knockdown of
miR-21 can inhibit angiogenesis in VEGFR2-luc mouse breast tumor
model and reverse EMT by targeting PTEN in breast cancer cells
(46,47). The significant decrease of miR-21
expression was confirmed by microRNA array and quantitative PCR
method in MAD-MB-231-prpM cells. Our data showed that the
upregulated expression of miR-21 could partially reverse the
inhibition of angiogenesis through the ERK1/2 pathway activation in
MAD-MB-231-prpM cells, MTDH knockdown inhibited angiogenesis via
decreasing the expression level of miR-21.
To further validate the relation of MTDH and
angiogenesis in breast cancer samples, we evaluated the angiogenic
markers CD31 by using immunohistochemistry. The result showed that
MTDH was related with CD31 and the CD31 decreased in samples with
the lower expression of MTDH, thus, demonstrating that low
expression of MTDH suppressed angiogenesis in breast cancer.
Anti-angiogenesis is considered to be a prospective
novel therapeutic strategy for malignant tumors. However, to find
an efficient target gene or molecule is still an unsolved problem.
As an important oncogene, MTDH plays an important role in tumor
progression and prognosis. In this study, we provided evidence that
the knockdown of MTDH significantly inhibited angiogenesis. Our
findings demonstrate that MTDH is a potential therapeutic target
for anti-angiogenesis in breast cancer.
Acknowledgements
This study was supported by National Natural Science
Foundation of China (nos. 30772133, 81172529 and 81272903) and
Shandong Science and Technology Development Plan (nos. 2012GZC22115
and 2013GRC31801) to Q.Y.
References
|
1
|
Fan L, Strasser-Weippl K, Li J-J, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar
|
|
3
|
Ellis LM and Fidler IJ: Angiogenesis and
Metastasis. Eur J Cancer. 32A:2451–2460. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bikfalvi A: Significance of angiogenesis
in tumour progression and metastasis. Eur J Cancer. 31A:1101–1104.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fox SB: Tumor angiogenesis and prognosis.
Histopathology. 30:294–301. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weidner NSJ, Welch WR and Folkman J: Tumor
angiogenesis and metastasis - correlation in invasive breast
carcinoma. N Engl J Med. 324:81991. View Article : Google Scholar
|
|
7
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao
W, Volsky DJ and Fisher PB: Identification and cloning of human
astrocyte genes displaying elevated expression after infection with
HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid
subtraction hybridization, RaSH. Oncogene. 21:3592–3602. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang C and Yang Q: Astrocyte elevated
gene-1 and breast cancer (Review). Oncol Lett. 2:399–405.
2011.PubMed/NCBI
|
|
10
|
Li X, Kong X, Huo Q, Guo H, Yan S, Yuan C,
Moran MS, Shao C and Yang Q: Metadherin enhances the invasiveness
of breast cancer cells by inducing epithelial to mesenchymal
transition. Cancer Sci. 102:1151–1157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Y, Kong X, Li X, Yan S, Yuan C, Hu W
and Yang Q: Metadherin mediates lipopolysaccharide-induced
migration and invasion of breast cancer cells. PLoS One.
6:e293632011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu C, Kong X, Wang H, et al: MTDH mediates
estrogen-independent growth and tamoxifen resistance by
down-regulating PTEN in MCF-7 breast cancer cells. Cell Physiol
Biochem. 33:1557–1567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang N, Wang X, Huo Q, Li X, Wang H,
Schneider P, Hu G and Yang Q: The oncogene metadherin modulates the
apoptotic pathway based on the tumor necrosis factor superfamily
member TRAIL (tumor necrosis factor-related apoptosis-inducing
ligand) in breast cancer. J Biol Chem. 288:9396–9407. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Zhang N, Song LB, et al: Astrocyte
elevated gene-1 is a novel prognostic marker for breast cancer
progression and overall patient survival. Clin Cancer Res.
14:3319–3326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoo BK, Emdad L, Su ZZ, Villanueva A,
Chiang DY, Mukhopadhyay ND, Mills AS, Waxman S, Fisher RA, Llovet
JM, et al: Astrocyte elevated gene-1 regulates hepatocellular
carcinoma development and progression. J Clin Invest. 119:465–477.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Su Z, Li G, et al: Increased
expression of metadherin protein predicts worse disease-free and
overall survival in laryngeal squamous cell carcinoma. Int J
Cancer. 133:671–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou J, Li J, Wang Z, Yin C and Zhang W:
Metadherin is a novel prognostic marker for bladder cancer
progression and overall patient survival. Asia Pac J Clin Oncol.
8:e42–e48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song L, Li W, Zhang H, Liao W, Dai T, Yu
C, Ding X, Zhang L and Li J: Over-expression of AEG-1 significantly
associates with tumour aggressiveness and poor prognosis in human
non-small cell lung cancer. J Pathol. 219:317–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tokunaga E, Nakashima Y, Yamashita N,
Hisamatsu Y, Okada S, Akiyoshi S, Aishima S, Kitao H, Morita M and
Maehara Y: Overexpression of metadherin/MTDH is associated with an
aggressive phenotype and a poor prognosis in invasive breast
cancer. Breast Cancer. 21:341–349. 2014. View Article : Google Scholar
|
|
20
|
Kong X, Moran MS, Zhao Y and Yang Q:
Inhibition of metadherin sensitizes breast cancer cells to AZD6244.
Cancer Biol Ther. 13:43–49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kikuno N, Shiina H, Urakami S, Kawamoto K,
Hirata H, Tanaka Y, Place RF, Pookot D, Majid S, Igawa M, et al:
Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer
progression through upregulation of FOXO3a activity. Oncogene.
26:7647–7655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng X, Brachova P, Yang S, Xiong Z, Zhang
Y, Thiel KW and Leslie KK: Knockdown of MTDH sensitizes endometrial
cancer cells to cell death induction by death receptor ligand TRAIL
and HDAC inhibitor LBH589 co-treatment. PLoS One. 6:e209202011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu G, Chong RA, Yang Q, Wei Y, Blanco MA,
Li F, Reiss M, Au JL, Haffty BG and Kang Y: MTDH activation by 8q22
genomic gain promotes chemoresistance and metastasis of
poor-prognosis breast cancer. Cancer Cell. 15:9–20. 2009.
View Article : Google Scholar :
|
|
24
|
Fang JH, Zhou HC, Zeng C, Yang J, Liu Y,
Huang X, Zhang JP, Guan XY and Zhuang SM: MicroRNA-29b suppresses
tumor angiogenesis, invasion, and metastasis by regulating matrix
metalloproteinase 2 expression. Hepatology. 54:1729–1740. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Zhang N, Huo Q and Yang Q:
Anti-angiogenic and antitumor activities of Huaier aqueous extract.
Oncol Rep. 28:1167–1175. 2012.PubMed/NCBI
|
|
26
|
Su P, Zhang Q and Yang Q:
Immunohistochemical analysis of Metadherin in proliferative and
cancerous breast tissue. Diagn Pathol. 5:382010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu J, Li X, Kong X, Moran MS, Su P,
Haffty BG and Yang Q: Testin is a tumor suppressor and prognostic
marker in breast cancer. Cancer Sci. 103:2092–2101. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Ach RA and Curry B: Direct and
sensitive miRNA profiling from low-input total RNA. RNA.
13:151–159. 2007. View Article : Google Scholar :
|
|
29
|
Du J, Xu R, Hu Z, Tian Y and YZ: PI3K and
ERK-induced Rac1 activation mediates hypoxia-induced HIF-1α
expression in MCF-7 breast cancer cells. PloS One. 6:e252132011.
View Article : Google Scholar
|
|
30
|
Liu L, Cao Y, Chen C, Zhang X, McNabola A,
Wilkie D, Wilhelm S, Lynch M and Carter C: Sorafenib blocks the
RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor
cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer
Res. 66:11851–11858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shan B, Li W, Yang S-Y and Li Z-R:
Estrogen up-regulates MMP2/9 expression in endometrial epithelial
cell via VEGF-ERK1/2 pathway. Asian Pac J Trop Med. 6:826–830.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu LZ, Li C, Chen Q, Jing Y, Carpenter R,
Jiang Y, Kung HF, Lai L and Jiang BH: MiR-21 induced angiogenesis
through AKT and ERK activation and HIF-1α expression. PLoS One.
6:e191392011. View Article : Google Scholar
|
|
34
|
Shapira I, Lee A, Vora R and Budman DR:
P53 mutations in triple negative breast cancer upregulate endosomal
recycling of epidermal growth factor receptor (EGFR) increasing its
oncogenic potency. Crit Rev Oncol Hematol. 88:284–292. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma J, Xue Y, Cui W, Li Y, Zhao Q, Ye W,
Zheng J, Cheng Y, Ma Y, Li S, et al: Ras homolog gene family,
member A promotes p53 degradation and vascular endothelial growth
factor-dependent angiogenesis through an interaction with murine
double minute 2 under hypoxic conditions. Cancer. 118:4105–4116.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li X, Kong X, Wang Y and Yang Q: 53BP1 is
a novel regulator of angiogenesis in breast cancer. Cancer Sci.
104:1420–1426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li C, Li R, Song H, Wang D, Feng T, Yu X,
Zhao Y, Liu J, Yu X, Wang Y, et al: Significance of AEG-1
expression in correlation with VEGF, microvessel density and
clinicopathological characteristics in triple-negative breast
cancer. J Surg Oncol. 103:184–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bartholomeusz C, Gonzalez-Angulo AM, Liu
P, Hayashi N, Lluch A, Ferrer-Lozano J and Hortobágyi GN: High ERK
protein expression levels correlate with shorter survival in
triple-negative breast cancer patients. Oncologist. 17:766–774.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chetram MA and Hinton CV: PTEN regulation
of ERK1/2 signaling in cancer. J Recept Signal Transduct Res.
32:190–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Anand S and Cheresh DA: Emerging role of
micro-RNAs in the regulation of angiogenesis. Genes Cancer.
2:1134–1138. 2011. View Article : Google Scholar
|
|
42
|
Yamada N, Tsujimura N, Kumazaki M,
Shinohara H, Taniguchi K, Nakagawa Y, Naoe T and Akao Y: Colorectal
cancer cell-derived microvesicles containing microRNA-1246 promote
angiogenesis by activating Smad 1/5/8 signaling elicited by PML
down-regulation in endothelial cells. Biochim Biophys Acta.
1839:1256–1272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang W, Ren F, Wu Q, Jiang D, Li H and Shi
H: MicroRNA-497 suppresses angiogenesis by targeting vascular
endothelial growth factor A through the PI3K/AKT and MAPK/ERK
pathways in ovarian cancer. Oncol Rep. 32:2127–2133.
2014.PubMed/NCBI
|
|
44
|
Zheng Y, Li S and Ding Y: The role of
miR-18a in gastric cancer angiogenesis. Hepatogastroenterology.
60:52013.
|
|
45
|
Si ML, Zhu S, Wu H, Lu Z, Wu F and Mo YY:
miR-21-mediated tumor growth. Oncogene. 26:2799–2803. 2007.
View Article : Google Scholar
|
|
46
|
Han M, Liu M and Wang Y: Antagonism of
miR-21 reverses epithelial-mesenchymal transition and cancer stem
cell phenotype through AKT/ERK1/2 inactivation by targeting PTEN.
PLoS One. 7:112012.
|
|
47
|
Zhao D, Tu Y, Wan L, Bu L, Huang T, Sun X,
Wang K and Shen B: In vivo monitoring of angiogenesis inhibition
via down-regulation of mir-21 in a VEGFR2-luc murine breast cancer
model using bioluminescent imaging. PLoS One. 8:e714722013.
View Article : Google Scholar : PubMed/NCBI
|