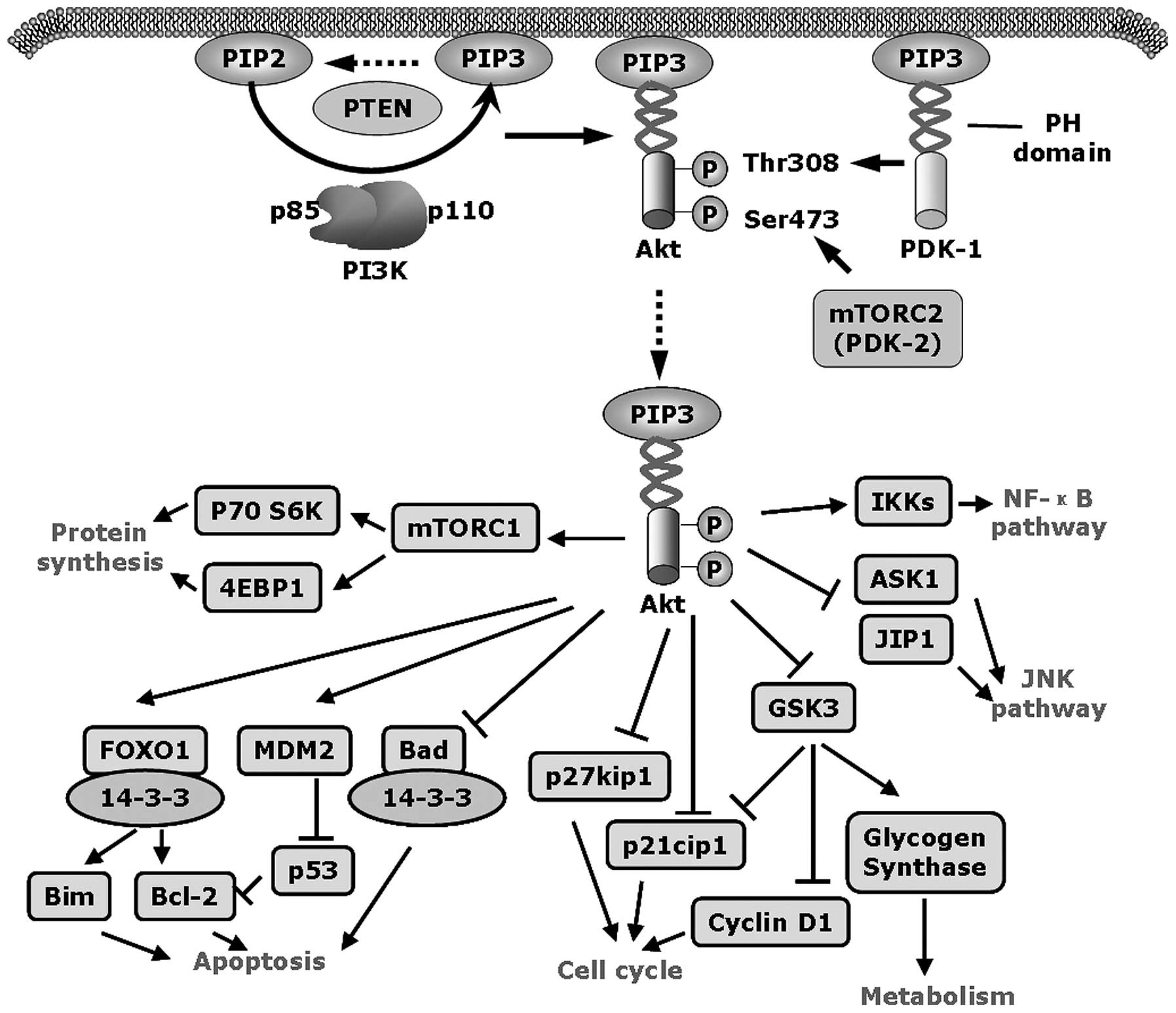
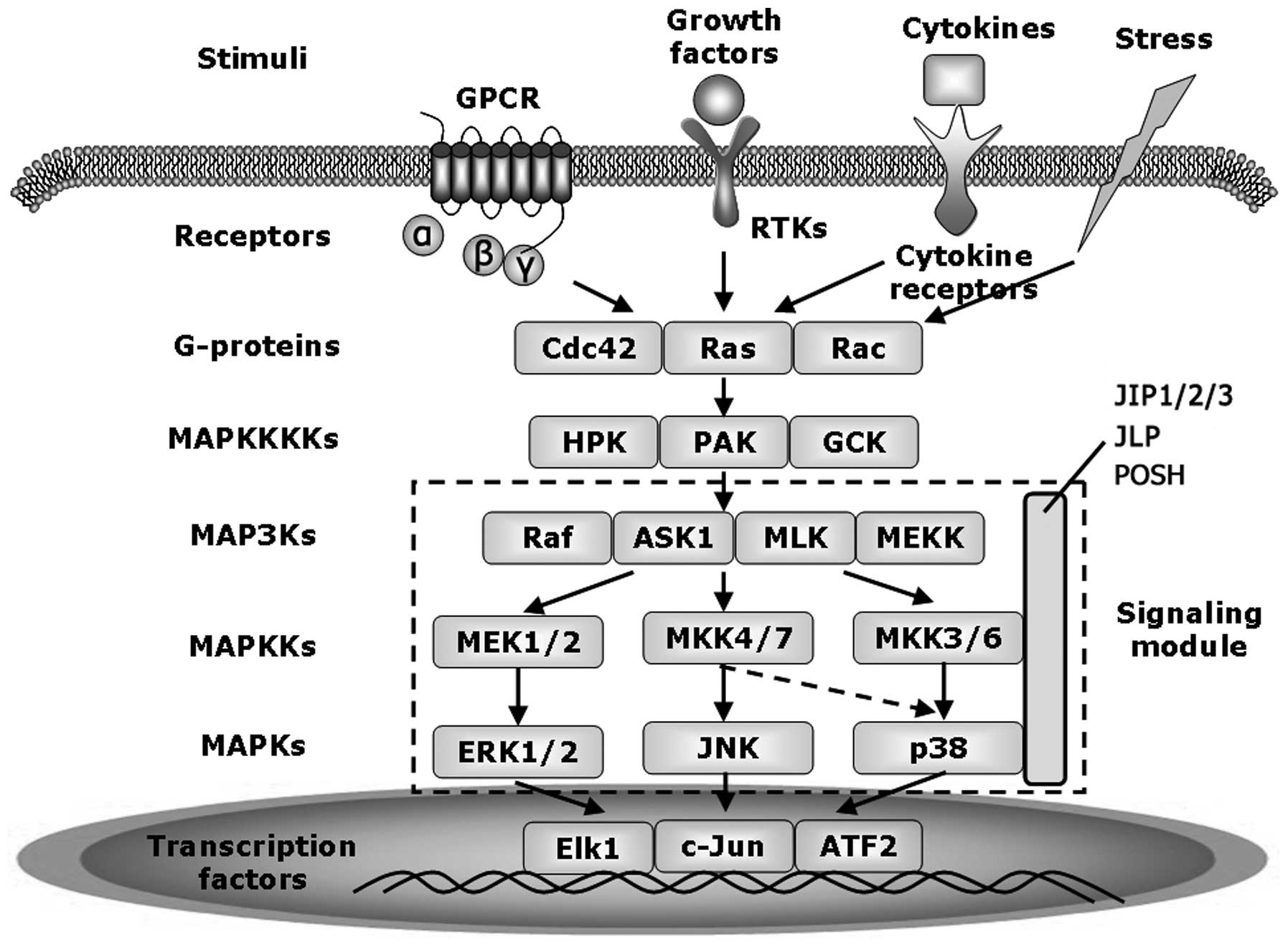
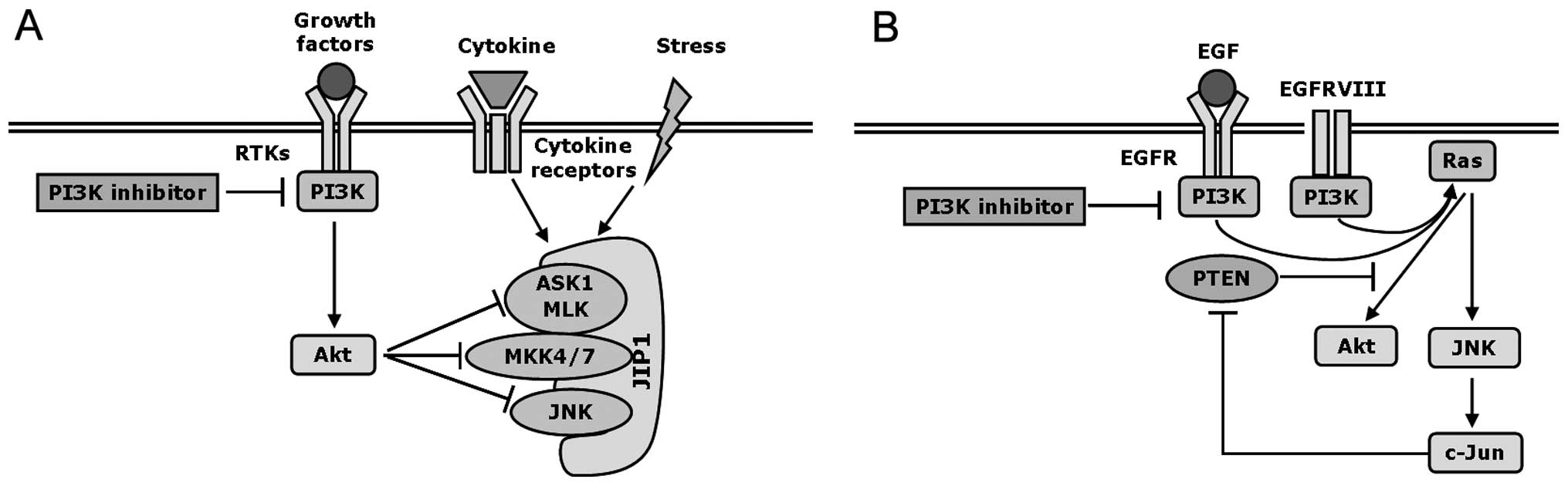
|
1
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katso R, Okkenhaug K, Ahmadi K, White S,
Timms J and Waterfield MD: Cellular function of phosphoinositide
3-kinases: Implications for development, homeostasis, and cancer.
Annu Rev Cell Dev Biol. 17:615–675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vanhaesebroeck B and Alessi DR: The
PI3K-PDK1 connection: More than just a road to PKB. Biochem J.
346:561–576. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toker A and Newton AC: Cellular signaling:
Pivoting around PDK-1. Cell. 103:185–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sarbassov DD, Ali SM and Sabatini DM:
Growing roles for the mTOR pathway. Curr Opin Cell Biol.
17:596–603. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun H, Lesche R, Li DM, Liliental J, Zhang
H, Gao J, Gavrilova N, Mueller B, Liu X and Wu H: PTEN modulates
cell cycle progression and cell survival by regulating
phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B
signaling pathway. Proc Natl Acad Sci USA. 96:6199–6204. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012.PubMed/NCBI
|
|
9
|
Srividya MR, Thota B, Shailaja BC,
Arivazhagan A, Thennarasu K, Chandramouli BA, Hegde AS and Santosh
V: Homozygous 10q23/ PTEN deletion and its impact on outcome in
glioblastoma: A prospective translational study on a uniformly
treated cohort of adult patients. Neuropathology. 31:376–383. 2011.
View Article : Google Scholar
|
|
10
|
Chong ML, Loh M, Thakkar B, Pang B,
Iacopetta B and Soong R: Phosphatidylinositol-3-kinase pathway
aberrations in gastric and colorectal cancer: Meta-analysis,
co-occurrence and ethnic variation. Int J Cancer. 134:1232–1238.
2014. View Article : Google Scholar
|
|
11
|
Garcia-Dios DA, Lambrechts D, Coenegrachts
L, Vandenput I, Capoen A, Webb PM, Ferguson K, Akslen LA, Claes B,
Vergote I, et al; ANECS. High-throughput interrogation of PIK3CA,
PTEN, KRAS, FBXW7 and TP53 mutations in primary endometrial
carcinoma. Gynecol Oncol. 128:327–334. 2013. View Article : Google Scholar
|
|
12
|
McConechy MK, Ding J, Senz J, Yang W,
Melnyk N, Tone AA, Prentice LM, Wiegand KC, McAlpine JN, Shah SP,
et al: Ovarian and endometrial endometrioid carcinomas have
distinct CTNNB1 and PTEN mutation profiles. Mod Pathol. 27:128–134.
2014. View Article : Google Scholar :
|
|
13
|
Jin G, Kim MJ, Jeon HS, Choi JE, Kim DS,
Lee EB, Cha SI, Yoon GS, Kim CH and Jung TH: PTEN mutations and
relationship to EGFR, ERBB2, KRAS, and TP53 mutations in non-small
cell lung cancers. Lung Cancer. 69:279–283. 2010. View Article : Google Scholar
|
|
14
|
Kang-Park S and Lee YI and Lee YI: PTEN
modulates insulin-like growth factor II (IGF-II)-mediated
signaling; the protein phosphatase activity of PTEN downregulates
IGF-II expression in hepatoma cells. FEBS Lett. 545:203–208. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yi HK, Kim SY, Hwang PH, Kim CY, Yang DH,
Oh Y and Lee DY: Impact of PTEN on the expression of insulin-like
growth factors (IGFs) and IGF-binding proteins in human gastric
adenocarcinoma cells. Biochem Biophys Res Commun. 330:760–767.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oki E, Baba H, Tokunaga E, Nakamura T,
Ueda N, Futatsugi M, Mashino K, Yamamoto M, Ikebe M, Kakeji Y, et
al: Akt phosphorylation associates with LOH of PTEN and leads to
chemoresistance for gastric cancer. Int J Cancer. 117:376–380.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang JM, He QY, Guo RX and Chang XJ:
Phosphorylated Akt overexpression and loss of PTEN expression in
non-small cell lung cancer confers poor prognosis. Lung Cancer.
51:181–191. 2006. View Article : Google Scholar
|
|
18
|
Vivanco I, Rohle D, Versele M, Iwanami A,
Kuga D, Oldrini B, Tanaka K, Dang J, Kubek S, Palaskas N, et al:
The phosphatase and tensin homolog regulates epidermal growth
factor receptor (EGFR) inhibitor response by targeting EGFR for
degradation. Proc Natl Acad Sci USA. 107:6459–6464. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weston CR and Davis RJ: The JNK signal
transduction pathway. Curr Opin Cell Biol. 19:142–149. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bode AM and Dong Z: The functional
contrariety of JNK. Mol Carcinog. 46:591–598. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang JY, Moulin N, van Bemmelen MX, Dubuis
G, Tawadros T, Haefliger JA, Waeber G and Widmann C: Splice
variant-specific stabilization of JNKs by IB1/JIP1. Cell Signal.
19:2201–2207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davis RJ: Signal transduction by the JNK
group of MAP kinases. Cell. 103:239–252. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barr RK and Bogoyevitch MA: The c-Jun
N-terminal protein kinase family of mitogen-activated protein
kinases (JNK MAPKs). Int J Biochem Cell Biol. 33:1047–1063. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tournier C, Dong C, Turner TK, Jones SN,
Flavell RA and Davis RJ: MKK7 is an essential component of the JNK
signal transduction pathway activated by proinflammatory cytokines.
Genes Dev. 15:1419–1426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haeusgen W, Herdegen T and Waetzig V: The
bottleneck of JNK signaling: Molecular and functional
characteristics of MKK4 and MKK7. Eur J Cell Biol. 90:536–544.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leicht DT, Balan V, Kaplun A, Singh-Gupta
V, Kaplun L, Dobson M and Tzivion G: Raf kinases: Function,
regulation and role in human cancer. Biochim Biophys Acta.
1773:1196–1212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ichijo H, Nishida E, Irie K, ten Dijke P,
Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K and Gotoh
Y: Induction of apoptosis by ASK1, a mammalian MAPKKK that
activates SAPK/JNK and p38 signaling pathways. Science. 275:90–94.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun BK, Kim JH, Nguyen HN, Oh S, Kim SY,
Choi S, Choi HJ, Lee YJ and Song JJ: MEKK1/MEKK4 are responsible
for TRAIL-induced JNK/p38 phosphorylation. Oncol Rep. 25:537–544.
2011.
|
|
29
|
Xu Z, Maroney AC, Dobrzanski P, Kukekov NV
and Greene LA: The MLK family mediates c-Jun N-terminal kinase
activation in neuronal apoptosis. Mol Cell Biol. 21:4713–4724.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lopez-Ilasaca M: Signaling from
G-protein-coupled receptors to mitogen-activated protein
(MAP)-kinase cascades. Biochem Pharmacol. 56:269–277. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shaulian E: AP-1 - The Jun proteins:
Oncogenes or tumor suppressors in disguise? Cell Signal.
22:894–899. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Whitmarsh AJ, Cavanagh J, Tournier C,
Yasuda J and Davis RJ: A mammalian scaffold complex that
selectively mediates MAP kinase activation. Science. 281:1671–1674.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Whitmarsh AJ, Kuan CY, Kennedy NJ, Kelkar
N, Haydar TF, Mordes JP, Appel M, Rossini AA, Jones SN, Flavell RA,
et al: Requirement of the JIP1 scaffold protein for stress-induced
JNK activation. Genes Dev. 15:2421–2432. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nihalani D, Meyer D, Pajni S and Holzman
LB: Mixed lineage kinase-dependent JNK activation is governed by
interactions of scaffold protein JIP with MAPK module components.
EMBO J. 20:3447–3458. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin A: Activation of the JNK signaling
pathway: Breaking the brake on apoptosis. BioEssays. 25:17–24.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin A and Dibling B: The true face of JNK
activation in apoptosis. Aging Cell. 1:112–116. 2002. View Article : Google Scholar
|
|
37
|
Besirli CG and Johnson EM Jr:
JNK-independent activation of c-Jun during neuronal apoptosis
induced by multiple DNA-damaging agents. J Biol Chem.
278:22357–22366. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huntwork-Rodriguez S, Wang B, Watkins T,
Ghosh AS, Pozniak CD, Bustos D, Newton K, Kirkpatrick DS and
Lewcock JW: JNK-mediated phosphorylation of DLK suppresses its
ubiquitination to promote neuronal apoptosis. J Cell Biol.
202:747–763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reno EM, Haughian JM, Jackson TA, Thorne
AM and Bradford AP: c-Jun N-terminal kinase regulates apoptosis in
endometrial cancer cells. Apoptosis. 14:809–820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Robitaille K, Daviau A, Lachance G,
Couture JP and Blouin R: Calphostin C-induced apoptosis is mediated
by a tissue trans-glutaminase-dependent mechanism involving the
DLK/JNK signaling pathway. Cell Death Differ. 15:1522–1531. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song J, Ko HS, Sohn EJ, Kim B, Kim JH, Kim
HJ, Kim C, Kim JE and Kim SH: Inhibition of protein kinase C α/βII
and activation of c-Jun NH2-terminal kinase mediate glycyrrhetinic
acid induced apoptosis in non-small cell lung cancer NCI-H460
cells. Bioorg Med Chem Lett. 24:1188–1191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim BJ, Ryu SW and Song BJ: JNK- and p38
kinase-mediated phosphorylation of Bax leads to its activation and
mitochondrial translocation and to apoptosis of human hepatoma
HepG2 cells. J Biol Chem. 281:21256–21265. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ambacher KK, Pitzul KB, Karajgikar M,
Hamilton A, Ferguson SS and Cregan SP: The JNK- and
AKT/GSK3β-signaling pathways converge to regulate Puma induction
and neuronal apoptosis induced by trophic factor deprivation. PLoS
One. 7:e468852012. View Article : Google Scholar
|
|
44
|
Zhao Z, Wang J, Tang J, Liu X, Zhong Q,
Wang F, Hu W, Yuan Z, Nie C and Wei Y: JNK- and Akt-mediated Puma
expression in the apoptosis of cisplatin-resistant ovarian cancer
cells. Biochem J. 444:291–301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu J and Zhang L: PUMA, a potent killer
with or without p53. Oncogene. 27(Suppl 1): S71–S83. 2008.
View Article : Google Scholar
|
|
46
|
Buschmann T, Potapova O, Bar-Shira A,
Ivanov VN, Fuchs SY, Henderson S, Fried VA, Minamoto T,
Alarcon-Vargas D, Pincus MR, et al: Jun NH2-terminal kinase
phosphorylation of p53 on Thr-81 is important for p53 stabilization
and transcriptional activities in response to stress. Mol Cell
Biol. 21:2743–2754. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jones EV, Dickman MJ and Whitmarsh AJ:
Regulation of p73-mediated apoptosis by c-Jun N-terminal kinase.
Biochem J. 405:617–623. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu C, Minemoto Y, Zhang J, Liu J, Tang F,
Bui TN, Xiang J and Lin A: JNK suppresses apoptosis via
phosphorylation of the proapoptotic Bcl-2 family protein BAD. Mol
Cell. 13:329–340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nishina H, Fischer KD, Radvanyi L,
Shahinian A, Hakem R, Rubie EA, Bernstein A, Mak TW, Woodgett JR
and Penninger JM: Stress-signalling kinase Sek1 protects thymocytes
from apoptosis mediated by CD95 and CD3. Nature. 385:350–353. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Potapova O, Gorospe M, Dougherty RH, Dean
NM, Gaarde WA and Holbrook NJ: Inhibition of c-Jun N-terminal
kinase 2 expression suppresses growth and induces apoptosis of
human tumor cells in a p53-dependent manner. Mol Cell Biol.
20:1713–1722. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cellurale C, Sabio G, Kennedy NJ, Das M,
Barlow M, Sandy P, Jacks T and Davis RJ: Requirement of c-Jun
NH(2)-terminal kinase for Ras-initiated tumor formation. Mol Cell
Biol. 31:1565–1576. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xiao L and Lang W: A dominant role for the
c-Jun NH2-terminal kinase in oncogenic ras-induced morphologic
transformation of human lung carcinoma cells. Cancer Res.
60:400–408. 2000.PubMed/NCBI
|
|
53
|
Nielsen C, Thastrup J, Bøttzauw T,
Jäättelä M and Kallunki T: c-Jun NH2-terminal kinase 2 is required
for Ras transformation independently of activator protein 1. Cancer
Res. 67:178–185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mathiasen DP, Egebjerg C, Andersen SH,
Rafn B, Puustinen P, Khanna A, Daugaard M, Valo E, Tuomela S,
Bøttzauw T, et al: Identification of a c-Jun N-terminal
kinase-2-dependent signal amplification cascade that regulates
c-Myc levels in ras transformation. Oncogene. 31:390–401. 2012.
View Article : Google Scholar
|
|
55
|
Johnson R, Spiegelman B, Hanahan D and
Wisdom R: Cellular transformation and malignancy induced by ras
require c-jun. Mol Cell Biol. 16:4504–4511. 1996.PubMed/NCBI
|
|
56
|
Shibata W, Maeda S, Hikiba Y, Yanai A,
Sakamoto K, Nakagawa H, Ogura K, Karin M and Omata M: c-Jun
NH2-terminal kinase 1 is a critical regulator for the development
of gastric cancer in mice. Cancer Res. 68:5031–5039. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chang Q, Chen J, Beezhold KJ, Castranova
V, Shi X and Chen F: JNK1 activation predicts the prognostic
outcome of the human hepatocellular carcinoma. Mol Cancer.
8:642009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang X, Chao L, Li X, Ma G, Chen L, Zang Y
and Zhou G: Elevated expression of phosphorylated c-Jun
NH2-terminal kinase in basal-like and ‘triple-negative’ breast
cancers. Hum Pathol. 41:401–406. 2010. View Article : Google Scholar
|
|
59
|
Li JY and Wang H, May S, Song X, Fueyo J,
Fuller GN and Wang H: Constitutive activation of c-Jun N-terminal
kinase correlates with histologic grade and EGFR expression in
diffuse gliomas. J Neurooncol. 88:11–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Takahashi R, Hirata Y, Sakitani K, Nakata
W, Kinoshita H, Hayakawa Y, Nakagawa H, Sakamoto K, Hikiba Y,
Ijichi H, et al: Therapeutic effect of c-Jun N-terminal kinase
inhibition on pancreatic cancer. Cancer Sci. 104:337–344. 2013.
View Article : Google Scholar
|
|
61
|
Chen P, O’Neal JF, Ebelt ND, Cantrell MA,
Mitra S, Nasrazadani A, Vandenbroek TL, Heasley LE and Van Den Berg
CL: Jnk2 effects on tumor development, genetic instability and
replicative stress in an oncogene-driven mouse mammary tumor model.
PLoS One. 5:e104432010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Das M, Garlick DS, Greiner DL and Davis
RJ: The role of JNK in the development of hepatocellular carcinoma.
Genes Dev. 25:634–645. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kennedy NJ, Sluss HK, Jones SN, Bar-Sagi
D, Flavell RA and Davis RJ: Suppression of Ras-stimulated
transformation by the JNK signal transduction pathway. Genes Dev.
17:629–637. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hübner A, Mulholland DJ, Standen CL,
Karasarides M, Cavanagh-Kyros J, Barrett T, Chi H, Greiner DL,
Tournier C, Sawyers CL, et al: JNK and PTEN cooperatively control
the development of invasive adenocarcinoma of the prostate. Proc
Natl Acad Sci USA. 109:12046–12051. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ying J, Li H, Cui Y, Wong AH, Langford C
and Tao Q: Epigenetic disruption of two proapoptotic genes
MAPK10/JNK3 and PTPN13/FAP-1 in multiple lymphomas and carcinomas
through hypermethylation of a common bidirectional promoter.
Leukemia. 20:1173–1175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yoshida S, Fukino K, Harada H, Nagai H,
Imoto I, Inazawa J, Takahashi H, Teramoto A and Emi M: The c-Jun
NH2-terminal kinase3 (JNK3) gene: Genomic structure, chromosomal
assignment, and loss of expression in brain tumors. J Hum Genet.
46:182–187. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jurewicz A, Matysiak M, Tybor K and Selmaj
K: TNF-induced death of adult human oligodendrocytes is mediated by
c-jun NH2-terminal kinase-3. Brain. 126:1358–1370. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dajas-Bailador F, Bantounas I, Jones EV
and Whitmarsh AJ: Regulation of axon growth by the JIP1-AKT axis. J
Cell Sci. 127:230–239. 2014. View Article : Google Scholar :
|
|
69
|
Kim AH, Sasaki T and Chao MV:
JNK-interacting protein 1 promotes Akt1 activation. J Biol Chem.
278:29830–29836. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Pan J, Pei DS, Yin XH, Hui L and Zhang GY:
Involvement of oxidative stress in the rapid Akt1 regulating a JNK
scaffold during ischemia in rat hippocampus. Neurosci Lett.
392:47–51. 2006. View Article : Google Scholar
|
|
71
|
Kim AH, Yano H, Cho H, Meyer D, Monks B,
Margolis B, Birnbaum MJ and Chao MV: Akt1 regulates a JNK scaffold
during excitotoxic apoptosis. Neuron. 35:697–709. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Cerezo A, Martínez-A C, Lanzarot D,
Fischer S, Franke TF and Rebollo A: Role of Akt and c-Jun
N-terminal kinase 2 in apoptosis induced by interleukin-4
deprivation. Mol Biol Cell. 9:3107–3118. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Levresse V, Butterfield L, Zentrich E and
Heasley LE: Akt negatively regulates the cJun N-terminal kinase
pathway in PC12 cells. J Neurosci Res. 62:799–808. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Okubo Y, Blakesley VA, Stannard B, Gutkind
S and Le Roith D: Insulin-like growth factor-I inhibits the
stress-activated protein kinase/c-Jun N-terminal kinase. J Biol
Chem. 273:25961–25966. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang Q, Zhang QG, Wu DN, Yin XH and Zhang
GY: Neuroprotection of selenite against ischemic brain injury
through negatively regulating early activation of ASK1/JNK cascade
via activation of PI3K/AKT pathway. Acta Pharmacol Sin. 28:19–27.
2007. View Article : Google Scholar
|
|
76
|
Kim AH, Khursigara G, Sun X, Franke TF and
Chao MV: Akt phosphorylates and negatively regulates apoptosis
signal-regulating kinase 1. Mol Cell Biol. 21:893–901. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Aikin R, Maysinger D and Rosenberg L:
Cross-talk between phosphatidylinositol 3-kinase/AKT and c-jun
NH2-terminal kinase mediates survival of isolated human islets.
Endocrinology. 145:4522–4531. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Xie D, Gore C, Zhou J, Pong RC, Zhang H,
Yu L, Vessella RL, Min W and Hsieh JT: DAB2IP coordinates both
PI3K-Akt and ASK1 pathways for cell survival and apoptosis. Proc
Natl Acad Sci USA. 106:19878–19883. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Park HS, Kim MS, Huh SH, Park J, Chung J,
Kang SS and Choi EJ: Akt (protein kinase B) negatively regulates
SEK1 by means of protein phosphorylation. J Biol Chem.
277:2573–2578. 2002. View Article : Google Scholar
|
|
80
|
Murakami T, Takagi H, Suzuma K, Suzuma I,
Ohashi H, Watanabe D, Ojima T, Suganami E, Kurimoto M, Kaneto H, et
al: Angiopoietin-1 attenuates H2O2-induced
SEK1/JNK phosphorylation through the phosphatidylinositol
3-kinase/Akt pathway in vascular endothelial cells. J Biol Chem.
280:31841–31849. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Barthwal MK, Sathyanarayana P, Kundu CN,
Rana B, Pradeep A, Sharma C, Woodgett JR and Rana A: Negative
regulation of mixed lineage kinase 3 by protein kinase B/AKT leads
to cell survival. J Biol Chem. 278:3897–3902. 2003. View Article : Google Scholar
|
|
82
|
Wen XR, Li C, Zong YY, Yu CZ, Xu J, Han D
and Zhang GY: Dual inhibitory roles of geldanamycin on the c-Jun
NH2-terminal kinase 3 signal pathway through suppressing the
expression of mixed-lineage kinase 3 and attenuating the activation
of apoptosis signal-regulating kinase 1 via facilitating the
activation of Akt in ischemic brain injury. Neuroscience.
156:483–497. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Song JJ and Lee YJ: Dissociation of Akt1
from its negative regulator JIP1 is mediated through the
ASK1-MEK-JNK signal transduction pathway during metabolic oxidative
stress: A negative feedback loop. J Cell Biol. 170:61–72. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Logan SK, Falasca M, Hu P and Schlessinger
J: Phosphatidylinositol 3-kinase mediates epidermal growth
factor-induced activation of the c-Jun N-terminal kinase signaling
pathway. Mol Cell Biol. 17:5784–5790. 1997.PubMed/NCBI
|
|
85
|
Vivanco I, Palaskas N, Tran C, Finn SP,
Getz G, Kennedy NJ, Jiao J, Rose J, Xie W, Loda M, et al:
Identification of the JNK signaling pathway as a functional target
of the tumor suppressor PTEN. Cancer Cell. 11:555–569. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Cui J, Han SY, Wang C, Su W, Harshyne L,
Holgado-Madruga M and Wong AJ: c-Jun NH(2)-terminal kinase 2alpha2
promotes the tumorigenicity of human glioblastoma cells. Cancer
Res. 66:10024–10031. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Tsuiki H, Tnani M, Okamoto I, Kenyon LC,
Emlet DR, Holgado-Madruga M, Lanham IS, Joynes CJ, Vo KT and Wong
AJ: Constitutively active forms of c-Jun NH2-terminal kinase are
expressed in primary glial tumors. Cancer Res. 63:250–255.
2003.PubMed/NCBI
|
|
88
|
Nitta RT, Del Vecchio CA, Chu AH, Mitra
SS, Godwin AK and Wong AJ: The role of the c-Jun N-terminal kinase
2-α-isoform in non-small cell lung carcinoma tumorigenesis.
Oncogene. 30:234–244. 2011. View Article : Google Scholar
|
|
89
|
Antonyak MA, Kenyon LC, Godwin AK, James
DC, Emlet DR, Okamoto I, Tnani M, Holgado-Madruga M, Moscatello DK
and Wong AJ: Elevated JNK activation contributes to the
pathogenesis of human brain tumors. Oncogene. 21:5038–5046. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Bost F, McKay R, Bost M, Potapova O, Dean
NM and Mercola D: The Jun kinase 2 isoform is preferentially
required for epidermal growth factor-induced transformation of
human A549 lung carcinoma cells. Mol Cell Biol. 19:1938–1949.
1999.PubMed/NCBI
|
|
91
|
Rong Y, Belozerov VE, Tucker-Burden C,
Chen G, Durden DL, Olson JJ, Van Meir EG, Mackman N and Brat DJ:
Epidermal growth factor receptor and PTEN modulate tissue factor
expression in glioblastoma through JunD/activator protein-1
transcriptional activity. Cancer Res. 69:2540–2549. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Bonavia R, Inda MM, Vandenberg S, Cheng
SY, Nagane M, Hadwiger P, Tan P, Sah DW, Cavenee WK and Furnari FB:
EGFRvIII promotes glioma angiogenesis and growth through the NF-κB,
interleukin-8 pathway. Oncogene. 31:4054–4066. 2012. View Article : Google Scholar
|
|
93
|
Gu J, Tamura M and Yamada KM: Tumor
suppressor PTEN inhibits integrin- and growth factor-mediated
mitogen-activated protein (MAP) kinase signaling pathways. J Cell
Biol. 143:1375–1383. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hettinger K, Vikhanskaya F, Poh MK, Lee
MK, de Belle I, Zhang JT, Reddy SA and Sabapathy K: c-Jun promotes
cellular survival by suppression of PTEN. Cell Death Differ.
14:218–229. 2007. View Article : Google Scholar
|