Introduction
Choriocarcinoma (CC) is a unique malignant
gestational trophoblastic tumor that occurs primarily in women of
reproductive age and is extremely prone to brain and/or lung
metastasis. CC can be derived either from a normal or pathological
pregnancy, such as molar pregnancies, ectopic pregnancies,
induced/spontaneous abortions and preterm deliveries (1). Despite established first-line
chemotherapy, 10–20% of CC patients presented drug-resistantance,
or relapsed due to solitary metastasis lesions. Thus, to understand
the underlying recurrence and metastasis mechanisms of CC remains
crucial.
Hypoxia has a critical role in carcinogenesis, tumor
progression, distant metastasis, angiogenesis and resistance to
chemotherapy and radiation therapy (2,3).
When oxygen demand of solid tumors exceed the oxygen-supplying
capacity of the vasculature, hypoxia is primarily mediated through
hypoxia inducible factors (HIFs) (4). HIFs comprise oxygen-sensitive
α-subunit (HIF-1α, HIF-2α and HIF-3α subunits) and constitutively
express β-subunit (HIF-β, known as ARNT). When stabilized in
hypoxia, the remarkably high expression of HIF-1α dimerizes with
HIF-β and activates downstream genes involved in the canonical
hypoxic response via HRE elements in their promoters (5,6).
Regulated post-translation-ally in normoxia, HIF-1α is rapidly
degraded through the von Hippel-Lindau (VHL)-dependent
ubiquitin-proteasome pathway (7).
It has been reported that the initiation of
metastasis involves EMT-like processes (8). EMT transforms tumor cells from a
polar, epithelial morphology to mesenchymal and invade tissues
(8). The EMT renders a more
aggressive phenotype with enhanced invasiveness and proliferation,
formation of metastases and higher mortality (9). Substantial evidence demonstrates that
activation of hypoxic signaling through HIFs is triggered by
modulators of EMT, which is accompanied by specific changes in gene
expression, such as downregulation of E-cadherin, CK18, CK19
(10–14). Notably, Snail-1, the zinc finger
transcription factor, represses the E-cadherin promoter and
effectively induces EMT (15).
However, the molecular mechanisms between hypoxia and EMT are still
elusive.
In mammals, the Notch signaling pathway involving
differentiation, proliferation, and survival, comprise a series of
ligands (Jagged-1 and -2, Delta-1, -3, and -4) and transmembrane
receptors (Notch-1 to -4) (16).
When ligand has integrated the Notch receptor susceptible to
cleavage, initially by TACE (TNF-α converting enzyme) and then by
the γ-secretase complex, which ultimately take the form of the
activated Notch intracellular domain (NICD). NICD enters the
nucleus and interacts with the DNA-binding protein CSL to regulate
expression of downstream genes, such as Hes and Hey (17).
Recently, connection between Notch signaling and
hypoxia was demonstrated in the control of cell proliferation, stem
cell differentiation and angiogenesis of missed abortion (18–20).
It was also indicated that Jagged-1 and DLL1 induces EMT through
repression of E-cadherin (21,22).
Furthermore, several studies demonstrated that Notch signaling
mediated molecular mechanisms underlying HIF-dependent regulation
of the EMT in ovarian carcinoma, breast cancer and melanoma,
respectively (22–24). Thus, we propose that the Notch
signaling pathway is a valuable candidate as a mediator between
hypoxia and EMT in CC cells. Hence, our study stably overexpressed
HIF-1α by Lentiviral vector to examine the effects of HIF-1α on
migration and invasion, and the HIF-1α/Notch/EMT pathway in CC
cells. We provide evidence for the role of Notch as a crucial
mediator of HIF-1α in enhanced tumor migration and
invasiveness.
Materials and methods
Cell culture
The human first trimester extravillous trophoblast
(EVT) cell line (HTR-8) cultured in DMEM/F12 and two human CC cell
lines (JAR and JEG-3) cultured in DMEM were obtained from the
American Type Culture Collection (Manassas, VA, USA). These cells
were supplemented with 10% FBS and incubated in a 37°C humidified
incubator containing 5% CO2. Notch activity was blocked
by a 10 μM concentration of DAPT (Sigma, St. Louis, MO, USA), added
at each day of culturing.
RNA extraction and real-time PCR
Total RNA was extracted from above-mentioned cell
lines according to RNeasy Mini kit (Qiagen, USA) manufacturer’s
protocol and quantitated by spectrophotometry. According to the
manufacturer’s protocol, mRNA was then reverse transcribed using
Revert Aid™ First Strand cDNA Synthesis kit (MBI Fermentas, St.
Leon-Rot, Germany). The SYBRR Premix Ex Taq™ II system (Takara,
Dalian, China) and the Bio-Rad CFX96™ Real-time system (Bio-Rad,
CA, USA) were used to perform real-time quantitative PCR. Following
the reverse transcription, 1 μl primer (10 μM, summarized in
Table I), 12.5 μl SYBRR Premix Ex
Taq II, 2 μl cDNA and 8.5 μl dH2O mixed together,
pre-degeneration for 95°C, 30 sec, one repeat, and PCR reaction,
95°C 5 sec followed by 60°C, 30 sec, 35 repeats, and the
dissociation stage, 95°C, 15 sec followed by 60°C, 30 sec, and
95°C, 15 sec, then the data were collected and analyzed. β-actin
(25) was used as the internal
control. The threshold cycle (Ct) value for triplicate reactions
was averaged. Melting curve for the primers was analyzed to confirm
the specificity of the PCR product. The relative expression of mRNA
for each target gene was calculated as follows: ΔCt = Ct(target) -
Ct(β-actin), ΔΔCt = ΔCt - ΔCt(calibrator), and the fold changes in
mRNAs were calculated through relative quantification
(2−ΔΔCt).
 | Table IPrimers for real-time PCR. |
Table I
Primers for real-time PCR.
| Gene ID | Gene | Primers |
|---|
| NM_005524.3 | Hes1 | Forward |
AGGCGGACATTCTGGAAATG |
| | Reverse |
TCGTTCATGCACTCGCTGA |
| NM_005985.3 | Snail-1 | Forward |
ATCCCTGGAAGCTGCTCTCT |
| | Reverse |
TCTGGTCCAGTGAGGGAG |
| NM_004360.3 | E-cadherin | Forward |
GGDCTGAAGTGACTCGTAACGA |
| | Reverse |
CAGCCGCTTTCAGATTTTCATC |
| NM_000224.2 | CK18 | Forward |
GGCATCCAGAACGAGAAGGAG |
| | Reverse |
ATTGTCCACAGTATTTGCGAAGA |
| NM_002276.4 | CK19 | Forward |
AACGGCGAGCTAGAGGTGA |
| | Reverse |
GGATGGTCGTGTAGTAGTGGC |
| NM_001530.3 | HIF-1α | Forward |
GTGTTATCTGTCGCTTTGAGTC |
| | Reverse |
GTCTGGCTGCTGTAATAATGTT |
| NM_001197325.1 | HIF-1β (ARNT) | Forward |
CTGCCAACCCCGAAATGACAT |
| | Reverse |
CGCCGCTTAATAGCCCTCTG |
| NM_017617.3 | Notch1 | Forward |
AAGCTGCATCCAGAGGCAAAC |
| | Reverse |
TGGCATACACACTCCGAGAACAC |
| NM_001127891.2 | MMP2 | Forward |
TACAGGATCATTGGCTACACAC |
| | Reverse |
GGTCACATCGCTCCAGACT |
| NM_004994.2 | MMP9 | Forward |
TGTACCGCTATGGTTACACTCG |
| | Reverse |
GGCAGGGACAGTTGCTTCT |
| NM_001101.3 | β-actin | Forward |
AAGAGATGGCCACGGCTG |
| | Reverse |
GAACCGCTCATTGCCAATG |
Western blotting
Cells were harvested at 80–90% confluence, and
washed with 4°C PBS three times. Total cellular protein lysates
were reconstituted with RIPA buffer [50 mM Tris (pH 8.0), 0.1% SDS,
150 mM NaCl, 1% NP40 and 0.5% sodium deoxycholate] and proteinase
inhibitors [1 mM PMSF (Sigma)]. The denatured protein samples was
separated by SDS-PAGE and electrophoretically transferred to
nitrocellulose (NC) membranes. The NC membranes were blocked with
5% skim milk at room temperature for 1 h, then incubated in
different monoclonal or polyclonal antibodies with different
dilutions by 5% BSA overnight at 4°C and washed with TBST three
times (with Tween-20, pH 7.6). Subsequently, membranes were
incubated in secondary antibodies (Licor, Rockford, IL, USA) in the
dark for 1 h. The NC membranes were scanned with Odyssey detection
system (Licor). MG-132 (Sigma) was applied to inhibit the
proteasome-dependent degradation if necessary (10 μM, 4 h before
the protein harvest) (26).
β-actin (25) were used as the
loading controls.
Production and transfection of
lentivirus-mediated vectors
The NCBI accession number for the Homo
sapiens HIF-1α gene sequence is NM_001530.3. The CDS of HIF-1α
was used to construct lentiviral vectors. HIF-1α cDNA was amplified
and cloned into the lentiviral (lv) vector GV358 (Genechem,
Shanghai, China), a lentiviral vector encoding the enhanced green
fluorescent protein (EGFP) gene cDNA downstream of the ubi promoter
(Lenti-ubiluc). To prepare lentiviral particles, the lentiviral
vector overexpressing HIF-1α gene (lv-HIF-1α), pHelper 1.0 plasmid
and pHelper 2.0 helper plasmid were co-transfected into 293T cells
according to the manufacturer’s protocol. The lentiviral vector
were used to infect JAR and JEG-3 cells at a multiplicity of
infection (MOI) of 40 and 100, respectively. Lentivirus vectors
only expressing EGFP gene (lv-NC) was used as a transfection
control. Seventy-two hours after infection, EGFP expression was
observed to evaluate the infection efficiency. Cells were harvested
96 h after infection. The expression level of HIF-1α was examined
by qPCR and western blotting. These transfectants were selected by
2 or 1 μg/ml puromycin respectively (Sigma) to establish the stable
clone cell lines and analyze with fluorescent microscopy. After the
DAPT treatment was performed, the cells were harvested for further
analysis.
Scratch wound assay
To assay cell migration, JAR and JEG-3 cells after
lentiviral transfection (lv-HIF-1α and lv-NC) were scratched in
6-well plates with pipette tip when the cells were at 80%
confluence. Cells were incubated in serum-free medium, and the
width of the scratches was measured at 0 and 24 h after scratching.
DMSO (0.1%) or 10 μM DAPT was added to medium.
Invasion assays
Cell invasion were performed by Boyden chamber
assay. The 8-mm pore size upper chamber with 50 μl Matrigel (Sigma)
was incubated for 4 h. For migration, the serum-free medium
suspension with 5×104 cells was added in the upper
chamber, and 800 μl medium with 20% serum was added in the lower
chamber of 24-well plate. Cells (5×104/well) were seeded
in the same medium in a well without chamber simultaneously. 0.1%
DMSO or 10 μM DAPT was added to medium in upper and lower chambers.
After 24 h, the chambers were washed with PBS (pH 7.4) three times.
The non-invaded cells of upper surface in chamber were removed with
a cotton swab. The cells of lower surface was fixed with 4%
formalin for 10 min, then stained with crystal violet (0.01% in
ethanol, Sigma) for 20 min followed by PBS washing three times.
Five random visions were taken by inverted microscope, and the cell
number was counted.
In vivo growth and metabolic activity
assay
Considering that the JAR cell line is more easily
infected by lentiviruses than the JEG-3 cell line (unpublished
data), the JAR cells transfected with lv-NC or lv-HIF-1α were used
in vivo. Cells were cultured and collected in a single-cell
suspension in PBS (pH 7.4). Suspension (200 μl) (5×106
lv-NC or lv-HIF-1α cells) was implanted under the dorsal skin of 4-
to 6-week-old female SCID mice to form a subcutaneous tumor. Based
on the references (27–29) and previous studies, the mice were
randomly divided into four groups (n=5): i) JAR (lv-NC) treated
with 0.1% DMSO diluted with saline; ii) JAR (lv-NC) treated with
DAPT (10 mg/kg); iii) JAR (lv-HIF-1α) treated with 0.1% DMSO
diluted with saline; iv) JAR (lv-HIF-1α) treated with DAPT (10
mg/kg). The mice were treated with DAPT or DMSO intraperitoneally
six times at 3-day intervals (on days 1, 4, 7, 10, 13 and 16).
Tumors were observed for growth, swelling, and diabrosis, and the
tumor volumes were measured daily. The following experiment was
conducted when the tumors reached 15–75 mm3 in size.
All mice were induced with 4% isoflurane and
maintained on 2–2.5% isoflurane in preparation for SA-PET/CT scans.
Subsequently, 18F-FDG tracer were injected intravenously
under general anesthesia. The PET/CT imaging was carried out for
evaluation of uptake in the subcutaneous tumors (30). A CT scan was obtained for
reconstruction and further analysis of the PET/CT data.
Standardized uptake value (SUV) were used to compare variations
among groups.
In vivo invasion and metastasis
assay
To establish a model for monitoring of Notch
signaling in choriocarcinoma metastasis, the JAR cells transfected
with lv-NC or lv-HIF-1α (200 μl, 5×106 cells) were
injected intravenously into femal SCID mice. The groups and
treatment were the same as the growth assay. Their metastasis was
monitored by using In Vivo Imaging System (IVIS; Xenogen, Alameda,
CA, USA) at the end of treatment. Animal studies were approved by
the Ethics Committee of our Hospital.
Statistical analysis
Each of the experiments were performed in triplicate
and repeated three times. Statistical analysis was carried out
using the SPSS 18.0 statistical software package (SPSS Inc.,
Chicago, IL, USA). Summary data are showed as means ± SEM. Group
means were compared using the appropriate version of the Student’s
unpaired t-test. P-values were 2-sided. Test results were
considered significant at P<0.05.
Results
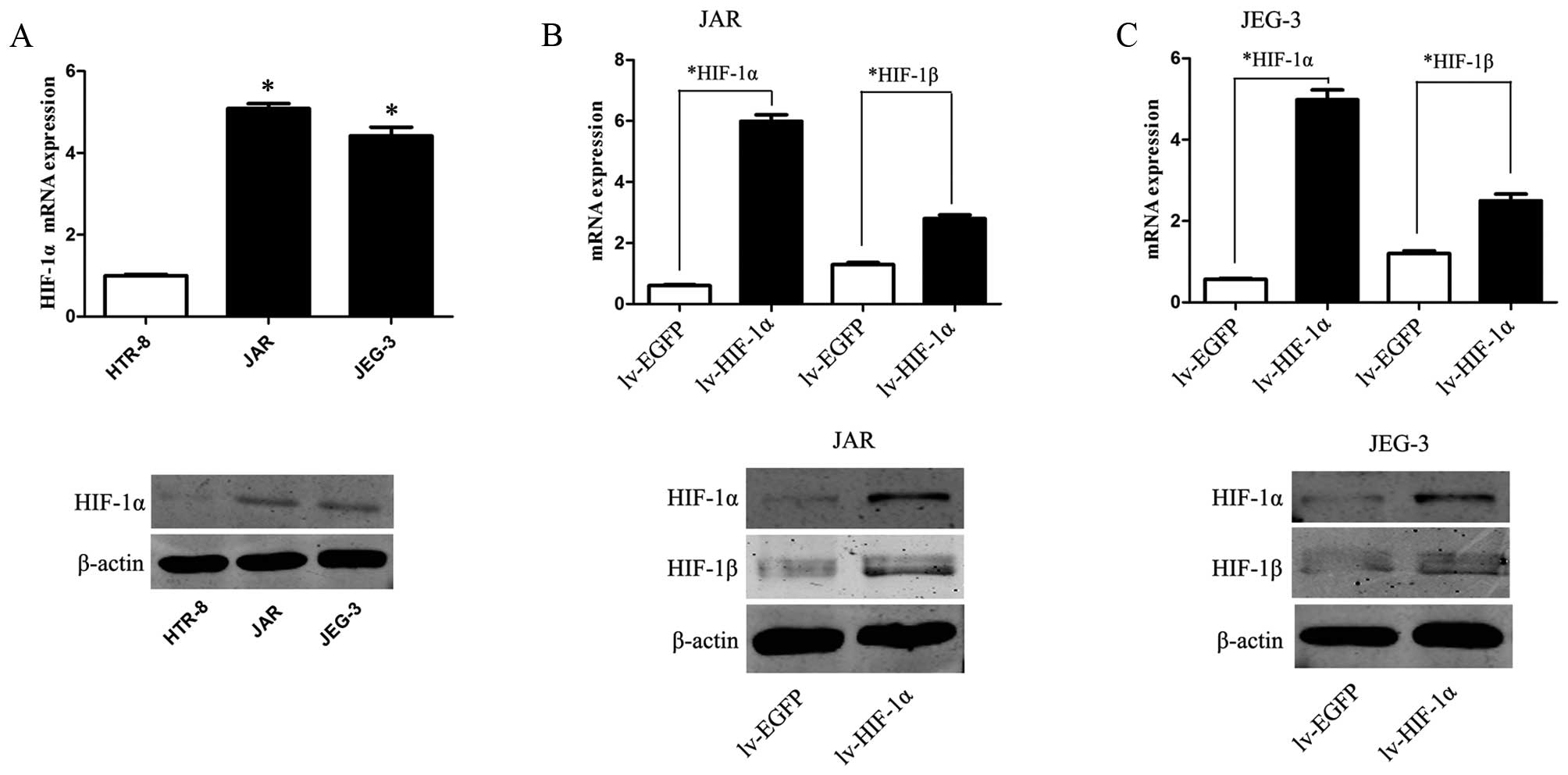
HIF-1α is upregulated in choriocarcinoma
cells
We examine the mRNA and protein expression levels of
HIF-1α in CC cell lines and HTR-8. The expression levels of HIF-1α
were markedly upregulated in JAR and JEG-3 cells, as compared to
the expression level of a control HTR-8 cell line (Fig. 1A, P<0.01).
Upregulation of HIF-1α enhances EMT and
the ability of migration/invasion, accompanied by the elevation of
HIF-1β
To mimic the hypoxic microenvironment and further
define our understanding of HIF-1α in choriocarcinoma, JAR and
JEG-3 cells were transfected with lentivirus of HIF-1α (lv-HIF-1α)
to establish stable cell lines that upregulated expression of
HIF-1α in CC cells. Simultaneously, EGFP expression was observed in
CC cells transfected with the corresponding lv-NC. Compared to the
lv-NC cells, the mRNA and protein level of HIF-1α were
significantly upregulated in lv-HIF-1α cells (P<0.01,
respectively, Fig. 1B and C).
Consistently, upregulation of HIF-1α promotes corresponding HIF-1β
expression on mRNA and protein level (P<0.01).
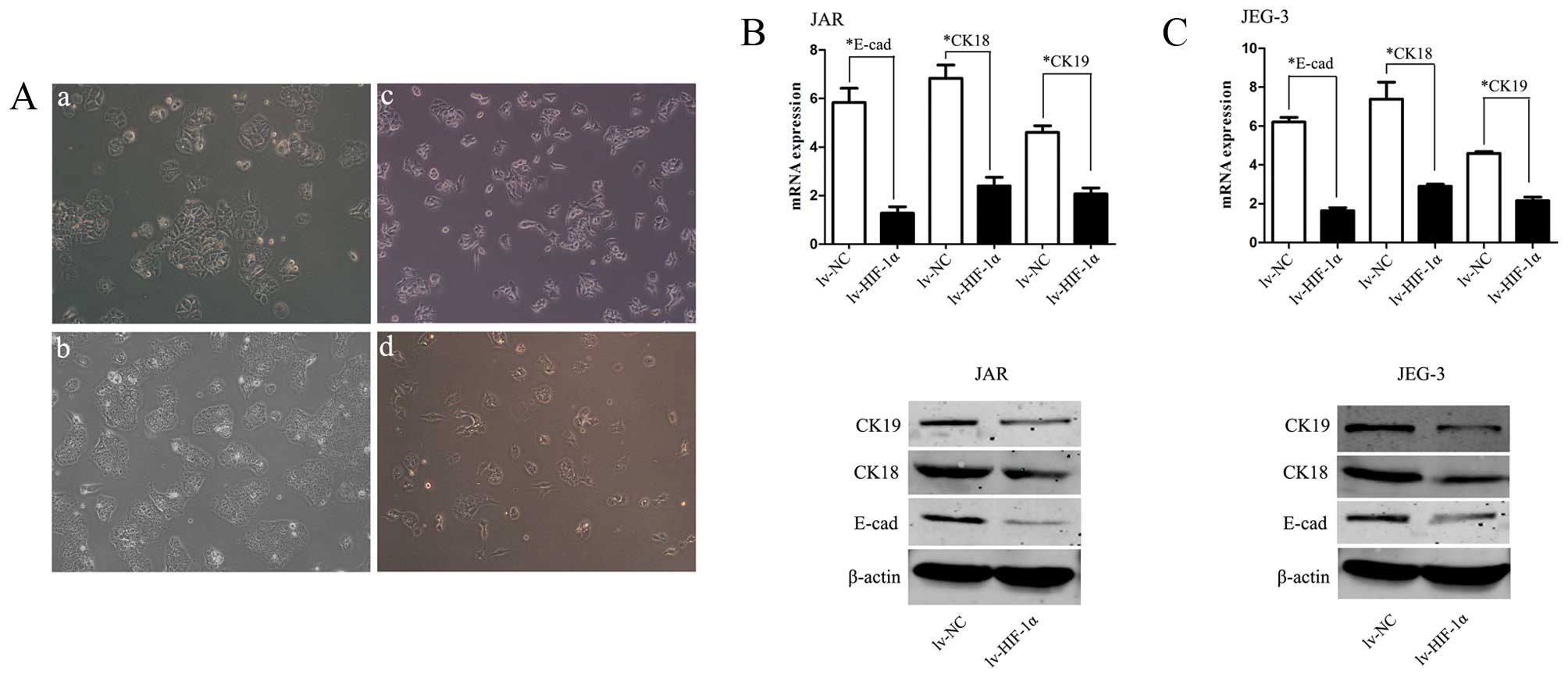
In order to investigate whether HIF-1α induces EMT
of CC cells, inverted microscopy was used revealing that Lv-HIF-1α
cells formed a spindle-like monolayer with more elongated and
larger gaps between cells than lv-NC cells which formed a
cobblestone-like monolayer (Fig.
2A). Western blot analyses further confirmed that the EMT
phenotype is induced by over-expression of HIF-1α in CC cells.
HIF-1α markedly decreased E-cadherin, CK-18 and CK-19 expression in
lv-HIF-1α cells, the reciprocal changes associated with the EMT
phenotypic transition (P<0.01, respectively, Fig. 2B and C).
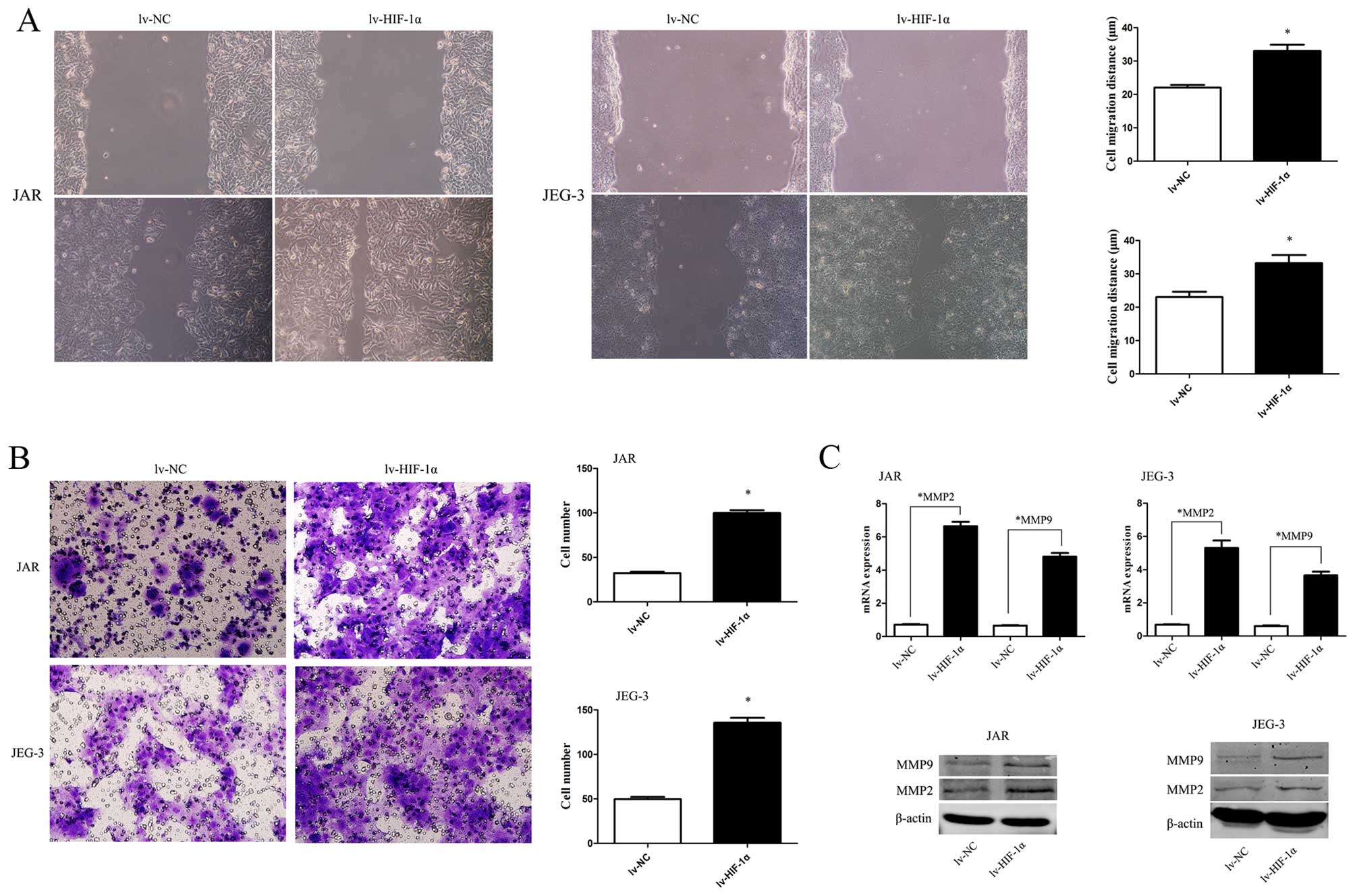
To study HIF-1α-induced EMT with malignant behavior,
the scratch wound-healing assay and Matrigel membrane invasion
assay were used. The extent of migration of CC cells with exogenous
HIF-1α into the scratched area was elevated (Fig. 3A, P<0.05). Similarly, the
invasive capability was significantly enhanced when HIF-1α was
upregulated (Fig. 3B, P<0.05).
Consistent with the data, MMP2 and MMP9 were upregulated by HIF-1α
overexpression (Fig. 3C,
P<0.01).
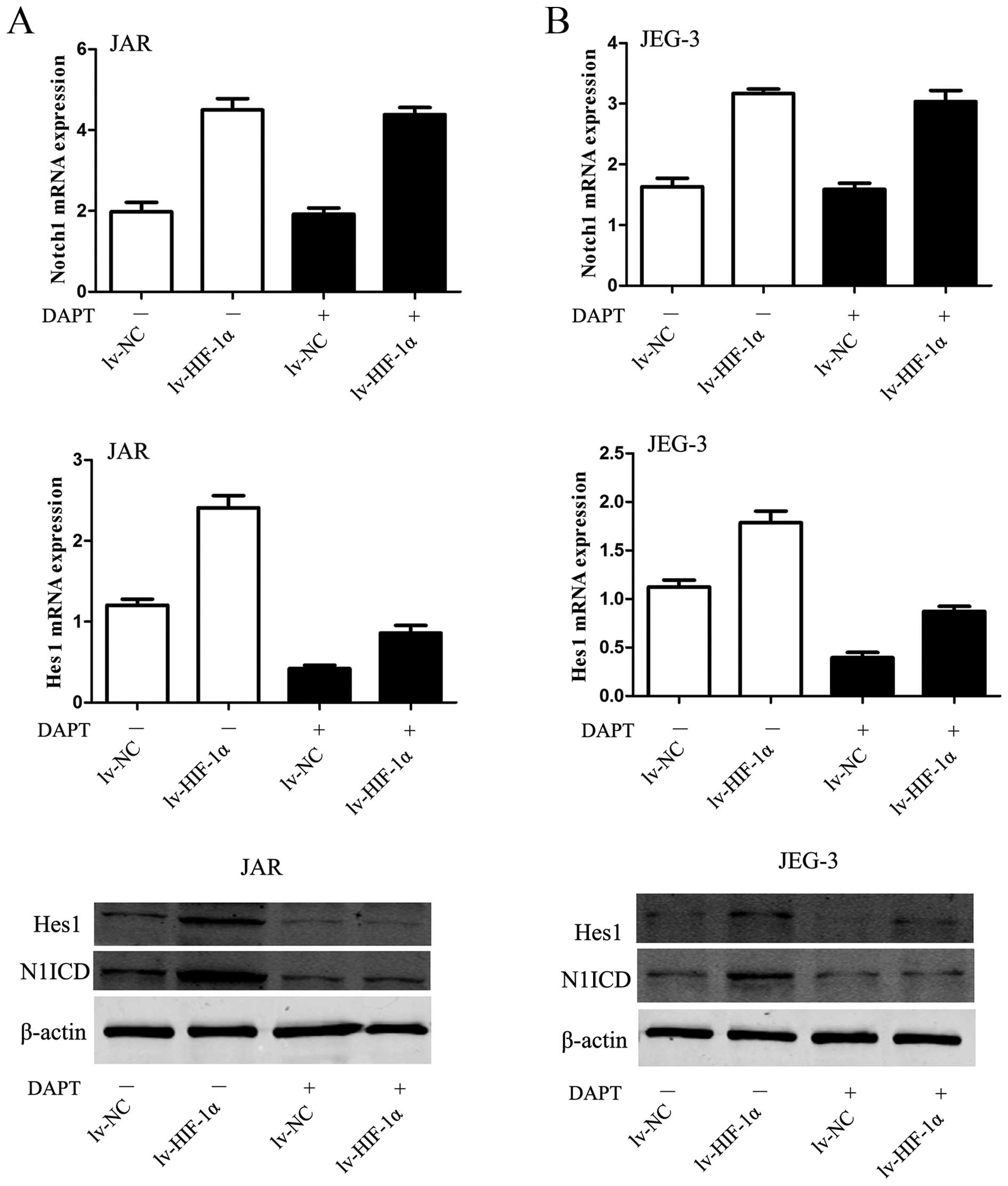
HIF-1α-induced upregulation of N1ICD and
Hes1 was abolished by DAPT
The elevated Notch response after mimicked hypoxia
was observed at two levels in the Notch signaling pathway. On the
receptor side, the level of N1ICD was elevated in JAR cells
subjected to HIF-1α. On the downstream side, upregulation of Hes1
mRNA and protein was observed in CC cells, simultaneously
(P<0.01, Fig. 4A). The DAPT
data would suggest that the inhibitor blocked the conformation of
N1ICD protein to abrogate Notch signaling, then Hes1 mRNA and
protein were decreased in JAR cells (P<0.01, Fig. 4A). Similar data were obtained for
the JEG-3 cell line (P<0.01, Fig.
4B).
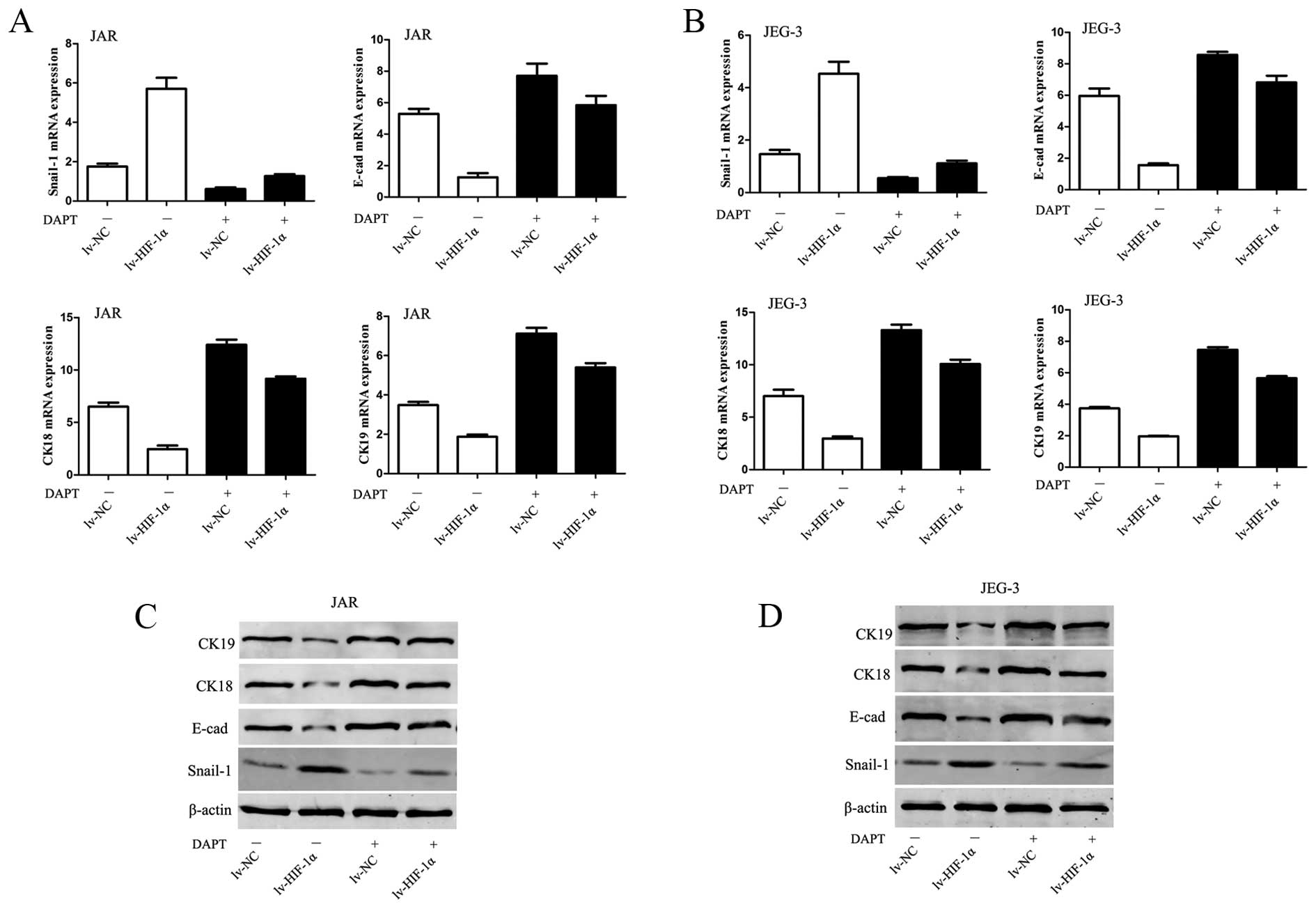
HIF-1α induces EMT through Notch
signaling
To evaluate the involvement of Notch in
HIF-1α-induced EMT in CC cells, 0.1% DMSO or 10 μM DAPT was added
in culture medium. Inhibition of Notch signaling abolished the
downregulation of E-cadherin, CK-18, CK-19 mRNA and protein levels
observed in lv-HIF-1α cells (P<0.01, respectively, Fig. 5). Because Snail-1 is the crucial
regulator of E-cadherin expression, the data would hint that
E-cadherin expression is mediated via regulating Snail-1 at the
transcriptional and protein level. Upregulation of Snail-1 by
exogenous HIF-1α was abrogated by DAPT treatment in CC cells
(P<0.01, Fig. 5).
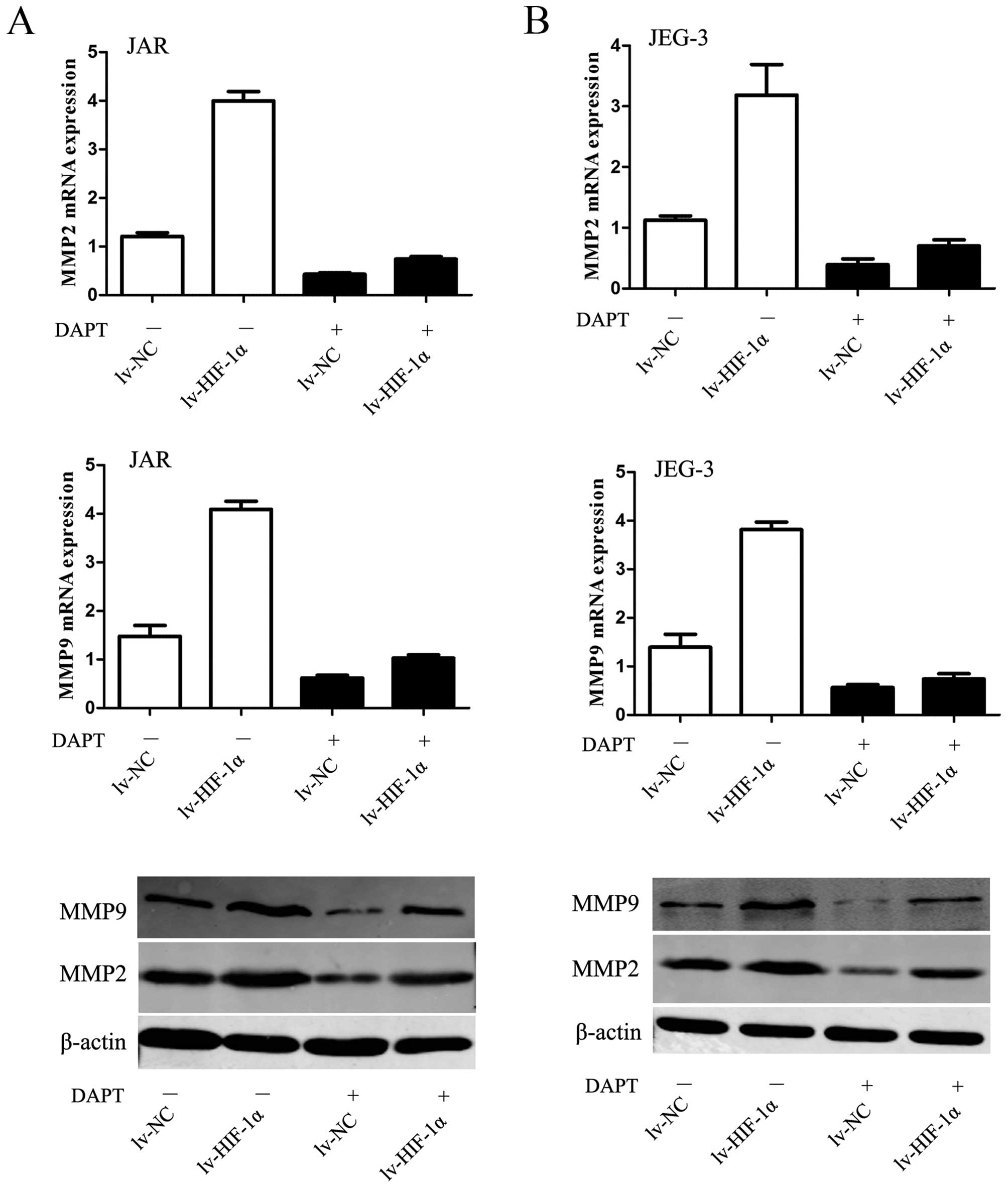
HIF-1α-induced upregulation of MMPs
requires Notch activation
Given the fundamental interaction between Notch
signaling and HIF-1α-induced EMT with enhanced ability of
migration/invasion, we considered whether HIF-1α-induced
upregulation of MMPs require Notch signaling. The data revealed
that MMP2 and MMP9 were upregulated by HIF-1α but substantially
blocked in the presence of DAPT (P<0.01, respectively, Fig. 6).
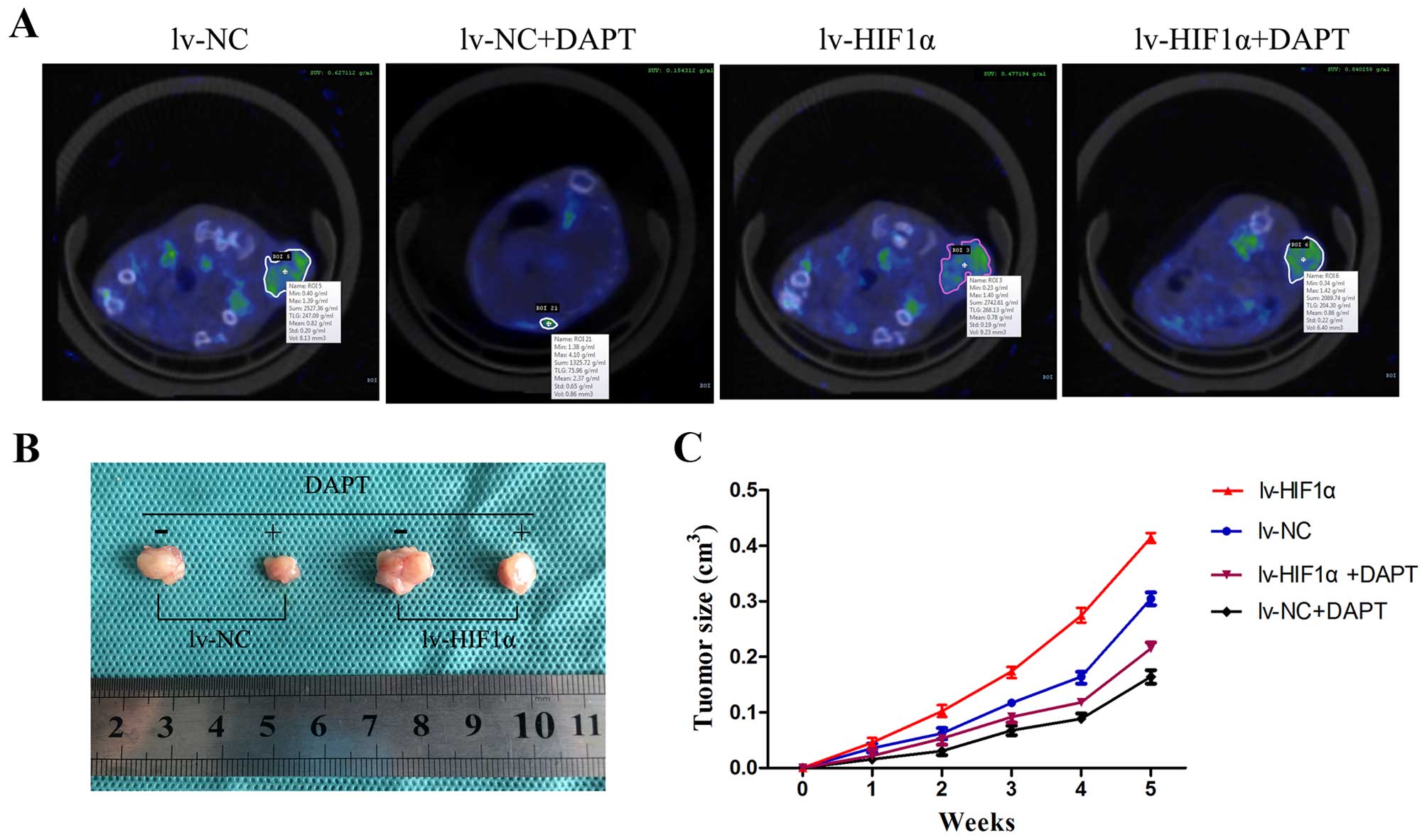
DAPT inhibits choriocarcinoma growth and
metabolic activity in vivo
To determine whether the observed antitumor activity
in vitro by Notch inhibitors could be extended in
vivo, DAPT was injected intraperitoneally into SCID mice with
tumor xenografts of the JAR cells transfected with lv-NC or
lv-HIF-1α six times at 3-day intervals. Here, SA-PET/CT scans of
subcutaneous tumors were performed on mice and SUV of
18F-FDG binding in vivo scans are provided in
Fig. 7A. 18F-FDG uptake
in nodules of the lv-HIF-1α mice treated with DMSO was high, while
the nodules of lv-NC mice treated with DMSO were at low levels.
DAPT-treated groups showed only slight 18F-FDG activity
in the tumors of the lv-NC group, unlike the high level of activity
in the lv-HIF-1α group (Fig. 7A).
After PET/CT scans, we removed the red-brown transplantation tumor
(Fig. 7B). The transplantation
tumors grew in elliptical shapes with the purplish-black covering
skin. The DMSO groups showed that HIF-1α alone significantly
promoted tumor growth. Compared with the DMSO group, DAPT treatment
significantly inhibited tumor growth (P<0.05, Fig. 7C).
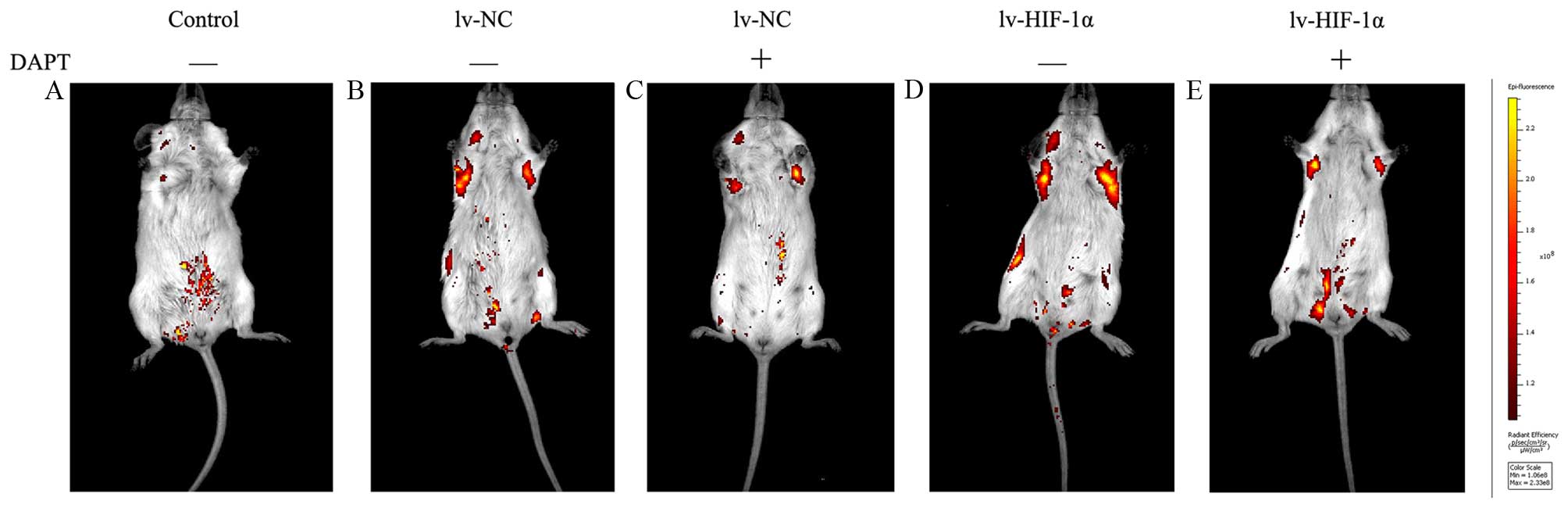
DAPT inhibits choriocarcinoma invasion
and metastasis in vivo
To assess the capacity of suppression of endogenous
Notch signaling as a treatment for choriocarcinoma cell metastasis,
JAR cells stably expressing EGFP were injected intravenously into
SCID mice as an in vivo model of metastasis. In mice treated
with DAPT or DMSO six times at 3-day intervals from the time of
cell injection. Under IVIS system, macroscopic appearance of
metastatic tumors were detected in lung of 85% (17/20) mice at 30
days after intravenous injection. Positive signals were detected in
100% (5/10, lv-HIF-1α) and 100% (5/5, lv-NC) of mice treated with
DMSO, respectively. Because there were multiple metastatic lesions
found in the lungs, positive signals from isolated lesions were
integrated for the measurement of photon counts in each mouse. The
DMSO groups showed that the levels of detectable EGFP signals for
the lv-HIF-1α mice were stronger than lv-NC mice. In contrast, 80%
(4/5, HIF-1α) and 60% (3/5, lv-NC) of mice treated with DAPT had
positive signals. In contrast to increased areas of detectable EGFP
in DMSO-treated lv-HIF-1α and lv-NC mice, the EGFP-positive areas
were limited in mice with DAPT treatment (Fig. 8). No side effect was observed
throughout the experiments in the tested mice.
Discussion
Choriocarcinoma is highly malignant and aggressive
epithelial tumor type comprising a series of heterogeneous diseases
arising from gestational trophoblasts that form the placenta. It
becomes a conspicuous tumor when it metastasizes to affected
organs, which induces symptoms such as shortness of breath from
lung involvement. Although CC is highly responsive to chemotherapy
with an overall survival rate of >90%, the mechanisms for
metastasis and resistance to conventional chemotherapy is still
unclear.
HIF-1α as a potential hypoxia marker is associated
with carcinogenesis and metastasis in various solid tumors
(26,31,32).
Since associated with chemotherapy failure, HIF-1α could be an
attractive therapeutic strategy crucial for tumor growth (33,34).
Although HIF-1α has been explored in several other gynecological
cancers, scarce date exist on the role of HIF-1α in CC. In this
study, we first detected the expression of HIF-1α in several cell
lines, and found that the expression of HIF-1α in human
choriocarcinoma cell lines was higher than that in the immortalized
normal human EVT cell line (HTR-8). The data show that
overexpression of HIF-1α is emerging as an significant factor in
carcinogenesis on the molecular level in CC. Generally, HIF-1β is
regarded as constitutively expressed and being present in excess
within the cell (4,35). However, several studies described
that HIF-1β was upregulated in a HIF-1α-dependent manner after
treatment with the hypoxia-mimetic cobalt chloride
(CoCl2) or exposure to hypoxia on both RNA and protein
levels (26,36,37).
To inspect the hypothesis that the upregulation of HIF-1β might be
mediated by HIF-1α, we demonstrated that overexpression of HIF-1α
was upregulated HIF-1β-dependently. The data confirm the leading
role of HIF-1α among HIF subunits.
In cell culture, animal models and human specimen
experiments, the evidence for EMT with increased invasion and
metastasis is irresistible (38).
In this study, the inseparable connection between hypoxia and EMT
has been recognized. Here we indicated that HIF-1α evidently
correlated with more aggressive and invasive behavior accompanied
by the EMT switch.
Notch signaling acts as a tumor promoter or a
suppressor depending on the cell type and context. As the first
evidence, Notch signaling is considered the main trigger of T-ALL
(39). However, Notch activation
in bladder cancer cells suppresses proliferation both in
vitro and in vivo (40). Recently, various research,
respectively, indicated that Notch signaling potentially mediated
molecular mechanisms underlying HIF-dependent regulation of the EMT
in solid tumors. To our knowledge, we show the involvement of the
Notch pathway during the process of HIF-1α-induced switch of EMT in
choriocarcinoma for the first time. Our finding that the Notch
signaling pathway respond to HIF-1α at various levels is consistent
with some other studies (22,33).
As γ-secretase inhibitor, DAPT interfers with Notch intracellularly
and NICD synthesis is a powerful blocker of Notch activity. In our
study, following treatment of CC cell lines with DAPT, the
expression of N1ICD and downstream Hes1, was significantly
decreased, whereas, E-cadherin, CK18 and CK19 were upregulated. In
addition, through the suppression of Notch signaling, a crucial
target reducing migration and invasion, may be the upregulation of
E-cadherin, CK18 and CK19 expression and downregulation of MMPs,
such as MMP2 and MMP9 during the acquisition of the epithelial
phenotype, which recovers cell-cell adhesion and stabilizes the
epithelial architecture. Above all, HIF-1α-induced EMT requires
Notch signaling and the decreased endogenous Notch signaling
blocked cells to EMT. Moody et al reported that upregulation
of Snail-1 induced metastasis and poor prognosis, whereas silencing
of Snail-1 suppressed tumor growth and invasiveness (41). In keeping with this notion,
Sahlgren et al showed that Notch regulated expression of
Snail-1 in two different but synergistic ways (22). Coincidentally, Snail-1, which is
activated during the acquisition of EMT was downregulated in CC
cells treated with DAPT.
The most common metastatic site of CC is the lungs,
which are affected in >80% patients and PET/CT scanning and can
aid in identifying sites of active disease, and select patients
with solitary lesions who may benefit from surgical resection of
chemotherapy-resistant metastases (42,43).
18F-FDG SA-PET is widely used for non-invasive in
vivo therapy assessment in cancer research (44). Using the present in vivo
model of CC, SA-PET/CT and IVIS system, we further confirmed
important roles of Notch1 signaling in overexpressed HIF-1α cell
invasion and metastasis. Consistent with in vitro studies,
suppression of endogenous Notch1 signaling by DAPT not only led to
inhibited CC growth and metabolic activity, but also decreased
their metastasis to lung, accompanied by suppressed growth of
metastatic tumors. Leong et al indicated that blocking Notch
signaling inhibits tumor growth and metastasis in an in vivo
tumor model (21). These findings
indicated that Notch signaling is a valuable target with a
therapeutic potential for both early and advanced stages of CC.
In conclusion, this study demonstrated that the
Notch1 signaling pathway is closely associated with the invasion
and metastasis of CC. Furthermore, the results demonstrated that
the suppression of Notch1 with the γ-secretase inhibitor restrains
the invasion and metastasis of CC by inhibiting EMT. Therefore,
purification of Notch inhibitors and a more local containment may
be an effective way toward improving therapy.
Acknowledgement
This study was supported by the National Natural
Science Foundation of China (no. 81172489).
References
|
1
|
Cheung AN, Zhang HJ, Xue WC and Siu MK:
Pathogenesis of choriocarcinoma: Clinical, genetic and stem cell
perspectives. Future Oncol. 5:217–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Semenza GL: HIF-1 and tumor progression:
Pathophysiology and therapeutics. Trends Mol Med. 8(Suppl):
S62–S67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen G, Li X, Jia YF, Piazza GA and Xi Y:
Hypoxia-regulated microRNAs in human cancer. Acta Pharmacol Sin.
34:336–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1)alpha: Its protein stability
and biological functions. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruick RK: Oxygen sensing in the hypoxic
response pathway: Regulation of the hypoxia-inducible transcription
factor. Genes Dev. 17:2614–2623. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Semenza GL: Hypoxia-inducible factor 1:
Oxygen homeostasis and disease pathophysiology. Trends Mol Med.
7:345–350. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang J, Tang YL and Liang XH: EMT: A new
vision of hypoxia promoting cancer progression. Cancer Biol Ther.
11:714–723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Christofori G: New signals from the
invasive front. Nature. 441:444–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ombrato L and Malanchi I: The EMT
universe: Space between cancer cell dissemination and metastasis
initiation. Crit Rev Oncog. 19:349–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou X, Hu Y, Dai L, Wang Y, Zhou J, Wang
W, Di W and Qiu L: MicroRNA-7 inhibits tumor metastasis and
reverses epithelial-mesenchymal transition through AKT/ERK1/2
inactivation by targeting EGFR in epithelial ovarian cancer. PLoS
One. 9:e967182014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ponnusamy MP, Lakshmanan I, Jain M, Das S,
Chakraborty S, Dey P and Batra SK: MUC4 mucin-induced epithelial to
mesenchymal transition: A novel mechanism for metastasis of human
ovarian cancer cells. Oncogene. 29:5741–5754. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Techasen A, Namwat N, Loilome W,
Bungkanjana P, Khuntikeo N, Puapairoj A, Jearanaikoon P, Saya H and
Yongvanit P: Tumor necrosis factor-α (TNF-α) stimulates the
epithelial-mesenchymal transition regulator Snail in
cholangiocarcinoma. Med Oncol. 29:3083–3091. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee
JE, Cho JY, Yoo JE, Choi JS and Park YN: Human hepatocellular
carcinomas with ‘Stemness’-related marker expression: Keratin 19
expression and a poor prognosis. Hepatology. 54:1707–1717. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barrallo-Gimeno A and Nieto MA: The Snail
genes as inducers of cell movement and survival: Implications in
development and cancer. Development. 132:3151–3161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kadesch T: Notch signaling: The demise of
elegant simplicity. Curr Opin Genet Dev. 14:506–512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gustafsson MV, Zheng X, Pereira T, Gradin
K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U and
Bondesson M: Hypoxia requires notch signaling to maintain the
undifferentiated cell state. Dev Cell. 9:617–628. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, De Marco MA, Graziani I, Gazdar
AF, Strack PR, Miele L and Bocchetta M: Oxygen concentration
determines the biological effects of NOTCH-1 signaling in
adenocarcinoma of the lung. Cancer Res. 67:7954–7959. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang Y, Yu S, Ma Y, Sun P, Ma D, Ji C and
Kong B: Association of Dll4/notch and HIF-1α-VEGF signaling in the
angiogenesis of missed abortion. PLoS One. 8:e706672013. View Article : Google Scholar
|
|
21
|
Leong KG, Niessen K, Kulic I, Raouf A,
Eaves C, Pollet I and Karsan A: Jagged1-mediated Notch activation
induces epithelial-to-mesenchymal transition through Slug-induced
repression of E-cadherin. J Exp Med. 204:2935–2948. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sahlgren C, Gustafsson MV, Jin S,
Poellinger L and Lendahl U: Notch signaling mediates
hypoxia-induced tumor cell migration and invasion. Proc Natl Acad
Sci USA. 105:6392–6397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen J, Imanaka N, Chen J and Griffin JD:
Hypoxia potentiates Notch signaling in breast cancer leading to
decreased E-cadherin expression and increased cell migration and
invasion. Br J Cancer. 102:351–360. 2010. View Article : Google Scholar :
|
|
24
|
Bedogni B, Warneke JA, Nickoloff BJ,
Giaccia AJ and Powell MB: Notch1 is an effector of Akt and hypoxia
in melanoma development. J Clin Invest. 118:3660–3670. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baddela VS, Baufeld A, Yenuganti VR,
Vanselow J and Singh D: Suitable housekeeping genes for
normalization of transcript abundance analysis by real-time RT-PCR
in cultured bovine granulosa cells during hypoxia and differential
cell plating density. Reprod Biol Endocrinol. 12:1182014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guan Z, Ding C, Du Y, Zhang K, Zhu JN,
Zhang T, He D, Xu S, Wang X and Fan J: HAF drives the switch of
HIF-1α to HIF-2α by activating the NF-κB pathway, leading to
malignant behavior of T24 bladder cancer cells. Int J Oncol.
44:393–402. 2014.
|
|
27
|
Xie M, He CS, Wei SH and Zhang L: Notch-1
contributes to epidermal growth factor receptor tyrosine kinase
inhibitor acquired resistance in non-small cell lung cancer in
vitro and in vivo. Eur J Cancer. 49:3559–3572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y, Yan X, Duan W, Yan J, Yi W, Liang
Z, Wang N, Li Y, Chen W, Yu S, et al: Pterostilbene exerts
antitumor activity via the Notch1 signaling pathway in human lung
adenocarcinoma cells. PLoS One. 8:e626522013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang S, Yang Y, Liang Z, Duan W, Yang J,
Yan J, Wang N, Feng W, Ding M, Nie Y, et al: Silybin-mediated
inhibition of Notch signaling exerts antitumor activity in human
hepatocellular carcinoma cells. PLoS One. 8:e836992013. View Article : Google Scholar
|
|
30
|
Aboagye EO: Positron emission tomography
imaging of small animals in anticancer drug development. Mol
Imaging Biol. 7:53–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Semenza GL: HIF-1 mediates metabolic
responses to intra-tumoral hypoxia and oncogenic mutations. J Clin
Invest. 123:3664–3671. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maroni P, Matteucci E, Drago L, Banfi G,
Bendinelli P and Desiderio MA: Hypoxia induced E-cadherin involving
regulators of Hippo pathway due to HIF-1alpha stabilization/nuclear
translocation in bone metastasis from breast carcinoma. Exp Cell
Res. 30:287–299. 2015. View Article : Google Scholar
|
|
33
|
Seeber LM, Horrée N, Vooijs MA, Heintz AP,
van der Wall E, Verheijen RH and van Diest PJ: The role of hypoxia
inducible factor-1alpha in gynecological cancer. Crit Rev Oncol
Hematol. 78:173–184. 2011. View Article : Google Scholar
|
|
34
|
Miyazawa M, Yasuda M, Fujita M, Hirasawa
T, Kajiwara H, Hirabayashi K, Ogane N, Shimizu M, Asanuma H,
Murakami M, et al: Association of hypoxia-inducible factor-1
expression with histology in epithelial ovarian tumors: A
quantitative analysis of HIF-1. Arch Gynecol Obstet. 279:789–796.
2009. View Article : Google Scholar
|
|
35
|
Semenza GL: Oxygen homeostasis. Wiley
Interdiscip Rev Syst Biol Med. 2:336–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mandl M, Kapeller B, Lieber R and Macfelda
K: Hypoxia-inducible factor-1β (HIF-1β) is upregulated in a
HIF-1α-dependent manner in 518A2 human melanoma cells under hypoxic
conditions. Biochem Biophys Res Commun. 434:166–172. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chilov D, Camenisch G, Kvietikova I,
Ziegler U, Gassmann M and Wenger RH: Induction and nuclear
translocation of hypoxia-inducible factor-1 (HIF-1):
Heterodimerization with ARNT is not necessary for nuclear
accumulation of HIF-1alpha. J Cell Sci. 112:1203–1212.
1999.PubMed/NCBI
|
|
38
|
Liang X: EMT: New signals from the
invasive front. Oral Oncol. 47:686–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Weng AP, Ferrando AA, Lee W, Morris JP IV,
Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT and Aster
JC: Activating mutations of NOTCH1 in human T cell acute
lymphoblastic leukemia. Science. 306:269–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rampias T, Vgenopoulou P, Avgeris M,
Polyzos A, Stravodimos K, Valavanis C, Scorilas A and Klinakis A: A
new tumor suppressor role for the Notch pathway in bladder cancer.
Nat Med. 20:1199–1205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Moody SE, Perez D, Pan TC, Sarkisian CJ,
Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD and
Chodosh LA: The transcriptional repressor Snail promotes mammary
tumor recurrence. Cancer Cell. 8:197–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dhillon T, Palmieri C, Sebire NJ, Lindsay
I, Newlands ES, Schmid P, Savage PM, Frank J and Seckl MJ: Value of
whole body 18FDG-PET to identify the active site of
gestational trophoblastic neoplasia. J Reprod Med. 51:879–887.
2006.PubMed/NCBI
|
|
43
|
Froeling FE and Seckl MJ: Gestational
trophoblastic tumours: An update for 2014. Curr Oncol Rep.
16:4082014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lasnon C, Dugue AE, Briand M,
Blanc-Fournier C, Dutoit S, Louis MH and Aide N: NEMA NU
4-optimized reconstructions for therapy assessment in cancer
research with the Inveon small animal PET/CT system. Mol Imaging
Biol. 17:403–412. 2015. View Article : Google Scholar
|