Introduction
Metronomic chemotherapy is the chronic
administration of low dose chemotherapy as opposed to the
conventional chemotherapy protocol of the pulsatile administration
of a maximum tolerated dose (1).
It is a promising protocol of chemotherapy with a low toxic profile
and encouraging results in certain clinical trials (2). Metronomic chemotherapy seems to have
pleiotropic effects (2). It can
target the cancer cell and modulate the immune system but it is
primarily considered anti-angiogenic (2). Antimitotics, including taxanes and
vinca alkaloids are lead drugs for metronomic treatment as they
inhibit angiogenesis through multiple mechanisms (3).
Vinorelbine is a semisynthetic vinca alkaloid with
the additional advantage of the oral formulation which favors its
use in the chronic administration protocol of metronomic
chemotherapy (4). Briasoulis et
al demonstrated that the metronomic administration of
vinorelbine, given three times a week, maintains low nanomolar
steady state concentrations in the blood (4) and yields objective responses of
prolonged duration with negligible toxicity (4,5). The
authors suggested that the antitumor efficacy is likely due to
anti-angiogenic action because of the profile of circulating
angiogenic biomarkers in responding patients, the low nanomolar
concentrations of the drug and the minimal toxicity (5).
Unfortunately, anti-angiogenic therapies have only
an ephemeral effect (6), since
after the initial response resistance develops leading to treatment
failure (6). Tumors acquire
resistance to VEGF-targeted agents through activation of different
modes of vascularization, upregulation of alternative
pro-angiogenic signaling pathways and recruitment of pro-angiogenic
cells (6–8). Anti-angiogenic treatment cut off the
tumor blood supply creating a hypoxic microenvironment.
Treatment-induced hypoxia is shown to be the initiating factor of
this secondary resistance to anti-VEGF therapies, as reviewed by
Loges et al (8).
Furthermore, hypoxia is associated with resistance to chemotherapy
(9–11). Hypoxia modulates the intrinsic
apoptotic pathway and alters cell cycle leading to refractoriness
to cell cycle specific agents (9).
Drugs with vascular disrupting properties, such as
microtubule targeting agents, can rapidly promote and sustain
conditions of severe hypoxia with partial oxygen pressure <2.5
mm Hg in the tumor center (12,13).
Considering the fact that hypoxia is the triggering factor of the
evasive resistance to certain anti-angiogenic therapies (6–8) and
it confers resistance to chemotherapy (9–11),
we questioned whether severe hypoxia can mediate resistance to the
anti-angiogenic action of metronomic vinorelbine.
The rational combination of metronomic chemotherapy
with a targeted agent can enhance the efficacy of metronomic
treatment (14). The Akt pathway
is a critical modulator of angiogenesis and cell survival (15). Both vinca alkaloids and Akt
converge to the intrinsic mitochondrial apoptotic pathway to
regulate cell death (16,17). We tested whether Akt inhibition
could sensitize endothelial cells to the anti-proliferative action
of metronomic vinorelbine.
In this study, we sought to determine whether the
clinically relevant metronomic concentration (5) of 10 nM is anti-angiogenic in
vitro and we compared it with the concentration of 1 μM which
simulates the peak plasma levels of the conventional chemotherapy
protocol (18). We show that 10 nM
vinorelbine inhibits the sequential steps of sprouting angiogenesis
(19) such as migration, tube
formation and proliferation. We found that severe hypoxia (0.1%
O2) confers resistance to the anti-proliferative action
of metronomic vinorelbine due to G1 arrest and attenuation of
apoptosis. The Bcl-2 protein family is implicated in the cell death
caused by the microtubule targeting agents (MTAs) (16) and we questioned whether Bcl-2 is
also regulated by severe hypoxia. Finally, we sought to find a way
to circumvent this hypoxic resistance and we report that
combination with Akt inhibition sensitizes HUVECs to the action of
10 nM vinorelbine.
Materials and methods
Cell culture and chemical compounds
Human umbilical vein endothelial cells (HUVECs),
supplied from Lonza, were cultured on culture dishes (Corning)
coated with gelatin (0.1% w/v) and were fed with endothelial basal
media supplemented with growth factors (EGM-2; Lonza). Incubation
in severe hypoxia (0.1% O2) was undertaken in an invivo2
400 hypoxic workstation (Ruskin Technologies). Vinorelbine (Tocris
Bioscience) was dissolved in dimethyl sulfoxide and used at the
indicated concentrations. Akt inhibitor V (Calbiochem) was
dissolved in dimethyl sulfoxide and used at a concentration of 10
μM.
Immunoblotting
Cells were lysed with RIPA buffer (Sigma-Aldrich),
supplemented with a cocktail of protease and phosphatase inhibitors
(Roche), by incubating on ice for 20 min. Cell lysate was clarified
by centrifugation for 10 min at 4°C and protein was quantified with
Bio-Rad protein assay. We probed for Bcl-2 (Santa Cruz, sc-509),
Bax (Santa Cruz, sc-493), p27 Kip (Cell Signaling, no. 2552) and
β-actin as loading control (anti-β-actin HRP conjugate,
Sigma-Aldrich, A3854). Quantification of the intensity of the
protein bands was performed with ImageJ.
Proliferation assay
Proliferation was determined with the
CyQUANT® assay (Life Technologies). Cells were plated on
96-well plates in a density of 2,000 cells/well and treated as
appropriate. Afterwards the media were aspirated and the reagent
was added according to the manufacturer’s protocol for 1 h.
Fluorescence was measured with SpectraMax M2 multimode microplate
reader. Proliferation was assessed by the relative fluorescent unit
(RFU) normalized to the untreated control.
Wound healing assay
Migration was assessed with the wound healing assay.
One hundred thousand cells/well were seeded on a 24-well plate
supplied by Essen BioScience. When the cell monolayer became
confluent, a scratch wound was performed at the time-point 0 (t:
0). An image of the wound was captured at t: 0 and the cells were
subsequently treated with vinorelbine in normoxia or severe hypoxia
for 6 h (t: 6 h). At t: 6 h a second image was taken. Images of the
wound were captured by the IncuCyte (Essen BioScience), analyzed
with the built in algorithm and quantified by assessing the wound
confluence parameter. The wound confluence value at t: 6 h was
corrected by subtracting the initial wound confluence at t: 0.
Matrigel assay
Tube formation was assessed with the Matrigel assay.
96-well plates were coated with 50 μl Matrigel (BD Matrigel™
basement membrane matrix) which was allowed to set for 30 min in
37°C. Fifteen thousand HUVECs/well were subsequently seeded on the
top of the matrix and the cells were treated with vinorelbine in
normoxia or severe hypoxia for 6 h. Phase contrast images were
taken with the EVOS Cell Imaging System (Life Technologies) at the
end of the treatment. Tube formation was determined by the number
of polygones of the tube network.
Hanging drop assay
Angiogenic sprouting was examined with the hanging
drop assay. Briefly, drops of HUVEC suspension with 750 cells/20 μl
were dispensed on the inner side of an inverted culture dish lid.
The lid was carefully placed back on the top of the dish and the
droplets were allowed to form spheroids overnight. The spheroids
were then pelleted, resuspended in fibrin solution (2 mg/ml)
containing 0.15 U/ml aprotinin and dispensed in 24-well plates
containing thrombin. The fibrin solution with HUVEC spheroids was
mixed gently with thrombin (0.625 U/ml) and allowed to clot for 20
min in 37°C. Media with or without vinorelbine were then added in
the well on the top of the clot. HUVEC spheroids were treated in
normoxia or severe hypoxia for 24 h. Phase contrast images were
taken at the end of the treatment with the EVOS Cell Imaging System
(Life Technologies). Sprouting was determined by quantifying the
area occupied by the sprout outgrowth at the end of the treatment.
Quantification of the sprout area was performed with ImageJ.
Cell cycle analysis
Cells were treated for 24 h in normoxia or severe
hypoxia and were subsequently harvested, washed with PBS and fixed
with cold 70% ethanol overnight at 4°C. Afterwards, the cells were
washed twice with PBS and then treated with a solution containing
ribonuclease I (20 μg/ml) and propidium iodine (PI) (100 μg/ml) at
room temperature. After incubation for 15 min the cells were
analyzed in a FACS Analyzer CyAn ADP on FL3 channel. Quantification
was done with FlowJo software v.10 by employing the built-in
algorithm.
Analysis of apoptosis
Cells were treated as appropriate and were
subsequently harvested and washed with PBS. Cells were resuspended
in binding buffer to bring 105 cells/100 μl and
incubated with PI in the final concentration of 1 μg/ml and Annexin
V conjugated with AlexaFluor 647 in the final dilution of 1/100.
After incubation for 15 min at room temperature, stained cells were
analyzed on FL3 channel for PI and FL8 for Annexin V by using a
FACS Analyzer CyAn ADP. Reagents were supplied by Molecular
ProbesR.
Statistical analyses
Statistical analyses and graphs were performed with
GraphPad prism v.5. Statistical comparisons were carried out by
using one-way ANOVA or un-paired t-test.
Results
Dose- and time-dependent effect of
vinorelbine on endothelial cell proliferation
To investigate whether metronomic vinorelbine is
anti-angiogenic, we first tested the effect on endothelial cell
proliferation which is one of the sequential steps of sprouting
angiogenesis (19). We compared
the effect of 10 nM, a clinically relevant metronomic concentration
(4,5) with the effect of 1 μM, which is close
to the transient peak plasma levels of the drug in maximum
tolerated dose chemotherapy (18).
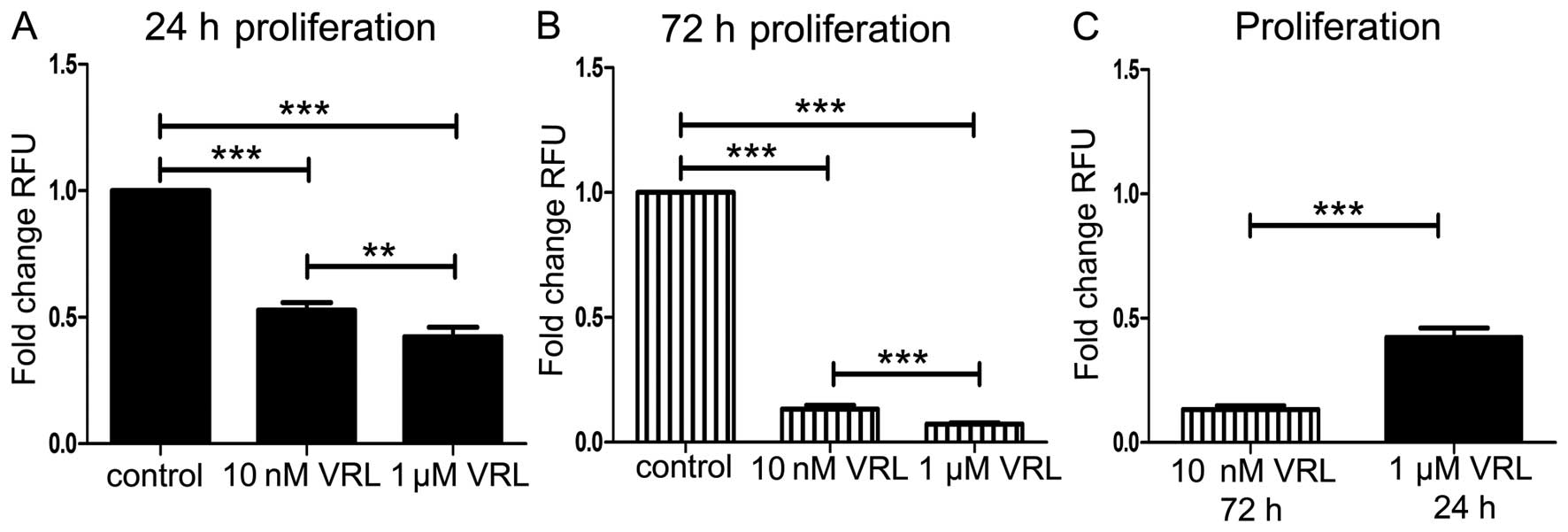
Ten nanomolar inhibited proliferation by 47% (P<0.001) and 1 μM
by 58% (P<0.001) at 24-h treatment (Fig. 1A). Ten nanomolar inhibited
proliferation by 87% (P<0.001) and 1 μM by 93% (P<0.001) at
72-h treatment (Fig. 1B).
Vinorelbine inhibited proliferation in a dose responsive manner but
the metronomic concentration of 10 nM was more effective at 72 h
than the concentration of 1 μM at 24 h (Fig. 1C). The latter indicates the
favorable effect of the prolonged metronomic treatment, compared to
the short-term treatment resembling conventional chemotherapy.
The metronomic concentration of 10 nM
vinorelbine inhibits migration, tube formation and sprouting
without affecting cell viability
To further examine the anti-angiogenic action of
metronomic vinorelbine, we investigated the effect of 10 nM on
migration, tube formation and sprouting in vitro and we
compared it with the concentration of 1 μM (18).
We assessed migration and tube formation in the
short-term treatment of 6 h to avoid the interference from the
anti-proliferative effect of metronomic vinorelbine.
Vinorelbine inhibited migration as determined by the
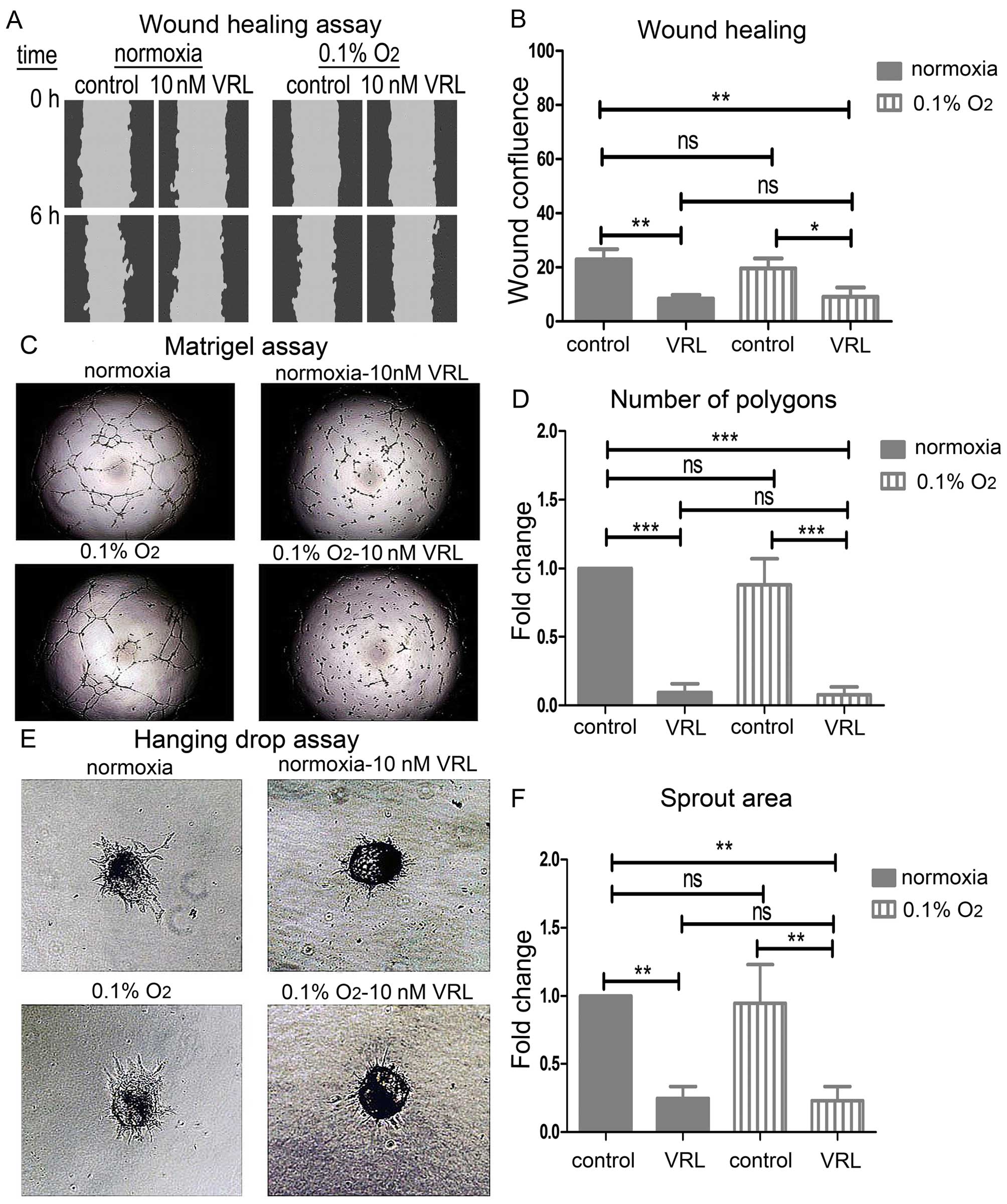
wound healing assay (Fig. 2A). Ten
nanomolar vinorelbine decreased the wound confluence by 1.8 times
after the scratch wound while 1 μM decreased it by 2.81 times
(Fig. 2B).
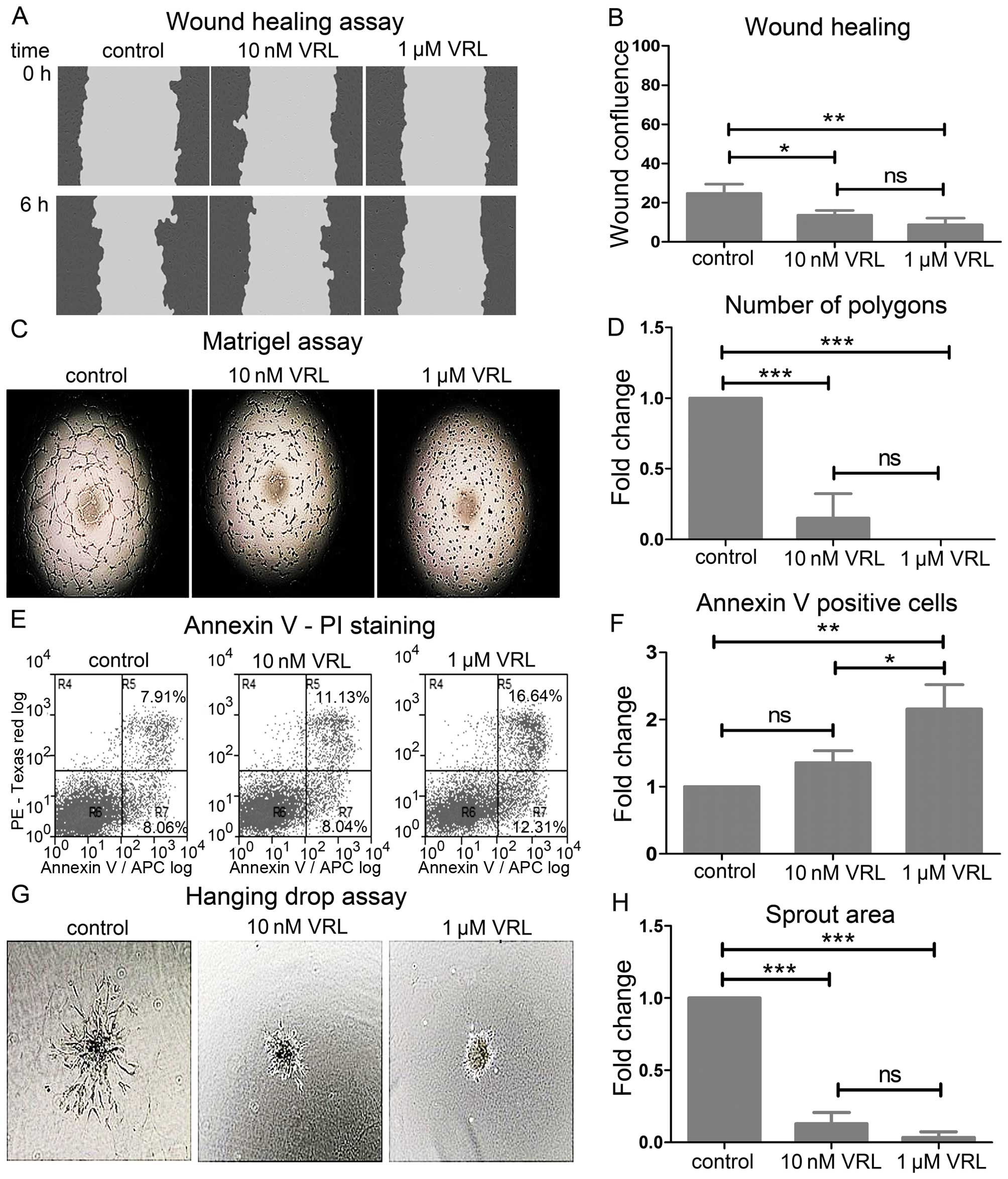 | Figure 2The effect of vinorelbine (VRL) on
migration, tube formation, viability and sprouting of HUVECs. (A
and B) Migration in the wound healing assay. (A) Representative
images showing the scratch wound mask at the time-points 0 and 6 h
(hours). Note the delay of the healing process with VRL at 6 h
compared to control. (B) The effect of VRL on wound healing as
determined by the wound confluence parameter. Results are expressed
as mean wound confluence ± SD of three independent experiments.
Error bars depict standard deviation. One-way ANOVA; ns, not
significant; *P<0.05; **P<0.01. (C and
D) Tube formation in the Matrigel™ assay. (C) Representative images
showing suppressed tube formation upon treatment with VRL. (D) The
effect of VRL on tube formation as determined by the number of
polygons formed. (E and F) Annexin V-PI staining analysis with FACS
after 6-h treatment with VRL. (E) Representative dual parametric
dot plot showing staining for Annexin V and/or PI. The values
represent percentages of the total cell number. R4, PI-positive
(necrotic cells); R5, Annexin V + PI-positive (late apoptotic
cells); R6, negative (viable cells); R7, Annexin V-positive (early
apoptotic cells). R5+R7, total apoptotic cells. (F) The effect of
VRL on cell death as determined by the Annexin V-positive cells. (G
and H) Angiogenic sprouting in the hanging drop assay. (G)
Representative images showing inhibition of the sprout outgrowth
upon treatment with VRL. (H) The effect of VRL on sprouting as
determined by the spout area. (D, F and H) Results are expressed as
mean fold change ± SD of three independent experiments. Error bars
represent standard deviation (SD). One-way ANOVA; ns, not
significant; *P<0.05; **P<0.01;
***P<0.001. |
Vinorelbine inhibited tube formation as determined
by the Matrigel assay (Fig. 2C).
Ten nanomolar vinorelbine decreased the number of polygons formed
within the tube network by 85% (P<0.001) 6 h after plating
HUVECs on Matrigel while 1 μM completely prevented the formation of
the tube network (P<0.001) (Fig.
2D).
The inhibition of the functions above by 10 nM
vinorelbine was not attributed to cell toxicity (Fig. 2E). Ten nanomolar vinorelbine did
not change significantly the cell percentage stained positive for
Annexin V at 6 h whereas 1 μM vinorelbine increased it by 2.16-fold
(P<0.01) (Fig. 2F).
Finally, we examined the overall effect on sprouting
angiogenesis with the hanging drop assay (Fig. 2G). Sprouting angiogenesis involves
a sequence of events starting with endothelial sprouting into tip
cells, tip cell migration, stalk cell proliferation, branching and
finally lumen formation (19). We
assessed sprouting in fibrin gel at 24 h. Ten nanomolar decreased
the area of the sprout outgrowth by 87% (P<0.001) while 1 μM
almost completely disrupted sprouting (P<0.001) (Fig. 2H).
Severe hypoxia does not interfere with
the inhibitory action of 10 nM vinorelbine on the endothelial cell
migration, tube formation or sprouting
Having shown that metronomic vinorelbine is
anti-angiogenic and considering that hypoxia, which is exacerbated
by anti-angiogenic therapy, is associated with treatment failure
(8), we investigated whether
severe hypoxia (0.1% O2) mediates resistance to
metronomic vinorelbine treatment. We show that the metronomic
concentration of 10 nM vinorelbine reduced the wound confluence to
the same extent (Fig. 3B) in
normoxia and severe hypoxia in the wound healing assay (Fig. 3A). Moreover, it reduced the number
of polygons formed within the tube network to the same degree
(Fig. 3D) in normoxia and severe
hypoxia in the Matrigel™ assay (Fig.
3C). Finally, the sprout area was reduced to the same extent
(Fig. 3F) in the hanging drop
assay (Fig. 3E). Severe hypoxia
did not change either the above functions under control
conditions.
Severe hypoxia confers resistance to the
anti-proliferative action of vinorelbine
We further addressed whether hypoxia mediates
resistance to the anti-angiogenic action of vinorelbine by
examining the effect of severe hypoxia (0.1% O2) on
endothelial cell proliferation (19).
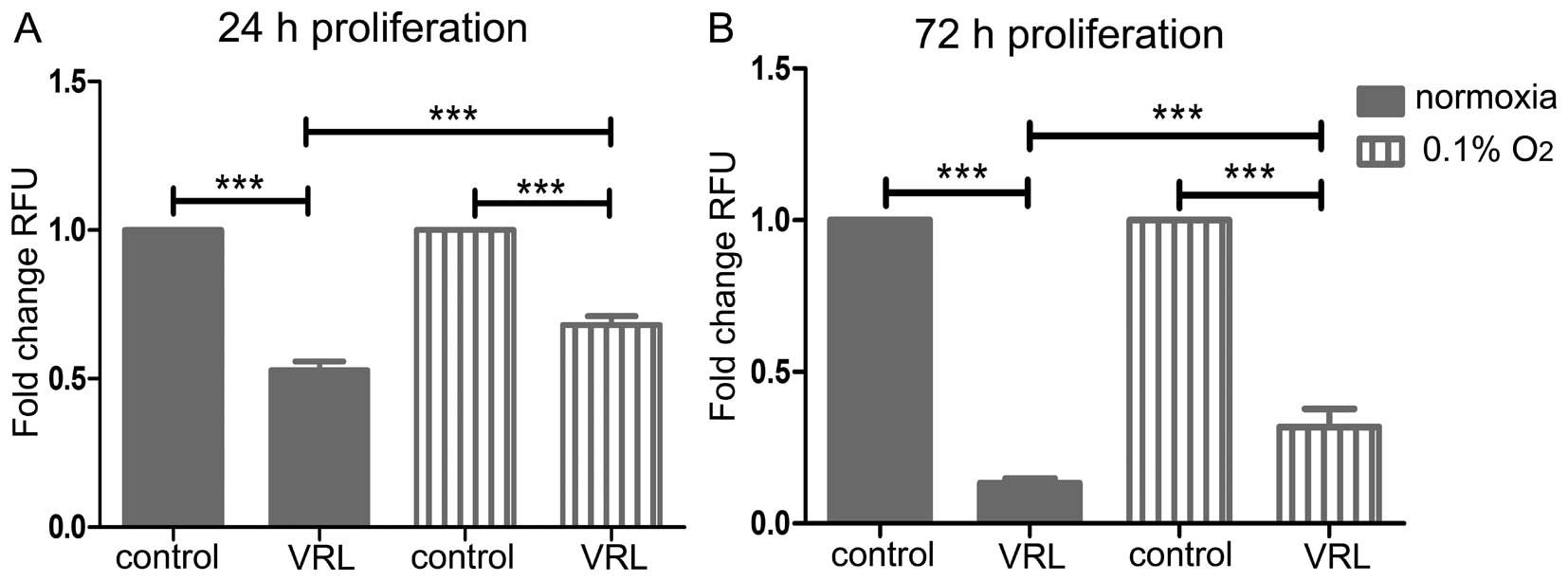
After 24-h treatment, 10 nM vinorelbine inhibited
proliferation more potently in normoxia than in severe hypoxia (47%
versus 32%, P<0.001) (Fig. 4A).
Likewise, after 72-h treatment, 10 nM vinorelbine inhibited
proliferation to a greater extent in normoxia than in severe
hypoxia (87% versus 68%, P<0.001) (Fig. 4B).
Resistance to the anti-proliferative
action of metronomic vinorelbine is due to attenuation of the
mitotic arrest and protection from apoptosis
To elaborate the mechanism of resistance to the
anti-proliferative action of metronomic vinorelbine, we examined
the effect of severe hypoxia on the cell cycle and apoptosis.
We hypothesized that severe hypoxia slows down
proliferation and alters the cell cycle, making vinorelbine less
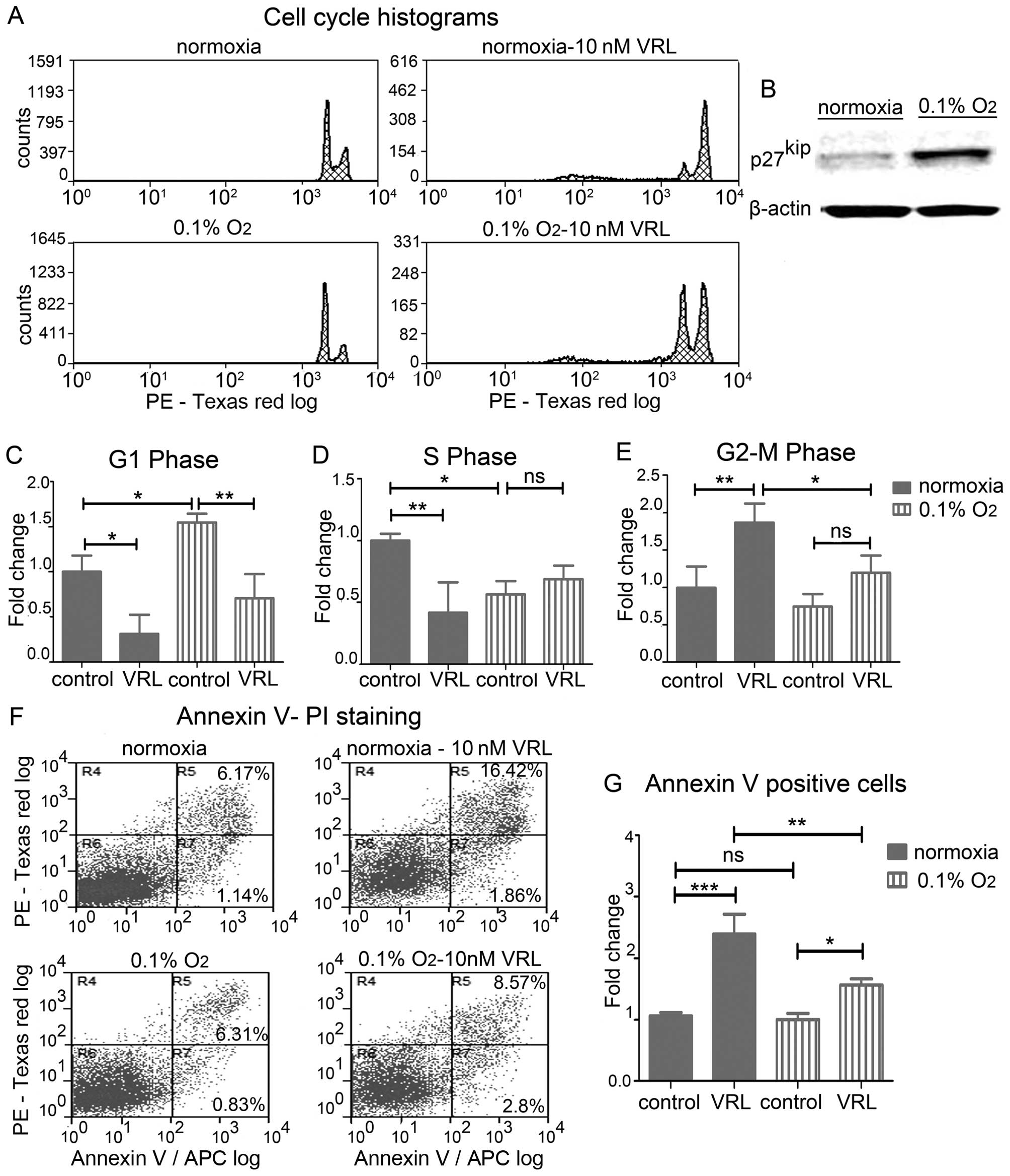
effective in targeting mitotic microtubules (20). We show that severe hypoxia for 24 h
upregulated the cyclin-dependent kinase (cdk) inhibitor
p27kip (Fig. 5B), which
is suggested to block G1/S transition and results in G1 arrest
(21). Cell cycle analysis
revealed alterations of the distribution of HUVECs in the different
cell cycle phases (Fig. 5A). In
particular, severe hypoxia increased the percentage of HUVECs in
the G1 phase by 1.55-fold (P<0.05) (Fig. 5C) causing a G1 phase arrest while
it concomitantly decreased the fraction of the cells in the DNA
synthesis S phase by 1.78-fold (P<0.05) (Fig. 5D). Metronomic vinorelbine induced
G2/M arrest in normoxia but severe hypoxia attenuated this effect
by 1.56-fold (P<0.05) (Fig.
5E).
We next questioned whether the effect of severe
hypoxia on the cell cycle leads to apoptotic cell death. Annexin V
staining and FACS (Fig. 5F) after
36 h of hypoxia revealed no change in apoptosis levels in untreated
cells, however, hypoxia decreased the effect of vinorelbine-induced
apoptosis by 1.53-fold (P<0.01) (Fig. 5G).
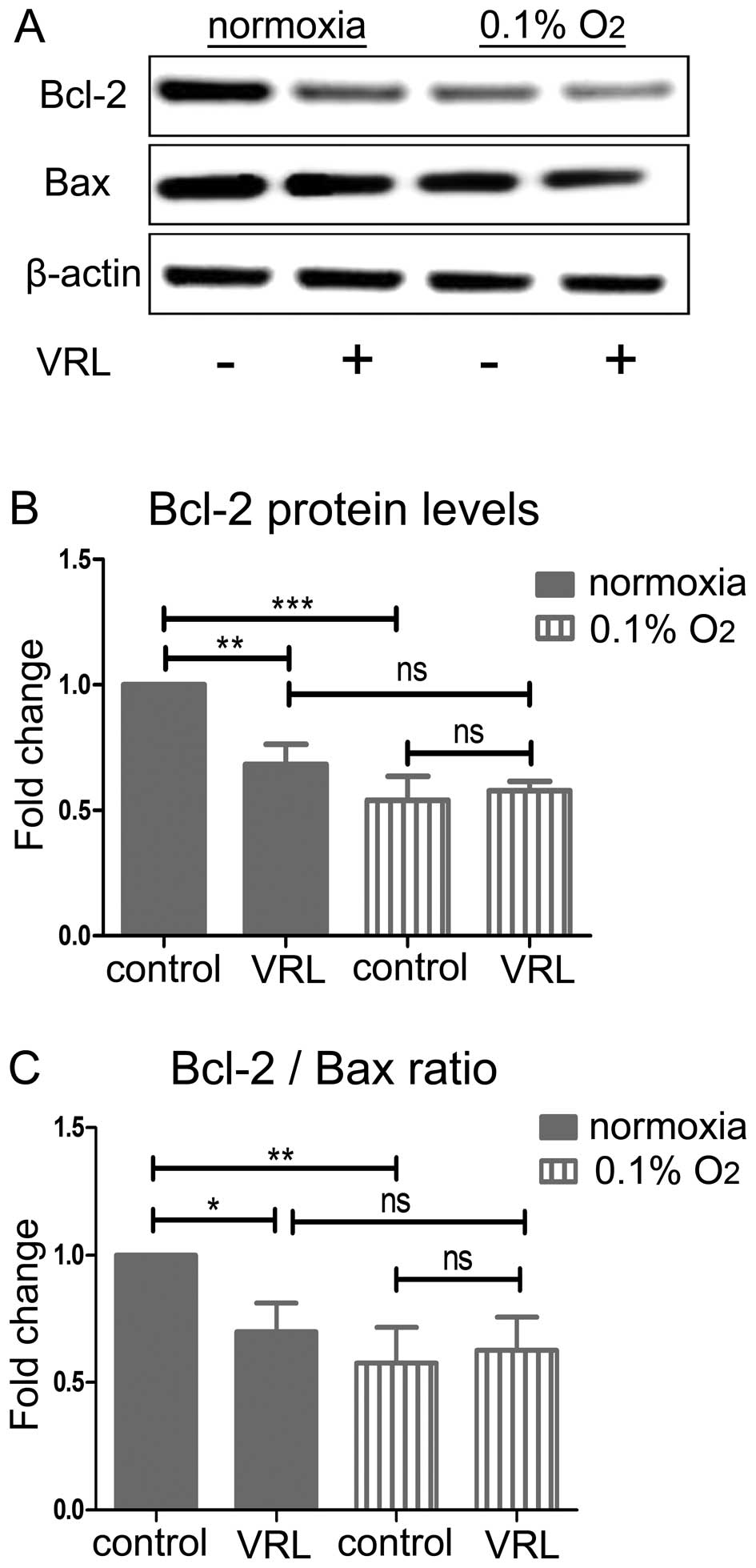
Ten nanomolar vinorelbine fails to
regulate the Bcl-2/Bax ratio in severe hypoxia
To determine the mechanism of protection from the
pro-apoptotic action of metronomic vinorelbine we investigated the
balance of the anti-apoptotic Bcl-2 and pro-apoptotic protein Bax
(Fig. 6A). Bcl-2 and Bax are
players of the intrinsic mitochondrial apoptotic pathway and a low
Bcl-2/Bax ratio leads to apoptotic cell death through mitochondrial
outer membrane permeabilization (22) (MOMP). Moreover, Bcl-2
downregulation has previously been implicated in the cell death
caused by vinorelbine (23). Ten
nanomolar vinorelbine downregulated the anti-apoptotic protein
Bcl-2 in normoxia by 32% (P<0.01) at 24 h. Severe hypoxia also
decreased Bcl-2 protein by 46% (P<0.001) but 10 nM vinorelbine
did not further reduce Bcl-2 under these conditions (Fig. 6B). Similar changes were seen in the
Bcl-2/Bax ratio (Fig. 6C). In
particular, 10 nM vinorelbine decreased the Bcl-2/Bax ratio by 30%
(P<0.05) in normoxia at 24 h, which is consistent with induction
of apoptosis. Severe hypoxia decreased the Bcl-2/Bax ratio by 42%
(P<0.01) while 10 nM vinorelbine did not have an additional
effect.
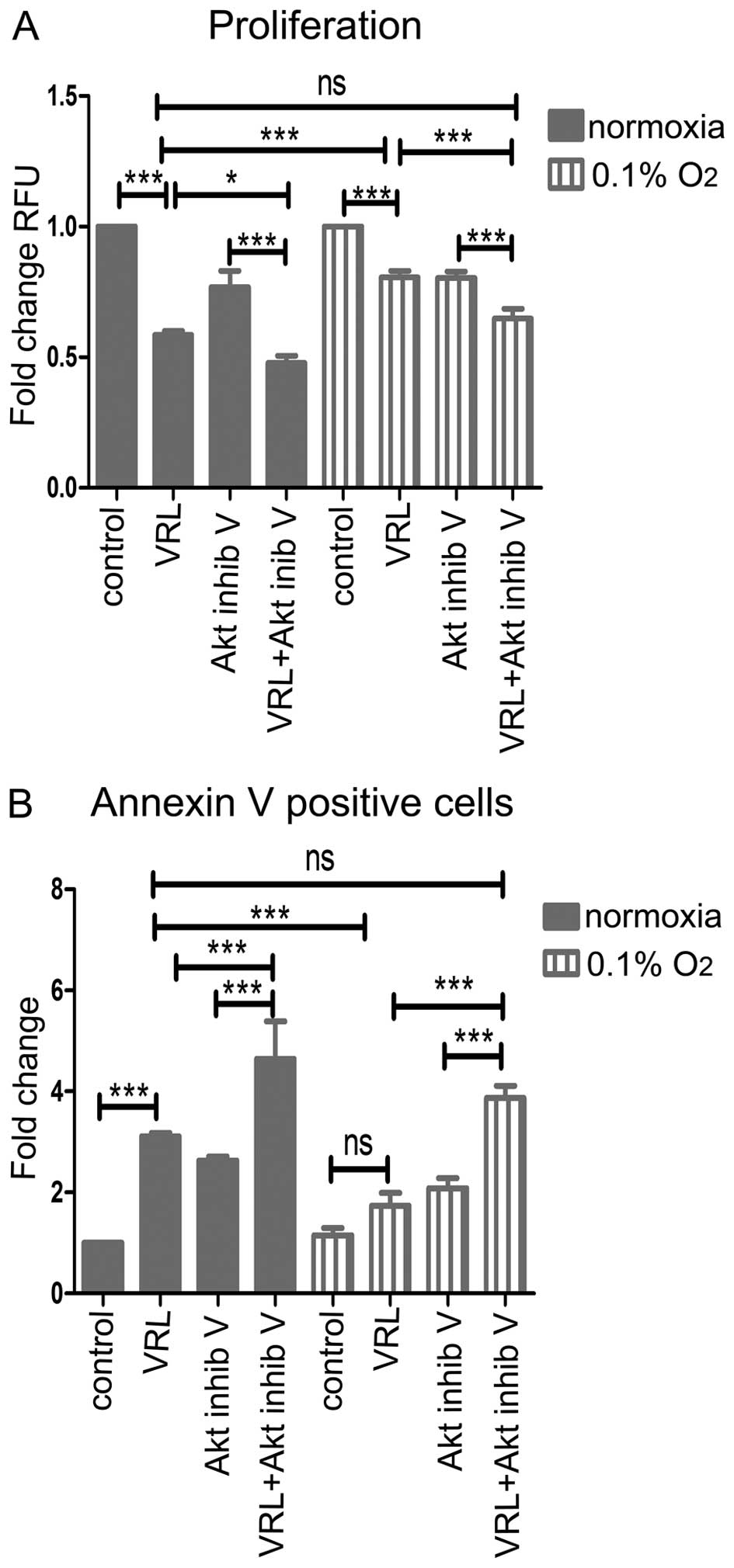
Akt inhibition sensitizes hypoxic
endothelial cells to the anti-proliferative and pro-apoptotic
action of metronomic vinorelbine
To circumvent the counterproductive effect of severe
hypoxia, we examined Akt inhibition as a possible means to reverse
hypoxic resistance in vitro.
Akt inhibition increased the anti-proliferative
effect of metronomic vinorelbine in severe hypoxia (Fig. 7A). In particular, 10 nM vinorelbine
plus Akt inhibitor V inhibited proliferation more potently than 10
nM vinorelbine alone (35% versus 20% inhibition, P<0.001) after
24-h treatment. Moreover, Akt inhibition in severe hypoxia restored
the anti-proliferative effect of vinorelbine to normoxic
levels.
Finally, Akt inhibition increased the pro-apoptotic
effect of metronomic vinorelbine in severe hypoxia (Fig. 7B). Ten nanomolar vinorelbine plus
Akt inhibitor V were more effective, by 2.48-fold (P<0.001),
compared to 10 nM vinorelbine alone in inducing apoptosis after
36-h treatment. Furthermore, Akt inhibition in severe hypoxia
restored the pro-apoptotic effect of vinorelbine to normoxic
levels.
Discussion
In this study, we demonstrated that the clinically
relevant metronomic concentration of vinorelbine, determined in
previous clinical trials (4,5),
inhibited endothelial cell proliferation. The prolonged treatment
with 10 nM metronomic vinorelbine was superior to the short-term
treatment with 1 μM. Given that 1 μM vinorelbine approximates the
transient peak plasma levels of the drug in pharmacokinetic studies
of conventional chemotherapy (18), the in vitro short exposure
to 1 μM could simulate the pulsatile administration of a maximum
tolerated dose. The fact that a treatment that simulates the
chronic low dose chemotherapy in vitro had greater effect
than a treatment that resembles conventional chemotherapy advocates
the use of vinorelbine in a metronomic regimen. These results are
in line with other studies which denote that endothelial cells are
more sensitive to metronomic than conventional chemotherapy.
Bertolini et al demonstrated that the viability of
circulating endothelial progenitors (CEP) in mice is reduced upon
treatment with metronomic cyclophosphamide while treatment with the
maximum tolerated dose increased their number during the drug-free
periods (24). Pasquier et
al reported that immortalized endothelial cells have impaired
ability to form vascular structures and increased sensitivity to
chemotherapy after continuous treatment with non-toxic
concentrations of vinblastine as opposed to prior treatment with a
maximum tolerated concentration (25).
Furthermore, we proved that the metronomic
concentration of vinorelbine is anti-angiogenic in vitro. We
showed that metronomic vinorelbine inhibited critical events of the
angiogenic process in a complete array of angiogenic assays. Apart
from endothelial cell proliferation, we demonstrated that
metronomic vinorelbine inhibited migration and tube formation
whereas the conventional concentration of 1 μM (18) inhibited these functions with
simultaneous induction of cell death. Moreover, we showed that
metronomic vinorelbine inhibited endothelial cell sprouting. Our
results agree with accumulated evidence regarding microtubule
targeting agents (MTAs) (26).
Vinflunine was shown to be anti-angiogenic in vitro at
concentrations that do not affect proliferation (27) and paclitaxel was shown to inhibit
functions of the endothelial cell biology at ultra low
concentrations (28). Our in
vitro evidence on the anti-angiogenic activity of metronomic
vinorelbine is in concert with the clinical evidence provided by
Briasoulis et al (4,5),
where patients that responded to metronomic vinorelbine treatment
expressed low levels of circulating pro-angiogenic biomarkers,
whereas patients that failed to respond expressed markers
associated with resistance to anti-angiogenesis.
Despite promising preclinical data, clinical
practice has shown that even responding patients become eventually
refractory to anti-angiogenic therapy (7). Treatment-induced hypoxia emerges as a
major mechanism of resistance to anti-angiogenic therapy (8). We found that vinorelbine inhibited
migration, tube formation and sprouting to the same extent in
normoxia and severe hypoxia. Interestingly, severe hypoxia did not
have any effect per se on these functions. The latter may be
explained by the fact that we cultivated HUVECs in media
supplemented with growth factors and fetal bovine serum (FBS).
Calvani et al demonstrated that hypoxia enhanced formation
of tube-like structures when they cultured HUVECs in media depleted
from growth factors due to the autocrine action of hypoxia induced
b-FGF (29). We did cultivate
HUVECs in growth media to simulate the complex tumor
microenvironment (6) rather than a
condition-dependent on b-FGF alone. Similarly, Calvani et al
showed that tube formation in hypoxia is comparable to that in
normoxia when HUVECs were challenged with growth factor containing
media (29).
However, we found that severe hypoxia mediated
resistance to the anti-proliferative action of vinorelbine. This in
line with observations from other investigators who showed that
hypoxia mediates resistance to the anti-proliferative action of
chemotherapeutics in cancer cells (10,11).
We demonstrated that severe hypoxia induces G1 arrest in HUVECs.
This is consistent with the upregulation of the cyclin-dependent
kinase (cdk) inhibitor p27kip that we detected. Hypoxia
is suggested to increase p27kip protein which arrests
cells in G1 phase by inhibiting CDK2 activity which prevents entry
to S phase as described by Gardner et al (21). Vinca alkaloids are suggested to
exert their activity by blocking mitosis and arresting cells in
G2/M phase (20). In particular,
vinorelbine suppresses microtubule dynamics and thus disorganizes
the mitotic spindle which fails to congress chromosomes during
mitosis, as reported by Ngan et al (30). The perturbation of this process
blocks the metaphase to anaphase transition (30). In accordance with this, we found
that metronomic vinorelbine induced G2/M arrest in endothelial
cells. However, we show that severe hypoxia attenuated the G2/M
block as it shifted the cells in G1 phase where they are
insensitive to vinorelbine which is a cell cycle specific agent
(20). We therefore propose that
severe hypoxia interferes with the action of metronomic vinorelbine
in blocking mitosis.
Besides altering the cell cycle, we demonstrated
that severe hypoxia lessened the pro-apoptotic action of metronomic
vinorelbine as determined with Annexin V staining. Activation of
the mitochondrial intrinsic pathway and downregulation of the Bcl-2
protein is suggested to be the mechanism of cell killing by
vinorelbine (23). Specifically,
the balance between the pro-apoptotic and anti-apoptotic proteins
of the Bcl-2 family dictates whether the cell undergoes apoptosis
or not (22). Consequently, we
examined the levels of the pro-apoptotic Bax and anti-apoptotic
Bcl-2 which are regulated by vinca alkaloids.
We show that metronomic vinorelbine decreased the
Bcl-2 protein and the Bcl-2/Bax ratio in normoxia that is
consistent with induction of apoptosis. Severe hypoxia decreased
the Bcl-2 protein and the Bcl-2/Bax ratio. Treatment with
metronomic vinorelbine did not further reduce the ratio compared to
the hypoxic control. Therefore, vinorelbine failed to regulate the
Bcl-2 protein in severe hypoxia as opposed to normoxia and this may
account for its decreased pro-apoptotic action. Although Bcl-2 is
anti-apoptotic, some authors suggest that the low Bcl-2 levels
correlate with poor response to microtubule targeting agents.
Esteve et al found that downregulation of Bcl-2 is
associated with resistance of ovarian cancer cells to vinflunine
(31). Moreover, Savry et
al demonstrated that the Bcl-2 overexpression enhances the
efficacy of vinorelbine and paclitaxel in lung and breast cancer
cells through upregulation of Bim (32). Therefore, we speculate that the low
Bcl-2 protein levels in hypoxic endothelial cells may contribute to
poor efficacy of vinorelbine in severe hypoxia.
We sought to find a way to overcome the resistance
in severe hypoxia. We found that the addition of the Akt inhibitor
V increased the sensitivity to the anti-proliferative effect of
vinorelbine in severe hypoxia. Moreover, Akt inhibition enhanced
the pro-apoptotic action of vinorelbine in normoxia and severe
hypoxia and restored the effect of vinorelbine in severe hypoxia to
normoxic levels.
The Akt pathway mediates cell survival through
multiple mechanisms (15). In
particular, it inhibits directly caspase 3 and 9 (33), the pro-apoptotic proteins Bad and
Bax (34) as well as GSK3β
(35). Akt suppresses
functionality of the pro-apoptotic proteins Bad and Bax, rendering
them unable to permeabilize mitochondrial membrane. Furthermore,
Akt inhibition leads to GSK3β activation and subsequently
disruption of hexokinase II (HK II) binding from the mitochondrial
membrane (36). Dissociation of HK
II triggers apoptosis through mitochondrial permeability transition
(MPT) (37) which is a distinct
mechanism of mitochondrial permeabilization that allows
mitochondrial swelling, outer membrane disruption and cytochrome
c (38) release
independently of the presence of Bax and Bak (35). Finally, Akt inactivates caspase 3
and 9 by a posttranslational modification (33). The caspase cascade is the final
step of apoptosis where breakdown of the cell takes place. Hence,
Akt inhibition seems to be a reasonable way to enhance the
sensitivity to apoptotic stimuli as it acts in multiple levels of
the apoptotic process.
In conclusion, we report that the clinically
relevant metronomic concentration of 10 nM vinorelbine is
anti-angiogenic in vitro and we speculate that its clinical
efficacy can be attributed at least in part to anti-angiogenesis.
Severe hypoxia, which is potentially induced by anti-angiogenic
treatment, has a counterproductive effect and can be a factor of
treatment failure. It confers resistance to its anti-proliferative
action by modulating cell cycle and apoptotic cell death. Akt
inhibition appears to be a promising target for combination in
order to circumvent hypoxic resistance and warrants further
investigation.
Acknowledgements
This study was supported by Cancer Research United
Kingdom (CR-UK). We would also like to thank Dr Vasiliki Mavroeidi
for useful discussion and critical reading of this manuscript.
References
|
1
|
Kerbel RS and Kamen BA: The
anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer.
4:423–436. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pasquier E, Kavallaris M and André N:
Metronomic chemotherapy: New rationale for new directions. Nat Rev
Clin Oncol. 7:455–465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pasquier E, Honore S and Braguer D:
Microtubule-targeting agents in angiogenesis: where do we stand?
Drug Resist Update. 9:74–86. 2006. View Article : Google Scholar
|
|
4
|
Briasoulis E, Pappas P, Puozzo C, Tolis C,
Fountzilas G, Dafni U, Marselos M and Pavlidis N: Dose-ranging
study of metronomic oral vinorelbine in patients with advanced
refractory cancer. Clin Cancer Res. 15:6454–6461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Briasoulis E, Aravantinos G, Kouvatseas G,
Pappas P, Biziota E, Sainis I, Makatsoris T, Varthalitis I,
Xanthakis I, Vassias A, et al: Dose selection trial of metronomic
oral vinorelbine monotherapy in patients with metastatic cancer: A
hellenic cooperative oncology group clinical translational study.
BMC Cancer. 13:2632013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bergers G and Hanahan D: Modes of
resistance to anti-angiogenic therapy. Nat Rev Cancer. 8:592–603.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Azam F, Mehta S and Harris AL: Mechanisms
of resistance to antiangiogenesis therapy. Eur J Cancer.
46:1323–1332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loges S, Schmidt T and Carmeliet P:
Mechanisms of resistance to anti-angiogenic therapy and development
of third-generation anti-angiogenic drug candidates. Genes Cancer.
1:12–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cosse JP and Michiels C: Tumour hypoxia
affects the responsiveness of cancer cells to chemotherapy and
promotes cancer progression. Anticancer Agents Med Chem. 8:790–797.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang L, Ao Q, Zhang Q, Yang X, Xing H, Li
F, Chen G, Zhou J, Wang S, Xu G, et al: Hypoxia induced paclitaxel
resistance in human ovarian cancers via hypoxia-inducible factor
1alpha. J Cancer Res Clin Oncol. 136:447–456. 2010. View Article : Google Scholar
|
|
11
|
Raz S, Sheban D, Gonen N, Stark M, Berman
B and Assaraf YG: Severe hypoxia induces complete antifolate
resistance in carcinoma cells due to cell cycle arrest. Cell Death
Dis. 5:e10672014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sersa G, Krzic M, Sentjurc M, Ivanusa T,
Beravs K, Cemazar M, Auersperg M and Swartz HM: Reduced tumor
oxygenation by treatment with vinblastine. Cancer Res.
61:4266–4271. 2001.PubMed/NCBI
|
|
13
|
Zhao D, Jiang L, Hahn EW and Mason RP:
Tumor physiologic response to combretastatin A4 phosphate assessed
by MRI. Int J Radiat Oncol Biol Phys. 62:872–880. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klement G, Baruchel S, Rak J, Man S, Clark
K, Hicklin DJ, Bohlen P and Kerbel RS: Continuous low-dose therapy
with vinblastine and VEGF receptor-2 antibody induces sustained
tumor regression without overt toxicity. J Clin Invest.
105:R15–R24. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rovini A, Savry A, Braguer D and Carré M:
Microtubule-targeted agents: When mitochondria become essential to
chemotherapy. Biochim Biophys Acta. 1807.679–688. 2011.
|
|
17
|
Kennedy SG, Kandel ES, Cross TK and Hay N:
Akt/protein kinase B inhibits cell death by preventing the release
of cytochrome c from mitochondria. Mol Cell Biol. 19:5800–5810.
1999.PubMed/NCBI
|
|
18
|
Marty M, Fumoleau P, Adenis A, Rousseau Y,
Merrouche Y, Robinet G, Senac I and Puozzo C: Oral vinorelbine
pharmacokinetics and absolute bioavailability study in patients
with solid tumors. Ann Oncol. 12:1643–1649. 2001. View Article : Google Scholar
|
|
19
|
Potente M, Gerhardt H and Carmeliet P:
Basic and therapeutic aspects of angiogenesis. Cell. 146:873–887.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gascoigne KE and Taylor SS: How do
anti-mitotic drugs kill cancer cells? J Cell Sci. 122:2579–2585.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gardner LB, Li Q, Park MS, Flanagan WM,
Semenza GL and Dang CV: Hypoxia inhibits G1/S transition through
regulation of p27 expression. J Biol Chem. 276:7919–7926. 2001.
View Article : Google Scholar
|
|
22
|
Chipuk JE and Green DR: How do BCL-2
proteins induce mitochondrial outer membrane permeabilization?
Trends Cell Biol. 18:157–164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bourgarel-Rey V, Savry A, Hua G, Carré M,
Bressin C, Chacon C, Imbert J, Braguer D and Barra Y:
Transcriptional down-regulation of Bcl-2 by vinorelbine:
Identification of a novel binding site of p53 on Bcl-2 promoter.
Biochem Pharmacol. 78:1148–1156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bertolini F, Paul S, Mancuso P,
Monestiroli S, Gobbi A, Shaked Y and Kerbel RS: Maximum tolerable
dose and low-dose metronomic chemotherapy have opposite effects on
the mobilization and viability of circulating endothelial
progenitor cells. Cancer Res. 63:4342–4346. 2003.PubMed/NCBI
|
|
25
|
Pasquier E, Tuset MP, Street J, Sinnappan
S, MacKenzie KL, Braguer D, Andre N and Kavallaris M:
Concentration- and schedule-dependent effects of chemotherapy on
the angiogenic potential and drug sensitivity of vascular
endothelial cells. Angiogenesis. 16:373–386. 2013. View Article : Google Scholar :
|
|
26
|
Schwartz EL: Antivascular actions of
microtubule-binding drugs. Clin Cancer Res. 15:2594–2601. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pourroy B, Honoré S, Pasquier E,
Bourgarel-Rey V, Kruczynski A, Briand C and Braguer D:
Antiangiogenic concentrations of vinflunine increase the interphase
microtubule dynamics and decrease the motility of endothelial
cells. Cancer Res. 66:3256–3263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Lou P, Lesniewski R and Henkin J:
Paclitaxel at ultra low concentrations inhibits angiogenesis
without affecting cellular microtubule assembly. Anticancer Drugs.
14:13–19. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Calvani M, Rapisarda A, Uranchimeg B,
Shoemaker RH and Melillo G: Hypoxic induction of an
HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in
human endothelial cells. Blood. 107:2705–2712. 2006. View Article : Google Scholar
|
|
30
|
Ngan VK, Bellman K, Hill BT, Wilson L and
Jordan MA: Mechanism of mitotic block and inhibition of cell
proliferation by the semisynthetic Vinca alkaloids vinorelbine and
its newer derivative vinflunine. Mol Pharmacol. 60:225–232.
2001.PubMed/NCBI
|
|
31
|
Estève MA, Carré M, Bourgarel-Rey V,
Kruczynski A, Raspaglio G, Ferlini C and Braguer D: Bcl-2
down-regulation and tubulin subtype composition are involved in
resistance of ovarian cancer cells to vinflunine. Mol Cancer Ther.
5:2824–2833. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Savry A, Carre M, Berges R, Rovini A,
Pobel I, Chacon C, Braguer D and Bourgarel-Rey V: Bcl-2-enhanced
efficacy of microtubule-targeting chemotherapy through Bim
overexpression: Implications for cancer treatment. Neoplasia.
15:49–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou H, Li XM, Meinkoth J and Pittman RN:
Akt regulates cell survival and apoptosis at a postmitochondrial
level. J Cell Biol. 151:483–494. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals to the cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Majewski N, Nogueira V, Bhaskar P, Coy PE,
Skeen JE, Gottlob K, Chandel NS, Thompson CB, Robey RB and Hay N:
Hexokinase-mitochondria interaction mediated by Akt is required to
inhibit apoptosis in the presence or absence of Bax and Bak. Mol
Cell. 16:819–830. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pastorino JG, Hoek JB and Shulga N:
Activation of glycogen synthase kinase 3beta disrupts the binding
of hexokinase II to mitochondria by phosphorylating
voltage-dependent anion channel and potentiates
chemotherapy-induced cytotoxicity. Cancer Res. 65:10545–10554.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chiara F, Castellaro D, Marin O,
Petronilli V, Brusilow WS, Juhaszova M, Sollott SJ, Forte M,
Bernardi P and Rasola A: Hexokinase II detachment from mitochondria
triggers apoptosis through the permeability transition pore
independent of voltage-dependent anion channels. PloS One.
3:e18522008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brenner C and Grimm S: The permeability
transition pore complex in cancer cell death. Oncogene.
25:4744–4756. 2006. View Article : Google Scholar : PubMed/NCBI
|