Introduction
Breast cancer is the largest group of malignancies
among women in the Western world (1) and the majority of breast tumors
express estrogen receptors (ER) (2). A common treatment strategy for these
patients is blocking estrogen action by tamoxifen (2–4). One
of the cellular responses of the ER block is cell cycle arrest in
the G1 phase by decreased expression of the cell cycle regulator
cyclin D1 (5). Tamoxifen treatment
is not efficient for all patients and many experience breast cancer
relapse. Endocrine drug resistance may be caused by several
mechanisms, such as elevated levels of co-activating proteins
involved in ER signaling or changes in tamoxifen metabolism
(6–10). Despite extensive research for
proteins involved in tamoxifen resistance, no breakthrough of
markers has been implicated for clinical practice.
The biological significance of the human epidermal
growth factor receptor (HER) 4 is not fully elucidated although it
has been associated with ER expression (11,12)
and favorable outcome of breast cancer (7,13).
Like in other receptor tyrosine kinases, activation results in
downstream signaling through pathways such as phosphoinositide
3-kinase (PI3K)/Akt -and Ras/mitogen activated protein kinase
(MAPK) (14). There are at least
four known isoforms of HER4, due to alternative splicing, having
either juxtamembrane domain JM-a or JM-b (15) connected to the cytoplasmic domains
CYT-1 or CYT-2 (16). These
isoforms give rise to opposing effects in mammary epithelia, making
it challenging to evaluate the clinical relevance (17,18).
Isoforms with the cleavable JM-a domain release an 80-kDa
intracellular region (4ICD/s80) into the cytoplasm where it remains
or is transported to the nucleus (19). The occurrence of 4ICD in the
cytoplasm has been associated to apoptosis, initiated by its
proapoptotic BH3-only domain (20–22)
while nuclear 4ICD acts as a co-activator for ERα stimulated gene
expression (23) and may therefore
promote proliferation of ER-positive cells. At least one cleavable
HER4 isoform (JM-a/CYT-2), shown to increase cyclin D1 in breast
epithelium, cause hyperplasia in mammary epithelia (17). According to current knowledge some
isoforms of HER4 may reverse the effect of tamoxifen thus
decreasing patient survival while on the other hand it is also
hypothesized that tamoxifen disrupts the ERα/4ICD complex in tumor
cells by mitochondrial accumulation of 4ICD and subsequent
apoptosis (20). In the present
study we evaluated the expression and localization of HER4 using
immunohistochemistry and the association to prognosis and clinical
parameters in 912 breast cancer patients randomized to tamoxifen or
to no endocrine treatment. We also examined the effect of estrogen
(β-estradiol, E2) and tamoxifen (4-hydroxy-tamoxifen, 4-OHT) on
HER4 cellular localization in vitro.
Materials and methods
Patients
We analyzed tissue from low risk breast cancer
patients registered in a randomized tamoxifen trial, conducted in
the Stockholm region between 1976 and 1990. This cohort has been
described in detail elsewhere (24). All patients (n=1,780) were female
and postmenopausal at the time of diagnosis. For inclusion, they
were required to have a tumor ≤30 mm with no infiltrating tumor
cells in axillary lymph nodes (N0). The patients were treated
either with modified radical mastectomy or with breast conserving
surgery plus radiation therapy (50 Gy/5 weeks). Patients were
randomized to tamoxifen therapy (40 mg/day) for 2 years (n=886) or
no adjuvant endocrine treatment (n=894). Tamoxifen treatment was
initiated within 2–4 weeks after surgery thus administered
concurrently with radiation therapy. Patients without recurrence
after 2 years were re-randomized to additional 3 years of tamoxifen
therapy, hence a total treatment period of 5 years, or no further
treatment. The mean follow-up period was 17 years and the patient
data were collected through regional population registers including
Swedish Cause of Death Registry. The study was approved by the
ethics committee at the Karolinska Institute and the experiments in
the present study were conducted according to the Declaration of
Helsinki.
Breast cancer tissue microarray
Archival breast tumor tissue from 912 of the 1,780
patients participating in the original study were collected. A
pathologist chose representative tissue from formalin-fixed,
paraffin-embedded breast tumor material as donor block for tissue
microarray (TMA). From each block a section was stained with
hematoxylin and eosin. Further, three morphologically
representative regions from each section were chosen and then
cylindrical cores with a diameter of 0.8 mm were taken and mounted
in a recipient block. For each TMA, cores from liver tissue were
mounted as internal controls. The TMA were constructed using a
manual arrayer (Beecher Instruments, Sun Prairie, WI, USA).
Hormone receptor status and HER2
The tumor data of hormone receptors and HER2 was
obtained from prior studies. In brief, ER status was initially
determined by cytosol assay and isoelectric focusing with a cut-off
level set to 0.05 fmol/μg DNA, according to earlier clinical
routine practice (24). In the
present study, ER data were collected from a re-evaluation using
immunohistochemistry (IHC). Briefly, the Ventana Benchmark system
with prediluted antibodies (anti-ER clone 6F11 and anti-PgR clone
16) was used to determine the ER and progesterone (PgR) status.
Tumors with >10% positively stained nuclei were considered
positive. For cases without immunohistochemical data, results from
cytosolic assay were used. Expression of HER2 was analyzed using
the Dako AO0485 polyclonal rabbit antibody (Dako, Glostrup,
Denmark). The tumor cells were graded (0, 1+, 2+ and 3+) and
patients having a score of 3+ were considered HER2-positive.
HER4 analysis
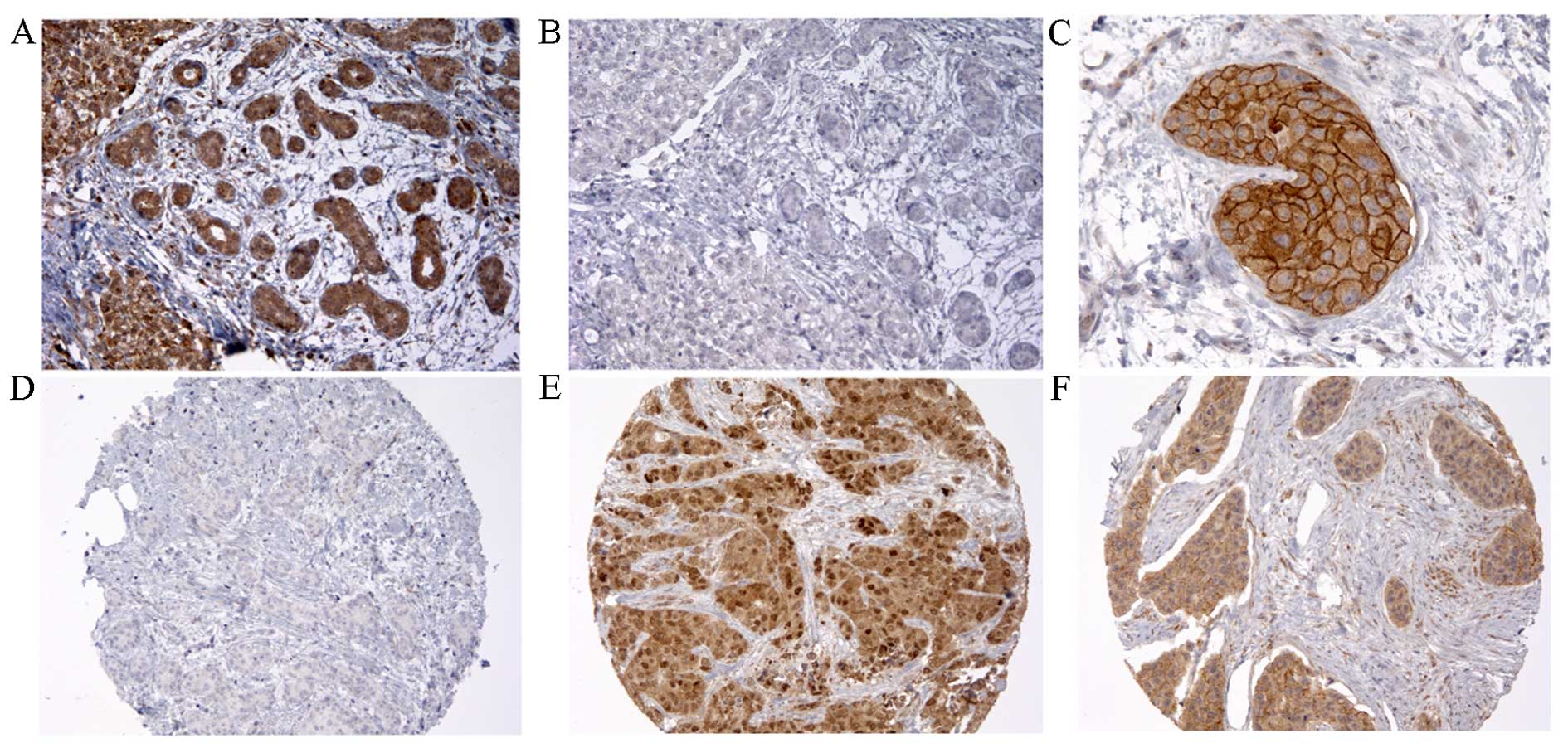
IHC specific monoclonal rabbit anti-HER4 antibody
#4792 (Cell Signaling Technology Inc, Beverly, MA, USA) was used at
dilution of 1:350. According to suppliers, the antibodies do not
cross-react with other EGFR family members. The specificity of the
antibody was evaluated by pre-incubation with a blocking peptide
#1022 (Cell Signaling Technology Inc.) for 1 h in room temperature
(RT) prior incubation of tissue (Fig.
1). In addition, the antibody was successfully tested using
tissue with known HER4 expression and western blotting. The TMA
slides were deparaffinized in xylene and rehydrated in decreasing
series of ethanol followed by MilliQ water. Heat-induced antigen
retrieval was performed in Target Retrieval Solution pH 9.0 (Dako)
for 1 h in a 99°C water bath and then cooled to RT. The slides were
blocked for endogenous peroxidase activity using 3%
H2O2 for 10 min following 1 h treatment in RT
with 5% horse serum diluted in phosphate-buffered saline (PBS). The
slides were incubated with primary antibody at 4°C overnight,
washed and forthcoming detection was made using the EnVision™
system (Dako) with secondary horseradish peroxidase (HRP) polymer
reagent for 20 min at RT and subsequently visualized with
3,3′-diaminobenzidine (DAB). Sections were counterstained with
hematoxylin and mounted. All washing between reactions was
performed using PBS with 0.1% Tween.
TMA evaluation
The TMA slides were examined in Olympus BX41 light
microscope (Olympus Life Science Europe GMBH, Hamburg, Germany)
connected to Leica DFC420 digital microscope camera (Leica
Microsystems, Heerbrugg, Switzerland). Two investigators (A.
Göthlin Eremo and P. Wegman) evaluated the slides independently and
unaware of clinical data and patients outcome, by grading the
staining intensity of cytoplasmic and nuclear HER4 (0, negative; 1,
weak/moderate; 2, strong). The cut-off level for a positive signal
was set at >10% of tumor cells. If results were not consistent
between investigators, a consensus score was set after
re-evaluation.
Cell culture and reagents
To investigate HER4 intracellular response from
estrogen and tamoxifen exposure, three epithelial breast cancer
cell lines were used; MCF7, ZR-75-1 and T-47D (American Type
Culture Collection, Manassas, VA, USA). All three cell lines are
considered ER-positive and in addition, ZR-75-1 cells also express
low levels of HER2 (25). MCF7
cells were cultured in Iscove's modified Dulbecco's medium (IMDM)
complemented with 10% FBS and 0.25% insulin. ZR-75-1 cells were
cultured in RPMI-1640 supplemented with 10% FBS and 2.5% HEPES
buffer. T-47D cells were grown in in RPMI-1640 supplemented with
10% FBS, 2.5% HEPES buffer and 0.25% insulin. All cell culture
media were free from phenol red. In between experiments, cells were
incubated in 75-cm2 culture flasks in a 5%
CO2 humidified atmosphere at 37°C. Cell culture media
and supplements were purchased from Invitrogen, Carlsbad, CA,
USA.
In vitro experiments
For cell experiments, the use of conventional FBS
was changed to 5% charcoal stripped FBS (csFBS) (Invitrogen) to
avoid influence from unknown concentrations of steroid hormone.
Culture conditions and drug concentrations were optimized prior to
experiments. The cells were seeded at a density of 20,000
cells/cm2 in 6-well plates and cultured for 24 h. Cells
were then exposed for 72 h to either 100 nM 4-hydroxytamoxifen
(4-OHT) (Sigma-Aldrich, Stockholm, Sweden), 1 nM β-estradiol (E2)
(Sigma-Aldrich), or both 4-OHT and E2 in combination. E2 and 4-OHT
were dissolved using ethanol (EtOH), hence control cells were
cultured in 0.1% EtOH. After exposure, cells were harvested by
trypsinization, using 0.5% trypsin/EDTA, and centrifuged at 300 × g
for 5 min for following procedures.
Analysis of HER4 and cyclin D1 expression
using quantitative real-time PCR (qPCR)
RNA was extracted, using the RNeasy Plus Micro kit
(Qiagen, Hilden, Germany) according to the manufacturer's protocol.
RNA concentrations were determined using the NanoDrop
Spectrophotometer ND-1000 (NanoDrop Technologies; Thermo Fisher
Scientific, Wilmington, DE, USA) and stored at −80°C until further
use. Later, samples were thawed and diluted with RNase-free water.
A total of 400 ng RNA was reverse transcribed in a 20-μl reaction
using the High Capacity cDNA RT kit (Applied Biosystems, Foster
City, CA, USA), according to the manufacturer's protocol. The cDNA
was synthesized in following conditions; 25°C for 10 min, 37°C for
120 min, 85°C for 5 min and cooled to 4°C by using the MJ Research
PTC-200 Peltier Thermal Cycler (GMI Inc., Ramsey, MI, USA). qPCR
was carried out in a solution containing 7.5 μl (2X)
TaqMan® Fast Advanced PCR Master Mix (Applied
Biosystems), 0.75 μl (20X) TaqMan Gene Expression Assays (Applied
Biosystems) (HER4: Hs00955525_m1, cyclin D1; Hs00765553_m1,
β-actin; Hs99999903_m1, ABL1; Hs01104728_m1), 6 μl RNase-free
H2O and 1.5 μl cDNA with the final volume of 15 μl. The
96-well plates were run, using the C1000 Touch™ Thermal Cycler
(CFX96™ Real-Time System, Bio-Rad, Solna, Sweden) in 50°C for 2
min, 95°C for 20 sec and 95°C for 1 sec and 60°C for 20 sec for 40
cycles. The fold change between expression of the target genes
(HER4 and cyclin D1) and house-keeping genes (β-actin and ABL1) was
obtained using the 2−ΔΔCt method.
Separation of nuclear and cytoplasmic
proteins
In order to analyze HER4 localization in response of
E2 and 4-OHT exposure, nuclear and cytoplasmic proteins were
isolated. Briefly, the cells were re-suspended in 1.5 ml PBS and
centrifuged at 500 × g for 5 min (4°C). The proteins were extracted
and separated through a series of centrifugation steps using
NE-PER® Nuclear and Cytoplasmic Extraction reagents
(Thermo Scientific, Rockford, IL, USA) according to the
manufacturer's protocol. Before use, 10 μl/ml of Protease Inhibitor
Cocktail (Sigma-Aldrich) was added to reagent CER I and NER.
Protein concentrations were determined by spectrophotometry using
DC Protein Assay (Bio-Rad) and 96-well plate reader Multiskan
Ascent (Thermo Labsystems, Helsinki, Finland) according to
instructions.
Western blot analysis of HER4 and cyclin
D1 protein expression
From the nuclear and cytoplasmic cell fractions, 40
μg of proteins were separated by SDS-PAGE using Any kD™
Mini-PROTEAN® TGX™ Precast Gel (Bio-Rad) and
subsequently transferred to Immun-blot™ PVDF (Bio-Rad) membranes.
The membranes were blocked for unspecific binding for 1 h at RT
using 2% ECL Advance Blocking Agent (GE Healthcare,
Buckinghamshire, UK) then incubated with rabbit monoclonal
anti-HER4 antibody #4795 (Cell Signaling) (1:1,000) at 4°C
overnight. For detection, the membranes were incubated with goat
anti-rabbit HRP conjugated antibody #AS09 602 (Agrisera, Vännäs,
Sweden) (1:50,000) for 1 h at RT and visualized using the ECL
Advance Western Blotting Detection kit (GE Healthcare) and ChemiDoc
charge-coupled device (CCD) camera (Bio-Rad) according to the
manufacturer's instructions. Antibodies were stripped off the
membrane by stripping buffer (0.1 M β-mercaptoethanol, 2% SDS, 62.5
mM Tris, pH 6.7) incubation at 50°C for 30 min. From the step of
blocking, the protocol was repeated using rabbit monoclonal
anti-cyclin D1 antibody #2978 (Cell Signaling) (1:1,000), rabbit
polyclonal anti-β actin antibody #ab8227 (Abcam, Cambridge, UK)
(1:2,500) and finally rabbit monoclonal anti-histone H3 antibody
#9717 (Cell Signaling) (1:1,000) for 1 h at RT.
Statistical analysis
To examine the relationship between the levels of
protein expression and tumor characteristics the Pearson's
Chi-square test was used. The differences in recurrence-free
survival were assessed using the log-rank test. Hazard ratios
(relative hazard, HR) with 95% confidence interval (95% CI), were
calculated using univariate and multivariate Cox proportional
hazards regression analysis, which was also used for interaction
tests. In addition, the fold changes in gene expression from qPCR
results were estimated using the 2−ΔΔCt method and the
differences (ΔCt) were tested using one-way ANOVA with Holm-Sidak
test for correction of multiple comparisons. All P-values ≤0.05
were considered significant. The Statistical Package for the Social
Sciences (SPSS) version 17.0 and GraphPad Prism version 6.0d were
used to perform the statistical analyses.
Results
HER4 protein expression and
localization
Protein expression of HER4 was assessed in tumor
tissue from 912 breast cancer patients and scoring was attainable
in 727 cases (79.7%). The distributions of tumor characteristics
for these cases were similar to those available on TMA and to the
original cohort (Table I). For
accessible cases, nuclear and cytoplasmic HER4 were evaluated
separately and graded (Table II).
Tissues with moderate and strong grade of immunoreactivity were
considered HER4-positive (HER4+). Two hundred and
thirty-five (32.3%) tumors were considered as HER4-negative
(HER4−), 28 (3.9%) had exclusively nuclear staining
(HER4N) and 388 (53.4%) had only cytoplasmic staining
(HER4C). Seventy-six patients (10.5%) had both nuclear
and cytoplasmic HER4 (HER4NC) and for 70 cases (9.6%), a
distinct membranous staining was found (Table II).
 | Table IComparison of the distribution of
characteristics for included patients with tumor tissue on TMA,
patients with tumors assessed for HER4 protein expression and the
patients from the original cohort.a |
Table I
Comparison of the distribution of
characteristics for included patients with tumor tissue on TMA,
patients with tumors assessed for HER4 protein expression and the
patients from the original cohort.a
| No. of patients
(%) |
|---|
|
|
|---|
| Patients in present
study (n =912) | Patients assessed
for HER4 expression (n =727) | Original randomized
study (n =1,780) | P-valueb |
|---|
| Estrogen
receptor |
| Positive | 684 (77) | 544 (77) | 1,183 (80) | 0.18 |
| Negative | 200 (23) | 162 (23) | 296 (20) | |
| Unavailable | 28 | 21 | | 301 |
| Progesterone
receptor |
| Positive | 379 (48) | 306 (47) | 590 (48) | 0.86 |
| Negative | 416 (52) | 342 (53) | 627 (52) | |
| Unavailable | 117 | 79 | 563 | |
| Tumor diameter |
| ≤20 mm | 697 (79) | 552 (78) | 1,393 (81) | 0.09 |
| >20 mm | 189 (21) | 159 (22) | 323 (19) | |
| Unavailable | 26 | 16 | 64 | |
| Tamoxifen
treatment |
| Yes | 473 (52) | 369 (51) | 886 (50) | 0.59 |
| No | 439 (48) | 358 (49) | 894 (50) | |
 | Table IIGrades of HER4-staining in different
cellular compartments, with statistical relationship to tumor
characteristics. |
Table II
Grades of HER4-staining in different
cellular compartments, with statistical relationship to tumor
characteristics.
| |
No. of
patients (%) |
|---|
| |
|
|---|
| | Nucleus | | Cytoplasm | | Membraneb | |
|---|
| |
| |
| |
| |
|---|
| Totalc | Negative | Moderate | Strong | P-valuea | Negative | Moderate | Strong | P-valuea | Undefined | Defined | P-valuea |
|---|
| Total | 727 (100) | 623 (85.7) | 98 (13.5) | 6 (0.8) | | 263 (36.2) | 399 (54.9) | 65 | (8.9) | | 657 (90.4) | 70 (9.6) | |
| ER | | | | | | | | | | | | |
| Positive | 544 | 455 (83.6) | 85 (15.6) | 4 (0.7) | 0.004 | 219 (40.3) | 297 (54.6) | 28 (5.1) | <0.0005 | 508 (93.4) | 36 (5.1) | <0.0005 |
| Negative | 162 | 151 (93.2) | 9 (5.6) | 2 (1.2) | | 40 (24.7) | 86 (53.1) | 36 (22.2) | | 128 (79.0) | 34 (21.0) | |
| PgR | | | | | | | | | | | | |
| Positive | 306 | 256 (83.7) | 46 (15.0) | 4 (1.3) | 0.32 | 137 (44.8) | 153 (50.0) | 16 (5.2) | <0.0005 | 294 (96.1) | 10 (3.3) | <0.0005 |
| Negative | 342 | 299 (87.4) | 41 (12.0) | 2 (0.6) | | 104 (30.4) | 193 (56.4) | 45 (13.2) | | 285 (83.3) | 57 (16.7) | |
| Tumor diameter | | | | | | | | | | | | |
| ≤20 mm | 552 | 473 (85.7) | 75 (13.6) | 4 (0.7) | 0.77 | 205 (37.1) | 309 (56.0) | 38 (6.9) | 0.001 | 505 (91.5) | 47 (8.5) | 0.026 |
| >20 mm | 159 | 137 (86.2) | 20 (12.6) | 2 (1.3) | | 55 (34.6) | 78 (49.1) | 26 (16.4) | | 136 (85.5) | 23 (14.5) | |
| HER2 | | | | | | | | | | | | |
| Positive | 77 | 67 (87.0) | 8 (10.4) | 2 (2.6) | 0.19 | 18 (23.4) | 47 (61.0) | 12 (15.6) | 0.008 | 40 (51.9) | 37 (48.1) | <0.0005 |
| Negative | 596 | 511 (85.7) | 81 (13.6) | 4 (0.7) | | 232 (38.9) | 316 (53.0) | 48 (8.1) | | 566 (95.0) | 30 (5.0) | |
| Tamoxifen
treatment | | | | | | | | | | | | |
| Yes | 369 | 310 (84.0) | 56 (15.2) | 3 (0.8) | 0.40 | 140 (37.9) | 200 (54.2) | 29 | (7.9) | 0.43 | 334 (90.5) | 35 (9.5) | 0.50 |
| No | 358 | 313 (87.4) | 42 (11.7) | 3 (0.8) | | 123 (34.4) | 199 (55.6) | 36 (10.1) | | 323 (90.2) | 35 (9.8) | |
Association between HER4 protein
expression and tumor characteristics
Different grades of HER4 staining were associated to
tumor characteristics (Table II).
Higher grades of nuclear expression (HER4N and
HER4NC tumors) were associated to ER-positivity
(P=0.004) and tumors having nuclear HER4 were more often
ER-positive compared to tumors without the presence of nuclear HER4
(HER4C and HER4−-tumors) (P=0.002, OR=2.69,
95% CI=1.40–5.16). Higher grades of cytoplasmic expression
(HER4C and HER4NC tumors) were associated
with poor prognostic factors such as ER-negativity (P<0.0005),
PgR-negativity (P=<0.0005), tumor size >20 mm (P=0.001) and
HER2-positivity (P=0.008). The tumors with defined membranous HER4
staining shared similar associations to poor prognostic factors
(Table II).
The associations between localization of HER4 and
tumor characteristics are shown in Table III where HER4C tumors
correlated negatively to ER (P<0.0005, OR=0.49, 95% CI=
0.33–0.72) and PgR (P<0.0005, OR= 0.54, 95% CI=0.39–0.74) and
were more often HER2-positive compared to HER4N and
HER4NC tumors (P=0.008, OR=2.09, 95% CI=1.20–3.63).
Finally, HER4− tumors were more often HER2-negative than
HER4-positive tumors (Table
III).
 | Table IIIStatistical association between
patients and tumor characteristics and HER4 localization. |
Table III
Statistical association between
patients and tumor characteristics and HER4 localization.
| No. of patients
(%) |
|---|
|
|
|---|
|
HER4− |
HER4N |
HER4C |
HER4NC | P-valuea |
|---|
| Total | 235 (32.3) | 28 (3.9) | 388 (53.4) | 76 (10.5) | |
| ER |
| Positive | 196 (83.4) | 23 (82.1) | 259 (66.8) | 66 (86.8) | <0.0005 |
| Negative | 36 (15.3) | 4 (14.3) | 115 (29.6) | 7 (9.2) | |
| Na | 3 (1.3) | 1 (3.6) | 14 (3.6) | 3 (3.9) | |
| PgR |
| Positive | 124 (52.8) | 13 (46.4) | 132 (34.0) | 37 (48.7) | <0.0005 |
| Negative | 92 (39.1) | 12 (42.9) | 207 (53.4) | 31 (40.8) | |
| Na | 19 (8.1) | 3 (10.3) | 49 (12.6) | 8 (10.5) | |
| Tumor diameter |
| ≤20 mm | 182 (77.4) | 23 (82.1) | 291 (75.0) | 56 (73.7) | 0.91 |
| >20 mm | 50 (21.3) | 5 (17.9) | 87 (22.4) | 17 (22.4) | |
| Na | 3 (1.3) | - | 10 (2.6) | 3 (3.9) | |
| HER2 |
| Positive | 15 (6.4) | 3 (10.7) | 52 (13.4) | 7 (9.2) | 0.035 |
| Negative | 208 (88.5) | 24 (85.7) | 303 (78.1) | 61 (80.3) | |
| Na | 12 (5.1) | 1 (3.6) | 33 (8.5) | 8 (10.5) | |
| Tamoxifen
treatment |
| Yes | 122 (51.9) | 18 (64.3) | 188 (48.5) | 41 (53.9) | 0.35 |
| No | 113 (48.1) | 10 (35.7) | 200 (51.5) | 35 (46.1) | |
Prognostic relevance of HER4 protein
expression
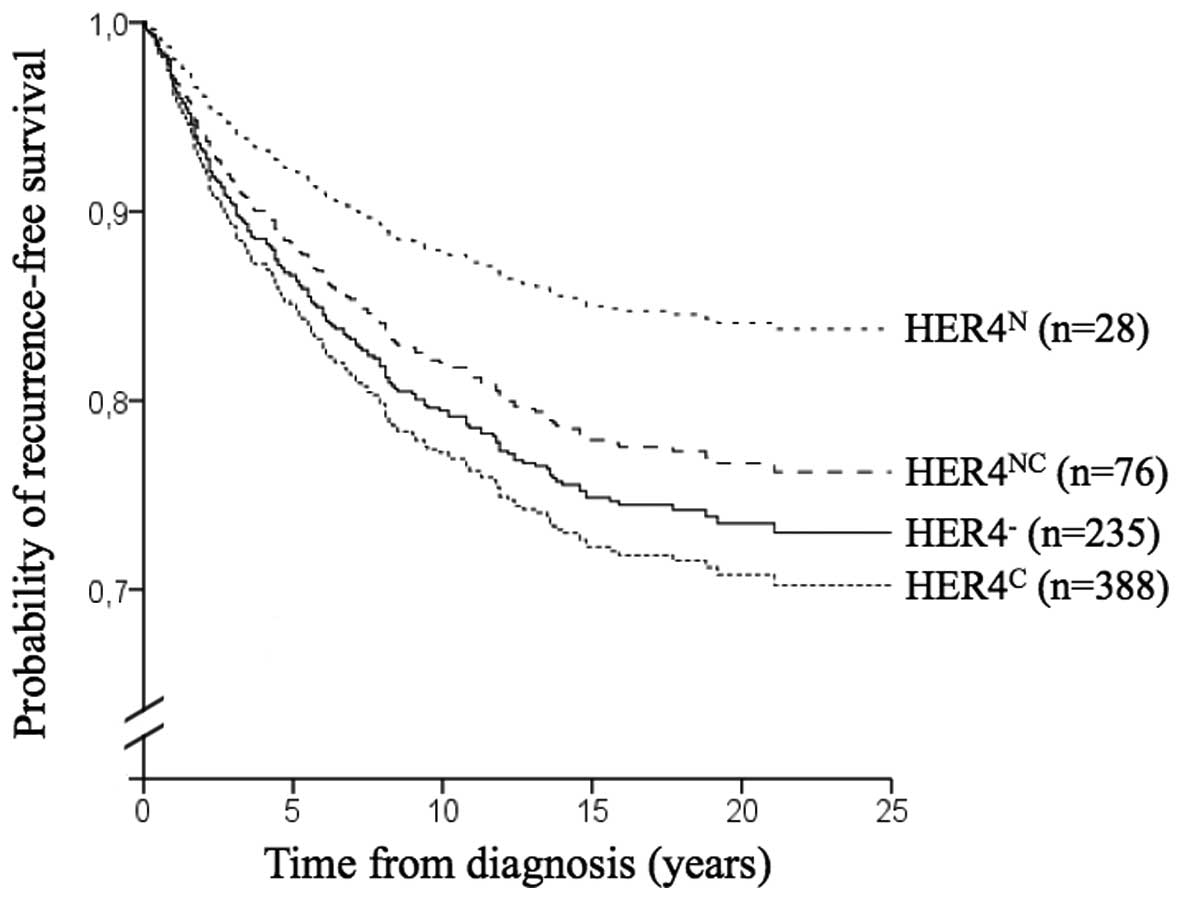
Using the Kaplan-Meier method and log-rank test, no
statistical differences in recurrence-free survival were found in
regard of HER4−, HER4N, HER4C or
HER4NC expression (Fig.
2). For the 70 cases with evident membranous HER4 staining,
recurrence-free survival was shorter than for all cases without
distinguishable membranous staining (P=0.023). Compared to
HER4− cases, the result was no longer significant
(P=0.063). In multivariate analysis including ER, PgR, HER2 and
tumor size, there was no impact of HER4 or HER4 localization on
recurrence-free survival. Multivariate test for interaction
including HER4 membrane showed no additional effect of HER2 on
survival.
HER4 protein expression and prediction of
tamoxifen treatment
Among ER-positive patients treated with adjuvant
tamoxifen there was no significant difference in recurrence-free
survival in regard of HER4 expression. In order to describe
tamoxifen benefit in the ER-positive patients of the present study,
65/361 (18%) of those treated with adjuvant tamoxifen had a
recurrence compared to 101/326 (31%) of those without adjuvant
tamoxifen (log-rank test P<0.0005). When sub-analyzing
ER-positive patients categorized by HER4 expression
(HER4−, HER4N, HER4C or
HER4NC) only HER4− patients showed
significant benefit from tamoxifen treatment (P<0.0005)
[HER4N (P=0.98), HER4C (P=0.058),
HER4NC (P=0.40) and membrane HER4 (P=0.14)].
Multivariate analysis using Cox regression, including ER, PgR, HER2
and tumor size showed no independent predictive significance of
HER4 in regard of tamoxifen treatment.
Analysis of HER4 and cyclin D1 mRNA and
protein expression in vitro
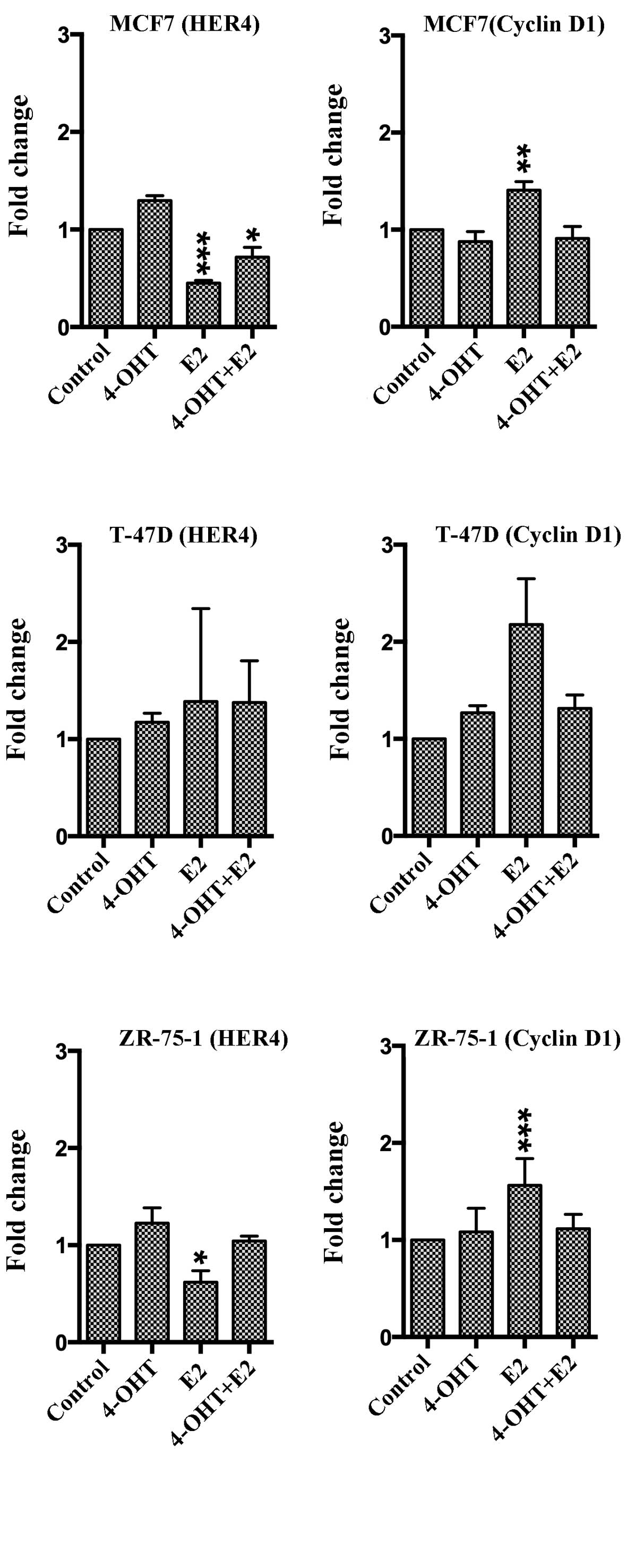
Three different epithelial breast cancer cell lines
were used to study HER4 and cyclin D1 expression after exposure to
4-OHT, E2 and 4-OHT + E2. Cyclin D1 was used to control endocrine
response. After 72-h exposure of 4-OHT, there were no significant
changes in gene expression of HER4 or cyclin D1 in either cell line
(Fig. 3). After exposure to E2,
HER4 mRNA was decreased in MCF7 cells (P=0.0001) and in ZR-75-1
cells (P=0.018) while E2 exposure resulted in increased cyclin D1
mRNA levels in MCF7 cells (P=0.0066) and in ZR-75-1 cells
(P=0.0007). For T-47D cells, there were no significant changes in
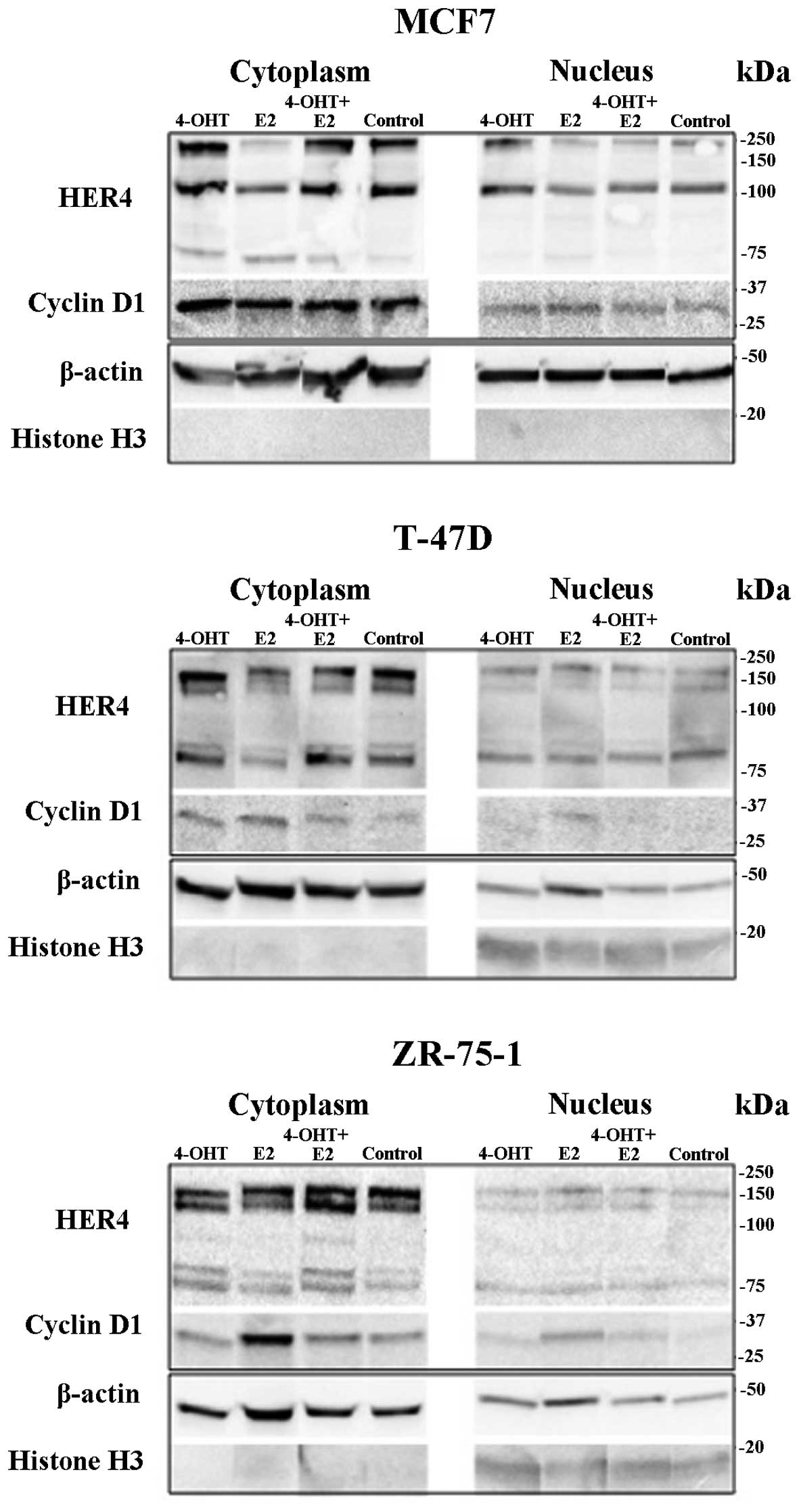
HER4 or cyclin D1 gene expression. As compared to results from
western blot analysis, exposure to 4-OHT resulted in a higher level
of nuclear HER4 (HER4N) in MCF7 cells, whereas
cytoplasmic HER4 (HER4C) was decreased after E2 exposure
in MCF7 cells as well as in T-47D cells (Fig. 4). E2 exposure induced cyclin D1
protein expression in all the cell lines, and the increase was
blocked by co-exposure with 4-OHT.
Discussion
Receptor tyrosine kinases are important for
epithelial growth in mammary tissue, and during carcinogenesis. The
family member HER4 has been suggested to play a role in breast
cancer growth, although opposing reports of HER4 activity has made
it difficult to evaluate its clinical importance. In the present
study, we assessed HER4 protein expression and its prognostic and
predictive relevance in breast cancer patients (n=912) randomized
to adjuvant tamoxifen treatment or no adjuvant endocrine treatment
(24). From the
immunohistochemical staining, HER4 was detected in the cytoplasm
(HER4C), in the nucleus (HER4N) or in both
locations (HER4NC) of tumor cells. In addition, a small
fraction of cases had a tumor with distinct HER4 membrane staining.
Generally, expression of HER4 is associated to ER-positivity and
may predict endocrine responsiveness because of an ERα
co-activating role of 4ICD (11,12,23,26).
We found a significant association between HER4N and
ER-positivity but no association to prognosis or prediction.
Expression of HER4C associated significantly to poor
prognostic markers such as ER-negativity, HER2-positivity,
PgR-negativity and to larger tumors. According to the hypothesis
that HER4C could signify increased 4ICD mitochondrial
access and apoptosis, these patients should have an improved
survival. In a study by Thor et al (22) cytoplasmic 4ICD correlated
positively to tumor cell apoptosis as well as to ER, PgR, and
improved breast cancer prognosis, which is contradictory to our
findings.
The tumors with membranous HER4 may hypothetically
express uncleavable JM-b-isoforms or the receptor may be
inactivated or truncated. Even so, cases with membranous HER4 were
shown to have a shorter recurrence-free survival than cases
without. Strong cytoplasmic staining could mask membranous staining
and we therefore estimated the influence of membrane HER4 in
comparison to HER4− cases, and the difference in
survival was no longer significant. More than half of the tumors
with a defined staining of HER4 membrane were HER2-positive,
raising the question whether HER4/HER2 dimerization occurs in these
tumors. However, co-expression of membrane HER4 and HER2 did not
affect recurrence-free survival according to our interaction
test.
In a more recent breast cancer study by Fujiwara
et al high intensity staining of HER4, or 4ICD as they
report, was observed in the nucleus, cytoplasm or in both (27). In accordance with our study, they
declare an association between cytoplasmic 4ICD and HER2-positivity
as well as PgR-negativity. Fujiwara et al did not find any
association of nuclear 4ICD to ER but to small tumors (≤20 mm), a
marker of good prognosis. In their cohort and among patients with
endocrine treatment, high nuclear HER4 expression (compared to low)
was significantly correlated to increased recurrence-free survival.
The tamoxifen treated patients in present study showed no
significant correlations between HER4 and recurrence-free survival
according to our analyses.
The original hypothesis by Naresh et al
stated that tamoxifen interaction to ER obstructs the binding of
4ICD to ER. This could increase 4ICD mitochondrial accumulation and
following apoptosis (20).
Expression of nuclear 4ICD in tumor cells could therefore involve a
mechanism for tamoxifen-induced apoptosis and consequently be an
advantage for tamoxifen-treated patients. HER4 absence has been
associated to tamoxifen resistance (13) and is proposed to predict recurrence
of ductal in situ carcinoma (28). In our results, tamoxifen was most
beneficial for the HER4-negative patients, which according to
Barnes et al is the most unfavorable population (28). The discordance between studies of
HER4 predicting outcome from tamoxifen treatment might be caused by
differential expression of HER4 isoforms. In mammary epithelia and
in breast cancer, the cleavable JM-a isoform is most prominent
(15,27) and according to Muraoka-Cook et
al CYT-2-expressing glands show increased cyclin D1 levels
(17) possibly affecting tumor
growth.
It is important to note that we did not use an
isoform-specific anti-HER4 antibody, however, a specific anti-HER4
antibody directing the carboxy-terminal which harbours 4ICD, the
principally target of interest, was used. In addition to IHC
analysis of HER4 protein in breast cancer tissue, Fujiwara et
al investigated what specific isoforms were expressed using
qPCR (27). They found that HER4
mRNA expression comprised of both CYT-1 and CYT-2 variants and that
CYT-2 was superior in terms of recurrence-free survival. Also, they
found a larger proportion of HER4/CYT-1 in nuclei. Other studies
claimed that overexpressed HER4/CYT-2 causes hyperplasia in mammary
epithelia and CYT-2 translocate to the nucleus more easily than
CYT-1 (17,19). HER4 expression is clearly
influenced by hormones as seen by the association to ER and in
in vitro results. The levels of HER4 mRNA decreased in two
out of three cell lines following E2 exposure. The cyclin D1 mRNA
increased in all cell lines showing that cells are responsive to
estrogenic growth stimulation. Following 4-OHT exposures, no
significant changes in gene expression were detected. One
explanation for the absence of tamoxifen effect could be that
cellular growth in hormone-depleted serum may cause the same effect
as estrogen blocking, thus masking the influence of tamoxifen.
Other researchers have found that HER4 increases in response to
endocrine treatment (such as 4-OHT and fulvestrant) and decreases
in response to E2 (29,30). HER4 is transcriptionally repressed
by estrogen stimulation (30),
explaining why tamoxifen exposure results in increased HER4. The
main reason for the in vitro experiments was to investigate
whether 4ICD locates predominantly to nucleus or cytoplasm
following exposure to E2 and 4-OHT. In the western blot results,
all three breast cancer cell lines revealed an 80 kDa band
corresponding to the size of the cleaved carboxy-terminal product
4ICD. When analyzing contents in isolated cellular compartments,
both HER4C and HER4N decreased after E2
exposure. In line with the qPCR results, cyclin D1 protein
expression increased. There was, however, no visual difference in
nuclear or cytoplasmic protein fractions.
In conclusion, the present study showed no
significance of HER4 expression in breast cancer survival, however,
an association to biological markers related to endocrine response
was evident. Using more sophisticated techniques, future studies
may reveal whether HER4 expression add prognostic or predictive
relevance for breast cancer outcome.
Acknowledgements
This study was supported by grants from
Nyckelfonden, Örebro University Hospital, Sweden and Lions Cancer
Research Foundation, Region Uppsala - Örebro, Sweden and the
Stockholm Cancer Society, Sweden. We thank Christine Pentz (MSc)
for help with cell culturing and Hanna Arnesson (ML) for assistance
with western blot analyses.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Burstein HJ, Temin S, Anderson H, Buchholz
TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Rowden D, Solky
AJ, et al: Adjuvant endocrine therapy for women with hormone
receptor-positive breast cancer: American Society of Clinical
Oncology clinical practice guideline focused update. J Clin Oncol.
32:2255–2269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lumachi F, Brunello A, Maruzzo M, Basso U
and Basso SM: Treatment of estrogen receptor-positive Breast
Cancer. Curr Med Chem. 20:596–604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Davies C, Godwin J, Gray R, Clarke M,
Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, et al;
Early Breast Cancer Trialists' Collaborative Group (EBCTCG).
Relevance of breast cancer hormone receptors and other factors to
the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of
randomised trials. Lancet. 378:771–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kilker RL, Hartl MW, Rutherford TM and
Planas-Silva MD: Cyclin D1 expression is dependent on estrogen
receptor function in tamoxifen-resistant breast cancer cells. J
Steroid Biochem Mol Biol. 92:63–71. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wegman P, Elingarami S, Carstensen J, Stål
O, Nordenskjöld B and Wingren S: Genetic variants of CYP3A5,
CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal
patients with breast cancer. Breast Cancer Res. 9:R72007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghayad SE, Vendrell JA, Ben Larbi S,
Dumontet C, Bieche I and Cohen PA: Endocrine resistance associated
with activated ErbB system in breast cancer cells is reversed by
inhibiting MAPK or PI3K/Akt signaling pathways. Int J Cancer.
126:545–562. 2010. View Article : Google Scholar
|
|
8
|
Lindberg K, Helguero LA, Omoto Y,
Gustafsson JA and Haldosén LA: Estrogen receptor β represses Akt
signaling in breast cancer cells via downregulation of HER2/HER3
and upregulation of PTEN: Implications for tamoxifen sensitivity.
Breast Cancer Res. 13:R432011. View
Article : Google Scholar
|
|
9
|
Normanno N, Di Maio M, De Maio E, De Luca
A, de Matteis A, Giordano A and Perrone F; NCI-Naple Breast Cancer
Group. Mechanisms of endocrine resistance and novel therapeutic
strategies in breast cancer. Endocr Relat Cancer. 12:721–747. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ring A and Dowsett M: Mechanisms of
tamoxifen resistance. Endocr Relat Cancer. 11:643–658. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Knowlden JM, Gee JM, Seery LT, Farrow L,
Gullick WJ, Ellis IO, Blamey RW, Robertson JF and Nicholson RI:
c-erbB3 and c-erbB4 expression is a feature of the endocrine
responsive phenotype in clinical breast cancer. Oncogene.
17:1949–1957. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujiwara S, Ibusuki M, Yamamoto S,
Yamamoto Y and Iwase H: Association of ErbB1-4 expression in
invasive breast cancer with clinicopathological characteristics and
prognosis. Breast Cancer. 21:472–481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guler G, Iliopoulos D, Guler N, Himmetoglu
C, Hayran M and Huebner K: Wwox and Ap2gamma expression levels
predict tamoxifen response. Clin Cancer Res. 13:6115–6121. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kainulainen V, Sundvall M, Määttä JA,
Santiestevan E, Klagsbrun M and Elenius K: A natural ErbB4 isoform
that does not activate phosphoinositide 3-kinase mediates
proliferation but not survival or chemotaxis. J Biol Chem.
275:8641–8649. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elenius K, Corfas G, Paul S, Choi CJ, Rio
C, Plowman GD and Klagsbrun M: A novel juxtamembrane domain isoform
of HER4/ErbB4. Isoform-specific tissue distribution and
differential processing in response to phorbol ester. J Biol Chem.
272:26761–26768. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elenius K, Choi CJ, Paul S, Santiestevan
E, Nishi E and Klagsbrun M: Characterization of a naturally
occurring ErbB4 isoform that does not bind or activate phosphatidyl
inositol 3-kinase. Oncogene. 18:2607–2615. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muraoka-Cook RS, Sandahl MA, Strunk KE,
Miraglia LC, Husted C, Hunter DM, Elenius K, Chodosh LA and Earp HS
III: ErbB4 splice variants Cyt1 and Cyt2 differ by 16 amino acids
and exert opposing effects on the mammary epithelium in vivo. Mol
Cell Biol. 29:4935–4948. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sundvall M, Veikkolainen V, Kurppa K,
Salah Z, Tvorogov D, van Zoelen EJ, Aqeilan R and Elenius K: Cell
death or survival promoted by alternative isoforms of ErbB4. Mol
Biol Cell. 21:4275–4286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sundvall M, Peri L, Määttä JA, Tvorogov D,
Paatero I, Savisalo M, Silvennoinen O, Yarden Y and Elenius K:
Differential nuclear localization and kinase activity of
alternative ErbB4 intracellular domains. Oncogene. 26:6905–6914.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naresh A, Thor AD, Edgerton SM, Torkko KC,
Kumar R and Jones FE: The HER4/4ICD estrogen receptor coactivator
and BH3-only protein is an effector of tamoxifen-induced apoptosis.
Cancer Res. 68:6387–6395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naresh A, Long W, Vidal GA, Wimley WC,
Marrero L, Sartor CI, Tovey S, Cooke TG, Bartlett JM and Jones FE:
The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein
promoting apoptosis of breast cancer cells. Cancer Res.
66:6412–6420. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thor AD, Edgerton SM and Jones FE:
Subcellular localization of the HER4 intracellular domain, 4ICD,
identifies distinct prognostic outcomes for breast cancer patients.
Am J Pathol. 175:1802–1809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rokicki J, Das PM, Giltnane JM, Wansbury
O, Rimm DL, Howard BA and Jones FE: The ERalpha coactivator,
HER4/4ICD, regulates progesterone receptor expression in normal and
malignant breast epithelium. Mol Cancer. 9:1502010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rutqvist LE and Johansson H: Long-term
follow-up of the randomized Stockholm trial on adjuvant tamoxifen
among post-menopausal patients with early stage breast cancer. Acta
Oncol. 46:133–145. 2007. View Article : Google Scholar
|
|
25
|
Subik K, Lee JF, Baxter L, Strzepek T,
Costello D, Crowley P, Xing L, Hung MC, Bonfiglio T, Hicks DG, et
al: The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and
AR by immunohistochemical analysis in breast cancer cell lines.
Breast Cancer (Auckl). 4:35–41. 2010.
|
|
26
|
Han W and Jones FE: HER4 selectively
coregulates estrogen stimulated genes associated with breast tumor
cell proliferation. Biochem Biophys Res Commun. 443:458–463. 2014.
View Article : Google Scholar :
|
|
27
|
Fujiwara S, Hung M, Yamamoto-Ibusuk CM,
Yamamoto Y, Yamamoto S, Tomiguchi M, Takeshita T, Hayashi M, Sueta
A and Iwase H: The localization of HER4 intracellular domain and
expression of its alternately-spliced isoforms have prognostic
significance in ER+ HER2− breast cancer.
Oncotarget. 5:3919–3930. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barnes NL, Khavari S, Boland GP, Cramer A,
Knox WF and Bundred NJ: Absence of HER4 expression predicts
recurrence of ductal carcinoma in situ of the breast. Clin Cancer
Res. 11:2163–2168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hutcheson IR, Goddard L, Barrow D,
McClelland RA, Francies HE, Knowlden JM, Nicholson RI and Gee JM:
Fulvestrant-induced expression of ErbB3 and ErbB4 receptors
sensitizes oestrogen receptor-positive breast cancer cells to
heregulin β1. Breast Cancer Res. 13:R292011. View Article : Google Scholar
|
|
30
|
Revillion F, Pawlowski V, Lhotellier V,
Louchez MM and Peyrat JP: mRNA expression of the type I growth
factor receptors in the human breast cancer cells MCF-7: Regulation
by estradiol and tamoxifen. Anticancer Res. 23B:1455–1460.
2003.
|