Introduction
Gastric cancer is one of the most common cancers in
the world (1). Up to date, the
castration-resistant gastric cancer has only limited curative
effect and has shown poor prognosis in clinical practice (2). New treatment strategy of targeting
driver pathways provides optional treatment for cancers. For
example, induced robust CD8+ T cell response against
tumor-associated macrophages (TAM) suggested a novel strategy
against breast cancer (3). In
tumor progression, TAM is increased and thereby remodels the tumor
microenvironment which promoted carcinogenesis (4). By secreting growth and proangiogenic
factors, TAM participates in tumor cell proliferation and
metastasis via regulating the function of fibroblasts in the tumor
stroma (5). Recent studies
suggested that anti-TAM effects by small molecule inhibitors could
depress tumor suppression, such as cytotoxic (6), biphosphonate compound (7) and zoledronic acid (7). However, these inhibitors showed low
infiltrate and limited effects on tumor growth. Thus, the
therapeutic targeting of TAM needs to be elucidated and more
inhibitors should be developed.
Vasoactive intestinal peptide (VIP) belongs to a
superfamily of peptides which also includes pituitary adenylate
cyclase-activating polypeptide (PACAP), secretin, and glucagon
(8). In recent studies, VIP was
shown to regulate the production of TNFα, IL-6, IL-12 and iNOS
(8–10). Moreover, VIP also inhibits
expression levels of cyclooxygenase-2 (COX2) and high mobility
group box-1 (HMGB1) in activated macrophages (11,12).
In peritonitis, VIP reduced recruitment of neutrophils, macrophages
and lymphocytes via controlling the expression of transcription
factors including AP-1, CREB and IRF-1 (12–14).
Although inhibiting the expression levels of signaling pathways,
VIP also induces the expression of toll-like receptors (TLRs)
(15,16), indicating the multiple-effects on
the immune system. In macrophages, similar to PACAP, VIP was able
to bind to specific membrane receptors, including PAC1 and VPAC
(17). The receptors of VIP
interact with G proteins, and mediate cAMP-dependent pathway as
well as calcium mobilization, protein kinase C, phosphoinositide
3-kinase (PI3-K) and mitogen-activated protein kinase MEK1/2
pathways (18,19). Thus, the expression of VIP in
organisms plays a crucial role in multiple biological actions
including immunomodulation, muscle relaxation, cell proliferation
and differentiation. Several studies have shown that VIP has
potential effects on increasing vessel formation in a xenograft
model providing insight into VIP treatment in clinical practice
(10,20). Similarly, Vacas and colleagues
indicated that VIP suppresses clear cell renal cell carcinoma by
inducing oxidative stress (9).
Therefore, VIP as deactivator of macrophages may also contribute to
the suppression of TAM. However, the molecular mechanism underlying
this suppression effect of VIP remains poorly understood.
The effects of VIP on expression of TNFα, IL-6,
IL-12 and iNOS in macrophages were reported previously (12). TNFα, IL-6, IL-12 and iNOS are
important regulators and indicators in the process of physiological
and pathological immune system (21–23).
Herein, we hypothesized that VIP may directly interact with TAM in
gastric cancer and modulate the activation of TAM by regulating
TNFα, IL-6, IL-12 and iNOS. The aim of the present study was to
understand the effects of VIP on TAM in gastric cancer and
illustrate the mechanism by which VIP represses the activation of
TAM. For this purpose, the increasing TAM profile in patients and
the depressive effects of VIP on TAM in gastric cancer were
studied. Furthermore, by in vivo and in vitro
experiments, the TNFα, IL-6, IL-12 and iNOS expression levels after
VIP treatment was also assayed.
Materials and methods
Patients
All the samples from gastric cancer were obtained
from Xiangya School of Medicine, Central South University
(Changsha, China). The experiments in the present study were
according to the ethical guidelines of Xiangya School of Medicine
Research Ethics Committee. All the patients signed informed consent
forms and the study was approved by the Hospital Ethics Committee.
In total, 38 patients with gastric cancer were involved in this
study. Tissues were collected during the operation. Tissue adjacent
to the tumors were determined under a microscope as normal control
tissues. The characteristics of the patients were shown in Table I.
 | Table IThe patient characteristics. |
Table I
The patient characteristics.
| Variables | Data |
|---|
| Sample size | 38 |
| Age (years) |
| Median | 53.69 |
| Range | 37–63 |
| Histology |
| Distal | 30 |
| Proximal | 8 |
| Size (mm) |
| <11 | 13 |
| 11–20 | 11 |
| 21–30 | 7 |
| 30–40 | 6 |
| >40 | 1 |
| Histological
grade |
| I | 21 |
| II | 11 |
| III | 6 |
| Stage |
| I | 14 |
| II | 12 |
| III | 7 |
| IV | 5 |
Cell culture and treatment
Human gastric cancer cell line MKN-45 was purchased
from Shanghai Cell Bank, Chinese Academy of Sciences. TAM was
induced from human monocytes THP1 as previously reported (24). Briefly, with 48-h treatment by 320
nmol/l phorbol myristate acetate (Merck Chemical Division, Rahway,
NJ, USA), the suspended cells were transferred into adherent cells.
Then the cells were treated with 20 ng/ml IL-4 and IL-13 for 72 h.
The induced TAM was demonstrated using flow cytometry by the
biomarkers CD14, CD68, CD206 and CD204.
The MKN-45 cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) (Gibco, Gaithersburg, MD, USA)
[supplemented with 10% fetal bovine serum (Gibco)] at 37°C in an
incubator with atmosphere of 5% CO2. For TAM, the medium
was supplemented with 500 U/ml IFN-γ and 100 ng/ml LPS
(Sigma-Aldrich, St. Louis, MO, USA).
To determine the effect of VIP on TAM, cells were
planted at 3×104 cells/ml in 6-well plates (Gibco). The
20 wells were randomly divided into five groups (n=4), including 0,
0.5, 1, 2 and 10 μM VIP supplemented groups. After 48-h culture,
the TAM responses to the VIP were detected by flow cytometry,
proliferation assay and colony formation. The maximal responses of
the VIP concentration was confirmed as 1.0 μM, and used to treat
the TAM in following analysis. Subsequently, to understand the
effects of TAM and VIP in the treated TAM on the gastric cancer
cells, the MKN-45 cells at 3×104 cells/ml were plated in
6-well plates (Gibco). The plated cells were divided into five
groups (n=4), including control, TAM+MKN-45, TAM+VIP (1 μM),
MKN-45+VIP (1 μM) and TAM+MKN-45+VIP (1 μM). The groups which
contained TAM and MKN-45 were co-cultured at concentration of
3×104 cells/ml for each cell type. For the groups with
VIP supplementation, the VIP was added within 48 h after plating.
Subsequently, the cells were analyzed within 96 h. Three
independent experiments were performed.
Real-time PCR
Total RNAs from tissues and cells were isolated
using RNA TRIzol (Invitrogen, Carlsbad, CA, USA). By agarose gel
electrophoresis and BioPhotometer Plus (Eppendorf AG, Hamburg,
Germany), the integrity and amount were assayed. Then 2 μg of total
RNA was reverse transcribed into first cDNA using reverse
transcriptase (Invitrogen) according to the manufacturer's
protocols. The primers of the present study were designed as shown
in Table II. The PCR was
performed on ABI 7500 Real-Time PCR system (Applied Biosystems,
Austin, TX, USA). The conditions were: 95°C for 3 min, 40 cycles at
95°C for 12 sec and 55°C for 40 sec. In this experiment, GAPDH mRNA
is the internal control gene for normalization. Tests without DNA
template were performed as negative control and melt curves were
performed to remove the DNA contamination. The relative expressions
of mRNAs were calculated using 2−ΔΔCt method.
 | Table IIPrimers used for real-time PCR. |
Table II
Primers used for real-time PCR.
| Gene names | Forward primers
(5′→3′) | Reverse primers
(5′→3′) |
|---|
| CD68 |
CGGAATTCTGCTGGGGCTACTGGCAG |
TGATCTAGAGTCCCCTGGGCTTTTGGCAG |
| TNF-α |
GGAGAAGGGTGACCGACTCA |
CTGCCCAGACTCGGCAA |
| IL-6 |
AGCACATTAAGTACATCCTCGGC |
CCAGATTGGAAGCATCCGTC |
| IL-12 |
TGGAGTGCCAGGAGGACAGT |
TCTTGGGTGGGTCAGGTTTG |
| iNOS |
GGATGACTTTCGAGGACATGC |
GGGCCCTCTGGTCATACTTTT |
| GAPDH |
TGCACCACCAACTGCTTAGC |
GGCATGGACTGTGGTCATGAG |
Western blotting
The protein expression levels were assayed using
western blotting. After homogenized in RIPA buffer (50 mM Tris pH
8.0, 150 mM NaCl, 1% NP-40, 0.5% DOC, 0.1% SDS, 1 mM DTT, protease
and phosphatase inhibitors), the protein were isolated and then the
quantity was determined by BCA protein assay kit (Beyotime, Wuhan,
China). For each sample, 20 μg total protein was separated by 10%
dodecyl sulfate polyacrylamide gel. The protein on gel was then
transferred into polyvinylidene fluoride membranes (Millipore,
Billerica, MA, USA). After blocking with 4% skim milk for 1 h at
room temperature, primary polyclonal antibodies of CD68 (IS613,
Dako, Denmark, produced in rabbit, 1:1,000), TNFα (T8300,
Sigma-Aldrich, produced in rabbit, 1:2,000), IL-6 (MFCD00162579,
Sigma-Aldrich, produced in rabbit, 1:1,000), IL-12 (I4153,
Sigma-Aldrich, produced in goat, 1:1,000), iNOS (SAB4502011,
Sigma-Aldrich, produced in rabbit, 1:1,000) and GAPDH (SAB2100894,
Sigma-Aldrich, produced in rabbit, 1:500) were incubated with
membranes at 4°C overnight. All the antibodies were purchased from
Sigma-Aldrich. After 3 times washing in TBST buffer (pH 7.6, 20 mM
Tris-HCl, 137 mM NaCl, 0.01% Tween-20), the second antibodies
(HRP-conjugated anti-rabbit IgG and HRP-conjugated anti-goat IgG,
Sigma-Aldrich, 1:2,000) and enhanced chemiluminescence (ECL,
Millipore) were used to visualized the protein signals.
Immunohistochemical staining (IHC)
Tissues were cut into 6-μm thick sections in
paraffin wax. After de-waxing, the sections were blocked with 4%
skim milk and incubated with primary antibodies (CD68, 1:200; TNFα,
1:200; IL-6, 1:500; IL-12, 1:200; iNOS, 1:200) at 4°C overnight.
Subsequently, the proteins were visualized after the second
antibody incubation at room temperature for 1 h and visualized
using streptavidin-biotinylated horseradish peroxidase complex kit
(Beyotime). The TAM and MKN-45 cells were labeled by antibodies of
FITC-CD68 (1:200) and cy5-MSI1 (1:200) (Bioss Co., China). Each
staining was repeated 3 times.
Colony formation assay
After treatment, 2,000 cells from each group were
plated into 6-well plates and incubated for 7 days at 37°C in an
incubator with atmosphere of 5% CO2. After washing with
phosphate buffer solution (PBS) three times, the cells were fixed
with methanol for 15 min and stained using 0.2% crystal violet for
15 min. The colonies were counted and mean values were calculated
from three independent experiments.
Flow cytometry
To analyze the apoptosis of the cells, we used flow
cytometry (Coulter, Luton, UK) and Annexin V-FITC and propidium
iodide (PI) (BioVision, Milpitas, CA, USA) staining according to
the manufacturer's instructions. At least 30,000 cells for each
sample were treated. The detection of biomarkers of TAM was
performed using antibodies of CD14, CD68, CD206 and CD204. The
cells were fixed using Cytofix/Cytoperm™ Fixation/Permeabilization
solution (BD Biosciences, Franklin Lakes, NJ, USA). The FITC-CD68,
FITC-CD14, PE-CD206, and PE-CD204 antibodies (Sigma-Aldrich) were
incubated with the cells and labeled with PE-conjugated goat
anti-mouse secondary antibody. Triplicate biological repeats were
measured for this experiment.
Tumor formation in a nude mouse
model
We used 5-week-old nude mice to generate the tumor
model. The animal experiments were approved of the ethics
committees of Xiangya School of Medicine, Central South University
(Changsha, China). The tested mice were randomly divided into three
groups (n=10 for each group), including control, VIP and VIP+TAM
group. The mice were first injected with 3×104 MKN-45
cells in 1 ml DMEM. The injections were performed every 2 days for
20 days until the tumor size was 100 mm3. The tumor size
was calculated as V=LxWxDx3.14/6. Subsequently, TAM and TAM+VIP (1
μM) were injected into the tumor tissues. Also, a blank group was
performed by injection with saline. The tumor volumes were measured
every 2 days until 20 days. After 20 days, the tumor tissues were
excised and assayed using real-time PCR, western blotting and
IHC.
Statistical analysis
The data are indicated as mean ± standard deviation
(SD). The difference among the groups were confirmed using
one-way-ANOVA analysis. The significant difference was determined
at P<0.05. All the statistical analysis were performed using
SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
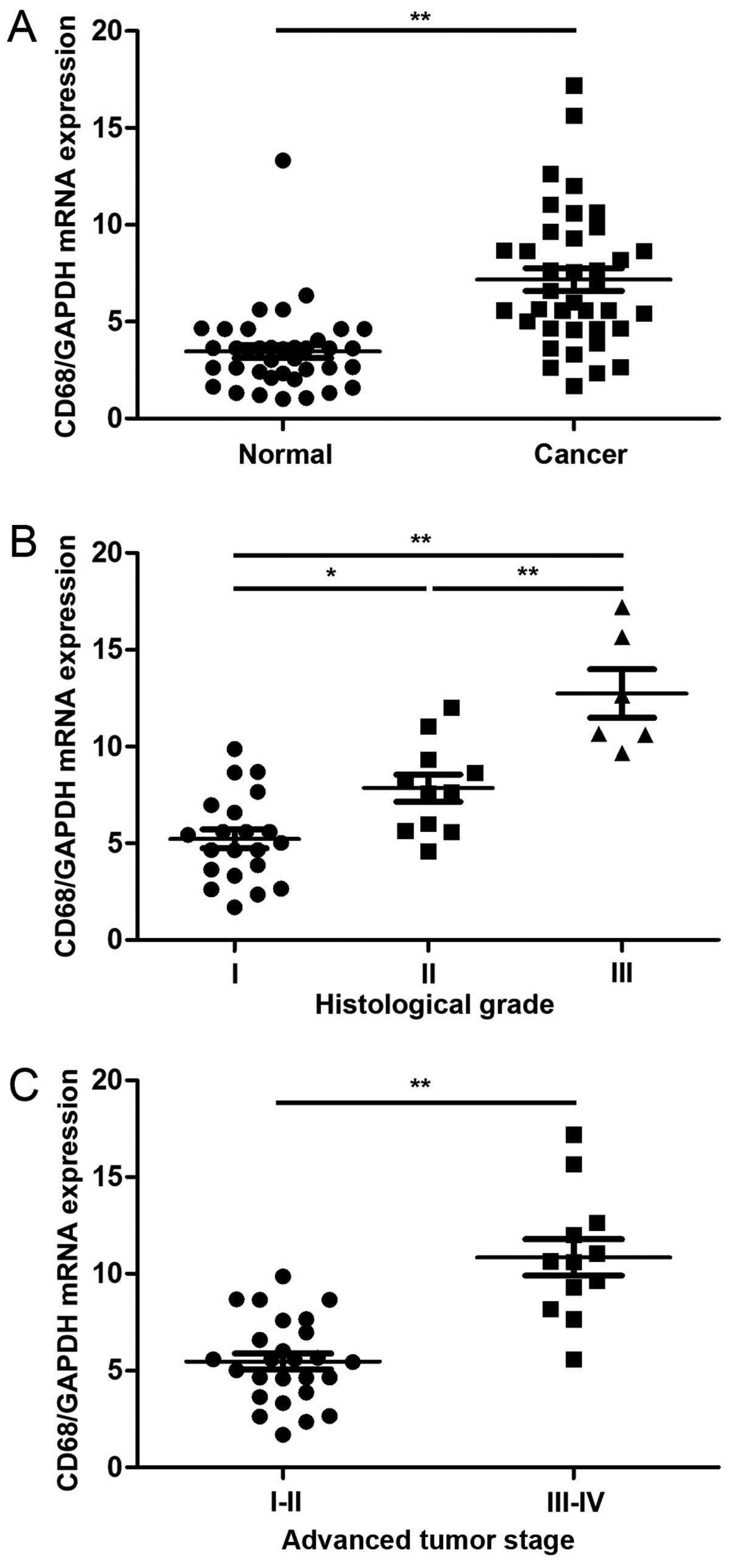
Increasing of TAM in gastric cancer
To assess the TAM level in gastric cancer, the
indicator of TAM, CD68 was detected using real-time PCR. Compared
to the adjacent normal tissues, the expression of CD68 was much
higher in cancer samples (P<0.001, Fig. 1A). High level of CD68 was observed
in the high histological grade (Fig.
1B) and advanced tumor stage (Fig.
1C). The results indicated high levels of TAM in gastric
cancer.
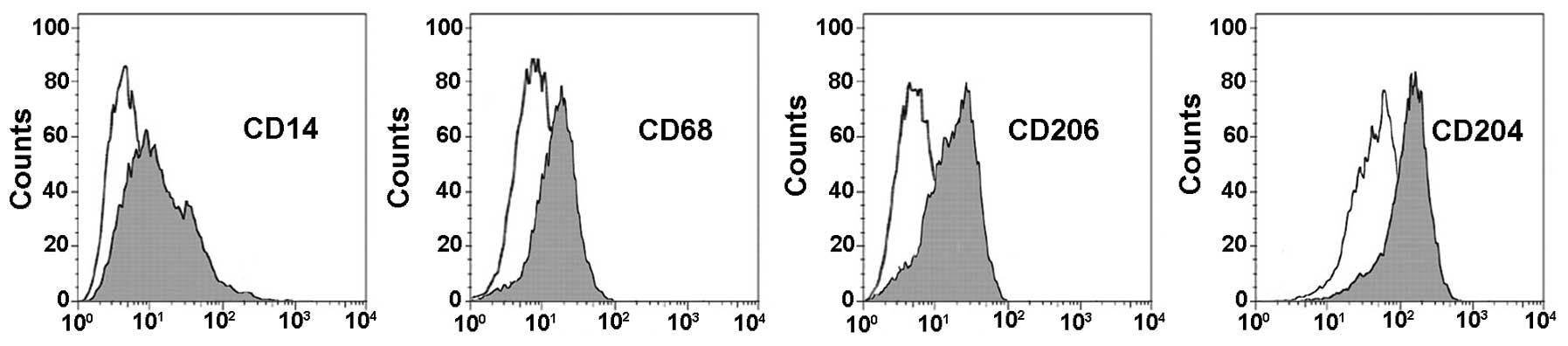
VIP depresses TAM activation
The TAM were induced by THP1 human monocytes. The
flow cytometry analysis of biomarkers including CD14 (marker for
monocyte differentiation), CD68 (marker for macrophages
differentiation), CD206, and CD204 (both markers for M2
macrophages) proved that the TAM were successfully induced
(Fig. 2).
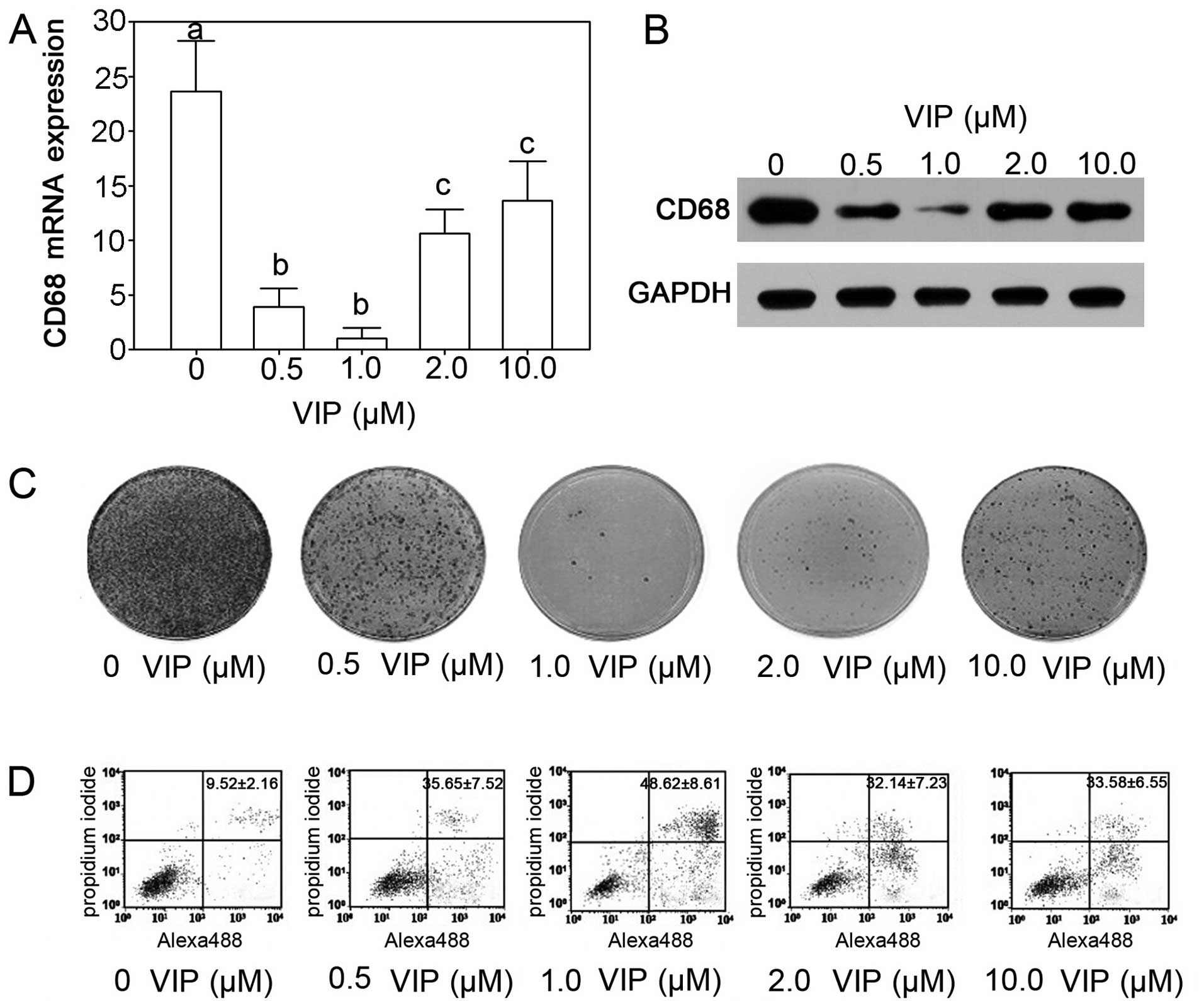
After VIP treatment, the CD68 mRNA levels in TAM
were significantly depressed (P<0.05). The 0.5 and 1.0 μM VIP
treatment showed the most significant effects on expression of CD68
mRNA indicating the inhibition of TAM by VIP (Fig. 3A). The protein expression levels
decreased significantly and was similar to the expression of mRNA.
The 1.0 μM VIP treatment showed the most efficient depressive
effects (Fig. 3B). The colony
formation assay suggested that treatment with 1.0 μM VIP inhibited
growth of TAM (Fig. 3C). By flow
cytometry, we also showed that VIP treatment stimulated the
apoptosis of TAM and 1.0 μM VIP induced apoptosis with the optimal
dosage (Fig. 3D).
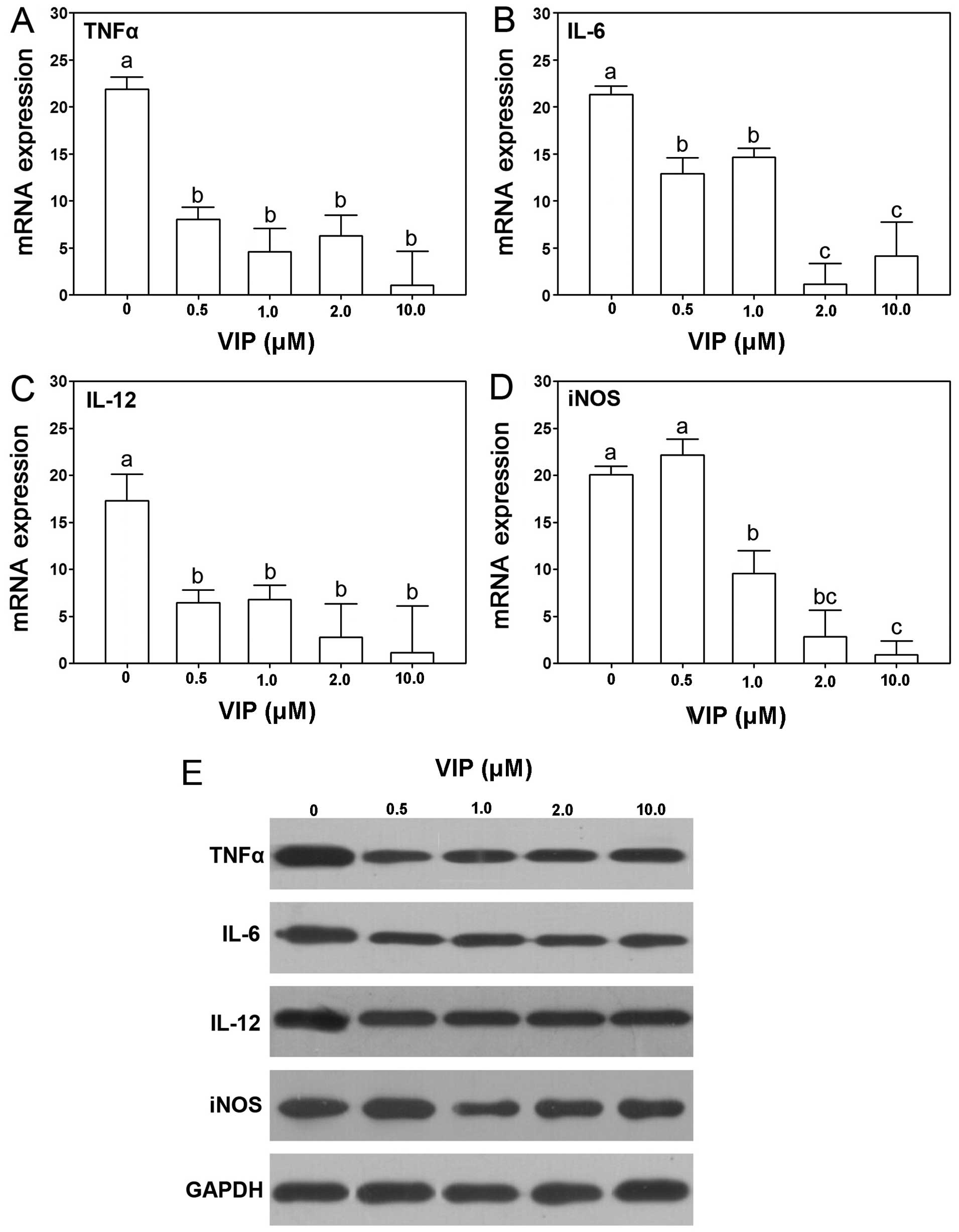
VIP inhibits TNFα, IL-6, IL-12 and iNOS
in TAM
VIP has been shown to depress expression of TNFα,
IL-6, IL-12 and iNOS in macrophages. In the present study, the
effect of VIP on expression of TNFα, IL-6, IL-12 and iNOS in TAM
was determined. The result showed that VIP depressed the expression
of TNFα, IL-6 and IL-12 in all the treatment groups, including 0.5,
1.0, 2.0 and 10.0 μM VIP treatment (Fig. 4A–C). For iNOS, except the 0.5 μM
VIP treatment, the other concentrations of VIP treatment, including
1.0, 2.0 and 10.0 μM VIP, inhibited the expression in TAM
significantly (Fig. 4D). Western
blotting showed similar changes (Fig.
4E). The protein expression of TNFα, IL-6, IL-12 and iNOS was
dcreased after VIP treatment. The 1.0 μM VIP treatment group showed
inhibition of these genes among all the groups, thus, the following
experiment was performed using 1.0 μM VIP in treatment of TAM.
The VIP-treated TAM depressed gastric
cancer cells
To examine the possible effects of VIP on gastric
cancer cells via TAM, TAM were co-cultured with human gastric
cancer cell line MKN-45 and treated with VIP. The co-culture of TAM
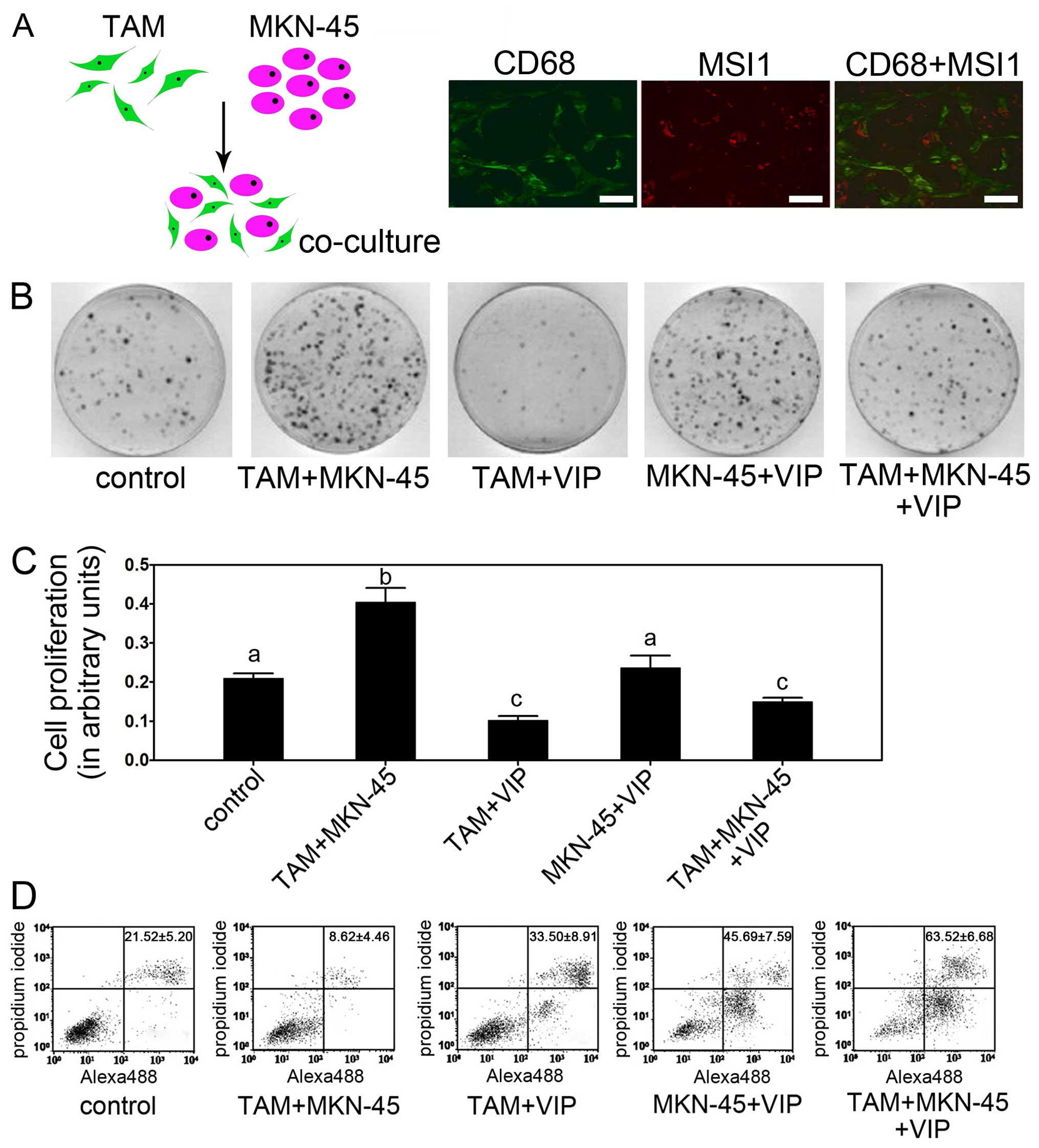
and MKN-45 is shown in Fig. 5A.
The CD68 and MSI1 as specific biomarkers for TAM and MKN-45 were
used to identify the cells. The result showed co-existence of TAM
and MKN-45 (Fig. 5A). Colony
formation assay showed that the VIP-treated TAM remarkably reduced
colony formation of gastric cancer cells (Fig. 5B). Moreover, without TAM, the
MKN-45+VIP group had no significant decrease compared to control,
which indicated the depressive effects of VIP on gastric cancer
cells is indirect and meditated by TAM (Fig. 5B). The proliferation assay showed
that the lowest cell viability of MKN-45 cells was observed in
TAM+VIP and TAM+MKN-45+VIP group (Fig.
5C). The TAM+MKN-45 group had the highest cell viability,
while, other groups showed medial cell viability. Apoptosis
demonstrated by flow cytometry showed similar results.
TAM+MKN-45+VIP group showed the highest apoptosis rate while
TAM+MKN-45 group had the lowest apoptosis rate (Fig. 5D).
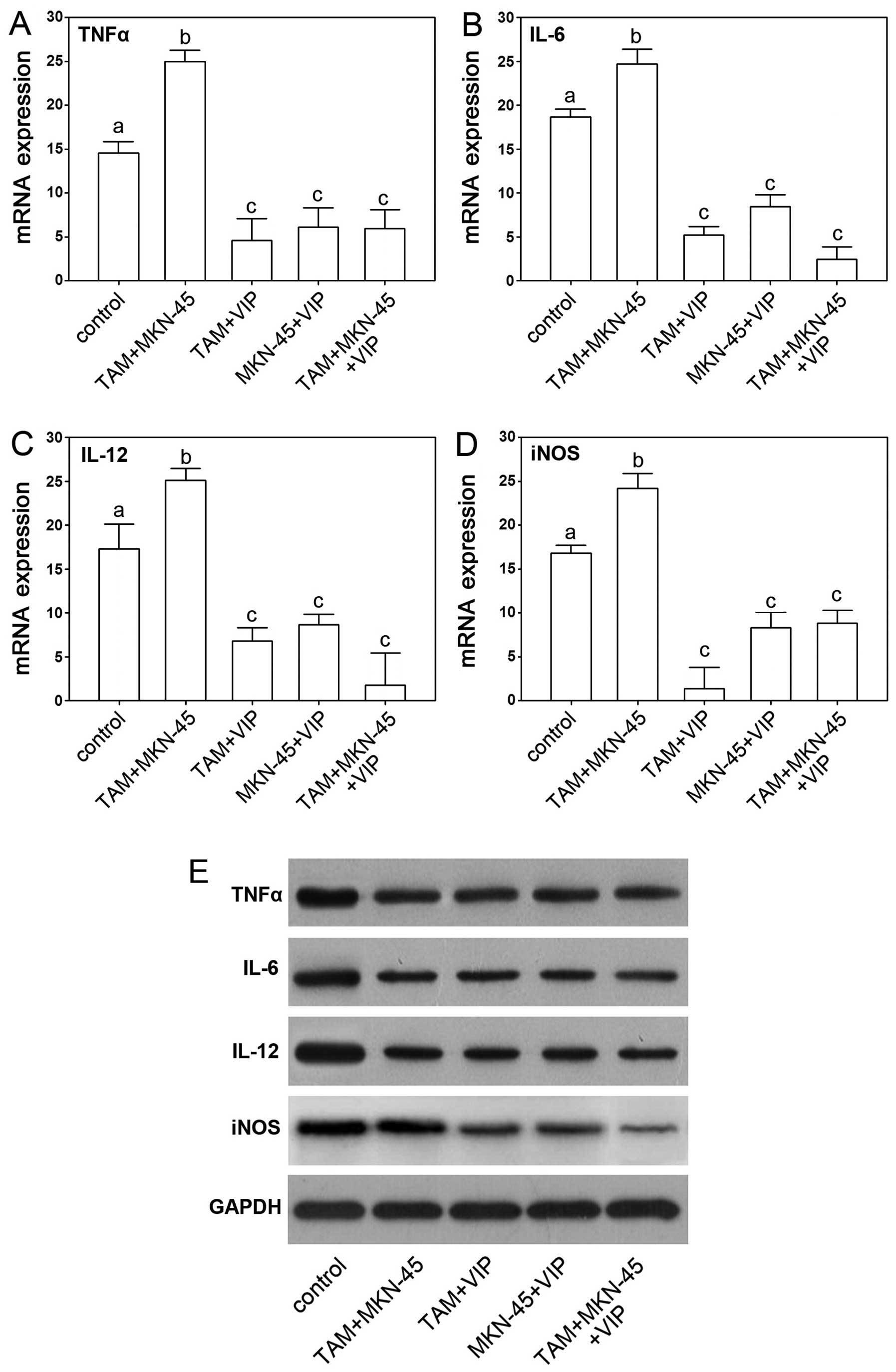
As the VIP depressed activation of gastric cancer
cells via TAM, we next determine if VIP affects gene expression
levels in cultured gastric cancer cells directly or indirectly. VIP
downregulated TNFα, IL-6, IL-12 and iNOS were found in TAM+VIP and
TAM+MKN-45+VIP groups while MKN-45+VIP group showed no significant
difference of expression compared to control, which suggested that
the depressive effects of TNFα, IL-6, IL-12 and iNOS was mediated
by TAM (Fig. 6).
VIP depresses TAM and tumor formation in
the nude mouse model
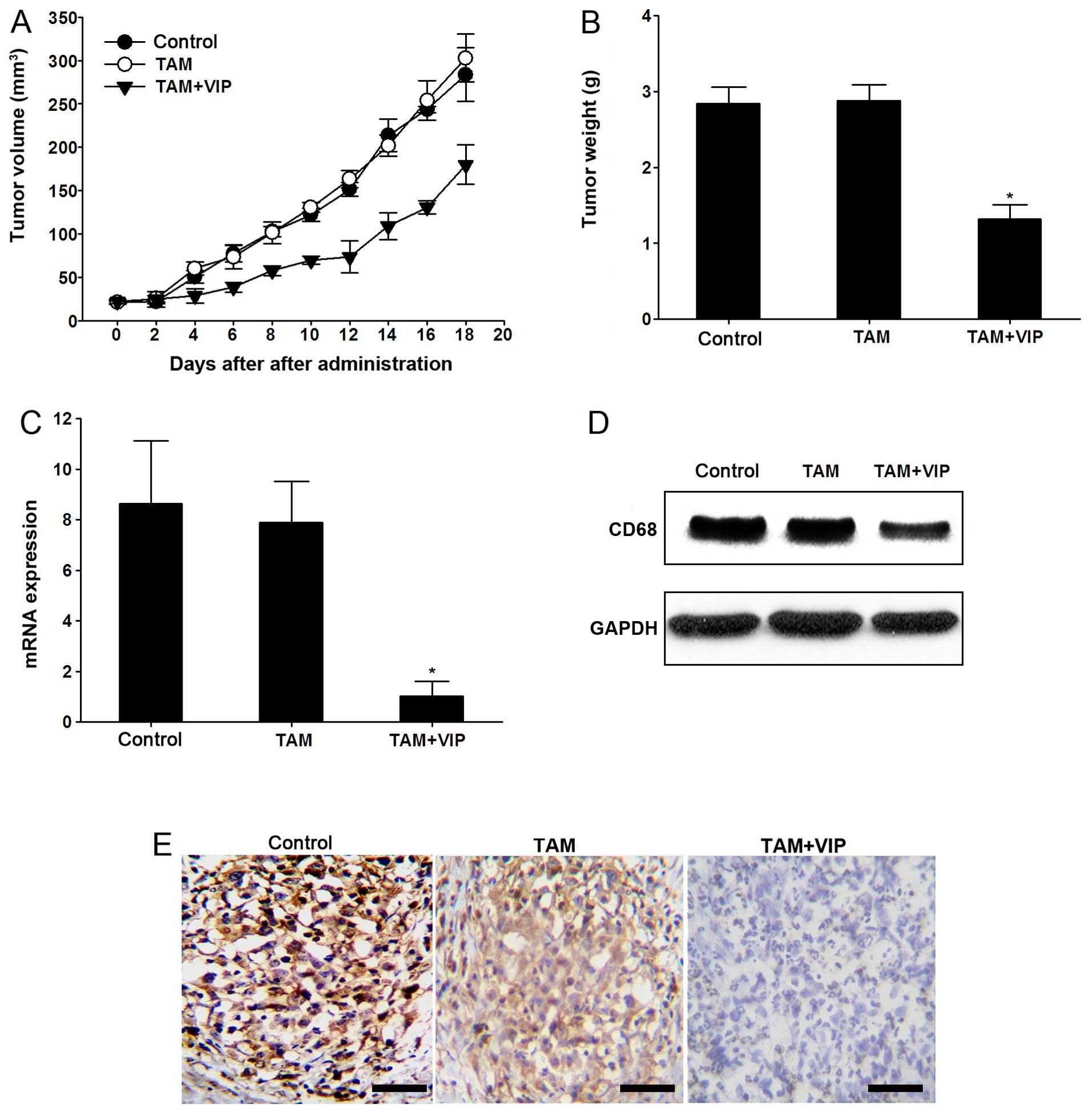
To illustrate the effect of VIP on gastric cancer
in vivo, the tumor formation in the nude mouse model was
constructed by 3×104 MKN-45 cell injections. After tumor
formation, the TAM and TAM+VIP were injected into the tumors. The
tumor volume and tumor weight of TAM+VIP group were significantly
lower compared with the TAM group (P<0.05 after 20 days)
(Fig. 7A and B). The CD68 was
increased accordingly in TAM groups while depressed CD68 was found
in TAM+VIP group indicating the depressive effect of TAM in
vivo (Fig. 7C–E).
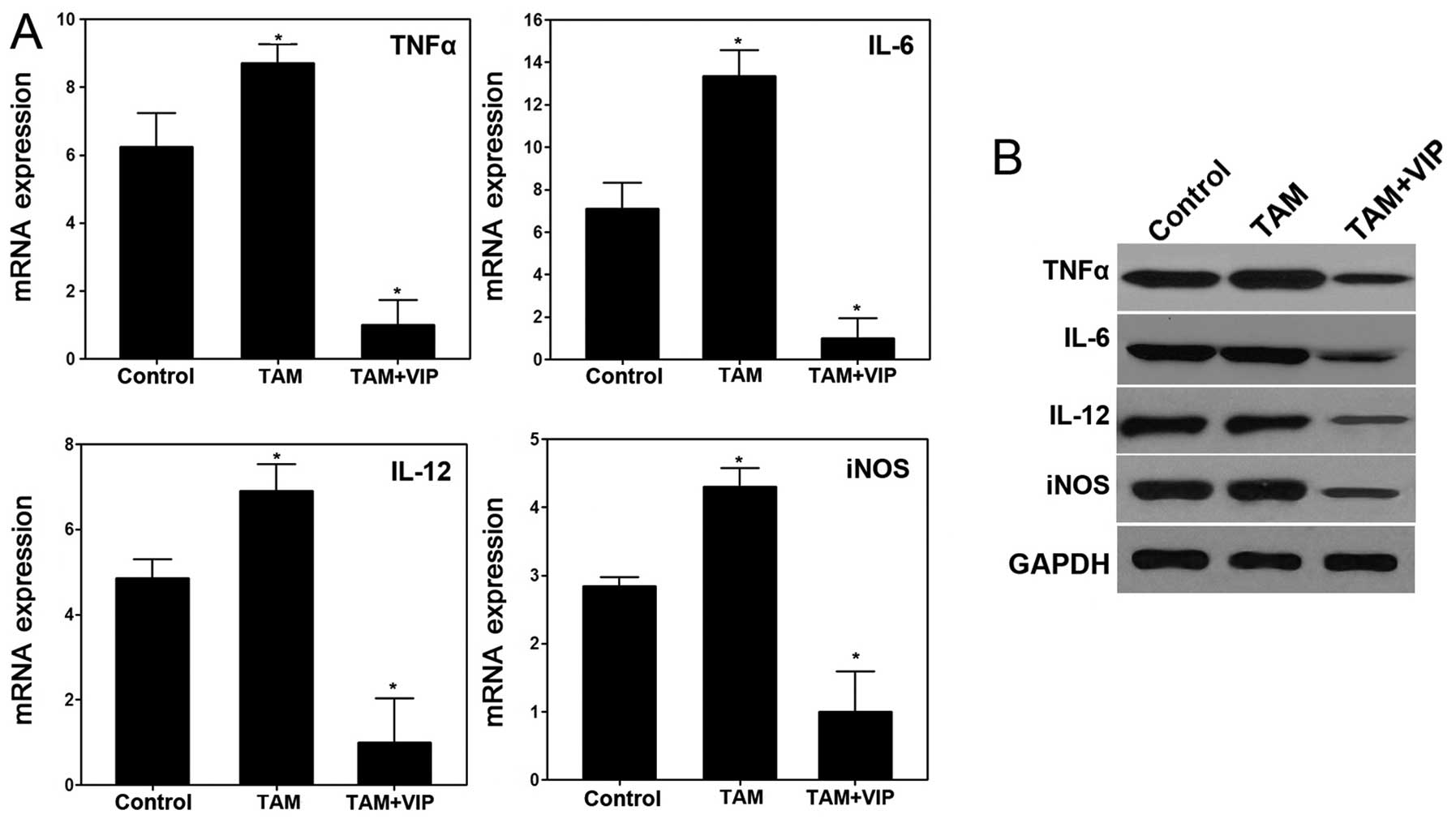
Consistent with the result in vitro, the
expression levels of TNFα, IL-6, IL-12 and iNOS in xenograft tumor
tissues were downregulated by VIP in TAM+VIP group compared with
the TAM group (Fig. 8). Thus, the
results of the xenograft model suggested that VIP inhibits the
tumor progression of gastric cancer mediated by TAM in vivo
via downregulating expression of TNFα, IL-6, IL-12 and iNOS.
Discussion
The present study showed the primary findings on the
VIP depressive effect on TAM. Moreover, the treatment with VIP
inhibits the expression of TNFα, IL-6, IL-12 and iNOS in TAM, which
results in deactivation of TAM. For cancer cells, TAM acts as
mediators for interacting growth factors, cytokines and chemokines
and change the tumor microenvironment to stimulate tumor
progression (25–27). We demonstrated that the VIP
inhibited gastric cancer via TAM both in cultured cells and in the
nude mouse model.
Macrophages originating from blood monocytes are
divided into M1 (classically activated) and M2 types (alternatively
activated) (28). TAM has been
regarded as M2 phenotype and play mostly pro-tumoral functions such
as promoting tumor cell survival, proliferation and invasion
(29). High levels of TAM are
correlated with poor prognosis (30). Simultaneously, CD68 as indicator of
TAM has been used as molecular signature to determine the prognosis
of cancer (31,32). With no surprise, as we found in the
present study, CD68 was highly expressed in gastric cancer tissues
compared to normal tissues showing similar results with previous
reports with higher level of TAM in tumor than normal tissues.
Accordingly, depression of TAM may be a potential therapy for
gastric cancer treatment. Further, we used VIP, a pleiotropic
peptides to intervene in TAM to demonstrate the possibility of VIP
as new therapeutic strategy.
Tumorigenesis as a complex process, results from
molecular and cellular variation with a variety of compounds
including oncoproteins and tumor proteins. During this process, TAM
are the promotional factor for tumorigenesis (33). VIP is a neuropeptide that exerts
multiple actions in different types of cells (8). Several studies showed that the
de-activated function of VIP in macrophages (11,12,34).
As we found in the present study, VIP depressed TAM as well. It is
known that VIP affects the expression of both pro- and
anti-inflammatory factors after LPS and IFNγ induced macrophages
(35,36). In the present study, the treatment
of VIP depressed activities of TAM by suppressing expression of
CD68 and colony formation as well as inducing apoptosis. Based on
these findings, it seems likely that the depressing effect of VIP
on the proliferation of macrophages also exists in TAM. However,
there is still a lack of straight forward answer as to how VIP
suppressed the TAM and whether VIP could inhibit tumorigenesis by
depressing TAM in vivo.
The molecular mechanism by which VIP exerts its
deactivated effects in macrophage is well studied. VIP has been
shown to regulate the expression and/or transactivating of
transcription factors such as AP-1, NFκB, CREB and IRF-1 (13,14,34,36)
and mediate the expression of chemokines, tumor necrosis factors,
COX2, interleukin and toll-like receptors (8,15).
TNFα showed tumor-promoting roles in previous studies and is
regarded as a target in malignant cancer (21,37).
Inhibition of TNFα reduces metastatic activity in tumors (37). The present study showed that VIP
inhibited production of TNFα and the incubation of VIP suppressed
the effects of TAM. These data are consistent with previous studies
showing that VIP reduced the growth of macrophage via regulating
the growth factor, and in macrophages, IL-6, IL-12 and iNOS could
be inhibited by VIP which showed deactivation effects of VIP on
macrophages (8,11,12).
IL-6 and IL-12 are synthesized by macrophages and participate in
inducing antibody secretion, acute phase reaction, hematopoiesis
and regulating production of IFN-γ and TNFα (8). iNOS, also participates in the immune
response by binding to calmodulin and produces NO as an immune
defense mechanism which also indicates the activities of
macrophages (23). The inhibited
expression levels of IL-6, IL-12 and iNOS by VIP showed decreased
activity of TAM. These results indicate that treatment with VIP
inhibits TNFα and IL-6, IL-12 and iNOS of TAM which then results in
depressed cell proliferation.
Our data showed that VIP inhibits progression of
TAM, and the role of TAM as cancer promoter has been demonstrated
previously. This evidence suggests TAM as potential therapeutic
target in human cancer and VIP could be an efficient inhibitor for
TAM. In the present study, VIP is found to inhibit gastric cancer
cells as well as tumor formation in the nude mouse model. We found
the depressive effects of VIP are indirect via first suppressing
TAM. The decreased expression of TNFα and IL-6, IL-12 and iNOS
after VIP treated required co-culture of TAM and MKN-45. In a
previous study, VIP suppressed metastatic human clear cell renal
cell carcinoma by inducing oxidative stress (9). Vacas et al demonstrated that
VIP inhibited invasion and metastasis of human clear cell renal
cell carcinoma via decreasing β-catenin (9). On the contrary, high expression of
VIP in pancreas could induce VIPoma which is a very rare type of
cancer that usually derived from pancreatic cells (38). Thus, the roles of VIP in cancer
progression seem contradictory in different types of cancer which
needs to be elucidated in further study. The results of our present
study showed that the VIP treatment with a proper dosage could
inhibit progression of gastric cancer by deactivating TAM which is
similar to the macrophages by decreasing expression levels of TNFα
and IL-6, IL-12 and iNOS.
In conclusion, we demonstrated that VIP inhibits
progression of gastric cancer mediated by TAM in the present study.
The antitumor action of VIP appears to be initiated by interaction
with TAM via depression of the expression levels of TNFα and IL-6,
IL-12 and iNOS. The presented data provide new insight into the
therapeutic application of VIP to inhibit gastric cancer both in
vivo and in vitro via TAM.
References
|
1
|
Roder DM: The epidemiology of gastric
cancer. Gastric Cancer. 5(Suppl 1): S5–S11. 2002. View Article : Google Scholar
|
|
2
|
Cervantes A, Roda D, Tarazona N, Roselló S
and Pérez-Fidalgo JA: Current questions for the treatment of
advanced gastric cancer. Cancer Treat Rev. 39:60–67. 2013.
View Article : Google Scholar
|
|
3
|
Mukhtar RA, Nseyo O, Campbell MJ and
Esserman LJ: Tumor-associated macrophages in breast cancer as
potential biomarkers for new treatments and diagnostics. Expert Rev
Mol Diagn. 11:91–100. 2011. View Article : Google Scholar
|
|
4
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:49–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Palma M and Lewis CE: Macrophage
regulation of tumor responses to anticancer therapies. Cancer Cell.
23:277–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bingle L, Brown NJ and Lewis CE: The role
of tumour-associated macrophages in tumour progression:
Implications for new anticancer therapies. J Pathol. 196:254–265.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giraudo E, Inoue M and Hanahan D: An
amino-bisphosphonate targets MMP-9-expressing macrophages and
angiogenesis to impair cervical carcinogenesis. J Clin Invest.
114:623–633. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Delgado M and Ganea D: Vasoactive
intestinal peptide: A neuropeptide with pleiotropic immune
functions. Amino Acids. 45:25–39. 2013. View Article : Google Scholar
|
|
9
|
Vacas E, Bajo AM, Schally AV,
Sánchez-Chapado M, Prieto JC and Carmena MJ: Vasoactive intestinal
peptide induces oxidative stress and suppresses metastatic
potential in human clear cell renal cell carcinoma. Mol Cell
Endocrinol. 365:212–222. 2013. View Article : Google Scholar
|
|
10
|
Yang J, Shi QD, Song TB, Feng GF, Zang WJ,
Zong CH and Chang L: Vasoactive intestinal peptide increases VEGF
expression to promote proliferation of brain vascular endothelial
cells via the cAMP/PKA pathway after ischemic insult in vitro.
Peptides. 42:105–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Delgado M and Ganea D: Vasoactive
intestinal peptide prevents activated microglia-induced
neurodegeneration under inflammatory conditions: Potential
therapeutic role in brain trauma. FASEB J. 17:1922–1924.
2003.PubMed/NCBI
|
|
12
|
Delgado M, Robledo G, Rueda B, Varela N,
O'Valle F, Hernandez-Cortes P, Caro M, Orozco G, Gonzalez-Rey E and
Martin J: Genetic association of vasoactive intestinal peptide
receptor with rheumatoid arthritis: Altered expression and signal
in immune cells. Arthritis Rheum. 58:1010–1019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Delgado M and Ganea D: Cutting edge: Is
vasoactive intestinal peptide a type 2 cytokine? J Immunol.
166:2907–2912. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Delgado M and Ganea D: Inhibition of
endotoxin-induced macrophage chemokine production by VIP and PACAP
in vitro and in vivo. Arch Physiol Biochem. 109:377–382. 2001.
View Article : Google Scholar
|
|
15
|
Gomariz RP, Arranz A, Abad C, Torroba M,
Martinez C, Rosignoli F, Garcia-Gómez M, Leceta J and Juarranz Y:
Time-course expression of Toll-like receptors 2 and 4 in
inflammatory bowel disease and homeostatic effect of VIP. J Leukoc
Biol. 78:491–502. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arranz A, Juarranz Y, Leceta J, Gomariz RP
and Martínez C: VIP balances innate and adaptive immune responses
induced by specific stimulation of TLR2 and TLR4. Peptides.
29:948–956. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laburthe M and Couvineau A: Molecular
pharmacology and structure of VPAC Receptors for VIP and PACAP.
Regul Pept. 108:165–173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brenneman DE: Neuroprotection: A
comparative view of vasoactive intestinal peptide and pituitary
adenylate cyclase-activating polypeptide. Peptides. 28:1720–1726.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Voice JK, Dorsam G, Chan RC, Grinninger C,
Kong Y and Goetzl EJ: Immunoeffector and immunoregulatory
activities of vasoactive intestinal peptide. Regul Pept.
109:199–208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Collado B, Carmena MJ, Clemente C, Prieto
JC and Bajo AM: Vasoactive intestinal peptide enhances growth and
angiogenesis of human experimental prostate cancer in a xenograft
model. Peptides. 28:1896–1901. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Balkwill F: TNF-α in promotion and
progression of cancer. Cancer Metastasis Rev. 25:409–416. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wong CK, Ho CY, Ko FW, Chan CH, Ho AS, Hui
DS and Lam CW: Proinflammatory cytokines (IL-17, IL-6, IL-18 and
IL-12) and Th cytokines (IFN-γ, IL-4, IL-10 and IL-13) in patients
with allergic asthma. Clin Exp Immunol. 125:177–183. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aktan F: iNOS-mediated nitric oxide
production and its regulation. Life Sci. 75:639–653. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao
YH, Chu CY, Tsai TF, Chiu HC, Dai YS, Inoue H, et al:
Tumor-associated macrophage-induced invasion and angiogenesis of
human basal cell carcinoma cells by cyclooxygenase-2 induction. J
Invest Dermatol. 129:1016–1025. 2009. View Article : Google Scholar
|
|
25
|
Liu J, Zhang N, Li Q, Zhang W, Ke F, Leng
Q and Wang H, Chen J and Wang H: Tumor-associated macrophages
recruit CCR6+ regulatory T cells and promote the
development of colorectal cancer via enhancing CCL20 production in
mice. PLoS One. 6:e194952011. View Article : Google Scholar
|
|
26
|
Caillou B, Talbot M, Weyemi U,
Pioche-Durieu C, Al Ghuzlan A, Bidart JM, Chouaib S, Schlumberger M
and Dupuy C: Tumor-associated macrophages (TAMs) form an
interconnected cellular supportive network in anaplastic thyroid
carcinoma. PLoS One. 6:e225672011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen JJ, Lin YC, Yao PL, Yuan A, Chen HY,
Shun CT, Tsai MF, Chen CH and Yang PC: Tumor-associated
macrophages: The double-edged sword in cancer progression. J Clin
Oncol. 23:953–964. 2005. View Article : Google Scholar
|
|
28
|
Martinez FO, Sica A, Mantovani A and
Locati M: Macrophage activation and polarization. Front Biosci.
13:453–461. 2008. View
Article : Google Scholar
|
|
29
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khorana AA, Ryan CK, Cox C, Eberly S and
Sahasrabudhe DM: Vascular endothelial growth factor, CD68, and
epidermal growth factor receptor expression and survival in
patients with Stage II and Stage III colon carcinoma: A role for
the host response in prognosis. Cancer. 97:960–968. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Strojnik T, Kavalar R, Zajc I, Diamandis
EP, Oikonomopoulou K and Lah TT: Prognostic impact of CD68 and
kallikrein 6 in human glioma. Anticancer Res. 29:3269–3279.
2009.PubMed/NCBI
|
|
33
|
Solinas G, Germano G, Mantovani A and
Allavena P: Tumor-associated macrophages (TAM) as major players of
the cancer-related inflammation. J Leukoc Biol. 86:1065–1073. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Delgado M, Abad C, Martinez C, Leceta J
and Gomariz RP: Vasoactive intestinal peptide prevents experimental
arthritis by downregulating both autoimmune and inflammatory
components of the disease. Nat Med. 7:563–568. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Delgado M and Ganea D: Inhibition of
IFN-γ-induced janus kinase-1-STAT1 activation in macrophages by
vasoactive intestinal peptide and pituitary adenylate
cyclase-activating polypeptide. J Immunol. 165:3051–3057. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ganea D and Delgado M: Vasoactive
intestinal peptide (VIP) and pituitary adenylate cyclase-activating
polypeptide (PACAP) as modulators of both innate and adaptive
immunity. Crit Rev Oral Biol Med. 13:229–237. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van Horssen R, Ten Hagen TL and Eggermont
AM: TNF-α in cancer treatment: Molecular insights, antitumor
effects, and clinical utility. Oncologist. 11:397–408. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Krejs GJ: VIPoma syndrome. Am J Med.
82B:37–48. 1987. View Article : Google Scholar
|