Introduction
Meningiomas represent the most common benign brain
tumors in adults, with an annual incidence of ~0.0013–0.0078%
(1). It accounts for 13–26% of
primary intracranial tumors and mainly classified into three
histology subtypes: classical (WHO I, >90%), atypical (WHO II,
5–7%) and anaplastic (WHO III, 1–3%) variants (2). Despite a large majority being
classified as benign lesions, the clinical implementation for
aggressive meningiomas (WHO II, III) yield depressing results and
patients with malignant meningiomas rarely achieve cure (3,4).
Therefore, it is urgent to identify new molecular biomarkers that
regulate malignant behavior of meninigomas and predict clinical
outcome of patients with high-grade meninigomas.
High-mobility group nucleosome-binding proteins
(HMGN/NSBP) are a family of ubiquitous nuclear proteins which
participate in various physiological process, including DNA repair,
replication, transcription and recombination (5). HMGN5, a typical member of the HMGN
family, can modulate the cellular epigenetic profile and impact
biological activities (5–7). Recently, aberrant high expression
level of HMGN5 has been found in several malignant tumors,
including bladder, prostate, renal cancers and glioma (8–11).
Importantly, there is a positive association between excessive
expression of HMGN5 and poor clinical outcomes in patients with
glioma (11), suggesting that
increased HMGN5 level may be critical to patient survival.
Furthermore, it has been reported that HMGN5 plays an oncogenic
role in clear cell renal cell carcinomas by promoting cell
proliferation and invasion, and could be utilized as a target for
cancer treatment (8). However, its
expression pattern, biological function, and clinical significance
in meningiomas are still unknown.
In this study, we show that HMGN5 overexpression is
correlated with advanced pathological grade and poorer prognosis.
Specially, we decreased HMGN5 expression in IOMM-Lee and CH157
cells by small interfering RNA (siRNA) to clarify the role of HMGN5
on cell apoptosis, proliferation and invasion in vitro.
Moreover, the effects of knocking down HMGN5 expression on
chemosensitivity to temozolomide (TMZ) in IOMM-Lee and CH157
meningioma cancer cells were also assessed. We believe HMGN5 could
be a novel molecular target for meninigioma therapy in future.
Materials and methods
Tissue samples
The meningioma specimens were obtained with the
approval from the Specialty Committee on Ethics of Biomedicine
Research, PLA General Hospital. Tissue specimens were collected
from 102 patients with complete clinical and follow-up information
who underwent surgery from August 2004 to July 2011 in PLA General
Hospital. The clinical characteristics of the patient cohort are
summarized in Table I. The
selection criteria were as follows: i) complete clinical data; ii)
the subject had a diagnosis of meningioma without history of other
tumors; ii) complete clinical data; iii) the subject underwent
evaluation by enhanced head MRI scans for tumor progression after
surgery at least once every six months.
 | Table ICorrelation between HMGN5
immunoreactivity and clinicopathologic characteristics of
meningioma patients. |
Table I
Correlation between HMGN5
immunoreactivity and clinicopathologic characteristics of
meningioma patients.
| | HMGN5 expression | |
|---|
| |
| |
|---|
| Characteristics | Value | Low | High | P-value |
|---|
| No. of patients | 102 | 57 | 45 | |
| Age, years | | | | 0.12 |
| <60 | 68 | 40 | 28 | |
| ≥60 | 34 | 17 | 17 | |
| Gender | | | | 0.15 |
| Male | 36 | 19 | 17 | |
| Female | 66 | 38 | 28 | |
| Tumor location | | | | 0.18 |
| Convexity | 32 | 16 | 16 | |
| Parasagittal
sinus | 25 | 11 | 14 | |
| Parafalcine | 20 | 12 | 8 | 0.23 |
| Skull base | 25 | 18 | 7 | |
| Tumor size | | | | 0.17 |
| <3 cm | 29 | 16 | 13 | |
| ≥3 cm | 73 | 41 | 32 | |
| Extent of
resection | | | | 0.11 |
| Simpson grade
I | 49 | 26 | 23 | |
| Simpson grade
II | 40 | 23 | 17 | |
| Simpson grade
III | 13 | 8 | 5 | |
| Histological
grade | | | | 0.008 |
| I (classical) | 70 | 45 | 25 | |
| II (atypical) | 18 | 10 | 8 | |
| III
(anaplastic) | 14 | 2 | 12 | |
| Recurrence | | | | 0.004 |
| Negative | 74 | 50 | 24 | |
| Positive | 28 | 7 | 21 | |
Cell culture and transfection
The meningioma cell lines IOMM-Lee, CH157 and
Ben-Men-1 were obtained from the Chinese Academy of Sciences
(Shanghai, China). The three cell lines were grown in MEM medium
with 10% fetal bovine serum and maintained in monolayer culture at
37°C in humidified air with 5% CO2. Cells were
transfected with small interfering RNA (siRNA) that targets HMGN5
(HMGN5 siRNA, Sigma-Aldrich, NM_030763) according to the
manufacturer's protocols. Three different synthetic interfering RNA
(siRNA) sequences were tested for inhibitory activity against HMGN5
expression by transient transfection into HEK293 cells. The most
effective sequence was cloned into the pLVTHM vector. This short
hairpin sequence specific for HMGN5 is 5′-GCAGTTGCTGAAACCAAGC-3′.
Green fluorescent protein (GFP) was used to create non-targeting
GFP-siRNA. Conditioned medium containing lentiviruses was harvested
48 h after transfection of HEK293 cells. This medium was filtered
and used to infect recipient cells in the presence of 8 μg/ml
polybrene. After 2–3 weeks, single independent clones were randomly
isolated and each individual clone was plated separately. After
clonal expansion, cells from each independent clone were tested for
HMGN5 expression by immunoblotting.
Immunohistochemistry and expression
analysis
All sections were incubated in non-immune serum at
4°C overnight in HMGN5 antibody (1:500; Sigma-Aldrich, HPA000511).
The positive percentage and the intensity of staining of cells were
divided as previously described (12). The final score of HMGN5 expression
was the product of the HMGN5 expression rate and intensity (−, 0;
+, 1–3; ++, 4–6; ++, 7–9). For statistical analysis, HMGN5
expression was divided into ‘high’ (++ and +++) vs. ‘low’ (+ and
−).
Cell proliferation assay
Cell viability was determined by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. MTT (5 mg/ml) was added to cells for 4 h at 37°C. Dimethyl
sulfoxide (DMSO) was used to dissolve the formazan crystals
produced from MTT by live cells. The microplate reader (Thermo
Scientific) was used to measure the optical density at 570 nm.
Quantitative RT-PCR analysis
The quantitative RT-PCR analysis for mRNA level was
performed as previously described (12). The PCR were conducted as follows: 5
min at 95°C followed by 40 cycles of 94°C for 30 sec, 50°C for 30
sec and 72°C for 30 sec. The primers for HMGN5, MMP-2, Bcl-2, Bcl-2
(B-cell lymphoma-2), caspase-3, and β-actin were designed as
follows: HMGN5, forward, 5′-GGTTGTCTG CTATGCTTGTG-3′; reverse,
5′-ACTGCTTCTTGCTTGGT TTC-3′. MMP-2, forward,
5′-AGATCTTCTTCTTCAAGGAC
CGGTT-3′;reverse,5′-GGCTGGTCAGTGGCTTGGGGTA-3′. Bcl-2, forward,
5′-CCGGGAGATCGTGATGAAGT-3′; reverse, 5′-ATCCCAGCCTCCGTTATCCT-3′.
Caspase-3, forward, 5′-ATGGAGAACAATAAAACCT-3′; reverse, 5′-CTAGTGAT
AAAAGTAGAGTTC-3′. β-actin, forward, 5′-TGACGTGGAC ATCCGCAAAG-3′;
reverse 5′-CTGGA AGGTGGACAGCG AGG-3′.
Protein extraction and western
blotting
Western blot analysis was conducted as described
previously. All operations were completed on ice at 4°C. The
primary antibodies used were HMGN5 (Sigma-Aldrich, SAB1305759,
1:1,000), Bcl-2 (Sigma-Aldrich, 1:500), caspase-3 (Santa Cruz,
1:1,000), and β-actin (Santa Cruz, 1:1,000). Western blot data were
quantified by normalizing the signal intensity of each sample to
that of β-actin.
Invasion assay
Equal number of untransfected,
HMGN5-siRNA-transfected and GFP-siRNA-transfected cells were plated
onto a 24-well culture plate. Adherent cells in the upper surface
of the filter were removed, and the cells on the lower surface were
fixed with 3.7% formaldehyde. The cells were stained with
hematoxylin and counted. Three independent experiments were
determined for the invasion rate.
Apoptosis assay
Annexin V-FITC apoptosis kit was used to measure
apoptosis according to the manufacturer's instructions
(Immunochemistry Technologies). For each experiment, ≥ 20,000 cells
were analyzed.
Statistical analyses
The data are expressed as mean ± SD for triplicate
determination, and analyzed using Student's t-test. Kaplan-Meier
analysis was used to investigate overall survival (OS) in
meningioma patients. SPSS 16.0 was used to conduct analyses and
P<0.05 was considered statistically significant.
Results
HMGN5 is overexpressed in human
meningiomas and correlates with poor prognosis
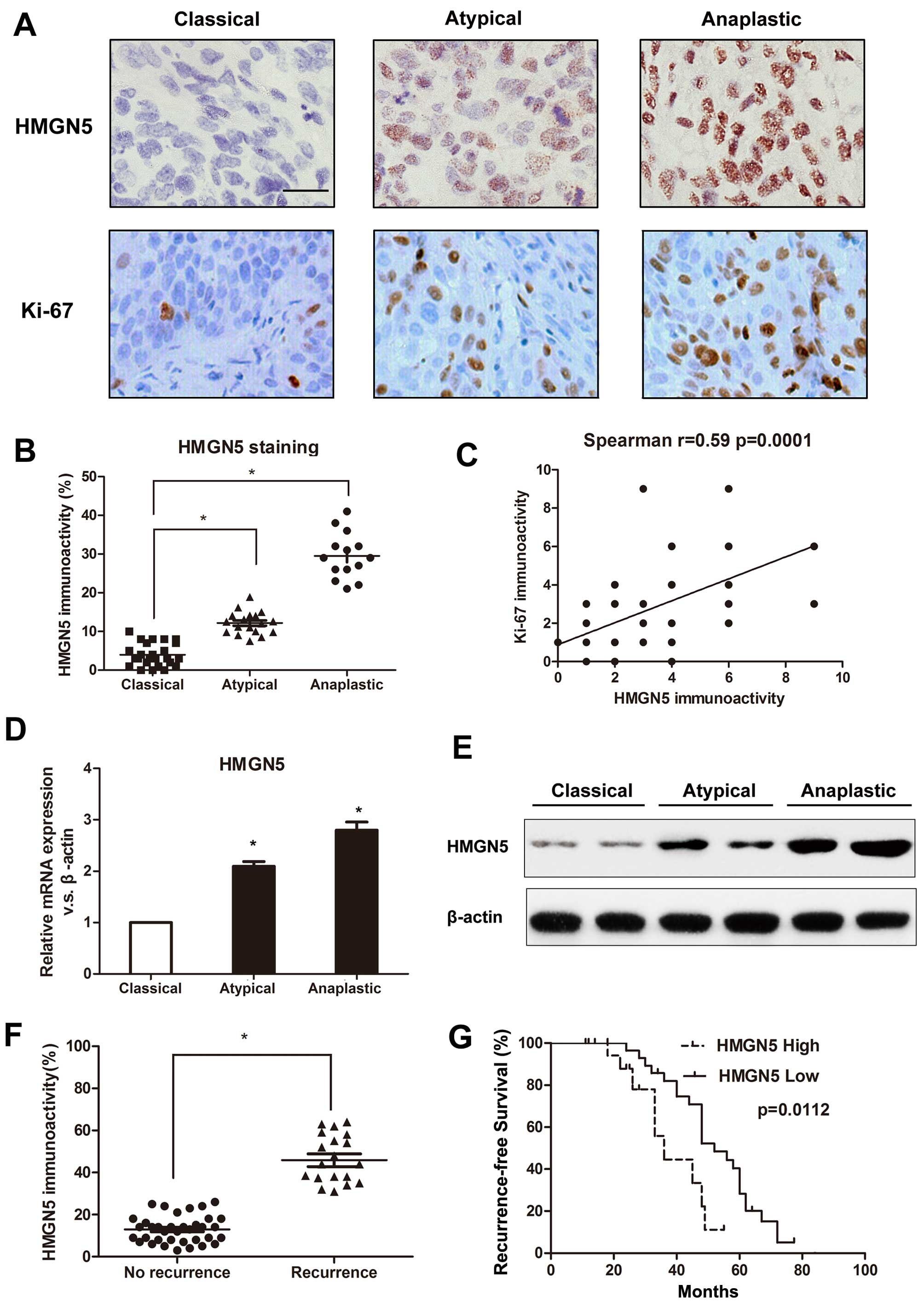
We first used immunohistochemical staining to
measure the expression levels of HMGN5 and Ki-67 in 102 human
meningioma specimens. Advanced meningiomas (WHO grade II and III)
showed greater immunoreactivity for HMGN5 and Ki-67 expressions
compared to classical meningiomas (WHO grade I) (Fig. 1A) and the expression level of HMGN5
correlated with the histological grading (P=0.008) (Fig. 1B). Statistical analyses indicated
that HMGN5 immunolabeling significantly associated with
immunolabeling for Ki-67 (P=0.0001) (Fig. 1C). Consistent with immunostaining
results, atypical and anaplastic meningiomas exhibited dramatically
higher HMGN5 mRNA expression than classical meningiomas (Fig. 1D). Western blotting confirmed the
similar results (Fig. 1E). In
addition, despite the benign tumors having low HMGN5 expression,
aberrant high levels of HMGN5 expression was observed in
meningiomas with recurrence (Fig.
1F), suggesting that HMGN5 expression may induce more
aggressive tumor behavior.
The demographic and clinicopathological
characteristics stratified by high HMGN5 and low HMGN5 are
summarized in Table I. We showed
that HMGN5 expression was positively correlated with histological
grade (P=0.008), and recurrence (P=0.004). However, no significant
associations were found between HMGN5 expression and other
clinicopathological features (Table
I). In addition, Kaplan-Meier analysis revealed that patients
in low HMGN5 expression group had significantly longer
recurrence-free survival than those in high HMGN5 expression group
(P=0.0112) (Fig. 1G). These
results suggested that HMGN5 might be a critical molecular
biomarker that predicts the recurrence of patients with
meningiomas.
Altered HMGN5 expression affects
proliferation and apoptosis in meningiomas
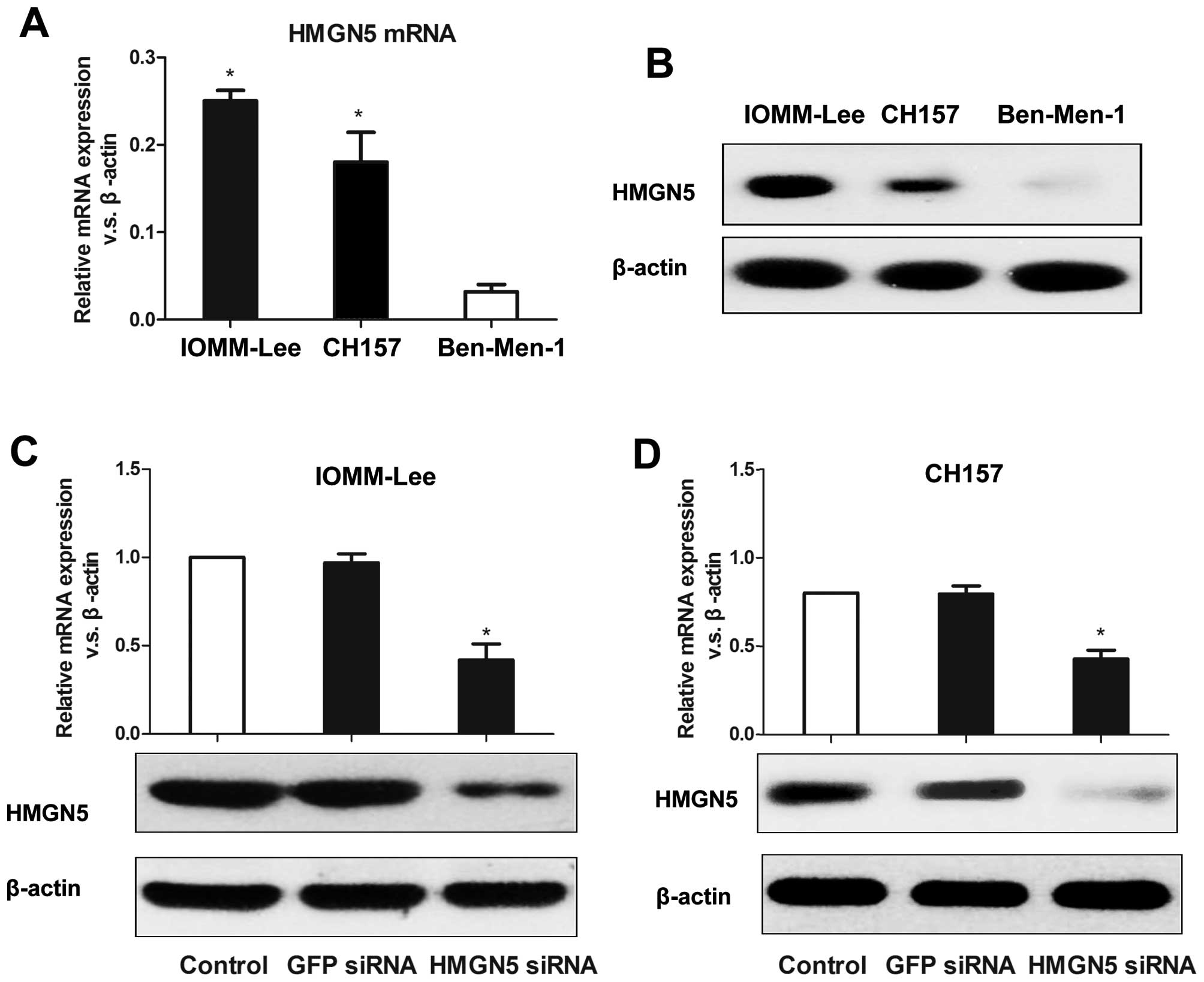
We first measured HMGN5 expression in three
meningioma cell lines (Ben-Men-1, IOMM-Lee, and CH157) by using
RT-PCR. The mRNA expression levels of HMGN5 normalized by β-actin
mRNA in IOMM-Lee, CH157, and Ben-Men-1 cells were 0.2538±0.0162
(P=0.0002 vs. Ben-Men-1), 0.1824±0.0241 (P=0.004 vs. Ben-Men-1),
and 0.0411±0.0134, respectively, which indicates that HMGN5 mRNA
levels in IOMM-Lee and CH157 cell lines were significantly higher
than those in Ben-Men-1 cells (Fig.
2A). Western blotting confirmed the same results (Fig. 2B). So IOMM-Lee and CH157 cells were
used to perform the following experiments. Next, we established
IOMM-Lee and CH157 cell lines stably expressing HMGN5-targeted
siRNA. As shown in Fig. 2C and D,
HMGN5 expression was significantly decreased on both mRNA and
protein levels in IOMM-Lee and CH157 cells. We then measured the
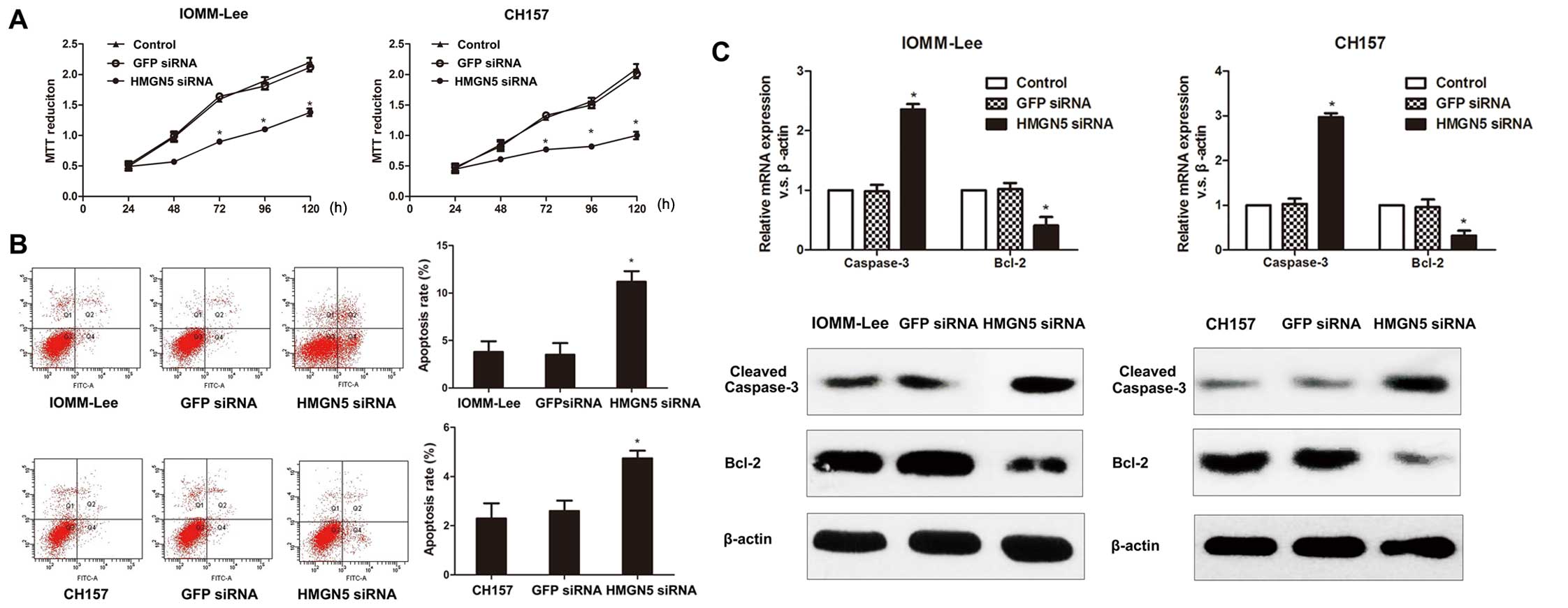
effects of HMGN5 expression on proliferation in IOMM-Lee and CH157
cells by using MTT assay and demonstrated that HMGN5 inhibition
significantly suppressed the cell growth in both cell lines
(Fig. 3A). In addition, flow
cytometry revealed that the apoptosis rate was significantly
enhanced in IOMM-Lee and CH157 cells underexpressing HMGN5 compared
to control cells (Fig. 3B). These
data indicate that knockdown of HMGN5 expression was able to
inhibit cell proliferation and enhance apoptosis in meningioma
cells.
Considering the increased rate of apoptosis in cells
transfected with HMGN5-siRNA, we measured the expression of Bcl-2
and caspase-3 in IOMM-Lee and CH157 cells to explore the mechanism.
Our data revealed that the mRNA and protein levels of Bcl-2 were
significantly decreased in cells underexpressing HMGN5 compared to
control cells (Fig. 3C).
Conversely, the mRNA and protein expressions of cleaved-caspase-3
were significantly upregulated (Fig.
3C). These results suggest the antitumor effects of HMGN5
inhibition on meningiomas are associated with enhanced
caspase-3-dependence and suppressed the Bcl-2-induced apoptotic
pathway.
Altered HMGN5 expression affects invasion
in meningiomas
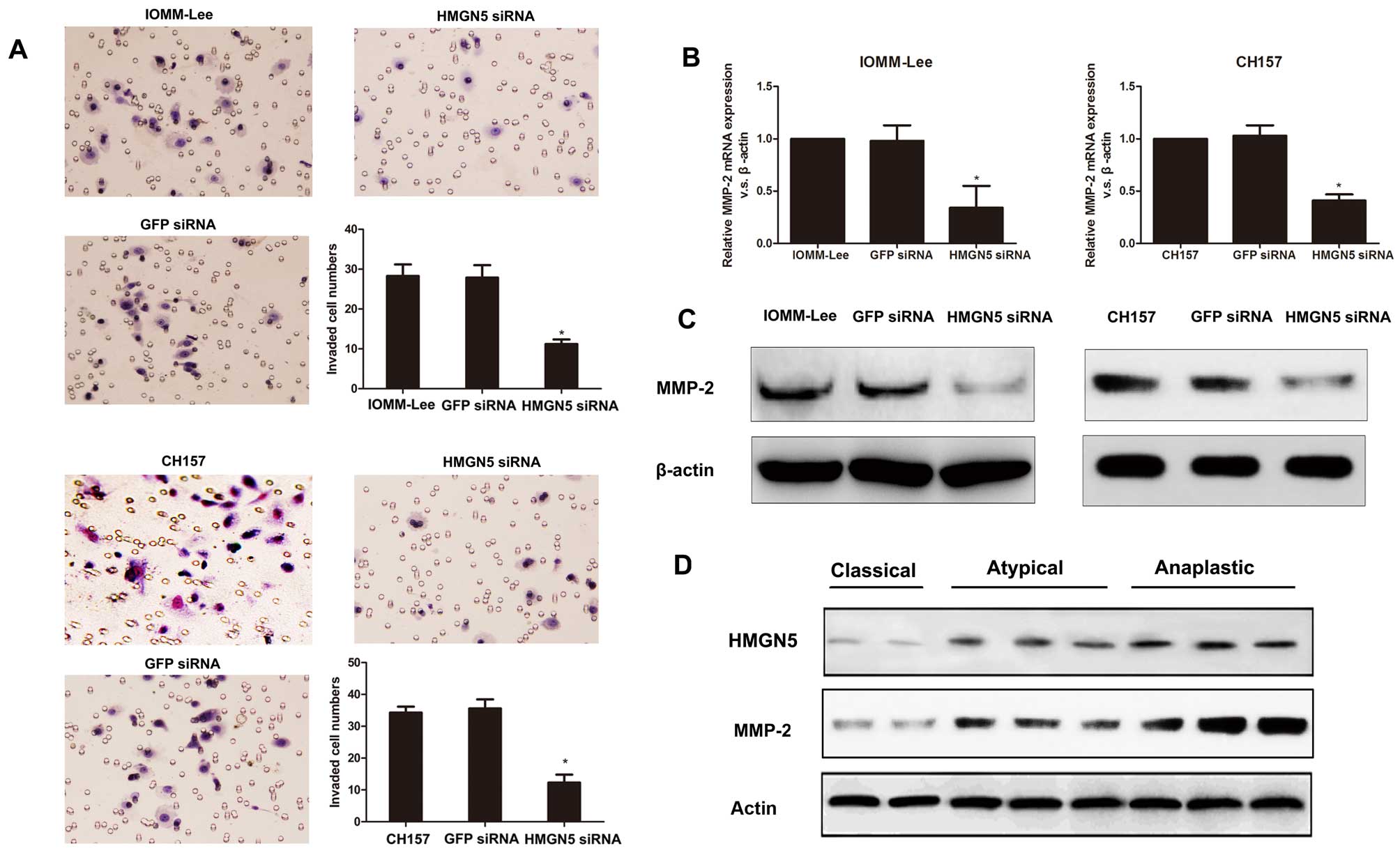
Invasion assay showed that knockdown of HMGN5
significantly suppressed the invasive activity of IOMM-Lee and
CH157 cells, whereas the transfection of GFP-siRNA had no
significant effects on the invasive activity of cells (Fig. 4A). Since the extracellular matrix
metalloproteinases (MMPs) play a critical role in cell invasion
processes, we measured MMP-2 expression to further determine the
mechanism of altered HMGN5 expression in meningioma cell invasion.
We found downregulation of HMGN5 expression in IOMM-Lee and CH157
cells significantly suppressed MMP-2 expression on mRNA and protein
levels (Fig. 4B and C). In
addition, we further found MMP-2 protein expression was
significantly increased in high grade meningiomas compared to low
grade meningiomas by using western blotting (Fig. 4D). Moreover, western blot analysis
indicated that MMP-2 expression associated positively with HMGN5
expression in human meningioma specimens (Fig. 4D). These data suggest that
knockdown of HMGN5 results in the suppression of MMP-2 expression
followed by a decrease of invasion.
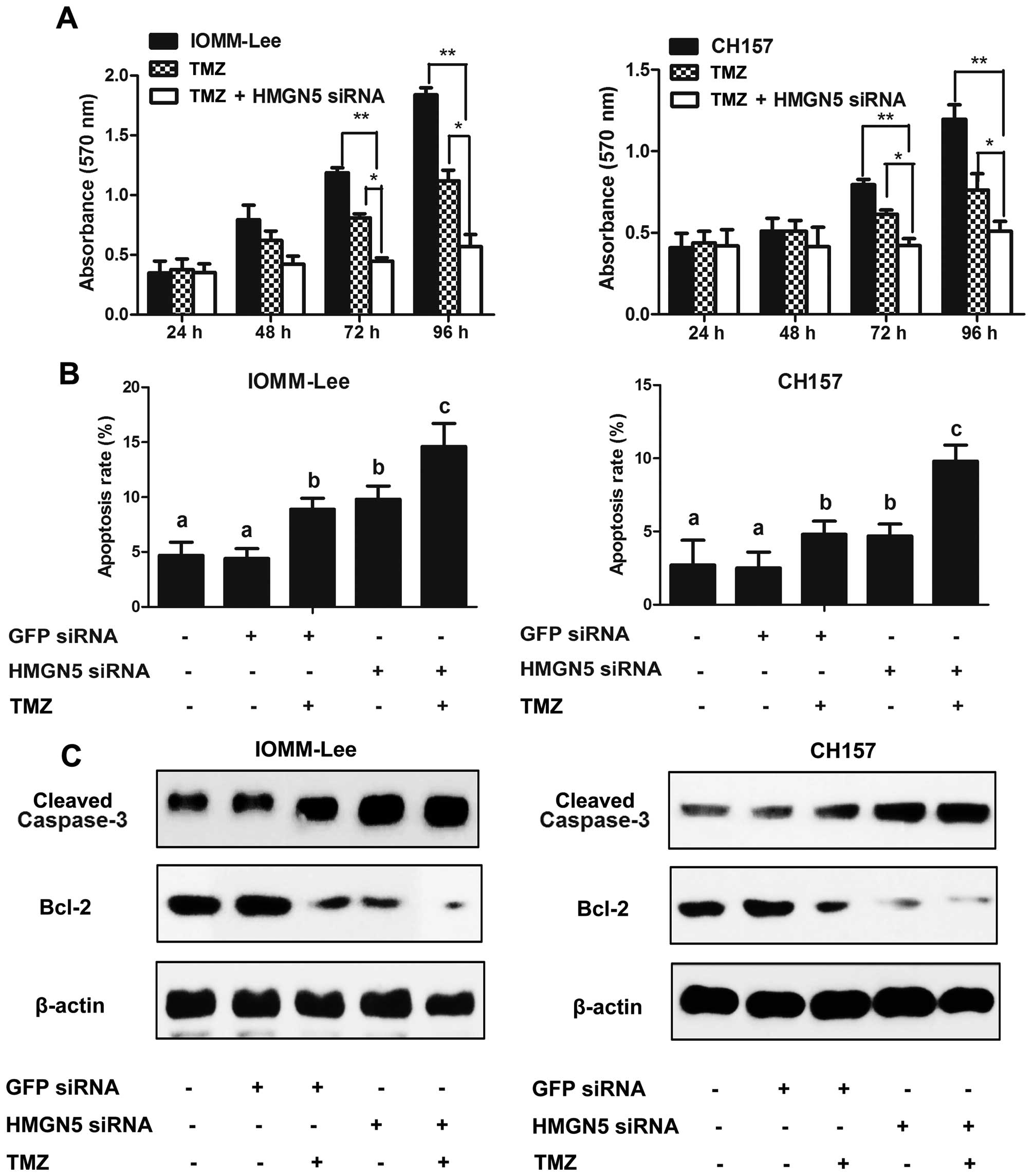
HMGN5 regulates TMZ-induced cytotoxicity
in meningiomas
We first measured the effects of HMGN5 expression on
the TMZ cytotoxicity to IOMM-Lee and CH157 cells by using MTT
assay. HMGN5 inhibition significantly increased TMZ-induced
cytotoxicity in a time-dependent manner in both cell lines
(Fig. 5A). We next examined the
apoptosis rate of TMZ-induced apoptosis by flow cytometric
analysis. As illustrated in Fig.
5B, HMGN5 siRNA alone could significantly enhance apoptosis
compared to meningioma cells with cells transfected with GFP siRNA.
When treated with 50 μM TMZ, the combination of HMGN5 siRNA and TMZ
significantly enhanced apoptosis of meningioma cells compared to
those treated with TMZ alone (Fig.
5B). These results suggest that HMGN5 inhibition was able to
sensitize TMZ-induced apoptosis.
We further found that HMGN5 inhibition significantly
decreased Bcl-2 protein expression and increased cleaved-caspase-3
expression in both IOMM-Lee and CH157 cells treated with TMZ when
compared with cells treated with TMZ alone, which might explain the
HMGN5 inhibition contributing to the improvement of chemoresitance
(Fig. 5C). Thus, these results
suggest that knockdown of HMGN5 expression sensitizes malignant
meningioma cells to TMZ-induced apoptosis via regulating the
expression of Bcl-2 and cleaved-caspase-3.
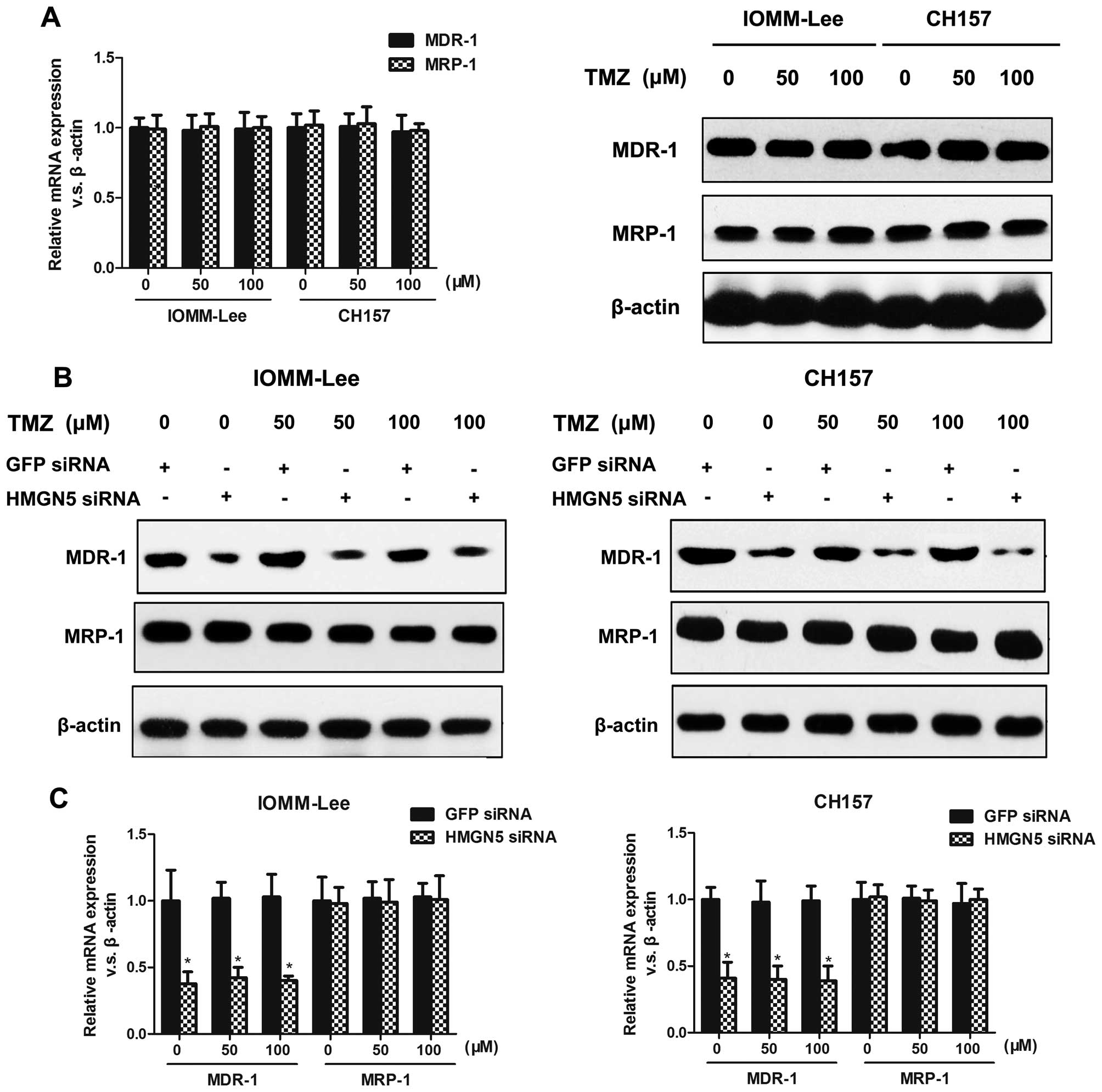
HMGN5 knockdown decreases MDR-1
expression without affecting MRP-1 expression
Considering that MDR-1 and MRP-1 proteins confer
chemoresistance to tumor cells, we investigated whether HMGN5
alters TMZ sensitivity by mediating the expression of these
proteins. We first confirmed that different doses of TMZ alone did
not affect MDR-1 or MRP-1 expression (Fig. 6A). Next, we demonstrated that HMGN5
inhibition suppressed MDR-1 expression without affecting MRP-1
expression on protein levels in IOMM-Lee and CH157 cells treated
with different doses of TMZ (Fig.
6B). Consistent with these western blot results, the mRNA
expression of MDR-1 was significantly suppressed in IOMM-Lee and
CH157 cells transfected with HMGN5-siRNA compared to controls,
whereas MRP-1 expression did not change significantly between
siRNA-transfected and GFP-siRNA-transfected meningioma cells
(Fig. 6C). These data indicate
that the increased susceptible to TMZ in meningioma cells
underexpressing HMGN5 was associated with decreased MDR-1
expression and a concomitant suppression in MDR-1-induced TMZ
efflux.
Discussion
Recent studies have suggested that HMGN5 is highly
expressed in various cells derived from breast, uterine cancer, as
well as gliomas (8–11), but little is known about its
expression and functional role in meningiomas. Here, we measured
HMGN5 expression levels in clinical meningioma specimens and
revealed that HMGN5 expression was positively correlated with
pathological grade and reccurence. Results from our study indicated
that aberrantly high HMGN5 expression was a prognostic parameter
for poor outcome. Immunohistochemical staining suggested that HMGN5
expression level could be used to predict the recurrence of
meningiomas. In addition, we demonstrated a significant correlation
between HMGN5 overexpression and elevated Ki-67 expression, a
cellular proliferative marker, in human meningioma specimens.
Furthermore, we showed that knockdown of HMGN5 expression in
meningioma cells resulted in significantly lower cell numbers at
72, 96, and 120 h compared with control cells. Thus, our study
provides both clinical and experimental evidence that HMGN5 may
play an important role in proliferation and growth in meningioma
cells.
We further found downregulation of HMGN5 enhanced
apoptosis in IOMM-Lee and CH157 cells as indicated by the increase
in the number of Annexin V/PI-positive cells measured by flow
cytometry. The Bcl-2 family members are predominantly responsible
for mediating the intrinsic apoptosis pathway (13). The most important member of this
family is Bcl-2 itself (14,15).
Deregulation of apoptosis during tumor development can be caused by
a disturbance in the homeostatic balance of the Bcl-2 family
members (16). Caspases are a
family of cysteine proteases with roles in apoptosis, inflammation,
and development (17). Caspase-3
plays a critical role in evoking many of the defining biochemical
and biophysical changes that occur during apoptosis (18). Previous studies have reported that
elevated caspase-3 is associated with shorten overall survival in
patients with malignant meningiomas (19). To explore the mechanisms of
HMGN5-induced apoptosis in meningioma cells, we measured Bcl-2 and
caspase-3 expression and suggested that reduced HMGN5 expression
was correlated with increased effector caspase-3 expression and
decreased anti-apoptotic Bcl-2 expression. Although there is no
direct interaction of HMGN5 with either Bcl-2 or caspase-3, it is
reasonable to speculate that HMGN5 plays a critical role in Bcl-2
and Cas-3 pathway. Additional studies are essential to fully
describe HMGN5-related Bcl-2 and Cas-3 pathway in future.
MMPs play critical a role in promoting angiogenesis,
tumor invasion, and tumor metastasis (20). Emerging evidence shows that
overexpression of MMP-2 is correlated with the recurrence of
intracranial meningiomas and predicts a poor prognosis in patients
with advanced meningiomas (21,22).
It is also noteworthy that knockdown of MMP-2 could significantly
suppress the invasive process in malignant brain tumors (23). Our results suggested that knockdown
of HMGN5 significantly inhibited the invasiveness of meningioma
cells. In addition, the mRNA and protein expression of MMP-2 in
IOMM-Lee and CH157 cells was also significantly reduced after HMGN5
suppression. Thus, it is reasonable to speculate that high
expression of HMGN5 could modulate matrix metalloproteinases
signaling pathways, and by doing so, ultimately contributes to
meningioma invasion.
TMZ is an oral alkylating chemotherapeutic agent
that exerts antitumor effects by creating DNA damage in tumor
cells. TMZ treatment has been reported to control
treatment-resistant recurrent meningioma (24). Li et al demonstrated that
the cytotoxic sensitivity to TMZ may be associated with its
pro-apoptotic function (25). In
this study, we found that that combined TMZ and HMGN5 inhibition
showed enhanced inhibition on proliferation compared to TMZ
treatment alone, indicating that HMGN5 is a promising therapeutic
target to enhance the antitumor efficacy of TMZ. We further found
that the combination of HMGN5 inhibition and TMZ significantly
decreased Bcl-2 expression and increased caspase-3 expression,
implying a functional interaction between HMGN5 and the apoptosis
signaling pathways. A previous study demonstrated that upregulation
of Bcl-2 may protect against chemical agent-induced apoptosis in
cancer cells (26). Thus, our data
suggest a potential molecular mechanism for TMZ resistance in
meningiomas that relies upon increased Bcl-2 expression secondary
to overactivation of the HMGN5 signaling pathway.
The ATP-binding cassette (ABC) superfamily of
ATP-dependent efflux pumps is one of the largest protein families.
Increased expression of ABC drug efflux transporters in tumors,
which reduces intracellular doses of cytotoxic drugs, results in
resistance to chemotherapy (27).
Two major efflux pump families P-glycoprotein (MDR-1) and multidrug
resistance associated proteins 1 (MRP-1) belong to the ABC
superfamily. Importantly, TMZ has been reported to be a substrate
of these ABC transporters (28).
In this study, we found that knockdown of HMGN5 by siRNA
significantly suppressed the MDR-1 expression without affecting
MRP-1 expression in IOMM-Lee and CH157 cells. Moreover, the
combination of HMGN5 siRNA and TMZ enhanced apoptosis when compared
with cells treated with TMZ alone. This is in agreement with
previous studies that MDR-1 may play a critical role in
chemoresistance in malignant meningiomas (29). Taken together, we believed that
HMGN5-dependent increase of MDR-1 may further suppress
chemosensitivity, whereas decreased HMGN5 signaling could inhibit
MDR-1 expression and drug efflux.
In conclusion, we demonstrated that knockdown of
HMGN5 in IOMM-Lee and CH157 cells enhances apoptosis through
increasing caspase-3 and decreasing Bcl-2 expression, inhibits
tumor invasiveness by suppressing MMP-2 expression and increases
chemosensitivity to TMZ via decreased MDR-1 expression without
affecting expression of MRP-1. These results suggest that
downregulation of HMGN5 expression may become an attractive
strategy to treat human malignant meningioma in the future.
References
|
1
|
Fathi AR and Roelcke U: Meningioma. Curr
Neurol Neurosci Rep. 13:3372013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dubel GJ, Ahn SH and Soares GM:
Contemporary endovascular embolotherapy for meningioma. Semin
Intervent Radiol. 30:263–277. 2013. View Article : Google Scholar :
|
|
3
|
Walcott BP, Nahed BV, Brastianos PK and
Loeffler JS: Radiation Treatment for WHO Grade II and III
meningiomas. Front Oncol. 3:2272013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saraf S, McCarthy BJ and Villano JL:
Update on meningiomas. Oncologist. 16:1604–1613. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rochman M, Malicet C and Bustin M:
HMGN5/NSBP1: A new member of the HMGN protein family that affects
chromatin structure and function. Biochim Biophys Acta. 1799:86–92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shirakawa H, Landsman D, Postnikov YV and
Bustin M: NBP-45, a novel nucleosomal binding protein with a
tissue-specific and developmentally regulated expression. J Biol
Chem. 275:6368–6374. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rochman M, Postnikov Y, Correll S, Malicet
C, Wincovitch S, Karpova TS, McNally JG, Wu X, Bubunenko NA,
Grigoryev S, et al: The interaction of NSBP1/HMGN5 with nucleosomes
in euchromatin counteracts linker histone-mediated chromatin
compaction and modulates transcription. Mol Cell. 35:642–656. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji SQ, Yao L, Zhang XY, Li XS and Zhou LQ:
Knockdown of the nucleosome binding protein 1 inhibits the growth
and invasion of clear cell renal cell carcinoma cells in vitro and
in vivo. J Exp Clin Cancer Res. 31:222012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wahafu W, He ZS, Zhang XY, Zhang CJ, Yao
K, Hao H, Song G, He Q, Li XS and Zhou LQ: The nucleosome binding
protein NSBP1 is highly expressed in human bladder cancer and
promotes the proliferation and invasion of bladder cancer cells.
Tumour Biol. 32:931–939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang N, Zhou LQ and Zhang XY:
Downregulation of the nucleosome-binding protein 1 (NSBP1) gene can
inhibit the in vitro and in vivo proliferation of prostate cancer
cells. Asian J Androl. 12:709–717. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu J, Yan R, Chen J, Xu T, Zhou J, Wang M,
Chen C, Yan Y and Lu Y: HMGN5: A potential oncogene in gliomas. J
Neurooncol. 104:729–736. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Q, Wang JY, Zhang XP, Lv ZW, Fu D, Lu
YC, Hu GH, Luo C and Chen JX: RLIP76 is overexpressed in human
glioblastomas and is required for proliferation, tumorigenesis and
suppression of apoptosis. Carcinogenesis. 34:916–926. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soane L and Fiskum G: Inhibition of
mitochondrial neural cell death pathways by protein transduction of
Bcl-2 family proteins. J Bioenerg Biomembr. 37:179–190. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Basu A, DuBois G and Haldar S:
Posttranslational modifications of Bcl2 family members - a
potential therapeutic target for human malignancy. Front Biosci.
11:1508–1521. 2006. View
Article : Google Scholar
|
|
16
|
Zeestraten EC, Benard A, Reimers MS,
Schouten PC, Liefers GJ, van de Velde CJ and Kuppen PJ: The
prognostic value of the apoptosis pathway in colorectal cancer: A
review of the literature on biomarkers identified by
immunohistochemistry. Biomark Cancer. 5:13–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Murray J and Renslo AR: Modulating caspase
activity: Beyond the active site. Curr Opin Struct Biol.
23:812–819. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McCracken JM and Allen LA: Regulation of
human neutrophil apoptosis and lifespan in health and disease. J
Cell Death. 7:15–23. 2014.PubMed/NCBI
|
|
19
|
Vranic A: Caspase-3 and survivin
expression in primary atypical and malignant meningiomas. ISRN
Neurosci. 2013:6262902013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shiraga M, Yano S, Yamamoto A, Ogawa H,
Goto H, Miki T, Miki K, Zhang H and Sone S: Organ heterogeneity of
host-derived matrix metalloproteinase expression and its
involvement in multiple-organ metastasis by lung cancer cell lines.
Cancer Res. 62:5967–5973. 2002.PubMed/NCBI
|
|
21
|
Mandara MT, Pavone S, Mandrioli L, Bettini
G, Falzone C and Baroni M: Matrix metalloproteinase-2 and matrix
metalloproteinase-9 expression in canine and feline meningioma. Vet
Pathol. 46:836–845. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okada M, Miyake K, Matsumoto Y, Kawai N,
Kunishio K and Nagao S: Matrix metalloproteinase-2 and matrix
metalloproteinase-9 expressions correlate with the recurrence of
intracranial meningiomas. J Neurooncol. 66:29–37. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kast RE and Halatsch ME: Matrix
metalloproteinase-2 and -9 in glioblastoma: A trio of old drugs -
captopril, disulfiram and nelfinavir - are inhibitors with
potential as adjunctive treatments in glioblastoma. Arch Med Res.
43:243–247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chamberlain MC, Tsao-Wei DD and Groshen S:
Temozolomide for treatment-resistant recurrent meningioma.
Neurology. 62:1210–1212. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li G, Zhang H, Liu Y, Kong L, Guo Q and
Jin F: Effect of temozolomide on livin and caspase-3 in U251 glioma
stem cells. Exp Ther Med. 9:744–750. 2015.PubMed/NCBI
|
|
26
|
Akay C, Thomas C III and Gazitt Y: Arsenic
trioxide and paclitaxel induce apoptosis by different mechanisms.
Cell Cycle. 3:324–334. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Q, Qian J, Wang J, Luo C, Chen J, Hu
G and Lu Y: Knockdown of RLIP76 expression by RNA interference
inhibits invasion, induces cell cycle arrest, and increases
chemosensitivity to the anticancer drug temozolomide in glioma
cells. J Neurooncol. 112:73–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schaich M, Kestel L, Pfirrmann M, Robel K,
Illmer T, Kramer M, Dill C, Ehninger G, Schackert G and Krex D: A
MDR1 (ABCB1) gene single nucleotide polymorphism predicts outcome
of temozolomide treatment in glioblastoma patients. Ann Oncol.
20:175–181. 2009. View Article : Google Scholar
|
|
29
|
Andersson U, Malmer B, Bergenheim AT,
Brännström T and Henriksson R: Heterogeneity in the expression of
markers for drug resistance in brain tumors. Clin Neuropathol.
23:21–27. 2004.PubMed/NCBI
|