Introduction
Oral cancer, which occurs in oral cavity and
oropharynx, is a leading cause of cancer-related death and
approximately 263,900 new cases were reported and approximately
128,000 of the patients died of oral cancer in 2011, in USA
(1). Oral cancer is one of most
common types of cancer and over 500,000 patients suffer from it
every year (2). Oral squamous cell
carcinoma (OSCC) which occurs in the lining of the epithelial cell
represents approximately 95% of head and neck cancer and is the
sixth most common malignant neoplasm worldwide (3–5).
This aggressive epithelial malignancy has a poor diagnosis and the
incidence rate of oral cancer has been elevated up to 50% over the
past two decades, with only a 50% 5-year survival rate in patients
with OSCC despite advanced medical treatment (6–10).
There are many chief factors for OSCC, including tobacco, alcohol,
and HPV infection (11–13). In addition, it was reported that
bacterial infections are associated with tumor site of OSCC because
of their ability to induce chronic inflammation.
Manumycin A (Manu A), a product of Streptomyces
parvulus, is a natural antibiotic and is known to be a
potential tumoricide. Many studies have demonstrated that Manu A
inhibits cell viability and induces cell apoptosis in many cancers,
such as prostate cancer, multiple myeloma, anaplastic thyroid
cancer and colon cancer (14–17).
Taking into consideration of possible correlation of bacterial
infection to OSCC, it is proposed that capability of Manu A to
directly suppress some prevalent bacteria (18) has also anticancer effect on
OSCC.
To induce apoptosis of cancer cells by targeting the
specific signal-transduction pathway could be an effective
anticancer therapy. Therefore, we investigated whether the Manu
A-induced cell apoptosis is related to Specificity protein 1 (Sp1),
a transcription factor that binds to a specific DNA sequence,
overexpressed in many cancer cells, such as bladder cancer
(19), breast cancer (20,21),
pancreatic cancer (22), gastric
cancer (23) and oral cancer
(24). Specificity protein 1 (Sp1)
has already been examined and plays important physiological roles
such as cell cycle regulation, cell proliferation, and cell
apoptosis (25). However, the
relationship between Manu A treatment and downregulation of Sp1 in
OSCC cells has not been studied yet. If Manu A can reduce Sp1
expression, it will be a potential candidate material for OSCC
therapy. In order to verify its therapeutic effect of Manu A, we
investigated the apoptotic effect of Manu A by downregulation of
Sp1 levels using the OSCC cell lines HN22 and HSC4.
Materials and methods
Reagents
All the solvents used in the experiments were of
extra pure grade. Hexane, ethyl acetate and acetonitrile were
purchased from J.T. Baker (Phillipsburg, NJ, USA). Silica gel for
Thin layer chromatography, precoated silica gel plate (Kieselgel
60F254, Merck, NJ, USA) was used. Silica gel for silica gel column,
Kieselgel 60 (70–230 mesh, Merck) was used to purify manumycin.
Purification of manumycin A
Streptomyces sp. CS392 was grown on rotary
shaker at 180 rpm in Emerson media for 2–3 days at 28°C. Culture
broth (3L) was centrifuged at 6,000 rpm for 20 min. Supernatant was
extracted two times with ethyl acetate (1:1, v/v). The extracted
ethyl acetate fraction was evaporated and dried using a rotary
evaporator at 50°C under reduced pressure. Purification of
antibiotic was carried out by silica gel column chromatography
(0.8×15 cm). After washing the column with hexane, active material
was eluted from the column with hexane-ethyl acetate (4:1). Active
fractions were collected and rechromatographed, using a reverse
phase-C18 silica gel column (1.0×15 cm) with 0.01% formic
acid-acetonitrile (4:6) to give manumycin A.
Cell culture
HN22 and HSC4 are human oral squamous cancer cell
lines. HN22 cells were provided by Dankook University (Cheonan,
Korea) and HSC4 cells were provided by Hokkaido University
(Hokkaido, Japan). HN22 and HSC4 cells were cultured in DMEM
containing 10% heat-inactivated FBS and 100 U/ml each of penicillin
and streptomycin at 37°C under 5% CO2 and humidified
condition.
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay
The result of the Manu A on HN22 and HSC4 cell
viability was observed using the Cell Titer 96® AQueous
One Solution Cell Proliferation Assay kit (Promega, Madison, WI,
USA) according to the manufacturer's instructions. The cells were
seeded in 96-well plates, grown for 24 h and treated with various
concentration of Manu A. After treatment with Manu A for 24 and 48
h, MTS solution was added to each well and the plates were
incubated for 2 h at 37°C. Its absorbance was read using an Enspire
Multimode Plate reader (Perkin-Elmer, USA) at 490 nm.
Cell cycle analysis
HN22 and HSC4 cells were seeded and treated with
Manu A (0, 2.5, 5, and 10 μM) for 48 h. The harvested cells were
washed with 1 ml PBS and 150 μl of Muse™ Cell cycle reagent (EMD
Millipore Corp. USA) was added. Additionally, cells were incubated
at RT for 30 min in the dark. Samples were analyzed by Muse Cell
Analyzer (Merck Millipore, Billerica, MA, USA) with Muse Cell cycle
kit (Merck Millipore).
(4′,6-diamidino-2-phenylindole) DAPI
staining
After treatment with Manu A, the cells were
harvested by trypsinization. The cells were washed with cold PBS,
and fixed in 100% methanol at room temperature for 20 min. The
cells were deposited on slides, stained with DAPI solution (2
μl/ml) and observed through a FluoView confocal laser microscope
(FluoView FV10i, Olympus Corp., Tokyo, Japan).
Reverse transcription-polymerase chain
reaction (RT-PCR)
To analyze the effect of Manu A on OSCC cell lines,
we performed RT-PCR using total RNAs and primers designed for the
specific gene. Total RNAs were harvested from OSCC cells treated
with or without Manu A using the Total RNA extraction (Life
Technologies, Carlsbad, CA, USA). With 2.5 μg of RNA, RT-PCR was
done using First-strand cDNA synthesis kit (Bioassay Co., Ltd.,
Korea) according to the kit instructions. We obtained cDNA using
actin-specific and Sp1-specific primers under the following PCR
condition (30 cycles: 1 min at 95°C, 1 min at 56°C and 1 min at
72°C). The actin primers used were: forward, 5′-GTG GGG CGC CCC AGG
CAC CA-3′; and reverse, 5′-CTC CTT AAT GTC ACG CAC GAT TTC-3′; and
the Sp1 primers were: forward, 5′-ATG CCT AAT ATT CAG TAT CAA
GTA-3′; and reverse, 5′-CCC TGA GGT GAC AGG CTG TGA-3′. Actin was
used as an internal control. The RT-PCR products were visualized
with ethidium bromide staining under UV light, after
electrophoresis on a 2% agarose gel.
Annexin V
HN22 and HSC4 cells were seeded and grown for 24 h.
After 48 h from treatment with various concentration of Manu A,
cells were harvested by trypsinization for analysis. The cells were
analyzed by Muse Cell Analyzer (Merck Millipore) with the Muse
Annexin V & Dead Cell kit (MCH100105, Merck Millipore). The
whole process of analysis followed the instructions of the kit. The
percentage of apoptotic and necrotic cells was calculated from each
triplicate sample by statistical analysis of the dot plot using
Muse 1.1.2 analysis software (Merck Millipore).
Western blotting
The lysates of treated cells were generated using
PRO-PREP™ Protein Extraction Solution (iNtRON Biotechnology,
Korea), followed by centrifugation and supernatant collection.
Proteins were separated using SDS-PAGE gel electrophoresis and
transferred onto a polyvinylidene fluoride (PVDF) membrane. After
blocking with PBS containing 0.1% Tween-20 and 5% skim milk,
membrane probed with primary antibody was shaken at 4°C overnight
and then incubated with the secondary antibody. The protein bands
were detected using ECL Plus Western Blotting Detection System
(Santa Cruz Biotechnology, USA).
Multi-Caspase
The process of the analysis followed the
instructions of the Muse Multi-Caspase kit (Muse Cell Analyzer,
Merck Millipore). OSCC cells including control and treatment groups
were incubated for appropriate time to induce caspase activity and
harvested. Cell samples in 1× caspase buffer with 50 μl of Muse
Multi-Caspase reagent working solution were incubated at 37°C for
30 min, then 150 μl of 7-AAD working solution was added to each
sample and triplicate samples were analyzed by Muse Cell Analyzer
(Merck Millipore).
Mitochondrial membrane potential assay
(MMP)
The whole process of the analysis followed the
instructions of the Muse MitoPotential kit (Merck Millipore).
Control cells and Manu A-treated (2.5, 5 and 10 μM) cells were
harvested. To investigate the mitochondrial membrane permeability,
these cells were incubated with 95 μl of Muse MitoPotential working
solution that is diluted with 1× assay buffer (1:1,000) for 20 min
in dark. Then, 5 μl of Muse 7-AAD was added and samples were
incubated at 37°C for 5 min. Finally, all samples were analyzed by
Muse Cell Analyzer (Merck Millipore).
Statistical analysis
Using Student's t-test, the statistical significance
was assessed. The results with a P-value <0.05, was considered
as statistically significant.
Results
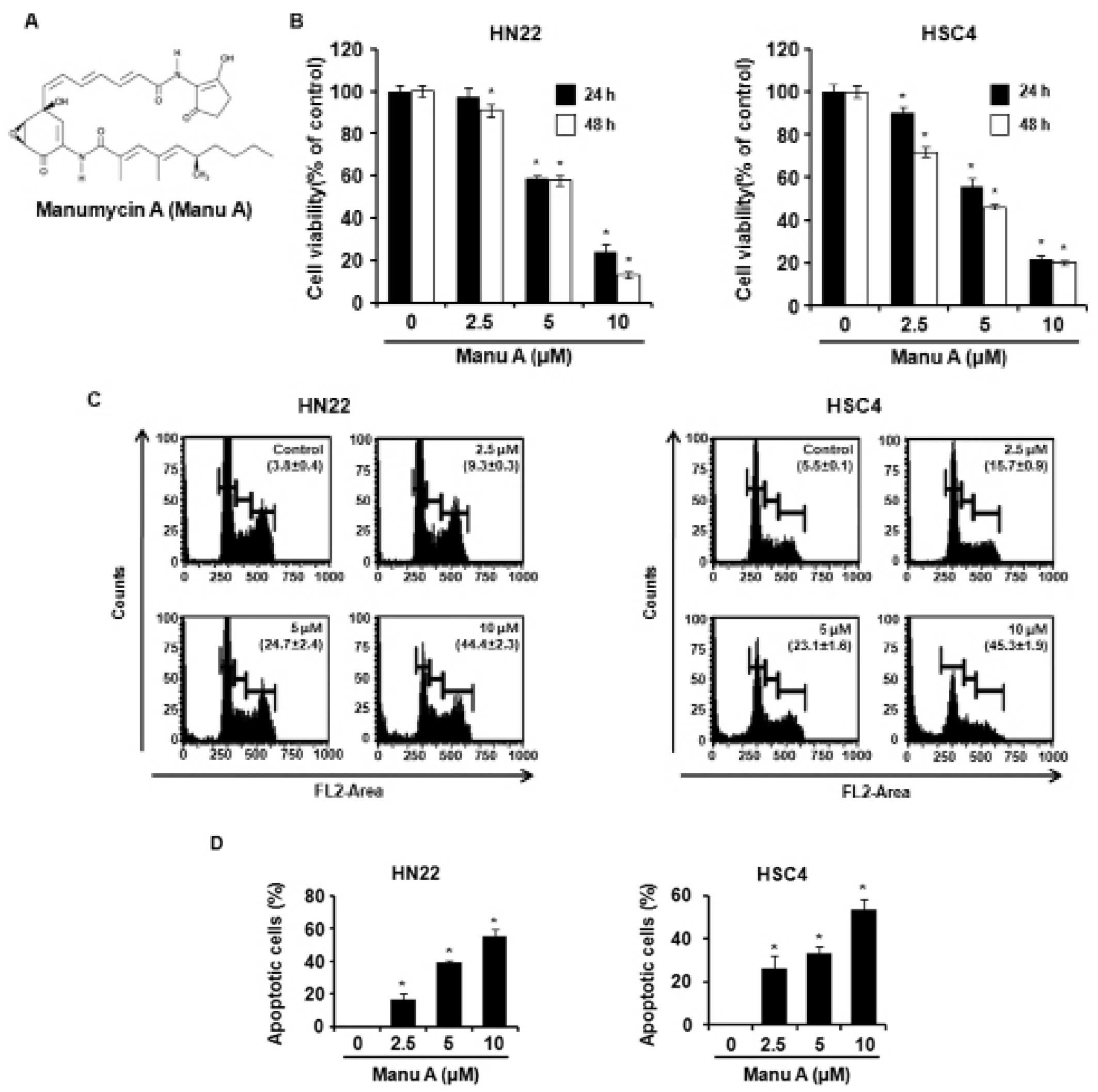
Manu A inhibits human OSCC cell
viability
Two OSCC cell lines HN22 and HSC4 were grown to
investigate the effect of Manu A on OSCC cells. Manu A treatment
significantly decreased cell viability in a dose-(2.5, 5, and 10
μM) and time-(24 and 48 h) dependent manner (Fig. 1B). Forty-eight hours after Manu A
treatment, the viabilities of HN22 cells were, respectively, 91,
57.8 and 13.3% at 2.5, 5 and 10 μM compared with control group and
it showed significant decrease of cell viability in a
dose-dependent manner. Similarly, the viabilities of HSC4 cells
were, respectively, 71.8, 46, and 19.9% at 2.5, 5, and 10 μM
compared with control cells and it also showed the same
dose-response as that in HN22. The IC50 values of Manu A
for cell viability were 6.38 and 4.6 μM in HN22 and HSC4,
respectively.
Manu A induces apoptosis in human OSCC
cells
We tested if Manu A could induce apoptosis of HN22
and HSC4 cells, using cell cycle analysis, DAPI staining, and
double-staining of 7-AAD and Annexin V. The OSCC cells were treated
with 2.5, 5 and 10 μM of Manu A for 48 h. Cell cycle analysis
showed that Manu A induced sub-G1 phase in HN22 and HSC4
cells in a dose-dependent manner (Fig.
1C). Especially, both of HN22 and HSC4 showed a significant
increase in sub-G1 phase and a decrease in G1
phase at 10 μM of Manu A, as compared to control groups. The
proportion of sub-G1 phase increased from 3.8±0.4
(control) to 44.4±2.3% (10 μM) in HN22 cells (Fig. 1C, left) and also increased from
5.5±0.1 (control) to 45.3±1.9% (10 μM) in HSC4 cells (Fig. 1C, right). Further DAPI staining
revealed the presence of nuclei condensation and apoptotic bodies
in Manu A-treated OSCC cells (Fig.
1D). Moreover, 7-AAD and Annexin V double-staining displayed an
increased percentage of apoptotic cells after treatment with Manu A
for 48 h in a dose-dependent manner (Fig. 5C and D).
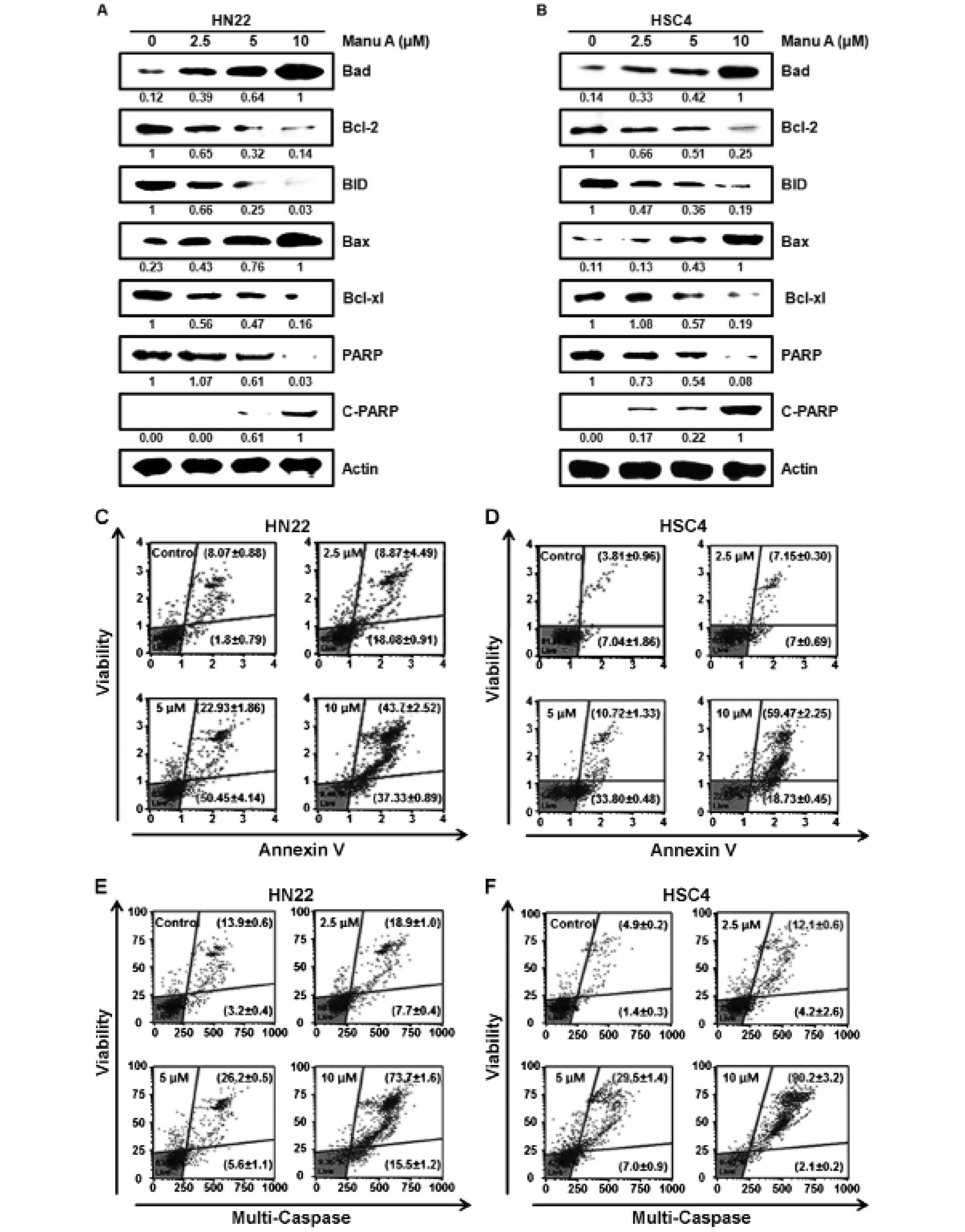
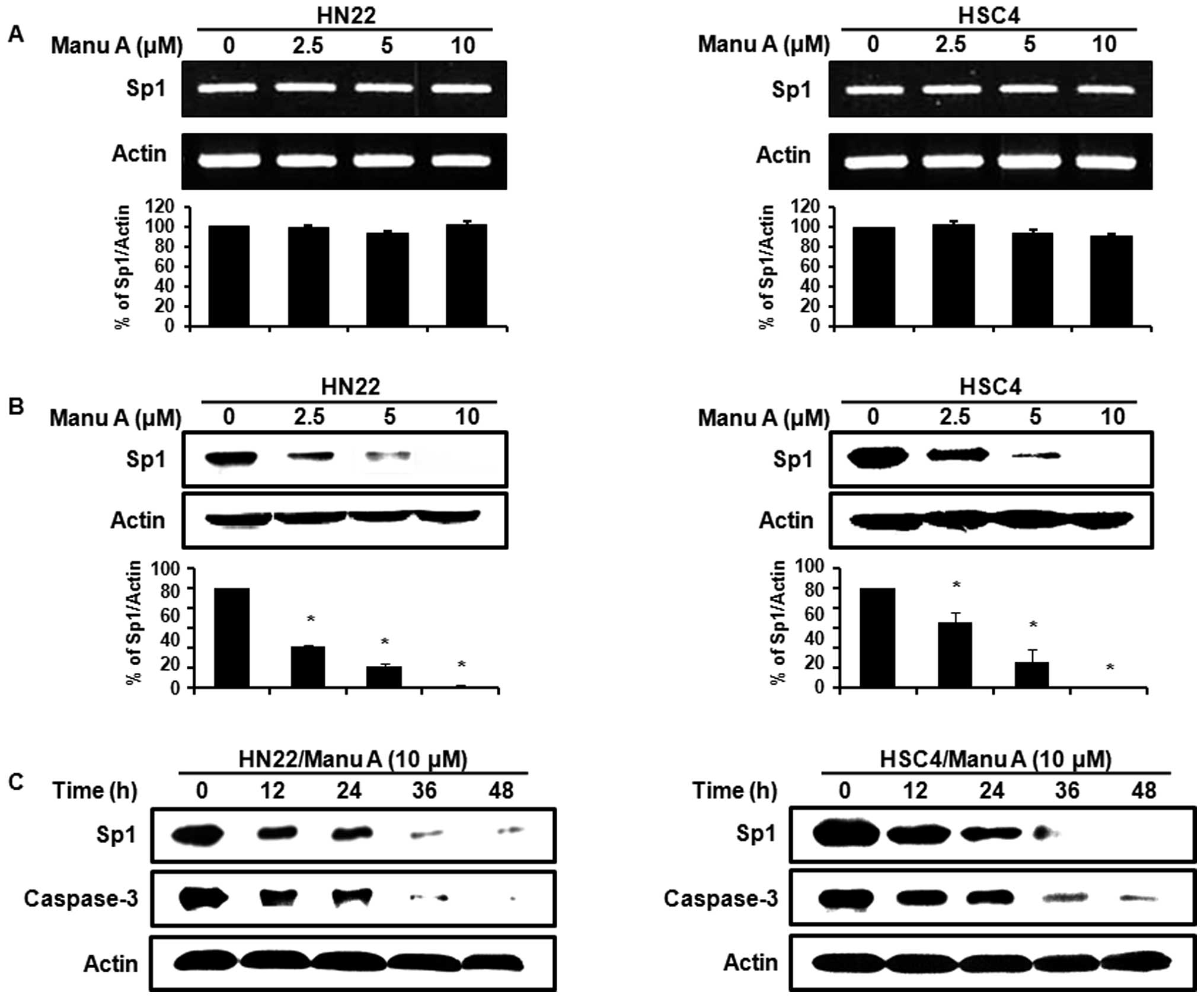
Manu A regulates Sp1 and its target
protein levels in human OSCC cells
Sp1 is a transcription factor of various genes that
are essential to the regulation of cell survival, cell growth, cell
cycle, and apoptosis (26–28). To investigate whether the
Sp1-mediated apoptosis of OSCC cells might be caused by Manu A
treatment or not, we used RT-PCR and western blotting in OSCC cells
treated with Manu A (2.5, 5, and 10 μM). As shown in Fig. 2A, there were no significant changes
in the expression of Sp1 mRNA. However, the Sp1 protein levels in
HN22 and HSC4 cells were decreased in a dose-dependent manner
(Fig. 2B). We also monitored the
protein levels of Sp1 and caspase-3 in OSCC cells (HN22 and HSC4)
treated with 10 μM of Manu A for various times (0, 12, 24, 36 and
48 h). The amounts of Sp1 were downregulated and also caspase-3
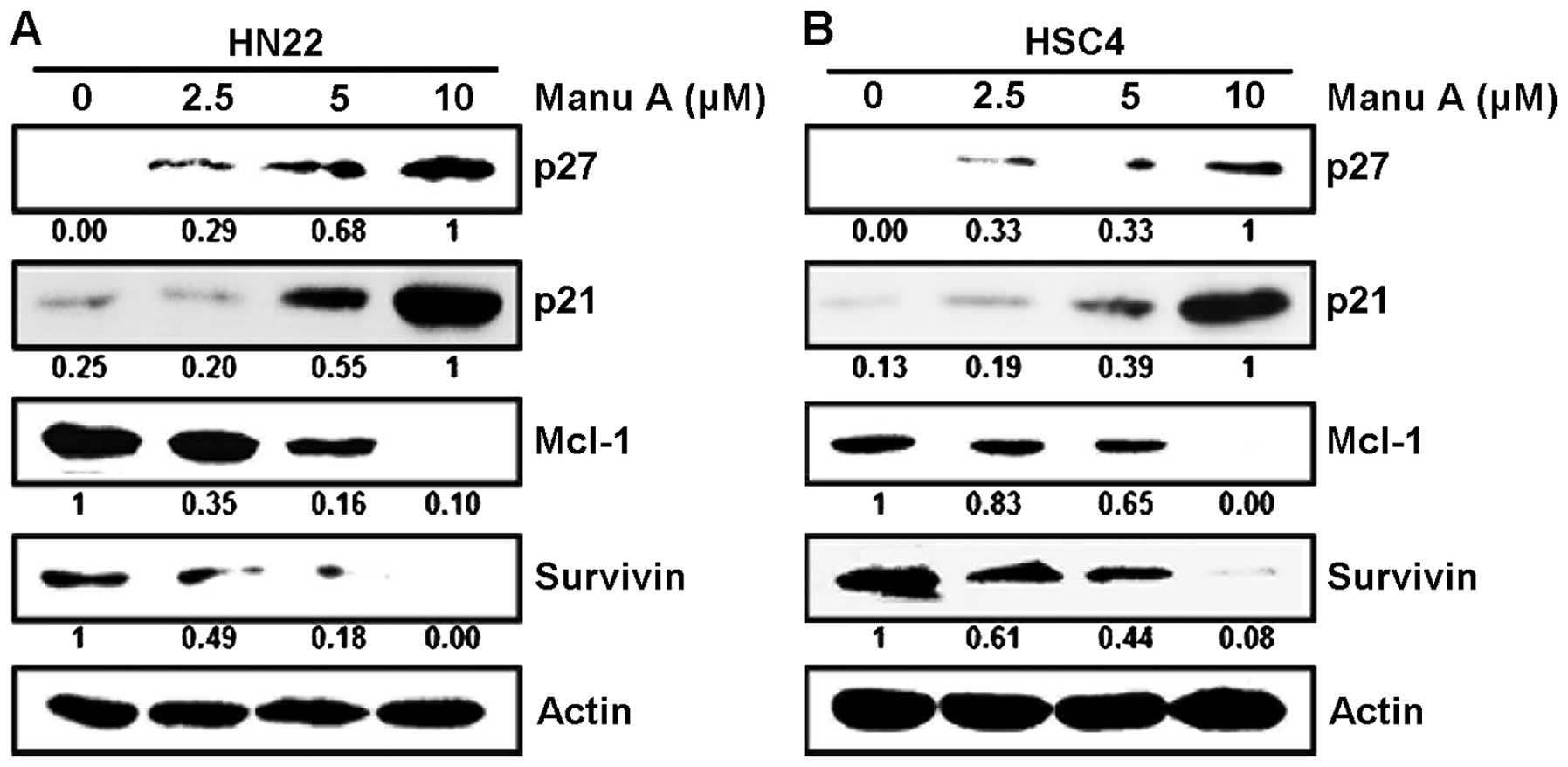
levels were significantly decreased with time by Manu A (Fig. 2C). Sp1 regulated the expression of
its downstream targets such as p27, p21, Mcl-1, and survivin. The
protein levels of cell cycle arrest proteins including p27 and p21
were elevated in HN22 (Fig. 3A)
and HSC4 (Fig. 3B) by increasing
doses of (2.5, 5 and 10 μM) Manu A. On the contrary, cell
proliferation-and survival-related proteins like Mcl-1 and survivin
were decreased in HN22 (Fig. 3A)
and HSC4 (Fig. 3B).
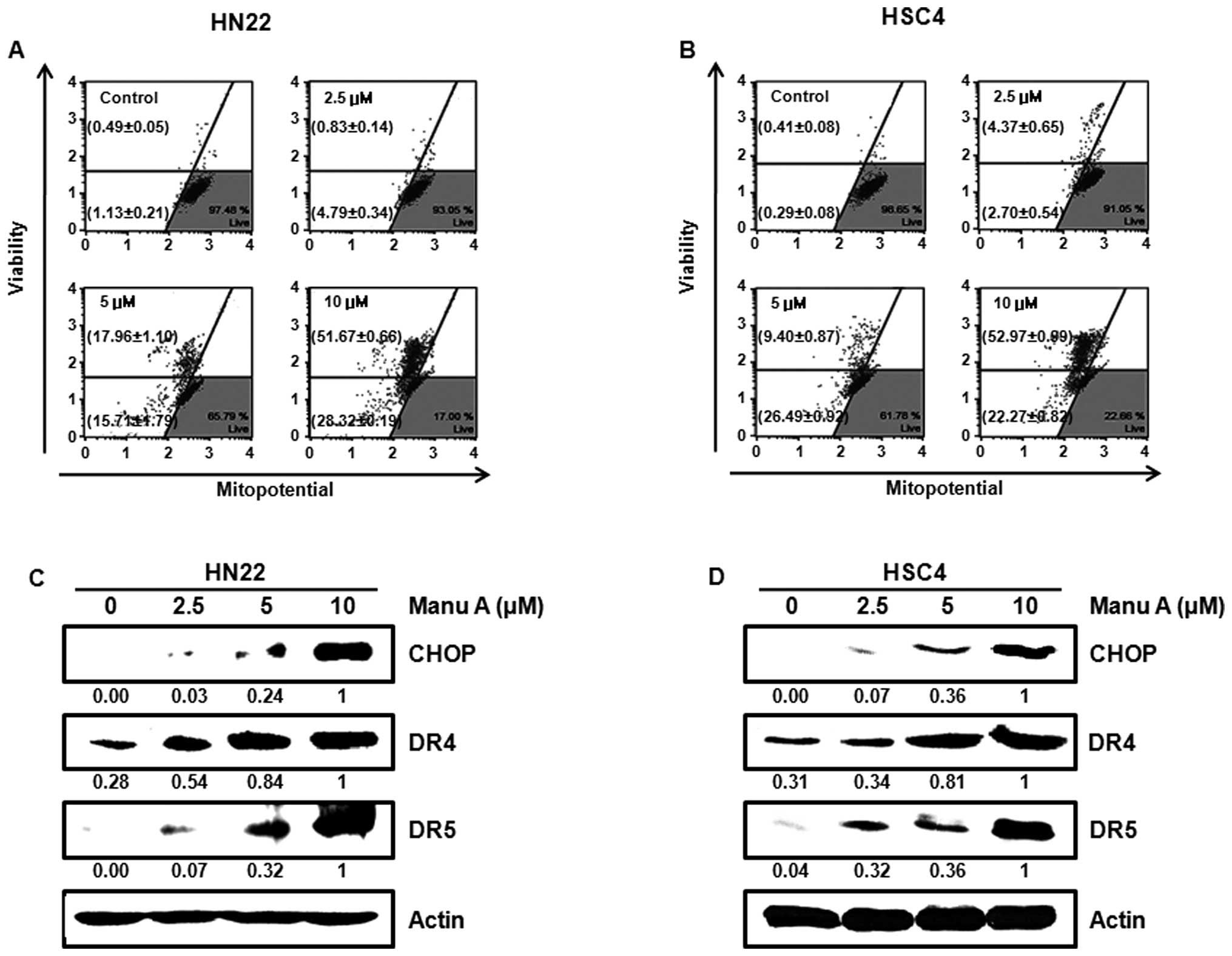
Manu A induces cell stress and controls
the mitochondrial membrane permeability during apoptosis
We investigated possible relationship between Manu
A-induced cell stress and mitochondrial integrity. CHOP, death
receptor 4 (DR4), and death receptor 5 (DR5) are related to
endoplasmic reticulum (ER) stress. In a previous study, CHOP
upregulated DRs (DR4 and DR5) by cell stress (29). The expression levels of CHOP, DR4,
and DR5 were significantly increased in HN22 cells (Fig. 4C) and HSC4 cells (Fig. 4D) by Manu A. Changes of
mitochondrial membrane permeability (MMP) are the common pathway of
stress, triggering cell apoptosis (30). The members of Bcl-2 family regulate
cell death by controlling the permeability of mitochondrial
membrane (31).As judged from
changes in MMP, total depolarized cell proportions were 5.6±0.2
(2.5 μM), 33.7±0.76 (5 μM), and 80.0±0.61% (10 μM) in HN22 cells
(Fig. 4A). In HSC4 cells (Fig. 4B), total depolarized cell
proportions were 7.1±1.0 (2.5 μM), 36.3±0.4 (5 μM) and 75.2±0.2%
(10 μM).
Manu A modulates apoptosis-related
proteins in OSCC cells
It has been reported that suppression of Sp1 induces
apoptosis of cancer cells (32–34).
To investigate molecular mechanism of Sp1-mediated apoptosis in
HN22 cells (Fig. 5A) and HSC4
cells (Fig. 5B), we carried out
western blot analysis of apoptosis-regulating proteins.
Consequently, there was a decrease in levels of Bcl-2, Bid, Bcl-xl,
and PARP in Manu A-treated OSCC cells. The levels of Bax and
cleavage of PARP were elevated in a dose-dependent manner by Manu
A. As shown in Fig. 5E and F,
there was an increase of multi-caspase activity in both HN22 and
HSC4 cells. As shown in Fig. 5E,
the proportion of Multi-Caspase-positive HN22 cells was increased
from 7.7±0.4 (2.5 μM) to 15.5±1.2% (10 μM) and the population of
caspase-positive/dead HN22 cells was increased from 18.9±1.0 (2.5
μM) to 73.7±1.6% (10 μM). In HSC4 (Fig. 5F), the proportion of
caspase-positive cells were 4.2±2.6, 7.0±0.9 and 2.1±0.2% of
control cells while caspase-positive/dead cells were 12.1±0.6,
29.5±1.4 and 90.2±3.2% of control cells at 2.5, 5 and 10 μM of Manu
A, respectively. As a whole, suppression of Sp1 by Manu A induces
apoptosis in OSCC cells.
Discussion
Oral cancer is a subtype of head and neck cancer and
its 5-year survival rate has been slightly improved over the last
few decades in spite of advanced cancer diagnosis or therapies
(radiotherapy, chemotherapy, and surgery) (35). Recent studies revealed that some
antibiotics not only reduce cell proliferation but also induce
apoptosis on human cancer cells (36,37).
We examined Manu A, a natural antibiotics and tumoricide, as a new
potential candidate substance for OSCC chemotherapy. In our study,
we investigated whether Manu A could reduce cell proliferation and
induce apoptosis through Sp1 regulation in OSCC cell lines (HN22
and HSC4). First of all, we tested anti-proliferation effect of
Manu A using MTS assay. Treatment of cells with various
concentration of Manu A exhibited a significant decrease in cell
viability in a dose-dependent manner. PI staining was performed to
find any link of Manu A-mediated cell cycle regulation to cell
apoptosis. We observed remarkable increase in proportion of
sub-G1 in a dose-dependent manner. Furthermore, both
cell anlayses of cells stained with 7-AAD and Annexin V and
measurement of caspase activity demonstrated dose-dependent
apoptotic effects of Manu A. Taken together, the data described
above, Manu A has biological effects on OSCC cells with respect to
cell growth and death.
Sp1 is a zinc finger transcription factor that binds
to GC-rich motifs of many promoters (38) and has been reported to affect the
tumorigenesis of many cancers including angiogenesis, cell cycle
progression and inflammation (38). To prove that the cell apoptosis by
Manu A is mediated by Sp1 regulation, we performed RT-PCR and
western blotting. Although the expression of Sp1 mRNA was not
decreased, the Sp1 protein levels were significantly downregulated
by Manu A in a dose- and time-dependent manner. To further
investigate molecular mechanism of Sp1-mediated cell apoptosis, we
also examined the expression levels of Sp1 target proteins such as
p21, p27, Mcl-1, and survivin. It was demonstrated that regulators
of cell cycle progression such as p21, p27 (39,40)
were increased. It is known that p27 binds to and prevents the
activation of cyclin E-cyclin-dependent kinase 2 (CDK2) or cyclin
D-cyclin-dependent kinase 4 (CDK4) complexes (41). During the cell division, p27 acts
as a cell cycle inhibitor. Similarly, p21 protein binds to and
inhibits the complexes of cyclin-CDKs (CDK2, CDK3, CDK4, or CDK6)
and thus acts as a cell cycle inhibitor (42). Anti-apoptosis factors such as
Mcl-1, and survivin were diminished by Manu A. As a member of Bcl-2
family, Mcl-1 is overexpressed in many human cancers and plays an
important role in acquiring resistance to apoptosis (43). The inhibitors of apoptosis (IAP),
survivin suppresses the apoptosis and its overexpression is
associated with development of human cancer (44). Accordingly, it can be summarized
that Manu A induces apoptotic pathways in OSCC cells through
regulation of Sp1 and its target proteins (p21, p27, Mcl-1 and
survivin).
In response to stress, cell initiates cell death
signaling through the intrinsic and the extrinsic pathways.
Intrinsic pathway involves mitochondrial involvement when exposed
to death stimuli. Mitochondria produce energy that the cell needs
by oxidative phosphorylation process on the inner membrane. In many
pathophysiological context, cell fate is dependent on Bcl-2 family
members (45). Bax is a
pro-apoptotic Bcl-2 family member and accelerates the opening of
voltage-dependent anion channel (VDAC) and Bad, pro-apoptotic Bcl-2
family member, allowing Bax-triggered apoptosis interacting with
Bcl-2 and Bcl-xl (46–48). Opening VDAC pore activates
death-driving proteolytic proteins known as caspase (30) beginning with cleavage of PARP
(49). Whereas, anti-apoptotic
Bcl-family members such as Bcl-2 and Bcl-xl inhibit opening the
VDAC pore (50). Manu A treatment
facilitated pro-apoptotic proteins (Bax and Bad) and cleaved PARP
while a decrease in anti-apoptotic proteins (Bcl-2 and Bcl-xl) was
also observed by Manu A treatment. Considering the data associated
with mitochondrial membrane potential, Manu A induces apoptosis
through the intrinsic pathway in OSCC cells, whereas, the extrinsic
pathway starts with stimulation of tumor necrosis factor (TNF)
receptor superfamily including TNF-related apoptosis-inducing
ligand (TRAIL) receptor (51). DR4
(TRAIL-R1 for TNF-related apoptosis-inducing ligand receptor-1) and
DR5 (TRAIL-R2) interact with its cognate ligand and share common
signaling mechanism that activates caspase-8 (52) and increased C/EBP homologous
protein (CHOP) elevates DR5 expression (53). Because caspase-8 catalyzes cleavage
of BH3-only protein (Bid) to t-Bid that facilitates the release of
mitochondrial proteins into cytosol and it has been reported that
CHOP downregulates Bcl-2 expression and sensitizes the cell to ER
stress (29), extrinsic pathway
incorporating the part of intrinsic apoptotic pathway (54). We found that CHOP, DR4, and DR5
were overexpressed in a dose-dependent manner while Bid was
downregulated in Manu A-treated OSCC cells. These results revealed
that Manu A induces cell apoptosis through not only the intrinsic
pathway, but also the extrinsic pathway.
Based on the effects of Manu A in OSCC cells, we
conclude that Manu A downregulates Sp1 protein levels, which in
turn induces cell apoptosis of OSCC cells (HN22 and HSC4) through
both the intrinsic and the extrinsic pathways. Therefore, the use
of Manu A may be a novel therapy for OSCC patients with
overexpression of Sp1 protein.
Acknowledgements
This study was supported by Basic Science Research
program through the National Research Foundation Korea (NRF) funded
by the Ministry of Education, Science and Technology
(2014R1A1A2053500) and the Next-Generation BioGreen 21 Program
(PJ01116401) from Rural Development Administration, Republic of
Korea.
Abbreviations:
|
OSCC
|
oral squamous cell carcinoma
|
|
Manu
|
manumycin
|
|
Sp1
|
specificity protein 1
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
PBS
|
phosphate-buffered saline
|
|
Mcl-1
|
myeloid cell leukemia-1
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
P/S
|
penicillin and streptomycin
|
|
MTS
|
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)
|
|
DAPI
|
4′-6-diamidino-2-phenylindole
|
|
PI
|
propidium iodide
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rivera C and Venegas B: Histological and
molecular aspects of oral squamous cell carcinoma (Review). Oncol
Lett. 8:7–11. 2014.PubMed/NCBI
|
|
3
|
Nagpal JK, Patnaik S and Das BR:
Prevalence of high-risk human papilloma virus types and its
association with P53 codon 72 polymorphism in tobacco addicted oral
squamous cell carcinoma (OSCC) patients of Eastern India. Int J
Cancer. 97:649–653. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Jia L, Kuang Z, Wu T, Hong Y, Chen
X, Leung WK, Xia J and Cheng B: The in vitro and in vivo antitumor
effects of clotrimazole on oral squamous cell carcinoma. PLoS One.
9:e988852014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson NW, Jayasekara P and Amarasinghe
AA: Squamous cell carcinoma and precursor lesions of the oral
cavity: Epidemiology and aetiology. Periodontol 2000. 57:19–37.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan P, Temam S, El-Naggar A, Zhou X, Liu
DD, Lee JJ and Mao L: Overexpression of podoplanin in oral cancer
and its association with poor clinical outcome. Cancer.
107:563–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Forastiere A, Koch W, Trotti A and
Sidransky D: Head and neck cancer. N Engl J Med. 345:1890–1900.
2001. View Article : Google Scholar
|
|
9
|
Sano D and Myers JN: Metastasis of
squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev.
26:645–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Silverman S Jr: Demographics and
occurrence of oral and pharyngeal cancers. The outcomes, the
trends, the challenge. J Am Dent Assoc. 132(Suppl): S7S–S11. 2001.
View Article : Google Scholar
|
|
11
|
Gervásio OL, Dutra RA, Tartaglia SM,
Vasconcellos WA, Barbosa AA and Aguiar MC: Oral squamous cell
carcinoma: A retrospective study of 740 cases in a Brazilian
population. Braz Dent J. 12:57–61. 2001.PubMed/NCBI
|
|
12
|
Bundgaard T, Bentzen SM and Wildt J: The
prognostic effect of tobacco and alcohol consumption in intra-oral
squamous cell carcinoma. Eur J Cancer B Oral Oncol. 30B:323–328.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lissowska J, Pilarska A, Pilarski P,
Samolczyk-Wanyura D, Piekarczyk J, Bardin-Mikolłajczak A, Zatonski
W, Herrero R, Munoz N and Franceschi S: Smoking, alcohol, diet,
dentition and sexual practices in the epidemiology of oral cancer
in Poland. Eur J Cancer Prev. 12:25–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou JM, Zhu XF, Pan QC, Liao DF, Li ZM
and Liu ZC: Manumycin inhibits cell proliferation and the Ras
signal transduction pathway in human hepatocellular carcinoma
cells. Int J Mol Med. 11:767–771. 2003.PubMed/NCBI
|
|
15
|
Frassanito MA, Cusmai A, Piccoli C and
Dammacco F: Manumycin inhibits farnesyltransferase and induces
apoptosis of drug-resistant interleukin 6-producing myeloma cells.
Br J Haematol. 118:157–165. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Paolo A, Danesi R, Nardini D, Bocci G,
Innocenti F, Fogli S, Barachini S, Marchetti A, Bevilacqua G and
Del Tacca M: Manumycin inhibits ras signal transduction pathway and
induces apoptosis in COLO320-DM human colon tumour cells. Br J
Cancer. 82:905–912. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li JG, She MR, Lu CY, Wei SS, Xia PF, Lu
ZS and Peng Q: Manumycin induces apoptosis in prostate cancer
cells. Onco Targets Ther. 7:771–777. 2014.PubMed/NCBI
|
|
18
|
Xiong Q and Rikihisa Y: The prenylation
inhibitor manumycin A reduces the viability of Anaplasma
phagocytophilum. J Med Microbiol. 60:744–749. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Devanand P, Kim SI, Choi YW, Sheen SS, Yim
H, Ryu MS, Kim SJ, Kim WJ and Lim IK: Inhibition of bladder cancer
invasion by Sp1-mediated BTG2 expression via inhibition of DNA
methyltransferase 1. FEBS J. 281:5581–5601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krishnan V, Wang X and Safe S: Estrogen
receptor-Sp1 complexes mediate estrogen-induced cathepsin D gene
expression in MCF-7 human breast cancer cells. J Biol Chem.
269:15912–15917. 1994.PubMed/NCBI
|
|
21
|
Chuang CW, Pan MR, Hou MF and Hung WC:
Cyclooxygenase-2 up-regulates CCR7 expression via AKT-mediated
phosphorylation and activation of Sp1 in breast cancer cells. J
Cell Physiol. 228:341–348. 2013. View Article : Google Scholar
|
|
22
|
Banerjee S, Sangwan V, McGinn O, Chugh R,
Dudeja V, Vickers SM and Saluja AK: Triptolide-induced cell death
in pancreatic cancer is mediated by O-GlcNAc modification of
transcription factor Sp1. J Biol Chem. 288:33927–33938. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo MM, Hu LH, Wang YQ, Chen P, Huang JG,
Lu N, He JH and Liao CG: miR-22 is down-regulated in gastric
cancer, and its overexpression inhibits cell migration and invasion
via targeting transcription factor Sp1. Med Oncol. 30:5422013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singha PK, Pandeswara S, Venkatachalam MA
and Saikumar P: Manumycin A inhibits triple-negative breast cancer
growth through LC3-mediated cytoplasmic vacuolation death. Cell
Death Dis. 4:e4572013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Courey AJ and Tjian R: Analysis of Sp1 in
vivo reveals multiple transcriptional domains, including a novel
glutamine-rich activation motif. Cell. 55:887–898. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu S and Archer MC: Sp1 coordinately
regulates de novo lipogenesis and proliferation in cancer cells.
Int J Cancer. 126:416–425. 2010. View Article : Google Scholar
|
|
27
|
Opitz OG and Rustgi AK: Interaction
between Sp1 and cell cycle regulatory proteins is important in
transactivation of a differentiation-related gene. Cancer Res.
60:2825–2830. 2000.PubMed/NCBI
|
|
28
|
Wang L, Wei D, Huang S, Peng Z, Le X, Wu
TT, Yao J, Ajani J and Xie K: Transcription factor Sp1 expression
is a significant predictor of survival in human gastric cancer.
Clin Cancer Res. 9:6371–6380. 2003.PubMed/NCBI
|
|
29
|
Yamaguchi H and Wang HG: CHOP is involved
in endoplasmic reticulum stress-induced apoptosis by enhancing DR5
expression in human carcinoma cells. J Biol Chem. 279:45495–45502.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimizu S, Narita M and Tsujimoto Y: Bcl-2
family proteins regulate the release of apoptogenic cytochrome c by
the mitochondrial channel VDAC. Nature. 399:483–487. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sankpal UT, Goodison S, Abdelrahim M and
Basha R: Targeting Sp1 transcription factors in prostate cancer
therapy. Med Chem. 7:518–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chintharlapalli S, Papineni S, Ramaiah SK
and Safe S: Betulinic acid inhibits prostate cancer growth through
inhibition of specificity protein transcription factors. Cancer
Res. 67:2816–2823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jutooru I, Chadalapaka G, Sreevalsan S,
Lei P, Barhoumi R, Burghardt R and Safe S: Arsenic trioxide
downregulates specificity protein (Sp) transcription factors and
inhibits bladder cancer cell and tumor growth. Exp Cell Res.
316:2174–2188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi ES, Cho SD, Jeon JG and Cho NP: The
apoptotic effect of the hexane extract of Rheum undulatum L. in
oral cancer cells through the down-regulation of specificity
protein 1 and survivin. Lab Anim Res. 27:19–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bhat UG, Halasi M and Gartel AL: Thiazole
antibiotics target FoxM1 and induce apoptosis in human cancer
cells. PLoS One. 4:e55922009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Basso AD, Solit DB, Munster PN and Rosen
N: Ansamycin antibiotics inhibit Akt activation and cyclin D
expression in breast cancer cells that overexpress HER2. Oncogene.
21:1159–1166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakano K, Mizuno T, Sowa Y, Orita T,
Yoshino T, Okuyama Y, Fujita T, Ohtani-Fujita N, Matsukawa Y,
Tokino T, et al: Butyrate activates the WAF1/Cip1 gene promoter
through Sp1 sites in a p53-negative human colon cancer cell line. J
Biol Chem. 272:22199–22206. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Toyoshima H and Hunter T: p27, a novel
inhibitor of G1 cyclin-Cdk protein kinase activity, is related to
p21. Cell. 78:67–74. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yoon MK, Mitrea DM, Ou L and Kriwacki RW:
Cell cycle regulation by the intrinsically disordered proteins p21
and p27. Biochem Soc Trans. 40:981–988. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee TH, Chang HC, Chuang LY and Hung WC:
Involvement of PKA and Sp1 in the induction of p27(Kip1) by
tamoxifen. Biochem Pharmacol. 66:371–377. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Neto AG, McCutcheon IE, Vang R, Spencer
ML, Zhang W and Fuller GN: Elevated expression of p21 (WAF1/Cip1)
in hormonally active pituitary adenomas. Ann Diagn Pathol. 9:6–10.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Quinn BA, Dash R, Azab B, Sarkar S, Das
SK, Kumar S, Oyesanya RA, Dasgupta S, Dent P, Grant S, et al:
Targeting Mcl-1 for the therapy of cancer. Expert Opin Investig
Drugs. 20:1397–1411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tanaka C, Uzawa K, Shibahara T, Yokoe H,
Noma H and Tanzawa H: Expression of an inhibitor of apoptosis,
survivin, in oral carcinogenesis. J Dent Res. 82:607–611. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Heibein JA, Goping IS, Barry M, Pinkoski
MJ, Shore GC, Green DR and Bleackley RC: Granzyme B-mediated
cytochrome c release is regulated by the Bcl-2 family members bid
and Bax. J Exp Med. 192:1391–1402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kelekar A, Chang BS, Harlan JE, Fesik SW
and Thompson CB: Bad is a BH3 domain-containing protein that forms
an inactivating dimer with Bcl-XL. Mol Cell Biol. 17:7040–7046.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hsu SY, Kaipia A, Zhu L and Hsueh AJ:
Interference of BAD (Bcl-xL/Bcl-2-associated death
promoter)-induced apoptosis in mammalian cells by 14-3-3 isoforms
and P11. Mol Endocrinol. 11:1858–1867. 1997.PubMed/NCBI
|
|
49
|
Kharbanda S, Pandey P, Schofield L,
Israels S, Roncinske R, Yoshida K, Bharti A, Yuan ZM, Saxena S,
Weichselbaum R, et al: Role for Bcl-xL as an inhibitor of cytosolic
cytochrome C accumulation in DNA damage-induced apoptosis. Proc
Natl Acad Sci USA. 94:6939–6942. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Marzo I, Brenner C, Zamzami N, Susin SA,
Beutner G, Brdiczka D, Rémy R, Xie ZH, Reed JC and Kroemer G: The
permeability transition pore complex: A target for apoptosis
regulation by caspases and bcl-2-related proteins. J Exp Med.
187:1261–1271. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Beurel E and Jope RS: The paradoxical pro-
and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic
apoptosis signaling pathways. Prog Neurobiol. 79:173–189. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Moon DO, Park SY, Choi YH, Ahn JS and Kim
GY: Guggulsterone sensitizes hepatoma cells to TRAIL-induced
apoptosis through the induction of CHOP-dependent DR5: Involvement
of ROS-dependent ER-stress. Biochem Pharmacol. 82:1641–1650. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|