Introduction
Plants are believed to contain chemical compounds
that inhibit the proliferation of cancer-derived cells in
vitro, and many attempts have been made to isolate anticancer
drugs from plants. For example, the diterpene paclitaxel is a
well-known anti-proliferative agent isolated from Taxus
brevifolia (1). Various groups
have conducted research on other diterpene-containing species, with
the aim of finding more effective agents for the treatment of
cancer (2,3).
Inula cappa is a subshrub of the genus
Inula. Its roots and/or whole plants have been used as
medicines because of their pharmacological effects such as
antitussive activity, promoting the expulsion of phlegm, promoting
blood circulation to restore menstrual flow and wound healing
(4). It is generally used as a
folk medicine by the Zhuang minority in the districts of Wenshan
and Xichou in Yunnan Province, China, for its anti-inflammatory and
detumescence effects. Inula cappa, named ‘Na Han’ by the Dai
nationality, was one of the primary ingredients of the ethnic
medicine formula ‘Ya Jiao Ha Dun San’, which was efficiently used
for the treatment of rheumatoid arthritis, laryngotracheitis,
irregular menstrual periods and abdominal pain. To date, the
chemical constituents such as sesquiterpenoids, triterpenoids,
steroids, anthraquinones, flavonoids, balmy compounds, amides, and
organic acids have been obtained from the roots of Inula
cappa (5). Among these, the
triterpenoids and steroids are the most predominant
constituents.
In the present study, the biological activity of the
sesquiterpene ineupatorolide B (InB) was examined using MTT assay,
TUNEL assay, luciferase reporter assay and western blotting using
human cancer cell lines. These results suggested the involvement of
protein kinase Cα (PKCα) in the cytotoxic acitivity of InB.
Materials and methods
Agents
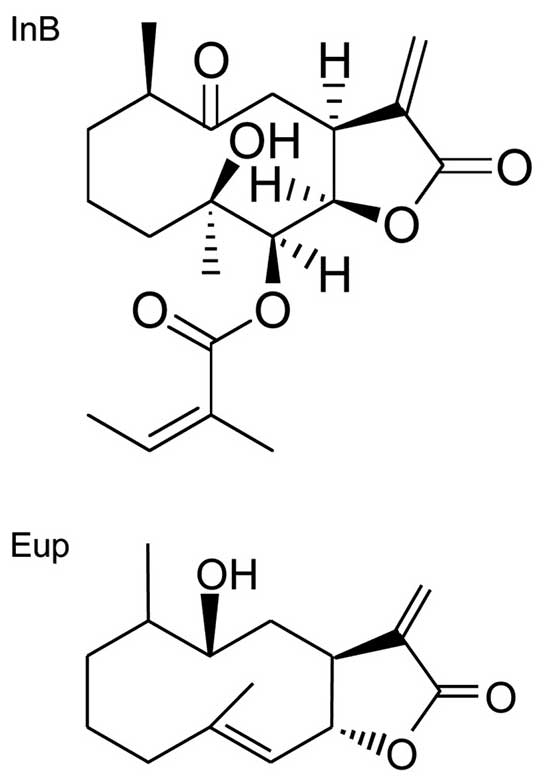
The structures of two sesquiterpenoids, InB and
eupatolide (Eup), have been analyzed using one-dimensional (1-D)
and two-dimensional (2-D) nuclear magnetic resonance (NMR) spectral
data and previously studied (6,7). The
compounds were dissolved in dimethyl sulfoxide (DMSO; Wako Pure
Chemical Industries, Ltd., Osaka, Japan).
Cells and culture conditions
The following human cancer derived cell lines were
used: HeLa (cervical cancer), HOC-21 (ovarian adenocarcinoma), T-98
(glioblastoma), U251SP (glioblastoma), A549 (lung carcinoma), QG-56
(lung carcinoma), PC-6 (lung carcinoma), HLE (hepatoma), and MM1-CB
(melanoma) (8). The cells were
cultured in Eagle's minimum essential medium (EMEM) supplemented
with 10% (v/v) calf serum (Thermo Fisher Scientific, Waltham, MA,
USA) or in Dulbecco's modified Eagle's medium (Wako Pure Chemical
Industries, Ltd.) supplemented with 10% fetal bovine serum
supplemented with antibiotics [100 μg/ml of streptomycin and
100 U/ml of penicillin G (both from Meiji Seika Kaisha, Ltd.,
Tokyo, Japan)] at 37°C in a humidified atmosphere containing 5%
CO2.
Measurement of cell viability
Cell viability was estimated using the MTT assay, as
previously described (9,10). In brief, logarithmically
proliferating cells were seeded (1×104 cells/well) in
96-well plates (Asahi Glass, Tokyo, Japan) with the medium
containing the test compounds at the indicated doses and then
cultured for 2 days. After the culture period, the activity of
mitochondrial succinic dehydrogenase was measured by further
incubation of the cells with 0.5 mg/ml
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich, St. Louis, MO, USA) for 4 h. After this incubation
period, the absorbance of each well was measured at 570 nm with a
reference wavelength at 655 nm. Cell survival was calculated from
this absorbance and presented as the percentage of surviving
cells.
TUNEL assay
We conducted in situ labeling of the
fragmented DNA using the TUNEL method (11,12).
In brief, HeLa cells were cultured in the presence of InB at 10 μM
for 12 h or 24 h. After culturing, we stained the fragmented DNA in
the cells using an apoptosis in situ detection kit (Wako
Pure Chemical Industries, Ltd.) in accordance with the
manufacturer's instructions.
Luciferase assay
The firefly luciferase reporter plasmid, PG13-Luc
(13), was provided by Dr Bert
Vogelstein (Howard Hughes Medical Institute). The plasmids,
pGL3-p21-Luc (14) and
pGL3-Bax-Luc (15) were provided
by Dr Mian Wu (University of Science and Technology of China). The
plasmid pGV-B2 hNoxa-Luc (16) was
provided by Dr Nobuyuki Tanaka (Nippon Medical School). SRE-Luc
(serum-responsive element), IgK-Luc (NF-κB), nuclear factor of
activated T-cell (NFAT)-Luc, CRE-Luc (cAMP-responsive element), and
control Renilla luciferase reporter SV40-Rluc were purchased
from Promega Corp. (Madison, WI, USA).
HeLa cells seeded in 24-well plates were then
transfected with test genes along with the firefly luciferase and
Renilla luciferase reporter plasmids using
Lipofectamine-Plus (Thermo Fisher Scientific). Two days after the
transfection, firefly and Renilla luciferase activities were
determined using the Dual-Luciferase Assay system (Promega Corp.)
and a luminescencer (Atto, Tokyo, Japan). Subsequently, the firefly
luciferase activities were normalized to the Renilla
luciferase control activities, as previously described (17). The inhibitory compounds used were
previously described (10,18).
Western blot analysis
Western blotting analysis was carried out as
previously described (19,20). After treatment with InB, the cells
were washed with phosphate-buffered saline and then lyzed by
incubation in a sodium dodecyl sulfate (SDS) sample buffer at 100°C
for 3 min. The whole cell lysate was then subjected to
SDS-polyacrylamide gel electrophoresis, followed by western
blotting using the specific antibodies targeting PKCα (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), phospho-specific PKCα
(p-PKCα) and PKCδ (p-PKCδ) (both from Cell Signaling Technology,
Inc., Danvers, MA, USA), PKCɛ (p-PKCɛ; Santa Cruz Biotechnology),
and PKCθ (p-PKCθ) and PKCλ (p-PKCλ) (both from Cell Signaling
Technology, Inc.), and β-actin (Santa Cruz Biotechnology,
Inc.).
Knockdown experiments
PKC subtype-specific siRNAs and a non-targeted
negative control siRNA were obtained from Qiagen (Hilden, Germany).
HeLa cells were transfected with siRNA using RNAiFect transfection
reagent (Qiagen). Two days after the transfection, the cells were
re-plated for the MTT assay. The subtype-specific knockdown of PKC
was confirmed by western blotting.
Statistical analysis
Statistical analyses were performed using the
Student's t-test with StatView software (version 4.5; Abacus
Concepts, Berkeley, CA, USA), as previously described (19).
Results
Growth-inhibitory activity of InB
Among the many compounds found in Inula
cappa, we selected two bioactive compounds, InB and Eup. The
chemical structures of InB and Eup are shown in Fig. 1. The cytotoxic potential of InB and
Eup was examined using the MTT assay after culture in the presence
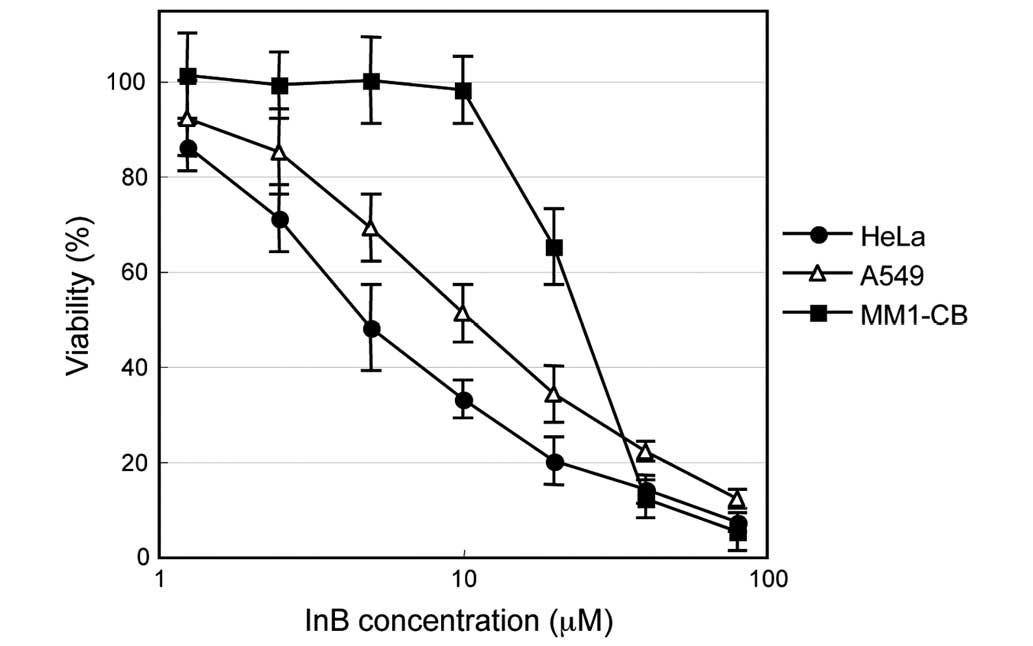
of each compound for 2 days. A representative result is shown in
Fig. 2. The IC50
concentrations of InB for HeLa, A549, and MM1-CB cells were 4.8,
10.8 and 23.2 μM, respectively. The IC50 values for the
other cells examined thus far are shown in Table I. InB exhibited a more potent
growth-inhibitory activity than Eup for all cell lines examined. We
further examined the cytotoxic activity of InB against HeLa cells,
which were among the most sensitive to InB.
 | Table IIC50 values of InB and
Eup. |
Table I
IC50 values of InB and
Eup.
| Cell lines |
|---|
|
|
|---|
| Agents | HeLa | HOC-21 | T-98 | U251SP | A549 | QG-56 | PC-6 | HLE | MM1-CB |
|---|
| InB | 4.8 | 7.9 | 7.1 | 13.8 | 10.8 | 4.4 | 17.7 | 21.6 | 23.2 |
| Eup | 64.4 | 46.5 | 67.7 | >100 | 70.9 | >100 | >100 | 30.2 | 26.0 |
Induction of apoptosis by InB
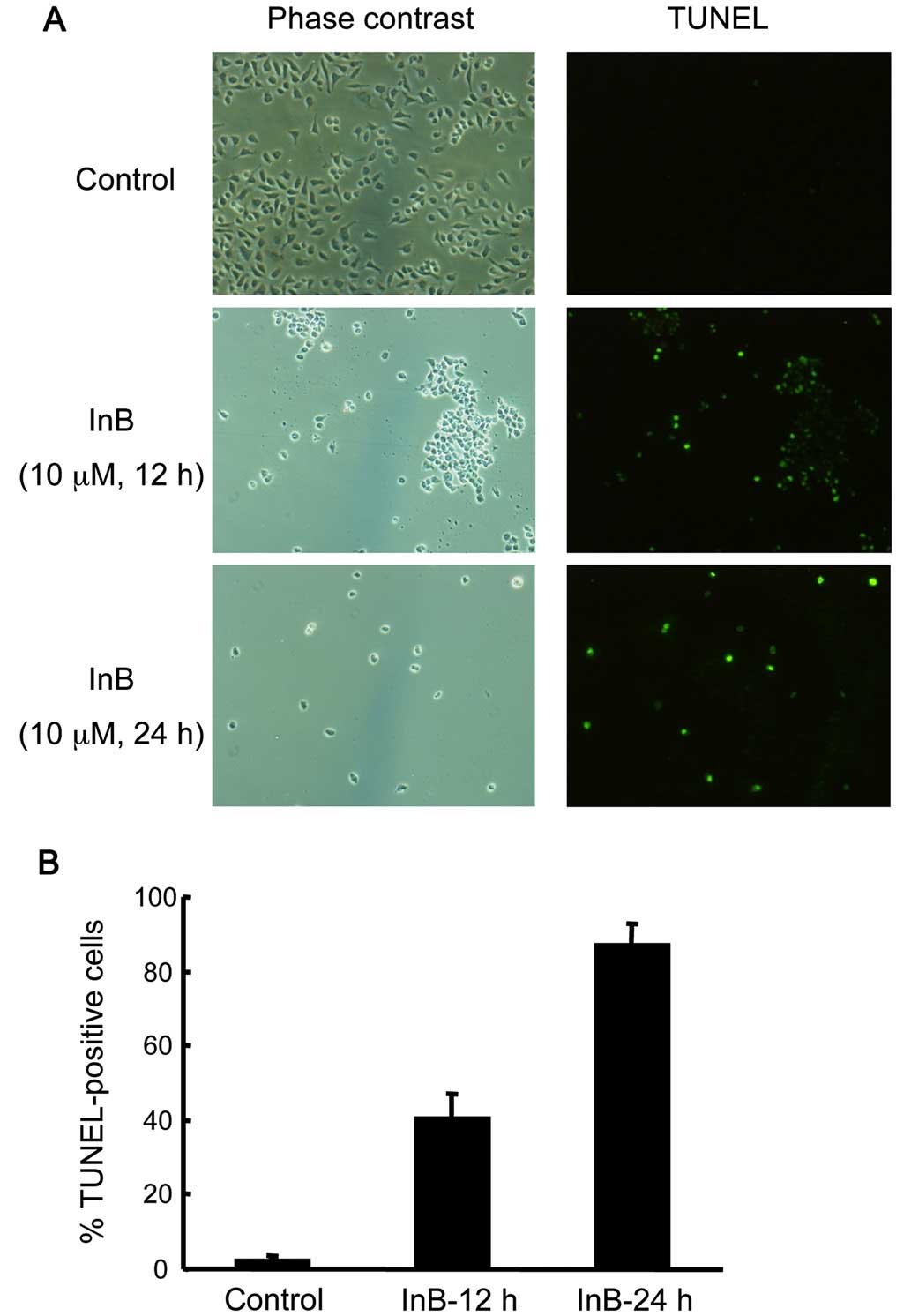
To examine whether the cytotoxic activity of InB was
due to the induction of apoptosis, we performed TUNEL staining,
which can detect the typical DNA fragmentation accompanied by
apoptosis. TUNEL-positive cell numbers increased as early as 12 h
after the addition of InB, and >80% of the cells were stained
after 24 h (Fig. 3). These results
suggest that the growth-inhibitory activity of InB was, at least in
part, due to the induction of apoptosis.
Activation of p53 and NFAT reporter
plasmids by InB
The transactivation ability of several major
transcription factors was examined using a luciferase reporter
assay. Among the reporters for p53 (PG13-Luc, p21-Luc, Bax-Luc, and
hNoxa-Luc), serum-responsive factor (SRE-Luc), NF-κB (IgK-Luc),
NFAT (NFAT-Luc) and CREB (CRE-Luc), the reporters PG13-Luc,
Bax-Luc, and NFAT-Luc were activated by InB in a dose-dependent
manner (Fig. 4A). The activation
of NFAT-Luc by InB in HeLa cells was greater than that observed in
InB-resistant MM1-CB cells whereas the activation of PG13-Luc and
Bax-Luc was similar between these cells (data not shown). Thus, the
elevation of NFAT transcriptional activity may be associated with
the cytotoxic activity of InB.
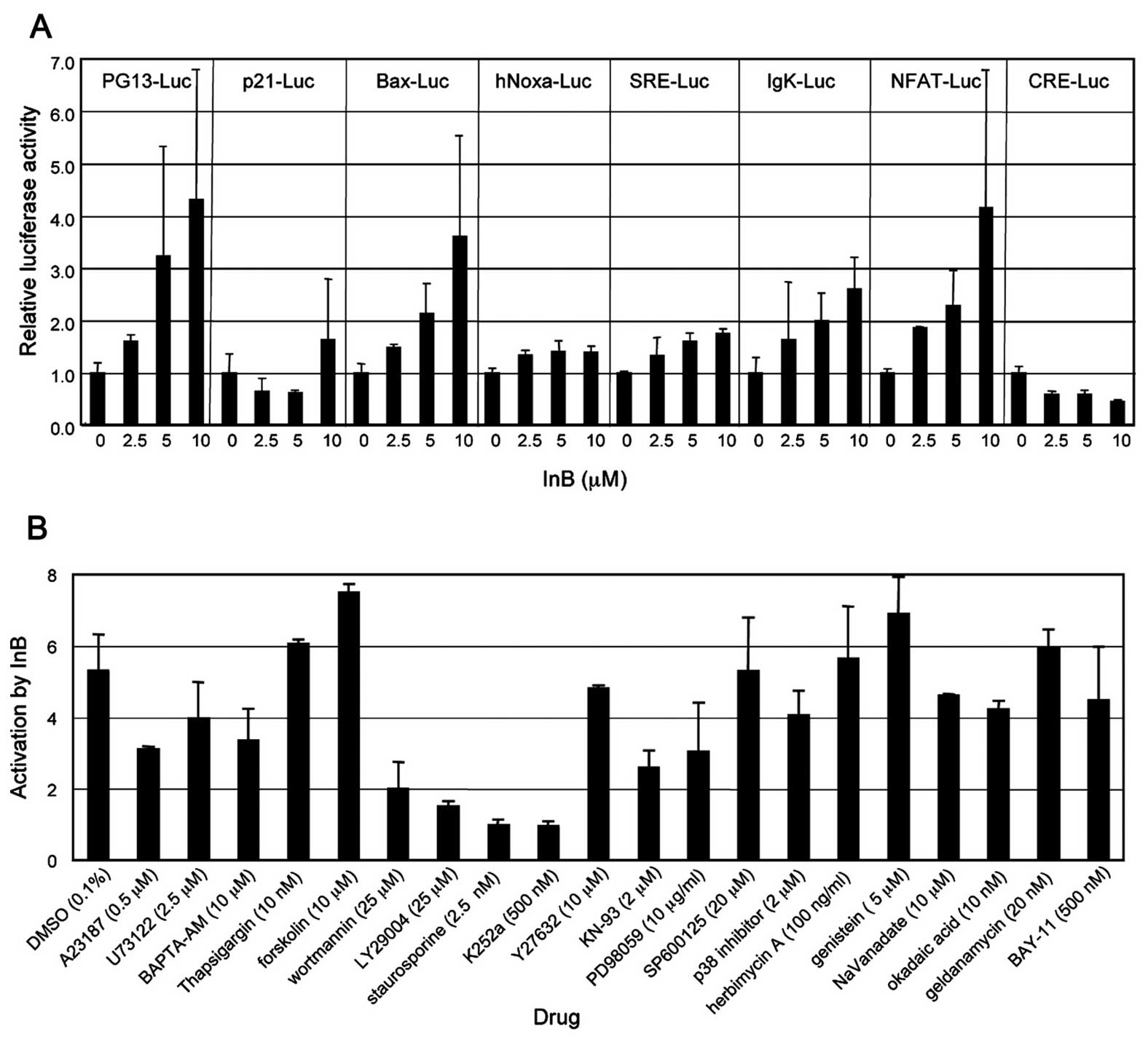 | Figure 4Activation of p53 and NFAT by
ineupatorolide B (InB). (A) HeLa cells were co-transfected with
firefly reporter plasmids such as pG13-Luc, p21-Luc, Bax-Luc,
hNoxa-Luc, SRE-Luc, IgK-Luc, NFAT-Luc and CRE-Luc, and the
Renilla reporter plasmid, SV40-Rluc, and then cultured for
48 h. The cells were then treated with InB at concentrations of 0,
2.5, 5 or 10 μM for 24 h and harvested. The firefly and
Renilla luciferase activities in the cell extracts were
sequentially measured. The activity of firefly luciferase was
normalized using Renilla luciferase activity. The error bars
represent SD. (B) Effects of inhibitory compounds on the activation
of NFAT by InB. The cells were treated as described above, except
that they were pretreated with various drugs at the concentrations
indicated for 1 h before the addition of InB. The relative
activation of luciferase activity in cells treated with InB at a
concentration of 10 μM vs. that of cells without InB treatment is
shown. |
PKC inhibitor suppression of the
InB-induced NFAT activation
We then examined the effects of various inhibitors
or activators on NFAT reporter activity. Preincubation with PKC
inhibitors such as staurosporine and K252a suppressed the
InB-induced activation of NFAT (Fig.
4B), suggesting the involvement of PKC. Wortmannin and
LY290004, both of which are inhibitors of phosphatidylinositol
3-kinase, also attenuated the NFAT activation, but less
effectively. Pretreatment with the other substances tested had
almost no effect on InB-induced activation of NFAT. These
substances included A23187 (calcium ionophore), U73122
(phospholipase C inhibitor), BAPTA-AM (intracellular calcium
chelator), forskolin (adenylate cyclase activator), Y27632 (ROCK
inhibitor), KN-93 (CaMK inhibitor), PD98059 (MEK inhibitor),
SP600125 (JNK inhibitor), p38 inhibitor (p38 MAPK inhibitor),
herbimycin A (Src kinase inhibitor), genistein (tyrosine kinase
inhibitor), vanadate (tyrosine phosphatase inhibitor), okadaic acid
(PP2A and PP1 inhibitor), geldanamycin (HSP90 inhibitor) and BAY-11
(NF-κB inhibitor) (10).
Activation of PKCα by InB
Activation of PKC is accompanied by phosphorylation,
which can be detected by western blotting using a phospho-specific
antibody. The phosphorylation level of PKCα, but not of other PKC
subtypes such as PKCδ, PKCɛ, PKCθ, and PKCλ, increased after
treatment with InB (Fig. 5). This
was not caused by an increase in the levels of protein expression
because the levels of total PKCα were not altered during the
incubation time of up to 24 h. An initial increase of
phosophorylated PKCα was observed as early as 10 min after the
addition of InB, and the level increased to a maximum 4 h after the
addition of InB. Thereafter, the phosphorylation level gradually
decreased; however, it maintained a higher level than the basal
level in non-treated cells 24 h after InB addition.
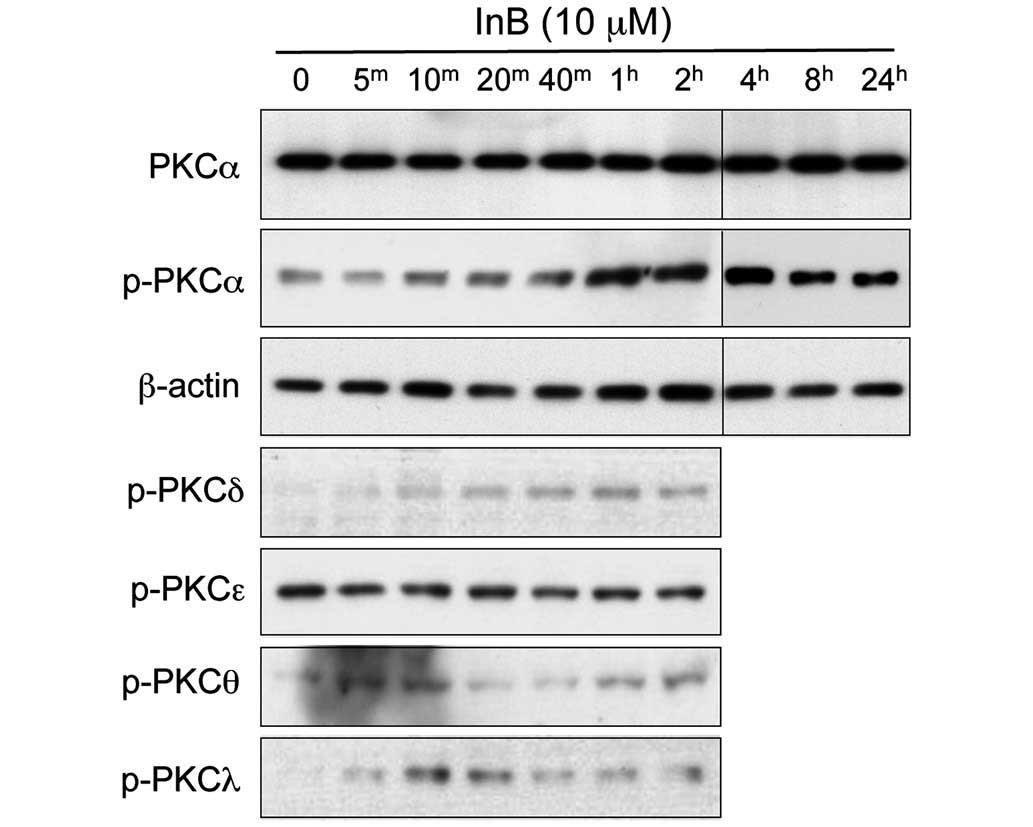 | Figure 5Activation of PKC by ineupatorolide B
(InB). HeLa cells were treated with 10 μM InB for 0, 5 min
(5m), 10 min (10m), 20 min (20m),
40 min (40m), 1 h (1h), 2 h (2h),
4 h (4h), 8 h (8h) and 24 h (24h),
and then a cell extract was prepared. Western blotting was
performed using anti-PKC, phospho-specific anti-PKCα,
phospho-specific anti-PKCδ, phospho-specific anti-PKCɛ,
phospho-specific anti-PKCθ, phospho-specific anti-PKCλ, and control
anti-β-actin antibodies. |
Knockdown of PKCα attenuates the
cytotoxic activity of InB
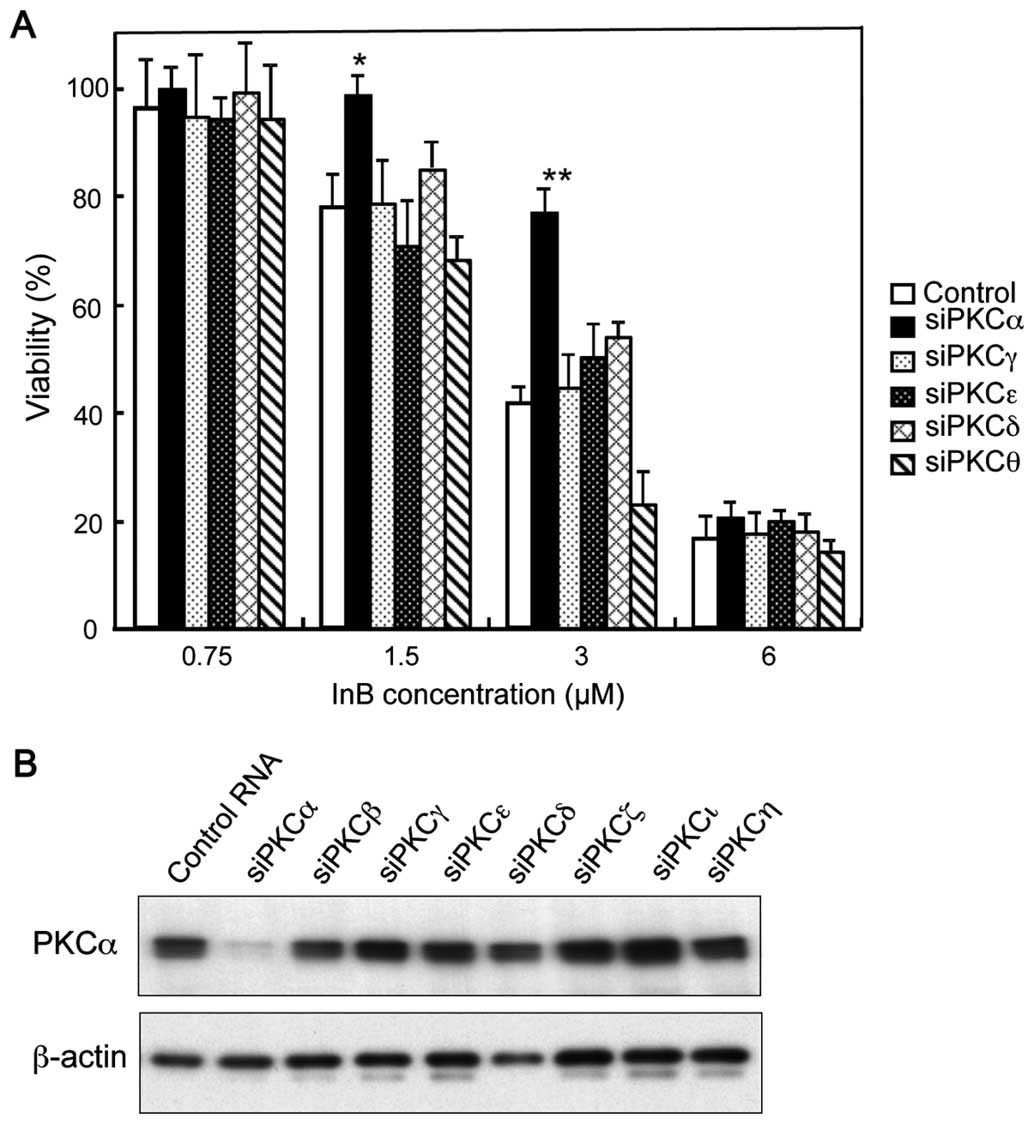
We then examined the effects of siRNAs targeting
various PKC subtypes on cellular survival in the presence of InB.
Knockdown of PKCα but not PKCγ, PKCɛ, PKCδ, or PKCθ attenuated the
cytotoxic activity caused by the addition of InB at concentrations
of up to 3 μM (Fig. 6A). The
expected knockdown of PKCα was confirmed by western blotting, which
showed an almost complete reduction of PKCα protein with no
apparent effects on the other PKC subtypes (Fig. 6B). The effects of siRNAs targeting
PKCβ, PKCγ, PKCζ, PKCι, and PKCη showed no apparent difference as
compared with those of control siRNAs (data not shown).
Consequently, it is suggested that InB can exhibit cytotoxic
effects via the activation of PKCα.
Discussion
Inula cappa contains many bioactive compounds
(6,7,21,22),
among which sesquiterpenoids may play a main role. In this study,
the effects of InB, one of sesquiterpenoids, on the proliferation
and survival of tumor cell lines were examined. Compared with A549
lung carcinoma and MM-CB melanoma cells, we found that HeLa
cervical cancer cells were highly sensitive to InB than (Fig. 2). The decrease of the cell survival
capacity after treatment with InB may mainly be attributable to the
induction of apoptosis (Fig. 3).
Luciferase reporter assay were performed to analyze the mechanism
of action of InB. The results showed the involvement of p53 and
NFAT/Ca2+ in the signaling pathway (Fig. 4A). Because the activation of NFAT
by InB was reduced in InB-resistant MM1-CB cells, we further
examined the NFAT signaling pathway. Using various inhibitors
(Fig. 4B) and siRNAs (Fig. 6), we found the involvement of PKCα
in the cytotoxic effects caused by InB.
The involvement of NFAT in apoptosis remains
obscure; however, a recent report verified the induction of
apoptosis by NFATc3 (23).
InB-induced transactivation ability of NFAT was inhibited by PKC
inhibitors such as staurosporine and K252a (Fig. 4B). This is consistent with a recent
report which stated that the staurosporine analogue GF109203X,
reduced NFAT activity in osteoclast progenitor cells (24).
The activation of PKCα was observed as early as 10
min after the addition of InB (Fig.
5). This alteration appeared to be the initial event induced by
InB, and therefore, it is probable that PKCα is the direct target
of InB. The tumor promoter,
12-O-tetradecanoylphorbol-13-acetate (TPA) (25) is a well-known diacylglycerol-like
PKC-activating compound. What could be the difference between
tumor-promoting TPA and cytotoxic InB? TPA is highly active because
of its direct activation of PKC at nM concentration, whereas InB
can cause effects at μM concentrations. TPA can activate the
conventional and novel subtypes of PKC such as PKCα, β, γ, δ, ɛ, η
and θ, however, InB activated PKCα exclusively (Fig. 5). After treatment with TPA, the
levels of the total protein amount of PKC were rapidly
downregulated to undetectable levels within 24 h (26). Conversely, phosphorylated PKCα was
still detectable and the total amount of PKCα was not altered after
a 24-h treatment period with InB (Fig.
5). Thus, the specific and continuous activation of PKCα by InB
may account for the cytotoxic effects leading to apoptosis.
In the reporter assay, p53 was also activated by
InB, although the activation of p53 in InB-sensitive HeLa cells was
similar to that in InB-resistant MM1-CB cells. Because p53 plays a
main role in the induction of apoptosis, adenoviral wild-type p53
has been used for gene therapy in esophageal squamous cell
carcinoma (27). InB as a
p53-activating compound is a promising and novel anticancer
drug.
Acknowledgements
The present study was supported, in part, by a
Grant-in-Aid for Scientific Research from the Ministry of
Education, Culture, Sports, Science and Technology of Japan as well
as grants from the Japan China Medical Association and Goho Life
Sciences International Fund. The authors thank Drs. Bert Vogelstein
(Howard Hughes Medical Institute), Mian Wu (University of Science
and Technology of China) and Nobuyuki Tanaka (Nippon Medical
School) for providing reporter plasmids. We also thank Professor
Masaki Takiguchi (Department of Biochemistry and Genetics, Graduate
School of Medicine, Chiba University) for valuable discussion.
References
|
1
|
Howat S, Park B, Oh IS, Jin YW, Lee EK and
Loake GJ: Paclitaxel: Biosynthesis, production and future
prospects. N Biotechnol. 31:242–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong M, Chen SP, Kita K, Ichimura Y, Guo
WZ, Lu S, Sugaya S, Hiwasa T, Takiguchi M, Mori N, et al:
Anti-proliferative and apoptosis-inducible activity of Sarcodonin G
from Sarcodon scabrosus in HeLa cells. Int J Oncol. 34:201–207.
2009.
|
|
3
|
Chen SP, Dong M, Kita K, Shi QW, Cong B,
Guo WZ, Sugaya S, Sugita K and Suzuki N: Anti-proliferative and
apoptosis-inducible activity of labdane and abietane diterpenoids
from the pulp of Torreya nucifera in HeLa cells. Mol Med Rep.
3:673–678. 2010.
|
|
4
|
Xie HG, Chen H, Cao B, Zhang HW and Zou
ZM: Cytotoxic germacranolide sesquiterpene from Inula cappa. Chem
Pharm Bull (Tokyo). 55:1258–1260. 2007. View Article : Google Scholar
|
|
5
|
Seca AM, Grigore A, Pinto DC and Silva AM:
The genus Inula and their metabolites: From ethnopharmacological to
medicinal uses. J Ethnopharmacol. 154:286–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baruah RN, Sharma RP, Thyagarajan G, Herz
W, Govindan SV and Blount JF: Unusual germacranolides from Inula
eupatorioides. J Org Chem. 45:4838–4843. 1980. View Article : Google Scholar
|
|
7
|
Lee J, Hwangbo C, Lee JJ, Seo J and Lee
JH: The sesquiterpene lactone eupatolide sensitizes breast cancer
cells to TRAIL through down-regulation of c-FLIP expression. Oncol
Rep. 23:229–237. 2010.
|
|
8
|
Kojima T, Suzuki N, Sugano I and Hayata I:
Enhancement of an anti-tumor effect of interferon by dipyridamole
in established human malignant melanoma cell lines. Int J Cancer.
46:853–857. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wano C, Kita K, Takahashi S, Sugaya S,
Hino M, Hosoya H and Suzuki N: Protective role of HSP27 against
UVC-induced cell death in human cells. Exp Cell Res. 298:584–592.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hiwasa T, Shimada H, Sakaida T, Kitagawa
M, Kuroiwa N, Ochiai T and Takiguchi M: Drug-sensitivity pattern
analysis for study of functional relationship between gene
products. FEBS Lett. 552:177–183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gavrieli Y, Sherman Y and Ben-Sasson SA:
Identification of programmed cell death in situ via specific
labeling of nuclear DNA fragmentation. J Cell Biol. 119:493–501.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hasegawa R, Kita K, Hasegawa R, Fusejima
K, Fukuzawa S, Wano C, Watanabe S, Saisho H, Masuda Y, Nomura F, et
al: Induction of apoptosis and ubiquitin hydrolase gene expression
by human serum factors in the early phase of acute myocardial
infarction. J Lab Clin Med. 141:168–178. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
el-Deiry WS, Kern SE, Pietenpol JA,
Kinzler KW and Vogelstein B: Definition of a consensus binding site
for p53. Nat Genet. 1:45–49. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
el-Deiry WS, Tokino T, Velculescu VE, Levy
DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW and
Vogelstein B: WAF1, a potential mediator of p53 tumor suppression.
Cell. 75:817–825. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyashita T and Reed JC: Tumor suppressor
p53 is a direct transcriptional activator of the human bax gene.
Cell. 80:293–299. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oda E, Ohki R, Murasawa H, Nemoto J,
Shibue T, Yamashita T, Tokino T, Taniguchi T and Tanaka N: Noxa, a
BH3-only member of the Bcl-2 family and candidate mediator of
p53-induced apoptosis. Science. 288:1053–1058. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shinmen N, Koshida T, Kumazawa T, Sato K,
Shimada H, Matsutani T, Iwadate Y, Takiguchi M and Hiwasa T:
Activation of NFAT signal by p53-K120R mutant. FEBS Lett.
583:1916–1922. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimada H, Ito M, Kagaya A, Shiratori T,
Kuboshima M, et al: Elevated serum antibody levels against cyclin
L2 in patients with esophageal squamous cell carcinoma. J Cancer
Sci Ther. 7:60–66. 2015.
|
|
19
|
Zhai L, Kita K, Wano C, Wu Y, Sugaya S and
Suzuki N: Decreased cell survival and DNA repair capacity after UVC
irradiation in association with down-regulation of GRP78/BiP in
human RSa cells. Exp Cell Res. 305:244–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumazawa T, Hiwasa T, Takiguchi M, Suzuki
O and Sato K: Activation of Ras signaling pathways by
pyrroloquinoline quinone in NIH3T3 mouse fibroblasts. Int J Mol
Med. 19:765–770. 2007.PubMed/NCBI
|
|
21
|
Al-Howiriny TA, Mossa JS and Ahmed B:
Beibersteneolides a and b: Two new sesquiterpene lactones from
Achillea beiberstenii. Indian J Chem B. 44B:2538–2544. 2005.
|
|
22
|
Daniewski WM, Danikiewicz W, Gumulka M,
Pankowska E, Krajewski J, Grabarczyk H and Wichacz M:
Sesquiterpenes of Cladanthus arabicus. Phytochemistry.
34:1639–1641. 1993. View Article : Google Scholar
|
|
23
|
Mojsa B, Mora S, Bossowski JP, Lassot I
and Desagher S: Control of neuronal apoptosis by reciprocal
regulation of NFATc3 and Trim17. Cell Death Differ. 22:274–286.
2015. View Article : Google Scholar
|
|
24
|
Yao J, Li J, Zhou L, Cheng J, Chim SM,
Zhang G, Quinn JM, Tickner J, Zhao J and Xu J: Protein kinase C
inhibitor, GF109203X attenuates osteoclastogenesis, bone resorption
and RANKL-induced NF-κB and NFAT activity. J Cell Physiol.
230:1235–1242. 2015. View Article : Google Scholar
|
|
25
|
Nishizuka Y: The molecular heterogeneity
of protein kinase C and its implications for cellular regulation.
Nature. 334:661–665. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohno S and Nishizuka Y: Protein kinase C
isotypes and their specific functions: Prologue. J Biochem.
132:509–511. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimada H, Shimizu T, Ochiai T, Liu TL,
Sashiyama H, Nakamura A, Matsubara H, Gunji Y, Kobayashi S, Tagawa
M, et al: Preclinical study of adenoviral p53 gene therapy for
esophageal cancer. Surg Today. 31:597–604. 2001. View Article : Google Scholar : PubMed/NCBI
|