Introduction
Apoptosis (also known as type I programmed cell
death) is a cellular suicide program critical for development and
tissue homeostasis (1). Excess
apoptosis is associated with degenerative disorders (2), while a failure of apoptosis
contributes to oncogenesis (3).
Anticancer agents can induce apoptosis in tumor cells that is
mediated by the common cell death machinery. Central to this is a
family of intracellular proteases, known as caspases. During
apoptosis, they can act either as initiators (caspases-8 and -9) in
response to apoptotic signals or as effectors (caspase-3, -6 and
-7) that finally cleave a number of vital proteins and lead to the
demise of the cells (1). ROS are
generated from the mitochondria and other cellular sources, and can
oxidize a wide range of cell constituents, including lipids,
proteins and DNA, thus damaging cell structures and compromising
function. When antioxidant mechanisms are overwhelmed by ROS and
subsequent oxidative stress occurs, cell damage and cell death are
induced (4). High levels of ROS
induce cell death, which often involves apoptosis through caspase
activation (5,6).
As a mode of type II programmed cell death,
autophagy is a series of biochemical steps through which eukaryotic
cells commit suicide by degrading their own cytoplasm and
organelles through a process in which these components are engulfed
and then digested in double membrane-bound vacuoles called
autophagosomes (7). However,
autophagy has recently gained much attention for its paradoxical
roles in cell survival and cell death, particularly in the
pathogenesis as well as the treatment of cancer (8,9).
Whether autophagy enables cells to survive or enhances their death
is context-driven, depending on the type of stimuli, nutrient
availability, and apoptotic status (10).
Laryngeal squamous cell carcinoma (LSCC) is the most
common squamous cell carcinoma of the head and neck, and there is a
steady annual increase in new cases and deaths (11). Epidemiological studies have
revealed that the incidence rates vary from country to country and
in different population groups (12). LSCC accounts for 1–8.4% of all
human cancers in China. However, only a minority of patients are
eligible for radical treatment aimed at a cure. The availability of
new cytotoxic drugs has led to steady improvements, but a paradigm
shift is required to significantly affect the poor prognosis of
most patients.
With a long history of cancer treatment, traditional
Chinese medicine (TCM) is recognized as a valuable source for
seeking bioactive anticancer compounds (13). Rabdosia rubescens (Hemsl.)
Hara (Lamiaceae), also known as Dong Ling Cao in TCM,
is commonly used in Chinese folk medicine to treat stomach aches,
pharyngitis, sore throats, coughs and wrestling injuries. This herb
has increasingly gained attention because of its use as an
antitumor herb and because of the antitumor activities of its
discovered bioactive constituents. The herb contains a variety of
active components, including diterpenoids, flavonoids, phenolic
acids, triterpenoids and volatile oils (14). Among these compounds, the
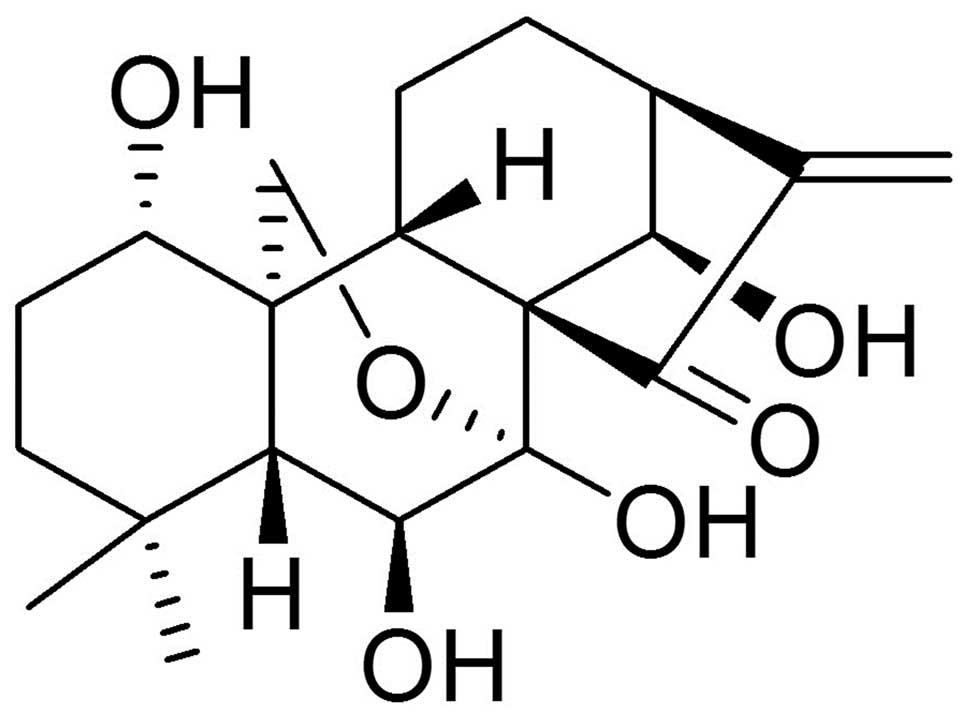
diterpenoid compound oridonin (Fig.
1) was reported to possess inhibitory effects on a variety of
cancer cell lines, such as leukemia, colorectal cancer, esophageal
cancer, liver cancer, epidermoid carcinoma, lung cancer and uterine
cervical cancer (15,16). However, no reports has yet been
found on its anticancer effects in human laryngeal cancer cells
until our laboratory demonstrated that oridonin could induce
apoptosis and G2-M phase arrest in human laryngeal squamous
carcinoma HEp-2 cells (17).
During the course of characterizing the role of the
mitochondrial pathway in oridonin-induced apoptosis in HEp-2 cells,
we made an unexpected observation that a selective inhibitor of
caspase-9 (C9i) could enhance (rather than retard) apoptosis in
response to selected stimuli. Interestingly, our group also found
that caspases did not mediate apoptosis, but protected L929 cells
from oridonin-induced cell death (18). Moreover, Shah et al also
found that a caspase-9 inhibitor could enhance stress-induced
apoptosis in B-lineage cells (19). Driven by the above-mentioned
interesting phenomena, we set out to delineate the signaling
pathways and specifically the role of caspase-9 in oridonin-induced
apoptosis in greater detail. Our data showed that caspase-9 played
an anti-apoptotic role in HEp-2 cells through inhibition of ROS
generation. Further, we discovered that caspase-9 promoted
oridonin-induced autophagy, and in this context, autophagy was a
protective mechanism against apoptosis.
Materials and methods
Cell culture and reagent treatment
Human laryngeal cancer HEp-2 cells were obtained
from the American Type Cell Culture Collection and were cultured as
previously described (20).
Oridonin was isolated from Rabdosia rubescens and was
identified by comparing its physical and spectroscopic
(1H NMR, 13C NMR) data with those reported in
the literature (21). The purity
was measured by HPLC (column: 4.6×150 mm, Inertsil ODS-SP, 5 μm;
solvent phase: methanol/H2O, 55:45) and determined to be
99.6%. Oridonin was dissolved in DMSO to obtain a stock solution.
The DMSO concentration was kept below 0.05% in all the cell
cultures so that it had no detectable effect on cell growth or cell
death.
Growth inhibition assay
HEp-2 cells were incubated in 96-well tissue culture
plates. After a 24-h incubation, the cells were treated with, or
without, pan-caspase inhibitor (z-VAD-fmk), caspase-9, -8, -3, -1
inhibitors [Ac-LEHD-cmk (C9i), z-IETD-fmk, z-DEVD-fmk or
Ac-YVAD-cmk], 3-MA or NAC (Sigma) at the given concentrations for 1
h and subsequently treated with oridonin for 24 h. The cytotoxic
effect was measured by MTT assay as described elsewhere (20).
Observation of morphological changes
HEp-2 cells were seeded into 6-well culture plates.
After a 24-h incubation, the cells were treated with, or without,
C9i or NAC at the given concentrations for 1 h and subsequently
incubated with oridonin for 24 h. The cellular morphology was
observed using a phase contrast microscope (Leica, Nussloch,
Germany).
Transmission electron microscopy
HEp-2 cells were treated with 36 μM oridonin for the
indicated time periods. The collected cells were fixed with PBS
containing 3% glutaraldehyde, and postfixed with PBS containing 1%
OsO4. The samples were dehydrated in graded alcohol
solutions, then embedded and sectioned. Ultrathin sections were
stained with uranyl acetate and lead citrate, and examined using a
JEM-1200 transmission electron microscope (Jeol, Tokyo, Japan)
(22).
Flow cytometric analysis of cell
apoptosis
After chemical treatment, 1×106 cells
were harvested. The collected cells were fixed in 70% ethanol,
stained for DNA content with propidium iodide (PI), and measured by
flow cytometry (Becton-Dickinson, Franklin Lakes, NJ, USA) as
previously described (20). The
sub-G1 DNA content was used as an indicator of apoptosis (23).
Measurement of intracellular ROS
generation
After treatment with 36 μM oridonin for the
indicated time periods, the cells were incubated with 10 mM DCF-DA
(Sigma) at 37°C for 30 min. The intracellular ROS caused oxidation
of DCF-DA to the fluorescent compound DCF. Then, the cells were
harvested and the pellets were suspended in 1 ml PBS. Samples were
analyzed at an excitation wavelength of 480 nm and an emission
wavelength of 525 nm by FACScan flow cytometry (18).
Determination of mitochondrial membrane
potential
The mitochondrial membrane potential was measured
using the fluorescent dye rhodamine-123 (Sigma) by flow cytometry
as previously described (20).
Measurement of autophagy
After incubation with 36 μM oridonin for the fixed
times, cells were cultured with 0.05 mM monodansylcadaverine (MDC)
at 37°C for 60 min. The cellular fluorescent changes were observed
under a fluorescence microscope (Olympus). The fluorescence
intensity of cells was analyzed by FACScan flow cytometry (24).
Assay for caspase-9 activity
A caspase-9 fluorimetric assay kit from Chemicon
International (Temecula, CA, USA) was used according to the
manufacturer's instructions. Briefly, cell lysates were incubated
with peptide substrate, LEHD-pNA (Ac-Leu-Glu-His-Asp-pNA), in assay
buffer [100 mM NaCl, 50 mM HEPES, 10 mM DTT, 1 mM EDTA, 10%
glycerol, 0.1% CHAPS (pH 7.4)] for 2 h at 37°C. The release of
p-nitroaniline was monitored at 405 nm. Caspase-9 activity in cell
lysates was expressed as the x-fold increase from baseline
controls.
RNA interference
HEp-2 cells were transfected with caspase-9 small
interfering RNA (siRNA) and control siRNA (Shanghai GenePharma,
China) by Lipofectamine 2000 (Invitrogen) according to the
manufacturer's instructions. The sequences of the sense strands of
the RNAs used in this study were as follows. Control siRNA: UUC UCC
GAA CGU GUC ACG. Caspase-9 siRNA-1: CGG UGA AAG GGA UUU AUA ATT.
Caspase-9 siRNA-2: CCA AAG UUG UCG AAG CCA ATT CGU UGA. Caspase-9
siRNA-3: GUG ACA UCU UUG UGU CCU ATT.
Transient transfection
HEp-2 cells at 70% confluence were transfected with
control pcDNA3.1-3FLAG and pcDNA3.1-hCASP9-3FLAG plasmid (Shanghai
GeneChem, China). Transfection of cells was performed by using
Lipofectamine 2000 reagent according to the manufacturer's
protocol. The effectiveness of transfection was detected by western
blot analysis.
Preparation of mitochondrial and
cytosolic extracts
HEp-2 cells were collected and then washed twice
with ice-cold PBS. The cell pellets were resuspended in ice-cold
homogenizing buffer (250 mM sucrose, 20 mM HEPES, 10 mM KCl, 1.5 mM
MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 mM
phenylmethanesulfonyl fluoride, 10 μg/ml pepstatin and 10 μg/ml
leupeptin). The cells were then homogenized and centrifuged at
14,000 g at 4°C for 60 min. The supernatant was used as the cytosol
fraction and the pellet was resuspended in lysis buffer as the
membrane fraction (25).
Western blot analysis
Western blot analysis was performed as previously
described (20) using
corresponding antibodies against Beclin 1, LC3, Bax, Bcl-2, AIF,
cytochrome c, ICAD, FADD, caspase-9, -8, -3, Hsp90, β-actin
(Santa Cruz). Representative blots from three independent
experiments are shown.
Statistical analysis of the data
All the presented data and results were confirmed in
at least three independent experiments. The data are expressed as
means ± SD. Statistical comparisons were made by Student's t-test
and P<0.05 was considered statistically significant.
Results
The apoptotic effects of oridonin in
HEp-2 cells are enhanced by an irreversible inhibitor of
caspase-9
Some of our preliminary studies have indicated that
oridonin is able to inhibit the proliferation of human laryngeal
squamous carcinoma HEp-2 cells, and the cell death is shown to be
typical of apoptosis (17,20). Since it is well known that caspase
family members play a pivotal role in the cell apoptosis process,
the effect of caspase inhibitors on oridonin-induced HEp-2 cell
death was examined. Unexpectedly, the C9i enhanced cell death due
to oridonin stimuli, while z-DEVD-fmk, z-IETD-fmk and Z-VAD-fmk
blocked the cytotoxity of the response to oridonin (Fig. 2A).
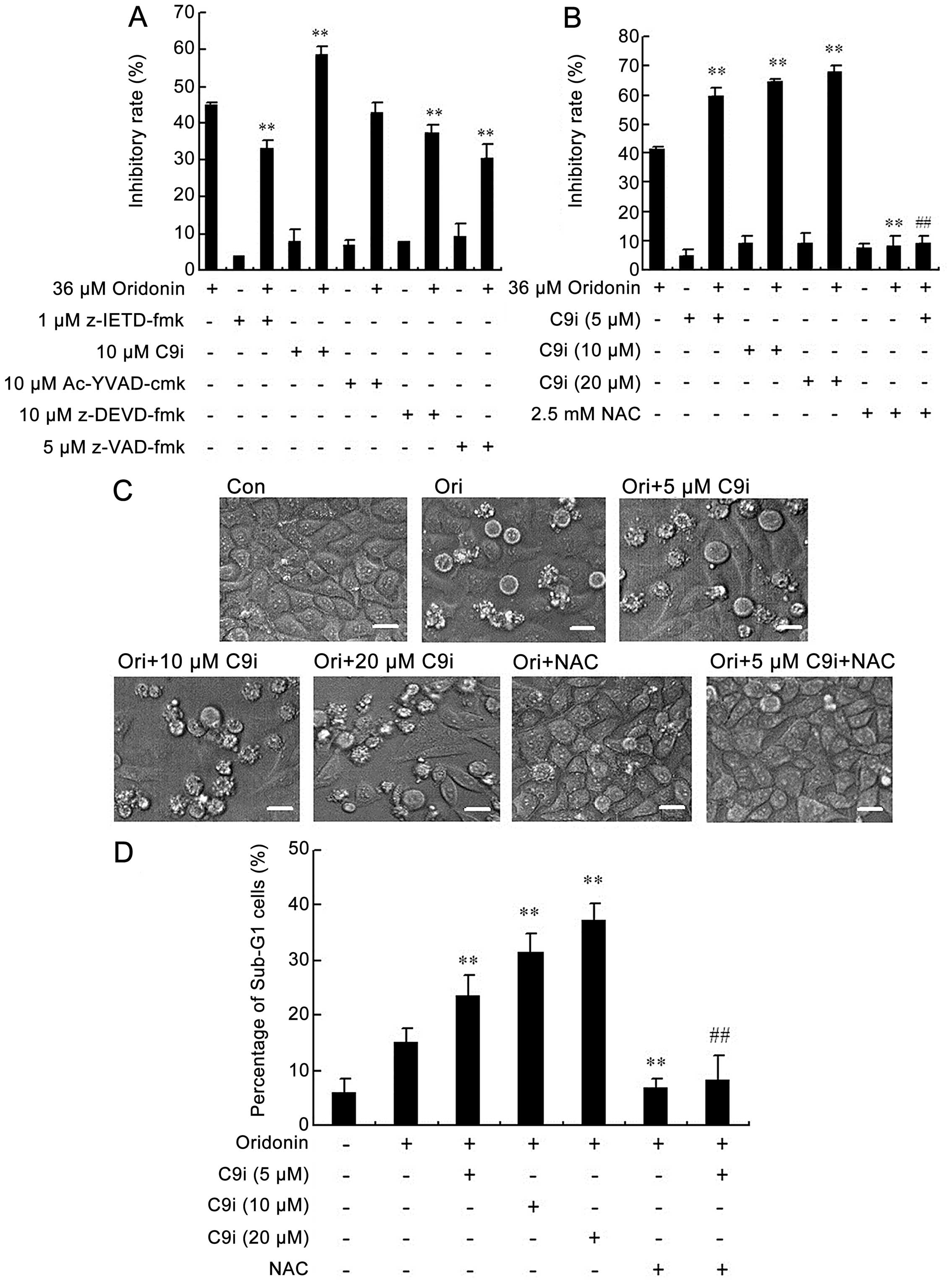 | Figure 2The growth-inhibitory ratio and
apoptotic ratio were enhanced by C9i but inhibited by NAC in
oridonin-treated HEp-2 cells. (A) The cells were treated with
oridonin for 24 h, in the presence or absence of caspase-9, -8, -3
and -1 inhibitors or pan-caspase inhibitor, and the inhibitory
ratio was measured by MTT assay, n=3, mean ± SD. After the cells
were incubated with oridonin in the absence or presence of C9i or
NAC for 24 h, the inhibitory ratio was measured by MTT assay, n=3,
mean ± SD (B), and the morphological changes were examined by phase
contrast microscopy; scale bar, 10 μm (C). The flow cytometric
quantification of apoptotic cells (sub-G1 fraction) is shown (D).
**P<0.01 vs oridonin alone-treated group;
##P<0.01 vs oridonin co-treated with 5 μM C9i
group. |
Further, we investigated whether inhibition of
caspase-9 affected drug sensitivity. As shown in Fig. 2B, pre-treatment of HEp-2 cells with
5–20 μM C9i markedly enhanced the inhibitory ratio in a
dose-dependent manner after treatment with oridonin. In addition,
we examined cellular morphological changes by phase contrast
microscopy. At 24 h, some of oridonin-treated HEp-2 cells became
round, with shrunken nuclei and membrane blebbing, while untreated
cells did not show these apoptotic characteristics (Fig. 2C). Whereas, the C9i-pre-treated
group showed more marked apoptotic changes in a dose-dependent
manner (Fig. 2C). Quantification
of apoptotic cells by flow cytometric analysis also showed that
combined treatment with oridonin and C9i caused more significant
apoptosis, and the apoptotic ratio was observed to be significantly
increased from 15.08% in oridonin alone-treated cells to 23.59,
31.54 and 37.26% in 5, 10 and 20 μM C9i pre-treated cells,
respectively (Fig. 2D).
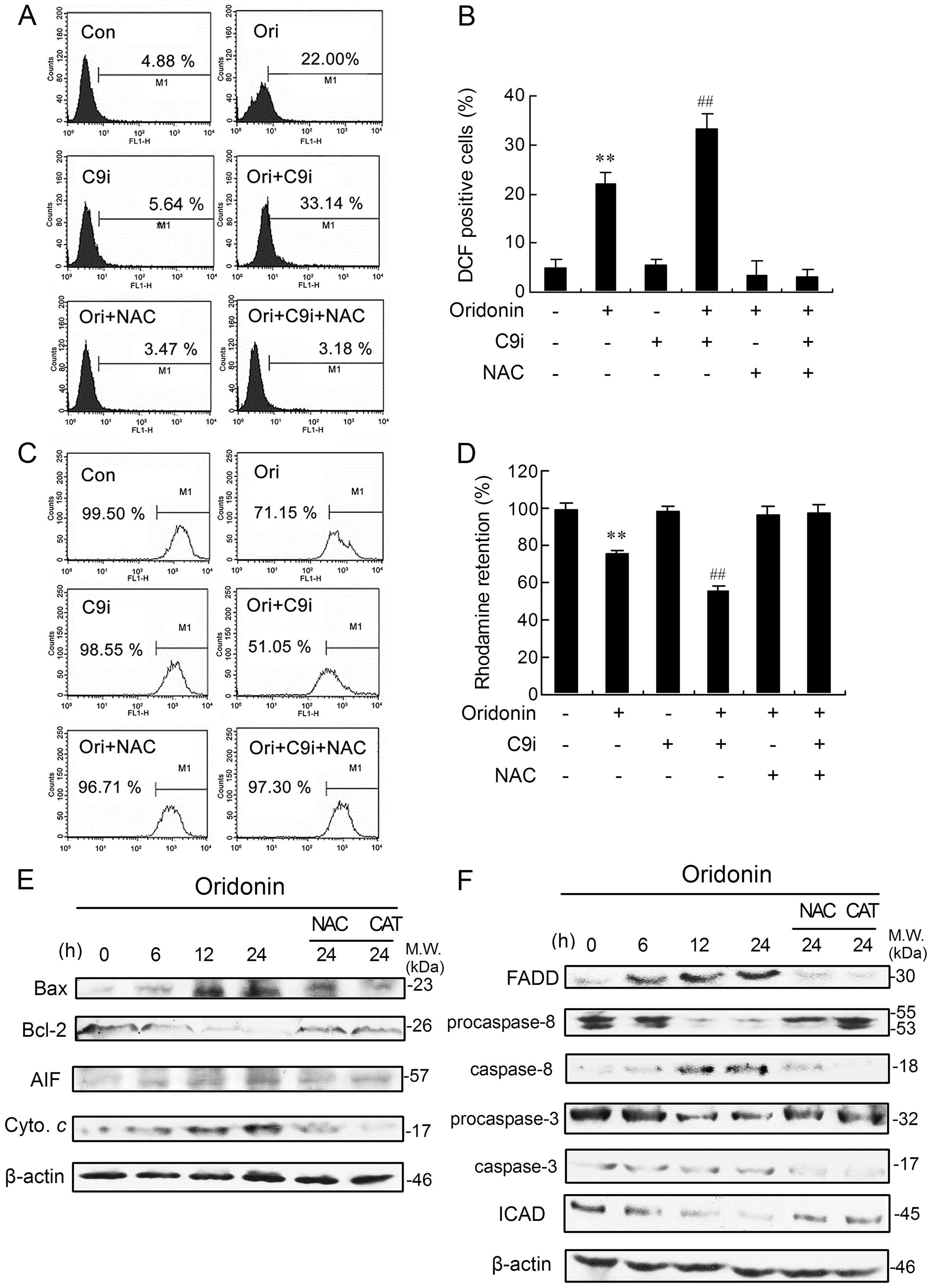
Oridonin-induced increase in ROS
generation is enhanced by inhibiting caspase-9
Dysregulation of cellular redox status can be a
potent mechanism of cell death (26). Therefore, we tested the possibility
that oridonin, with or without C9i, induces apoptosis through ROS
accumulation. Compared with the control group, ROS generation was
significantly increased after exposure to oridonin for 24 h. C9i
effectively increased oridonin-induced intracellular ROS generation
(Fig. 3A and B). As expected, the
use of NAC, a well known ROS scavenger (27), almost completely blocked ROS
generation induced by oridonin alone or in combination with C9i
(Fig. 3A and B). Whereas, NAC not
only completely prevented HEp-2 cell apoptosis induced by oridonin
alone, but also reversed the cell apoptosis induced by combined
treatment with oridonin and C9i (Fig.
2C and D). Collectively, these results demonstrated that
caspase-9 inhibition amplified oridonin-induced oxidative stress,
which leads to enhanced apoptosis.
Oridonin triggers a marked loss of ΔΨm
and caspase-9 inhibition produces a progressive loss of ΔΨm
It has been reported that ROS generation can lead to
mitochondrial damage and membrane depolarization (28). Therefore, we investigated whether
ROS targeted the mitochondria and thereby decreased the ΔΨm
in our model. As shown in Fig. 3C and
D, exposure of HEp-2 cells to oridonin for 24 h caused a marked
loss of ΔΨm compared with the control group and C9i
pre-treatment produced a progressive loss of ΔΨm. Notably,
pre-treatment with NAC reversed the disruption in ΔΨm
induced by oridonin alone or in combination with C9i (Fig. 3C and D). These results suggested
that the oridonin, with or without C9i, was capable of inducing
mitochondrial dysfunction and that the loss of ΔΨm might be
affected directly by ROS generation.
Oridonin-induced Bax activation, Bcl-2
degradation, cytochrome c and AIF elevation are markedly blocked by
NAC and CAT treatment
To determine the possible causes and consequences of
the ΔΨm decrease, we studied the presence of two proteins,
pro-apoptotic protein Bax and anti-apoptotic protein Bcl-2, which
are involved in the regulation of mitochondrial permeability and
two other proteins, cytochrome c and AIF, which are usually
released from mitochondria during apoptosis. We found that oridonin
treatment significantly increased and decreased levels of Bax and
Bcl-2 proteins, respectively, in a time-dependent manner (Fig. 3E). The expression of cytochrome
c was markedly elevated. Simultaneously, a visible increase
in AIF levels was also detectable after 12- and 24-h incubation
(Fig. 3E). However, NAC and
catalase (CAT, hydrogen peroxide decomposer) pre-treatment
completely reversed the changes in Bax, Bcl-2, cytochrome c
and AIF (Fig. 3E).
Changes stimulated by oridonin in FADD,
caspase-8, caspase-3 and ICAD expression are inhibited by NAC and
CAT
To assess whether the Fas-mediated pathway was
activated in oridonin-treated cells, the expressions of
Fas-associated protein with death domain (FADD), caspase-8 and -3
were determined by western blot analysis. The expression of FADD
was markedly elevated and there was clear cleavage of procaspase-8
after oridonin administration. A time-dependent cleavage of
procaspase-3 was also observed in oridonin-treated cells (Fig. 3F). ICAD, an inhibitor of
caspase-activated DNase (CAD), is a substrate for caspases such as
caspase-3. When caspase-3 is activated by apoptotic stimuli, ICAD
is cleaved, resulting in the release of CAD which appears to cause
DNA fragmentation in the nuclei (29). As shown in Fig. 3F, oridonin induced degradation of
ICAD in HEp-2 cells in a time-dependent manner. Addition of NAC and
CAT markedly inhibited the changes in FADD, caspase-8, -3 and ICAD
(Fig. 3F).
Caspase-9 facilitates oridonin-induced
autophagy
We first investigated the effect of oridonin on cell
autophagy. The ultrastructural details displayed that the
oridonin-treated cells possessed typical characteristics of
autophagy. These characteristic changes included extensive
cytoplasmic vacuolization, and some autophagic vacuoles contained
degraded organelles (Fig. 4A). The
formation of autophagic vacuoles was further assessed by MDC, a
fluorescent dye specifically staining autophagosomes. As shown in
Fig. 4A, control cells presented
diffused staining, and oridonin treatment resulted in an extensive
punctuate MDC staining pattern. Next, the levels of Beclin 1 and
LC3, two important proteins involved in autophagy (10,30),
were examined by western blot analysis. As shown in Fig. 4D, the level of Beclin 1 and
conversion from LC3-I to LC3-II both increased with time after
oridonin administration. These findings apparently reveal that
autophagy is induced as a response of HEp-2 cells to oridonin
treatment.
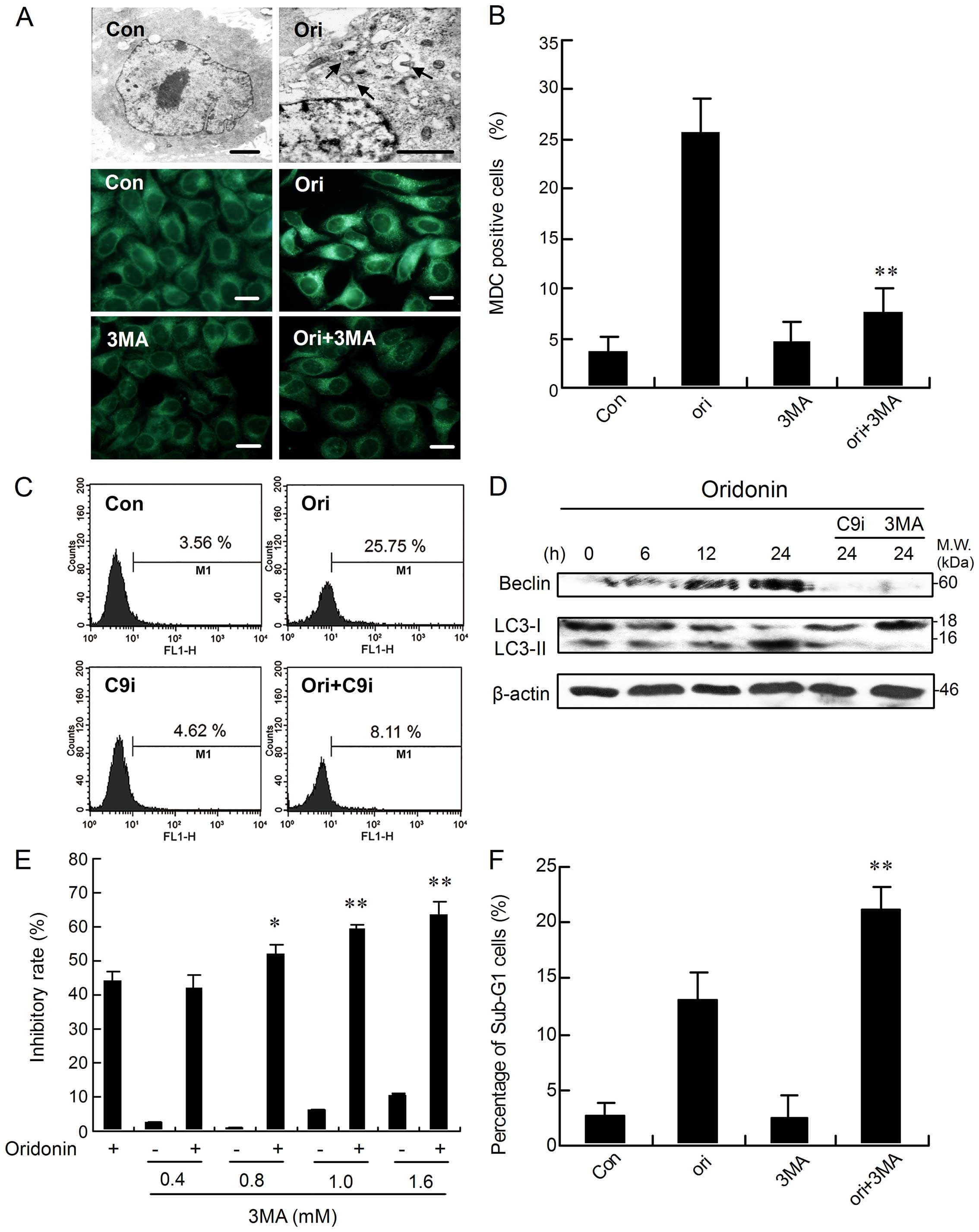 | Figure 4Caspase-9 facilitates
oridonin-induced autophagy, and inhibition of autophagy upregulates
apoptosis. (A) The cells were incubated with oridonin for 0 or 24
h, or co-incubated with 3-MA for 24 h. Then, the cellular
morphological changes were observed by transmission electron
microscopy (arrow indicates autophagic vacuoles; scale bar, 1 μm)
or by fluorescence microscopy with MDC staining (scale bar, 10 μm).
The cells were cultured in the presence of oridonin or co-incubated
with 1 mM 3-MA (B) or 5 μM C9i (C) for 24 h. The corresponding
column or flow cytometric histograms of autophagic level changes
are represented. (D) The cells were treated with oridonin in the
presence or absence of 3-MA or C9i for the indicated time periods,
followed by western blot analysis for Beclin 1 and LC3 levels. The
results shown here are representative of at least three independent
experiments. (E) The cells were treated with oridonin for 24 h, in
the presence or absence of 0.4–1.6 mM 3-MA, and the inhibitory
ratio was measured by MTT assay, n=3, mean ± SD. (F) The cells were
treated with oridonin for 24 h in the presence or absence of 1 mM
3-MA. The sub-G1 fraction was analyzed by flow cytometry.
*P <0.05 or **P<0.01 vs group treated
with oridonin alone. |
To explore the involvement of caspase-9 in the
modulation of autophagy, the autophagic ratio was measured by flow
cytometry. Compared with the oridonin treatment group, the
autophagic ratio was significantly reduced by the combined use of
oridonin and C9i (Fig. 4C).
Accordingly, the treatment of C9i markedly suppressed Beclin 1
upregulation and the conversion from LC3-I to LC3-II (Fig. 4D), suggesting the
autophagy-promoting effects of caspase-9.
Inhibition of autophagy upregulates
apoptosis induced by oridonin in HEp-2 cells
To investigate the role of autophagy in
oridonin-induced apoptosis in HEp-2 cells, 3-MA, a specific
inhibitor of autophagy, was introduced. Treatment with 3-MA prior
to the addition of oridonin induced a significant reduction in the
number of MDC-labeled fluorescent particles in the cells (Fig. 4A). Flow cytometric analysis also
indicated that pre-treatment with 3-MA markedly reduced the
autophagic ratio compared with the group treated with oridonin
alone (Fig. 4B). Simultaneously,
oridonin-induced Beclin 1 activation as well as the modification of
LC3-I to LC3-II were markedly blocked when the cells were
pre-treated with 3-MA (Fig. 4D).
However, pre-treatment of HEp-2 cells with 0.4–1.6 mM of 3-MA
dramatically increased the inhibitory rate of cell growth in a
dose-dependent manner after oridonin treatment (Fig. 4E). Further, inhibition of autophagy
increased the oridonin-induced sub-G1 cell proportion (an index of
apoptosis) in HEp-2 cells (Fig.
4F). Collectively, these results suggested that 3-MA inhibited
oridonin-mediated autophagy and it also sensitized the cells to the
cytotoxic actions of oridonin with induction of apoptosis. In
short, oridonin-induced autophagy inhibits apoptosis in our
model.
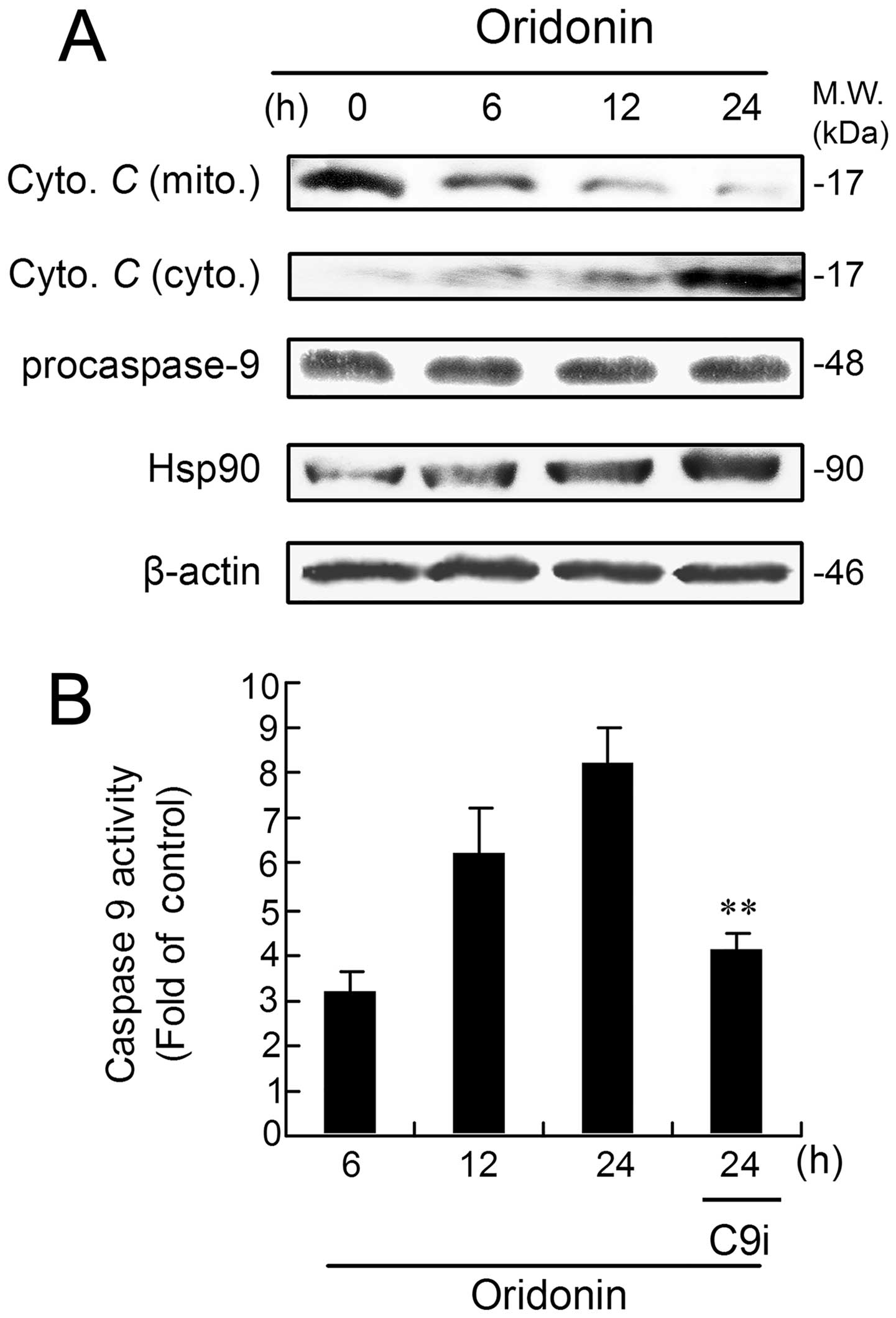
Evaluation of the expression pattern of
critical apoptosome complex-related proteins
In the cytosol, cytochrome c binds to Apaf-1,
allowing recruitment of caspase-9 and formation of apoptosome,
resulting in caspase-9 activation and execution of cell death
(31,32). Here, the expression of critical
apoptosome complex-related proteins was examined by means of
western blot analysis. As shown in Fig. 5A, the level of cytochrome c
in mitochondria began to decrease at 6 h, which was consistent with
the increase in cytochrome c in the cytosol. However, no
apparent change was observed in the protein level of pro-caspase-9,
and cleaved-caspase-9 could not be detected (Fig. 5A). Recently, several investigators
have described negative regulation of the apoptosome complex by
Hsp90 (33,34). Therefore, the effect of oridonin on
expression of Hsp90 was examined. The result shows that oridonin
treatment increased levels of Hsp90 in a time-dependent manner in
HEp-2 cells (Fig. 5A).
Next, we directly examined the effect of drug
treatments on caspase-9 activation using functional assays. The
result shows that caspase-9 activity was activated in a
time-dependent manner, and the maximal activity was seen after a
24-h incubation (Fig. 5B). As
expected, marked suppression of the high levels of caspase-9 was
noted in cells subjected to combination-treatment with C9i
(Fig. 5B).
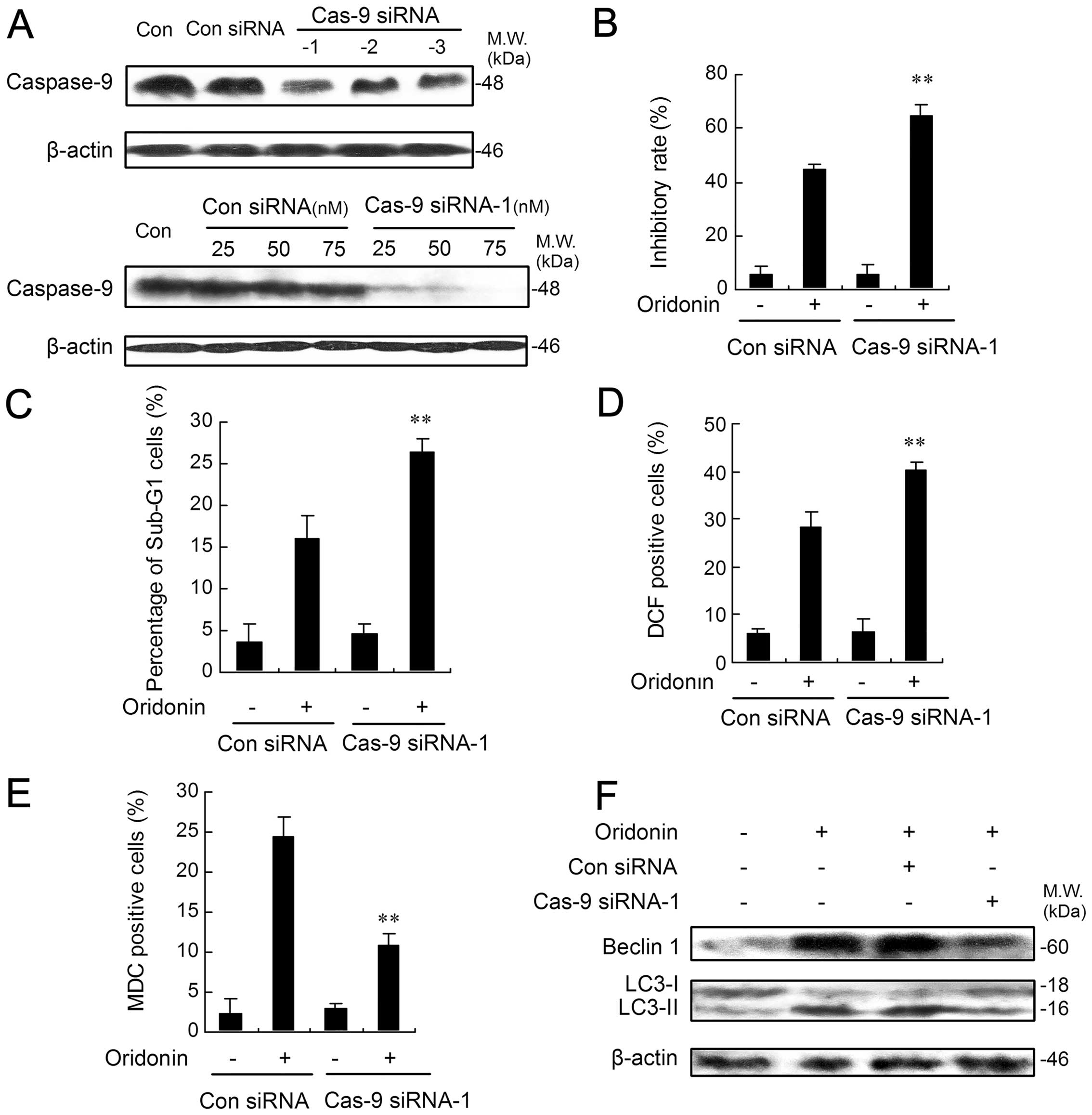
Lower expression of caspase-9 augments
apoptosis in oridonin-treated HEp-2 cells
To determine whether the results obtained with C9i
could be corroborated by another method, we turned to RNA
interference using siRNA. Here, the role of caspase-9 in
oridonin-mediated apoptosis was studied by knock-down of caspase-9
using 3 different specific siRNAs. The expression of capase-9 was
markedly suppressed in HEp-2 cells when transfected with caspase-9
siRNA-1 while only slight downregulation was found with the other
two siRNAs (Fig. 6A). Therefore,
caspase-9 siRNA-1 was selected as a valid candidate for the
subsequent investigation. As shown in Fig. 6A, caspase-9 siRNA-1 effectively and
specifically suppressed HEp-2 caspase-9 protein in a dose-dependent
manner. In contrast, an identical amount of control siRNA had no
effect on caspase-9. We then tested whether caspase-9-deficient
HEp-2 cells would undergo a heightened response to apoptotic
stimuli. As shown in Fig. 6B,
caspase-9-deficient HEp-2 cells were significantly more sensitive
to oridonin than control siRNA-treated cells. Quantification of
apoptotic cells by flow cytometric analysis showed that the
incidence of apoptosis after oridonin treatment was increased by
>10% in caspase-9 siRNA-1-transfected cells when compared with
control siRNA (Fig. 6C). Next, the
degree of ROS generation was also determined by flow cytometric
analysis. The percentage of DCF-positive cells was highest in
caspase-9 siRNA-1-transfected cells treated with oridonin (Fig. 6D). However, caspase-9-deficient
HEp-2 cells showed a much reduced autophagic ratio after oridonin
treatment when compared with those transfected with control siRNA
(Fig. 6E). Accordingly, oridonin
treatment, with or without control siRNA, enhanced both the
expression level of Beclin 1 and the conversion from LC3-I to
LC3-II, while these enhancements were markedly suppressed in HEp-2
cells transfected with caspase-9 siRNA-1 (Fig. 6F).
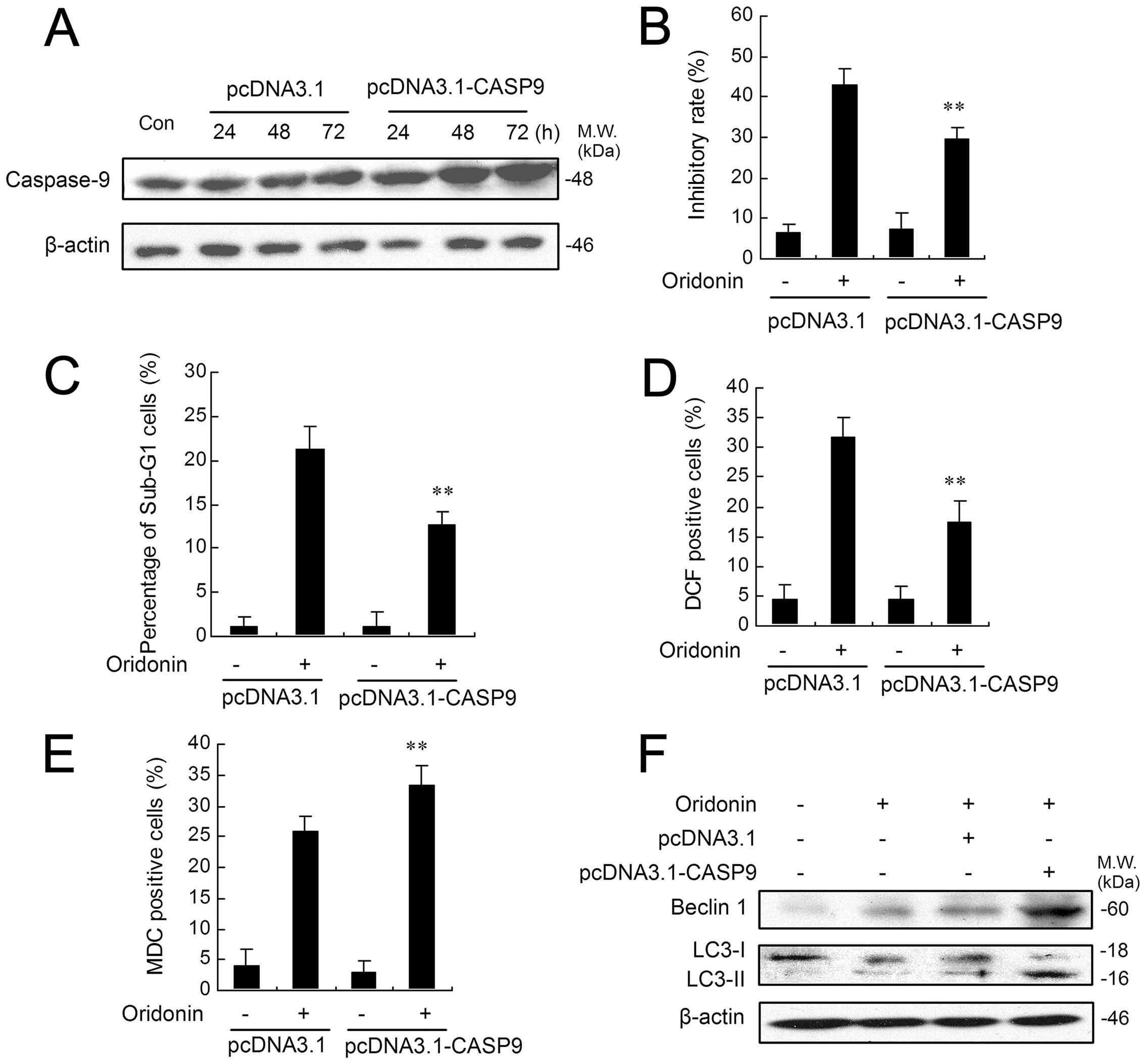
Caspase-9 overexpression attenuates
apoptosis in oridonin-treated HEp-2 cells
To test the ability of caspase-9 to inhibit
oridonin-induced apoptosis, we transfected HEp-2 cells with a
CAS9-expressing plasmid (pcDNA3.1-hCASP9-3FLAG) or control plasmid
(pcDNA3.1-3FLAG). After 24, 48 or 72 h of transfection, the
effectiveness of transfection was detected by western blotting. As
shown in Fig. 7A, caspase-9
protein level was significantly increased in a time-dependent
manner in the HEp-2 cells transiently transfected with the
CAS9-expressing plasmid. In contrast, the control plasmid had no
effect on caspase-9 expression. We then tested whether increased
caspase-9 expression would promote survival and diminish apoptosis
in oridonin-treated HEp-2 cells. As shown in Fig. 7B, the inhibitory ratio of control
plasmid-transfected cells is 42.80%, while, the inhibitory ratio of
CAS9-expressing plasmid-transfected cells is 29.64%, thus
demonstrating caspase-9-mediated increase in survival. Moreover,
quantification of apoptotic cells by flow cytometric analysis
showed that the incidence of apoptosis after oridonin treatment was
decreased by nearly 10% in CAS9-expressing plasmid-transfected
cells when compared with control plasmid (Fig. 7C). Next, the degree of ROS
generation was also determined by flow cytometric analysis. The
percentage of DCF-positive cells was significantly decreased in
CAS9-expressing plasmid-transfected cells compared with control
plasmid (Fig. 7D). However,
CAS9-expressing plasmid-transfected HEp-2 cells showed a much
increased autophagic ratio after oridonin treatment when compared
with those transfected with control plasmid (Fig. 7E). Accordingly, oridonin treatment,
with or without control plasmid, enhanced both the expression level
of Beclin 1 and the conversion from LC3-I to LC3-II, while these
enhancements were markedly augmented in HEp-2 cells transfected
with CAS9-expressing plasmid (Fig.
7F).
Discussion
Some plant-derived agents have been shown to inhibit
cell growth and induce apoptosis in numerous cancer cell types
(35). Oridonin, an active
diterpenoid isolated from R. Rubescens, has been found to
exert apoptotic effects in human laryngeal cancer HEp-2 cells by
our group (17,20). In this study, we made unexpected
observations that the C9i enhanced apoptosis to oridonin stimuli.
The C9i effect was dose-dependent and could be detected at
concentrations as low as 5 μM. This study also provides evidence
that sensitization to oridonin-mediated apoptosis by C9i is
dependent on the amplification of ROS production induced by
oridonin.
ROS, the products of cellular oxidative stress, have
been suggested to regulate the process involved in the initiation
of apoptotic signaling (26).
Several studies have shown that ROS are responsible for the
execution of the mitochondrial pathway of apoptosis (36–38).
In this study, we observed that oridonin treatment resulted in a
significant increase in Bax expression and a decrease in Bcl-2
expression. Our findings also showed a collapse of ΔΨm and a
substantial increase in AIF and cytochrome c. AIF will
translocate to the nucleus where it is capable of inducing nuclear
chromatin condensation and large scale DNA fragmentation to mediate
a caspase-independent mitochondrial apoptotic pathway (39). Cytochrome c normally
functions via its association with other molecules to form a
caspase-9-activating complex which plays a key role in the
caspase-dependent apoptotic pathway (31). However, in this study, it was
interesting to note that cleaved caspase-9 was not detected by
western blot analysis in oridonin-treated cells, although increased
expression of cytosolic cytochrome c was observed. Moreover,
inhibition of caspase-9 in HEp-2 cells did not protect the cells
from oridonin-induced apoptosis, indicating that the apoptosis
occurred via a caspase-9-independent mitochondrial pathway.
Pre-treatment with NAC and CAT not only reversed the expression of
Bax, Bcl-2, AIF and cytochrome c, but also resulted in the
complete inhibition of oridonin-induced ΔΨm collapse,
suggesting that ROS was capable of functioning as an initial
mediator in the caspase-9-independent mitochondrial apoptotic
pathway.
Fas receptor-mediated apoptotic signaling is one of
the most important extrinsic apoptotic pathways in cells (40). ROS generation has been reported to
induce the death receptor pathway and increase the activity of
caspase-8 (41). In the present
study, the increasing expression of FADD, cleaved caspase-8 and
cleaved caspase-3 in the oridonin-treated HEp-2 cells suggested the
involvement of the death receptor pathway. The activated caspase-3
was further supported by downregulation of ICAD, resulting in the
release of CAD which caused DNA fragmentation in the nuclei
(29). ROS scavengers NAC and CAT
significantly inhibited all the participants in the death receptor
pathway in the cells. These results support the notion that ROS
plays a primary role in triggering apoptosis through activation of
the extrinsic pathway in HEp-2 cells.
Despite the many studies on the relationship between
autophagy and apoptosis, the functional relationship between
caspases and autophagy is not well understood (42). Recent studies show that caspase-6
and -8 cleavage of p62 can inhibit autophagy (43). On the other hand, the cleavage of
Beclin 1 has been shown to be a critical event whereby caspases
inhibit autophagy (44,45). Moreover, clinical therapies
involving caspase inhibitors may arrest apoptosis but also have the
unanticipated effect of promoting autophagic cell death (46). Thus, caspase, the hallmark of
apoptosis, may be involved in the execution of autophagy, pointing
to major cross-talk between the two lethal subroutines. In the
present study, the results indicated that caspase-9 participated in
the autophagy process and acted as a promoting factor in
oridonin-induced autophagy. The reason why we were interested in
the role of the regulators of apoptosis, such as caspase-9, in
oridonin-induced autophagy was that, depending on the
circumstances, autophagy can protect cells from apoptosis (47) or kill cells by promoting apoptosis
(48). It is known in this regard
that autophagy may act as a regulator of oridonin-induced apoptosis
in HEp-2 cells. Here, inhibition of autophagy increased the
apoptotic ratio in oridonin-induced HEp-2 cells, suggesting that
autophagy has an anti-apoptotic function. Recently, Jeong et
al showed that treatment of breast cancer MCF-7 cells with the
NSAID FR122047 led to caspase-mediated apoptosis and simultaneously
stimulated cytoprotective autophagy (42). In the present study we have also
identified a novel caspase-9-dependent mechanism that suppresses
oridonin-induced apoptosis of HEp-2 cells by promoting
autophagy.
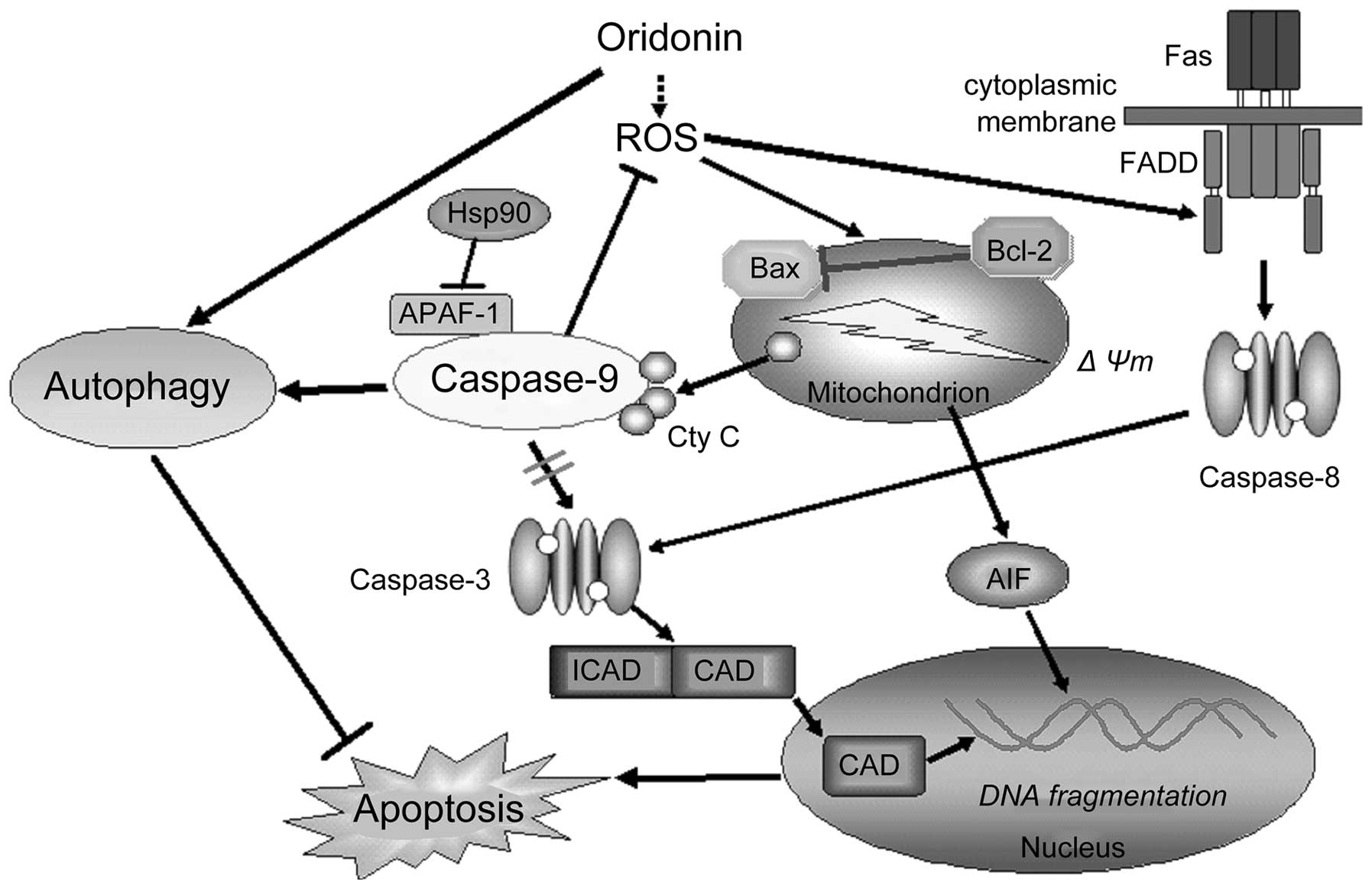
Of note, the release of cytochrome c from
mitochondria results in the formation of an Apaf-1-caspase-9
apoptosome and induces the apoptotic protease cascade (31,32).
Here, it is interesting to note that the HEp-2 cells treated with
oridonin are deficient in their ability to cleave procaspase-9 in
the presence of cytochrome c. The regulation and activation
of caspase-9 in the apoptosome complex is poorly understood.
Inhibitors of the apoptosome, such as Hsp90, have been described
recently (33,34). Hsp90 has been shown to inhibit
cytochrome c-mediated oligomerization of Apaf-1 and
subsequent activation of procaspase-9 (33). In this study, Hsp90 is
overexpressed in oridonin-treated HEp-2 cells. It is possible that
Hsp90, as an inhibitory factor, interferes with the oligomerization
of Apaf-1 to inhibit caspase-9 activation. The detailed mechanisms
by which pro-caspase-9 was not activated by proteolysis in
oridonin-treated HEp-2 cells remain to be determined. Next, we
focussed on whether the defect in the pro-caspase-9 cleavage was
the equivalent of the absence of caspase-9 enzymatic activity.
Therefore, we directly measured the enzyme activity of caspases-9
by spectrofluorometric assay. The enzyme activity of caspase-9
peaked at ~24 h, while C9i markedly inhibited caspase-9 activity.
Obviously, in this study, there must be another mechanism to
achieve the catalytically competent form because proteolysis is not
required for activation of caspase-9. Other investigators have
described the presence of an alternative mechanism in which
caspase-9 requires association with specific cofactors, such as
Apaf-1, for its activation (49,50).
Apaf-1 increases the catalytic activity of caspase-9 by allosteric
regulation (50). The competent
form of caspase-9 subsequently reduces sensitivity to apoptotic
stimuli through ROS-suppressing and autophagy-promoting methods in
our model, which is certainly different from the rule that protease
caspase-9 is the central participant in apoptosis (Fig. 8).
Given the limitations of using a short peptide
inhibitor such as C9i, we used RNA interference to more directly
assess the role of caspase-9. HEp-2 cells deficient in caspase-9
exhibited enhanced growth inhibitory as well as an apoptotic
response to oridonin, consistent with the results obtained with
C9i. Moreover, caspase-9-deficient HEp-2 cells also exhibited a
significantly enhanced level of ROS and a much reduced autophagic
ratio after oridonin treatment. Overall, our findings support the
idea that the promotion of ROS, but the repression of autophagy
might contribute to the failure to reduce apoptotic sensitivity in
caspase-9-deficient HEp-2 cells. By contrast, overexpression of
caspase-9 enhanced autophagy and suppressed ROS production, showing
a protective role on oridonin-induced apoptosis in HEp-2 cells.
In conclusion, our data indicate that targeting
caspase-9-dependent signals can cooperate in promoting cell death
induced by anticancer drugs. Thus, the combination of oridonin and
those leading to a reduction of caspase-9 in tumor cells with
agents, such as C9i and/or reduction in caspase-9, could represent
a novel approach to human laryngeal cancer treatment.
Acknowledgements
This study was supported by National Natural Science
Foundation of China (no. 81102855), China Postdoctoral Science
Foundation (no. 2013M541192) and China Postdoctoral Science Special
Foundation (no. 2014T70224).
Abbreviations:
|
ROS
|
reactive oxygen species
|
|
C9i
|
caspase-9 inhibitor
|
|
NAC
|
N-acetylcysteine
|
|
3-MA
|
3-methyladenine
|
|
LSCC
|
laryngeal squamous cell carcinoma
|
|
PI
|
propidium iodide
|
|
MDC
|
monodansylcadaverine
|
|
siRNA
|
small interfering RNA
|
|
CAT
|
catalase
|
|
FADD
|
Fas-associated protein with death
domain
|
|
CAD
|
caspase-activated DNase
|
|
ICAD
|
inhibitor of caspase-activated
DNase
|
References
|
1
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuan J and Yankner BA: Apoptosis in the
nervous system. Nature. 407:802–809. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nathan C: Specificity of a third kind:
Reactive oxygen and nitrogen intermediates in cell signaling. J
Clin Invest. 111:769–778. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pelicano H, Carney D and Huang P: ROS
stress in cancer cells and therapeutic implications. Drug Resist
Updat. 7:97–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reggiori F and Klionsky DJ: Autophagy in
the eukaryotic cell. Eukaryot Cell. 1:11–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amaravadi RK and Thompson CB: The roles of
therapy-induced autophagy and necrosis in cancer treatment. Clin
Cancer Res. 13:7271–7279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marioni G, Marchese-Ragona R, Cartei G,
Marchese F and Staffieri A: Current opinion in diagnosis and
treatment of laryngeal carcinoma. Cancer Treat Rev. 32:504–515.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rafferty MA, Fenton JE and Jones AS: The
history, aetiology and epidemiology of laryngeal carcinoma. Clin
Otolaryngol Allied Sci. 26:442–446. 2001. View Article : Google Scholar
|
|
13
|
Wang Z, Wang N, Chen J and Shen J:
Emerging glycolysis targeting and drug discovery from Chinese
medicine in cancer therapy. Evid Based Complement Alternat Med.
2012:8731752012.PubMed/NCBI
|
|
14
|
Yang YC, Wei MC and Huang TC: Optimisation
of an ultrasound-assisted extraction followed by RP-HPLC separation
for the simultaneous determination of oleanolic acid, ursolic acid
and oridonin content in Rabdosia rubescens. Phytochem Anal.
23:627–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujita T, Takeda Y, Sun HD, Minami Y,
Marunaka T, Takeda S, Yamada Y and Togo T: Cytotoxic and antitumor
activities of Rabdosia diterpenoids. Planta Med. 54:414–417. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang J, Wu L, Tashiro S, Onodera S and
Ikejima T: Reactive oxygen species mediate oridonin-induced HepG2
apoptosis through p53, MAPK, and mitochondrial signaling pathways.
J Pharmacol Sci. 107:370–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang N, Zhang JH, Qiu F, Chen S, Tashiro
S, Onodera S and Ikejima T: Induction of G(2)/M phase arrest and
apoptosis by oridonin in human laryngeal carcinoma cells. J Nat
Prod. 73:1058–1063. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu JN, Huang J, Yang J, Tashiro S, Onodera
S and Ikejima T: Caspase inhibition augmented oridonin-induced cell
death in murine fibrosarcoma l929 by enhancing reactive oxygen
species generation. J Pharmacol Sci. 108:32–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shah N, Asch RJ, Lysholm AS and Lebien TW:
Enhancement of stress-induced apoptosis in B-lineage cells by
caspase-9 inhibitor. Blood. 104:2873–2878. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang N, Zhang JH, Qiu F, Tashiro S,
Onodera S and Ikejima T: Inhibition of EGFR signaling augments
oridonin-induced apoptosis in human laryngeal cancer cells via
enhancing oxidative stress coincident with activation of both the
intrinsic and extrinsic apoptotic pathways. Cancer Lett.
294:147–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu Y, Sun C and Pan Y: Isolation and
purification of oridonin from Rabdosia rubescens using upright
counter-current chromatography. J Sep Sci. 29:314–318. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu C and Wang L, Lv B, Lu Y, Zeng L, Chen
Y, Ma D, Shi T and Wang L: TMEM74, a lysosome and autophagosome
protein, regulates autophagy. Biochem Biophys Res Commun.
369:622–629. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim RH, Coates JM, Bowles TL, McNerney GP,
Sutcliffe J, Jung JU, Gandour-Edwards R, Chuang FY, Bold RJ and
Kung HJ: Arginine deiminase as a novel therapy for prostate cancer
induces autophagy and caspase-independent apoptosis. Cancer Res.
69:700–708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Wu Y, Cheng Y, Zhao Z, Tashiro S,
Onodera S and Ikejima T: Fas-mediated autophagy requires JNK
activation in HeLa cells. Biochem Biophys Res Commun.
377:1205–1210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang HJ, Tashiro S, Onodera S and Ikejima
T: Inhibition of insulin-like growth factor 1 receptor signaling
enhanced silibinin-induced activation of death receptor and
mitochondrial apoptotic pathways in human breast cancer MCF-7
cells. J Pharmacol Sci. 107:260–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schumacker PT: Reactive oxygen species in
cancer cells: Live by the sword, die by the sword. Cancer Cell.
10:175–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sablina AA, Budanov AV, Ilyinskaya GV,
Agapova LS, Kravchenko JE and Chumakov PM: The antioxidant function
of the p53 tumor suppressor. Nat Med. 11:1306–1313. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong YT, Ruan R and Tay FE: Relationship
between levels of oxidative DNA damage, lipid peroxidation and
mitochondrial membrane potential in young and old F344 rats. Free
Radic Res. 40:393–402. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sakahira H, Enari M and Nagata S:
Functional differences of two forms of the inhibitor of
caspase-activated DNase, ICAD-L, and ICAD-S. J Biol Chem.
274:15740–15744. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Trejo-Solís C, Jimenez-Farfan D,
Rodriguez-Enriquez S, Fernandez-Valverde F, Cruz-Salgado A,
Ruiz-Azuara L and Sotelo J: Copper compound induces autophagy and
apoptosis of glioma cells by reactive oxygen species and JNK
activation. BMC Cancer. 12:1562012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cain K, Bratton SB, Langlais C, Walker G,
Brown DG, Sun XM and Cohen GM: Apaf-1 oligomerizes into
biologically active approximately 700-kDa and inactive
approximately 1.4-MDa apoptosome complexes. J Biol Chem.
275:6067–6070. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu Y, Benedict MA, Ding L and Núñez G:
Role of cytochrome c and dATP/ATP hydrolysis in Apaf-1-mediated
caspase-9 activation and apoptosis. EMBO J. 18:3586–3595. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pandey P, Saleh A, Nakazawa A, Kumar S,
Srinivasula SM, Kumar V, Weichselbaum R, Nalin C, Alnemri ES, Kufe
D, et al: Negative regulation of cytochrome c-mediated
oligomerization of Apaf-1 and activation of procaspase-9 by heat
shock protein 90. EMBO J. 19:4310–4322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu JR, Opipari AW, Tan L, Jiang Y, Zhang
Y, Tang H and Nuñez G: Dysfunctional apoptosome activation in
ovarian cancer: Implications for chemoresistance. Cancer Res.
62:924–931. 2002.PubMed/NCBI
|
|
35
|
Lee KH: Anticancer drug design based on
plant-derived natural products. J Biomed Sci. 6:236–250.
1999.PubMed/NCBI
|
|
36
|
Herrera B, Alvarez AM, Sánchez A,
Fernández M, Roncero C, Benito M and Fabregat I: Reactive oxygen
species (ROS) mediates the mitochondrial-dependent apoptosis
induced by transforming growth factor (beta) in fetal hepatocytes.
FASEB J. 15:741–751. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Herrera B, Fernández M, Alvarez AM,
Roncero C, Benito M, Gil J and Fabregat I: Activation of caspases
occurs downstream from radical oxygen species production, Bcl-xL
down-regulation, and early cytochrome C release in apoptosis
induced by transforming growth factor beta in rat fetal
hepatocytes. Hepatology. 34:548–556. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Q, Chai YC, Mazumder S, Jiang C,
Macklis RM, Chisolm GM and Almasan A: The late increase in
intracellular free radical oxygen species during apoptosis is
associated with cytochrome c release, caspase activation, and
mitochondrial dysfunction. Cell Death Differ. 10:323–334. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Daugas E, Nochy D, Ravagnan L, Loeffler M,
Susin SA, Zamzami N and Kroemer G: Apoptosis-inducing factor (AIF):
A ubiquitous mitochondrial oxidoreductase involved in apoptosis.
FEBS Lett. 476:118–123. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mitsiades CS, Poulaki V, Fanourakis G,
Sozopoulos E, McMillin D, Wen Z, Voutsinas G, Tseleni-Balafouta S
and Mitsiades N: Fas signaling in thyroid carcinomas is diverted
from apoptosis to proliferation. Clin Cancer Res. 12:3705–3712.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chandra J, Samali A and Orrenius S:
Triggering and modulation of apoptosis by oxidative stress. Free
Radic Biol Med. 29:323–333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jeong HS, Choi HY, Lee ER, Kim JH, Jeon K,
Lee HJ and Cho SG: Involvement of caspase-9 in autophagy-mediated
cell survival pathway. Biochim Biophys Acta. 1813.80–90. 2011.
|
|
43
|
Norman JM, Cohen GM and Bampton ET: The in
vitro cleavage of the hAtg proteins by cell death proteases.
Autophagy. 6:1042–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Young MM, Takahashi Y, Khan O, Park S,
Hori T, Yun J, Sharma AK, Amin S, Hu CD, Zhang J, et al:
Autophagosomal membrane serves as platform for intracellular
death-inducing signaling complex (iDISC)-mediated caspase-8
activation and apoptosis. J Biol Chem. 287:12455–12468. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cho DH, Jo YK, Hwang JJ, Lee YM, Roh SA
and Kim JC: Caspase-mediated cleavage of ATG6/Beclin-1 links
apoptosis to autophagy in HeLa cells. Cancer Lett. 274:95–100.
2009. View Article : Google Scholar
|
|
46
|
Wang ZH, Xu L, Duan ZL, Zeng LQ, Yan NH
and Peng ZL: Beclin 1-mediated macroautophagy involves regulation
of caspase-9 expression in cervical cancer HeLa cells. Gynecol
Oncol. 107:107–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lum JJ, Bauer DE, Kong M, Harris MH, Li C,
Lindsten T and Thompson CB: Growth factor regulation of autophagy
and cell survival in the absence of apoptosis. Cell. 120:237–248.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Crighton D, Wilkinson S, O'Prey J, Syed N,
Smith P, Harrison PR, Gasco M, Garrone O, Crook T and Ryan KM:
DRAM, a p53-induced modulator of autophagy, is critical for
apoptosis. Cell. 126:121–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rodriguez J and Lazebnik Y: Caspase-9 and
APAF-1 form an active holoenzyme. Genes Dev. 13:3179–3184. 1999.
View Article : Google Scholar
|
|
50
|
Stennicke HR, Deveraux QL, Humke EW, Reed
JC, Dixit VM and Salvesen GS: Caspase-9 can be activated without
proteolytic processing. J Biol Chem. 274:8359–8362. 1999.
View Article : Google Scholar : PubMed/NCBI
|