Introduction
Colorectal carcinoma (CRC) is a major global health
concern, with over one million new cases and more than half a
million deaths annually (1,2). The
pathogenic mechanisms mediating CRC development are complicated,
with involvement of multiple intracellular signaling transduction
cascades including mitogen-activated protein kinase (MAPK),
serine-threonine kinase Akt, p70S6 kinase (p70S6K) and p53 pathways
(3–11). Aberrant activation of these
pathways modulates the expression of many key genes, resulting in
the imbalance between cell proliferation and apoptosis, and
eventually the induction and progression of cancer. Moreover, these
pathways usually function redundantly and form a complicated
compensatory network via cross-talk. Thus, most currently-used
antitumor agents that are designed for single target might not be
always effective and their long-term use might generate drug
resistance (12–14).
Naturally-occurring products, including traditional
Chinese medicines (TCM), have gained increasing attention in the
field of anticancer treatment since they contain relatively few
side effects as compared to modern chemotherapeutics (15–17).
Oleanolic acid (OA), a natural pentacyclic triterpene acid, is an
active compound present in many well-known TCM medicinal herbs,
such as Hedyotic diffusa, Patrinia scabiosaefolia and
Scutellaria barbata. These herbs have long been used in
China to clinically treat various types of human malignancies
including CRC (18–26). Previous reports suggest OA may
contain anti-inflammatory, antioxidant, and antitumor activities
(27–29). However, the precise mechanisms of
tumorcidal activity of OA remain largely unclear. Using a CRC mouse
xenograft model and the cell line HT-29, we evaluated the effect of
OA on tumor growth in vivo and in vitro, and
investigated the underlying molecular mechanisms.
Materials and methods
Cell culture
Human colon carcinoma HT-29 cells were obtained from
the Cell Bank of Chinese Academy of Sciences (Shanghai, China) and
grown in DMEM supplemented with 10% (v/v) FBS, 100 U/ml penicillin
and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA, USA). Cells
were cultured at 37°C, in 5% CO2 humidified
incubator.
Oleanolic acid preparation
Oleanolic αcid (OA, ≥90%) was purchased from
Sigma-Aldrich (St. Louis, MO, USA). For cell-based experiments,
stock solution of OA was prepared by dissolving OA powder in DMSO
to a concentration of 20 mM. The working concentrations of OA were
made by diluting the stock solution in the culture medium. The
final concentrations of DMSO in the medium were 0.1%. For animal
experiments, OA was dissolved in saline.
Nude mouse xenograft study
Six-week-old male BALB/c athymic (nude) mice (20–22
g) were obtained from Shanghai SLAC Laboratory Animal Co., Ltd.
(Shanghai, China) and housed in the specific pathogen-free
controlled conditions (22°C, a 12-h light/dark cycle). Food and
water were given ad libitum. HT-29 CRC xenograft mice were
produced as previously described (19). Briefly, 2.5×106 of HT-29
cells were subcutaneously injected into the right flank of mice to
initiate tumor growth. At day 3, following xenograft implantation,
mice were randomly divided into two groups (n=10) and
intraperitoneally injected with 12.5 mg/kg of OA or saline, daily,
6 days per week for 16 days. Tumor volume was measured and
calculated by the formulation of ‘π/6 × L × W2’, where
‘L’ and ‘W’ refer to length and width, respectively. All animal
experiments were approved by the Institutional Animal Care and Use
Committee of Fujian University of Traditional Chinese Medicine.
Immunohistochemistical analysis
Tumor tissues were analyzed by immunohistochemistry
staining as described before (19). Briefly, 8 tumors were randomly
selected from OA-treatment or control groups. Tumor tissues were
fixed in 10% formaldehyde for 12 h, paraffin-embedded, sectioned
and placed on slides. The slides were subjected to antigen
retrieval and endogenous peroxidase activity was quenched with
hydrogen peroxide. Non-specific binding was blocked with normal
serum in PBS (0.1% Tween-20). Rabbit polyclonal antibody against
PCNA at dilution of 1:200 (Santa Cruz Biotechnology, CA, USA) was
used to detect the relevant proteins. The binding of the primary
antibody was demonstrated with a biotinylated secondary antibody,
horse-radish peroxidase (HRP)-conjugated streptavidin (Dako) and
diaminobenzidine (DAB) as the chromogen. The tissues were
counterstained with diluted Harris hematoxylin. After staining,
five high-power fields (at magnification of ×400) were randomly
selected in each slide and the proportion of positive cells in each
field was determined using a true color multi-functional cell image
analysis management system (Image-Pro Plus, Media Cybernetics,
USA). PBS was used to replace the primary antibody as a
non-specific control.
MTT assay
HT-29 cells were seeded into 96-well plates at
1.0×104 cells per well and treated with various
concentrations of OA for 24 h or 50 μM of OA for different
time-point. After treatment, MTT assay was performed as previously
described (20). Briefly, 10 μl
MTT [5 mg/ml in phosphate-buffered saline (PBS)] was added to each
well, and the samples were incubated for an additional 4 h at 37°C.
The purple-blue MTT formazan precipitate was dissolved in 100 μl
DMSO. The cell viability was measured at 570 nm with a Model ELX800
Microplate Reader (BioTek, VT, USA).
Colony formation assay
HT-29 cells were seeded into 6-well plates at a
density of 2×105 cells/well and treated with various
concentrations of OA for 24 h. The cells were harvested,
resuspended in medium and re-seeded into 6-well plates at a density
of 1.0×103 cells/well. After incubation for 10 days in a
37°C humidified incubator with 5% CO2, colonies were
counted by light microscopy.
Cell cycle analysis
HT-29 cells were seeded into 6-well plates at a
density of 2×105 cells/well and treated with indicated
concentrations of OA for 24 h. The cells were harvested,
resuspended and adjusted to a concentration of 1×106
cells/ml and fixed in 70% ethanol at 4°C overnight. The fixed cells
were washed twice with cold PBS and incubated for 30 min with RNase
(8 μg/ml) and PI (10 μg/ml). The cell cycle phases were analyzed by
flow cytometry (Caliber, Becton-Dickinson, CA, USA) as previously
described (22). The fluorescent
signal was detected through the FL2 channel and the proportion of
DNA in different phases was analyzed using ModfitLT Version 3.0
(Verity Software House, Topsham).
TUNEL assay
Paraffin-embedded sections of tumors (4-μm thick)
were analyzed by TUNEL staining with TumorTACS in situ kit (R&D
Systems, Minneapolis, MN, USA) according to the manufacturer's
recommended protocol. The proportion of apoptotic cells were
recorded as DAB-positive cells (brown stained). Five high-power
fields (x400) were randomly selected in each slide and the average
proportion of positive cells in each field was counted.
Apoptosis analysis in cells with Annexin
V/PI staining
HT-29 cells were seeded into 6-well plates at a
density of 2×105 cells/well and treated with various
concentrations of OA for 24 h. The cells were harvested,
resuspended and stained with Annexin V/PI (Becton-Dickinson, CA,
USA) according to the manufacturer's instructions followed by flow
cytometry analysis. The percentage of cells in early apoptosis was
calculated by Annexin V-positivity and PI-negativity and the
percentage of cells in late apoptosis was calculated by Annexin
V-positivity and PI-positivity.
Western blot analysis
The protein expression was detected by western blot
analysis. Protein of tumor tissues or HT-29 cells was extracted
using RIPA protein extraction kit (Tiangen Biotech, Beijing,
China), respectively. The protein were separated by SDS-PAGE and
transferred onto PVDF membranes. The membranes were blocked for 1 h
with 5% non-fat milk and incubated with primary antibody against
Cyclin D1, CDK4, p21, Bcl-2, Bax or β-actin (all in 1:1,000
dilutions; Cell Signaling Technology, Beverly, MA, USA) overnight
at 4°C. The HRP-conjugated secondary antibodies (1:2,000 dilution)
were added. Then the levels of protein expression were detected
with enhanced chemiluminescence detection.
Bio-Plex phosphoprotein assay
The phosphorylation of multiple proteins was
simultaneously detected by Bio-Plex phosphoprotein assay as
described before (22). Briefly,
5×105 of HT-29 cells were seeded into 25-cm2
flasks with 5 ml medium and treated with 50 μM of OA for 24 h.
Eight tumors were randomly selected from each group and
homogenized. After lysis of treated cells or tumor tissues the
expression of pAkt, pErk1/2, pJNK, pp38, pp70S6K and pp53 was
examined using the Bio-Plex 200 suspension array system Bio-Plex
Phosphoprotein assay (Bio-Rad, Hercules, CA, USA) according to the
manufacturer's protocol.
Statistical analysis
The data were analyzed using SPSS for Windows
(Version 17.0) and shown as average with SD. Statistical analysis
was performed with Student's t-test or ANOVA.
Results and Discussion
OA inhibited CRC growth in vivo and in
vitro
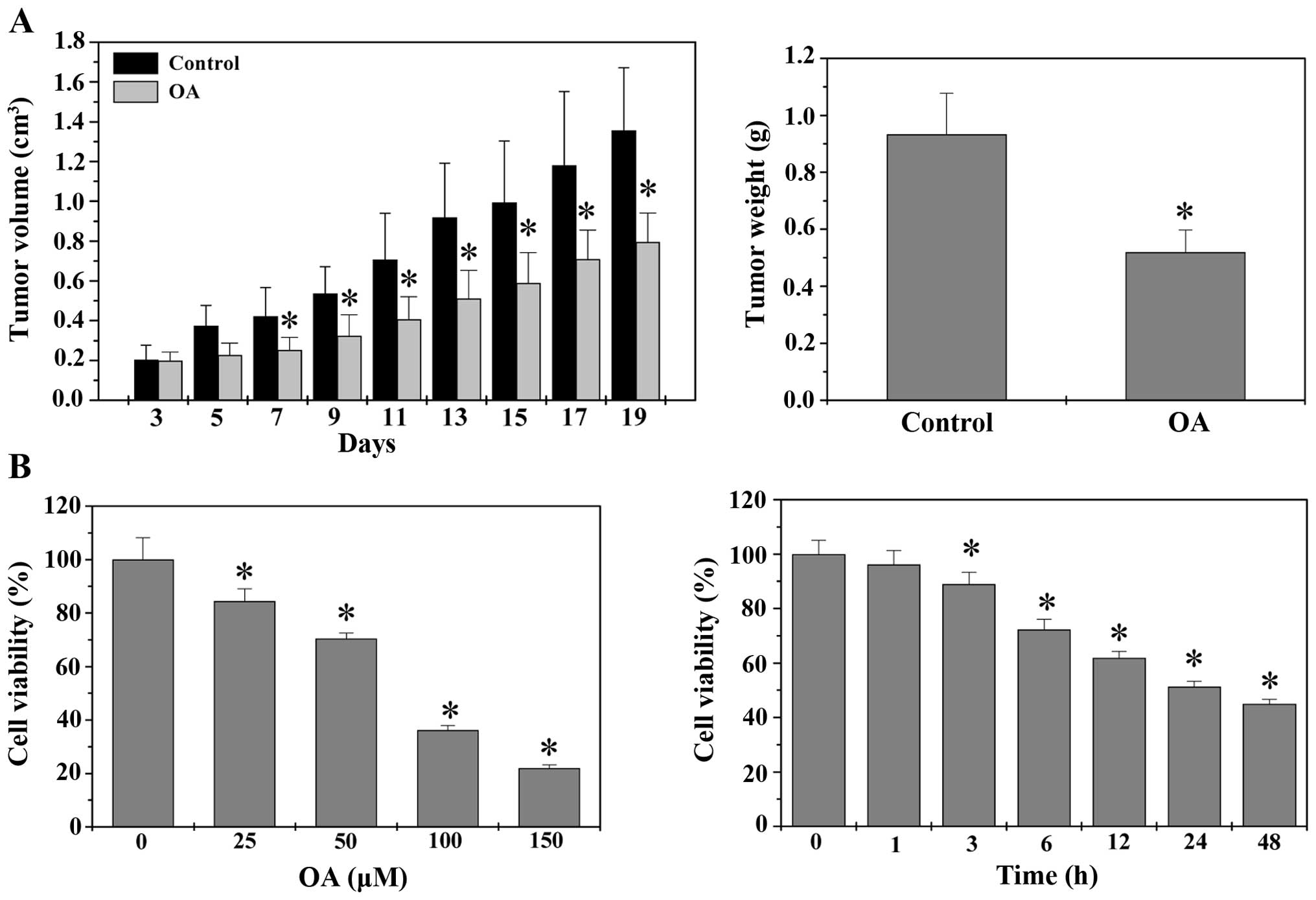
Tumor growth in vivo was evaluated by
measuring the tumor volume and tumor weight in CRC xenograft mice.
As shown in Fig. 1A,
administration with OA significantly decreased both tumor volume
and tumor weight. At the end of experiment, the tumor volume and
tumor weight per mouse in control group were 1.36±0.32
cm3 and 0.94±0.14 g, whereas those in OA-treated group
were 0.79±0.14 cm3 and 0.51±0.08 g (P<0.05). The
in vitro effect of OA on CRC growth was determined by MTT
assay to compare the relative viability of HT-29 cells in
OA-treated monolayers and untreated controls. As shown in Fig. 1B, treatment with 25–150 μM of OA
for 24 h reduced HT-29 cell viability by 16–78% compared to
untreated control cells; and 50 μM of OA gradually decreased cell
viability with the increase of exposure time (P<0.05). Taken
together, it is suggested that OA possesses inhibitory effects on
CRC growth both in vivo and in vitro, in a dose- and
time-dependent manner.
OA inhibited CRC cell proliferation
through blockage of cell cycle G1 to S progression
Cancers are typically characterized by an
uncontrolled increase of cell proliferation (30); which therefore is a critical target
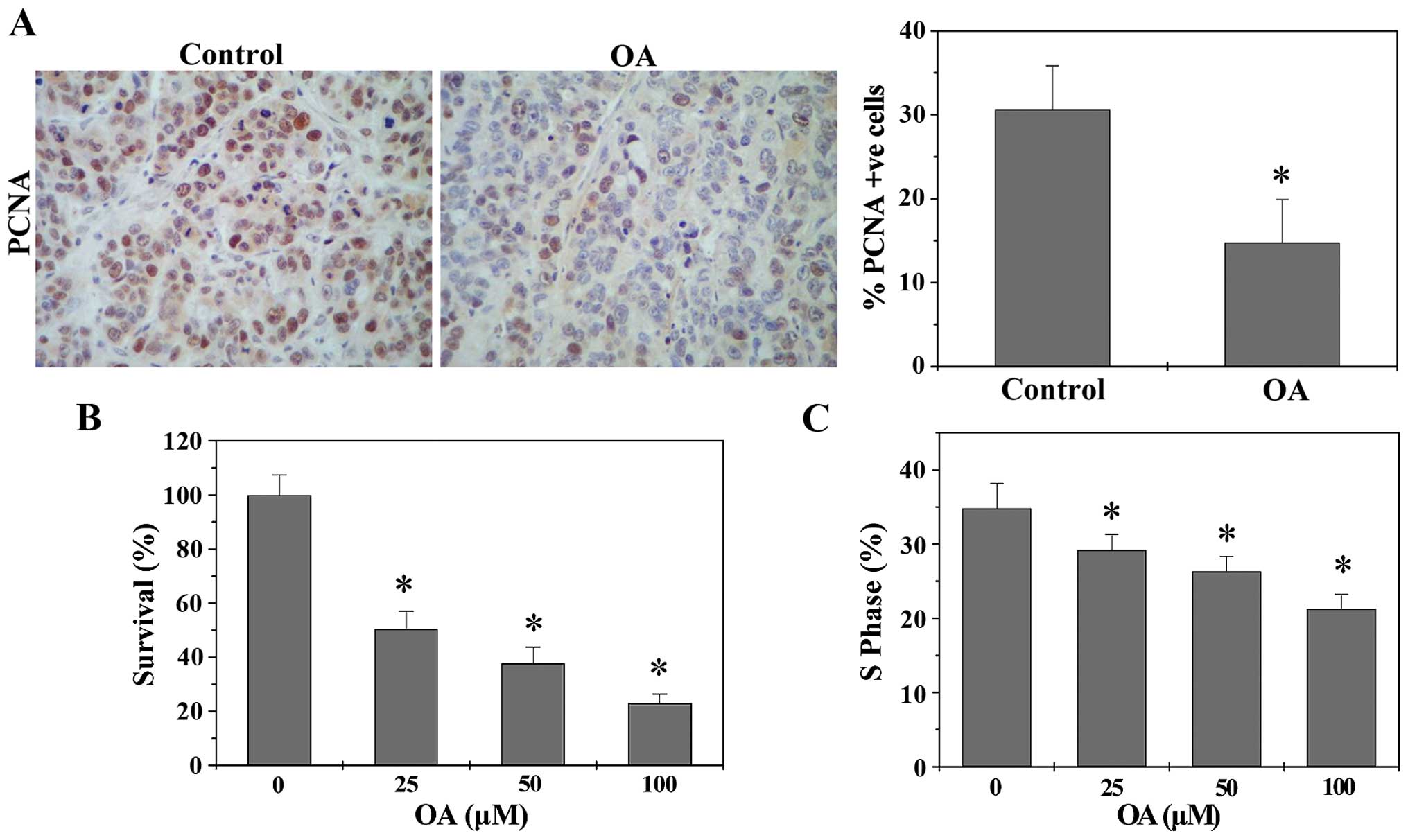
of anticancer treatment. To determine the in vivo effect of
OA on cancer cell proliferation, we performed immunohistochemical
staining (IHS) to evaluate the expression level of a proliferation
marker PCNA in CRC tumor tissues. As shown in Fig. 2A, OA treatment significantly
decreased the protein expression of PCNA in CRC xenograft mice. The
percentage of PCNA-positive cells in tumors from control and
OA-treated CRC mice was 30.64±5.18 and 14.75±5.17%, respectively
(P<0.05). The effect of OA on CRC cell proliferation in
vitro was assessed by colony formation assay. As shown in
Fig. 2B, OA significantly and
dose-dependently decreased the survival rate of HT-29 cells
(P<0.05). Eukaryotic cell proliferation is tightly regulated by
the cell cycle, and G1/S transition is a main checkpoint critical
for the control of the cell cycle progress. To investigate the
mechanism of anti-proliferative effect of OA, we performed FACS
analysis with PI staining to evaluate cell cycle. As shown in
Fig. 2C, OA treatment
significantly and dose-dependently reduced the S-phase proportion
of HT-29 cells. Collectively, OA can inhibit CRC cell proliferation
via G1/S cell cycle arrest.
OA promoted CRC cell apoptosis via the
mitochondrion-dependent pathway
Apoptosis eliminates abnormal cells and hence is
essential for tissue homeostasis. Dysregulation of apoptosis
represents a major causative factor of tumorigenesis (31). Therefore, promoting cell apoptosis
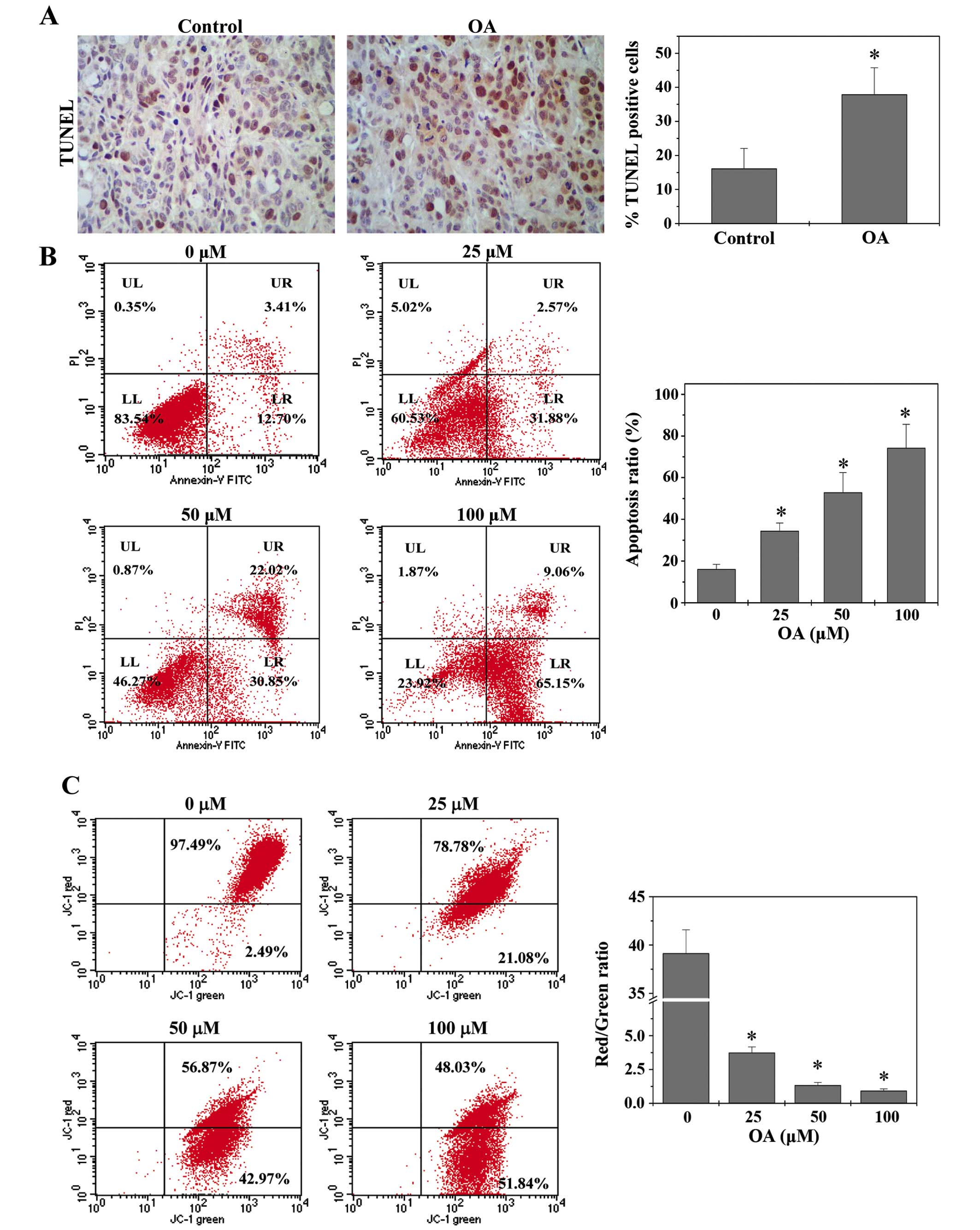
has been a major focus for antitumor therapies. The in vivo
apoptosis was evaluated by TUNEL assay. As shown in Fig. 3A, treatment with OA significantly
enhanced the percentage of TUNEL-positive cells in tumors from CRC
mice. HT-29 cell apoptosis in vitro was examined using
Annexin V/PI staining followed by FACS analysis. As shown in
Fig. 3B, OA treatment increased
the percentage of cells undergoing apoptosis in a dose-dependent
manner.
The mitochondrion-dependent pathway is the most
common apoptotic pathway in vertebrate animal cells. Mitochondrial
outer membrane permeabilization (MOMP) results in the release of
numerous apoptogenic proteins and eventually induces apoptosis,
which thereby is a key commitment step in the induction of cellular
apoptosis. During the process of MOMP the electrochemical gradient
across the mitochondrial membrane collapses. Therefore, the loss of
mitochondrial membrane potential is a hallmark for apoptosis. Since
the membrane-permeant JC-1 dye displays potential-dependent
accumulation in mitochondria, indicated by a fluorescence emission
shift from green (525 nm) to red (590 nm), collapse of
mitochondrial potential during apoptosis can be represented by a
decrease in the ratio of red/green fluorescence intensity of JC-1.
As shown in Fig. 3C, OA treatment
significantly and dose-dependently reduced JC-1 red/green
fluorescent ratio or mitochondrial membrane potential in HT-29
cells (P<0.05). These data together suggest that OA induces CRC
cell apoptosis in vivo and in vitro via the
mitochondrion-dependent pathway.
OA regulates the expression of Cyclin D1,
CDK4, p21, Bcl-2 and Bax
G1/S progression is highly mediated by Cyclin D1 and
its catalytic partner CDK4 (32,33).
An unchecked or hyperactivated Cyclin D1/CDK4 complex often leads
to uncontrolled increase in cell proliferation (34,35).
As a proliferation inhibitor, p21 protein plays a role in G1 arrest
by binding to and inhibiting the activity of Cyclin-CDK complexes
(36). Bcl-2 family proteins are
critical mediators of mitochondrion-dependent apoptosis,
functioning as either promoters (e.g., Bax) or inhibitors (e.g.,
Bcl-2) (37). The ratio of Bcl-2
to Bax is critical for determining the fate of cells. Higher
Bcl-2/Bax ratio is commonly found in various types of cancer,
conferring a survival advantage to cancer cells (38–40).
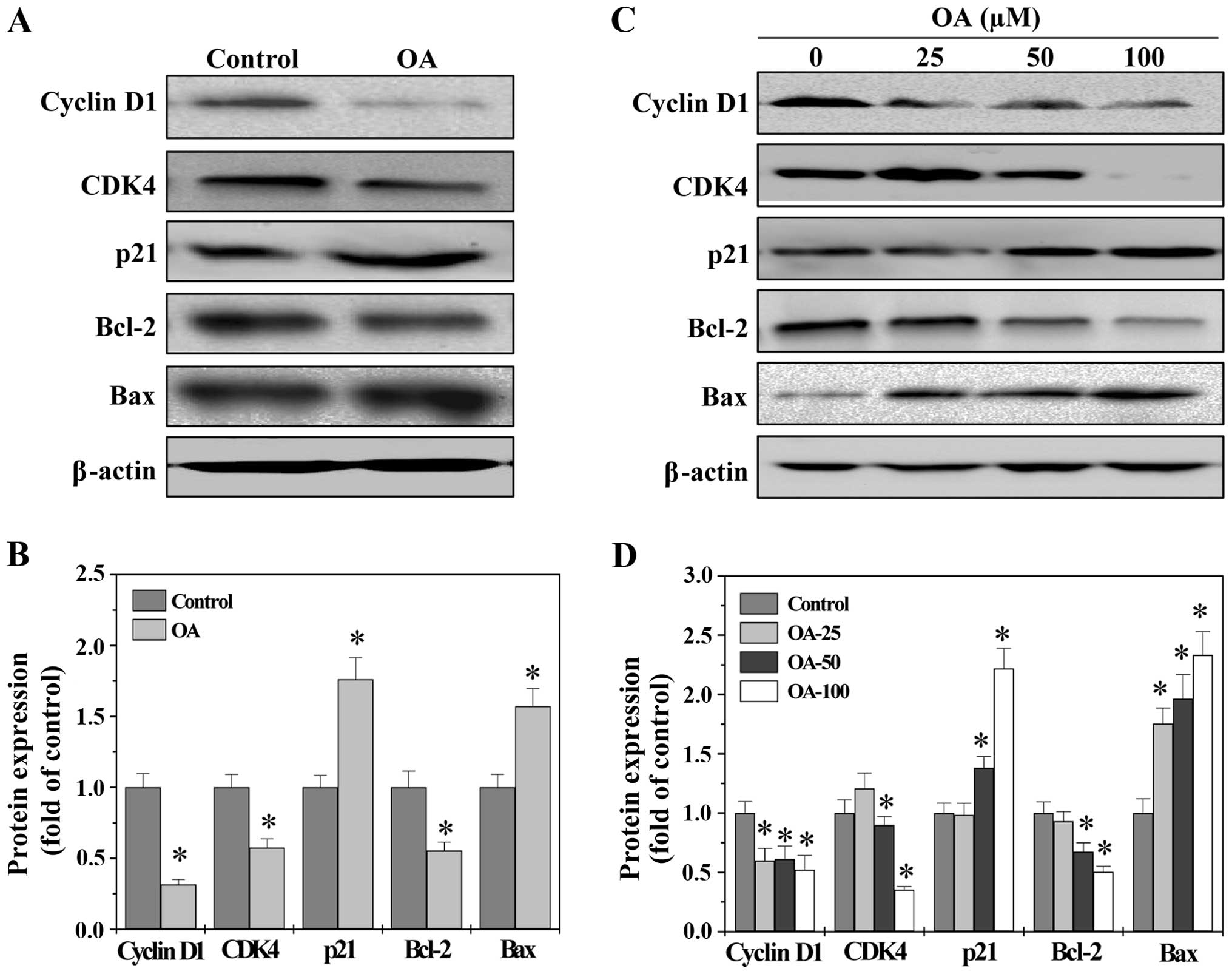
To explore the mechanisms whereby OA exerts its
anti-proliferative and pro-apoptotic activities, we performed
western blot analysis to determine the protein expression of the
above-mentioned factors. As shown in Fig. 4, OA significantly reduced the
protein level of pro-proliferative Cyclin D1 and CKD4 as well as
anti-apoptotic Bcl-2 in both CRC tumors and HT-29 cells, whereas
that of anti-proliferative p21 and pro-apoptotic Bax was
significantly increased after OA treatment. Taken together, these
data demonstrate that OA exerts its antitumor activities through
modulating the expression of critical genes involved in cell
proliferation and apoptosis.
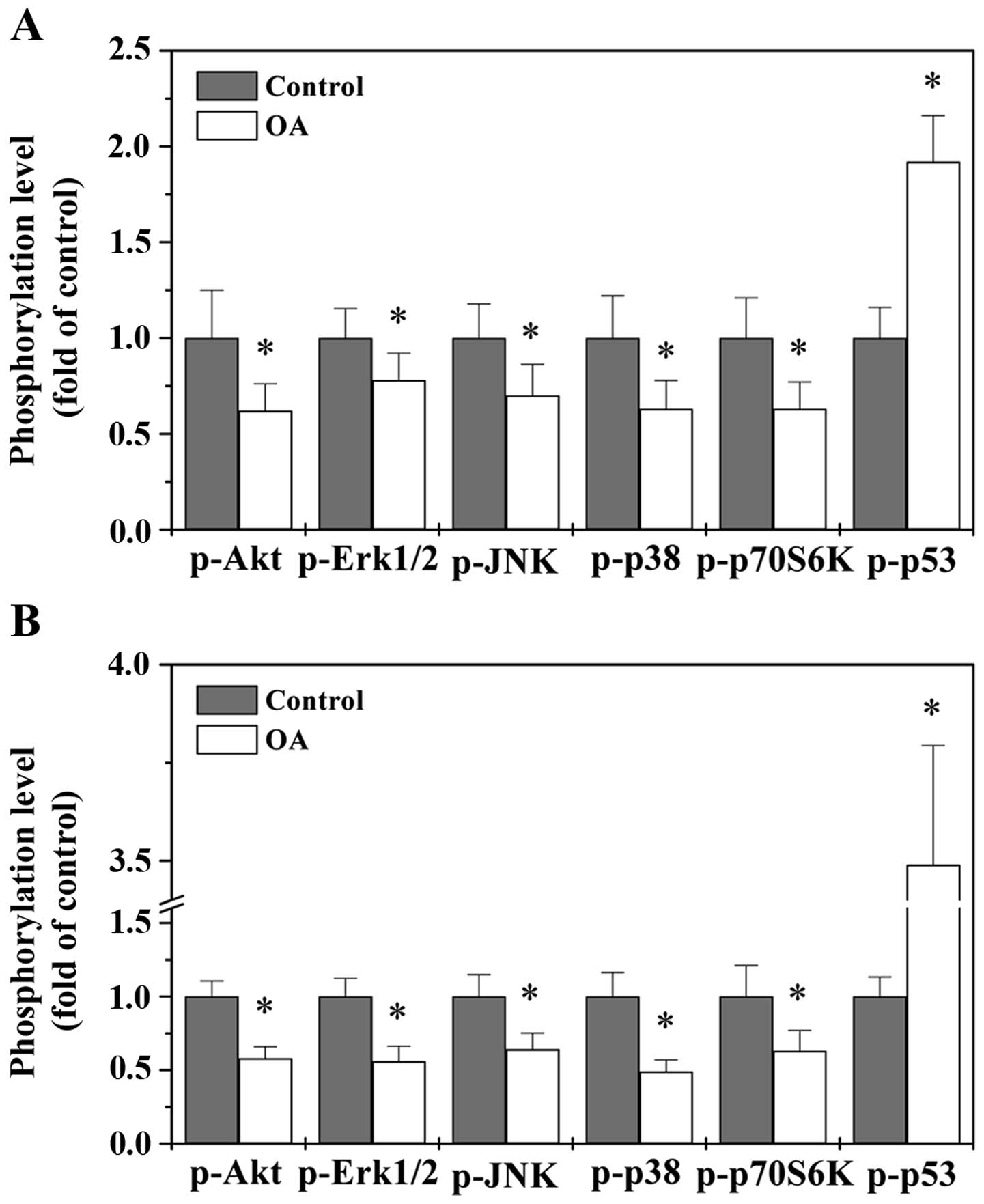
OA modulates the activation of multiple
CRC-related signaling pathways
The development of malignant tumors including CRC is
strongly associated with multiple intracellular signal transduction
cascades such as MAPK, Akt, p70S6K and p53 pathways (3–11).
After activation by PI3K, Akt phosphorylates mTOR which in turns
regulates p70S6K phosphorylation and activation. The
Akt-mTOR-p70S6K signaling pathway is considered as a central
regulatory pathway of protein expression involved in regulating
cell proliferation and survival. Mammals have three major
subfamilies of MAPK, including ERK, JNK and p38. The MAPK signaling
proceeds via a three-tiered kinase core consisting of MAPK kinase
kinase, MAPK kinase, and ultimately a given MAPK. Signaling of both
Akt and MAPK are major cell-survival and proliferation pathways and
have been shown to be activated in several cancers including CRC.
In addition, Akt and MAPK pathways can influence the tumor
suppressor p53, a transcription factor involved in many cellular
processes such as DNA repair, cell cycle arrest and apoptosis. p53
is one of the most frequently mutated genes in human cancers. To
further elucidate the mechanisms of antitumor activity of OA, we
used Bio-Plex Phosphoprotein assay to examine the
activation/phosphorylation of multiple proteins in CRC xenograft
tumor tissues and HT-29 cells. As shown in Fig. 5, OA treatment significantly reduced
the phosphorylation levels of Akt, ERK, JNK, p38 and p70S6K both
in vivo and in vitro, while p53 phosphorylation was
remarkably increased after OA treatment, suggesting that OA
profoundly modulates the activation of multiple CRC-related
signaling pathways in vivo and in vitro.
In conclusion, OA exerts its antitumor effects via
modulation of multiple cellular targets. Results from this study
may provide a strong scientific foundation for the development of
novel multi-targeted anticancer agents from the bioactive
ingredients in medicinal herbs.
Acknowledgements
This study was sponsored by the Research Fund for
the Doctoral Program of Higher Education of China (20133519110003),
the Natural Science Foundation of Fujian Province (2015J01337), and
the Developmental Fund of Chen Keji Integrative Medicine
(CKJ2014013 and CKJ2015007).
Abbreviations:
|
OA
|
oleanolic acid
|
|
CRC
|
colorectal cancer
|
|
TUNEL
|
terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labeling
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
MAPK
|
mitogen-activated protein kinase
|
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clarke RB: p27KIP1
phosphorylation by PKB/Akt leads to poor breast cancer prognosis.
Breast Cancer Res. 5:162–163. 2003. View
Article : Google Scholar :
|
|
4
|
Chang F, Lee JT, Navolanic PM, Steelman
LS, Shelton JG, Blalock WL, Franklin RA and McCubrey JA:
Involvement of PI3K/Akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: A target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo
P, Hu LS, Anderson MJ, Arden KC, Blenis J and Greenberg ME: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pratheeshkumar P, Budhraja A, Son YO, Wang
X, Zhang Z, Ding S, Wang L, Hitron A, Lee JC, Xu M, et al:
Quercetin inhibits angiogenesis mediated human prostate tumor
growth by targeting VEGFR-2 regulated AKT/mTOR/P70S6K signaling
pathways. PLoS One. 7:e475162012. View Article : Google Scholar
|
|
7
|
Li W, Tan D, Zhang Z, Liang JJ and Brown
RE: Activation of Akt-mTOR-p70S6K pathway in angiogenesis in
hepatocellular carcinoma. Oncol Rep. 20:713–719. 2008.PubMed/NCBI
|
|
8
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwartsmann G, Di Leone LP, Dal Pizzol F
and Roesler R: MAPK pathway activation in colorectal cancer: A
therapeutic opportunity for GRP receptor antagonists. Lancet Oncol.
6:444–445. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Quartuccio SM, Lantvit DD, Bosland MC and
Burdette JE: Conditional inactivation of p53 in mouse ovarian
surface epithelium does not alter MIS driven Smad2-dominant
negative epithelium-lined inclusion cysts or teratomas. PLoS One.
8:e650672013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Cutsem E and Costa F: Progress in the
adjuvant treatment of colon cancer: Has it influenced clinical
practice? JAMA. 294:2758–2760. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Longley DB, Allen WL and Johnston PG: Drug
resistance, predictive markers and pharmacogenomics in colorectal
cancer. Biochim Biophys Acta. 1766:184–196. 2006.PubMed/NCBI
|
|
14
|
Lippman SM: The dilemma and promise of
cancer chemoprevention. Nat Clin Pract Oncol. 3:5232006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma X and Wang Z: Anticancer drug discovery
in the future: An evolutionary perspective. Drug Discov Today.
14:1136–1142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Newman DJ, Cragg GM and Snader KM: The
influence of natural products upon drug discovery. Nat Prod Rep.
17:215–234. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin J, Wei L, Shen A, Cai Q, Xu W, Li H,
Zhan Y, Hong Z and Peng J: Hedyotis diffusa Willd extract
suppresses Sonic hedgehog signaling leading to the inhibition of
colorectal cancer angiogenesis. Int J Oncol. 42:651–656. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai Q, Lin J, Wei L, Zhang L, Wang L, Zhan
Y, Zeng J, Xu W, Shen A, Hong Z, et al: Hedyotis diffusa Willd
inhibits colorectal cancer growth in vivo via inhibition of STAT3
signaling pathway. Int J Mol Sci. 13:6117–6128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin J, Chen Y, Wei L, Chen X, Xu W, Hong
Z, Sferra TJ and Peng J: Hedyotis Diffusa Willd extract induces
apoptosis via activation of the mitochondrion-dependent pathway in
human colon carcinoma cells. Int J Oncol. 37:1331–1338.
2010.PubMed/NCBI
|
|
21
|
Lin J, Chen Y, Cai Q, Wei L, Zhan Y, Shen
A, Sferra TJ and Peng J: Scutellaria Barbata D Don inhibits
colorectal cancer growth via suppression of multiple signaling
pathways. Integr Cancer Ther. 13:240–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei L, Lin J, Wu G, Xu W, Li H, Hong Z and
Peng J: Scutellaria barbata D. Don induces G1/S arrest via
modulation of p53 and Akt pathways in human colon carcinoma cells.
Oncol Rep. 29:1623–1628. 2013.PubMed/NCBI
|
|
23
|
Wei L, Lin J, Xu W, Cai Q, Shen A, Hong Z
and Peng J: Scutellaria barbata D. Don inhibits tumor angiogenesis
via suppression of Hedgehog pathway in a mouse model of colorectal
cancer. Int J Mol Sci. 13:9419–9430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen L, Liu L, Ye L, Shen A, Chen Y,
Sferra TJ and Peng J: Patrinia scabiosaefolia inhibits colorectal
cancer growth through suppression of tumor angiogenesis. Oncol Rep.
30:1439–1443. 2013.PubMed/NCBI
|
|
25
|
Liu L, Shen A, Chen Y, Wei L, Lin J,
Sferra TJ, Hong Z and Peng J: Patrinia scabiosaefolia induces
mitochondrial-dependent apoptosis in a mouse model of colorectal
cancer. Oncol Rep. 30:897–903. 2013.PubMed/NCBI
|
|
26
|
Peng J, Chen Y, Lin J, Zhuang Q, Xu W,
Hong Z and Sferra TJ: Patrinia scabiosaefolia extract suppresses
proliferation and promotes apoptosis by inhibiting the STAT3
pathway in human multiple myeloma cells. Mol Med Rep. 4:313–318.
2011.PubMed/NCBI
|
|
27
|
Liu J: Oleanolic acid and ursolic acid:
Research perspectives. J Ethnopharmacol. 100:92–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Bai H, Zhang X, Liu J, Cao P, Liao
N, Zhang W, Wang Z and Hai C: Inhibitory effect of oleanolic acid
on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest
and mitochondrial-dependent apoptosis. Carcinogenesis.
34:1323–1330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lúcio KA, Rocha GG, Monção-Ribeiro LC,
Fernandes J, Takiya CM and Gattass CR: Oleanolic acid initiates
apoptosis in non-small cell lung cancer cell lines and reduces
metastasis of a B16F10 melanoma model in vivo. PLoS One.
6:e285962011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen Y, Robles AI, Martinez LA, Liu F,
Gimenez-Conti IB and Conti CJ: Expression of G1 cyclins,
cyclin-dependent kinases, and cyclin-dependent kinase inhibitors in
androgen-induced prostate proliferation in castrated rats. Cell
Growth Differ. 7:1571–1578. 1996.PubMed/NCBI
|
|
33
|
Graña X and Reddy EP: Cell cycle control
in mammalian cells: Role of cyclins, cyclin dependent kinases
(CDKs), growth suppressor genes and cyclin-dependent kinase
inhibitors (CKIs). Oncogene. 11:211–219. 1995.PubMed/NCBI
|
|
34
|
Chung DC, Brown SB, Graeme-Cook F, Seto M,
Warshaw AL, Jensen RT and Arnold A: Overexpression of cyclin D1
occurs frequently in human pancreatic endocrine tumors. J Clin
Endocrinol Metab. 85:4373–4378. 2000.PubMed/NCBI
|
|
35
|
Keum JS, Kong G, Yang SC, Shin DH, Park
SS, Lee JH and Lee JD: Cyclin D1 overexpression is an indicator of
poor prognosis in resectable non-small cell lung cancer. Br J
Cancer. 81:127–132. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Adams JM and Cory S: Bcl-2-regulated
apoptosis: Mechanism and therapeutic potential. Curr Opin Immunol.
19:488–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peng J, Ding J, Tan C, Baggenstoss B,
Zhang Z, Lapolla SM and Lin J: Oligomerization of membrane-bound
Bcl-2 is involved in its pore formation induced by tBid. Apoptosis.
14:1145–1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Peng J, Tan C, Roberts GJ, Nikolaeva O,
Zhang Z, Lapolla SM, Primorac S, Andrews DW and Lin J: tBid elicits
a conformational alteration in membrane-bound Bcl-2 such that it
inhibits Bax pore formation. J Biol Chem. 281:35802–35811. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yip KW and Reed JC: Bcl-2 family proteins
and cancer. Oncogene. 27:6398–6406. 2008. View Article : Google Scholar : PubMed/NCBI
|