Introduction
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL/APO2L) is a member of the tumor necrosis factor (TNF)
family of cytokines and an effective inducer of apoptosis in cancer
cells (1). The interaction of
TRAIL with death receptors (DRs), including the death receptors
TRAILR1 (also known as DR4 and TNFRSF10A) and TRAILR2 (also known
as DR5, KILLER and TNFRSF10B), on the surface of cancer cells can
trigger apoptotic cell death signaling through death
receptor-mediated apoptosis pathway without any harmful effects to
normal cells (2,3). TRAIL binding to DRs causes
conformational changes in DRs, which leads to the recruitment of
the adaptor protein Fas-associated death domain (FADD) and
caspase-8 and -10 through the cytoplasmic death domain (DD). This
forms the so-called death-inducing signaling complex (DISC).
Normally, the DISC fully activates caspase-8 and triggers apoptosis
by directly activating the executive caspases, such as caspase-3,
-6 and -7, also found in Fas type I cancer cells. However,
TRAIL-mediated apoptosis can also induce the mitochondria-mediated
apoptosis pathway through the implication of mitochondrial
dysfunction and caspase-9 activation via the cleavage of Bid
(BH3-interacting domain death agonist) protein into truncated Bid
(tBid) by caspase-8. tBid is capable of inducing mitochondrial
outer membrane permeabilisation (MOMP) in cells in which the ratio
of pro- and anti-apoptotic Bcl-2 family members allows it to do so
leading to mitochondrial dysfunction in TRAIL-treated cancer cells,
also found in Fas type II cancer cells (4–7).
However, some cancer cells are resistant to
TRAIL-induced apoptosis, especially some highly malignant tumors
such as pancreatic cancer, melanoma, neuroblastoma, prostate cancer
and colon cancer (8,9). Failure to undergo apoptosis has been
implicated in the resistance of cancer cells to TRAIL surveillance
and tumor development. The mechanism of resistance to TRAIL-induced
apoptosis in cancer can occur at different points in the signaling
pathways of TRAIL-induced apoptosis. Dysfunctions or low expression
of the DRs can lead to resistance. The defects in FADD and
caspase-8 can lead to TRAIL resistance. Another cause of this
defect is the high expression of cellular FADD-like
interleukin-1β-converting enzyme-inhibitory protein (cFILP) which
correlates with TRAIL resistance in several types of cancers
because it can bind to FADD and/or caspase-8 and death receptors.
This interaction in turn prevents DISC formation and subsequently
suppresses the activation of caspase cascade (10,11).
High expression of apoptosis inhibitors have been reported to
result in TRAIL resistance in mitochondria-dependent type II cancer
cells (9). Thus, developing
strategies to overcome the TRAIL resistance are the topics of
interest. Several observations suggest that the combination of
TRAIL with effective small molecule compounds can sensitize the
resistant cancer to TRAIL-induced apoptosis. Therefore, it has been
assumed as strategy to potentiate the cytotoxicity of TRAIL and its
therapeutic applications.
The combined compounds synergize TRAIL-induced
apoptosis in cancer through two pathways. First, to increase the
death receptors DR4/DR5 expression and trigger its translocation to
cell membranes thus increasing TRAIL binding resulted in extrinsic
apoptosis pathway. Several chemotherapeutic agents and natural
compounds, such as CDDP (12),
etoposide (13), PS-341
(bortezomib) (14), tunicamycin
(15), rottlerin (16), brandisianins (17), sodium butyrate (18), inostamycin (19) were reported to upregulate the death
receptor expression and subsequent sensitization of TRAIL-resistant
cancer cells to TRAIL-induced apoptosis. Second, resistant
mechanism of TRAIL-induced apoptosis is disrupted through
downregulation of cFLIP expression. Natural compounds such as
kurarinone (20), icaritin
(21), withanolide E (22) were reported to downregulate cFLIP
expression and subsequent sensitization of TRAIL-resistant cancer
cells to TRAIL-induced apoptosis. Natural compounds, such as
silibinin (23), gingerol
(24) and indomethacin (25) were reported to possess both
mechanisms of sensitizing TRAIL-resistant cancer cells.
The LoVo colorectal cancer (CRC) cell line is
derived from left supraclavicular region; stage Dukes' C (26). The CRC is the second most and the
third most common cancer in women (representing 9.2% of the total)
and men (representing 10.0% of the total) worldwide, respectively
(27). The CRC cell lines which
resist to TRAIL-induced apoptosis remains a problem in the
treatment of these cancers, thus the approaches for enhancing
TRAIL-induced apoptosis are urgently required. The LoVo cell line
was used as a model of TRAIL-refractory colorectal cancer cells in
this study as they were reported to express significantly lower
level of cell surface DR5 than the other colon cancer cell lines
resulting in resistance to TRAIL treatment (8). Thus, finding the strategy to overcome
the TRAIL-insensitive cancer cells is of importance.
Goniothalamin is a major bioactive styryl-lactone
compound found in plant Goniothalamus macrophyllus (Blume)
Hook. f. & Thomson, indigenous to South East Asia (28). Many reports suggested that
goniothalamin showed cytotoxic activity against various cancer cell
lines, such as liver, breast, and cervix (29–34).
Interestingly, our preliminary studies indicated that goniothalamin
could increase DR5 expression while decrease cFILP expression in
LoVo cells. These preliminary results suggested that goniothalamin
has a potential use for combination with TRAIL treatment in
TRAIL-resistant LoVo cells. In this study, the mechanisms to
overcome the resistance to TRAIL-induced apoptosis were
investigated using goniothalamin combining with TRAIL in
TRAIL-resistant LoVo cells. This indicated the potential
application of goniothalamin as a synergistic agent for combining
with TRAIL treatment in colorectal cancer.
Materials and methods
Chemical and antibodies
Goniothalamin (IUPAC name:
(2R)-2-[(E)-2-phenylethenyl]-2,3-dihydropyran-6-one) was obtained
from Dr Wilawan Mahabusarakam, Faculty of Science, Prince of
Songkla University, Thailand in purified powder form. The stems of
Goniothalamus macrophyllus were collected from Songkhla
province in the southern part of Thailand, in September, 2007.
Identification was made by Mr. Ponlawat Pattarakulpisutti,
Department of Biology, Faculty of Science, Prince of Songkla
University. The specimen (Uraiwan 01) has been deposited in the
Herbarium of Department of Biology, Faculty of Science, Prince of
Songkla University, Thailand. Recombinant TRAIL was purchased from
Merck Millipore Corp. (Merck KGaA, Darmstadt, Germany). Chemicals
for cell viability assay including MTT
(3-(4,5-dimethyl)-2,5-diphenyl tetrazolium bromide) and
dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich Corp.
(St. Louis, MO, USA). Chemicals for flow cytometry analysis
including Guava Cell Cycle® reagent and Guava
Nexin® reagent were purchased from Merck Millipore Corp.
(Merck KGaA), and PE-conjugated DR5 antibody was purchased from
eBioscience, Inc. (San Diego, CA, USA). Chemical for fluorescence
microscope observation Hoechst 33342 dye was purchased from Fisher
Scientific, Inc. (Invitrogen™, Waltham, MA, USA). Chemical for mRNA
extraction and cDNA synthesis were purchased from Qiagen N.V.
(QIAzol™ lysis reagent, Venlo, LI, The Netherlands) and Thermo
Fisher Scientific, Inc. (RevertAid™ First Strand cDNA Synthesis
kit, Fermentas™, Waltham, MA, USA), respectively. Chemical for
quantitative PCR was obtained from Thermo Fisher Scientific, Inc.
(SYBR® Select Master Mix, Applied Biosystems™, Waltham,
MA, USA). Antibodies (Abs) for immunoblot analysis including mouse
monoclonal Abs against CHOP, and rabbit monoclonal Abs against DR5,
PARP, caspase-3, caspase-8, caspase-9, Bcl2, Bax, Bid, Mcl1, and
anti-mouse immunoglobulin G and anti-rabbit immunoglobulin G
horseradish peroxidase-conjugated secondary antibodies were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA),
and mouse monoclonal Abs against phospho-histone H2AX at Ser139
(γ-H2AX), β-actin and rabbit monoclonal Abs against cFLIP were
obtained from Merck Millipore Corp. (Merck KGaA).
Cell culture
Human colorectal cancer, LoVo cell line, was
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA). It was maintained in RPMI-1640 medium (Gibco Life
Technologies, Carlbad, CA, USA) supplemented with 10% fetal bovine
serum (GE Healthcare Life Science, Little Chalfont, UK), 100 U/ml
penicillin and 100 μg/ml streptomycin (GE Healthcare Life Science,
Inc., Little Chalfont, UK) at 37°C in a humidified 5%
CO2 atmosphere and used for assays during exponential
phase of growth.
Cell viability assay
Cells/well (5×103) were seeded in a
96-well plate. After adherence, culture medium containing 10 and
100 ng/ml of TRAIL alone and in combination with different
goniothalamin concentrations 5, 15, 25 and 50 μM were incubated for
24 h at 37°C with 5% CO2. The control group was treated
with 0.5% DMSO. Cytotoxicity of goniothalamin was determined by
cell proliferation analysis using MTT assay as described by Denizot
and Lang (35). Briefly, after the
indicated treatment, 0.5 mg/ml of MTT solution dissolved in culture
medium was added and the cells were incubated for 2 h at 37°C with
5% CO2 in the incubator, the MTT solution was removed
and 100 μl of DMSO was added to dissolve the formazan crystals, a
product of cell respiration as for viable cells, and the absorbance
at 540 nm was quantified on Epoch™ Microplate Spectrophotometer and
analyzed by Gen5™ Data Analysis software (BioTek, CA, USA).
Chromatin condensation
Cells/well (8×104) were seeded in a
12-well plate. After adherence, culture medium containing 10 and
100 ng/ml of TRAIL alone and in combination with 15μM goniothalamin
were incubated for 24 h at 37°C with 5% CO2. The control
group was treated with 0.5% DMSO. Chromatin condensation, a
character of apoptosis, was detected by cell staining with a
fluorescent dye Hoechst 33342 modified from Oberhammer et al
(36). After treatment, the
treated cells were washed and fixed with fixative solution (4%
paraformaldehyde) for 15 min at room temperature. The fixed cells
were washed and then stained with chromatin staining solution (5
μg/ml of Hoechst 33342) for 15 min. After staining, the stained
cell were washed and then the plates were observed using a
fluorescence microscope IX73 model (Olympus, Tokyo, Japan) with
U-MWU2 mirror units for ultraviolet excitation.
Cell cycle determination
Cells/well (2×105) were seeded in each
6-well plate. After adherence, culture medium containing 10 and 100
ng/ml of TRAIL alone and in combination with 15 μM goniothalamin
were incubated for 24 h at 37°C with 5% CO2. The control
group was treated with 0.5% DMSO. After treatment, the whole cells
were collected and stained according to the manufacturer's
instructions (Guava Cell Cycle® reagent from Merck
Millipore Corp.; Merck KGaA). The stained cells were then sorted
and analyzed for DNA content by a Guava easyCyte™ flow cytometer
and GuavaSoft™ software (Merck Millipore Corp.; Merck KGaA),
respectively.
Cell surface phosphatidyl-serine
determination
Cells/well (2×105) were seeded in a
6-well plate. After adherence, culture medium containing 10 and 100
ng/ml of TRAIL alone and in combination with 15 μM goniothalamin
were incubated for 24 h at 37°C with 5% CO2. The control
group was treated with 0.5% DMSO. After treatment, whole cells were
collected and stained according to the manufacturer's instructions
(Guava Nexin® reagent from Merck Millipore Corp.; Merck
KGaA). The stained cells were sorted and analyzed for cell surface
phosphatidyl-serine content a Guava easyCyte™ flow cytometer and
GuavaSoft™ software (Merck Millipore Corp.; Merck KGaA),
respectively.
Cell surface DR5 determination
Cells/well (2×105) were seeded in a
6-well plate. After adherence, culture medium containing different
goniothalamin concentrations of 1, 5, 15 and 25 μM was incubated
for 24 h at 37°C with 5% CO2. The control group was
treated with 0.5% DMSO. After treatment, the whole cells were
collected and resuspended in PBS buffer containing PE-conjugated
DR5 antibody, then incubated in the dark for 1 h at room
temperature. The cells were washed and resuspended in PBS solution
then sorted and analyzed for cell surface DR5 by a Guava easyCyte™
flow cytometer and GuavaSoft software (Merck Millipore Corp.; Merck
KGaA), respectively.
mRNA expression analysis
Cells/well (8×104)were seeded in a
12-well plate. After adherence, culture medium containing 15μM
goniothalamin was incubated for 24 h at 37°C with 5%
CO2. The control group was treated with 0.5% DMSO.
Analysis of mRNA expression was performed using the two step
quantitative reverse transcriptase (RT)-PCR. After treatment, the
whole cells were collected and RNA was extracted by using QIAzol
lysis reagent (Qiagen N.V.) and cDNA synthesis by reverse
transcription according RevertAid First Strand cDNA Synthesis kit
(Fermentas, Thermo Fisher Scientific) with 2 μg of total RNA of
each sample. In quantitative PCR step, it was performed with SYBR
Select Master Mix (Applied Biosystems, Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The primers used for amplification were:
DR5 (forward 5′-CACCAGGTGTGATTCAGGTG-3′ and reverse
5′-TACGGCTGCAACTGTGACTC-3′), CHOP (forward
5′-GCGCATGAAGGAGAAAGAAC-3′ and reverse 5′-TCACCATTCGGTCAATCAGA-3′),
cFILPL (forward 5′-ATTGCATTGGCAATGAGACAGAGC-3′ and
reverse 5′-TCGGTGCT CGGGCATACAGG-3′), cFILPS (forward
5′-GGGCCGAG GCAAGATAAGCAAGG-3′ and reverse 5′-TCAGGACAAT
GGGCATAGGGTGT-3′), and GAPDH (forward 5′-AGGTCG GAGTCAACGGATTT-3′
and reverse 5′-TAGTTGAGGTC AATGAAGGG-3′). The PCR amplification was
analyzed by CFX96 Touch™ Real-Time PCR Detection system with CFX
Manager™ software (Bio-Rad Laboratories, Inc., CA, USA). All steps
were performed according to the manufacturer's instructions.
Protein expression analysis by
immunoblotting
Cells/well (2×105) were seeded in a
6-well plate. After adherence, the cells were treated with
appropriate condition. After treatment, the cells were lysed with
RIPA lysis buffer (50 mM Tris-HCl, pH 7.4, 1% NP-40, 0.5%
C24H39NaO4, 0.1% SDS, 150 mM NaCl,
2 mM EDTA, 50 mM NaF). The extracted proteins were separated on
8–15% acrylamind gel and transferred onto a polyvinylidene fluoride
(PVDF) membrane (Merck Millipore Corp., Merck KGaA). Then, the
membranes were blocked with 5% skimmed-milk in TBS-Tween buffer for
1 h at room temperature and incubated with mouse monoclonal Abs
against CHOP, γ-H2AX, and rabbit monoclonal Abs against DR5, PARP,
caspase-3, caspase-8, caspase-9, Bcl2, Bax, Bid), cFLIP, β-actin
overnight at 4°C. Following incubation with anti-mouse
immunoglobulin G or anti-rabbit immunoglobulin G horseradish
peroxidase-conjugated secondary antibodies for 1 h a room
temperature, the signals were developed using Immobilon™ Western
chemiluminescent HRP substrate (Merck Millipore Corp., Merck KGaA,
Darmstadt, Germany) and detected under Chemiluminescent Imaging
system (GeneGnome gel documentation, Synoptics Ltd., Cambridge,
UK).
Statistical analysis
To compare the data from different treatments,
Student's t-test was used. All data presented were obtained from at
least three independent experiments and presented as mean ±
standard deviation (SD). A p-value of 0.05 was taken as minimum
basis for assigning significance.
Results
Enhanced TRAIL-induced apoptosis in LoVo
cells by co-treatment with goniothalamin
Our preliminary study showed that LoVo cells were
insensitive to goniothalamin treatment with high IC50
value at 65.25±1.85 μM. However, goniothalamin induced increased
DR5 expression at lower concentration than IC50 value
indicating that goniothalamin in combination with TRAIL may have a
potential to trigger apoptosis induction via the death
receptor-TRAIL mediated apoptosis pathway. Thus, we tried to
investigate the mechanisms of apoptosis induction upon combination
of TRAIL and goniothalamin in LoVo cells.
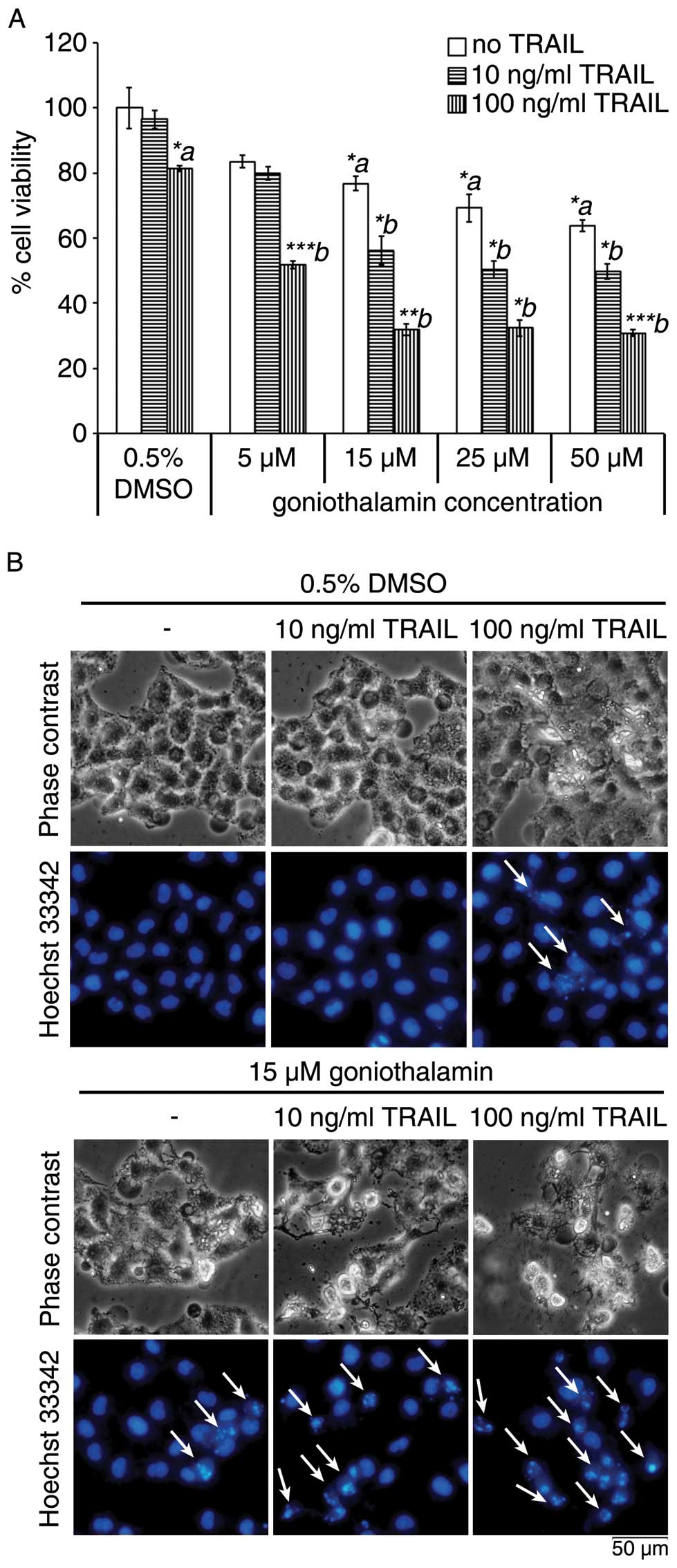
In this study, we first found that co-treatment of
goniothalamin and TRAIL enhanced cytotoxicity induction in LoVo
cells. We confirmed this cytotoxic effects and apoptosis induction
using the MTT assay and Hoechst 33342 staining to assess chromatin
condensation as shown in Fig. 1A and
B, respectively. Treatment of LoVo cells with 10 and 100 ng/ml
of TRAIL for 24 h showed >80% cell viability, while combined
treatment with 15 μM goniothalamin resulted in enhanced
cytotoxicity in LoVo cells. The increased chromatin condensation is
shown in Fig. 1B upon combining
treatment of 15 μM goniothalamin and 10 and 100 ng/ml of TRAIL as
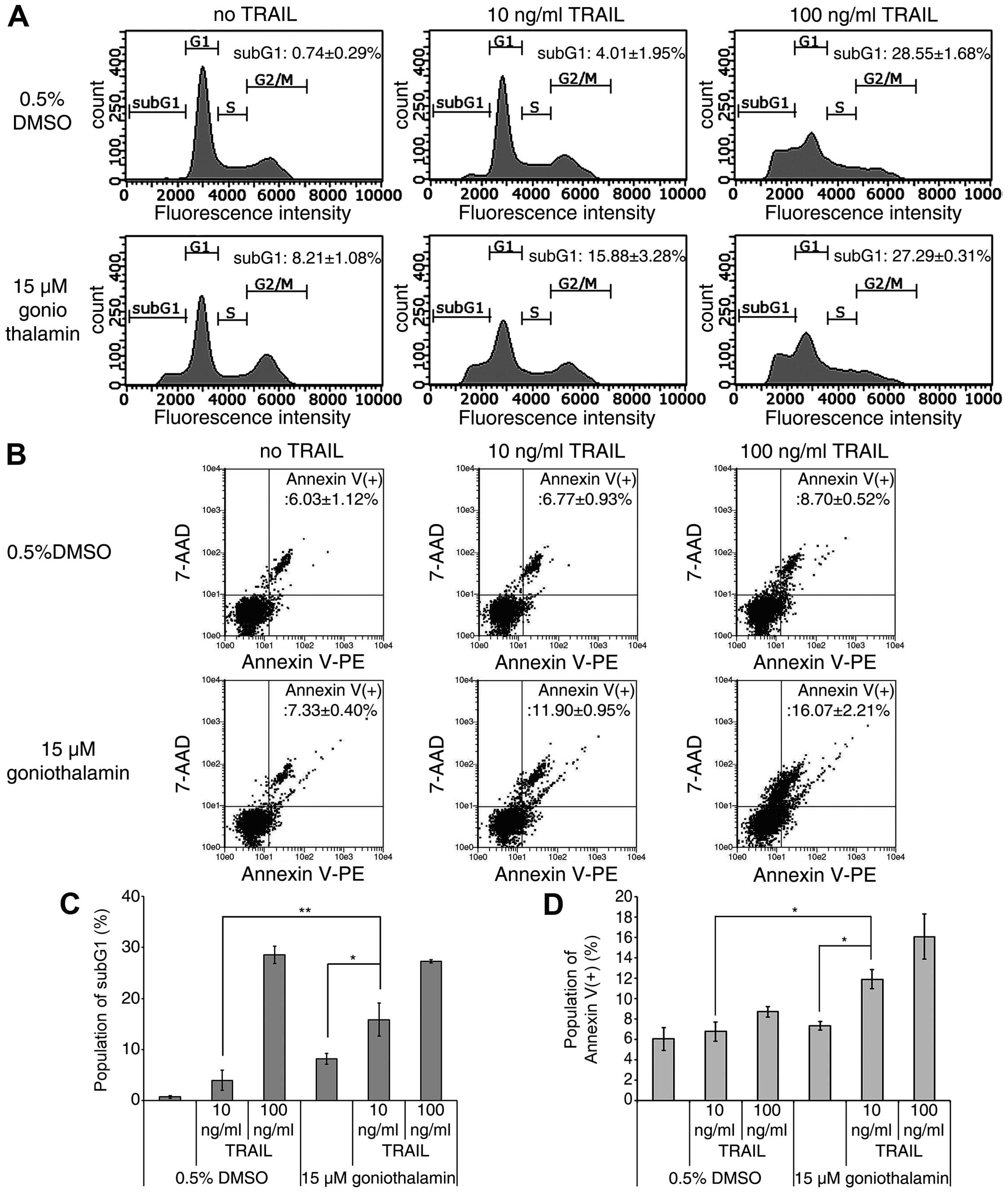
compared to a single treatment. Moreover, other apoptotic
characteristics, accumulation of subG1 phase population and cell
surface phosphatidyl-serine presentation, were studied using flow
cytometry technique. As shown in Fig.
2A and C, a significant increased accumulation of a subG1 phase
population was detected upon treatment with 15 μM goniothalamin and
10 ng/ml TRAIL as compared to a single treatment, but not for the
combination of 15 μM goniothalamin and 100 ng/ml TRAIL as compared
to a single 100 ng/ml TRAIL treatment. In addition, the significant
increased cell surface phosphatidyl-serine presentation was
detected upon combined treatment with both 10 and 100 ng/ml of
TRAIL as compared to a single treatment (Fig. 2B and D). Thus, these results
indicated that the combined treatment of goniothalamin and TRAIL
enhanced cytotoxicity and apoptosis induction in LoVo cells,
especially at 15 μM goniothalamin and 10 ng/ml TRAIL, which was
selected for use in the next steps to assess apoptosis pathway.
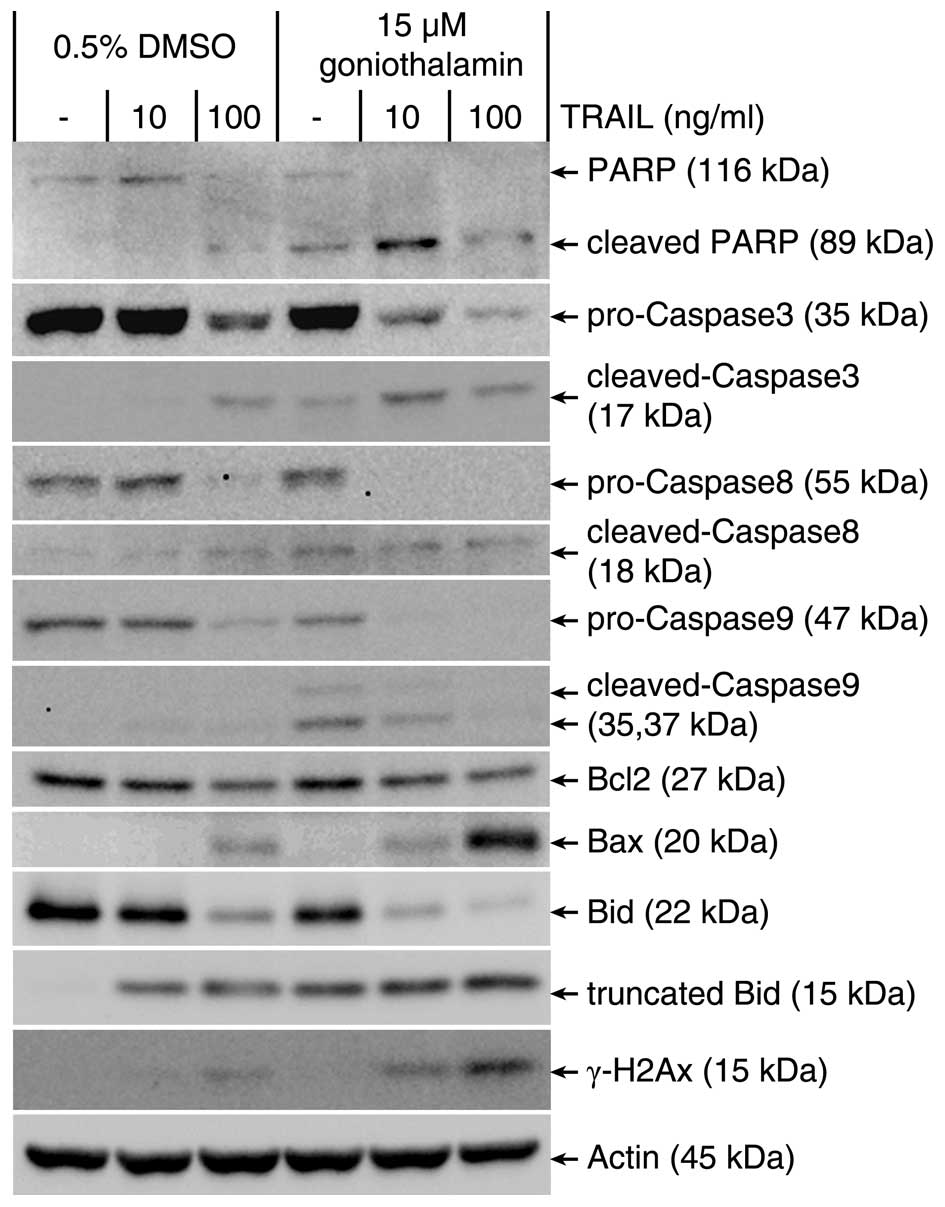
Combined treatment with goniothalamin and
TRAIL accelerate apoptosis induction by a caspase
activation-dependent pathway involved with both death receptor- and
mitochondrial-mediated apoptosis pathways in LoVo cells
To observe whether the combination of goniothalamin
and TRAIL leads to activation of caspase-activated apoptosis in
TRAIL-resistant LoVo cells, apoptotic-related protein was assessed
by immunoblot analysis. As shown in Fig. 3, caspase-activated apoptotic
mediators including PARP and caspase-3 as executive apoptosis,
caspase-8 and Bid as death receptor mediated apoptosis pathway,
caspase-9, Bcl2 and Bax as mitochondrial mediated apoptosis pathway
were determined. The results indicated that both extrinsic and
intrinsic pathway were enhanced upon combined treatment of 15 μM
goniothalamin and 10 or 100 ng/ml of TRAIL, as confirmed by
increasing the cleaved form of PARP, caspase-3, caspase-8,
caspase-9, Bid, decreased antiapoptotic Bcl2 and increased
proapoptotic Bax expression. Moreover, increased phosphorylation of
histone the so-called γ-H2AX, was observed upon the combined
treatments, these results indicated that goniothalamin induced DNA
double-strand breaks and triggered apoptosis-associated γ-H2AX
accumulation, which is one of apoptotic characteristics. The
results indicated that these combined treatment can induce
cytotoxicity resulting in DNA double-strand break in LoVo
cells.
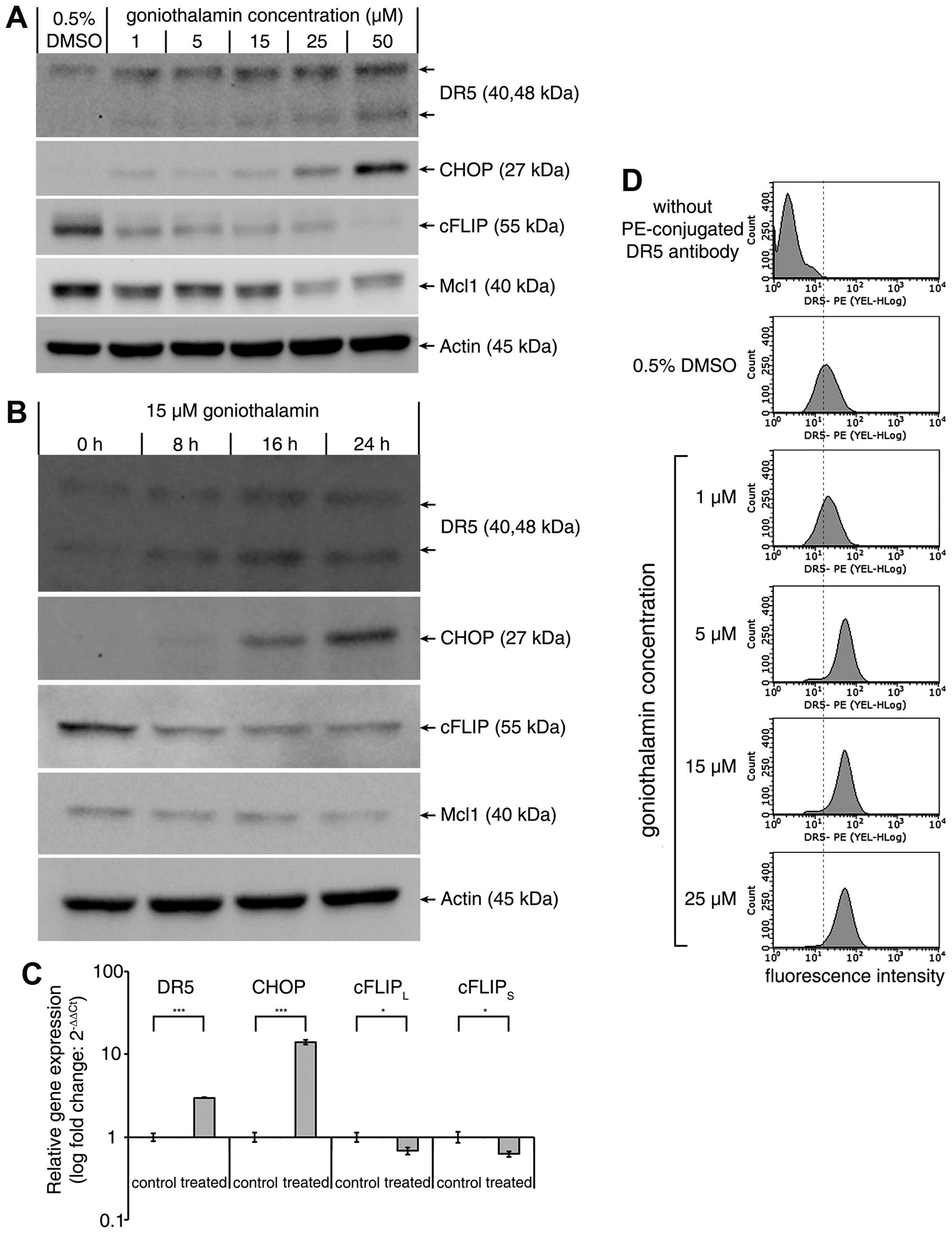
Goniothalamin enhances TRAIL-induced
apoptosis through DR5 upregulation and cFLIP downregulation
TRAIL-stimulated death signal is initiated by the
binding of TRAIL to DR5 resulted in the subsequent caspase-8
activation. As shown in Fig. 4,
immunoblot analysis showed that goniothalamin dramatically
upregulated the DR5 and CHOP protein but downregulated the cFLIP
and Mcl1 protein in a dose-dependent manner (Fig. 4A). Treatment with 15 μM
goniothalamin at various time points showed that goniothalamin
upregulated the DR5 and CHOP protein while downregulated the
antiapoptotic cFLIP and Mcl1 in a time-dependent manner (Fig. 4B). These results corresponded with
quantitative RT-PCR analysis (Fig.
4C) indicating that DR5 and CHOP mRNA were upregulated whereas
cFLIPL and cFLIPS mRNA was significantly
downregulated. Moreover, the translocation of DR5 to cell surface
was analyzed and the results indicated that goniothalamin increased
cell surface DR5 expression in a dose-dependent manner (Fig. 4D). These results implied that DR5
upregulation induced by increased CHOP expression together with the
downregulation of cFLIP and Mcl1 contributed to enhanced TRAIL
sensitization.
Discussion
TRAIL, also known as Apo-2L, is a typical member of
TNF ligand family that induces apoptosis via death-receptor
mediated pathway. TRAIL has potential benefits in cancer therapy
because of its potent ability to be selectively toxic in cancer
cells. Unlike the other death ligands such as TNF-α or FasL, the
treatment of TRAIL causes less toxic in normal cells (37,38).
Furthermore, the combined treatment of TRAIL and genotoxic
chemotherapeutic agents synergistically inhibited cancer cell
growth which are otherwise resistant or less toxicity to treatment
with TRAIL or chemotherapy alone (37–39).
There are several recombinant TRAIL and TRAIL-receptor agonists as
an anticancer therapy that have been tested in phase I and II
trials in patients with advanced cancer. Clinical studies in
TRAIL-receptor agonist are being investigated using combination
treatment in patients with advanced cancer stage (40). However, the single TRAIL treatment
probably is not feasible since the majority of cancer cells are
resistant to TRAIL. Thus, the combination treatment with TRAIL and
chemotherapy is essential for use in TRAIL-resistant cancers. We
also analyzed in detail that TRAIL combined treatment with
cytotoxic agent goniothalamin may enhance cytotoxicity and
apoptosis induction in colorectal cancer cells, indicating a
potential use for cancer therapy.
In this study, we demonstrated for the first time
that goniothalamin upon combined treatment with TRAIL-regulated
expression of antiapoptotic- and proapoptotic-related death
receptor-mediated apoptotic molecules, including upregulation of
DR5 and CHOP, downregulation of cFLIP and Mcl1 resulting in
enhancement of the ability of TRAIL in TRAIL refractory LoVo cells.
Various studies have reported the increased transcriptional
activation of DR5 gene by the upregulation of CHOP expression
(41–45), these correlated to the upregulation
of CHOP expression in goniothalamin treatment. Another mechanism
which is involved in sensitization to TRAIL-induced apoptosis is
downregulation of cFLIP and Mcl1. cFLIP is the major protein that
prevents caspase-8 from activation by death receptors through
binding to FADD and/or caspase-8 and TRAIL receptor DR5 in a
ligand-dependent and -independent manner and forms an apoptosis
inhibitory complex (AIC), then prevents death-inducing signaling
complex (DISC) formation and subsequently suppress the activation
of caspase cascade (46–53). Mcl1 is an antiapoptotic protein
involved in death receptor mediated pathway cross-link to
mitochondrial mediated pathway by interacting with truncated Bid
(tBid) and then strongly inhibits tBid-induced cytochrome c
release in mitochondrial mediated apoptosis pathway (54–57).
Downregulation of cFLIP and Mcl1 expression sensitizes
TRAIL-induced apoptosis in various TRAIL refractory cancers. Thus,
we speculated that goniothalamin plus TRAIL might play a critical
role in goniothalamin-stimulated TRAIL-mediated apoptosis in LoVo
cells through both upregulation of DR5 and CHOP and downregulation
of cFLIP and Mcl1 expression enhancing TRAIL ability to be
selectively cytotoxic to TRAIL-refractory LoVo cells. Moreover, DR5
translocation to cell surface was increased by goniothalamin
treatment that may increase potent death receptor-mediated
apoptosis induction by TRAIL through binding to DR5 (58–60).
Caspase-dependent pathways of these TRAIL-mediated
apoptosis are involved in this combination treatment, resulting in
a strong enhancement of PARP, caspase-3, -8 and -9 activation.
TRAIL triggered death receptor mediated apoptosis pathway via
binding to death receptor DR5 resulting in induction cleavage of
Bid to tBid, then crosslinking to mitochondria-mediated apoptosis
activation (4–7,61),
supported by downregulation of Mcl1 in goniothalamin treatment. Our
results showed that apoptotic related molecules triggered
activation in both death receptor- and mitochondrial-mediated
apoptosis pathways under combination treatment of goniothalamin and
TRAIL. Moreover, the increased accumulation of subG1 phase
population in cell cycle, increased cell surface
phosphatidyl-serine presentation and increased phosphorylation of
H2AX were observed under the combined treatment of TRAIL and
goniothalamin, indicating apoptosis induction in LoVo cells as
compared to a single treatment with TRAIL or goniothalmin alone
(62–65). Similar reports of other compounds
sensitize TRAIL-induced apoptosis include inostamycin (19), delphinidin (66), and parthenolide (67). Therefore, the combined treatment of
TRAIL and goniothalamin enhanced cytotoxicity in TRAIL refractory
LoVo cells through caspase-dependent apoptosis pathway in both
death receptor- and mitochondrial-mediated pathways.
In conclusion, this is the first report that the
combination of TRAIL and goniothalamin was able to effectively
enhance TRAIL mediated apoptosis induction in TRAIL refractory
colorectal cancer, LoVo cells. In addition, we found that
goniothalamin enhanced TRAIL sensitization in LoVo cells associated
with caspase cascade activation via induction of the DR5 pathway
and decreased expression level of antiapoptotic proteins which
related to DR5 pathway and subsequently increased apoptosis. From
these results, we speculate that combined treatment of TRAIL and
goniothalamin provides a possible therapeutic application for
treatment of colorectal cancer that are resistant to TRAIL.
Acknowledgements
This study was supported by The Royal Golden Jubilee
(RGJ) Ph.D. Program from Thailand Research Fund (TRF) Thailand and
the Strategic Wisdom and Research Institute, Srinakharinwirot
University, Bangkok, Thailand.
References
|
1
|
Fukuda M, Hamao A, Tanaka A, Kitada M,
Suzuki S, Kusama K and Sakashita H: Tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL/APO2L) and its receptors
expression in human squamous cell carcinoma of the oral cavity.
Oncol Rep. 10:1113–1119. 2003.PubMed/NCBI
|
|
2
|
Kichev A, Rousset CI, Baburamani AA,
Levison SW, Wood TL, Gressens P, Thornton C and Hagberg H: Tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL) signaling
and cell death in the immature central nervous system after
hypoxia-ischemia and inflammation. J Biol Chem. 289:9430–9439.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walczak H, Miller RE, Ariail K, Gliniak B,
Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schug ZT, Gonzalvez F, Houtkooper RH, Vaz
FM and Gottlieb E: BID is cleaved by caspase-8 within a native
complex on the mitochondrial membrane. Cell Death Differ.
18:538–548. 2011. View Article : Google Scholar :
|
|
7
|
Kantari C and Walczak H: Caspase-8 and
bid: Caught in the act between death receptors and mitochondria.
Biochim Biophys Acta. 1813:558–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galligan L, Longley DB, McEwan M, Wilson
TR, McLaughlin K and Johnston PG: Chemotherapy and TRAIL-mediated
colon cancer cell death: The roles of p53, TRAIL receptors, and
c-FLIP. Mol Cancer Ther. 4:2026–2036. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L and Fang B: Mechanisms of
resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther.
12:228–237. 2005. View Article : Google Scholar
|
|
10
|
Lemke J, von Karstedt S, Zinngrebe J and
Walczak H: Getting TRAIL back on track for cancer therapy. Cell
Death Differ. 21:1350–1364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grambihler A, Higuchi H, Bronk SF and
Gores GJ: cFLIP-L inhibits p38 MAPK activation: An additional
anti-apoptotic mechanism in bile acid-mediated apoptosis. J Biol
Chem. 278:26831–26837. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagane M, Pan G, Weddle JJ, Dixit VM,
Cavenee WK and Huang HJ: Increased death receptor 5 expression by
chemotherapeutic agents in human gliomas causes synergistic
cytotoxicity with tumor necrosis factor-related apoptosis-inducing
ligand in vitro and in vivo. Cancer Res. 60:847–853.
2000.PubMed/NCBI
|
|
13
|
Sheikh MS, Burns TF, Huang Y, Wu GS,
Amundson S, Brooks KS, Fornace AJ Jr and el-Deiry WS: p53-dependent
and -independent regulation of the death receptor KILLER/DR5 gene
expression in response to genotoxic stress and tumor necrosis
factor alpha. Cancer Res. 58:1593–1598. 1998.PubMed/NCBI
|
|
14
|
Liu X, Yue P, Chen S, Hu L, Lonial S,
Khuri FR and Sun SY: The proteasome inhibitor PS-341 (bortezomib)
up-regulates DR5 expression leading to induction of apoptosis and
enhancement of TRAIL-induced apoptosis despite up-regulation of
c-FLIP and survivin expression in human NSCLC cells. Cancer Res.
67:4981–4988. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shiraishi T, Yoshida T, Nakata S, Horinaka
M, Wakada M, Mizutani Y, Miki T and Sakai T: Tunicamycin enhances
tumor necrosis factor-related apoptosis-inducing ligand-induced
apoptosis in human prostate cancer cells. Cancer Res. 65:6364–6370.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lim JH, Park JW, Choi KS, Park YB and Kwon
TK: Rottlerin induces apoptosis via death receptor 5 (DR5)
upregulation through CHOP-dependent and PKC delta-independent
mechanism in human malignant tumor cells. Carcinogenesis.
30:729–736. 2009. View Article : Google Scholar
|
|
17
|
Kikuchi H, Ohtsuki T, Koyano T,
Kowithayakorn T, Sakai T and Ishibashi M: Brandisianins A-F,
isoflavonoids isolated from Millettia brandisiana in a screening
program for death-receptor expression enhancement activity. J Nat
Prod. 70:1910–1914. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim YH, Park JW, Lee JY and Kwon TK:
Sodium butyrate sensitizes TRAIL-mediated apoptosis by induction of
transcription from the DR5 gene promoter through Sp1 sites in colon
cancer cells. Carcinogenesis. 25:1813–1820. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamamoto K, Makino M, Watanapokasin R,
Tashiro E and Imoto M: Inostamycin enhanced TRAIL-induced apoptosis
through DR5 upregulation on the cell surface. J Antibiot (Tokyo).
65:295–300. 2012. View Article : Google Scholar
|
|
20
|
Zhou W, Cao A, Wang L and Wu D: Kurarinone
synergizes TRAIL-induced apoptosis in gastric cancer cells. Cell
Biochem Biophys. 72:241–249. 2014. View Article : Google Scholar
|
|
21
|
Han H, Xu B, Hou P, Jiang C, Liu L, Tang
M, Yang X, Zhang Y and Liu Y: Icaritin sensitizes human
glioblastoma cells to TRAIL-induced apoptosis. Cell Biochem
Biophys. 72:533–542. 2015. View Article : Google Scholar
|
|
22
|
Henrich CJ, Brooks AD, Erickson KL, Thomas
CL, Bokesch HR, Tewary P, Thompson CR, Pompei RJ, Gustafson KR,
McMahon JB and Sayers TJ: Withanolide E sensitizes renal carcinoma
cells to TRAIL-induced apoptosis by increasing cFLIP degradation.
Cell Death Dis. 6:e16662015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Son YG, Kim EH, Kim JY, Kim SU, Kwon TK,
Yoon AR, Yun CO and Choi KS: Silibinin sensitizes human glioma
cells to TRAIL-mediated apoptosis via DR5 up-regulation and
down-regulation of c-FLIP and survivin. Cancer Res. 67:8274–8284.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee DH, Kim DW, Jung CH, Lee YJ and Park
D: Gingerol sensitizes TRAIL-induced apoptotic cell death of
glioblastoma cells. Toxicol Appl Pharmacol. 279:253–265. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tse AK, Cao HH, Cheng CY, Kwan HY, Yu H,
Fong WF and Yu ZL: Indomethacin sensitizes TRAIL-resistant melanoma
cells to TRAIL-induced apoptosis through ROS-mediated upregulation
of death receptor 5 and downregulation of survivin. J Invest
Dermatol. 134:1397–1407. 2014. View Article : Google Scholar
|
|
26
|
Ahmed D, Eide PW, Eilertsen IA, Danielsen
SA, Eknæs M, Hektoen M, Lind GE and Lothe RA: Epigenetic and
genetic features of 24 colon cancer cell lines. Oncogenesis.
2:e712013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
28
|
Wattanapiromsakul C, Wangsintaweekul B,
Sangprapan P, Itharat A and Keawpradub N: Goniothalamin, a
cytotoxic compound, isolated from Goniothalamus macrophyllus
(Blume) Hook. f & Thomson var macrophyllus Songklanakarin. J
Sci Technol. 27:479–487. 2005.
|
|
29
|
Inayat-Hussain SH, Annuar BO, Din LB, Ali
AM and Ross D: Loss of mitochondrial transmembrane potential and
caspase-9 activation during apoptosis induced by the novel
styryl-lactone goniothalamin in HL-60 leukemia cells. Toxicol In
Vitro. 17:433–439. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chan KM, Rajab NF, Ishak MH, Ali AM,
Yusoff K, Din LB and Inayat-Hussain SH: Goniothalamin induces
apoptosis in vascular smooth muscle cells. Chem Biol Interact.
159:129–140. 2006. View Article : Google Scholar
|
|
31
|
Chen WY, Wu CC, Lan YH, Chang FR, Teng CM
and Wu YC: Goniothalamin induces cell cycle-specific apoptosis by
modulating the redox status in MDA-MB-231 cells. Eur J Pharmacol.
522:20–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Fátima A, Kohn LK, Antônio MA, de
Carvalho JE and Pilli RA: (R)-Goniothalamin: Total syntheses and
cytotoxic activity against cancer cell lines. Bioorg Med Chem.
13:2927–2933. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alabsi AM, Ali R, Ali AM, Al-Dubai SA,
Harun H, Abu Kasim NH and Alsalahi A: Apoptosis induction, cell
cycle arrest and in vitro anticancer activity of gonothalamin in a
cancer cell lines. Asian Pac J Cancer Prev. 13:5131–5136. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Petsophonsakul P, Pompimon W and
Banjerdpongchai R: Apoptosis induction in human leukemic
promyelocytic HL-60 and monocytic U937 cell lines by goniothalamin.
Asian Pac J Cancer Prev. 14:2885–2889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Denizot F and Lang R: Rapid colorimetric
assay for cell growth and survival. Modifications to the
tetrazolium dye procedure giving improved sensitivity and
reliability. J Immunol Methods. 89:271–277. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oberhammer FA, Hochegger K, Fröschl G,
Tiefenbacher R and Pavelka M: Chromatin condensation during
apoptosis is accompanied by degradation of lamin A+B, without
enhanced activation of cdc2 kinase. J Cell Biol. 126:827–837. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi J, Zheng D, Man K, Fan ST and Xu R:
TRAIL: A potential agent for cancer therapy. Curr Mol Med.
3:727–736. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nagane M, Huang HJ and Cavenee WK: The
potential of TRAIL for cancer chemotherapy. Apoptosis. 6:191–197.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shankar S and Srivastava RK: Enhancement
of therapeutic potential of TRAIL by cancer chemotherapy and
irradiation: Mechanisms and clinical implications. Drug Resist
Updat. 7:139–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Buchsbaum DJ, Forero-Torres A and LoBuglio
AF: TRAIL-receptor antibodies as a potential cancer treatment.
Future Oncol. 3:405–409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Krajarng A, Imoto M, Tashiro E, Fujimaki
T, Shinjo S and Watanapokasin R: Apoptosis induction associated
with the ER stress response through up-regulation of JNK in HeLa
cells by gambogic acid. BMC Complement Altern Med. 15:262015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Trivedi R, Maurya R and Mishra DP:
Medicarpin, a legume phytoalexin sensitizes myeloid leukemia cells
to TRAIL-induced apoptosis through the induction of DR5 and
activation of the ROS-JNK-CHOP pathway. Cell Death Dis.
5:e14652014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pennati M, Sbarra S, De Cesare M,
Lopergolo A, Locatelli SL, Campi E, Daidone MG, Carlo-Stella C,
Gianni AM and Zaffaroni N: YM155 sensitizes triple-negative breast
cancer to membrane-bound TRAIL through p38 MAPK- and CHOP-mediated
DR5 upregulation. Int J Cancer. 136:299–309. 2015. View Article : Google Scholar
|
|
44
|
Yi L, Zongyuan Y, Cheng G, Lingyun Z,
Guilian Y and Wei G: Quercetin enhances apoptotic effect of tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL) in
ovarian cancer cells through reactive oxygen species (ROS) mediated
CCAAT enhancer-binding protein homologous protein (CHOP)-death
receptor 5 pathway. Cancer Sci. 105:520–527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yoon MJ, Kang YJ, Kim IY, Kim EH, Lee JA,
Lim JH, Kwon TK and Choi KS: Monensin, a polyether ionophore
antibiotic, overcomes TRAIL resistance in glioma cells via
endoplasmic reticulum stress, DR5 upregulation and c-FLIP
downregulation. Carcinogenesis. 34:1918–1928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang S, Shen HM and Ong CN:
Down-regulation of c-FLIP contributes to the sensitization effect
of 3,3′-diindolylmethane on TRAIL-induced apoptosis in cancer
cells. Mol Cancer Ther. 4:1972–1981. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
García-García C, Fumarola C, Navaratnam N,
Carling D and López-Rivas A: AMPK-independent down-regulation of
cFLIP and sensitization to TRAIL-induced apoptosis by AMPK
activators. Biochem Pharmacol. 79:853–863. 2010. View Article : Google Scholar
|
|
48
|
Lin Y, Liu X, Yue P, Benbrook DM, Berlin
KD, Khuri FR and Sun SY: Involvement of c-FLIP and survivin
down-regulation in flexible heteroarotinoid-induced apoptosis and
enhancement of TRAIL-initiated apoptosis in lung cancer cells. Mol
Cancer Ther. 7:3556–3565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Day TW, Huang S and Safa AR: c-FLIP
knockdown induces ligand-independent DR5-, FADD-, caspase-8-, and
caspase-9-dependent apoptosis in breast cancer cells. Biochem
Pharmacol. 76:1694–1704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Safa AR and Pollok KE: Targeting the
anti-apoptotic protein c-FLIP for cancer therapy. Cancers (Basel).
3:1639–1671. 2011. View Article : Google Scholar
|
|
51
|
Safa AR: c-FLIP, a master anti-apoptotic
regulator. Exp Oncol. 34:176–184. 2012.PubMed/NCBI
|
|
52
|
Safa AR: Roles of c-FLIP in apoptosis,
necroptosis, and autophagy. J Carcinog Mutagen. (Suppl 6): pii:
003. 2013.PubMed/NCBI
|
|
53
|
Wilson TR, McLaughlin KM, McEwan M, Sakai
H, Rogers KM, Redmond KM, Johnston PG and Longley DB: c-FLIP: A key
regulator of colorectal cancer cell death. Cancer Res.
67:5754–5762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lee SJ, Noh HJ, Sung EG, Song IH, Kim JY,
Kwon TK and Lee TJ: Berberine sensitizes TRAIL-induced apoptosis
through proteasome-mediated downregulation of c-FLIP and Mcl-1
proteins. Int J Oncol. 38:485–492. 2011. View Article : Google Scholar
|
|
55
|
Murphy AC, Weyhenmeyer B, Noonan J,
Kilbride SM, Schimansky S, Loh KP, Kögel D, Letai AG, Prehn JH and
Murphy BM: Modulation of Mcl-1 sensitizes glioblastoma to
TRAIL-induced apoptosis. Apoptosis. 19:629–642. 2014. View Article : Google Scholar :
|
|
56
|
Kim SH, Ricci MS and El-Deiry WS: Mcl-1: A
gateway to TRAIL sensitization. Cancer Res. 68:2062–2064. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Clohessy JG, Zhuang J, de Boer J,
Gil-Gómez G and Brady HJ: Mcl-1 interacts with truncated Bid and
inhibits its induction of cytochrome c release and its role in
receptor-mediated apoptosis. J Biol Chem. 281:5750–5759. 2006.
View Article : Google Scholar
|
|
58
|
Ozören N and El-Deiry WS: Cell surface
death receptor signaling in normal and cancer cells. Semin Cancer
Biol. 13:135–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen JJ, Mikelis CM, Zhang Y, Gutkind JS
and Zhang B: TRAIL induces apoptosis in oral squamous carcinoma
cells - a crosstalk with oncogenic Ras regulated cell surface
expression of death receptor 5. Oncotarget. 4:206–217. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ren YG, Wagner KW, Knee DA, Aza-Blanc P,
Nasoff M and Deveraux QL: Differential regulation of the TRAIL
death receptors DR4 and DR5 by the signal recognition particle. Mol
Biol Cell. 15:5064–5074. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wei MC, Lindsten T, Mootha VK, Weiler S,
Gross A, Ashiya M, Thompson CB and Korsmeyer SJ: tBID, a
membrane-targeted death ligand, oligomerizes BAK to release
cytochrome c. Genes Dev. 14:2060–2071. 2000.PubMed/NCBI
|
|
62
|
Pietenpol JA and Stewart ZA: Cell cycle
checkpoint signaling: Cell cycle arrest versus apoptosis.
Toxicology. 181–182:475–481. 2002. View Article : Google Scholar
|
|
63
|
van Engeland M, Nieland LJ, Ramaekers FC,
Schutte B and Reutelingsperger CP: Annexin V-affinity assay: A
review on an apoptosis detection system based on phosphatidylserine
exposure. Cytometry. 31:1–9. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kuo LJ and Yang LX: Gamma-H2AX - a novel
biomarker for DNA double-strand breaks. In Vivo. 22:305–309.
2008.PubMed/NCBI
|
|
65
|
Rogakou EP, Nieves-Neira W, Boon C,
Pommier Y and Bonner WM: Initiation of DNA fragmentation during
apoptosis induces phosphorylation of H2AX histone at serine 139. J
Biol Chem. 275:9390–9395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ko H, Jeong MH, Jeon H, Sung GJ, So Y, Kim
I, Son J, Lee SW, Yoon HG and Choi KC: Delphinidin sensitizes
prostate cancer cells to TRAIL-induced apoptosis, by inducing DR5
and causing caspase-mediated HDAC3 cleavage. Oncotarget.
6:9970–9984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Trang KT, Kim SL, Park SB, Seo SY, Choi
CH, Park JK, Moon JC, Lee ST and Kim SW: Parthenolide sensitizes
human colorectal cancer cells to tumor necrosis factor-related
apoptosis-inducing ligand through mitochondrial and caspase
dependent pathway. Intest Res. 12:34–41. 2014. View Article : Google Scholar : PubMed/NCBI
|