Introduction
Of all clinical cancers, pancreatic cancer has one
of the poorest prognoses, with an overall 5-year survival rate of
~6% (1). Therefore, detection of
the tumor and its micrometastases at an early stage, curative
resectability of the tumor, and development of effective drugs for
its treatment are desired.
In present diagnostics, several non-invasive imaging
technologies, such as magnetic resonance imaging (MRI), positron
emission tomography (PET), single photon emission computed
tomography (SPECT) and computed tomography (CT) are available for
clinical use (2). To discriminate
the tumor from normal tissues, several monoclonal antibody
(mAb)-based probes have been developed by exploiting tumor-specific
molecules. MAbs have also been used to treat some types of cancer
(e.g., malignant lymphoma, breast cancer) (3,4).
Moreover, antibody-drug conjugates (ADCs), by which an anticancer
drug as a payload is preferentially delivered to the tumor tissue
with minimal adverse effects, are under development. Thus, mAbs
with high and specific affinity for tumor antigens are potentially
excellent tools for cancer diagnosis and treatment.
It is well-known that cancer invasion is accompanied
by an activation of blood coagulation (5). Recently, several clinical studies
have revealed that cancer patients are at a high risk for the
development of thrombosis (6).
Hemorrhage from the tumor vessels by the invading tumor cells and
subsequent fibrin clot formation to stop the bleeding occur
repeatedly in tumor tissues (7).
Moreover, this state lasts in solid tumor tissues for as long as
the tumor cells survive in the body (7). Tissue factor (TF) is a 47-kDa
transmembrane glycoprotein that is known to initiate the extrinsic
coagulation cascade and to play a critical role in hemostasis.
Therefore, it is conceivable that high expression levels of TF may
be one of the major causes of the hypercoagulable state in tumors.
TF is well known to be expressed at high levels in many types of
solid tumors, such as pancreatic cancer, glioma, colorectal cancer,
non-small cell lung cancer, ovarian cancer, prostate cancer and
breast cancer (8–10). TF is also known to be expressed in
the tumor stromal cells (11–13).
Some studies have indicated that activation of TF signaling in
tumor cells enhances tumor growth, metastasis, inflammation and
angiogenesis (14–18). Furthermore, TF expression has been
shown to be correlated with a poor prognosis in patients with
cancer (19–22).
We hypothesized that TF may be a promising target
for imaging probes or delivery of anticancer drugs into tumor
tissues, e.g., pancreatic tumor tissues. We and other groups have
already reported the usefulness of anti-TF mAb in cancer imaging
and therapy, including the Fab and scFv fragments (23–29).
However, the optimum size as a molecular probe has not been fully
evaluated from the perspective of biochemical characteristics and
bio-distribution within the tumor tissue.
In the present study, we prepared an anti-TF mAb and
its Fab fragment, and investigated their biochemical and
pharmacological characteristics in vitro and in vivo,
in order to determine their suitability for application in the
diagnosis and treatment of cancer.
Materials and methods
Antibodies and cell line
We developed the clone 1849 rat mAb IgG2b
which reacts with human TF antigen but not with mouse TF antigen.
As an isotype control antibody, we also developed clone 372 rat mAb
IgG2b which did not react with human and mouse TF antigens
(30). The human pancreatic cancer
cell line BxPC3 was purchased from the American Type Culture
Collection (ATCC, Manassas, VA, USA). The cell line was maintained
in RPMI-1640 medium (Wako, Osaka, Japan) supplemented with 10%
fetal bovine serum (FBS, Gibco, Grand Island, NY, USA) and 100 U/ml
of penicillin G, 100 μg/ml of streptomycin, and 0.25 μg/ml of
amphotericin B (Wako) in a 5% CO2 atmosphere at
37°C.
Generation of the Fab fragment
The 1849 and control mAbs were digested with
papain (Worthington Biochemical, Freehold, NJ, USA) at a
protein/enzyme ratio of 250:1, in a reaction buffer containing 100
mM sodium phosphate, 2 mM EDTA, and 10 mM Cysteine-HCl, pH 7.0, for
2 h at 37°C. The reaction buffer was then changed to 5 mM sodium
phosphate buffer (pH7.5) for removing Cysteine-HCl using Amicon
Ultra (Merck-Millipore, Darmstadt, Germany). The 1849-Fab
and control-Fab were purified in a CHT ceramic hydroxyapatite
column using a salt concentration gradient (Bio-Rad, Hercules, CA,
USA).
Purity of the antibodies
The purity of the antibodies was evaluated by
SDS-PAGE and Bioanalyzer analysis (Agilent Technologies, Santa
Clara, CA, USA) under non-reducing conditions. In SDS-PAGE, the
samples were loaded (2 μg/lane) on a 4–15% polyacrylamide gradient
gel (Bio-Rad). The gel was then stained with Coomassie Brilliant
Blue R-250 and scanned with ChemiDoc XRS+ (Bio-Rad). The purity and
molecular size of the antibodies were measured using an Agilent
bioanalyzer, as prescribed in the manual, using the Agilent Protein
230 kit.
Particle size determination and surface
plasmon resonance (SPR) analysis
Particle size was determined using DelsaNano HC
(Beckman Coulter, Brea, CA, USA) on the basis of photon correlation
spectroscopy (PCS) and dynamic light scattering (DLS). Biacore T200
(GE Healthcare, Uppsala, Sweden) was used to assess the binding of
the 1849 antibodies to recombinant human TF (rhTF) antigen.
Following standard amine chemistry protocols, rhTF antigen was
diluted to 1 μg/ml in 10 mM sodium acetate buffer (pH 5.0) and
immobilized on the CM5 sensor chips (GE Healthcare) at lower
density (17.7 resonance units) to only allow monovalent binding. We
used the single-cycle kinetics to measure the affinity of the
antibodies. Antibodies in PBS were passed over the sensor chips,
and the interactions were monitored for 30 sec. The sensor surface
was washed with PBS to detect dissociation and then regenerated
with 10 mM Glycine-HCl (pH 1.5) at the end of each experiment.
Antibody labeling
The 1849-whole IgG, 1849-Fab,
control-whole IgG and control-Fab were chemically labeled with the
fluorescent dyes Alexa-Fluor-647 (AF647, Invitrogen, Eugene, OR,
USA), according to the manufacturer's protocol (MP 00143,
Amine-Reactive Probes, Invitrogen). Following the labeling
reaction, the antibody concentration and degree of labeling were
measured by determining the absorbance values at 280 nm and 650 nm
with a Nano Drop ND-1000 spectrometer (Thermo Fisher Scientific,
Wilmington, DE, USA).
The final concentration of the antibodies-AF647 was
1.0 mg/ml, and the final dye molarities of 1849-whole
IgG-AF647, 1849-Fab-AF647, control-whole IgG-AF647 and
control-Fab-AF647 were 19.5, 18.2, 21.9 and 21.5 μM,
respectively.
Confocal fluorescence microscopy
analysis
BxPC3 cells were planted on BD Falcon 4-well chamber
slide at 1×105 cells/well. After 24 h of incubation,
cells were incubated with 500 μl of RPMI medium containing 0.1 μM
1849-whole IgG-AF647, 1849-Fab-AF647, control-whole
IgG-AF647 or control-Fab-AF647 for 1 h at 37°C. Cells were washed
three times with PBS, then fixed with 4% paraformaldehyde in PBS
for 15 min at RT and stained with DAPI solution (1 μg/ml) for 5 min
at RT. The slides were covered with Fluoromount-G (SouthernBiotech,
Birmingham, AL, USA). Fluorescence images were obtained with a
fluorescence microscope, BIOREVO BZ9000 (Keyence, Osaka,
Japan).
Flow cytometric analysis
The cell-binding activities of the antibodies-AF647
to BxPC3 cells were evaluated by flow cytometry. Briefly, BxPC3
cells were harvested and suspended in PBS with 0.5% bovine serum
albumin (BSA, Wako) and 2 mM EDTA (B.E.PBS) at a density of
4×105 cells/ml. The BxPC3 cells were then incubated with
the antibodies-AF647 at each concentration (1, 10 and 100 μM) for
30 min on ice, and washed three times with B.E.PBS. Subsequently,
propidium iodide (0.4 μl/ml; PI, Invitrogen) was added, and the
cell binding activities were analyzed with the guava easyCyte
(Millipore) using FlowJo analysis software (Tree Star Inc.,
Ashland, OR, USA). As a negative control, we added the fraction
containing PI and not the antibodies-AF647.
Animal models
Four-week-old female BALB/c nude mice were purchased
from SLC Japan (Shizuoka, Japan). A week later, the mice were
inoculated subcutaneously in the right flank with 1×106
BxPC3 cells suspended in 100 μl PBS. The test antibodies were
injected intravenously into the tail vein of the mice when the
tumor volume reached ~100–150 mm3, as measured with
calipers, and calculated by the formula: volume = length ×
(width)2 × 1/2. All animal procedures and experiments
were conducted with the approval of the Committee for Animal
Experimentation of the National Cancer Center, Japan. These
guidelines meet the ethical standards required by law and also
comply with the guidelines for the use of experimental animals in
Japan.
In vivo and ex vivo fluorescence
imaging
In vivo and ex vivo fluorescence
imaging were performed using IVIS Kinetic imaging system and
analyzed using IVIS Living Imaging 3.0 software (Caliper Life
Sciences, Hopkinton, MA, USA). A filter set (excitation at 605 nm,
emission at 640 nm) was used for acquiring the fluorescence of the
AF647 conjugated antibodies. All fluorescence images were acquired
in identical illumination settings and normalized as photons per
second per centimeter square per steradian (p/s/cm2/sr).
Quantitative data were obtained from ROI (regions of interest)
analysis of the fluorescence images. The mice were injected with
~100 μg of antibodies-AF647 via the tail vein in both studies.
In vivo imaging was performed at 1, 2, 3, 6,
9, 12, 24 and 72 h post-injection. The mean fluorescence intensity
of the left flank region, on the side contralateral to the tumor,
was used as the background value in order to investigate the
tumor-to-background ratio (TBR) of 1849-whole IgG-AF647 and
1849-Fab-AF647. The TBR was determined as the mean
fluorescence intensity of the tumor divided by the mean background
intensity. Statistical analysis were performed using Student's
t-test. P-values of <0.05 and <0.01 were considered
statistically significant. In the ex vivo study, the mice
were euthanized at 1, 3, 6, 24 and 72 h post-injection, followed by
excision of the tumor and major organs from the mice. The tumor and
the major organs, excluding blood, were washed with PBS and placed
on a dish with no fluorescence intensity in the measurement range.
The mean fluorescence intensity of the tumor and major organs
obtained from the mice which were not injected with antibodies was
used as the background value.
Distribution of antibodies
In the distribution study of antibodies, the mice
were injected with ~300 μg of 1849-whole IgG-AF647 and
1849-Fab-AF647 via the tail vein. The mice were euthanized
under deep anesthesia at 3 h after the injection, and the tumors
were excised. The tumors were then embedded in Tissue-Tec
optimal-cutting-temperature compound (Sakura Finetek, Tokyo, Japan)
and frozen at −80°C until use. Frozen sections, 6-μm-think, of the
tumors were fixed with 4% paraformaldehyde in PBS for 15 min at RT
and blocked with 5% skim milk in PBS for 1 h at RT. The sections
were then incubated with goat anti-mouse CD31 polyclonal antibody
(2 μg/ml; R&D Systems, Minneapolis, MN, USA) for 1 h at RT, and
then with AF555 conjugated anti-goat polyclonal antibody (4 μg/ml;
Invitrogen) for 1 h at RT. Subsequently, the sections were stained
with DAPI solution (1 μg/ml) for 5 min at RT and the slides were
covered with Fluoromount-G. Fluorescence images were obtained with
BIOREVO BZ9000.
Results
Generation and purity of the
antibodies
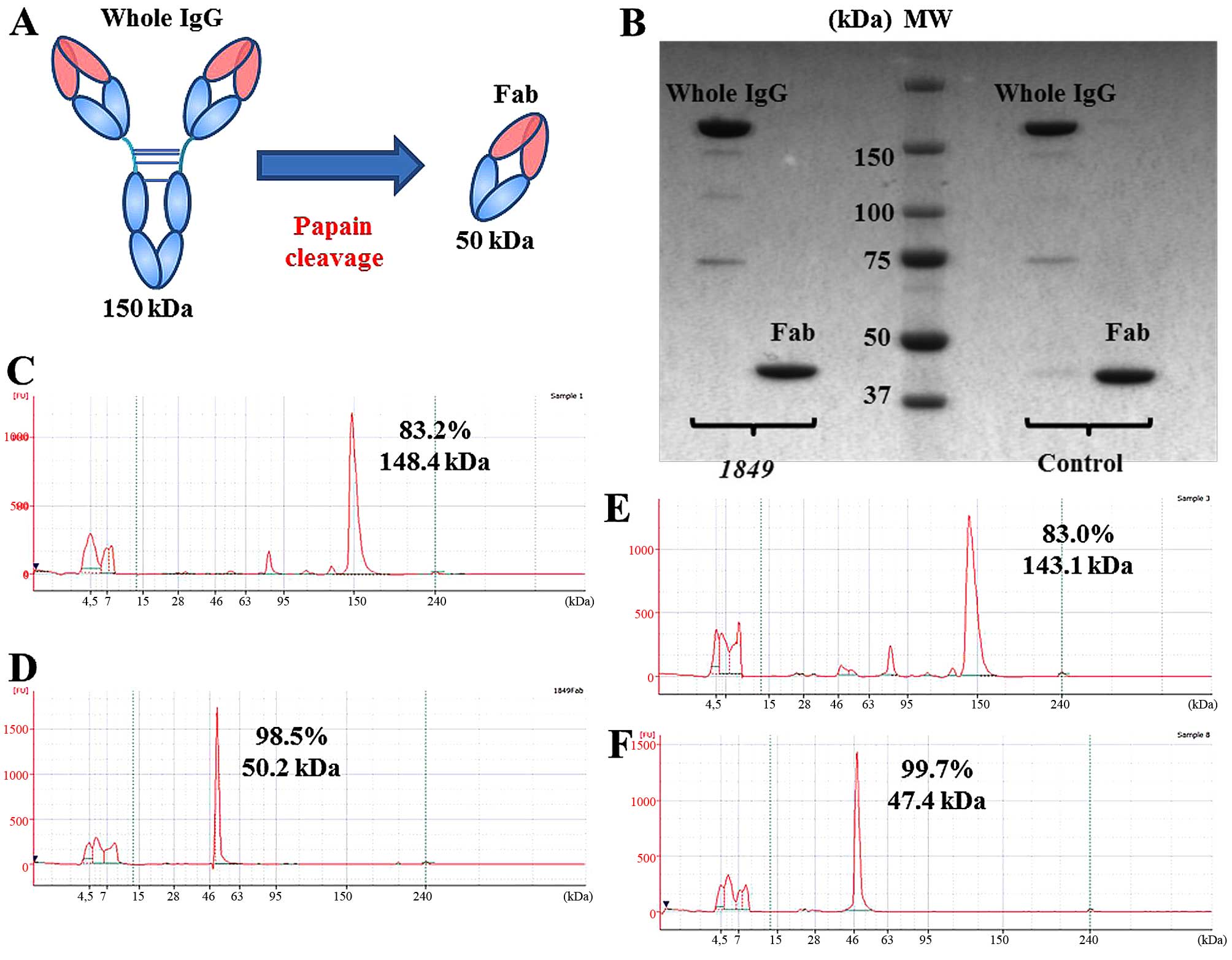
Fig. 1A shows a
schematic diagram of production of the Fab fragment from the whole
IgG. The Fab fragments were visualized as an almost single band on
SDS-PAGE, which indicated the high purity of the fragments
(Fig. 1B). The results of the
bioanalyzer analysis also confirmed the high purity (Fig. 1C–F).
Biochemical characteristics of the
antibodies
The particle sizes of the antibodies were correlated
with their molecular weights (Table
I). There were no significant differences in the molecular
weight between 1849-whole IgG and control-whole IgG, or
1849-Fab and control-Fab. In the SPR analysis, while the
association rate constant (Ka) values of 1849-whole IgG and
1849-Fab were almost the same, the dissociation rate
constant (Kd) value of 1849-Fab was ~4.2-fold higher than
that of 1849-whole IgG (Table
I). Consequently, the binding affinity constant (KD) value of
1849-Fab was ~5.5-fold higher than that of the
1849-whole IgG because of high dissociation.
 | Table IParticle size and binding parameters
of antibodies. |
Table I
Particle size and binding parameters
of antibodies.
| Particle size
(nm) | KD (M) | Ka (1/Ms) | Kd (1/s) |
|---|
| 1849 whole IgG | 13.1±4.6 |
3.665×10−10 |
3.770×104 |
1.382×10−5 |
| 1849 Fab | 5.0±2.4 |
2.001×10−9 |
2.895×104 |
5.795×10−5 |
| Control whole
IgG | 13.0±4.8 | N/A | N/A | N/A |
| Control Fab | 5.5±1.8 | N/A | N/A | N/A |
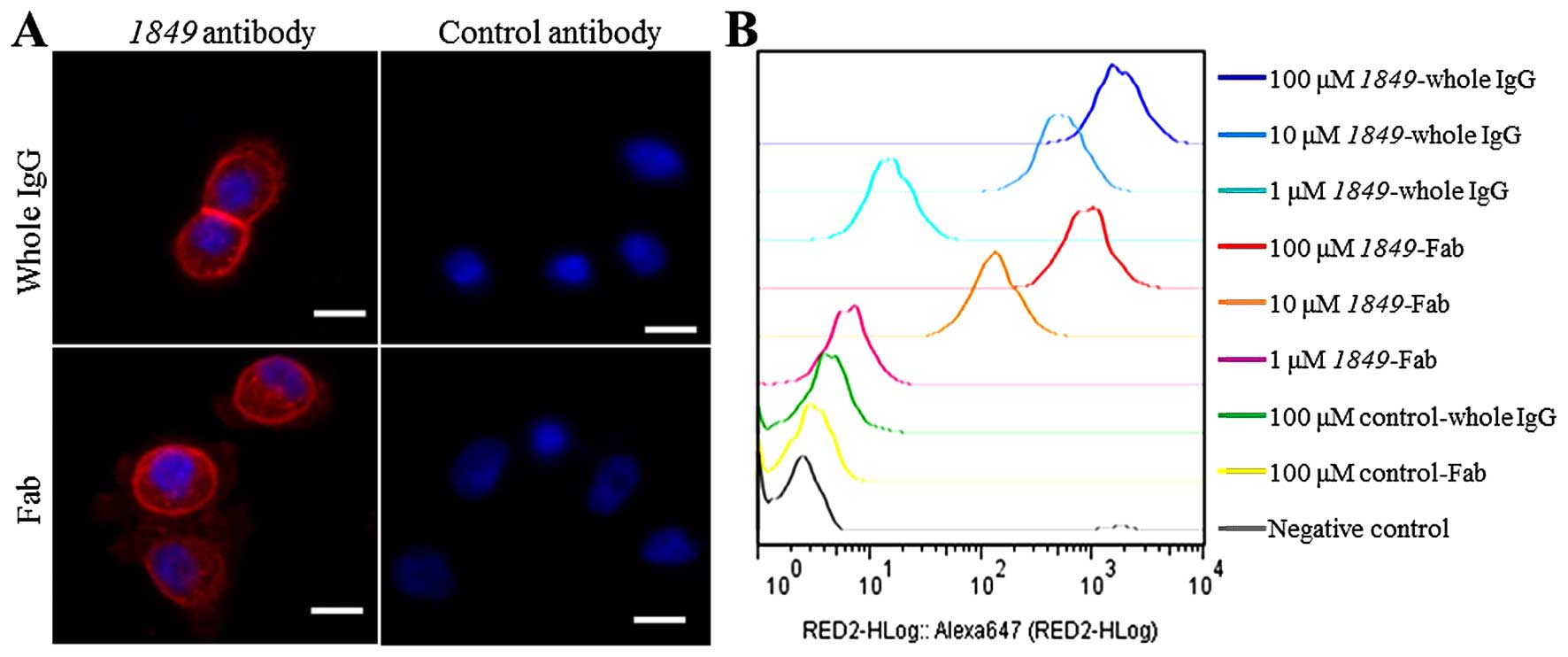
Confocal fluorescence microscopy analysis revealed
that 1849-whole IgG-AF647 and 1849-Fab-AF647 bound to the
membrane of BxPC3 cells and internalized into cells through TF
antigen on the membrane (Fig. 2A).
On the other hand, control-whole IgG-AF647 and control-Fab-AF647
did not bind to the BxPC3 cells. Flow cytometric analysis confirmed
a high affinity of 1849-antibodies-AF647 to BxPC3 cells
depending on the concentration (Fig.
2B).
In vivo imaging
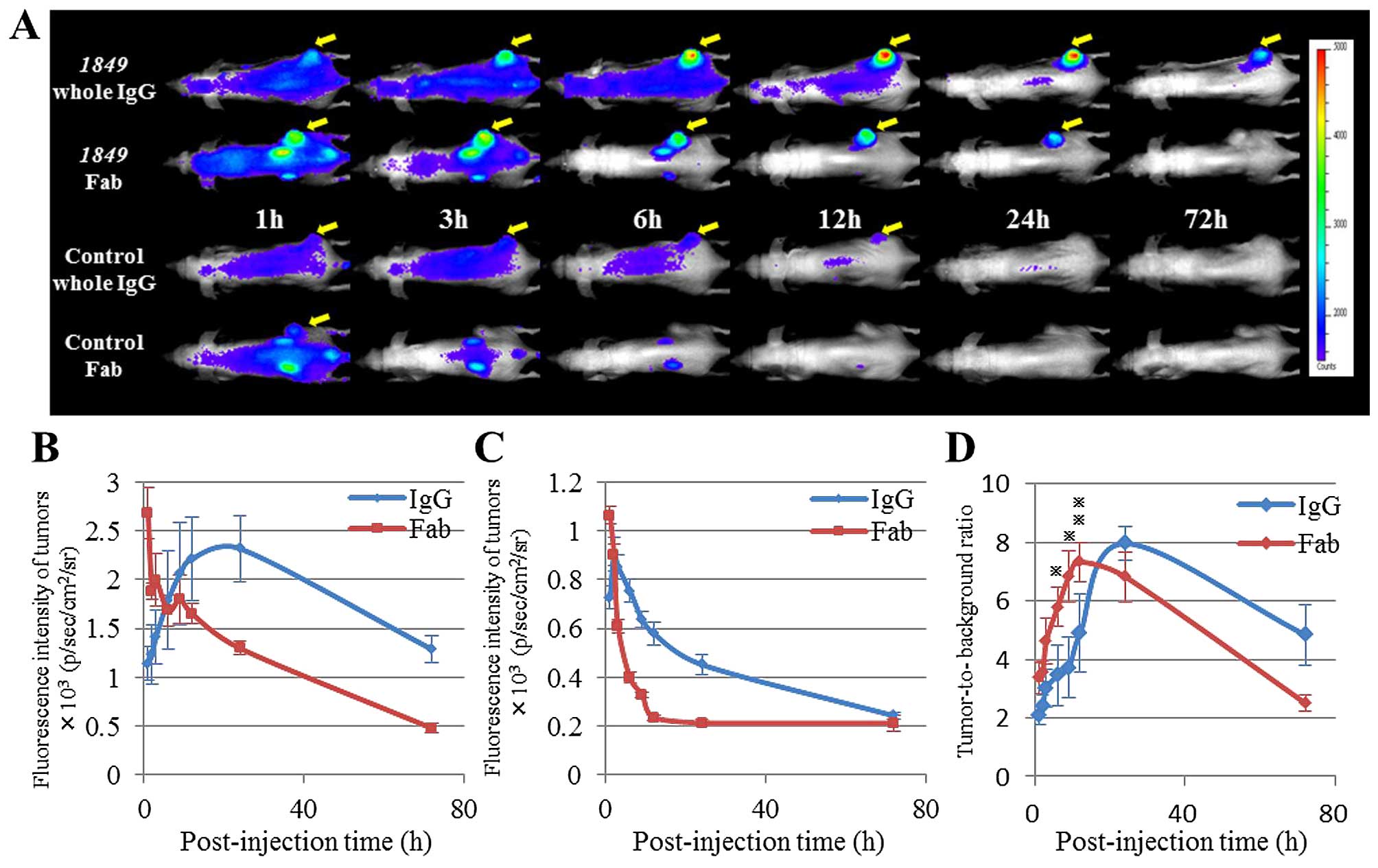
The biodistribution and tumor targeting efficacy of
each of the antibodies-AF647 were measured in mice bearing BxPC3
tumors by our in vivo imaging system (Fig. 3A–C). At all time-points, both
1849-whole IgG-AF647 and 1849-Fab-AF647 showed
notably higher tumor accumulation than control antibodies-AF647.
The tumor accumulation of 1849-whole IgG-AF647 reached its
peak at 24 h after the injection, whereas that of
1849-Fab-AF647 reached its maximum at 1 h after the
injection. Both 1849-Fab-AF647 and control-Fab-AF647 showed
high distribution into the kidneys in the early phase. Based on
these fluorescence images, the TBR values of 1849-whole
IgG-AF647 and 1849-Fab-AF647 were determined at all the
time-points examined (Fig. 3D).
The TBRs of 1849-whole IgG-AF647 and 1849-Fab-AF647
reached their maximum at 24 and 12 h after the injection,
respectively. The TBR of 1849-Fab-AF647 was higher than that
of 1849-whole IgG-AF647 at any time-points within 12 h after
the injection (3 and 6 h, P<0.05. 9 h, P<0.01). On the other
hand, the TBR of 1849-whole IgG-AF647 was higher than that
of 1849-Fab-AF647 at any time-points after 24 h (Fig. 3D).
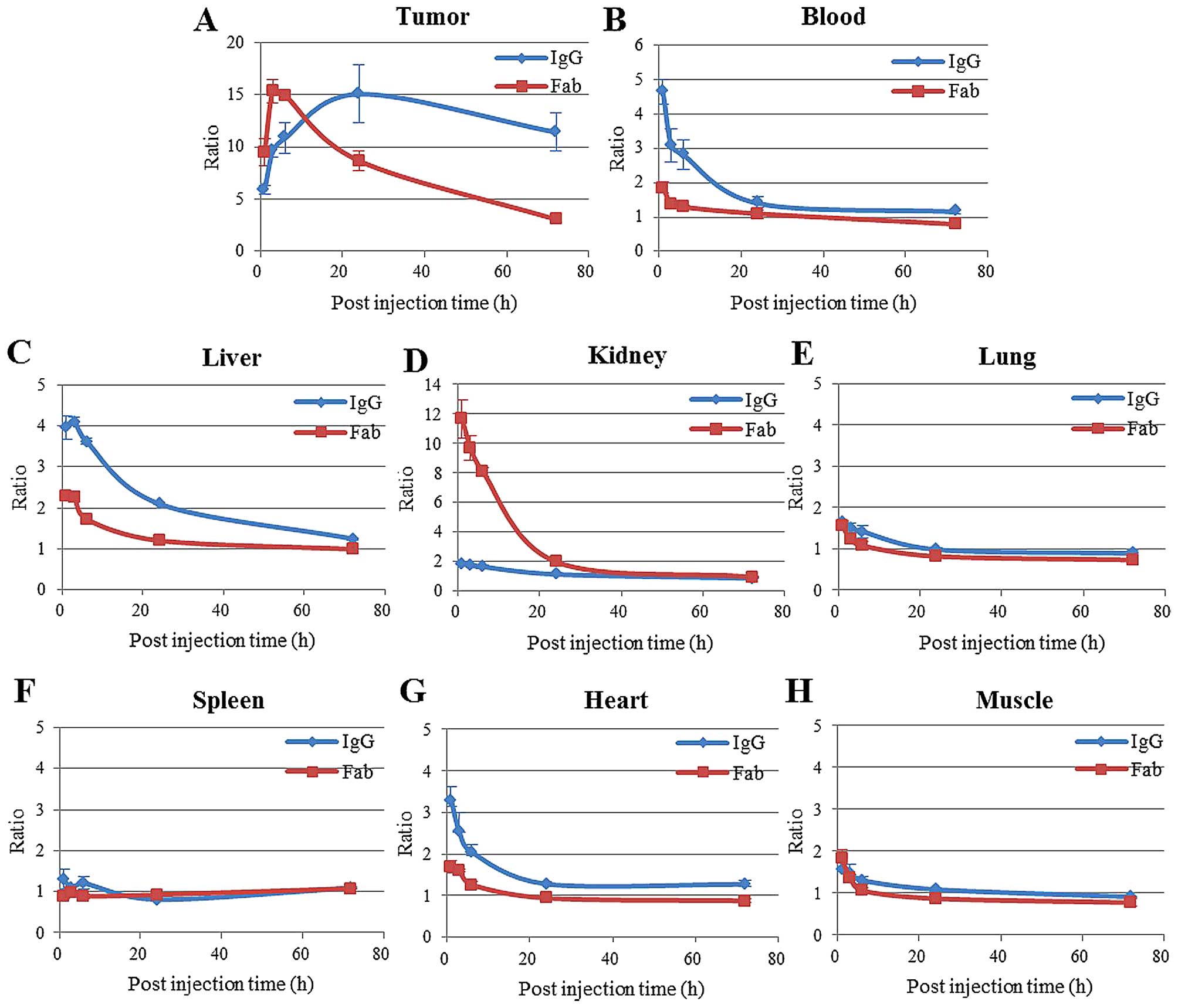
Ex vivo studies
The accumulation of antibodies-AF647 into the tumors
was almost consistent with those observed by in vivo
fluorescence imaging (Fig. 4A).
The blood, liver and heart, the tissue-to-background ratios of
1849-whole IgG-AF647 were higher than those of
1849-Fab-AF647 (Fig. 4B, C and
G), while the kidney tissue-to-background ratio was notably
higher for 1849-Fab-AF647 than for 1849-whole
IgG-AF647 at all time-points (Fig.
4D). Moreover, the uptake and distribution of these antibodies
in other normal tissues such as the lung, spleen and muscle were
significantly low (Fig. 4E, F and
H).
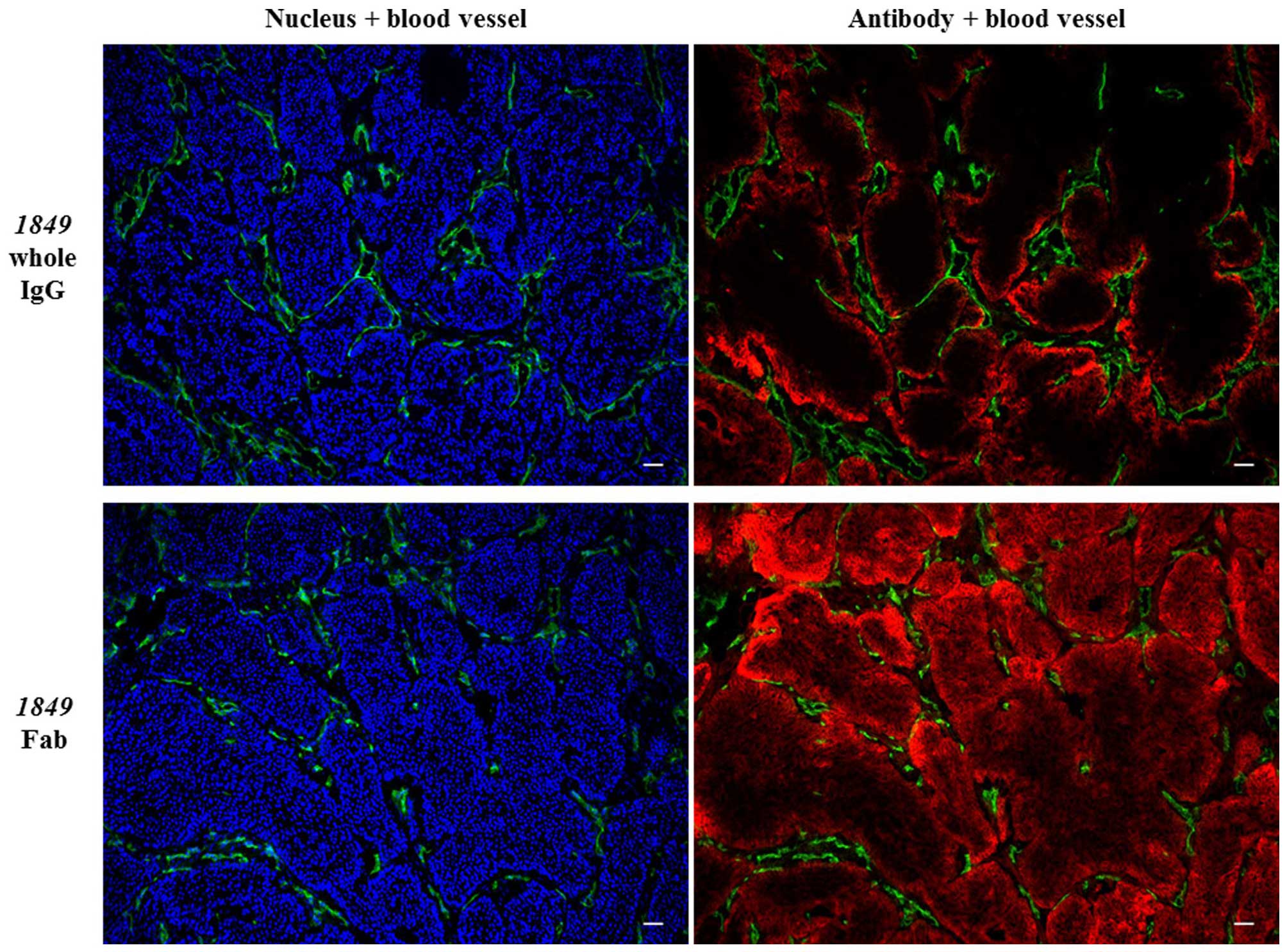
Distribution of the antibodies in the
tumor tissues
To analyze the intratumoral distribution of
1849-whole IgG-AF647 and 1849-Fab-AF647,
immunofluorescence staining was performed (Fig. 5). At 3 h after the injection, the
fluorescence intensity of 1849-Fab-AF647 in the BxPC3 tumor
tissue was higher than that of 1849-whole IgG-AF647. This
result was consistent with both the results of the in vivo
fluorescence imaging and ex vivo study. In addition, there
was a clear difference in intratumoral distribution between
1849-whole IgG-AF647 and 1849-Fab-AF647.
1849-whole IgG-AF647 was localized mainly in the periphery
of the tumor cell clusters, while 1849-Fab-AF647 penetrated
into the center of the tumor clusters.
Discussion
A large body of basic and clinical studies has shown
overexpression of TF in various types of solid tumor tissues
(8–10). We hypothesized that TF might
therefore be a promising target for cancer diagnosis and therapy
and developed a rat mAb (clone 1849) against human TF.
The purpose of this study was to characterize
1849-whole IgG and 1849-Fab in vitro and in
vivo, in order to clarify the suitability of their application
for diagnostic or therapeutic purpose, which would enhance the
potential of their application to clinical studies. We used a mouse
xenograft model of human pancreatic cancer as the TF-overexpressing
tumor.
First, SPR analysis showed that 1849-Fab
could dissociate from antigen more rapidly than 1849-whole
IgG (Table I). Although rhTF
antigen was coated to the chip at lower density, this result is
explained by the presence in 1849-whole IgG of two
antigen-binding sites, so one Fab-arm can still bind the antigen
even if the other is detached, in contrast to only one site in
1849-Fab. Confocal fluorescence microscopy analysis and Flow
cytometric analysis showed that 1849-antibodies-AF647 had a
high affinity and specificity to the TF antigen expressed in BxPC3
cells.
In the in vivo imaging study, the 1849
antibodies-AF647 showed higher accumulation in the tumors at
all-time-points as compared to control antibodies having passive
targeting which depends on the enhanced permeability and retention
(EPR) effect (31). It was clear
that the EPR effect combined with the active targeting against
human TF antigen contributed to the high tumor accumulation of
1849 antibodies as compared to that of control isotype IgG.
Furthermore, the in vivo imaging showed that the kinetics of
whole IgG and Fab changed due to the differences of their molecular
sizes and affinities. In the presence of the Fc domain, both
1849-whole IgG-AF647 and control-whole IgG-AF647 showed
prolonged systemic circulation in the body (32). The property of IgG of having a high
molecular weight (150 kDa), above the renal clearance threshold of
~65 kDa, permits it to escape renal clearance, which facilitates
prolonged circulation (33). In
the imaging using whole IgG, the prolonged circulation time causes
a sustained high background signal. On the other hand, because Fab
lacks the Fc domain and is smaller in size (~50 kDa), Fab can pass
through the renal glomeruli, resulting in rapid clearance from the
body and a low background signal. Moreover, the tumor signal
intensity of 1849-Fab-AF647 appeared to reach its peak
immediately after the injection, whereas that of 1849-whole
IgG-AF647 reached its peak at about 20 h after the injection. These
properties led to the differences in the TBR between
1849-whole IgG-AF647 and 1849-Fab-AF647, namely, a
maximum peak of 1849-whole IgG-AF647 at 24 h and of
1849-Fab-AF647 at 12 h after the injection. The results of
the in vivo imaging were confirmed by the results of the
ex vivo study. In brief, 1849-Fab-AF647 showed rapid
tumor accumulation and rapid blood clearance, whereas in contrast,
1849-whole IgG-AF647 showed sustained high tumor
accumulation and prolonged circulation in the blood. This study
also showed that 1849-whole IgG-AF647 and
1849-Fab-AF647 were mainly excreted by the liver and kidney,
respectively.
The present immunofluorescence staining study showed
that 1849-Fab-AF647 penetration was deeper into the tumors
than 1849-whole IgG-AF647. It was clear that the smaller
size of 1849-Fab-AF647 allowed more efficient tumor
penetration. In the histological examination, the BxPC3 cells at
the tumor front showed stronger TF expression than those in the
central region (30). Because the
1849-whole IgG-AF647 with its high affinity for the antigen
may be trapped and kept by TF-overexpressing cells at the tumor
front, it hardly penetrates into the tumor center at the early
phase. On the other hand, 1849-Fab-AF647, with its fast
dissociation rate and low internalization efficiency, may be able
to penetrate the tumor center more easily (34). Although it is recognized that
homogeneous penetration of the imaging probe is less important than
achieving a high TBR value (35),
improved tumor penetration would increase the sensitivity of
detection of small-sized tumors or micrometastases.
In conclusion, the properties of 1849-Fab,
namely, fast dissociation from TF antigen, rapid tumor
accumulation, efficient tumor penetration, and rapid body clearance
make it potentially suitable for diagnostic applications. A
diagnostic test with the Fab probe would provide a high contrast
and fine tumor imaging within half a day. Fab can also minimize
several of the adverse effects (e.g., hypersensitivity) of whole
IgG. In addition, it allows use of a short-half-life radionuclide
(e.g., 99mTc, half-life 6 h) (36), which can shorten the scan time and
reduce the total body radiation dose. These features would benefit
both outpatients and healthy people as the target populations for
diagnostic application. On the other hand, the properties of
1849-whole IgG, namely, high avidity, high tumor
accumulation and prolonged biological half-life, make it more
suitable for therapeutic applications. We thus propose the
importance of the developmental strategy of selecting a suitable
antibody depending on whether the intended application is
diagnostic or therapeutic. For clinical application of our
1849 antibodies, further investigation, i.e., PET or SPECT
imaging for diagnostic application and ADC for therapeutic
application using an appropriate experimental tumor model with high
TF expression and abundant tumor stroma, is needed.
Acknowledgements
This study was supported by a Grant-in-Aid from the
Japan Society for the Promotion of Science (JSPS) through the
Funding Program for World-Leading Innovative R&D on Science and
Technology (FIRST Program) initiated by the Council for Science and
Technology Policy (CSTP) (to Y. Matsumura) and the National Cancer
Center Research and Development Fund (23-A-45 to Y. Matsumura). We
thank Mrs. M. Kanzaki for supporting the animal experiments and Ms.
M. Nakayama for secretarial help.
Abbreviations:
|
MRI
|
magnetic resonance imaging
|
|
PET
|
positron emission tomography
|
|
SPECT
|
single photon emission computed
tomography
|
|
CT
|
computed tomography
|
|
mAb
|
monoclonal antibody
|
|
ADCs
|
antibody-drug conjugates
|
|
TF
|
tissue factor
|
|
SPR
|
surface Plasmon resonance
|
|
PCS
|
photon correlation spectroscopy
|
|
DLS
|
dynamic light scattering
|
|
TBR ratio
|
tumor-to background ratio
|
|
Ka
|
the association rate constant
|
|
Kd
|
the dissociation rate constant
|
|
KD
|
the binding affinity constant
|
|
EPR effect
|
enhanced permeability and retention
effect
|
References
|
1
|
Howlader N, Noone AM, Krapcho M, Garshell
J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,
et al: National Cancer Institute: SEER Cancer Statistics Review,
1975–2010. http://seer.cancer.gov/csr/1975_2010/.
Accessed June 14, 2013
|
|
2
|
Kaur S, Venktaraman G, Jain M, Senapati S,
Garg PK and Batra SK: Recent trends in antibody-based oncologic
imaging. Cancer Lett. 315:97–111. 2012. View Article : Google Scholar :
|
|
3
|
Verma S, Miles D, Gianni L, Krop IE,
Welslau M, Baselga J, Pegram M, Oh DY, Diéras V, Guardino E, et al;
EMILIA Study Group. Trastuzumab emtansine for HER2-positive
advanced breast cancer. N Engl J Med. 367:1783–1791. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ricart AD and Tolcher AW: Technology
insight: Cytotoxic drug immunoconjugates for cancer therapy. Nat
Clin Pract Oncol. 4:245–255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rickles FR, Levine M and Edwards RL:
Hemostatic alterations in cancer patients. Cancer Metastasis Rev.
11:237–248. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stein PD, Beemath A, Meyers FA, Skaf E,
Sanchez J and Olson RE: Incidence of venous thromboembolism in
patients hospitalized with cancer. Am J Med. 119:60–68. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsumura Y: Cancer stromal targeting
(CAST) therapy. Adv Drug Deliv Rev. 64:710–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Callander NS, Varki N and Rao LV:
Immunohistochemical identification of tissue factor in solid
tumors. Cancer. 70:1194–1201. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kasthuri RS, Taubman MB and Mackman N:
Role of tissue factor in cancer. J Clin Oncol. 27:4834–4838. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van den Berg YW, Osanto S, Reitsma PH and
Versteeg HH: The relationship between tissue factor and cancer
progression: Insights from bench and bedside. Blood. 119:924–932.
2012. View Article : Google Scholar
|
|
11
|
Contrino J, Hair G, Kreutzer DL and
Rickles FR: In situ detection of tissue factor in vascular
endothelial cells: Correlation with the malignant phenotype of
human breast disease. Nat Med. 2:209–215. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vrana JA, Stang MT, Grande JP and Getz MJ:
Expression of tissue factor in tumor stroma correlates with
progression to invasive human breast cancer: Paracrine regulation
by carcinoma cell-derived members of the transforming growth factor
beta family. Cancer Res. 56:5063–5070. 1996.PubMed/NCBI
|
|
13
|
Ueno T, Toi M, Koike M, Nakamura S and
Tominaga T: Tissue factor expression in breast cancer tissues: Its
correlation with prognosis and plasma concentration. Br J Cancer.
83:164–170. 2000.PubMed/NCBI
|
|
14
|
Versteeg HH and Ruf W: Emerging insights
in tissue factor-dependent signaling events. Semin Thromb Hemost.
32:24–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Langer F and Bokemeyer C: Crosstalk
between cancer and haemostasis. Implications for cancer biology and
cancer-associated thrombosis with focus on tissue factor.
Hamostaseologie. 32:95–104. 2012. View Article : Google Scholar
|
|
16
|
Khorana AA, Ahrendt SA, Ryan CK, Francis
CW, Hruban RH, Hu YC, Hostetter G, Harvey J and Taubman MB: Tissue
factor expression, angiogenesis, and thrombosis in pancreatic
cancer. Clin Cancer Res. 13:2870–2875. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Milsom CC, Yu JL, Mackman N, Micallef J,
Anderson GM, Guha A and Rak JW: Tissue factor regulation by
epidermal growth factor receptor and epithelial-to-mesenchymal
transitions: Effect on tumor initiation and angiogenesis. Cancer
Res. 68:10068–10076. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hjortoe GM, Petersen LC, Albrektsen T,
Sorensen BB, Norby PL, Mandal SK, Pendurthi UR and Rao LV: Tissue
factor-factor VIIa-specific up-regulation of IL-8 expression in
MDA-MB-231 cells is mediated by PAR-2 and results in increased cell
migration. Blood. 103:3029–3037. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Regina S, Valentin JB, Lachot S, Lemarié
E, Rollin J and Gruel Y: Increased tissue factor expression is
associated with reduced survival in non-small cell lung cancer and
with mutations of TP53 and PTEN. Clin Chem. 55:1834–1842. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamashita H, Kitayama J, Ishikawa M and
Nagawa H: Tissue factor expression is a clinical indicator of
lymphatic metastasis and poor prognosis in gastric cancer with
intestinal phenotype. J Surg Oncol. 95:324–331. 2007. View Article : Google Scholar
|
|
21
|
Kaido T, Oe H, Yoshikawa A, Mori A, Arii S
and Imamura M: Tissue factor is a useful prognostic factor of
recurrence in hepatocellular carcinoma in 5-year survivors.
Hepatogastroenterology. 52:1383–1387. 2005.PubMed/NCBI
|
|
22
|
Nitori N, Ino Y, Nakanishi Y, Yamada T,
Honda K, Yanagihara K, Kosuge T, Kanai Y, Kitajima M and Hirohashi
S: Prognostic significance of tissue factor in pancreatic ductal
adenocarcinoma. Clin Cancer Res. 11:2531–2539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sato R, Obonai T, Tsumura R, Tsumoto K,
Koga Y, Yasunaga M and Matsumura Y: Preparation and
characterization of anti-tissue factor single-chain variable
fragment antibody for cancer diagnosis. Cancer Sci. 105:1631–1637.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koga Y, Manabe S, Aihara Y, Sato R,
Tsumura R, Iwafuji H, Furuya F, Fuchigami H, Fujiwara Y, Hisada Y,
et al: Antitumor effect of antitissue factor antibody-MMAE
conjugate in human pancreatic tumor xenografts. Int J Cancer. Feb
20–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hong H, Zhang Y, Nayak TR, Engle JW, Wong
HC, Liu B, Barnhart TE and Cai W: Immuno-PET of tissue factor in
pancreatic cancer. J Nucl Med. 53:1748–1754. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Versteeg HH, Schaffner F, Kerver M,
Petersen HH, Ahamed J, Felding-Habermann B, Takada Y, Mueller BM
and Ruf W: Inhibition of tissue factor signaling suppresses tumor
growth. Blood. 111:190–199. 2008. View Article : Google Scholar
|
|
27
|
Harter PN, Dützmann S, Drott U, Zachskorn
C, Hattingen E, Capper D, Gessler F, Senft C, Seifert V, Plate KH,
et al: Anti-tissue factor (TF9-10H10) treatment reduces tumor cell
invasiveness in a novel migratory glioma model. Neuropathology.
33:515–525. 2013.PubMed/NCBI
|
|
28
|
Breij EC, de Goeij BE, Verploegen S,
Schuurhuis DH, Amirkhosravi A, Francis J, Miller VB, Houtkamp M,
Bleeker WK, Satijn D, et al: An antibody-drug conjugate that
targets tissue factor exhibits potent therapeutic activity against
a broad range of solid tumors. Cancer Res. 74:1214–1226. 2014.
View Article : Google Scholar
|
|
29
|
Shi S, Hong H, Orbay H, Graves SA, Yang Y,
Ohman JD, Liu B, Nickles RJ, Wong HC and Cai W: ImmunoPET of tissue
factor expression in triple-negative breast cancer with a
radiolabeled antibody Fab fragment. Eur J Nucl Med Mol Imaging. Mar
24–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saito Y, Hashimoto Y, Kuroda J, Yasunaga
M, Koga Y, Takahashi A and Matsumura Y: The inhibition of
pancreatic cancer invasion-metastasis cascade in both cellular
signal and blood coagulation cascade of tissue factor by its
neutralisation antibody. Eur J Cancer. 47:2230–2239. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsumura Y and Maeda H: A new concept for
macromolecular therapeutics in cancer chemotherapy: Mechanism of
tumoritropic accumulation of proteins and the antitumor agent
smancs. Cancer Res. 46:6387–6392. 1986.PubMed/NCBI
|
|
32
|
Gurbaxani B, Dostalek M and Gardner I: Are
endosomal trafficking parameters better targets for improving mAb
pharmacokinetics than FcRn binding affinity? Mol Immunol.
56:660–674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schneider DW, Heitner T, Alicke B, Light
DR, McLean K, Satozawa N, Parry G, Yoo J, Lewis JS and Parry R: In
vivo biodistribution, PET imaging, and tumor accumulation of
86Y-and 111In-antimindin/RG-1, engineered antibody
fragments in LNCaP tumor-bearing nude mice. J Nucl Med. 50:435–443.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Adams GP, Schier R, McCall AM, Simmons HH,
Horak EM, Alpaugh RK, Marks JD and Weiner LM: High affinity
restricts the localization and tumor penetration of single-chain fv
antibody molecules. Cancer Res. 61:4750–4755. 2001.PubMed/NCBI
|
|
35
|
Olafsen T and Wu AM: Antibody vectors for
imaging. Semin Nucl Med. 40:167–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kampmeier F, Williams JD, Maher J, Mullen
GE and Blower PJ: Design and preclinical evaluation of a
99mTc-labelled diabody of mAb J591 for SPECT imaging of
prostate-specific membrane antigen (PSMA). EJNMMI Res. 4:132014.
View Article : Google Scholar : PubMed/NCBI
|