Introduction
Osteosarcoma is the most commonly diagnosed primary
malignant tumor of the bone in children and adolescents (1,2).
High-dose chemotherapy and surgical intervention have improved
long-term prognosis for non-metastatic disease to ~50–80% (3). However, a high number of patients
with osteosarcoma have a strong tendency of lung metastasis.
Metastatic osteosarcoma exhibits resistance to standard
chemotherapy (4).
To establish new chemotherapies to improve
prognosis, many investigators have researched the molecular
mechanisms of osteosarcoma growth and metastasis. We and others
have investigated the function of the Hedgehog pathway in
osteosarcoma. The Hedgehog pathway plays important roles in
embryonic development and adult organ homeostasis and regeneration
(5,6). Binding of Hedgehog ligands (sonic,
Indian, and desert Hedgehog) to patched1 (PTCH1) promotes release
of smoothened (SMO), which then leads to nuclear translocation and
activation of GLI transcription factors (GLI1/-2/-3). GLI promotes
transcription of target genes, including GLI1, HHIP, PTCH1, CYCLIN
D, BCL2 and SNAIL (7). We and
others reported that inhibition of smoothened (SMO) or GLI family
zinc finger 2 (GLI2) prevents osteosarcoma growth and invasion
in vitro and in vivo (8–12).
Arsenic trioxide (ATO) is a Food and Drug
Administration (FDA)-approved reagent that is used to treat acute
promyelocytic leukemia (APL) patients (13). Recently, ATO was proposed as a
potentially useful inhibitor of Hedgehog-driven cancers including
osteosarcoma (11,14,15).
Furthermore, an SMO inhibitor, vismodegib, has been approved for
basal cell carcinoma treatment (16). To apply our previous findings in
clinical settings, we examined the effects of Hedgehog inhibitors
combined with standard anticancer agents. Our findings showed that
combination of Hedgehog inhibitors or combination of ATO and
standard anticancer agents prevent osteosarcoma growth in
vitro and in vivo.
Materials and methods
Cell lines and reagents
The human osteosarcoma cell lines 143B and Saos-2
were obtained from the American Type Culture Collection (ATCC;
Manassas, VA, USA). Cell lines were cultured at 37°C in 5%
CO2. Cisplatin (CDDP), ifosfamide (IFO), and doxorubicin
(DOX) were purchased from Sigma-Aldrich (St. Louis, USA), (Shionogi
& Co., Ltd., Osaka, Japan). ATO (Trisenox) was purchased from
Nippon Shinyaku Co., Ltd. (Tokyo, Japan). Vismodegib (GDC-0449) was
purchased from LC Laboratories (Woburn, MA, USA).
Analysis of cell viability
Cells were treated with ADM, CDDP, IFO, vismodegib
and ATO. After 48 h, cell viability was evaluated by colorimetric
assay for mitochondrial dehydrogenase activity, as described
previously (15) (WST-1; Roche,
Basel, Switzerland).
Animal studies
Examinations of xenograft models were performed as
previously reported (9,15). For ATO and CDDP or ATO and IFO
examinations, 143B cells (1×106) were suspended in 100
μl of Matrigel (BD, NJ, USA). Suspensions of 143B osteosarcoma
cells were subcutaneously inoculated in 5-week-old nude mice. For
ATO and vismodegib examination, 143B cell (1×106)
suspensions were inoculated into the left knee joint of 5-week-old
nude mice. Tumor volume was calculated using the formula
LW2/2 (L and W indicating the length and width of
tumors).
Xenograft models were randomly treated with ATO,
CDDP, IFO, and vismodegib or an equal volume of vehicle as control.
All animal experiments were performed in compliance with the
guidelines of the Institute of Laboratory Animal Sciences, Graduate
School of Medical and Dental Sciences, Kagoshima University. Every
effort was employed to minimize both the number of animals used and
animal pain.
Drug combination studies
Synergism after treatment with Hedgehog inhibitors
and standard chemotherapeutic reagents was evaluated using CalcuSyn
software (Biosoft, Ferguson, MO, USA), which is based on the
median-effect principle applied by Chou and Talalay (17). From the fraction affected by the
dose (Fa) obtained from cell proliferation assays and the dose of
drug (D), the software draws a dose effect curve and calculates the
median-effect dose (Dm). Dm is same as ED50. For each
combinative dose effect, a combination-index (CI) was generated.
The effects of the combinations were then transformed into and
displayed as fraction-affected CI plots. If the data of
single-agent and combination use is inputted, the software
calculates the CI, which represents of the pharmacological
interaction of two drugs. A CI value of 1 indicated an additive
effect between the two agents, whereas a CI<1 or CI>1
indicated synergism or antagonism, respectively.
Statistical analysis
Statistical analyses were performed using
Kruskal-Wallis tests with Excel Statistics 2012 and 2015 (SSRI,
Tokyo, Japan). P-values of <0.05 were considered statistically
significant.
Results
Combination of Hedgehog inhibitors and
standard anticancer agents prevents proliferation of osteosarcoma
cells
We evaluated the effect of anticancer agents
including CDDP, IFO, and DOX, which are standard chemotherapeutic
reagents for osteosarcoma patients in Japan (18). To determine whether ATO and
standard anticancer agents prevent the proliferation of
osteosarcoma cells, we performed WST-1 assays using ATO, CDDP, IFO,
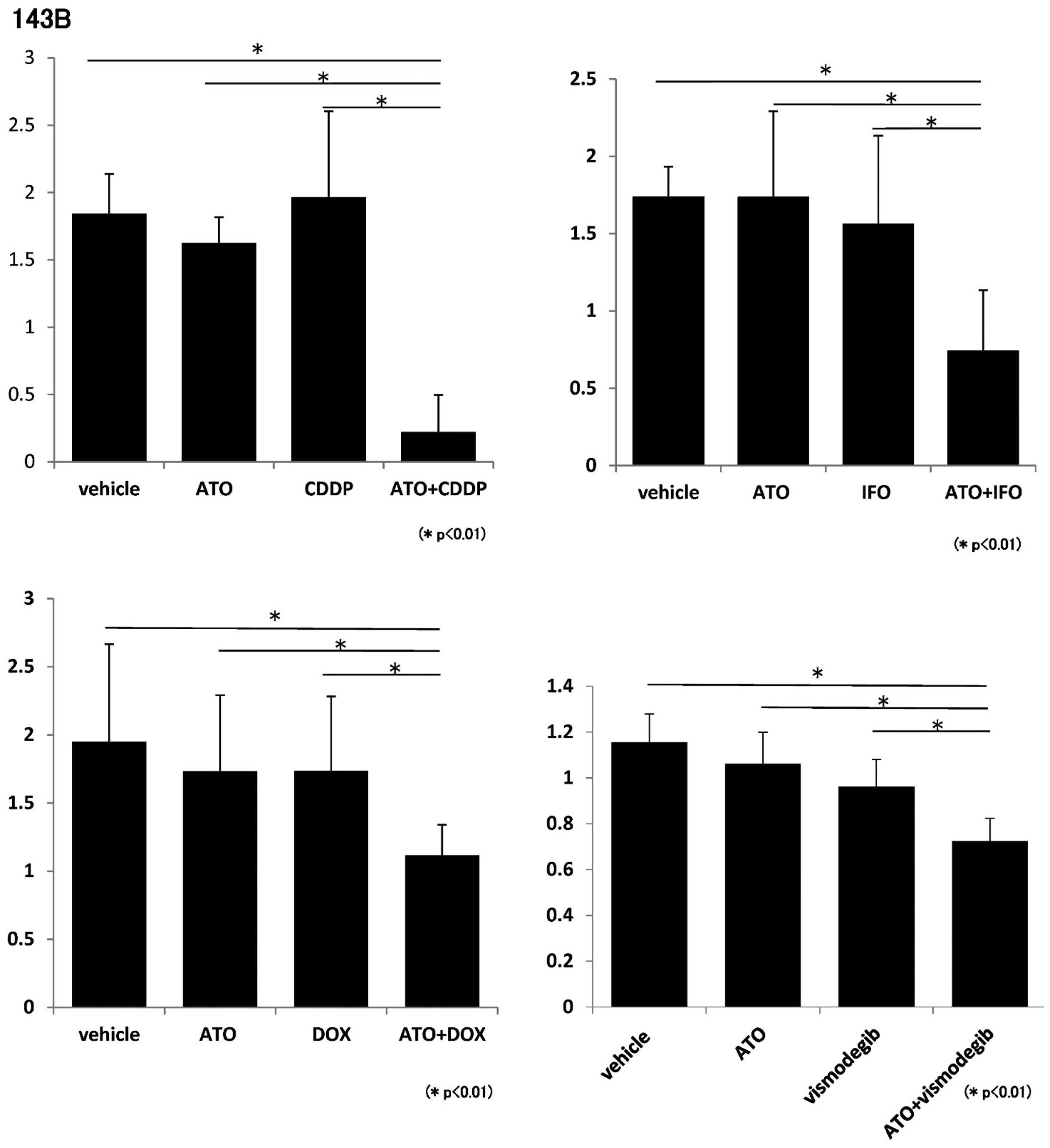
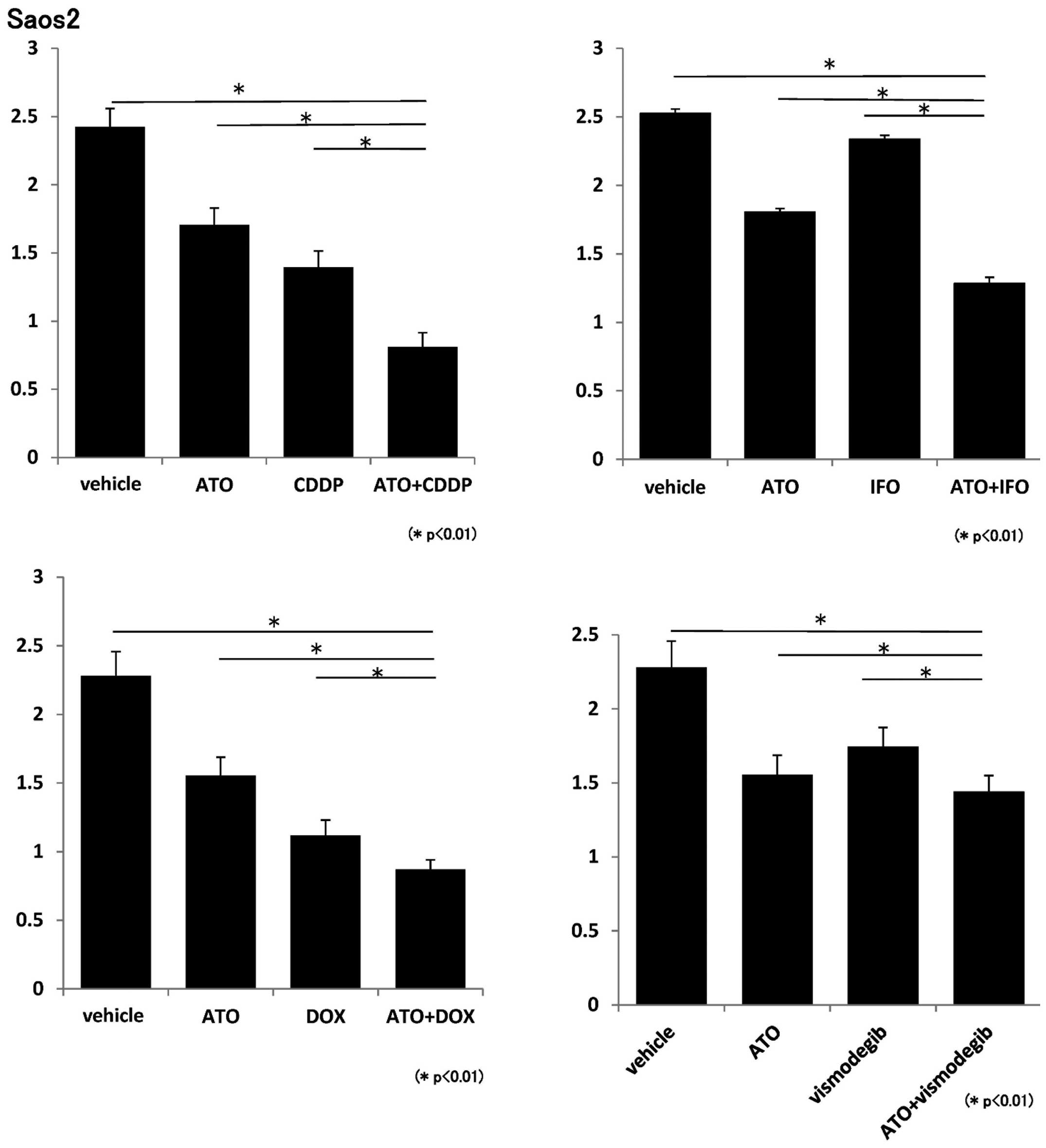
and DOX. WST-1 assays showed that proliferation of 143B and Saos2
cells was inhibited by ATO and each standard chemotherapeutic
reagent (Figs. 1 and 2). We next evaluated the effects of a
combination of Hedgehog inhibitors using ATO and vismodegib. SMO
inhibitor, vismodegib, was approved for the treatment of basal cell
carcinoma (BCC) in 2012 and is undergoing clinical trials for
metastatic cancer and advanced cancer (16,19).
WST-1 assays showed that 143B and Saos2 cell proliferation was
inhibited by ATO and vismodegib (Figs.
1 and 2). These findings
showed that combination of ATO and chemotherapeutic reagents
prevents proliferation of osteosarcoma cells in vitro.
Combination of Hedgehog inhibitors and
standard chemotherapeutic reagents prevent osteosarcoma cell
proliferation synergistically
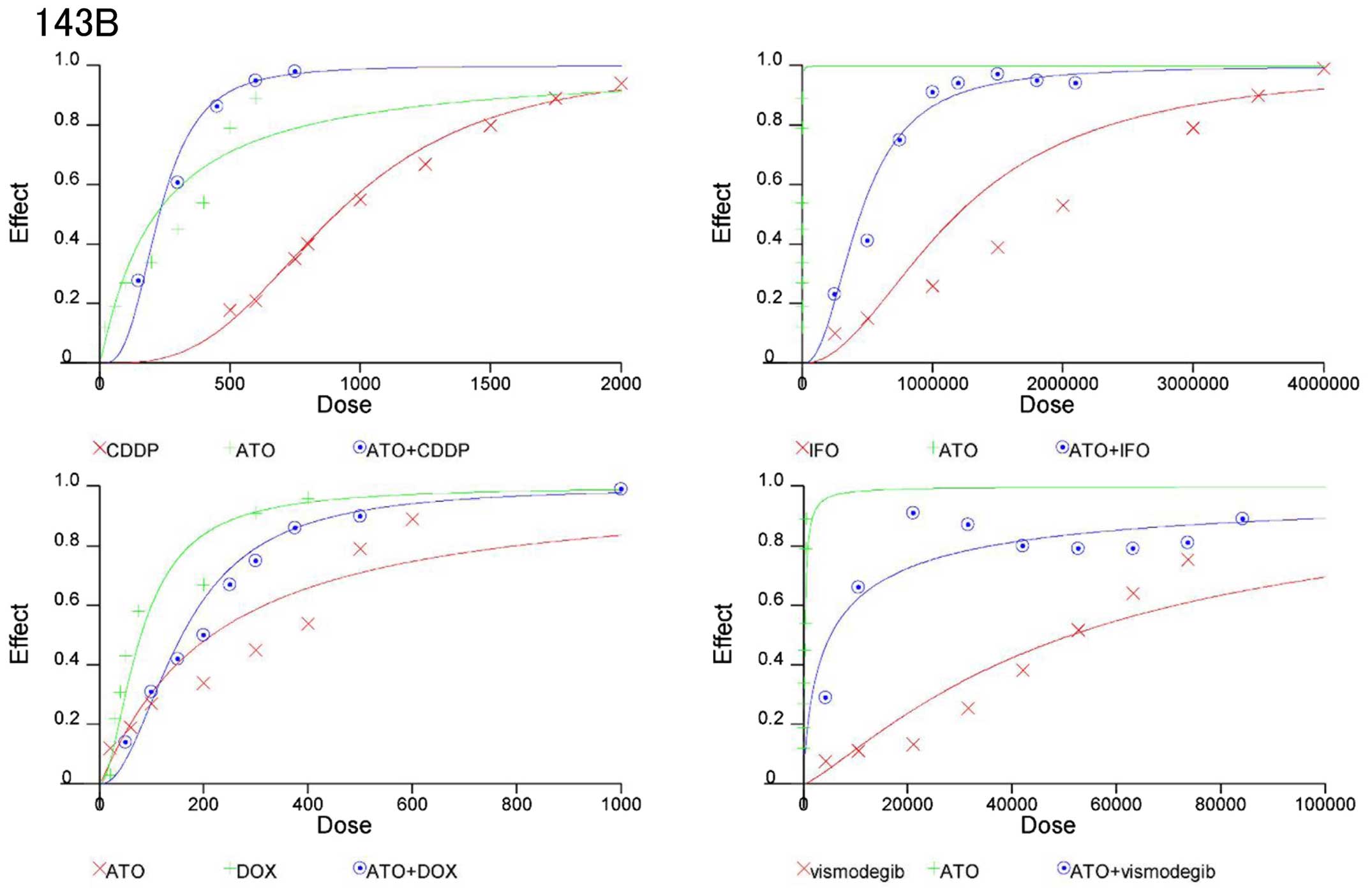
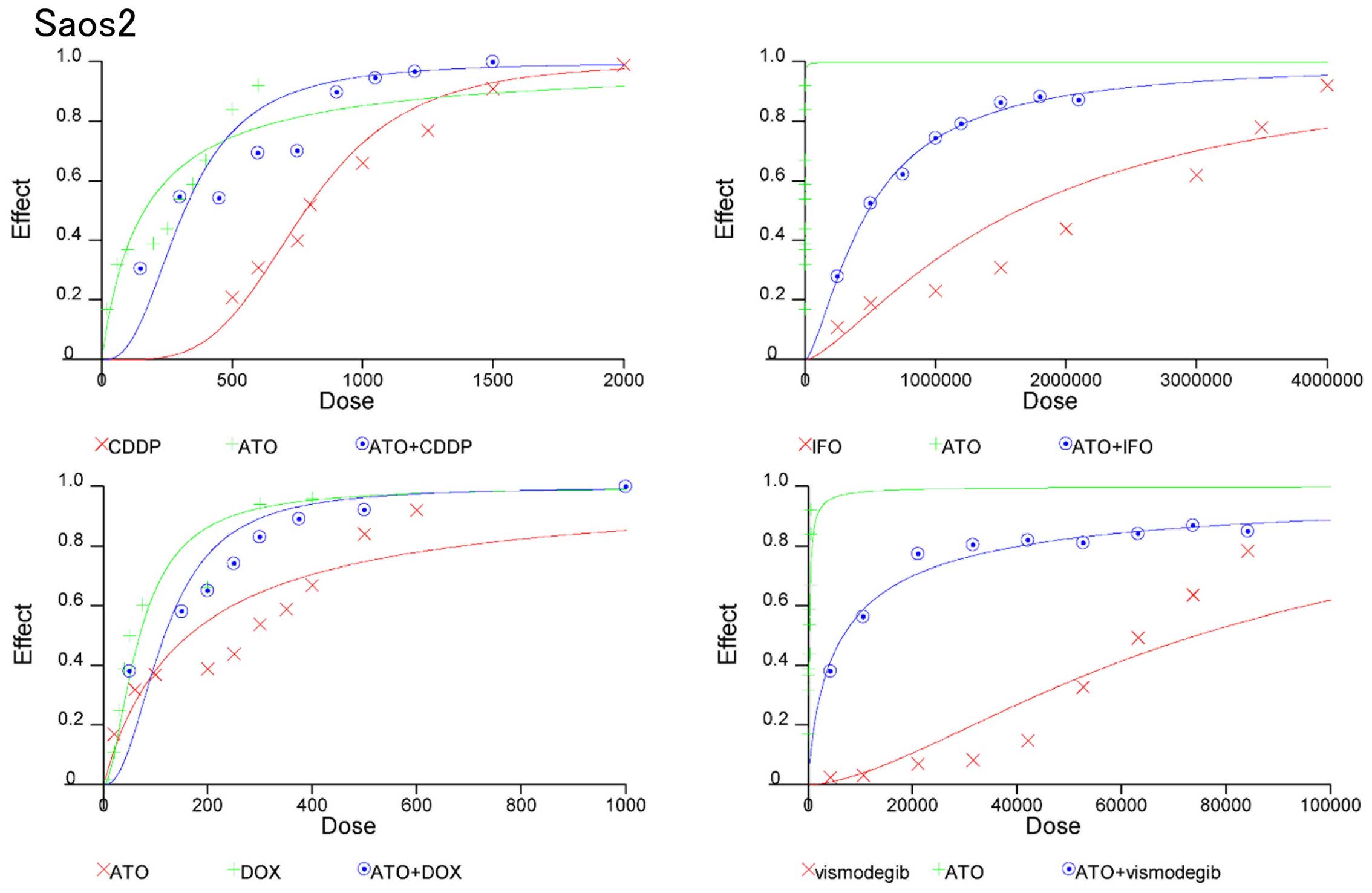
On the basis of these findings, we evaluated whether
combinations of ATO and standard chemotherapeutic reagents could be
synergistic to prevent osteosarcoma growth. CIs were used to
examine synergism after treatment with Hedgehog inhibitors and
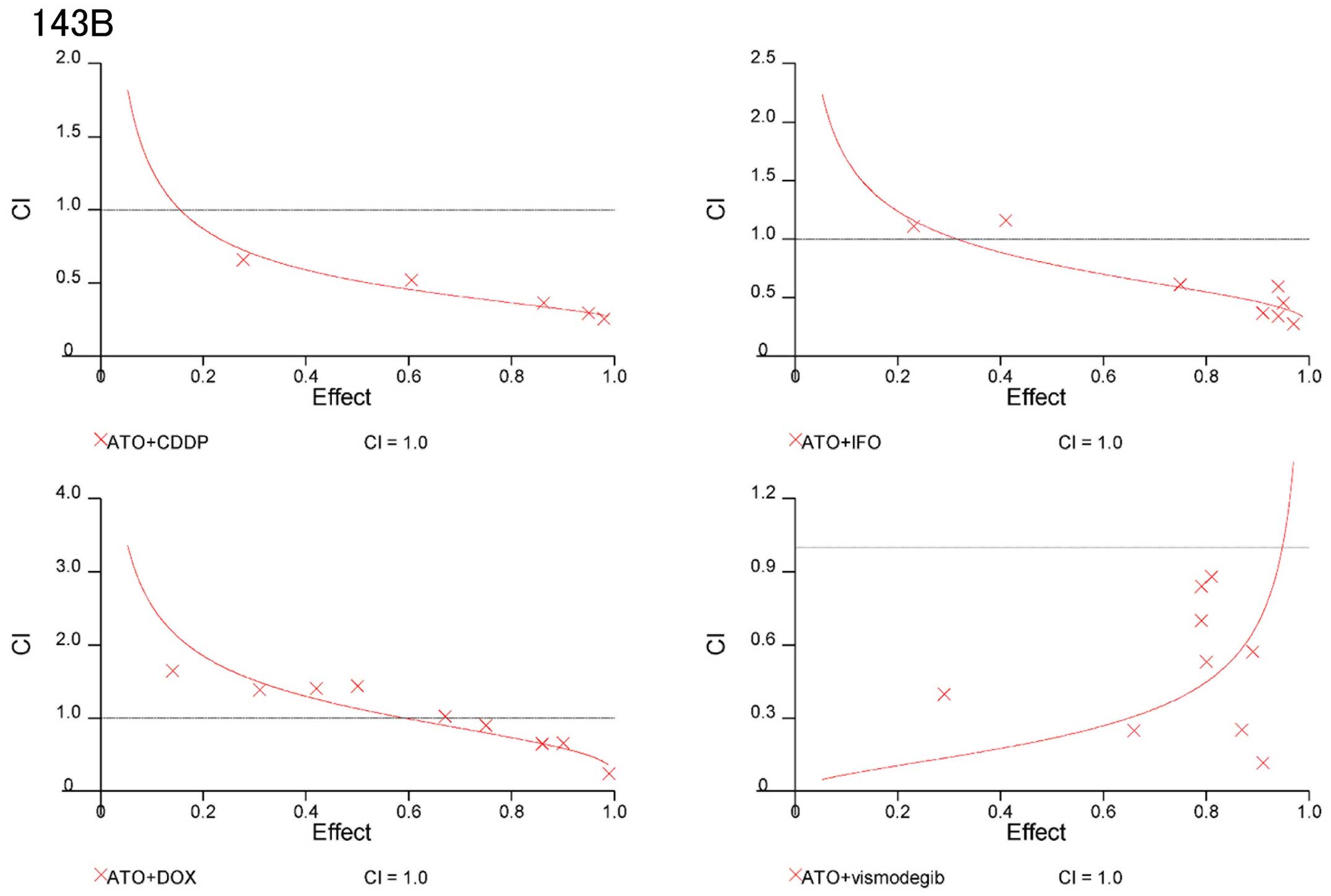
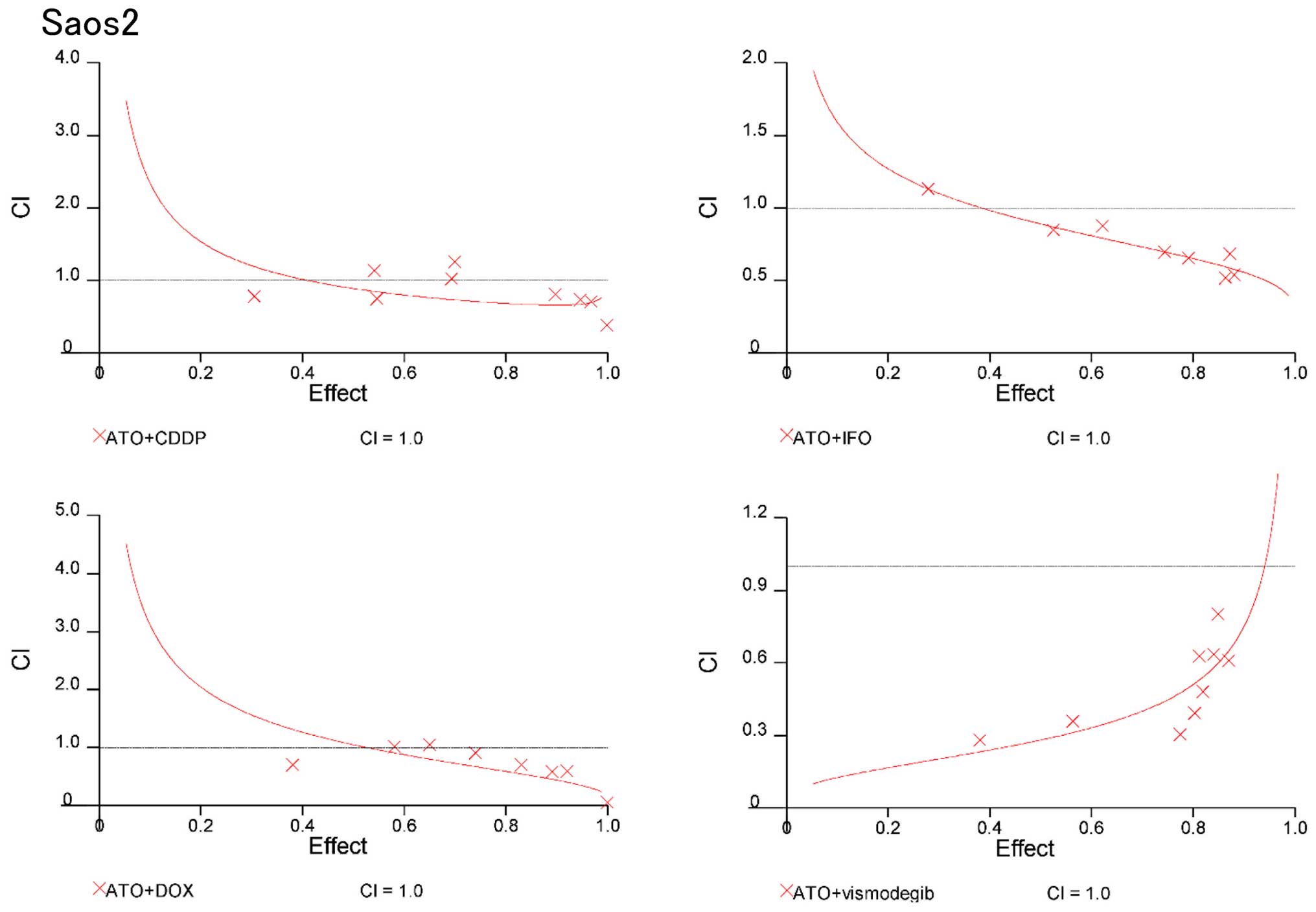
standard chemotherapeutic reagents (17). CI values and the degree of drug
interaction were evaluated by CalcuSyn software. The effects of the
combinations in 143B and Saos2 cells were then calculated and
presented in a dose-effect plot (Figs.
3 and 4) and fraction-affected
CI plots (Figs. 5 and 6). CIs of ATO with CDDP, IFO, or DOX at
ED50–90 (the average CI at ED50 to
ED90) of 143B cells were 0.41, 061, and 0.84,
respectively (Table IA). CIs of
ATO and CDDP, IFO, or DOX for ED50–90 of Saos2 were
0.75, 0.71, and 0.72, respectively (Table IB). Synergistic effects were
observed for all concentrations except for combination of DOX at
the ED50 in 143B and Saos2 cells. Next, we examined the
combination effects of Hedgehog inhibitors using ATO and
vismodegib. Synergic effects were observed at ED50 to
ED90 of 143B and Saos2 cells (Table I). These findings suggest that
combination of Hedgehog inhibitors and standard chemotherapeutic
reagents prevent osteosarcoma cell proliferation
synergistically.
 | Table ICombination index (CI) for
chemotherapy drugs. |
Table I
Combination index (CI) for
chemotherapy drugs.
| A, 143B |
|---|
|
|---|
| Drugs | CI
ED50 | CI
ED75 | CI
ED90 | CI
ED50–90 |
|---|
| ATO + CDDP
(CR;1:4) | 0.52 | 0.39 | 0.32 | 0.41 |
| ATO + IFO
(CR;1:5,000) | 0.79 | 0.59 | 0.47 | 0.61 |
| ATO + DOX
(CR;5:1) | 1.13 | 0.80 | 0.60 | 0.84 |
| ATO + Vis
(CR:1:200) | 0.22 | 0.39 | 0.69 | 0.43 |
|
| B, Saos2 |
|
| Drugs | CI
ED50 | CI
ED75 | CI
ED90 | CI
ED50–90 |
|
| ATO + CDDP
(CR;1:4) | 0.89 | 0.70 | 0.66 | 0.75 |
| ATO + IFO
(CR;1:5,000) | 0.89 | 0.69 | 0.56 | 0.71 |
| ATO + DOX
(CR;5:1) | 1.06 | 0.67 | 0.44 | 0.72 |
| ATO + Vis
(CR:1:200) | 0.28 | 0.45 | 0.75 | 0.49 |
Combination therapy of Hedgehog pathway
inhibitors and standard chemotherapeutic reagents in osteosarcoma
cells in vivo
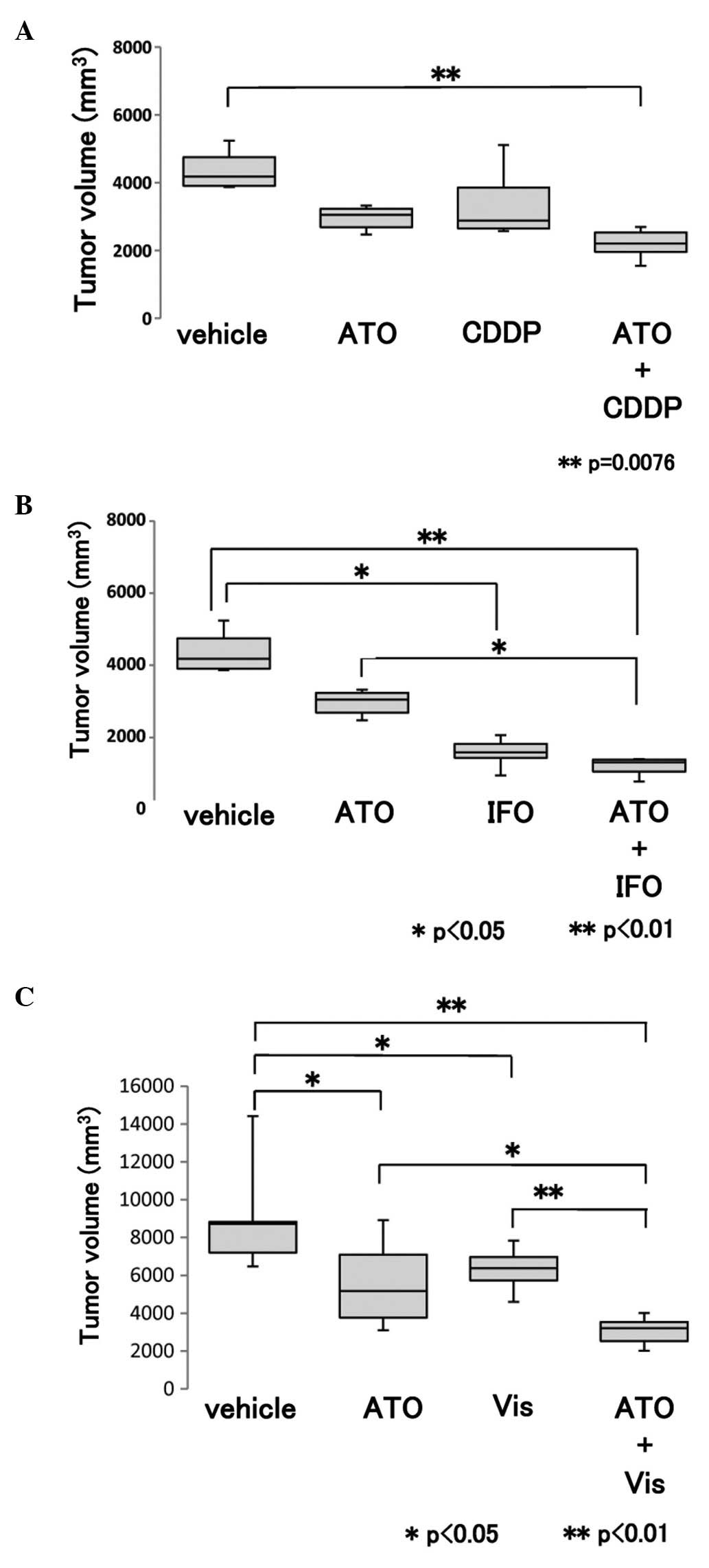
Nude mice were inoculated with 143B osteosarcoma
cells. ATO, IFO, CDDP, or vismodegib were intraperitoneally
administered, as previously reported (11,15,20).
Because intraperitoneal DOX treatment induces severe peritonitis,
intravenous administration of DOX is recommended (20). Our preliminary experiment showed
intravenous treatment could not achieve reproducibility because of
vascular leak and vascular occlusion. Compared with vehicle
treatment, treatment with ATO and CDDP or IFO significantly
prevented tumor growth (Fig. 7A and
B). Next, we examined the combined effect of Hedgehog
inhibitors, ATO and vismodegib. Compared with the vehicle control,
ATO, or vismodegib treatment, combination of ATO and vismodegib
significantly prevented tumor growth (Fig. 7C). These findings suggest that the
combined administration of ATO and standard chemotherapeutic
reagents might be effective in the treatment of osteosarcoma.
Discussion
Aberrant activation of the Hedgehog pathway due to
mutations of regulatory components or without driver mutations have
been reported in many malignancies (21–28).
Although treatment with vismodegib substantially benefited patients
with advanced basal cell carcinoma (29), there have been disappointing
reports for vismodegib from trials in colorectal and ovarian
cancers (30,31) and clinical trials of saridegib (a
SMO inhibitor) were halted because of failures in pancreatic
cancer, myelofibrosis and chondrosarcoma (28). In addition, mutations in SMO and
Sufu heterozygous mice have been shown to be unresponsive to SMO
inhibitors (28,32). Non-Hedgehog pathway-mediated
activation of GLI transcription has been also reported (28). These findings suggest that
additional approaches should be considered for Hedgehog inhibition
therapy for malignancy. Accordingly, ATO, which inhibits GLI
transcription, may be a more effective Hedgehog targeting
agent.
We and others reported that SMO and GLI2 were
overexpressed in human osteosarcoma specimens (8,9,12).
Upregulated expression levels of GLI2 correlated with lung
metastasis and poor survival of osteosarcoma patients (11,12).
Inhibition of GLI2 or SMO prevents osteosarcoma growth both in
vitro and in vivo (8,9). ATO
inhibits the activation of Hedgehog signaling and promotes
apoptotic cell death in osteosarcoma cells (15). We used ATO at 10 mg/kg body weight
intraperitoneally, as previously reported (33). However, the concentration of ATO
used in vivo was two-fold greater than that used for human
APL therapy (34). To decrease the
ATO concentration, combinations of Hedgehog inhibitors and standard
anticancer drugs were examined. Our findings showed that combined
treatment of ATO with CDDP, IFO, or DOX prevents osteosarcoma
proliferation synergistically in vitro. In vivo
examinations showed that statistical differences in tumor size were
observed between the vehicle and combinations of ATO with CDDP or
ATO with IFO. These findings suggest that combination therapy might
decrease the effective concentration of each drug. The lower levels
of reagents that could be used in these combinations might reduce
toxicities associated with effective use of a single drug.
Kim et al reported that combined use of ATO
and itraconazole, a SMO inhibitor, enables a reduced dose of ATO
and itraconazol to prevent medulloblastoma and basal cell carcinoma
growth (35). Consistent with this
report, we showed that combined use of ATO with vismodegib permits
a reduced dose of both ATO and vismodegib, and inhibited
osteosarcoma growth synergistically in vitro and in
vivo. Although the majority of side effects of vismodegib are
not so severe, >50% of patients discontinued their drug
treatment because of side effect concerns for long-term compliance
(16,36). Combination of ATO with vismodegib
might decrease the effective concentration of each drug. The lower
levels of reagents that could be used in these combinations might
reduce toxicity.
Because osteosarcoma is an extremely heterogeneous
and genomically unstable tumor (37), preselection of osteosarcoma
patients with activated Hedgehog is required before treatment with
Hedgehog inhibitors. Shou et al developed a five-gene
Hedgehog signature for patient preselection for Hedgehog inhibitor
therapy in medulloblastoma (38).
This method might be useful for other Hedgehog-activated
malignancies including osteosarcoma.
There is a possibility that the pleotropic effect of
ATO and off-target effects of SMO might affect the growth
inhibition of osteosarcoma. Nonetheless, combinations of ATO with
vismodegib or standard anticancer reagents showed promising
therapeutic efficacy for osteosarcoma.
Taken together, these findings indicate that
combination of Hedgehog pathway inhibitors and standard
FDA-approved anticancer agents with established safety profiles for
human use may be an attractive therapeutic method for treating
osteosarcoma.
Acknowledgements
We are grateful to Hui Gao for her excellent
technical assistance. We wish to thank the joint research
laboratory of Kagoshima University Graduate School of Medical and
Dental Sciences. This study was supported by Grants-in-Aid for
Scientific Research (KAKENHI) (C) 19591725, (C) 20591786, (C)
21591919, (C) 21591920, (C) 22591663, and (C) 23592195, a
Grant-in-Aid from the Ministry of Health, Labour and Welfare of
Japan for the Third Term Comprehensive Control Research for Cancer,
and Scientific Research on Priority Areas 201201976 to H. Nagao
from the Grants-in-Aid for JSPS Fellows. We thank for Edanz Group
Japan for English Editing service.
Abbreviations:
|
ATO
|
arsenic trioxide
|
|
PTCH1
|
patched1
|
|
SMO
|
smoothened
|
|
SUFU
|
suppressor of fused
|
|
GLI
|
glioma-associated oncogene homolog
|
|
FDA
|
Food and Drug Administration
|
|
APL
|
acute promyelocytic leukemia
|
|
ACTB
|
actin-beta
|
References
|
1
|
Sweetnam R: Osteosarcoma. Br J Hosp Med.
28:112116–121. 1982.PubMed/NCBI
|
|
2
|
Dorfman HD and Czerniak B: Bone cancers.
Cancer. 75(Suppl): 203–210. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hegyi M, Semsei AF, Jakab Z, Antal I, Kiss
J, Szendroi M, Csoka M and Kovacs G: Good prognosis of localized
osteosarcoma in young patients treated with limb-salvage surgery
and chemotherapy. Pediatr Blood Cancer. 57:415–422. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He JP, Hao Y, Wang XL, Yang XJ, Shao JF,
Guo FJ and Feng JX: Review of the molecular pathogenesis of
osteosarcoma. Asian Pac J Cancer Prev. 15:5967–5976. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nybakken K and Perrimon N: Hedgehog signal
transduction: Recent findings. Curr Opin Genet Dev. 12:503–511.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petrova R and Joyner AL: Roles for
Hedgehog signaling in adult organ homeostasis and repair.
Development. 141:3445–3457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Briscoe J and Thérond PP: The mechanisms
of Hedgehog signalling and its roles in development and disease.
Nat Rev Mol Cell Biol. 14:416–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirotsu M, Setoguchi T, Sasaki H,
Matsunoshita Y, Gao H, Nagao H, Kunigou O and Komiya S: Smoothened
as a new therapeutic target for human osteosarcoma. Mol Cancer.
9:52010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagao H, Ijiri K, Hirotsu M, Ishidou Y,
Yamamoto T, Nagano S, Takizawa T, Nakashima K, Komiya S and
Setoguchi T: Role of GLI2 in the growth of human osteosarcoma. J
Pathol. 224:169–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagao-Kitamoto H, Setoguchi T, Kitamoto S,
Nakamura S, Tsuru A, Nagata M, Nagano S, Ishidou Y, Yokouchi M,
Kitajima S, et al: Ribosomal protein S3 regulates GLI2-mediated
osteosarcoma invasion. Cancer Lett. 356B:855–861. 2015. View Article : Google Scholar
|
|
11
|
Nagao-Kitamoto H, Nagata M, Nagano S, et
al: GLI2 is a novel therapeutic target for metastasis of
osteosarcoma. Int J Cancer. 136:1276–1284. 2015. View Article : Google Scholar
|
|
12
|
Yang W, Liu X, Choy E, Mankin H, Hornicek
FJ and Duan Z: Targeting hedgehog-GLI-2 pathway in osteosarcoma. J
Orthop Res. 31:502–509. 2013. View Article : Google Scholar
|
|
13
|
Nasr R, Guillemin MC, Ferhi O, Soilihi H,
Peres L, Berthier C, Rousselot P, Robledo-Sarmiento M,
Lallemand-Breitenbach V, Gourmel B, et al: Eradication of acute
promyelocytic leukemia-initiating cells through PML-RARA
degradation. Nat Med. 14:1333–1342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beauchamp EM, Ringer L, Bulut G, Sajwan
KP, Hall MD, Lee YC, Peaceman D, Ozdemirli M, Rodriguez O,
Macdonald TJ, et al: Arsenic trioxide inhibits human cancer cell
growth and tumor development in mice by blocking Hedgehog/GLI
pathway. J Clin Invest. 121:148–160. 2011. View Article : Google Scholar :
|
|
15
|
Nakamura S, Nagano S, Nagao H, Ishidou Y,
Yokouchi M, Abematsu M, Yamamoto T, Komiya S and Setoguchi T:
Arsenic trioxide prevents osteosarcoma growth by inhibition of GLI
transcription via DNA damage accumulation. PLoS One. 8:e694662013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sekulic A, Migden MR, Oro AE, Dirix L,
Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander
PA, et al: Efficacy and safety of vismodegib in advanced basal-cell
carcinoma. N Engl J Med. 366:2171–2179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iwamoto Y and Tanaka K: The activity of
the Bone and Soft Tissue Tumor Study Group of the Japan Clinical
Oncology Group. Jpn J Clin Oncol. 42:467–470. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keating GM: Vismodegib: In locally
advanced or metastatic basal cell carcinoma. Drugs. 72:1535–1541.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Budach W, Budach V, Stuschke M, Schmauder
B, Reipke P and Scheulen ME: Efficacy of ifosfamide, dacarbazine,
doxorubicin and cisplatin in human sarcoma xenografts. Br J Cancer.
70:29–34. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gailani MR, Ståhle-Bäckdahl M, Leffell DJ,
Glynn M, Zaphiropoulos PG, Pressman C, Undén AB, Dean M, Brash DE,
Bale AE, et al: The role of the human homologue of Drosophila
patched in sporadic basal cell carcinomas. Nat Genet. 14:78–81.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie J, Murone M, Luoh SM, Ryan A, Gu Q,
Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, et al: Activating
smoothened mutations in sporadic basal-cell carcinoma. Nature.
391:90–92. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Slade I, Murray A, Hanks S, Kumar A,
Walker L, Hargrave D, Douglas J, Stiller C, Izatt L and Rahman N:
Heterogeneity of familial medulloblastoma and contribution of
germline PTCH1 and SUFU mutations to sporadic medulloblastoma. Fam
Cancer. 10:337–342. 2011. View Article : Google Scholar
|
|
24
|
Raffel C, Jenkins RB, Frederick L, Hebrink
D, Alderete B, Fults DW and James CD: Sporadic medulloblastomas
contain PTCH mutations. Cancer Res. 57:842–845. 1997.PubMed/NCBI
|
|
25
|
Reifenberger J, Wolter M, Weber RG,
Megahed M, Ruzicka T, Lichter P and Reifenberger G: Missense
mutations in SMOH in sporadic basal cell carcinomas of the skin and
primitive neuroectodermal tumors of the central nervous system.
Cancer Res. 58:1798–1803. 1998.PubMed/NCBI
|
|
26
|
Taylor MD, Liu L, Raffel C, Hui CC,
Mainprize TG, Zhang X, Agatep R, Chiappa S, Gao L, Lowrance A, et
al: Mutations in SUFU predispose to medulloblastoma. Nat Genet.
31:306–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scales SJ and de Sauvage FJ: Mechanisms of
Hedgehog pathway activation in cancer and implications for therapy.
Trends Pharmacol Sci. 30:303–312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amakye D, Jagani Z and Dorsch M:
Unraveling the therapeutic potential of the Hedgehog pathway in
cancer. Nat Med. 19:1410–1422. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Basset-Seguin N, Hauschild A, Grob JJ,
Kunstfeld R, Dréno B, Mortier L, Ascierto PA, Licitra L, Dutriaux
C, Thomas L, et al: Vismodegib in patients with advanced basal cell
carcinoma (STEVIE): A pre-planned interim analysis of an
international, open-label trial. Lancet Oncol. 16:729–736. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Berlin J, Bendell JC, Hart LL, Firdaus I,
Gore I, Hermann RC, Mulcahy MF, Zalupski MM, Mackey HM, Yauch RL,
et al: A randomized phase II trial of vismodegib versus placebo
with FOLFOX or FOLFIRI and bevacizumab in patients with previously
untreated metastatic colorectal cancer. Clin Cancer Res.
19:258–267. 2013. View Article : Google Scholar
|
|
31
|
Kaye SB, Fehrenbacher L, Holloway R, Amit
A, Karlan B, Slomovitz B, Sabbatini P, Fu L, Yauch RL, Chang I, et
al: A phase II, randomized, placebo-controlled study of vismodegib
as maintenance therapy in patients with ovarian cancer in second or
third complete remission. Clin Cancer Res. 18:6509–6518. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee Y, Kawagoe R, Sasai K, Li Y, Russell
HR, Curran T and McKinnon PJ: Loss of suppressor-of-fused function
promotes tumorigenesis. Oncogene. 26:6442–6447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim J, Lee JJ, Kim J, Gardner D and Beachy
PA: Arsenic antagonizes the Hedgehog pathway by preventing ciliary
accumulation and reducing stability of the Gli2 transcriptional
effector. Proc Natl Acad Sci USA. 107:13432–13437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM,
Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, et al: Use of arsenic
trioxide (As2O3) in the treatment of acute
promyelocytic leukemia (APL): II. Clinical efficacy and
pharmacokinetics in relapsed patients. Blood. 89:3354–3360.
1997.PubMed/NCBI
|
|
35
|
Kim J, Aftab BT, Tang JY, Kim D, Lee AH,
Rezaee M, Kim J, Chen B, King EM, Borodovsky A, et al: Itraconazole
and arsenic trioxide inhibit Hedgehog pathway activation and tumor
growth associated with acquired resistance to smoothened
antagonists. Cancer Cell. 23:23–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang JY, Mackay-Wiggan JM, Aszterbaum M,
Yauch RL, Lindgren J, Chang K, Coppola C, Chanana AM, Marji J,
Bickers DR, et al: Inhibiting the hedgehog pathway in patients with
the basal-cell nevus syndrome. N Engl J Med. 366:2180–2188. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kuijjer ML, Hogendoorn PC and
Cleton-Jansen AM: Genomewide analyses on high-grade osteosarcoma:
making sense of a genomically most unstable tumor. Int J Cancer.
133:2512–2521. 2013.PubMed/NCBI
|
|
38
|
Shou Y, Robinson DM, Amakye DD, Rose KL,
Cho YJ, Ligon KL, Sharp T, Haider AS, Bandaru R, Ando Y, et al: A
five-gene hedgehog signature developed as a patient preselection
tool for hedgehog inhibitor therapy in medulloblastoma. Clin Cancer
Res. 21:585–593. 2015. View Article : Google Scholar
|