Introduction
Vigorous research has shown that the intestinal
epithelial stem cells are located at the bottom of the crypt base
(1) and consist of proliferative
and quiescent types. It is widely accepted that the proliferative
stem cells are crypt base columnar (CBC) cells and are positive for
the leucine-rich repeat-containing G-protein-coupled receptor 5
(Lgr5) (2) and that quiescent stem
cells are located at +4 position from the bottom of the crypt base
(3) and positive for Bmi-1
(4), Hopx (5), mTert (6) and Lrig1 (7). The intestinal stem cells are thought
to be supported by their adjacent Paneth cells in small intestine
(8) through Wnt (9), Notch (10) and epidermal growth factor (EGF)
(11) signaling and adversely
influenced by villus cells through bone morphogenetic protein (BMP)
signaling (12).
Carbonic anhydrase 9 (CA9) is a membrane-bound
isozyme of 12 enzymatically active CAs in human, and catalyzes the
reversible reaction between carbon dioxide (CO2) and
water to the bicarbonate ion and protons at its extracellular
catalytic site. High expression of CA9 has been reported in limited
cell types of normal tissues (13,14)
and the relationship between CA9 and advanced status of cancer has
been intensely studied (15–19).
However, CA9 expression in normal human intestinal epithelial cells
and early stage colorectal cancer (CRC) remains incompletely
understood.
In this study, we assessed the characteristics and
distribution pattern of CA9 positive cells in human intestinal
epithelial cells using clinical samples and in T1 CRC.
Materials and methods
Tissue samples
Surgically resected human adult intestinal normal
tissues and T1 CRC tissues were obtained from 20 patients with CRC
(2 cecum, 5 ascending, 2 transverse, 3 descending, 5 sigmoid colon,
3 rectum; 54 to 84 years old, 6 female and 14 male, 14 normal
mucosa, 3 adenoma and 3 T1 CRC tissues) after informed consent from
Osaka University Medical Hospital with approval of the Research
Ethics Board. Normal intestinal epithelia were collected from
patients without evidence of symptomatic or microscopic
inflammation, and distance of >3 cm to the tumors.
Histopathology, immunohistochemical and
immunofluorescent analyses of intestinal tissue
Human colorectal tissue was fixed in 10% buffered
formalin and embedded in paraffin. Sequential 5-μm sections were
stained with hematoxylin and eosin (H&E) for histopathological
analyses, and for immunohistological analyses with antibodies to
CA9 (2D3, 1:500; Abcam, Cambridge, MA, USA and EPR4151, 1:200;
Epitomics, Burlingame, CA, USA), neuron specific enolase (NSE)
(1:100; Assaybiotech, Sunnyvale, CA, USA), CD68 (PG-M1; Dako,
Carpinteria, CA, USA) and polypyrimidine tract-binding protein 1
(PTBP1) (M01, 3 μg/ml; Abnova, Taipei, Taiwan). Antigen retrieval
(10 mmol/l citrate buffer, pH 6 at 100°C for 40 min) was performed
on paraffin-embedded tissues. Visualization was performed using
either fluorescent-conjugated species-specific secondary antibodies
or the avidin-biotin-peroxidase method (Vectastain Elite ABC
reagent kit; Vector Laboratories, Burlingame, CA, USA). Nuclear
counterstaining was performed with hematoxylin or ProLong Gold
antifade reagent with DAPI (Invitrogen, Carlsbad, CA, USA).
All-in-one type fluorescence microscopy (BZ-8000; Keyence, Osaka,
Japan) with digital photographic capability was used to visualize
cells at several magnifications.
Calculation of the frequency of
CA9+ cells of the intestinal crypts
The frequency of CA9+ cells at specific
positions relative to the crypt bottom were evaluated using crypts
which intact overall longitudinal sections were available in
sections of immunohistochemical staining with anti-CA9 antibody.
The counting of intestinal epithelial CA9+ and
CA9− cells was performed three times independently using
20, 70 and 150 crypts for ileum, right colon and left colon,
respectively. The frequency of CA9+ cells was calculated
by the ratio of the total number of CA9+ cells to the
total number of CA9− and CA9+ cells at each
cell position relative to crypt bottom.
Crypt isolation and cell
dissociation
The intestinal tissues were washed with cold
phosphate-buffered saline (PBS) until the supernatant was clear.
Next, they were incubated in 8 mmol/l ethylenediaminetetraacetic
acid (EDTA) cold chelation buffer (distilled water with 5.6 mmol/l
Na2HPO4, 8.0 mmol/l
KH2PO4, 96.2 mmol/l NaCl, 1.6 mmol/l KCl,
43.4 mmol/l sucrose, 54.9 mmol/l D-sorbitol, 0.5 mmol/l
DL-dithiothreitol) (20) for 10
min on ice and the intestinal crypts were stripped and collected
with angled Debakey forceps under a stereomicroscope (SZX10;
Olympus, Tokyo, Japan). After washing with cold chelation buffer,
the isolated crypts were incubated with TrypLE Express (Invitrogen)
including 2,000 U/ml DNase (Sigma-Aldrich, St. Louis, MO, USA) for
60 min at 37°C, passed through 40 μm cell strainers, treated with
BD Pharm Lyse (BD Biosciences, San Jose, CA, USA) for the lysis of
red blood cells and then washed with cold chelation buffer.
Flow cytometry
Dissociated intestinal crypt cells were blocked with
FcR blocking reagent (BD Biosciences) and incubated with antibodies
as follows; anti-human CA9 (APC-conjugated; R&D Systems,
Minneapolis, MN, USA), anti-human CD31 (FITC-conjugated;
eBiosciences, San Diego, CA, USA), anti-human CD44 (PE-conjugated)
and lineage cocktail 1 (Lin1) (FITC-conjugated) (both from BD
Biosciences). 7-AAD (BD Biosciences) was used to eliminate dead
cells. Cells were analyzed and isolated by using FACSAria II
equipped with FACSDiva software (BD Biosciences). The live single
epithelial crypt base cells (21,22)
(7-AAD−/CD31−/Lin1−/CD44+)
were evaluated for the CA9 expression and sorted accordingly.
RNA preparation and quantitative
real-time-polymerase chain reaction (qRT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen). In all cases, 400 ng of total RNA was
reverse-transcribed with High Capacity RNA-to-cDNA Master Mix
(Applied Biosystems, Foster City, CA, USA) following the
manufacturer's instructions. For quantitative assessments,
quantitative real-time reverse transcriptase analysis was performed
with the LightCycler TaqMan master kit (Roche Diagnostics, Tokyo,
Japan) and the LightCycler 480 system (Roche Applied Science,
Indianapolis, IN, USA). Primers are listed in Table I.
 | Table IPrimer sequences and TaqMan probes
used for quantitative real-time RT-PCR. |
Table I
Primer sequences and TaqMan probes
used for quantitative real-time RT-PCR.
| Gene | Primer | UPL probe |
|---|
| GAPDH
(NM_002046.3) |
5′-AGCCACATCGCTCAGACAC-3′
5′-GCCCAATACGACCAAATCC-3′ | 60 |
| ENO2
(NM_001975.2) |
5′-ACTTTGTCAGGGACTATCCTGTG-3′
5′-TCCCTACATTGGCTGTGAACT-3′ | 27 |
| AXIN2
(NM_004655.3) |
5′-AGAGCAGCTCAGCAAAAAGG-3′
5′-CCTTCATACATCGGGAGCAC-3′ | 88 |
Statistical analysis
The relationships among gene expressions, cell
counts, and tumor volume were analyzed using ANOVA. All tests were
analyzed with GraphPad Prism 6 software (GraphPad Software, San
Diego, CA, USA). A value of P<0.05 was taken as statistically
significant.
Results
CA9 protein expression in human
intestinal crypt base
To assess anatomical pattern of CA9 expression in
human normal intestinal epithelia, formalin-fixed paraffin-embedded
serial sections of ileum, right colon and left colon were stained
with H&E, CA9, CD68 and NSE (Fig.
1). The CA9 expression was confined to cell membrane and was
mainly observed in slender cells at the bottom of small intestinal
and in colon crypts with mosaic pattern. CA9+ cells
commonly contained eosinophilic structure in basal cytoplasm. Among
those evaluated, none of the CA9+ cells located in the
intestinal epithelia were positive for CD68, a marker for
macrophage, which means that although intestinal lamina propria
contains abundant macrophages (23), macrophages were not the source of
CA9+ cells. Almost all of the CA9+ cells in
the crypt base were also stained with NSE.
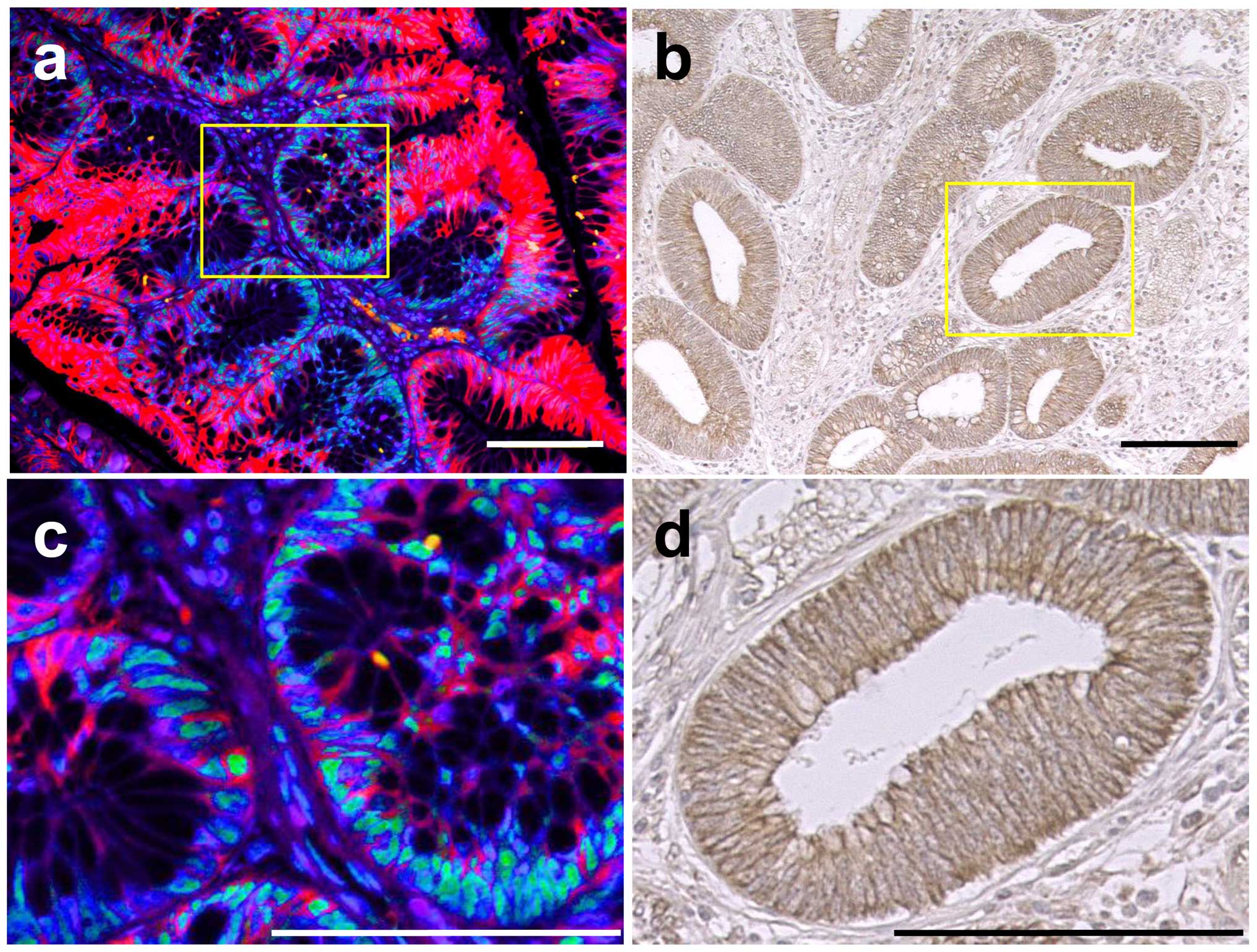
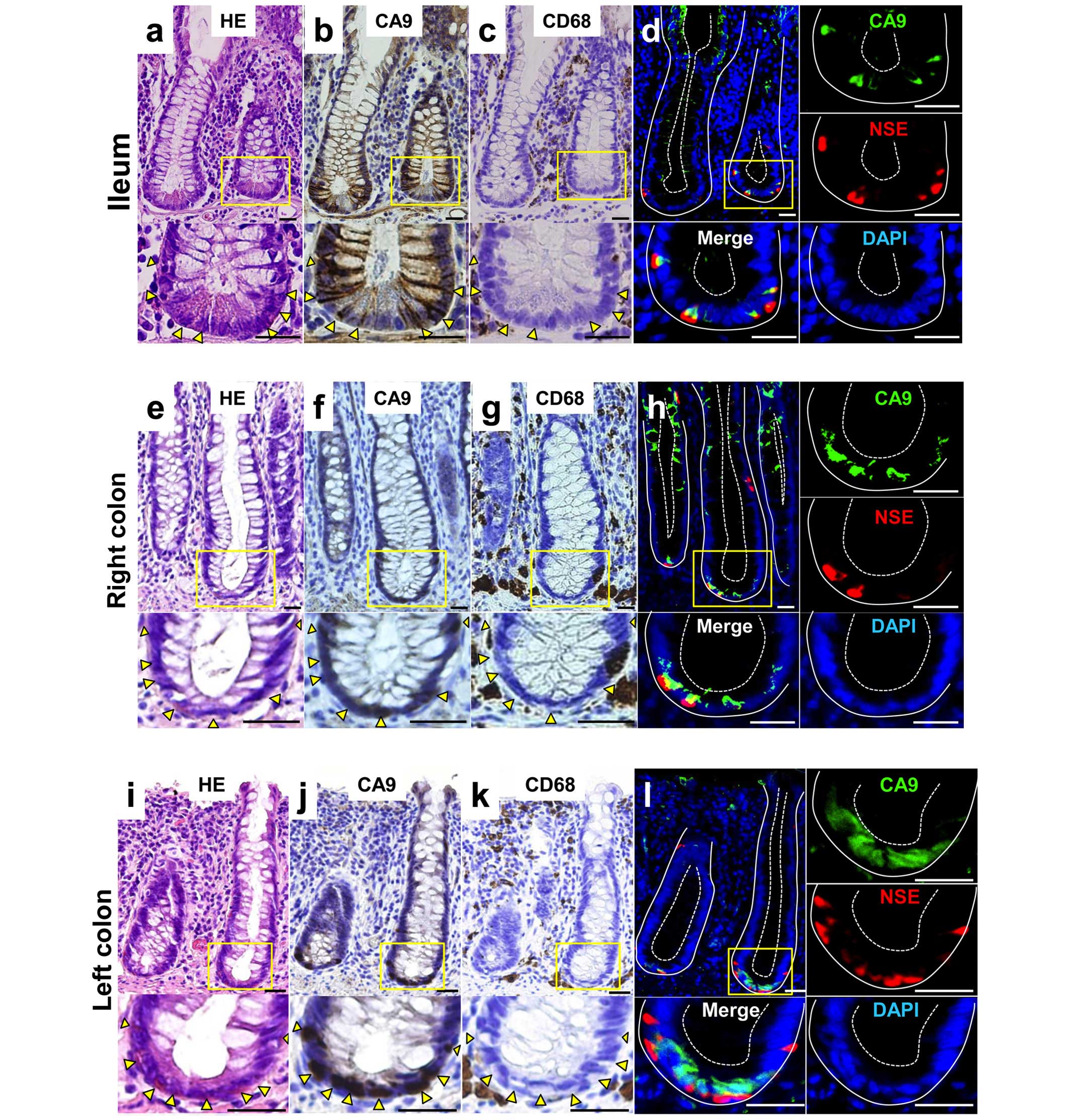 | Figure 1CA9 expression pattern in the human
ileum and colon. Representative staining patterns of human ileum
(a–d), right colon (e–h) and left colon (i–l). (a, e and i)
Hematoxylin and eosin (H&E) staining. (b, f and j)
Immunohistochemical (IHC) staining with anti-CA9 antibody. (c, g
and k) IHC staining with anti-CD68, a macrophage marker. (d, h and
l) Immunofluorescent staining with antibodies to CA9 (green),
neuron specific enolase (NSE) (red) and counterstained with DAPI
(blue). Larger magnification views of the crypt base boxed in
yellow are shown at the bottom on the right. IHC staining sections
were visualized with diaminobenzidine (brown) and counterstained
with hematoxylin. Arrowheads point to cells which express CA9.
White solid lines demarcate epithelialmesenchymal boundary, and
dashed lines mark the apical epithelial surface in (d, h and l).
Scale bar, 25 μm. Original magnification, top, ×20; bottom,
×40. |
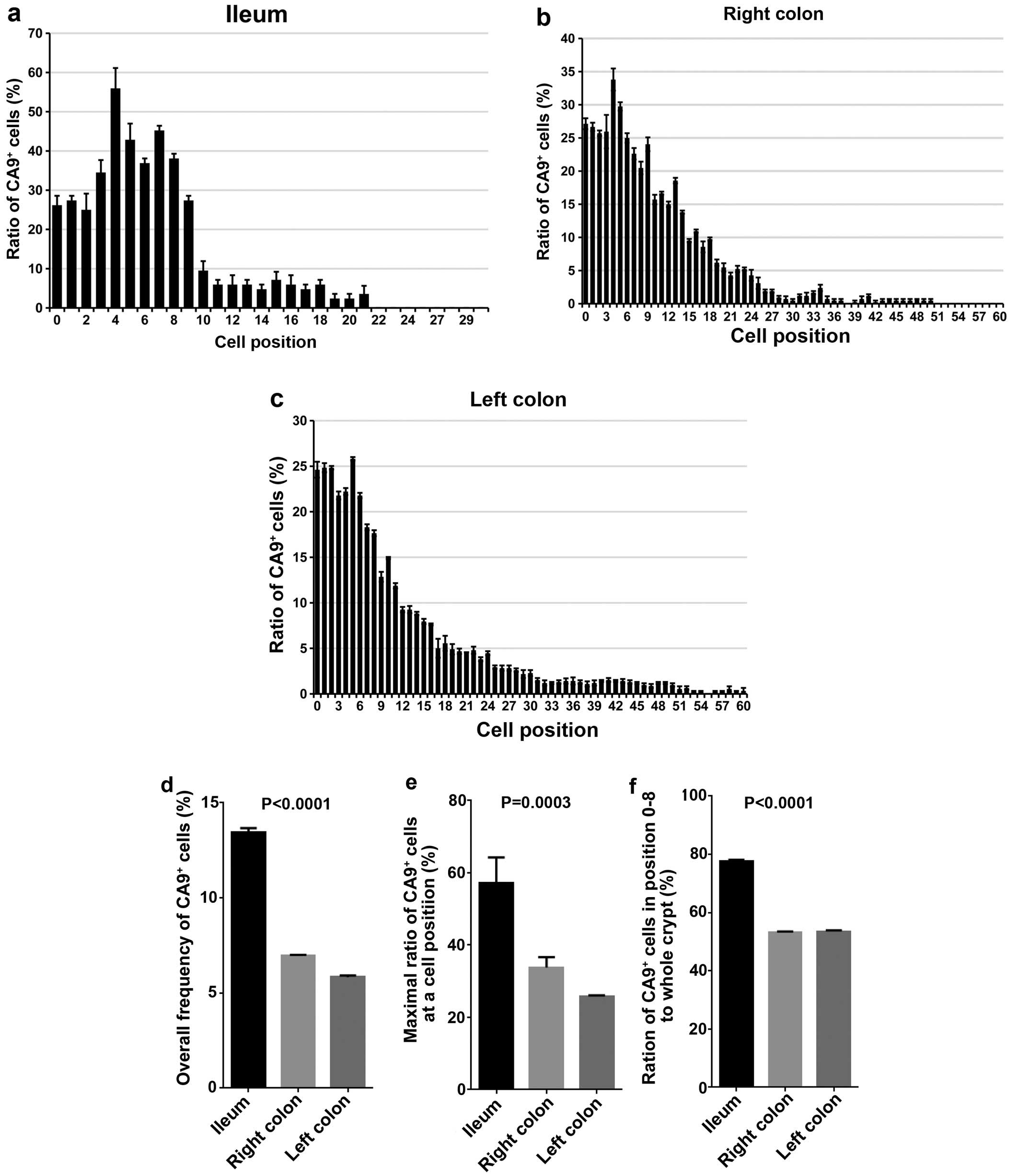
Frequency of CA9+ intestinal
epithelial cells in association with their position
To quantify the frequency of the CA9+
intestinal epithelial cells in association with their position
relative to crypt bottom, the counting of CA9− and
CA9+ cells of intestinal epithelia in ileum, right colon
and left colon was performed (Fig.
2a–c). Total frequency of CA9+ cells was 13.5±0.2,
7.0±0.1 and 5.9±0.1%, the maximal frequency of CA9+
cells in relation to cell position was 56.0±9.0% (cell position 3),
33.8±2.9% (cell position 4), 25.8±0.3% (cell position 5) and the
ratio of CA9+ cells in crypt base (cell position 0–8)
was 77.7±0.5, 53.1±0.4 and 53.5±0.4 in ileum, right colon and left
colon, respectively (Fig. 2d–f).
These data indicate that CA9+ cells are mainly located
in the crypt base, and ileum contains more CA9+ cells in
the crypt base than right or left colon.
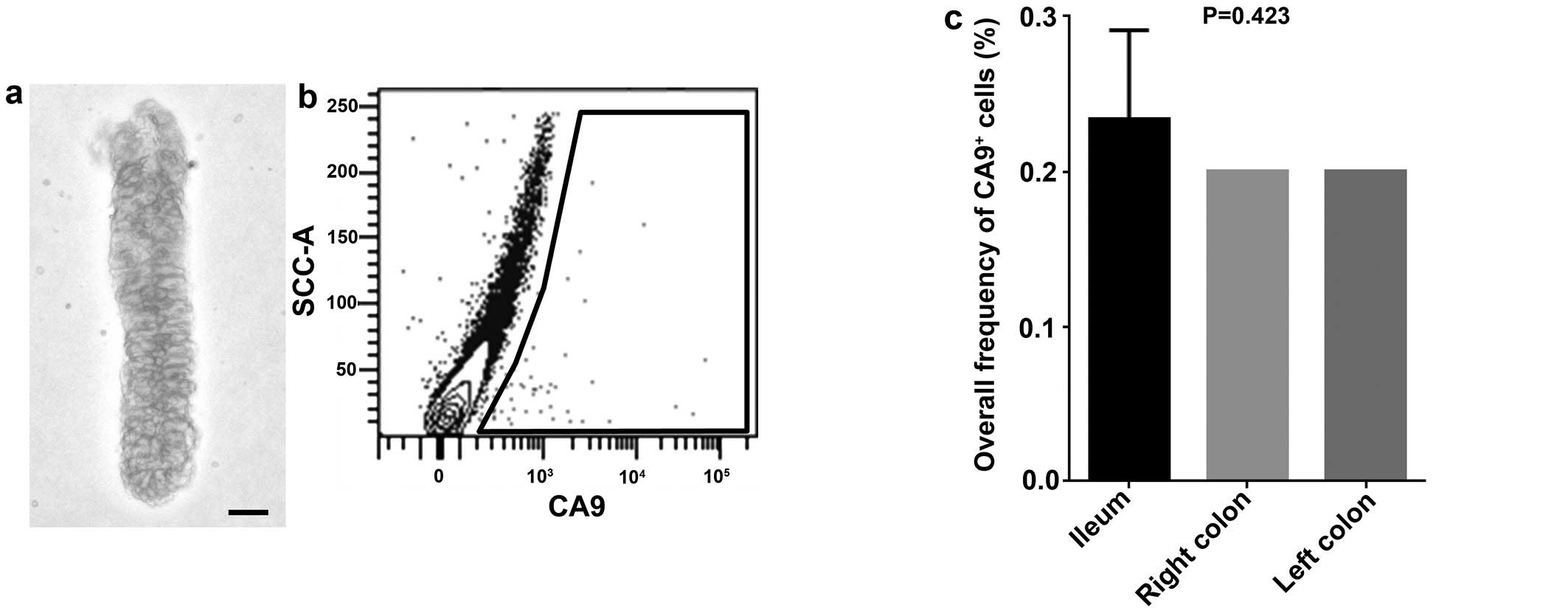
CA9+ intestinal epithelial
cells in fresh human clinical samples
To investigate the biological function of
CA9+ cells in intestinal epithelial crypt base, flow
cytometric analysis was performed on freshly isolated human
intestinal epithelial cells. The percentage of CA9+
cells was 0.23±0.06, 0.20±0.00 and 0.20±0.00%, in ileum, right
colon and left colon, respectively, without significant difference
among the locations (P=0.4226) (Fig.
3).
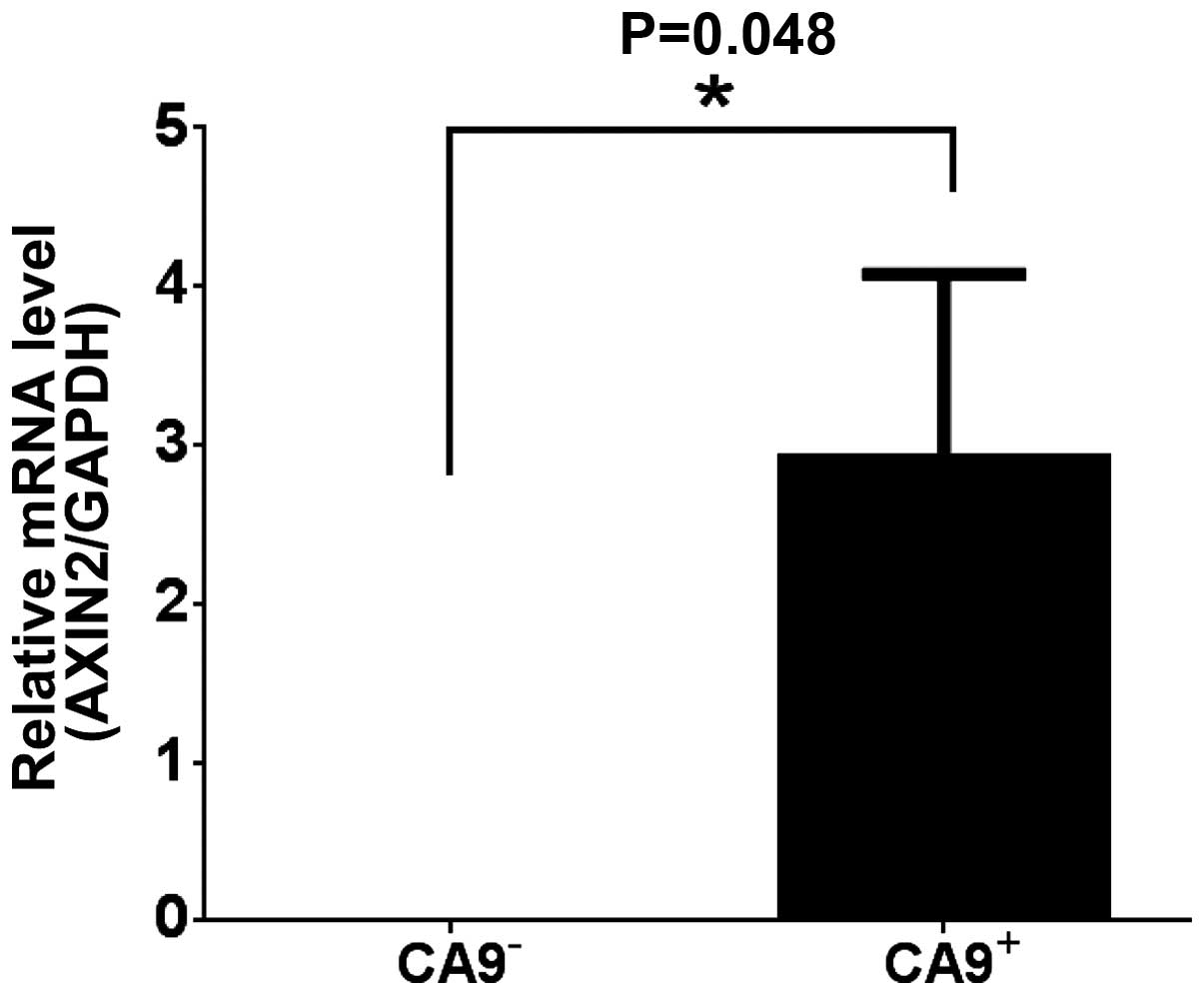
Correlation of CA9 and the Wnt pathway
gene AXIN2
To elucidate the characteristic of the
CA9+ cells in intestinal epithelial cells, expression of
AXIN2, a direct target gene of Wnt pathway (24), was analyzed on freshly isolated
CA9− and CA9+ dissociated human intestinal
epithelial cells by qRT-PCR. The expression of AXIN2 in
CA9+ cell population was significantly higher than that
of CA9− (P=0.048) (Fig.
4).
CA9 protein expression in adenoma and T1
CRC
To investigate the relationship between CA9
expression and tumorigenesis, formalin-fixed paraffin-embedded
sections were stained with CA9 in adenoma (3 samples) and T1 CRC (3
samples). Three (100%) and 3 (100%) were positive for CA9 in
adenoma and T1 CRC, respectively. In adenomas, the CA9 expression
was confined to cell membrane and was observed with mosaic-like
pattern at the bottom of crypt-like structures where PTBP1, a
hypoxia-related protein (25), is
abundantly observed (Fig. 5a and
c). In T1 cancer, CA9 expression was also confined to cell
membrane but almost all cells were positive for CA9, and there was
no apparent difference of staining positivity in the same tumor
tissue (Fig. 5b and d).
Discussion
In this study, we precisely revealed that
CA9+ cells exist in human adult crypt base of ileum,
right colon and left colon epithelia, and we also showed that the
CA9+ cells in the crypt base are suspected to be
associated with intestinal stem cells morphologically and
functionally. We also showed possible association of CA9 expression
with carcinogenesis.
In human adult normal intestinal epithelial crypt,
CA9+ cells were slender and mainly distributed with
mosaic pattern, which is consistent with morphological
characteristic of previously reported intestinal epithelial stem
cells (2–8). It is noteworthy that the CA9
expressed in normal colorectal epithelia is reported to be a
splicing variant lacking C-terminal part of the catalytic domain
and it is different from the full-length form expressed in CRC and
increased by hypoxia (26). This
may explain the reason CA9+ cells are arranged in
intestinal crypt bases with mosaic pattern regardless of the
distance from blood vessels. The antibodies, which we used in this
study, can also used in flow cytometry under non-denatered
condition. Since the reported splicing variant of CA9 is lacking
trans-membranous domain, the C-terminal of the full-length protein,
the CA9 protein levels which we analyzed in this study contains
both forms of CA9. Although NSE is commonly considered to be a
marker for enteroendocrine cells (27,28),
intestinal quiescent stem cells were recently shown to be the
precursors which were committed to mature into differentiated
secretory cells of the Paneth and enteroendocrine lineage (29). In addition, NSE functions as a
glycolytic enzyme by converting 2-phospho-D-glycerate into
phosphoenolpyruvate and exhibits proliferative and protective
effects on cultured neuron cells (30). These facts imply that it is
reasonable for the morphologically stem-like cells in the
intestinal crypt base to express NSE in this study. It was notable
that CA9+ cells in crypt base expressed AXIN2, a
direct target gene of Wnt pathway (24), which indicates that they have
increased activity of Wnt pathway, a critical pathway for
intestinal epithelial stem cells (2). Based on these findings, it can be
supposed that the CA9+ cells in human adult normal
intestinal epithelia are associated with stemness, although CA9 has
been associated with hypoxia in embryonic and fetal intestinal
epithelia (14), and CA9 per se is
not imperative in the development or maintenance of intestinal
epithelia under normal condition (31).
In the process of carcinogenesis and progression,
cancer cells are required to overcome hypoxia and acidosis caused
by over-population and increased distance from blood vessels
(32). CA9 is induced by hypoxia
(33–35) and regulates pH of microenvironment
(36). In addition, CA9 has been
shown to support carcinogenesis itself, promote cell migration,
invasion angiogenesis and metastasis (37–41)
and to be associated with cancer stemness (42) and resistance to the therapies
(41–44). All these aspects are consistent
with our results yielded from clinical samples and data, where the
expression of CA9 is mostly associated with poor prognosis or
progressed stage (16–19,44–49).
In this study, we showed that the colorectal adenomas had mosaic
pattern of CA9 expression in basal region, similar to but more
aberrant than normal epithelia, and that T1 CRC had CA9 expression
in entire area of the tumor, contrary to the normal intestinal
epithelia which CA9+ ratio is as low as 10%. Thus, it
could be reasonable to suspect that the CA9+ cells are
associated with carcinogenesis.
This study have some limitations as follows. First,
we analyzed the CA9+ cells only in immunohistrochemistry
and flow cytometry. In addition, analyzed sample number is small
for reading strong evidence. Second, although the morphology of
CA9+ cells were same as the CBC cells which were
reported as the stem cells of mouse small intestine, there has not
been any evidence that the same event was also justified in human
samples. Third, although there was a gap of the ratio of
CA9+ cells among the anatomical location, we were unable
to explain its meaning. The gaps of the ratio of CA9+
cells between IHC and flow cytometry may be explained by the fact
that the sensitivity of the antibodies used for flow cytometry and
those for IHC was different (50)
and that the methodology of calculation of positivity was
two-dimensional in IHC and three-dimensional in flow cytometry.
Forth, although in flow cytometry, we could detect CA9 high cells
in mucosal epithelial cells, in immunohistochemistry, we could not
classify the epithelial cells according to CA9 staining intensity.
However, accoding to Fig. 1b,
epithelial cells on villi were weakly positive and CBC cells were
highly positive for CA9 suggesting that CA9+ cells would
be morphologically identical for CBC cells. Further studies would
be needed for understanding the CA9 fuctions. This study propose
the possibility that CA9 could be a new marker of human adult
intestinal epithelial stem cells and that it is associated with
carcinogenesis in CRC.
Acknowledgements
This study was supported by a Grant-in-Aid for
Cancer Research from the Ministry of Education, Science, Sports and
Culture Technology, Japan, to H.Y. (grant no. 21390360).
References
|
1
|
Cheng H and Leblond CP: Origin,
differentiation and renewal of the four main epithelial cell types
in the mouse small intestine. V Unitarian Theory of the origin of
the four epithelial cell types. Am J Anat. 141:537–561. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barker N, van Es JH, Kuipers J, Kujala P,
van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H,
Peters PJ, et al: Identification of stem cells in small intestine
and colon by marker gene Lgr5. Nature. 449:1003–1007. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Potten CS: Extreme sensitivity of some
intestinal crypt cells to X and gamma irradiation. Nature.
269:518–521. 1977. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sangiorgi E and Capecchi MR: Bmi1 is
expressed in vivo in intestinal stem cells. Nat Genet. 40:915–920.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu
MM and Epstein JA: Interconversion between intestinal stem cell
populations in distinct niches. Science. 334:1420–1424. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Montgomery RK, Carlone DL, Richmond CA,
Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs
DM, Fogli LK, Algra S, et al: Mouse telomerase reverse
transcriptase (mTert) expression marks slowly cycling intestinal
stem cells. Proc Natl Acad Sci USA. 108:179–184. 2011. View Article : Google Scholar :
|
|
7
|
Powell AE, Wang Y, Li Y, Poulin EJ, Means
AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE,
et al: The pan-ErbB negative regulator Lrig1 is an intestinal stem
cell marker that functions as a tumor suppressor. Cell.
149:146–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sato T, van Es JH, Snippert HJ, Stange DE,
Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M
and Clevers H: Paneth cells constitute the niche for Lgr5 stem
cells in intestinal crypts. Nature. 469:415–418. 2011. View Article : Google Scholar
|
|
9
|
Korinek V, Barker N, Moerer P, van
Donselaar E, Huls G, Peters PJ and Clevers H: Depletion of
epithelial stem-cell compartments in the small intestine of mice
lacking Tcf-4. Nat Genet. 19:379–383. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pellegrinet L, Rodilla V, Liu Z, Chen S,
Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J and Radtke F:
Dll1- and dll4-mediated notch signaling are required for
homeostasis of intestinal stem cells. Gastroenterology.
140:1230–1240. e1231–1237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato T, Vries RG, Snippert HJ, van de
Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters
PJ, et al: Single Lgr5 stem cells build crypt-villus structures in
vitro without a mesenchymal niche. Nature. 459:262–265. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haramis AP, Begthel H, van den Born M, van
Es J, Jonkheer S, Offerhaus GJ and Clevers H: De novo crypt
formation and juvenile polyposis on BMP inhibition in mouse
intestine. Science. 303:1684–1686. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ivanov S, Liao SY, Ivanova A,
Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ,
Proescholdt MA, Oldfield EH, Lee J, et al: Expression of
hypoxia-inducible cell-surface transmembrane carbonic anhydrases in
human cancer. Am J Pathol. 158:905–919. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao SY, Lerman MI and Stanbridge EJ:
Expression of trans-membrane carbonic anhydrases, CAIX and CAXII,
in human development. BMC Dev Biol. 9:222009. View Article : Google Scholar
|
|
15
|
Hussain SA, Ganesan R, Reynolds G, Gross
L, Stevens A, Pastorek J, Murray PG, Perunovic B, Anwar MS,
Billingham L, et al: Hypoxia-regulated carbonic anhydrase IX
expression is associated with poor survival in patients with
invasive breast cancer. Br J Cancer. 96:104–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ilie M, Mazure NM, Hofman V, Ammadi RE,
Ortholan C, Bonnetaud C, Havet K, Venissac N, Mograbi B, Mouroux J,
et al: High levels of carbonic anhydrase IX in tumour tissue and
plasma are biomarkers of poor prognostic in patients with non-small
cell lung cancer. Br J Cancer. 102:1627–1635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saarnio J, Parkkila S, Parkkila AK,
Haukipuro K, Pastoreková S, Pastorek J, Kairaluoma MI and Karttunen
TJ: Immunohistochemical study of colorectal tumors for expression
of a novel transmembrane carbonic anhydrase, MN/CA IX, with
potential value as a marker of cell proliferation. Am J Pathol.
153:279–285. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jubb AM, Turley H, Moeller HC, Steers G,
Han C, Li JL, Leek R, Tan EY, Singh B, Mortensen NJ, et al:
Expression of delta-like ligand 4 (Dll4) and markers of hypoxia in
colon cancer. Br J Cancer. 101:1749–1757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Korkeila E, Talvinen K, Jaakkola PM, Minn
H, Syrjänen K, Sundström J and Pyrhönen S: Expression of carbonic
anhydrase IX suggests poor outcome in rectal cancer. Br J Cancer.
100:874–880. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato T, Stange DE, Ferrante M, Vries RG,
Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J,
Siersema PD, et al: Long-term expansion of epithelial organoids
from human colon, adenoma, adenocarcinoma, and Barrett's
epithelium. Gastroenterology. 141:1762–1772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rothenberg ME, Nusse Y, Kalisky T, Lee JJ,
Dalerba P, Scheeren F, Lobo N, Kulkarni S, Sim S, Qian D, et al:
Identification of a cKit(+) colonic crypt base secretory cell that
supports Lgr5(+) stem cells in mice. Gastroenterology.
142:1195–1205 e1196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gracz AD, Fuller MK, Wang F, Li L,
Stelzner M, Dunn JC, Martin MG and Magness ST: Brief report: CD24
and CD44 mark human intestinal epithelial cell populations with
characteristics of active and facultative stem cells. Stem Cells.
31:2024–2030. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith PD, Smythies LE, Shen R,
Greenwell-Wild T, Gliozzi M and Wahl SM: Intestinal macrophages and
response to microbial encroachment. Mucosal Immunol. 4:31–42. 2011.
View Article : Google Scholar
|
|
24
|
Jho EH, Zhang T, Domon C, Joo CK, Freund
JN and Costantini F: Wnt/beta-catenin/Tcf signaling induces the
transcription of Axin2, a negative regulator of the signaling
pathway. Mol Cell Biol. 22:1172–1183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang K, Peng X, Zhang X, Wang Y, Zhang L,
Gao L, Weng T, Zhang H, Ramchandran R, Raj JU, et al: MicroRNA-124
suppresses the transactivation of nuclear factor of activated T
cells by targeting multiple genes and inhibits the proliferation of
pulmonary artery smooth muscle cells. J Biol Chem. 288:25414–25427.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barathova M, Takacova M, Holotnakova T,
Gibadulinova A, Ohradanova A, Zatovicova M, Hulikova A, Kopacek J,
Parkkila S, Supuran CT, et al: Alternative splicing variant of the
hypoxia marker carbonic anhydrase IX expressed independently of
hypoxia and tumour phenotype. Br J Cancer. 98:129–136. 2008.
View Article : Google Scholar
|
|
27
|
Radu I: Morphological aspects of endocrine
cells in human fetal gastrointestinal mucosa. Microscopical,
electronmicroscopical and immunohistochemical studies. Rom J
Morphol Embryol. 40:93–98. 1994.PubMed/NCBI
|
|
28
|
Rindi G, Leiter AB, Kopin AS, Bordi C and
Solcia E: The ‘normal’ endocrine cell of the gut: Changing concepts
and new evidences. Ann NY Acad Sci. 1014:1–12. 2004. View Article : Google Scholar
|
|
29
|
Buczacki SJ, Zecchini HI, Nicholson AM,
Russell R, Vermeulen L, Kemp R and Winton DJ: Intestinal
label-retaining cells are secretory precursors expressing Lgr5.
Nature. 495:65–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hattori T, Takei N, Mizuno Y, Kato K and
Kohsaka S: Neurotrophic and neuroprotective effects of
neuron-specific enolase on cultured neurons from embryonic rat
brain. Neurosci Res. 21:191–198. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leppilampi M, Karttunen TJ, Kivelä J, Gut
MO, Pastoreková S, Pastorek J and Parkkila S: Gastric pit cell
hyperplasia and glandular atrophy in carbonic anhydrase IX knockout
mice: Studies on two strains C57/BL6 and BALB/C. Transgenic Res.
14:655–663. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gatenby RA and Gillies RJ: A
microenvironmental model of carcinogenesis. Nat Rev Cancer.
8:56–61. 2008. View Article : Google Scholar
|
|
33
|
Wykoff CC, Beasley NJ, Watson PH, Turner
KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell
PH, et al: Hypoxia-inducible expression of tumor-associated
carbonic anhydrases. Cancer Res. 60:7075–7083. 2000.
|
|
34
|
Kaluz S, Kaluzová M, Chrastina A, Olive
PL, Pastoreková S, Pastorek J, Lerman MI and Stanbridge EJ: Lowered
oxygen tension induces expression of the hypoxia marker MN/carbonic
anhydrase IX in the absence of hypoxia-inducible factor 1 alpha
stabilization: A role for phosphatidylinositol 3′-kinase. Cancer
Res. 62:4469–4477. 2002.PubMed/NCBI
|
|
35
|
Kaluz S, Kaluzová M and Stanbridge EJ:
Expression of the hypoxia marker carbonic anhydrase IX is
critically dependent on SP1 activity. Identification of a novel
type of hypoxia-responsive enhancer. Cancer Res. 63:917–922.
2003.PubMed/NCBI
|
|
36
|
Swietach P, Hulikova A, Vaughan-Jones RD
and Harris AL: New insights into the physiological role of carbonic
anhydrase IX in tumour pH regulation. Oncogene. 29:6509–6521. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim BR, Shin HJ, Kim JY, Byun HJ, Lee JH,
Sung YK and Rho SB: Dickkopf-1 (DKK-1) interrupts FAK/PI3K/mTOR
pathway by interaction of carbonic anhydrase IX (CA9) in
tumorigenesis. Cell Signal. 24:1406–1413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rasheed S, Harris AL, Tekkis PP, Turley H,
Silver A, McDonald PJ, Talbot IC, Glynne-Jones R, Northover JM and
Guenther T: Assessment of microvessel density and carbonic
anhydrase-9 (CA-9) expression in rectal cancer. Pathol Res Pract.
205:1–9. 2009. View Article : Google Scholar
|
|
39
|
Svastová E, Zilka N, Zat'ovicová M,
Gibadulinová A, Ciampor F, Pastorek J and Pastoreková S: Carbonic
anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via
interaction with beta-catenin. Exp Cell Res. 290:332–345. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shin HJ, Rho SB, Jung DC, Han IO, Oh ES
and Kim JY: Carbonic anhydrase IX (CA9) modulates tumor-associated
cell migration and invasion. J Cell Sci. 124:1077–1087. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lou Y, McDonald PC, Oloumi A, Chia S,
Ostlund C, Ahmadi A, Kyle A, Auf dem Keller U, Leung S, Huntsman D,
et al: Targeting tumor hypoxia: Suppression of breast tumor growth
and metastasis by novel carbonic anhydrase IX inhibitors. Cancer
Res. 71:3364–3376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lock FE, McDonald PC, Lou Y, Serrano I,
Chafe SC, Ostlund C, Aparicio S, Winum JY, Supuran CT and Dedhar S:
Targeting carbonic anhydrase IX depletes breast cancer stem cells
within the hypoxic niche. Oncogene. 32:5210–5219. 2013. View Article : Google Scholar
|
|
43
|
Sansone P, Storci G, Tavolari S, Guarnieri
T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P,
Marcu KB, et al: IL-6 triggers malignant features in mammospheres
from human ductal breast carcinoma and normal mammary gland. J Clin
Invest. 117:3988–4002. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Proescholdt MA, Merrill MJ, Stoerr EM,
Lohmeier A, Pohl F and Brawanski A: Function of carbonic anhydrase
IX in glioblastoma multiforme. Neuro Oncol. 14:1357–1366. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chia SK, Wykoff CC, Watson PH, Han C, Leek
RD, Pastorek J, Gatter KC, Ratcliffe P and Harris AL: Prognostic
significance of a novel hypoxia-regulated marker, carbonic
anhydrase IX, in invasive breast carcinoma. J Clin Oncol.
19:3660–3668. 2001.PubMed/NCBI
|
|
46
|
Driessen A, Landuyt W, Pastorekova S,
Moons J, Goethals L, Haustermans K, Nafteux P, Penninckx F, Geboes
K, Lerut T, et al: Expression of carbonic anhydrase IX (CA IX), a
hypoxia-related protein, rather than vascular-endothelial growth
factor (VEGF), a pro-angiogenic factor, correlates with an
extremely poor prognosis in esophageal and gastric adenocarcinomas.
Ann Surg. 243:334–340. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bui MH, Seligson D, Han KR, Pantuck AJ,
Dorey FJ, Huang Y, Horvath S, Leibovich BC, Chopra S, Liao SY, et
al: Carbonic anhydrase IX is an independent predictor of survival
in advanced renal clear cell carcinoma: Implications for prognosis
and therapy. Clin Cancer Res. 9:802–811. 2003.PubMed/NCBI
|
|
48
|
Malentacchi F, Vinci S, Della Melina A,
Kuncova J, Villari D, Giannarini G, Nesi G, Selli C and Orlando C:
Splicing variants of carbonic anhydrase IX in bladder cancer and
urine sediments. Urol Oncol. 30:278–284. 2012. View Article : Google Scholar
|
|
49
|
Beasley NJ, Wykoff CC, Watson PH, Leek R,
Turley H, Gatter K, Pastorek J, Cox GJ, Ratcliffe P and Harris AL:
Carbonic anhydrase IX, an endogenous hypoxia marker, expression in
head and neck squamous cell carcinoma and its relationship to
hypoxia, necrosis, and microvessel density. Cancer Res.
61:5262–5267. 2001.PubMed/NCBI
|
|
50
|
Tokunaga T, Tomita A, Sugimoto K, Shimada
K, Iriyama C, Hirose T, Shirahata-Adachi M, Suzuki Y, Mizuno H,
Kiyoi H, et al: De novo diffuse large B-cell lymphoma with a CD20
immunohistochemistry-positive and flow cytometry-negative
phenotype: Molecular mechanisms and correlation with rituximab
sensitivity. Cancer Sci. 105:35–43. 2014. View Article : Google Scholar
|