Introduction
Hepatocellular carcinoma (HCC) is a significant
cause of death worldwide. It has an insidious onset, with a high
degree of malignancy and very poor prognosis. Palliative
chemotherapy is one of the most important treatments for locally
advanced and metastatic HCCs, which are unresectable. In recent
years, the third-generation platinum compounds have been proposed
as potential active for HCC (1,2). The
positive results from the randomized phase III clinical trial EACH
(3), further support the role of
systemic oxaliplatin (OXA)-based regimen in advanced HCC (4). However, clinical benefits induced by
OXA are limited (5), and HCC is
traditionally believed to be insensitive to chemotherapy (6). The mechanisms underlying low
chemotherapeutic response of HCC are poorly understood.
Gap junction (GJ), a protein channel connecting two
adjacent cells, is composed of special proteins called connexins
(Cxs). Distinct Cx subtype is named based on its molecular weight.
Six Cxs form a hemi-channel (also called connexon) docking with its
counterpart in the neighboring cell to form an integral GJ channel.
These channels mediate direct intercellular molecular signaling,
allowing intercellular exchange of signaling molecules such as
inorganic ions, second messengers and other regulatory substances.
The intercellular molecular exchange mediates various cellular
events, including metabolism, homeostasis, cell proliferation and
differentiation, and carcinogenesis (7,8).
Evidence demonstrates that cytotoxicity of multiple
drugs is amplified via bystander effect (BE), which is partially
mediated by cell-cell communication mediated by GJs (9). It is noteworthy that GJ increases the
cytotoxicity of platinum drugs. Jensen et al (10) found that cisplatin (CIS)-induced
apoptosis produced death signals, which are transmitted to
neighboring cells via GJs. CIS toxicity in bladder cancer cells was
enhanced after GJ was upregulated by Cx26 transfection (11), and also strengthened in breast
cancer cells by quinolines (GJ enhancer) in vivo or in
vitro (12). Supersensitivity
of spinal astrocytes to OXA was accompanied by the upregulation of
Cx43 and GJ (13). Association of
chemo-resistant phenotypes with GJs in a three-dimensional culture
model of soft sarcoma has also been documented recently (14).
According to the BE mechanism, the efficacy of
platinum drugs positively correlated with GJ level in tumor cells.
However, tumor cells (including hepatic) were often accompanied by
a decline or loss of GJ (15–17).
For instance, normal liver tissue is abundant in GJs, mainly
expressing Cx26, Cx32 and Cx43 (18,19).
In rat hepatic tumorigenesis induced by oncogenic drugs, GJ and its
composed Cx were downregulated (20). The expression of Cx32 and Cx43 in
HCC tissues was found to be lower than in normal hepatic tissues
(21). Aberrant expression and
localization of Cx32 was observed during HCC development and
progression (22). Based on these
findings, we inferred that when GJ function decreased or was
lacking in HCC, cytotoxic signal was not transmitted to adjacent
cells, leading to decreased BE, and low chemosensitivity of HCC
cells to cytotoxic drugs including OXA. Additionally, the Cx
isoform determines the function of GJ channel and also the type of
signal transduction (23,24). Therefore, our study systematically
investigated the functional status of Cx and GJs in HCC at both
histologic and cytologic levels, and further focused on the effect
of GJs on OXA cytotoxicity in vitro. The effective GJ
components participated in the process were also explored. The
study may offer new insight into the mechanisms of low response of
HCC to chemotherapeutic drugs, and also provide novel potential
approaches and strategies to enhance OXA chemosensitivity in liver
cancer.
Materials and methods
Materials
SP kits were obtained from Fuzhou Maixin Biotech.
Co., Ltd (Fujian, China). OXA, 18-α-glycyrrhetinic acid (18-α-GA),
all-trans retinoid acid (ATRA), dimethyl sulfoxide (DMSO),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
anti-Cx32 and anti-Cx43 mouse IgG were purchased from Sigma (St.
Louis, MO, USA). DMEM, fetal bovine serum, TRIzol, Lipofectamine™
2000, DAPI, DiI-CM, calcein-AM, and anti-Cx26 mouse IgG were
procured from Invitrogen (Carlsbad, CA, USA). HPR and FITC-labeled
goat anti-mouse secondary antibody were obtained from Amersham
Biosciences Corp. (Piscataway, NJ, USA). All other reagents were
from Sigma unless stated otherwise.
Cell line and cell culture
Human normal liver cell line LO2 was obtained from
KeyGEN BioTECH (Nanjing, China). Human HCC cell line SMMC-7721 was
provided by Chinese Type Culture Collection (Shanghai, China). Both
cell lines were grown at 37°C in a humidified atmosphere containing
5% (v/v) CO2 in DMEM supplemented with 10% fetal bovine
serum, 100 U/ml streptomycin and 100 mg/ml penicillin. Cells in the
exponential phase of growth were selected for experiment.
Immunohistochemistry
Samples
Without any preoperative treatment, 76 archived HCC
paraffin samples were collected from patients following resection
at the First Affiliated Hospital of Bengbu Medical College from
January 2008 to December 2013. Twenty normal liver tissues obtained
via hepatic resection following hepatic trauma or accidental death
(healthy prior to death) served as controls. For the HCC samples,
61 were male and 15 were female, with a median age of 50 years old
(range: 22–76 years). The diagnosis was confirmed histologically in
all cases. Histology grade was based on the criteria proposed by
Edmondson and Steiner. Histological grade I–II accounted for 63%
(48 samples), and grade III–IV accounted for 37% (28 samples). The
nontumorous liver adjacent to tumor showed cirrhosis or chronic
hepatitis in 60 (79%) samples. Staging at the time of diagnosis was
based on the tumor-node-metastasis (TNM) classification. Tumors of
stage I–II accounted for 71% (54 samples), and tumors of stage
III–IV accounted for 29% (22 samples). Tumor size and lymph node
status were evaluated separately.
Immunohistochemical staining
Detail experimental procedures were described in our
previous studies (25,26). Specific procedures were strictly
performed according to the manufacturer's instructions. PBS instead
of primary antibody was used as the negative control. Positive
staining and cellular localization were observed and photographed
with an optical microscope.
Immunohistochemical evaluation
Cx staining was detected on the membrane or in the
cytoplasm. Yellow particles in cells were considered positive. We
calculated 500 tumor or normal liver cells for every section under
high magnification. The distribution of Cx-positive spots was
considered as ‘internalization’ if the spots were removed (or
partially removed) from the cell surface and detected in the
cytoplasm, and the internalization rate was calculated as follows:
(The number of Cx internalization cases/the total number of Cx
positive expression cases) ×100%.
RNA isolation and reverse
transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent
according to the manufacturer's instructions. cDNA was synthesized
by reverse transcription with a random primer. The specific Cx
upstream and downstream primers were added to the reverse
transcription cocktail to perform PCR. The following primers were
used: for human Cx26, 5′-GCT GCAAGAACGTGTGCTAC-3′ (upstream),
5′-TGGGTTTTGATCTCCTCGAT-3′ (downstream), product size 196 bp; for
human Cx32, 5′-TCCCTGCAGCTCATCCTAGT-3′ (upstream),
5′-CCCTGAGATGTGGACCTTGT-3′ (downstream), product size 156 bp; for
human Cx43, 5′-GGTCTGAGTGCCTGAACTTGCCT-3′ (upstream),
5′-AGCCACACCTTCCCTCCAGCA-3′ (downstream), product size 184 bp.
Human β-actin was used as an internal reference, with the primer
sequence 5′-TCCTCCTGAGCGCAAGTACTC-3′ (upstream), and
5′-GCATTTGCGGTGGACGAT-3′ (downstream), and product size 130 bp. The
reaction volume of PCR was 20 μl. PCR was initiated at 94°C for 3
min followed by 30 cycles consisting of 45 sec at 94°C, 45 sec at
55°C, and 45 sec at 72°C, with the final cycle extended to 10 min
at 72°C, followed by termination at 4°C. PCR amplicons (5 μl) were
analyzed on an ethidium bromide-stained 1.5% agarose gel.
Western blotting
LO2 and SMMC-7721 cells were harvested, centrifuged
(12000 × g/min at 4°C for 30 min), and supernatants were obtained.
Protein concentration was determined using a DC protein assay kit
(Bio-Rad Co., Hercules, CA, USA). Samples (50 μg) from cells were
transferred to SDS-PAGE, followed by electrophoresis and blotting.
Monoclonal antibodies against Cxs (Cx26-1:1000; Cx32-1:2000;
Cx43-1:4000) or β-actin (1:10,000) were used. The immunoreactive
bands were visualized using Amersham ECL™ Plus Western Blotting
Detection kit (GE Healthcare, Piscataway, NJ, USA).
Immunofluorescence
Cells in exponential growth phase were collected,
and fixed with 0.1% Triton X-100-4% paraformaldehyde for 30 min at
room temperature. Coverslips were blocked with 2% BSA in PBS and
probed with the primary antibodies (Cx26-1:200; Cx32-1:100;
Cx43-1:200; diluted in 2% BSA) overnight at 4°C. A secondary
antibody FITC anti-mouse IgG (1:200, diluted in 2% BSA) was added,
and incubated for 2 h in the dark, at room temperature. Nuclear
staining was performed with DAPI at 37°C for 5 min. After rinsing,
the coverslips were mounted on slides, and the cells were observed
and photographed under a fluorescence microscope (Olympus).
Determination of GJ function:
‘Parachute’ dye-coupling assay
‘Parachute’ dye-coupling protocols were described in
our previous studies (27,28). Briefly, LO2 and SMMC-7721 cells
were grown to 80%–85% confluence. Donor cells from one well were
incubated with a freshly made solution of 5 μM calcein-AM and 2.5
μM CM-DiI in growth medium at 37°C for 30 min. Calcein-AM was
converted intracellularly into the small GJ-permeable dye calcein,
while CM-DiI was too large to spread to coupled cells through GJs.
Unincorporated dye was removed by three gentle washes with PBS. The
donor cells were then trypsinized and seeded onto the receiver
cells at a density of 500–800 cells/ml, and cultured at 37°C for 4
h. The small molecule calcein (green fluorescence) entered adjacent
recipient cells through GJs when stable GJs were formed. The index
of GJ function was defined by counting the number of recipient
cells containing calcein around one donor cell under fluorescence
microscope.
MTT assay
OXA toxicity was assessed by MTT assay. SMMC-7721
cells were seeded at a density of 8×103/well into
96-well plates for 24 h. OXA of different concentrations were then
added for the indicated time periods. MTT (5 mg/ml) was added to
each well, and dishes were cultured at 37°C for 4 h. The medium
containing MTT was then removed, and 150 μl DMSO was added into
each well to fully dissolve formazan crystals in the viable cells.
The absorbance at 490 nm of each well was read using a microplate
ELISA reader (MRX II; Dynex Technologies, Chantilly, VA, USA). Cell
viability was calculated as follows: (OD of experimental group - OD
of blank group) / (OD of control group - OD of blank group).
Modulation of GJ function
SMMC-7721 cells were seeded at high
(8×103/well) and low (0.5×103/well) density
into 96-well plates to obtain GJ-formed and non-GJ formed cells,
corresponding to conditions in which junctional channel formation
was permitted or not, respectively (10). After cells at high density were
grown to 80–85% confluence, the cells under both culture conditions
were then exposed to a defined dose of OXA for 24 h, to enable the
effect of GJ presence on OXA toxicity to be observed. The changes
in OXA cytotoxicity were also investigated in high density-cultured
cells pretreated with GJ tool drugs, ATRA (10 μM) and 18-α-GA (5
μM), for 24 h and 1 h, respectively.
Specific regulation of Cx expression
by RNA interference and Cx26 overexpression
The siRNA fragments of different Cxs were
synthesized and provided by Shanghai GenePharma Co., Ltd.
(Shanghai, China). Three specific interfering sequences for each Cx
gene were synthesized as listed in Table I. Cx26 was overexpressed in
SMMC-7721 cells using a pEX-2/hCx26 vector (Shanghai GenePharma
Co.). Negative control siRNA (NC in the figures) and empty vector
negative control were also maintained. Transfection into SMMC-7721
cells was carried out using Lipofectamine 2000 according to the
manufacturer's instructions. Knockdown and upregulation of Cx
expression were confirmed by western blotting.
 | Table IsiRNA sequences for human Cx26, Cx32
and Cx43. |
Table I
siRNA sequences for human Cx26, Cx32
and Cx43.
| | Sequence |
|---|
| |
|
|---|
| Genbank ID | Gene | Sense (5′-3′) | Antisense
(5′-3′) |
|---|
| NM_004004.5 | Human Cx26 |
GAGGCUCAGAUUGUAAUAUTT |
AUAUUACAAUCUGAGCCUCTT |
| |
CCACGUUAAAGGUGAACAUTT |
AUGUUCACCUUUAACGUGGTT |
| |
CCCAGUUGUUAGAUUAAGATT |
UCUUAAUCUAACAACUGGGTT |
| NM_001097642.2 | Human Cx32 |
GCUCCCUGAAAGACAUACUTT |
AGUAUGUCUUUCAGGGAGCTT |
| |
GCCGUCUUCAUGUAUGUCUTT |
AGACAUACAUGAAGACGGCTT |
| |
GCAACACAUAGAGAAGAAATT |
UUUCUUCUCUAUGUGUUGCTT |
| NM_000165.4 | Human Cx43 |
GGCCUUGAAUAUCAUUGAATT |
UUCAAUGAUAUUCAAGGCCTT |
| |
GCCGCAAUUACAACAAGCATT |
UGCUUGUUGUAAUUGCGGCTT |
| |
GGAAGCACCAUCUCUAACUTT |
AGUUAGAGAUGGUGCUUCCTT |
Statistical analysis
Results were analyzed with SPSS version 17.0
software (Chicago, IL, USA). Differences between two groups in
immunohistochemistry experiments were evaluated by χ2
test and Fisher's exact test where appropriate. Numerical data are
presented as means ± SEM and compared with unpaired Student's t
test using Sigmaplot 10.0 software (Jandel Scientific, San Rafael,
CA, USA). Differences at p<0.05 were considered significant.
Results
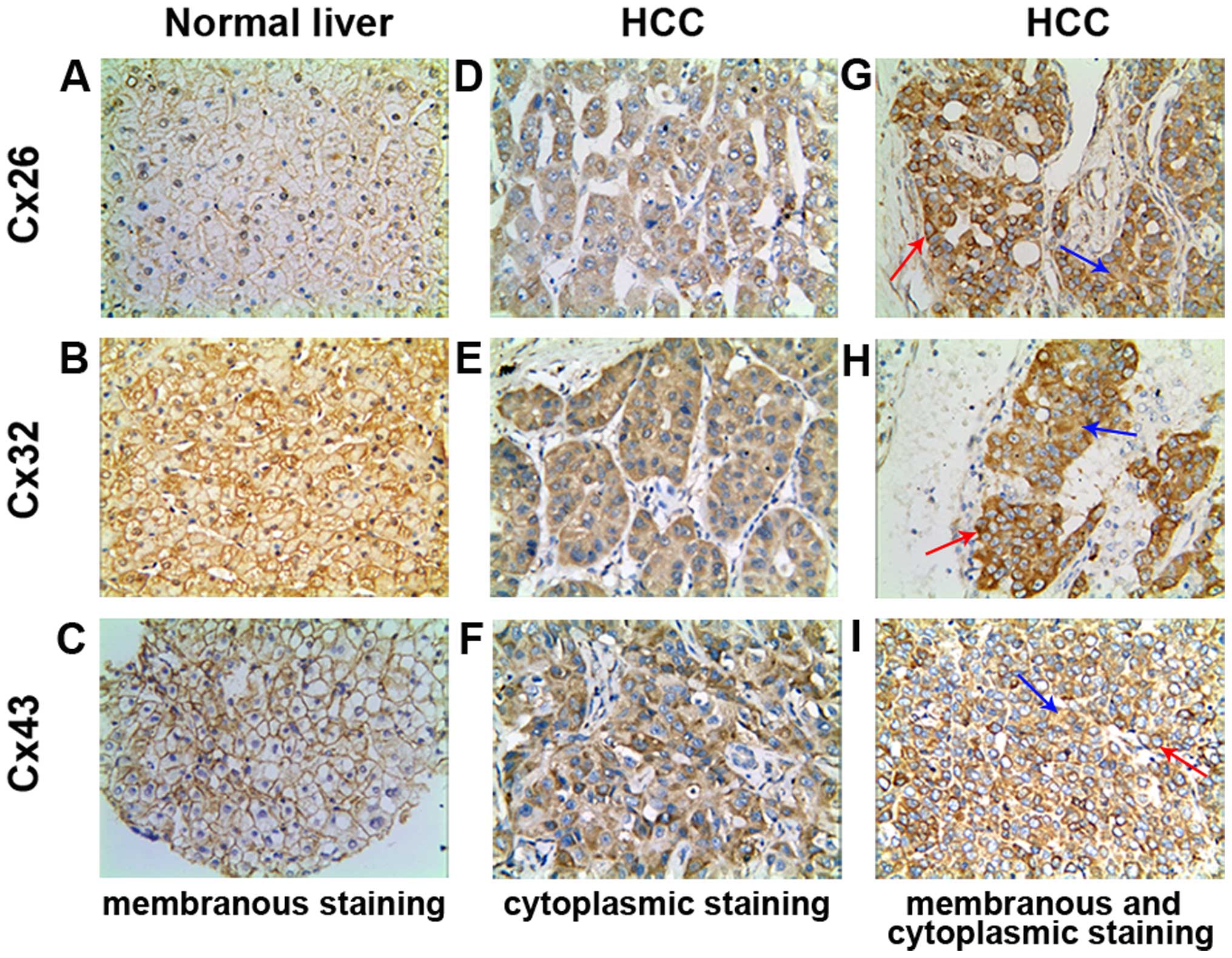
Cx expression in human normal and HCC
liver tissues
As shown in Table
II, Cx26 and Cx32 was 100% and Cx43 showed 90.00% positive
expression in normal liver tissues. However, the positive rate for
the three Cxs was significantly reduced to 47.37, 43.42 and 60.53%
in HCC tissues, respectively (χ2=18.045, p=0.000;
χ2=20.497, p=0.000; χ2=6.189, p=0.015). In Cx
localization, while the positive particles were distributed mainly
linearly on membrane in normal liver tissues (Fig. 1A–C), in HCC tissues Cxs stained
positive mostly in the cytoplasm (Fig.
1D–F), and occasionally on the membrane of cancer cells,
concurrently in certain cases (Fig.
1G–I). The phenomenon of Cx removal from membrane to cytoplasm
is known as ‘internalization’. The internalization rates of the
three Cx proteins in HCC group were 100% and statistically
significant compared with the normal liver tissue group
(χ2=43.938, p=0.000; χ2=31.392, p=0.000;
χ2=41.358, p=0.000, Table
III).
 | Table IIExpression of Cx26, Cx32 and Cx43 in
human normal liver and HCC tissues. |
Table II
Expression of Cx26, Cx32 and Cx43 in
human normal liver and HCC tissues.
| | Cx26 | Cx32 | Cx43 |
|---|
| |
|
|
|
|---|
| Group | n | − | + | − | + | − | + |
|---|
| Normal liver | 20 | 0 | 20 | 0 | 20 | 2 | 18 |
| HCC | 76 | 40 | 36 | 43 | 33 | 30 | 46 |
|
χ2-value | | 18.045 | 20.497 | 6.189 |
| p-value | | 0.000 | 0.000 | 0.015 |
 | Table IIIInternalization of Cx expression in
human normal liver and HCC tissues. |
Table III
Internalization of Cx expression in
human normal liver and HCC tissues.
| Cytoplasmic
Cx26 | Cytoplasmic
Cx32 | Cytoplasmic
Cx43 |
|---|
|
|
|
|
|---|
| Group | n | − | + | n | − | + | n | − | + |
|---|
| Normal liver | 20 | 17 | 3 | 20 | 14 | 6 | 18 | 14 | 4 |
| HCC | 36 | 0 | 36 | 33 | 0 | 33 | 46 | 0 | 46 |
|
χ2-value | 43.938 | 31.392 | 41.358 |
| p-value | 0.000 | 0.000 | 0.000 |
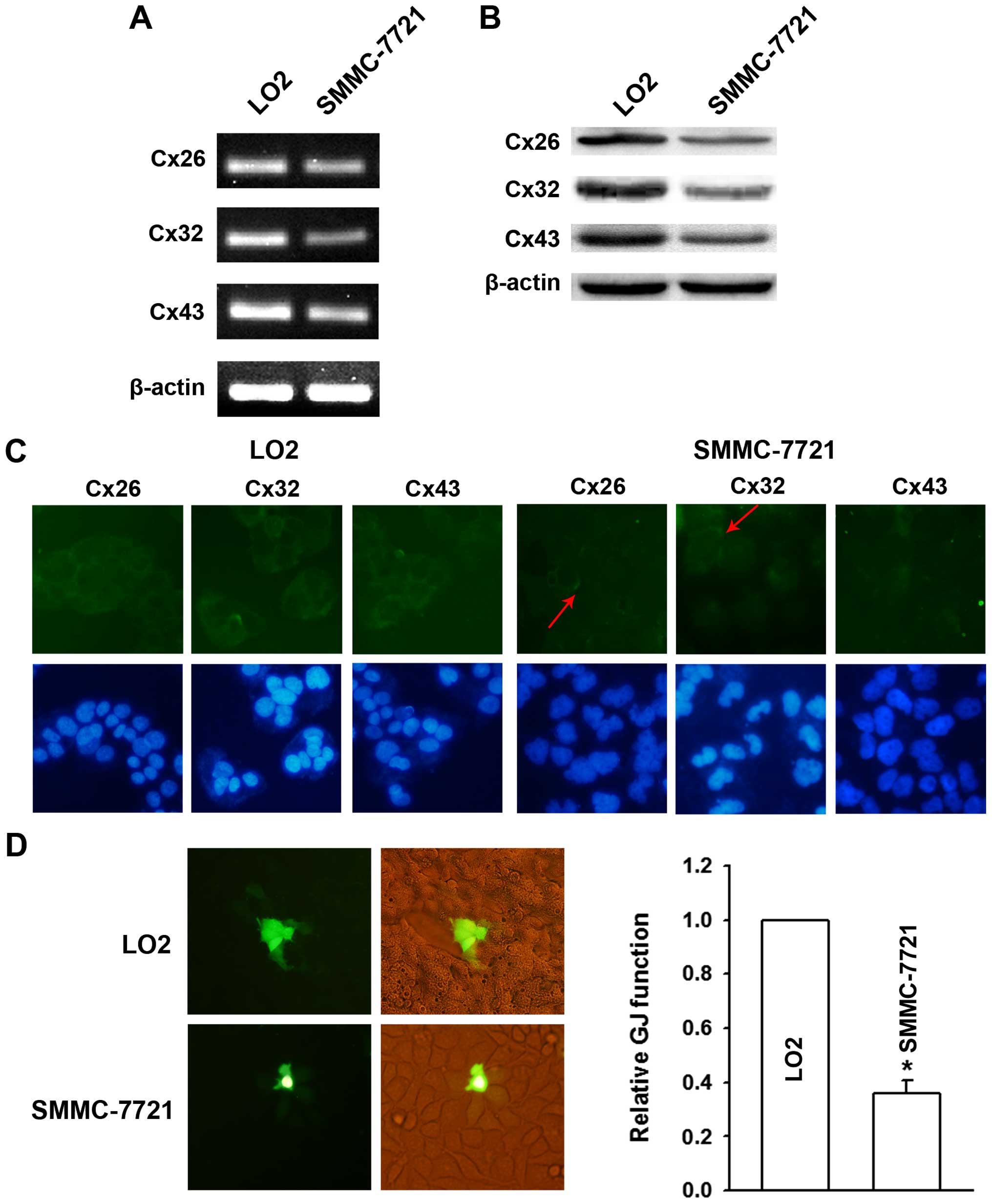
Cx expression and GJ function in human
normal liver LO2 and hepatoma SMMC-7721 cells
The reduced levels and cytoplasmic localization of
Cx protein suggested a decreased GJ function in HCC carcinogenesis.
To further validate the histologic results of the Asian population,
two Asian-derived human cell lines including the normal liver cell
line LO2 and HCC cell line SMMC-7721 were used in vitro. The
RT-PCR and western blot results revealed a positive expression of
Cx26, Cx32 and Cx43 at both mRNA and protein levels. Compared with
LO2 cells, the expression of Cxs was markedly reduced in SMMC-7721
cells (Fig. 2A and B).
Immunofluorescence analysis revealed a clear membranous
localization of the three Cxs in LO2 cells. While in SMMC-7721
cells, Cx26 and Cx32 were detected mainly in the cytoplasm and only
a partial expression on membrane; and for Cx43 only cytoplasmic
staining was observed (Fig. 2C).
Thus cytologic and histologic results were highly consistent.
Subsequent ‘parachute’ dye-coupling assay in vitro revealed
that GJ was abundant in LO2 cells, and was attenuated to ~36% in
SMMC-7721 cells (Fig. 2D).
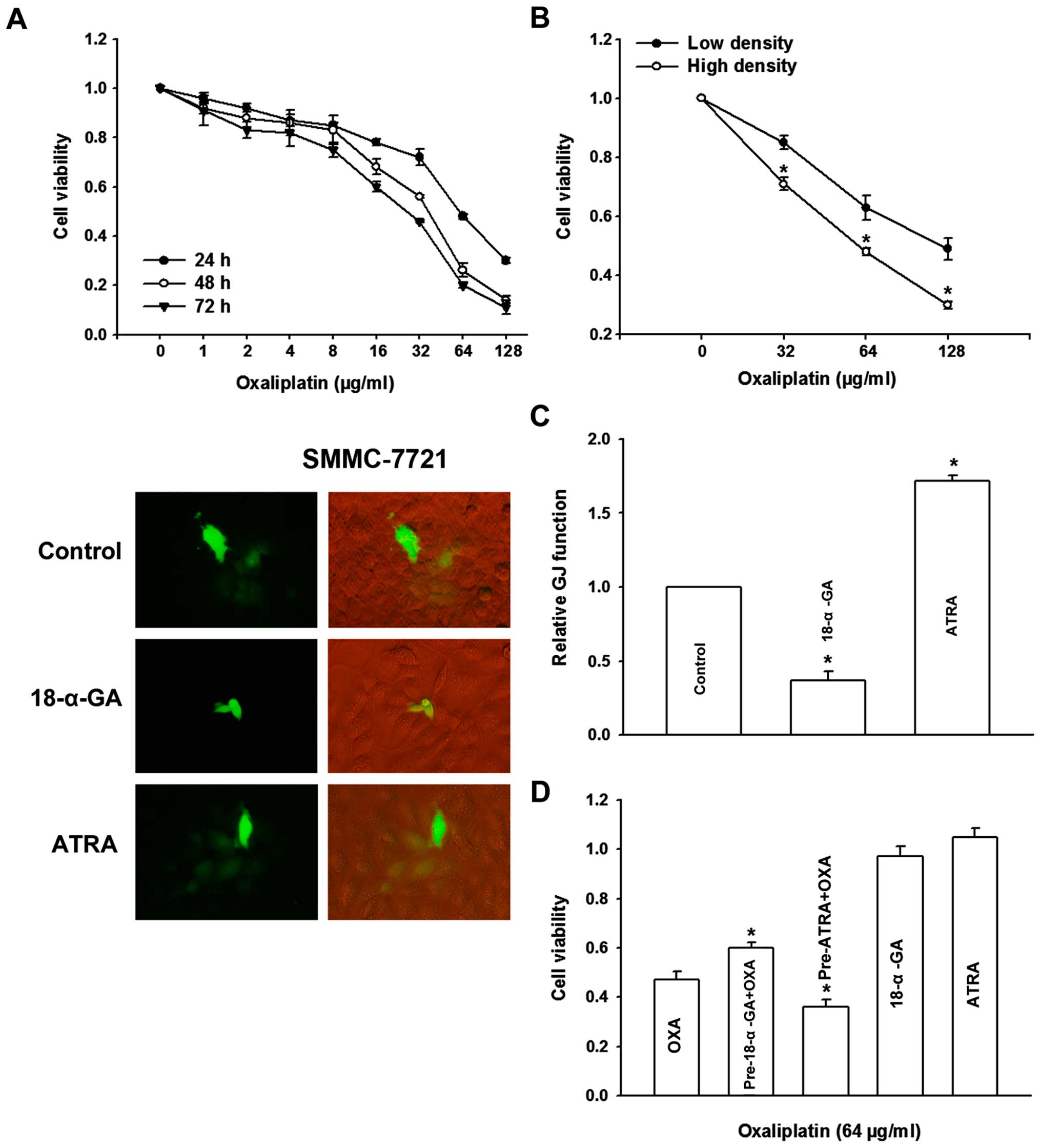
GJ modulates OXA cytotoxicity in
SMMC-7721 cells
As shown in Fig.
3A, results of MTT indicated that cell viability of SMMC-7721
cells decreased with increase in OXA concentration and treatment
time. The effects of 32, 64 and 128 μg/ml dose groups were
significant, and the inhibition rates for 24 h were 28, 52 and 70%,
respectively. In the experiments involving high-density and
low-density cell seeding, we found that OXA cytotoxicity of
high-density cultures was substantially greater than that of
low-density cultures (p<0.05, Fig.
3B). The formation of GJs is just one of the several potential
differences between low and high density cultures, and therefore,
GJ coupling was further manipulated in the cultures by chemical
drugs.
18-α-GA, recognized as a GJ channel inhibitor
(29,30), and ATRA as an enhancer of
reinforced Cx expression in various tumor cells (31,32),
have been widely used in the GJ analysis. We then observed the
alteration of GJ function in SMMC-7721 cells by these two drugs. As
shown in Fig. 3C, 18-α-GA (5 μM)
treatment for 1 h significantly inhibited GJ function by about 63%;
conversely, ATRA (10 μM) treatment for 24 h markedly strengthened
GJ function by about 72%. Subsequent studies revealed that 18-α-GA
(5 μM) and ATRA (10 μM) alone had no obvious effect on SMMC-7721
cell growth. However, upon pretreatment with 18-α-GA for 1 h to
inhibit GJ function, OXA toxicity was reduced. On the contrary,
upon pretreatment with ATRA for 24 h to increase GJ function, OXA
toxicity was increased (p<0.05, Fig. 3D). These results suggested a
potential role of GJ in modulating OXA cytotoxicity in SMMC-7721
cells.
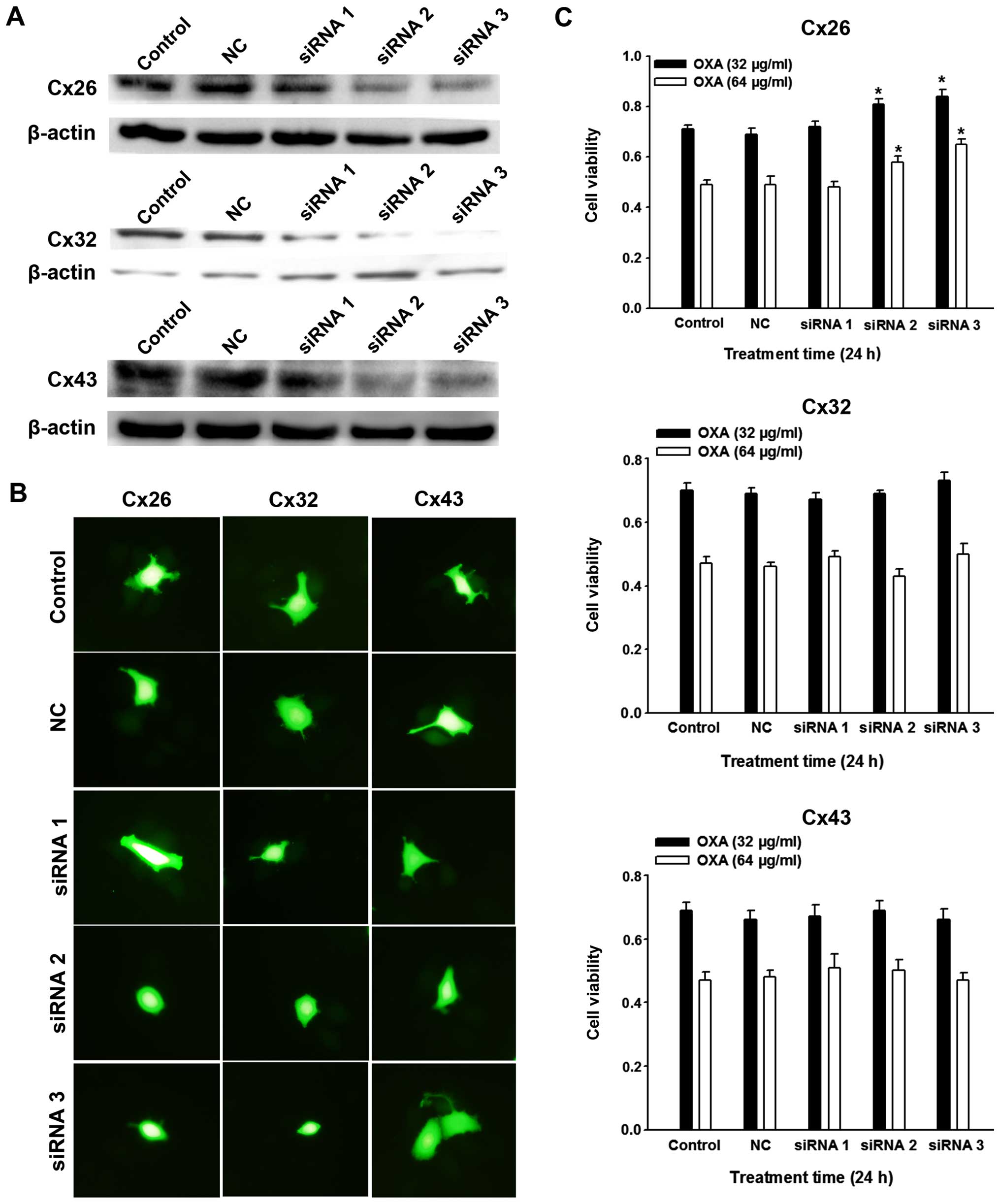
Effect of specific regulation of
SMMC-7721 Cx expression on OXA cytotoxicity
To verify that the effects of cell density and the
drugs on OXA cytotoxicity were due to GJ-mediated cell-cell
communication, RNA interference of the dominant Cxs was conducted
in SMMC-7721 cells. After inhibition of Cx26, Cx32 and Cx43
expression was confirmed by western blot analysis (Fig. 4A), ‘parachute’ dye transfer assay
demonstrated that Cx26 and Cx32 downregulation led to decreased
GJs, but not Cx43 (Fig. 4B). These
observations are important as they indicate the effective component
of GJs in SMMC-7721 cells. Further studies showed that only the
knockdown of Cx26, but not Cx32 or Cx43 expression depressed OXA
toxicity (Fig. 4C). We further
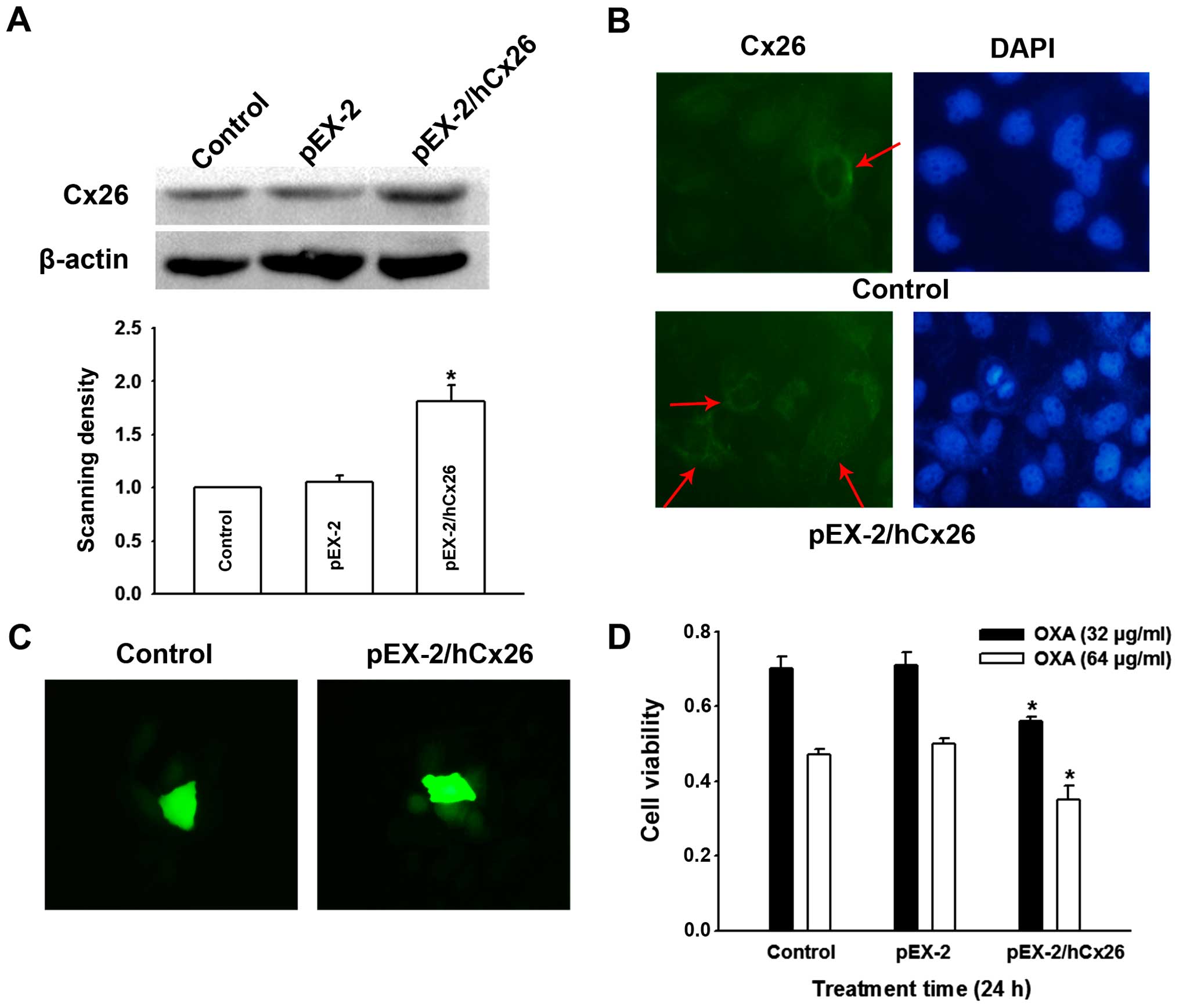
explored the effect of overexpression of Cx26 by transfection of
pEX-2/hCx26. Western blot confirmed that the expression of Cx26 was
markedly enhanced relative to its untreated counterparts and pEX-2
negative controls (Fig. 5A).
Accompanied by Cx26 upregulation, more Cx26 particles were located
on SMMC-7721 cell membrane (Fig.
5B), and ‘parachute’ dye-coupling assay confirmed an increased
GJ function (Fig. 5C). Expectedly,
SMMC-7721 OXA cytotoxicity was finally strengthened by the
upregulation of Cx26 expression (Fig.
5D). These results together demonstrate that the Cx26 protein
component was specifically responsible for the role of SMMC-7721
GJs in OXA toxicity.
Discussion
Studies have demonstrated that injury or death
signals induced by cytotoxic drugs such as platinum-based agents
have the potential to be transferred between adjacent tumor cells
via GJs, leading to the cytotoxicity amplified (10–12).
Therefore, the final anti-tumor effect of chemotherapeutic drugs
depends largely on the tumor GJ levels. However, Cx and its
composed GJs are frequently reduced or absent in cancer cells
compared with the original normal tissue (33,34).
GJ abnormalities compromise the regulatory framework of the
organism to certain initial transformed cells, resulting in
malignant tumors followed by uncontrolled excessive proliferation.
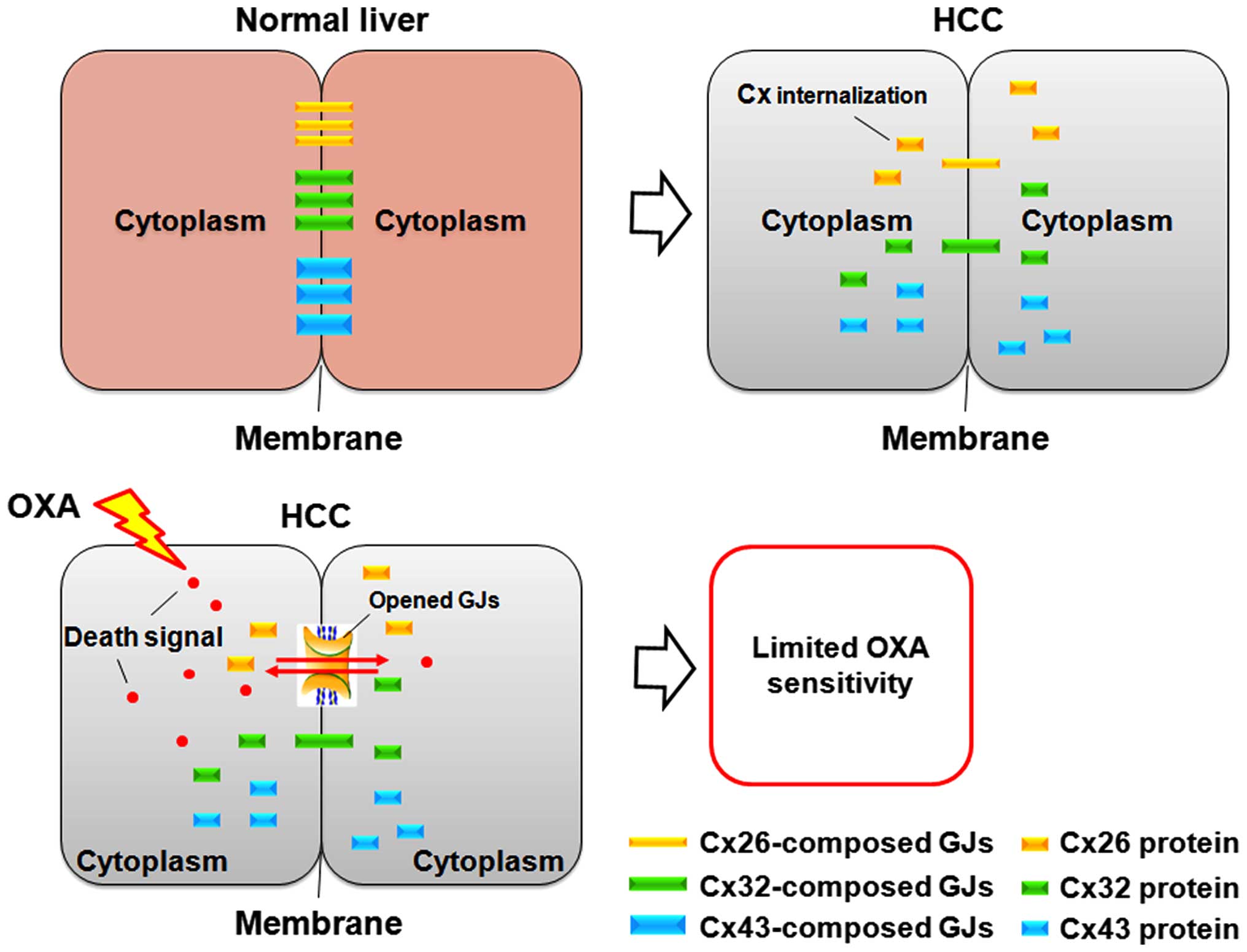
Based on these findings, it is plausible that GJ decline limits the
transmission of toxic signal induced by chemotherapeutic drugs
between adjacent cells, resulting in restricted drug sensitivity or
even drug resistance in its targeted cells (Fig. 6). It may be a mechanism underlying
the poor chemotherapeutic response in tumors such as HCC. To verify
the hypothesis, we first investigated the differences in Cx
expression between HCC and normal liver tissues in histological
specimens using a relatively large number of samples. Human normal
liver cell line and HCC cell line were then used in vitro to
confirm the Cx expression profile and its GJ status, and the role
of GJs in mediating OXA cytotoxicity was also explored.
The present study showed that expressions of Cx26,
Cx32 and Cx43 decreased significantly in human HCC tissues compared
with normal liver tissues, which was in accordance with the results
of liver cancer animal model (20). Further, we noted marked
localization changes for the three Cxs: in normal liver tissues
they were located mainly on the intercellular membranes of
hepatocytes. In HCC tissues, they were located in the cytoplasm
mainly, due to ‘internalization’, which was discovered in a prior
study focused on the role of Cx32 in HCC development (22). Considering the dominant liver Cx
proteins exhibit a similar pattern, Cx internalization may be a
significant marker of hepatocarcinogenesis. GJs channels are formed
by the end-to-end docking of two hemichannels in adjacent cells,
and functional GJs occur only when Cxs are located on the membrane.
Thus, an internalization of Cx proteins may lead to depressed or
deficient GJs. Note is that Cx membranous localization is still
retained in a few HCC cases, suggesting a theoretical possibility
of GJ formation. We then indeed confirmed in subsequent in
vitro experiments that functional GJs were reduced in human HCC
cell line SMMC-7721 compared with the normal liver cell line LO2,
due to reduced Cx expression and aberrant localization of the
dominant Cx proteins. From the localization of Cx by
immunofluorescence assay, we inferred Cx43 was not the main
effective component of SMMC-7721 GJs.
Human liver cancer tissues or cells retain partial
GJs enabling observation of the influence of GJ regulation on OXA
cytotoxicity. GJ is the direct channel communicating with the
adjacent cytoplasm, suggesting that the growth and confluence were
proportional to GJ formation in cells with Cx expression. We found
that OXA cytotoxicity in high density cultures (with GJ formation)
was substantially greater than that of low density cultures
(without GJ formation), showing that cell density affected OXA
cytotoxicity. In addition to promoting GJ formation, the intimate
contact between cells occurred via other mechanisms such as
increased adherent proteins or vascular endothelial growth factor
(VEGF) affecting cell growth and drug action (35,36).
Subsequent experiments of pharmacologic modulation of GJs by
chemical agents elucidated an additional positive relationship
between GJs and OXA toxicity. To more directly assess the role of
GJs in cell density-dependent OXA sensitivity, and also determine
its effective component, we explored the effects of specific
alteration of SMMC-7721 Cx expression using knockdown of Cxs
expression with RNA interference and Cx overexpression by gene
transfection. Data showed that knockdown of Cx26 expression
depressed OXA toxicity. Conversely, upregulation of Cx26 expression
enhanced OXA toxicity. The differences of OXA cytotoxicity mediated
by Cx and cell density were similar, suggesting that OXA
cytotoxicity was mediated by Cx26-composed GJs.
The role of GJs composed of different Cx subtypes in
regulating OXA cytotoxicity was distinct. We found that
specifically the inhibition of Cx26, not Cx32 or Cx43 expression,
decreased OXA cytotoxicity. Additionally, upregulation of Cx26
expression enhanced GJ formation and ultimately OXA toxicity. It is
not surprising that Cx43 expression knockdown did not affect the
anti-tumor effect of OXA, as it may not be the main Cx isoform for
GJs in SMMC-7721 cells. Its deficiency in modulating OXA toxicity,
together with the results from initial cytologic study, strongly
suggested a non-GJ-dependent Cx43 function in SMMC-7721 cells. Cx26
and Cx32 both have the potential to form liver GJs based on
histologic and cytologic results, however, Cx32 did not exert any
effect on OXA toxicity. The reason may be that the GJ channels,
composed of different Cxs, mediate varying signal transduction
(23,24). For example, Cx expression is cell-
and tissue-specific, with multiple Cxs associated with unique
functions (7). The transfection of
Cx26 gene into HepG2 hepatoma cells localized to the membrane,
inducing the recovery of GJ function, and reduced the malignant
phenotype. However, Cx32 did not exhibit this function (37). The result was similar to our
present study, confirming the idea that different Cx proteins
perform different physiological and pathological functions
(38). Similarly, the Cx component
determines the selectivity of GJ channels for signaling ligands,
and may thus result in the differential permeability to death or
injury signal molecules induced by OXA.
In the present study, the toxic signals induced by
OXA were propagated by Cx26 channels more easily or were
specifically permeable to this channel, which may explain the
discrepancy of OXA toxicity following siRNA knockdown of distinct
Cx in targeted SMMC-7721 cells (Fig.
6). We believe that these signals include the toxic OXA itself
and its metabolites, or the molecules mediating the cellular death
pathway. OXA and its cytoplasmic species have a molecular mass of
about 400 Da, lower than the upper limit of GJ permeability.
Alternatively, following OXA treatment of SMMC-7721 cells for 24 h,
the induction and transfer of relevant proteins such as cyclins,
apoptosis-related proteins and DNA injury-linked proteins cannot be
ruled out (39,40). However, further studies are needed
to explore the explicit signal molecules mediating the process.
In conclusion, histologic and cytologic data
consistently demonstrate reduced expression and aberrant
localization of the three dominant Cx proteins including Cx26, Cx32
and Cx43, abrogating the function of GJs in HCC tissues and cells
significantly. Downregulated GJs comprising Cx26 specifically, but
not Cx32 or Cx43 limited OXA cytotoxicity. Targeting Cx26 and even
transiently increasing its expression, or reversing its
internalization may represent an effective therapeutic strategy in
liver cancer treatment.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81402514), the grant from the
Natural Science Foundation of Anhui Province (no. 1408085QH166),
the Natural Science Research key Project of Education Office of
Anhui Province (no. KJ2014A152), and internal grant from Bengbu
Medical College (no. Bykf13A12).
Abbreviations:
|
18-α-GA
|
18-α-glycyrrhetinic acid
|
|
ATRA
|
all-trans retinoid acid
|
|
CIS
|
cisplatin
|
|
Cx
|
connexin
|
|
GJ
|
gap junction
|
|
HCC
|
hepatocellular carcinoma
|
|
OXA
|
oxaliplatin
|
References
|
1
|
Wu Q, Qin SK, Teng FM, Chen CJ and Wang R:
Lobaplatin arrests cell cycle progression in human hepatocellular
carcinoma cells. J Hematol Oncol. 3:432010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zaanan A, Williet N, Hebbar M, Dabakuyo
TS, Fartoux L, Mansourbakht T, Dubreuil O, Rosmorduc O, Cattan S,
Bonnetain F, et al: Gemcitabine plus oxaliplatin in advanced
hepatocellular carcinoma: A large multicenter AGEO study. J
Hepatol. 58:81–88. 2013. View Article : Google Scholar
|
|
3
|
Qin S, Bai Y, Lim HY, Thongprasert S, Chao
Y, Fan J, Yang TS, Bhudhisawasdi V, Kang WK, Zhou Y, et al:
Randomized, multicenter, open-label study of oxaliplatin plus
fluorouracil/leucovorin versus doxorubicin as palliative
chemotherapy in patients with advanced hepatocellular carcinoma
from Asia. J Clin Oncol. 31:3501–3508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrelli F, Coinu A, Borgonovo K, Cabiddu
M, Ghilardi M, Lonati V and Barni S: Oxaliplatin-based
chemotherapy: A new option in advanced hepatocellular carcinoma. a
systematic review and pooled analysis. Clin Oncol (R Coll Radiol).
26:488–496. 2014. View Article : Google Scholar
|
|
5
|
Abdel-Rahman O: Revisiting
oxaliplatin-based regimens for advanced hepatocellular carcinoma.
Curr Oncol Rep. 16:3942014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miao J, Chen GG, Chun SY, Chak EC and Lai
PB: Bid sensitizes apoptosis induced by chemotherapeutic drugs in
hepatocellular carcinoma. Int J Oncol. 25:651–659. 2004.PubMed/NCBI
|
|
7
|
Vinken M, Vanhaecke T, Papeleu P, Snykers
S, Henkens T and Rogiers V: Connexins and their channels in cell
growth and cell death. Cell Signal. 18:592–600. 2006. View Article : Google Scholar
|
|
8
|
Brockmeyer P, Jung K, Perske C,
Schliephake H and Hemmerlein B: Membrane connexin 43 acts as an
independent prognostic marker in oral squamous cell carcinoma. Int
J Oncol. 45:273–281. 2014.PubMed/NCBI
|
|
9
|
Krutovskikh VA, Piccoli C and Yamasaki H:
Gap junction inter-cellular communication propagates cell death in
cancerous cells. Oncogene. 21:1989–1999. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jensen R and Glazer PM:
Cell-interdependent cisplatin killing by Ku/DNA-dependent protein
kinase signaling transduced through gap junctions. Proc Natl Acad
Sci USA. 101:6134–6139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka M and Grossman HB: Connexin 26 gene
therapy of human bladder cancer: Induction of growth suppression,
apoptosis, and synergy with Cisplatin. Hum Gene Ther. 12:2225–2236.
2001. View Article : Google Scholar
|
|
12
|
Shishido SN and Nguyen TA: Gap junction
enhancer increases efficacy of cisplatin to attenuate mammary tumor
growth. PLoS One. 7:e449632012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoon SY, Robinson CR, Zhang H and
Dougherty PM: Spinal astrocyte gap junctions contribute to
oxaliplatin-induced mechanical hypersensitivity. J Pain.
14:205–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bai C, Yang M, Fan Z, Li S, Gao T and Fang
Z: Associations of chemo- and radio-resistant phenotypes with the
gap junction, adhesion and extracellular matrix in a
three-dimensional culture model of soft sarcoma. J Exp Clin Cancer
Res. 34:582015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mesnil M: Connexins and cancer. Biol Cell.
94:493–500. 2002. View Article : Google Scholar
|
|
16
|
Cronier L, Crespin S, Strale PO, Defamie N
and Mesnil M: Gap junctions and cancer: New functions for an old
story. Antioxid Redox Signal. 11:323–338. 2009. View Article : Google Scholar
|
|
17
|
Vinken M, De Kock J, Oliveira AG, Menezes
GB, Cogliati B, Dagli ML, Vanhaecke T and Rogiers V: Modifications
in connexin expression in liver development and cancer. Cell Commun
Adhes. 19:55–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang JT and Nicholson BJ: The topological
structure of connexin 26 and its distribution compared to connexin
32 in hepatic gap junctions. J Membr Biol. 139:15–29. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maes M, Decrock E, Cogliati B, Oliveira
AG, Marques PE, Dagli ML, Menezes GB, Mennecier G, Leybaert L,
Vanhaecke T, et al: Connexin and pannexin (hemi)channels in the
liver. Front Physiol. 4:4052014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Plante I, Charbonneau M and Cyr DG:
Decreased gap junctional intercellular communication in
hexachlorobenzene-induced gender-specific hepatic tumor formation
in the rat. Carcinogenesis. 23:1243–1249. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma XD, Sui YF and Wang WL: Expression of
gap junction genes connexin 32, connexin 43 and their proteins in
hepatocellular carcinoma and normal liver tissues. World J
Gastroenterol. 6:66–69. 2000. View Article : Google Scholar
|
|
22
|
Nakashima Y, Ono T, Yamanoi A, El-Assal
ON, Kohno H and Nagasue N: Expression of gap junction protein
connexin32 in chronic hepatitis, liver cirrhosis, and
hepatocellular carcinoma. J Gastroenterol. 39:763–768. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bevans CG, Kordel M, Rhee SK and Harris
AL: Isoform composition of connexin channels determines selectivity
among second messengers and uncharged molecules. J Biol Chem.
273:2808–2816. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ayad WA, Locke D, Koreen IV and Harris AL:
Heteromeric, but not homomeric, connexin channels are selectively
permeable to inositol phosphates. J Biol Chem. 281:16727–16739.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng R, Wang J, Wu Q, Wang Z, Ou Y, Ma L,
Wang M, Wang J and Yang Y: Expression of ALDH1 and TGFβ2 in benign
and malignant breast tumors and their prognostic implications. Int
J Clin Exp Pathol. 7:4173–4183. 2014.
|
|
26
|
He XD, Wang Y, Wu Q, Wang HX, Chen ZD,
Zheng RS, Wang ZS, Wang JB and Yang Y: Xuebijing protects rats from
sepsis challenged with acinetobacter baumannii by promoting Annexin
A1 expression and inhibiting proinflammatory cytokines secretion.
Evid Based Complement Alternat Med. 2013:8049402013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Y, Qin SK, Wu Q, Wang ZS, Zheng RS,
Tong XH, Liu H, Tao L and He XD: Connexin-dependent gap junction
enhancement is involved in the synergistic effect of sorafenib and
all-trans retinoic acid on HCC growth inhibition. Oncol Rep.
31:540–550. 2014.
|
|
28
|
Yang Y, Cao MH, Wang Q, Yuan DD, Li L and
Tao L: The effects of 2-aminoethoxydiphenyl borate and
diphenylboronic anhydride on gap junctions composed of Connexin43
in TM(4) sertoli cells. Biol Pharm Bull. 34:1390–1397. 2011.
View Article : Google Scholar
|
|
29
|
Eugenín EA, Eckardt D, Theis M, Willecke
K, Bennett MV and Saez JC: Microglia at brain stab wounds express
connexin 43 and in vitro form functional gap junctions after
treatment with interferon-gamma and tumor necrosis factor-alpha.
Proc Natl Acad Sci USA. 98:4190–4195. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Garg S, Md Syed M and Kielian T:
Staphylococcus aureus-derived peptidoglycan induces Cx43 expression
and functional gap junction intercellular communication in
microglia. J Neurochem. 95:475–483. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sáez CG, Velásquez L, Montoya M, Eugenín E
and Alvarez MG: Increased gap junctional intercellular
communication is directly related to the anti-tumor effect of
all-trans-retinoic acid plus tamoxifen in a human mammary cancer
cell line. J Cell Biochem. 89:450–461. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang J, Dai Y, Huang Y, Chen X, Wang H,
Hong Y, Xia J and Cheng B: All-trans retinoic acid restores gap
junctional intercellular communication between oral cancer cells
with upregulation of Cx32 and Cx43 expressions in vitro. Med Oral
Patol Oral Cir Bucal. 18:e569–e577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mesnil M, Crespin S, Avanzo JL and
Zaidan-Dagli ML: Defective gap junctional intercellular
communication in the carcinogenic process. Biochim Biophys Acta.
1719:125–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leithe E, Sirnes S, Omori Y and Rivedal E:
Downregulation of gap junctions in cancer cells. Crit Rev Oncog.
12:225–256. 2006. View Article : Google Scholar
|
|
35
|
Dejana E, Orsenigo F, Molendini C, Baluk P
and McDonald DM: Organization and signaling of endothelial
cell-to-cell junctions in various regions of the blood and
lymphatic vascular trees. Cell Tissue Res. 335:17–25. 2009.
View Article : Google Scholar
|
|
36
|
Lampugnani MG and Dejana E: The control of
endothelial cell functions by adherens junctions. Novartis Found
Symp. 283:4–13; discussion 13–17, 238–241. 2007. View Article : Google Scholar
|
|
37
|
Yano T, Hernandez-Blazquez FJ, Omori Y and
Yamasaki H: Reduction of malignant phenotype of HEPG2 cell is
associated with the expression of connexin 26 but not connexin 32.
Carcinogenesis. 22:1593–1600. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamasaki H and Naus CC: Role of connexin
genes in growth control. Carcinogenesis. 17:1199–1213. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Z, Zhou J, Fan J, Qiu SJ, Yu Y, Huang
XW, Sun J, Tan CJ and Dai Z: Oxaliplatin induces apoptosis in
hepatocellular carcinoma cells and inhibits tumor growth. Expert
Opin Investig Drugs. 18:1595–1604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao J, Wang R, Yang Q, Chen C and Wu Q:
Effect of Oxaliplatin on cell cycle of hepatocellular carcinoma
cell line HepG2. Zhejiang Da Xue Xue Bao Yi Xue Ban. 42:437–442.
2013.(In Chinese). PubMed/NCBI
|