Introduction
The plasminogen activator (PA) system consists of
plasminogen activator inhibitor type 1 (PAI-1), urokinase-type
plasminogen activator and its receptor (uPA and uPAR) (1). PAI-1 inhibits the activation of uPA
(which converts plasminogen to plasmin), and the PA system is
involved in proteolysis (1).
Dysregulation of the PA system is related to disorders such as
thrombosis, atherosclerosis and type 2 diabetes (2–5). In
addition, it has been reported that PAI-1 is involved in cancer
invasion and metastasis, by remodeling the extracellular matrix
(ECM) through regulating plasmin (6–12).
Some studies reported that overexpression of PAI-1 correlated with
poor prognosis in cancer patients, however, it is controversial
(13–16).
Cancer stem cells (CSCs) are a small subset of cells
within tumors and possess abilities similar to normal stem cells;
the ability to self-renew and differentiate (17,18).
This concept was first demonstrated in leukemia and increasing
evidence supports this model in various types of solid tumors
including cervical cancer (19–24).
CSCs are thought to be involved in tumor recurrence and metastasis;
failure to treat CSCs by surgery or chemotherapy would result in
relapse (17). Considering these
facts, investigating the impact of PAI-1 on CSCs could give insight
into CSC features in terms of metastasis.
In this study, we investigated the significance of
PAI-1 in CSCs and non-CSCs. In cervical cancer, ALDH1-positive
cells, like other solid tumors, are known to be more tumorigenic
than negative ones, and we used ALDH1 as a marker of cervical CSCs
(25–34). First, using the cervical cancer
cell line CaSki we confirmed that the expression of PAI-1 was
significantly increased by changing coatings of culture plates as
described (35), especially
collagen IV-coating, confirmed by enzyme-linked immunosorbent assay
(ELISA). These results were also obtained after sorting CaSki cells
into ALDH1-high cells and ALDH1-low cells, and in addition, the
expression levels themselves in the supernatants from ALDH1-high
cells and ALDH1-low cells were different.
Secondly, we investigated the significance of PAI-1
in degradation of the ECM, by investigating gelatin zymography
assays and collagenase activity assays. We found that matrix
metalloproteinase-2 (MMP-2) was involved, and confirmed that the
activity to degrade the ECM was increased by exposing ALDH1-low
cells to TM5275 (a small molecule inhibitor of PAI-1). This result
was suggestive that PAI-1 was indeed involved in maintaining the
ECM, especially around non-CSCs. In conclusion, we investigated the
significance of PAI-1 in CSCs and non-CSCs. The expression levels
of PAI-1 were different from ALDH1-high cells to ALDH1-low cells,
and also different depending on culture plates. Our study could be
an explanation of conflicting reports, where some researchers found
the impacts of PAI-1 expression on poor clinical outcomes and
others not, by considering the concept of CSCs.
Materials and methods
Cell culture
The cervical cancer cell lines CaSki and SiHa were
obtained from American Type Culture Collection (ATCC, USA) and
cultured in Dulbecco's modified Eagle's medium (DMEM; Wako, Japan)
supplemented with 10% fetal bovine serum (FBS; Invitrogen,
Carlsbad, CA, USA) and sub-cultured by 0.25% trypsin/EDTA (Wako)
detachment. The cells were grown in a humidified atmosphere at 37°C
and 5% CO2.
Coatings
Fibronectin solution, vitronectin solution and
laminin solution were purchased from Wako (Cat# 063–05591,
220–02041 and 120–05751, respectively), and were used according to
manufacturer's instructions. Collagen I and collagen IV-coated
plates were obtained from Corning (Acton, MA, USA).
Reagents
Human PAI-1 peptide was purchased from Abcam
(Cambridge, MA, USA). TM5275 was purchased from Axon Medchem
(USA).
RNA extraction and RT-quantitative PCR
(RT-qPCR)
Total RNA was extracted with Tissue Total RNA kit
(Favorgen Biotech Corp., Taiwan) according to manufacturer's
protocols. First-strand cDNA was synthesized from 500 ng of total
RNA using ReverTra Ace (Toyobo, Japan). qPCR was performed with
SYBR Green PCR master mix (Roche) according to manufacturer's
instructions. Denaturation was performed at 95°C for 2 min,
followed by 35 cycles at 98°C for 10 sec, at 65°C for 10 sec and at
68°C for 10 sec. β-actin was used as a housekeeping gene and the
results are presented as fold change relative to β-actin expression
(2−ΔΔCt). Each experiment was performed in triplicate.
The sequences of the primer pairs used in this study are shown in
Table I.
 | Table IPrimer sequences used in this
study. |
Table I
Primer sequences used in this
study.
| Gene name | Primer
sequence | Size (bp) |
|---|
| PAI-1 |
GCACCACAGACGCGATCTT
ACCTCTGAAAAGTCCACTTGC | 112 |
| uPA |
GCCATCCCGGACTATACAGA
AGGCCATTCTCTTCCTTGGT | 417 |
| uPAR |
CTGGAGCTGGTGGAGAAAAG
TGTTGCAGCATTTCAGGAAG | 406 |
| β-actin |
CATGTACGTTGCTATCCAGGC
CTCCTTAATGTCACGCACGAT | 250 |
Enzyme-linked immunosorbent assay
(ELISA)
Culture supernatants were assayed for active PAI-1
and active uPA by ELISA kits (Innovation Research Inc., USA, Cat#
IHPAIKT and IHUPAKT) according to manufacturer's protocol. Cells
(2×105) were seeded into 6-well plates and were cultured
for 24 h. After that, medium was switched to DMEM without FBS for
24 h, and then the supernatants were collected. Immediately after
collection, these supernatants were centrifuged for 10 min at 300 ×
g to remove cell debris and were stored at −80°C. For detection of
intracellular active PAI-1, cells were permeabilized with 1 ml of
0.5% Triton X-100 for 20 min. Plates were read on an ELISA reader,
EPOCH (BioTek, Winooski, VT, USA).
Flow cytometry
The ALDH enzymatic activity of the cells was
measured as described previously (34), using the Aldefluor kit (StemCell
Technologies, Vancouver, BC, Canada). CaSki cells (5×106
cells) were suspended in Aldefluor assay buffer containing ALDH
substrate. The brightly fluorescent ALDH-positive cells were
detected using MoFlo XDP (Beckman Coulter, Inc., Brea, CA, USA). As
a negative control, cells were stained under identical conditions
after treatment with the specific ALDH inhibitor
N,N-diethylaminobenzaldehyde (DEAB). After sorting,
1×105 cells were seeded into 6-well plates and were
cultured for 24 h. Then medium was changed under each experimental
condition. Experiment was repeated at least three times.
Gelatin zymograhpy assays
Gelatin zymography assays (Cosmobio, Japan, Cat#
AK45) were performed as previously described (36). Collected supernatants (10 μl) were
mixed with 2X sample buffer and electrophoresed according to
manufacturer's instructions. Subsequent enzymatic reactions were
performed at 37°C for 40 h.
Collagenase activity assays
Collagenase activity was detected with EnzChek
Gelatinase/Collagenase Assay kit (Molecular Probes, USA, Cat#
E-12055) according to manufacturer's instructions. The supernatant
(100 μl) was used, and incubated with DQ gelatin solution for 24 h
at room temperature. The fluorescence intensity was measured in a
fluorescence microplate reader, Fluoroskan Ascent FL (Thermo Fisher
Scientific, Waltham, MA, USA), set for excitation at 485 nm and
emission detection at 538 nm. The fluorescence intensity was
corrected for background fluorescence by subtracting the value
derived from the no-enzyme control. Clostridium collagenase
(provided with the kit) was used as a positive control. Experiment
was repeated three times.
Statistical analysis
ANOVA test was used for comparing the influence of
the ECM. Student t-test was used to compare the means of expression
levels of PAI-1. Wilcoxon rank-sum test was used to investigate the
effects of TM5275. p-values <0.05 were considered statistically
significant. JMP/SAS Institute software was used for statistical
analysis.
Results
Expression levels of PAI-1 are
significantly different depending on the coating
In advance, we confirmed the expression of PAI-1,
uPA and uPAR of CaSki and SiHa by RT-PCR (data not shown). The
concentration of active PAI-1 in the supernatants from CaSki and
SiHa is shown in Fig. 1A. The
expression levels of active PAI-1 in the supernatants from CaSki
cells were significantly different depending on the coating, and we
selected CaSki cells for further experiment. Besides, we decided to
investigate the relationship between collagen IV and PAI-1, because
i) We detected high levels of PAI-1 when culturing CaSki cells on
collagen IV-coated plates as shown in Fig. 1A. ii) Few studies have investigated
the relationship between PAI-1 and collagen IV, while most studies
investigated the relationship between PAI-1 and fibronectin,
vitronectin and laminin (1,8,11,35).
iii) Collagen IV is known to be rich in basal membrane (where
cervical cancer occurs) (37), and
collagen IV could be important for CSCs. As shown in Fig. 1B, culturing CaSki cells on collagen
IV-coated plates increased the expression of PAI-1 at mRNA
levels.
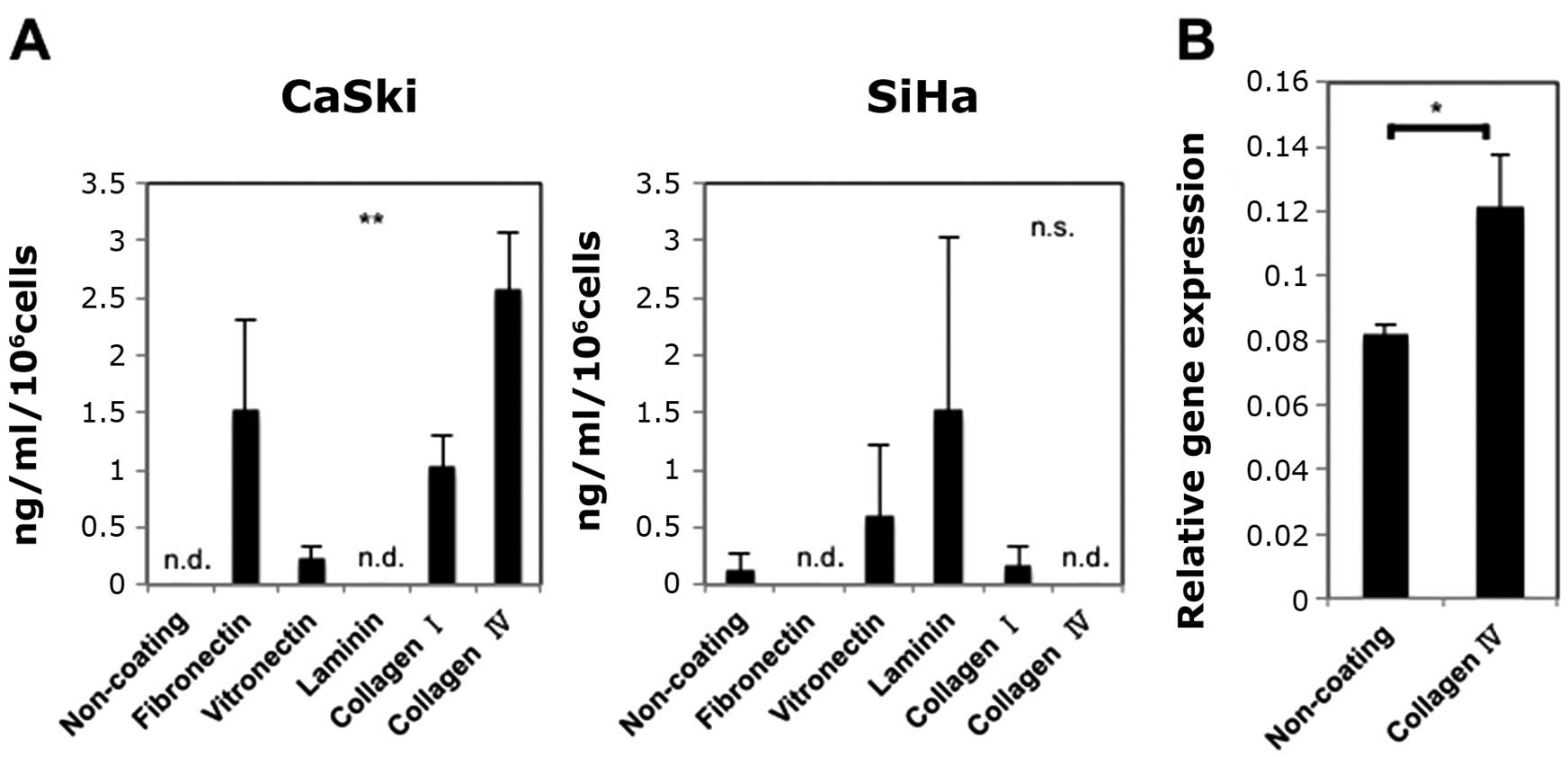 | Figure 1Influence of types of coatings on
PAI-1 expression. (A) Influence of types of coatings on active
PAI-1 expression in the supernatants from CaSki and SiHa. Active
PAI-1 levels in the supernatants from CaSki were significantly
increased (ELISA). Experiments were performed in duplicate and
repeated three times (n=3). The values shown represent the mean ±
SE. (B) Influence of collagen IV on PAI-1 mRNA expression of CaSki.
Culturing CaSki cells on collagen IV-coted plates increased their
expression levels of PAI-1 mRNA (RT-qPCR). Experiments were
performed in triplicate and repeated three times (n=3). The values
shown represent the mean ± SE. **p<0.01,
*p<0.05; n.s., not significant. n.d., not detected.
(A) X-axis, non-coating, fibronectin, vitronectin, laminin,
collagen I or collagen IV. Y-axis, ng/ml/106 cells. (B)
X-axis, non-coating or collagen IV. Y-axis, relative gene
expression. |
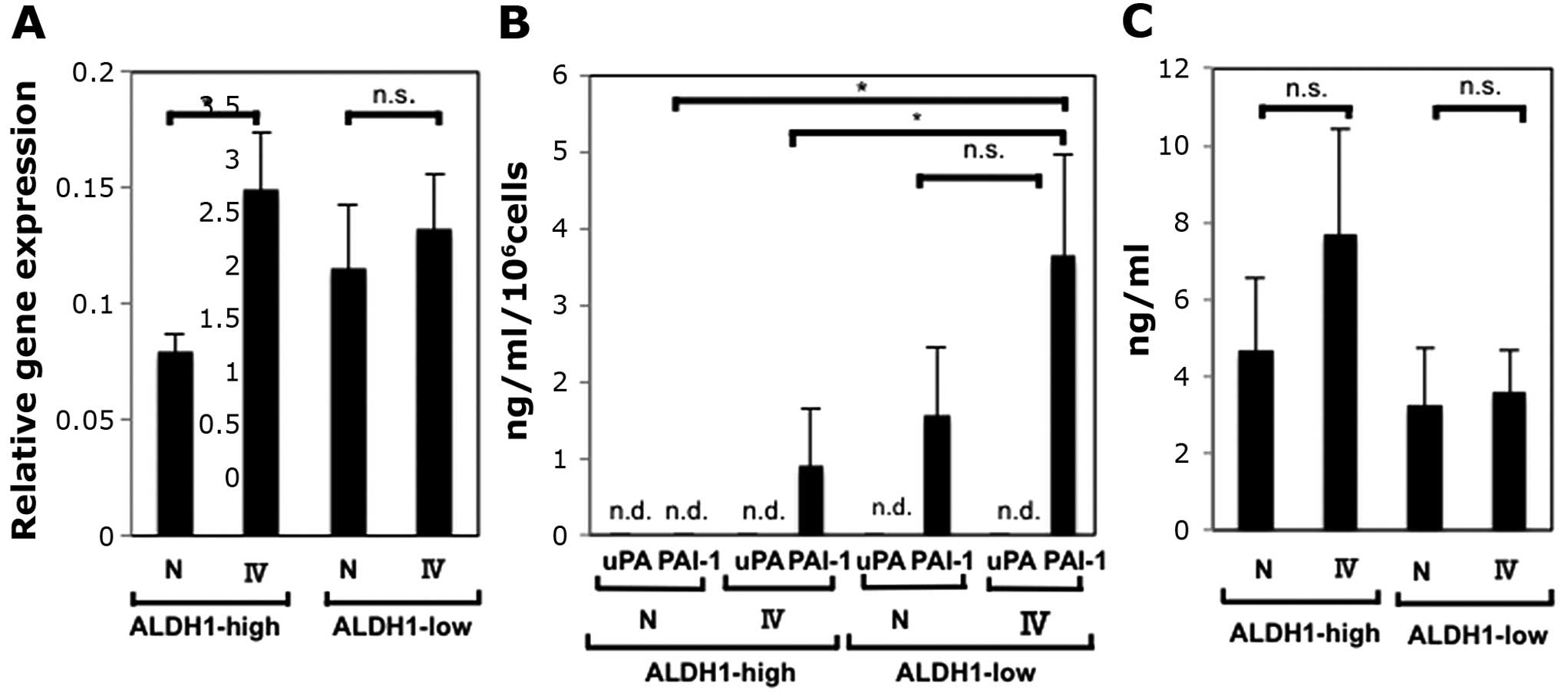
Expression levels of PAI-1 are different
from ALDH1-high cells to ALDH1-low cells
We performed the same procedures after sorting CaSki
cells into ALDH1-high cells and ALDH1-low cells. As shown in
Fig. 2A, culturing ALDH1-high
cells and ALDH1-low cells on collagen IV-coated plates increased
the expression levels of PAI-1 mRNA. The increase of ALDH1-high
cells was statistically significant, and it was suggestive that
ALDH1-high cells contributed to the increase of PAI-1 mRNA
expression levels (Fig. 1A).
PAI-1 plays a role in maintaining the ECM when
secreted (1), and we detected
secreted active PAI-1 in the supernatants of cultured cells using
ELISA. In addition, since the balance of uPA and PAI-1 is important
rather than the concentration of PAI-1 itself (1,2,15),
we investigated secreted active uPA as well (Fig. 2B). We found that the expression of
PAI-1 is higher when cells were cultured on collagen IV-coated
plates than when cultured on non-coating plates, and that the
expression of PAI-1 of ALDH1-low cells was higher than that of
ALDH1-high cells.
Of note, the patterns of secreted PAI-1 and PAI-1
mRNA expression levels were apparently different. In the context of
maintaining the ECM, not only active PAI-1 but also many factors,
such as non-active PAI-1, uPA, tPA and uPAR, and their combination
are important (1,7,10,15,38–40).
Considering these facts, the expression levels of PAI-1 mRNA do not
have to accord with the concentration of secreted active PAI-1,
however, we further detected intracellular active PAI-1 in order to
obtain insights into the inconsistency. Of interest, the
concentration of intracellular active PAI-1 was similar to the
expression patterns of PAI-1 mRNA (Fig. 2A and C). These results can be
interpreted as follows: The amount of active PAI-1 is consistent
with the expression of PAI-1 mRNA, however, PAI-1 from ALDH1-high
cells is more difficult to be secreted than PAI-1 from ALDH1-low
cells [due to transportation system or membrane permiability, for
instance (41)].
ALDH1-high cells and ALDH1-low cells
express pro-MMP-2
We then speculated on the impact of PAI-1 in
maintenance of the ECM. With gelatin zymography assays, we
investing the ability of each collected supernatant to degrade the
general ECM, gelatin. As shown in Fig.
3, each supernatant expressed only pro-MMP2. This result was
acceptable since pro-MMP-2 is the substrate of plasmin (1,42),
although we could not find significant difference of pro-MMP-2
expression among these conditions.
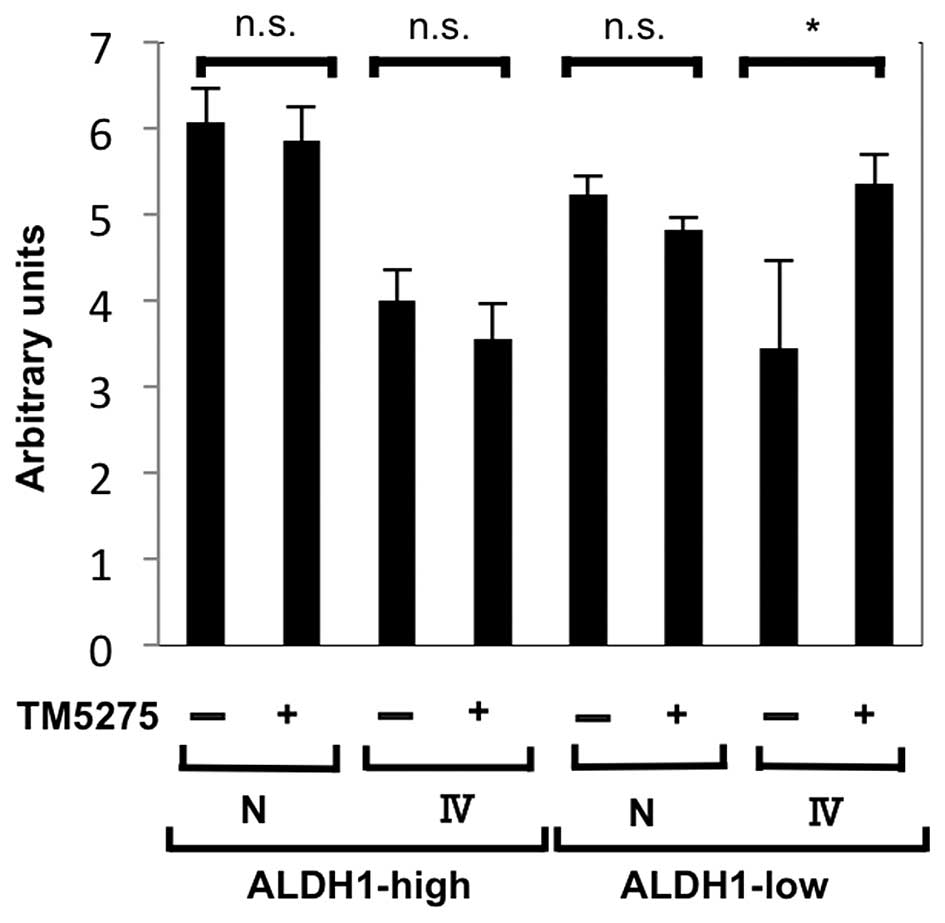
Exposing ALDH1-low cells to TM5275, a
small molecule inhibitor of PAI-1, increases collagenase activity
of the supernatants
In order to quantify the activity of degrading the
ECM, we proceeded to perform collagenase activity assays. After
sorting, 1×105 cells were seeded and cultured for 24 h.
Then, medium was changed to DMEM with or without 10 nM TM5275 for
24 h, and the supernatants were collected (43). As shown in Fig. 4, collagenase activity was increased
only when ALDH1-low cells were exposed to TM5275. The intensities
ranged from the intensity of 0.02 U/ ml of clostridium collagenase
to that of 0.2 U/ml of clostridium collagenase.
Discussion
In the present study, we investigated the
significance of PAI-1 in cervical CSCs and non-CSCs, and its impact
on the ECM maintenance. PAI-1 inhibits the activation of uPA (which
converts plasminogen to plasmin), and it is involved in cancer
invasion and metastasis, by remodeling the ECM through regulating
plasmin (1,6–12).
The studies investigating CSCs are now increasing in various types
of solid tumors including cervical cancer, and CSCs are thought to
be involved in tumor recurrence and metastasis (17,18).
Putting these facts together, we aimed to investigate the
relationship between PAI-1 and cervical CSCs.
First, using the cervical cancer cell line CaSki we
confirmed that the expression levels of PAI-1 were significantly
increased when the cells were cultured on collagen IV-coated plates
compared to when cultured on non-coated plates, employing ELISA and
RT-qPCR. When we use collagen IV as the representative of the ECM,
we can put a focus on proteolytic activity of PAI-1 unlike using
fibronectin and vitrinectin (non-proteolytic, or direct interaction
between PAI-1 and the ECM is known) (1). At the same time, we have to consider
the protein downstream of uPA and plasmin such as MMP-2, because
the substrate of uPA itself is not collagen IV but fibronectin
(44). This is why we performed
gelatin zymography assays. With gelatin zymography assays, we found
the involvement of pro-MMP-2.
Secondly, we investigated the significance of PAI-1
in maintenance of the ECM, by investigating collagenase activity.
We confirmed that the activity to degrade the ECM was increased by
exposing ALDH1-low cells to TM5275 (a small molecule inhibitor of
PAI-1). On the contrary, adding recombinant PAI-1 (the final
concentration of 1 μg/ml), to the supernatants from ALDH1-high
cells cultured on non-coating plates, reduced its fluorescence
intensity by 7% (data not shown). These results were suggestive
that PAI-1 was indeed involved in maintaining the ECM in CSCs and
non-CSCs.
The limitation of our experiment is that we could
not assess to what extent PAI-1 was involved in maintaining the
ECM. The question remains from that we could not show a clear
inverse correlation between concentration of PAI-1 and fluorescence
intensity (Figs. 2B and 3). This is due to the complexity of the
PA system, and considering only PAI-1 would not be enough in the
context of degradation of the ECM (10). However, we may say that we shed
light on the necessity to consider the concept of CSCs when
investigating the PA system.
ALDH1-low cells expressed PAI-1 more highly than
ALDH1-high cells (Fig. 2A). PAI-1
is known to be related to senescence, and it is more highly
expressed in ‘old cells’ than in ‘young cells’, and ‘more passaged
cell line’ than ‘less passaged cell line’ (45–47).
Considering the direction of differentiation from ALDH1-high cells
to ALDH1-low cells, it is reasonable to think that ALDH1-low cells
express PAI-1 more highly than ALDH1-high cells.
Although the increase of PAI-1 expression in the
supernatants from SiHa cells when cultured on laminin-coated plates
was not statistically significant (Fig. 1A), it is suggestive that PAI-1 is
important for the interaction between cancer cells and basal
membrane, considering that laminin is a major component of basal
membrane of uterine cervix as well as collagen IV (37).
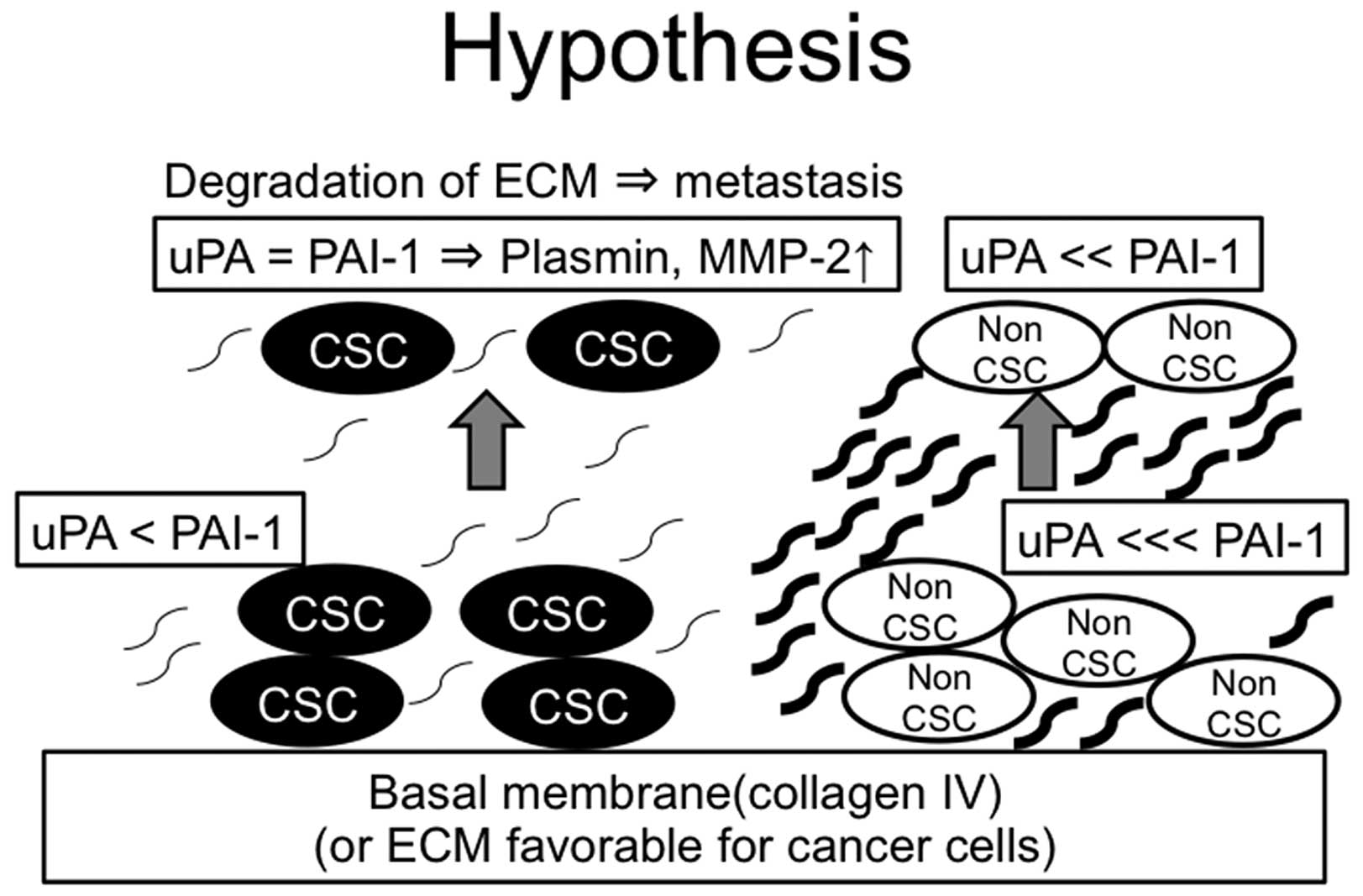
In conclusion, we investigated the significance of
PAI-1 in CSCs and non-CSCs. The expression levels of PAI-1 of
ALDH1-high and ALDH1-low cells were both increased when cultured on
collagen IV-coated plates, however, the basic expression levels of
PAI-1 of ALDH1-high were lower than those in ALDH1-low cells. This
result was suggestive that the ECM surrounding CSCs (especially
distant from basal membrane) is more susceptible to degrade due to
its low expression levels of PAI-1 (hypothetic schema from the data
we obtained is shown in Fig. 5).
Although verification to other kinds of CSC markers, and other
types of cancers are needed, our study could be an explanation of
conflicting reports, where some researchers found negative impacts
of PAI-1 expression on clinical outcomes and others not, by
considering the concept of CSCs.
Acknowledgements
We are grateful to Stem Cell Laboratory of Medical
Research Institute in Tokyo Medical and Dental University, for kind
assistance in flow cytometric sorting.
References
|
1
|
Czekay RP, Wilkins-Port CE, Higgins SP,
Freytag J, Overstreet JM, Klein RM, Higgins CE, Samarakoon R and
Higgins PJ: PAI-1: An integrator of cell signaling and migration.
Int J Cell Biol. 2011:5624812011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jankun J: Plasminogen activator
inhibitor-1 in kidney pathology (Review). Int J Mol Med.
31:503–510. 2013.PubMed/NCBI
|
|
3
|
Klinger KW, Winqvist R, Riccio A,
Andreasen PA, Sartorio R, Nielsen LS, Stuart N, Stanislovitis P,
Watkins P, Douglas R, et al: Plasminogen activator inhibitor type 1
gene is located at region q21.3-q22 of chromosome 7 and genetically
linked with cysticfibrosis. Proc Natl Acad Sci USA. 84:8548–8552.
1987. View Article : Google Scholar
|
|
4
|
Krause MP, Moradi J, Nissar AA, Riddell MC
and Hawke TJ: Inhibition of plasminogen activator inhibitor-1
restores skeletal muscle regeneration in untreated type 1 diabetic
mice. Diabetes. 60:1964–1972. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ricart JM, Ramón LA, Vayá A, España F,
Santaolaria ML, Todolí J, Castelló R, Fontcuberta J and Estellés A:
Fibrinolytic inhibitor levels and polymorphisms in Behcet disease
and their association with thrombosis. Br J Haematol. 141:716–719.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bajou K, Noël A, Gerard RD, Masson V,
Brunner N, Holst-Hansen C, Skobe M, Fusenig NE, Carmeliet P, Collen
D, et al: Absence of host plasminogen activator inhibitor 1
prevents cancer invasion and vascularization. Nat Med. 4:923–928.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fabre-Guillevin E, Malo M, Cartier-Michaud
A, Peinado H, Moreno-Bueno G, Vallée B, Lawrence DA, Palacios J,
Cano A, Barlovatz-Meimon G, et al: PAI-1 and functional blockade of
SNAI1 in breast cancer cell migration. Breast Cancer Res.
10:R1002008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smit JW, van der Pluijm G, Romijn HA,
Löwik CW, Morreau H and Goslings BM: Degradation of extracellular
matrix by metastatic follicular thyroid carcinoma cell lines: role
of the plasmin activation system. Thyroid. 9:913–919. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maillard C, Jost M, Rømer MU, Brunner N,
Houard X, Lejeune A, Munaut C, Bajou K, Melen L, Dano K, et al:
Host plasminogen activator inhibitor-1 promotes human skin
carcinoma progression in a stage-dependent manner. Neoplasia.
7:57–66. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Riddick AC, Shukla CJ, Pennington CJ, Bass
R, Nuttall RK, Hogan A, Sethia KK, Ellis V, Collins AT, Maitland
NJ, et al: Identification of degradome components associated with
prostate cancer progression by expression analysis of human
prostatic tissues. Br J Cancer. 92:2171–2180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vial D and McKeown-Longo PJ: PAI1
stimulates assembly of the fibronectin matrix in osteosarcoma cells
through crosstalk between the alphavbeta5 and alpha5beta1
integrins. J Cell Sci. 121:1661–1670. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Woo M, Park K, Nam J and Kim JC: Clinical
implications of matrix metalloproteinase-1, -3, -7, -9, -12, and
plasminogen activator inhibitor-1 gene polymorphisms in colorectal
cancer. J Gastroenterol Hepatol. 22:1064–1070. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanekom GS, Stubbings HM and Kidson SH:
The active fraction of plasmatic plasminogen activator inhibitor
type 1 as a possible indicator of increased risk for metastatic
melanoma. Cancer Detect Prev. 26:50–59. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lara PC, Lloret M, Valenciano A, Clavo B,
Pinar B, Rey A and Henríquez-Hernández LA: Plasminogen activator
inhibitor-1 (PAI-1) expression in relation to hypoxia and
oncoproteins in clinical cervical tumors. Strahlenther Onkol.
188:1139–1145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Malinowsky K, Wolff C, Berg D, Schuster T,
Walch A, Bronger H, Mannsperger H, Schmidt C, Korf U, Höfler H, et
al: uPA and PAI-1-related signaling pathways differ between primary
breast cancers and lymph node metastases. Transl Oncol. 5:98–104.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sternlicht MD, Dunning AM, Moore DH,
Pharoah PD, Ginzinger DG, Chin K, Gray JW, Waldman FM, Ponder BA
and Werb Z: Prognostic value of PAI1 in invasive breast cancer:
evidence that tumor-specific factors are more important than
genetic variation in regulating PAI1 expression. Cancer Epidemiol
Biomarkers Prev. 15:2107–2114. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meacham CE and Morrison SJ: Tumour
heterogeneity and cancer cell plasticity. Nature. 501:328–337.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X, Rycaj K, Liu X and Tang DG: New
insights into prostate cancer stem cells. Cell Cycle. 12:579–586.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dick JE: Stem cell concepts renew cancer
research. Blood. 112:4793–4807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
López J, Valdez-Morales FJ,
Benítez-Bribiesca L, Cerbón M and Carrancá AG: Normal and cancer
stem cells of the human female reproductive system. Reprod Biol
Endocrinol. 11:532013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Skibinski A and Kuperwasser C: The origin
of breast tumor heterogeneity. Oncogene. 34:5309–5316. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Triscott J, Rose Pambid M and Dunn SE:
Concise review: bullseye: targeting cancer stem cells to improve
the treatment of gliomas by repurposing disulfiram. Stem Cells.
33:1042–1046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeki SS, Graham TA and Wright NA: Stem
cells and their implications for colorectal cancer. Nat Rev
Gastroenterol Hepatol. 8:90–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hirata N, Yamada S, Shoda T, Kurihara M,
Sekino Y and Kanda Y: Sphingosine-1-phosphate promotes expansion of
cancer stem cells via S1PR3 by a ligand-independent Notch
activation. Nat Commun. 5:48062014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alonso-Alconada L, Muinelo-Romay L,
Madissoo K, Diaz-Lopez A, Krakstad C, Trovik J, Wik E, Hapangama D,
Coenegrachts L, Cano A, et al: Molecular profiling of circulating
tumor cells links plasticity to the metastatic process in
endometrial cancer. Mol Cancer. 13:2232014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma I and Allan AL: The role of human
aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell
Rev. 7:292–306. 2011. View Article : Google Scholar
|
|
28
|
Penumatsa K, Edassery SL, Barua A,
Bradaric MJ and Luborsky JL: Differential expression of aldehyde
dehydrogenase 1a1 (ALDH1) in normal ovary and serous ovarian
tumors. J Ovarian Res. 3:282010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pisanu ME, Noto A, De Vitis C, Masiello
MG, Coluccia P, Proietti S, Giovagnoli MR, Ricci A, Giarnieri E,
Cucina A, et al: Lung cancer stem cell lose their stemness default
state after exposure to microgravity. Biomed Res Int.
2014:4702532014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pors K and Moreb JS: Aldehyde
dehydrogenases in cancer: An opportunity for biomarker and drug
development? Drug Discov Today. 19:1953–1963. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Raha D, Wilson TR, Peng J, Peterson D, Yue
P, Evangelista M, Wilson C, Merchant M and Settleman J: The cancer
stem cell marker aldehyde dehydrogenase is required to maintain a
drug-tolerant tumor cell subpopulation. Cancer Res. 74:3579–3590.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Warrier S, Bhuvanalakshmi G, Arfuso F,
Rajan G, Millward M and Dharmarajan A: Cancer stem-like cells from
head and neck cancers are chemosensitized by the Wnt antagonist,
sFRP4, by inducing apoptosis, decreasing stemness, drug resistance
and epithelial to mesenchymal transition. Cancer Gene Ther.
21:381–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Watanabe Y, Yoshimura K, Yoshikawa K,
Tsunedomi R, Shindo Y, Matsukuma S, Maeda N, Kanekiyo S, Suzuki N,
Kuramasu A, et al: A stem cell medium containing neural stimulating
factor induces a pancreatic cancer stem-like cell-enriched
population. Int J Oncol. 45:1857–1866. 2014.PubMed/NCBI
|
|
34
|
Liu SY and Zheng PS: High aldehyde
dehydrogenase activity identifies cancer stem cells in human
cervical cancer. Oncotarget. 4:2462–2475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Isogai C, Laug WE, Shimada H, Declerck PJ,
Stins MF, Durden DL, Erdreich-Epstein A and DeClerck YA:
Plasminogen activator inhibitor-1 promotes angiogenesis by
stimulating endothelial cell migration toward fibronectin. Cancer
Res. 61:5587–5594. 2001.PubMed/NCBI
|
|
36
|
Taguchi A, Kawana K, Tomio K, Yamashita A,
Isobe Y, Nagasaka K, Koga K, Inoue T, Nishida H, Kojima S, et al:
Matrix metalloproteinase (MMP)-9 in cancer-associated fibroblasts
(CAFs) is suppressed by omega-3 polyunsaturated fatty acids in
vitro and in vivo. PLoS One. 9:e896052014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goldberg I, Davidson B, Lerner-Geva L,
Gotlieb WH, Ben-Baruch G, Novikov I and Kopolovic J: Expression of
extracellular matrix proteins in cervical squamous cell carcinoma -
a clinicopathological study. J Clin Pathol. 51:781–785. 1998.
View Article : Google Scholar
|
|
38
|
Han B, Nakamura M, Zhou G, Ishii A,
Nakamura A, Bai Y, Mori I and Kakudo K: Calcitonin inhibits
invasion of breast cancer cells: Involvement of urokinase-type
plasminogen activator (uPA) and uPA receptor. Int J Oncol.
28:807–814. 2006.PubMed/NCBI
|
|
39
|
Hildenbrand R and Schaaf A: The
urokinase-system in tumor tissue stroma of the breast and breast
cancer cell invasion. Int J Oncol. 34:15–23. 2009.
|
|
40
|
Roomi MW, Kalinovsky T, Niedzwiecki A and
Rath M: Modulation of uPA, MMPs and their inhibitors by a novel
nutrient mixture in human glioblastoma cell lines. Int J Oncol.
45:887–894. 2014.PubMed/NCBI
|
|
41
|
Chung CL, Sheu JR, Liu HE, Chang SC, Chou
YC, Chen WL, Chou DS and Hsiao G: Dynasore, a dynamin inhibitor,
induces PAI-1 expression in MeT-5A human pleural mesothelial cells.
Am J Respir Cell Mol Biol. 40:692–700. 2009. View Article : Google Scholar
|
|
42
|
Baramova EN, Bajou K, Remacle A, L'Hoir C,
Krell HW, Weidle UH, Noel A and Foidart JM: Involvement of
PA/plasmin system in the processing of pro-MMP-9 and in the second
step of pro-MMP-2 activation. FEBS Lett. 405:157–162. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Izuhara Y, Yamaoka N, Kodama H, Dan T,
Takizawa S, Hirayama N, Meguro K, van Ypersele de Strihou C and
Miyata T: A novel inhibitor of plasminogen activator inhibitor-1
provides antithrombotic benefits devoid of bleeding effect in
nonhuman primates. J Cereb Blood Flow Metab. 30:904–912. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Marchina E and Barlati S: Degradation of
human plasma and extracellular matrix fibronectin by tissue type
plasminogen activator and urokinase. Int J Biochem Cell Biol.
28:1141–1150. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ota H, Akishita M, Eto M, Iijima K, Kaneki
M and Ouchi Y: Sirt1 modulates premature senescence-like phenotype
in human endothelial cells. J Mol Cell Cardiol. 43:571–579. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Calvanese V, Lara E, Suárez-Alvarez B, Abu
Dawud R, Vázquez-Chantada M, Martínez-Chantar ML, Embade N,
López-Nieva P, Horrillo A, Hmadcha A, et al: Sirtuin 1 regulation
of developmental genes during differentiation of stem cells. Proc
Natl Acad Sci USA. 107:13736–13741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wan YZ, Gao P, Zhou S, Zhang ZQ, Hao DL,
Lian LS, Li YJ, Chen HZ and Liu DP: SIRT1-mediated epigenetic
downregulation of plasminogen activator inhibitor-1 prevents
vascular endothelial replicative senescence. Aging Cell.
13:890–899. 2014. View Article : Google Scholar : PubMed/NCBI
|