Introduction
Acute promyelocytic leukemia (APL) accounts for
~5–10% of acute myeloid leukemia (AML) (1). It is identified as a distinct entity
among the AML by its consistent chromosomal translocation t(l5;17)
with corresponding PML-RARα and RARA-PML fusion genes (2). APL accounted for high mortality rates
during induction therapy by chemotherapy drugs because of bleeding
diathesis in the pre-all transretinoic acid (ATRA) era (3). However, the discovery in the late
1980s of the clinical efficacy of ATRA, which promotes the terminal
differentiation of malignant promyelocytes to mature neutrophils,
changed the natural history of APL (4,5).
Regimens using a combination of ATRA and anthracyclines (such as
idarubicin or daunorubicin) have been shown to achieve very high
remission rates of up to 90% and prolong event-free survival in
patients with APL (6–8). Now it has become the standard
treatment for induction and consolidation in APL. The use of
arsenic trioxide (ATO) since early 1990s further improved the
clinical outcome of refractory or relapsed as well as newly
diagnosed APL (9,10). While death during the induction
phase from causes such as haemorrhage, differentiation syndrome
(DS) and infection poses a significant challenge in early
treatment, resistance to therapy is an uncommon cause of induction
failure (11). However, so far
only ATRA alone, or combined with ATO, was able to induce CR in
most of patients with APL, and they do not work on the other types
of patients with AML. Therefore, scientists have begun to explore
new agents and strategies to induce differentiation or apoptosis of
leukemia cells. The combination of traditional Chinese medicine and
Western medicine is possibly an ideal choice to treat patients with
AML.
Oridonin is a diterpenoid compound isolated from the
Chinese medicinal herb Isodon rubescens, and possesses a variety of
biological effects such as anti-inflammatory, antiviral and
immunoregulatory functions (12).
To date, oridonin has been demonstrated to be an effective
anti-tumor agent with significant effects on some malignancies of
different pathological types, included acute leukemia (13,14).
A recent study showed that oridonin induced potent growth
inhibition, cell cycle arrest and apoptosis induction by increasing
histone hyperacetylation (H3 and H4) and regulation of p16, p21,
p27 and c-myc (15). Another
report revealed that oridonin greatly enhanced apoptosis induced by
As2O3 in hepatocellular carcinoma cells by
increasing intracellular reactive oxygen species (ROS) level,
decreasing mitochondrial membrane potential (MMP), and relocating
Bax and cytochrome c (16).
Histone deacetylase (HDAC) inhibitors have emerged
recently as promising antineoplastic agents (17). By promoting histone acetylation,
HDAC inhibitors permit chromatin to assume a more relaxed state,
thereby allowing transcription of genes involved in cell cycle
arrest, differentiation and (or) apoptosis. Valproic acid (VPA), as
a well-tolerated agent for neurological disorders, has been safely
used for >30 years. VPA has been shown to be a histone
deacetylase inhibitor which binds to and directly inhibits HDAC
(18,19). Accumulating evidence demonstrates
that VPA can induce apoptosis or differentiation of leukemia cells
either alone or in combination with other anti-leukemic agents
(20,21).
Therefore, prompted by the above reports and based
on effect of oridonin and VPA on histone acetylation, we determined
whether oridonin in conjunction with VPA would produce even more
encouraging synergistic effect than each of them alone, which has
not been reported so far. The results indicated that combination of
oridonin plus VPA could potentially be a promising regimen for
treatment of AML.
Materials and methods
Reagents
Oridonin was kindly provided by Dr Xiao Wang
(Shandong Academy of Sciences). It was dissolved in DMSO at a stock
concentration of 5 mg/ml and was stored at −20°C. Valproic acid
sodium salt (VPA) was from Sigma. Caspase-inhibitor(Z-VAD-fmk),
JNKinhibitor(SP600125), p38inhibitor (SB203580), ERK inhibitor
(PD98059) and Hoechst 33342 was obtained from Beyotime
Biotechnology, Inc. (Nantong, China). Annexin V fluorescein
isothiocyanate (FITC) kit was obtained from BD Biosciences (San
Diego, CA, USA). Antibodies for detecting Bax, Bcl-2, cleaved
caspase-3, caspase-8, caspase-9, Fas, FasL, ERK, p38,
phosphorylated-JNK, phosphorylated-ERK, phosphorylated-p38 were
purchased from Cell Signaling Technology (Beverly, MA, USA). GAPDH
antibody was purchased from Proteintech Group, Inc. (Chicago, IL,
USA). JNK antibody was purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Horseradish peroxidase-labeled IgG
anti-mouse and anti-rabbit antibodies were supplied by Zhongshan
Golden Bridge Biotechnology Co. (Beijing, China). Cell Counting
Kit-8 (CCK-8) was obtained from Dojindo Laboratories (Kumamoto,
Japan).
Cell culture
Human acute myeloid leukemia HL-60 cells were
cultured in RPMI-1640 medium supplemented with 10% newborn calf
serum (NCS, Sijiqing Biotechnology Co. Hangzhou, China).
Logarithmically growing cells were exposed to the indicated drugs
for the indicated time-points.
Cell proliferation assay
Cell proliferation was detected by CCK-8 assay
according to the manufacturer's instructions. In brief, cells were
plated in 96-well plates at a density of 1×104
cells/well in 100 μl of medium in triplicate and treated with
oridonin (3, 6, 9 and 12 μM) or VPA (0.5, 1 and 2 mM) or in
combination. Cells exposed to RPMI-1640 medium only were used as
control. Following incubation for 24 h, 10 μl of CCK-8 solution was
added to each well in the assay plate and incubated for an
additional 2 h at 37°C. Absorbance was measured at 450 nm using a
microplate reader (Model 550; Bio-Rad, USA). Each group had
triplicate samples. The inhibition rate was calculated as the
following formula: inhibition rate (%) = 1 − average absorbance of
treated group/average absorbance of control group × 100%. Data were
indicated as the means ± SD of triplicate samples. The 50%
inhibitory concentration (IC50) was calculated by the
software for IC50 calculation.
Determination of drug interactions
Drug interaction between oridonin and VPA was
assessed by CCK-8. The combination index (CI) was calculated by
Chou-TC association index. CI<1, CI=1, and CI>1 indicated
synergistic, additive, and antagonistic effects, respectively
(22,23).
Morphological detection of apoptosis
HL-60 cells were exposed to 6 μM oridonin and/or 1
mM VPA for 24 h, the cell morphology was observed by light
microscopy. For nuclear morphology, cells were washed twice with
PBS, stained with Hoechst 33342 (10 μg/ml) for 5 min at room
temperature in the dark and subjected to a Nikon Eclipse Ti
fluorescence microscope (Nikon, Japan).
Annexin V/PI assay
After treatment with drugs for 24 h, HL-60 cells
were harvested, washed with cold PBS twice and re-suspended in 100
μl of 1X binding buffer containing 5 μl Annexin V and 10 μl PI for
15 min at room temperature in the dark. Flow cytometry measurements
were made on a Beckman Coulter Epics XL cytometer.
Reverse transcription (RT)-PCR
analysis
The total RNA was extracted with TRIzol agent
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer's
protocol. The RNA concentration was measured by spectrophotometry.
RT-PCR assay was performed as previously described (24). The PCR products were
electrophoresed in 1.5% agarose gels. The primers were all
synthesized by Sangon Co., Ltd. (Shanghai, China). The sequences
are listed as Table I.
 | Table IPrimers for RT-PCR. |
Table I
Primers for RT-PCR.
| Gene | Sequence of
primers | Size of products
(bp) |
|---|
| β-actin | Sense: |
5′-GTGGGGCGCCCCAGGCACCA-3′ | 540 |
| Antisense: |
5′-CTCCTTAATGTCACGCACGATTTC-3′ |
| Bax | Sense: |
5′-CTGACATGTTTTCTGACGGC -3′ | 289 |
| Antisense: |
5′-TCAGCCCATCTTCTTCCAGA -3′ |
| Bcl-2 | Sense: |
5′-AGGCACCCAGGGTGATGCAA-3′ | 304 |
| Antisense: |
5′-GTGGAGGAGCTCTTCAGGGA-3′ |
Western blot analysis
HL-60 cells were washed twice with cold PBS and
lysed in extraction buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1
mM PMSF, 1 mM EDTA, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS)
for 30 min on ice. The lysates were centrifuged at 12,000 × g for
15 min and quantified using Bradford protein assay. Total proteins
(50 μg) were separated by SDS-PAGE and electroblotted onto PVDF
membranes. The membranes were blocked with 5% milk for 1 h and
incubated with antibodies against caspase-8 (1:1,000), caspase-9
(1:1,000), cleaved caspase-3 (1:1,000), Fas (1:1,000), FasL
(1:1,000), Bcl-2 (1:1,000), Bax (1:1,000), Cyt-C, JNK (1:1,000),
ERK (1:1,000), p38, P-JNK (1:1,000), P-ERK (1:1,000), P-p38
(1:1,000) (Cell Signaling Technology) overnight at 4°C followed by
incubation with HRP-conjugated secondary antibodies (Zhongshan
Golden Bridge Biotechnology Co.) for 1 h, visualized using ECL
detection system (Millipore, Billerica, USA) and pictured by
LAS-4000 mini luminescent image analyzer (Fujifilm, Tokyo,
Japan).
Antitumor effect in vivo
All management procedures were approved by the
Institutional Animal Care and Use Committee of the Shandong Academy
of Medical Science. BALBc nude mice were established by
subcutaneous inoculation of 1×107 HL-60 cells as
previously described. Then the nude mice were randomly assigned to
two groups: control, and oridonin plus VPA group (n=6). The
treatments began 10 days later with 15 mg/kg/d oridonin and/or 100
mg/kg/d VPA for 14 days, and the nude mice in control group were
treated with the same volume of saline. Tumor volume and weights of
the nude mice were measured daily.
TUNEL assay
TUNEL assay was performed to detect in situ
apoptosis using a TUNEL assay kit (Roche Co., USA). Briefly, fresh
cleaned specimens were fixed in 4% formalin and embedded in
paraffin, then cut into 3-μm thick sections, affixed to
silane-coated slides and stained with TUNEL assay kit (Roche Co.)
according to the manufacturer's instructions.
Statistical analysis
Data are presented as the means ± SD from at least
three independent experiments. Statistical analyses were performed
using one-way analysis of variance (ANOVA) followed by Tukey's test
using SPSS 13.0 (SPSS, Chicago, IL, USA). P<0.05 was considered
to indicate statistically significant differences.
Results
Combination treatment of oridonin with
VPA synergistically inhibited the proliferation of HL-60 cells
First, we detected the effects of oridonin and VPA
on the proliferation of HL-60 cells at the indicated concentration
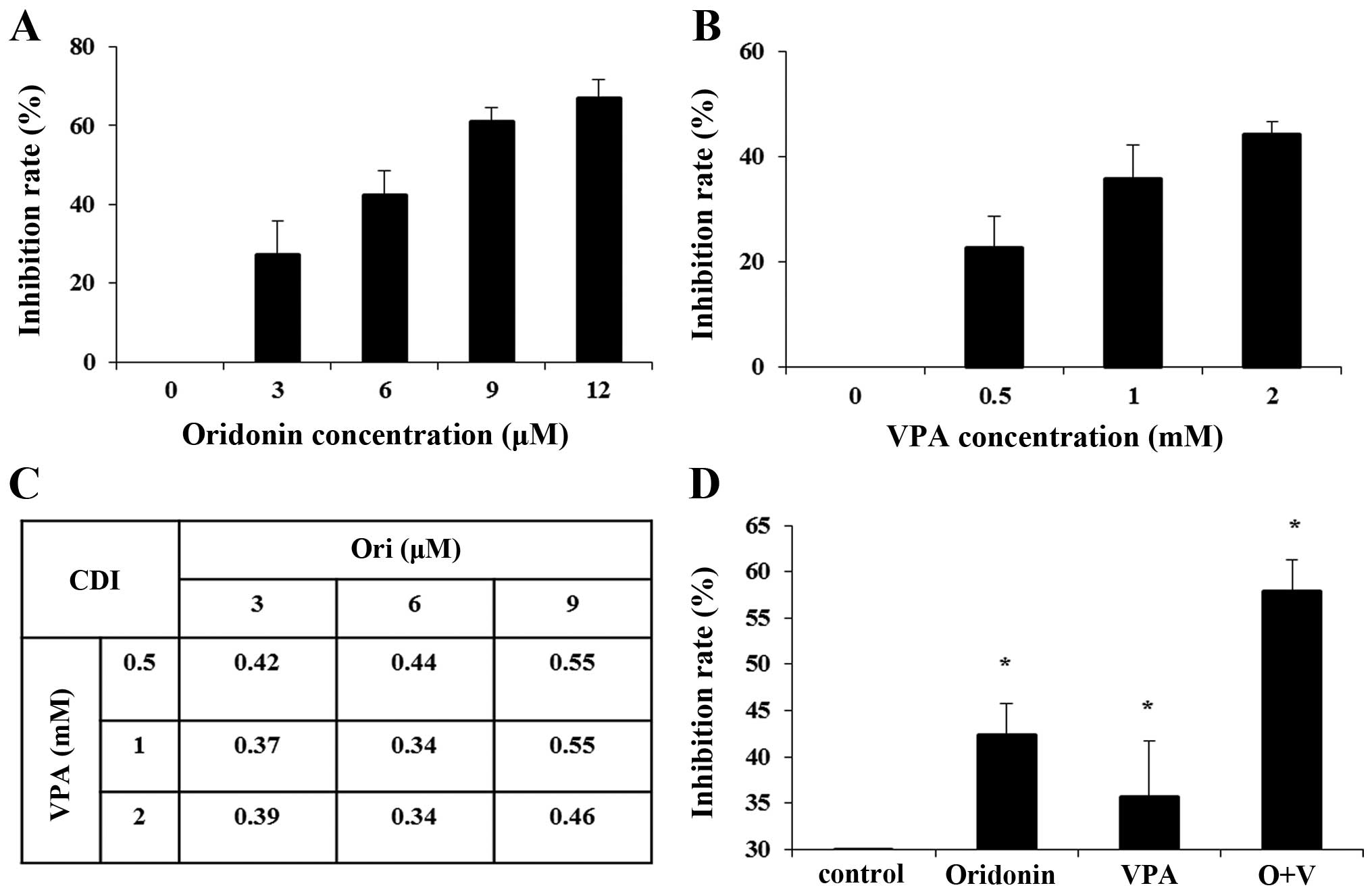
scope. As shown in Fig. 1A,
oridonin inhibited the proliferation of HL-60 cells in a
dose-dependent manner with an IC50 of 6.85 μM at 24 h.
Similarly, CCK-8 assay showed that VPA inhibited cell growth in a
dose-dependent manner with an IC50 of 2.59 mM (Fig. 1B). To detect the inhibitory effect
of the combination treatment, HL-60 cells were exposed to oridonin
(3, 6 and 9 μM) and VPA (0.5, 1 and 2 mM) concurrently for 24 h.
The data showed the CI values were 0.34–0.55 (CI<1), which
implied a synergistic anti-proliferative effect of the combination
group on HL-60 cells (Fig. 1C).
When the concentration of oridonin and VPA was 6 and 1 mM,
respectively, the CI was the minimum value. Thus, we used oridonin
(6 μM) and VPA (1 mM) as the optimal concentration for the
forthcoming experiment. After HL-60 cells were treated with 6 μM
oridonin plus 1 mM VPA for 24 h, the inhibition rate strikingly
increased from 42.34±6.04% for 6 μM oridonin alone, 35.70±6.59% for
1 mM VPA, to 57.94±4.83% (P<0.01) for the combination, which
confirmed that combination treatment exerted a synergistic
inhibitory effect on the proliferation of HL-60 cells (Fig. 1D).
Combination of oridonin plus VPA
synergistically induced the apoptosis of HL-60 cells
To determine the effect of oridonin plus VPA on
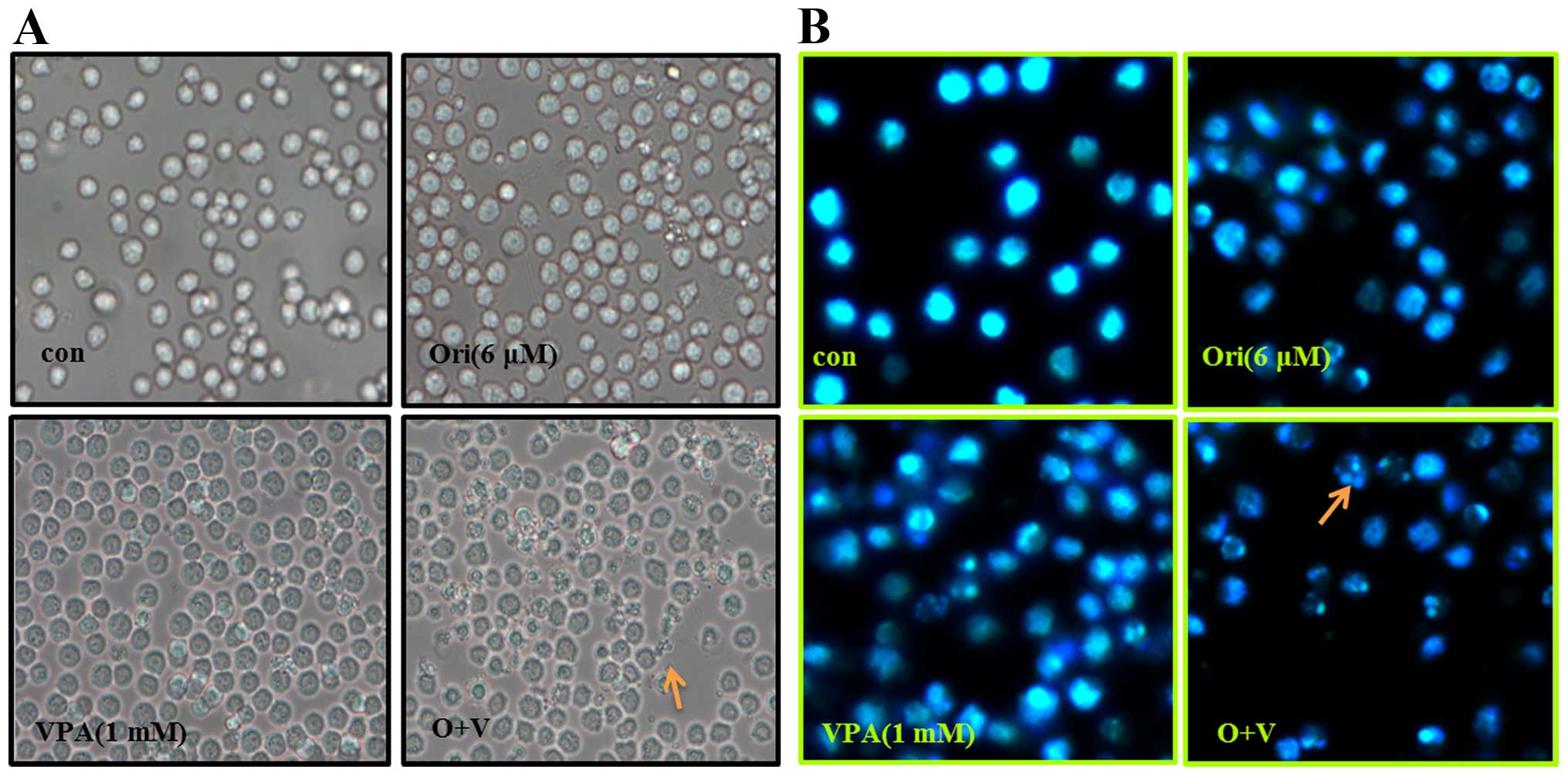
apoptosis in HL-60 cells, morphological changes were observed by
light microscopy, and an inverted fluorescence microscope after
Hoechst-33342 staining. It was noted that part of the cells treated
by oridonin or VPA alone exhibited cell shrinkage, or (and)
apoptotic bodies, which are typical morphological characteristics
of apoptosis (Fig. 2A). The
phenomenon was more evident in cells treated with oridonin and VPA.
In parallel, Hoechst-33342 staining results showed that the nuclei
of control cells were round and exhibited homogeneous blue
fluorescence. While cells treated with oridonin or VPA alone for 24
h appeared with condensed or fragmented nuclei which is
characteristic of cell apoptosis. Moreover, the apoptosis events in
the combination group were more distinguished than each agent alone
(Fig. 2B).
To confirm the enhanced apoptosis induced by
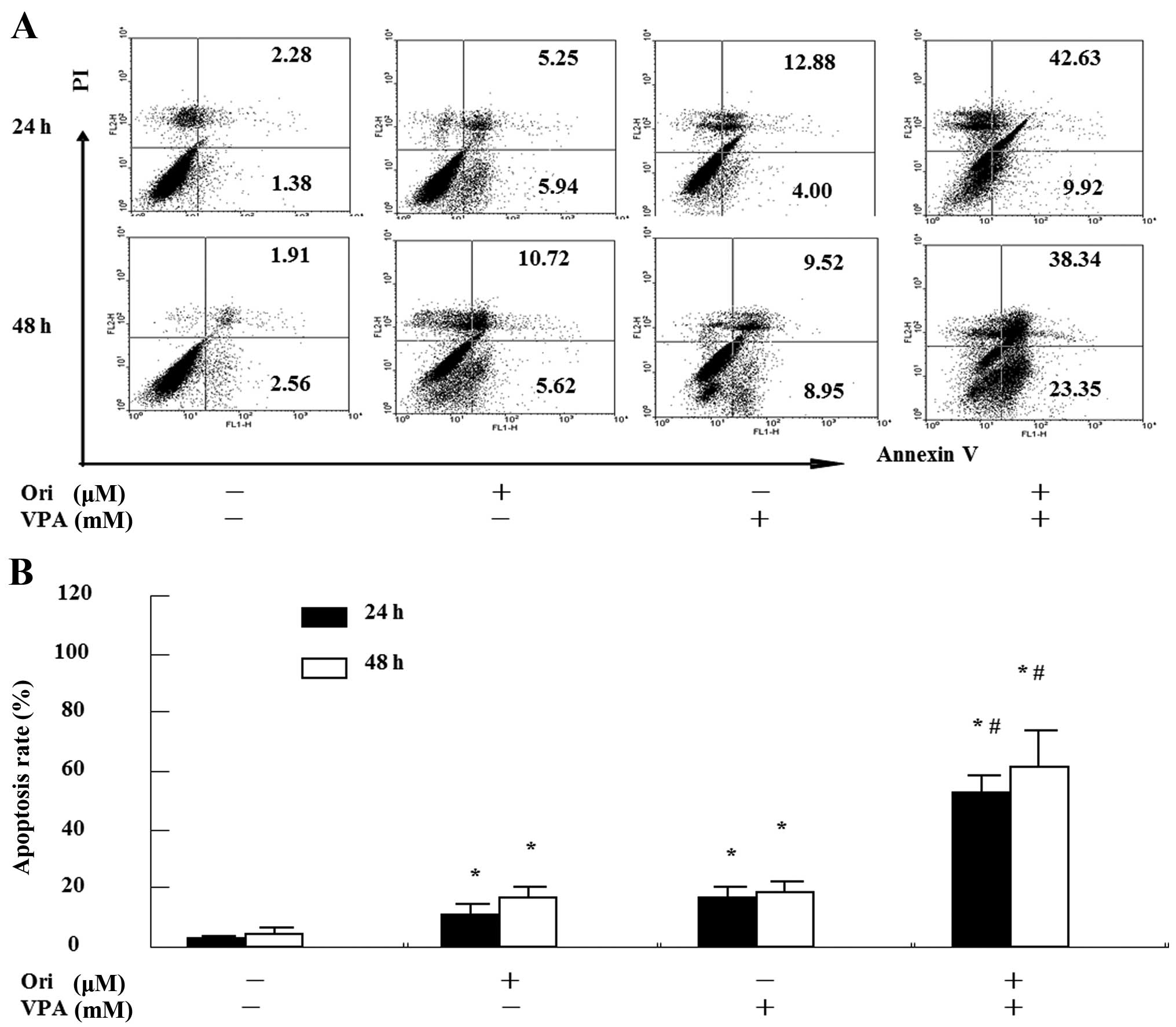
combination treatment, Annexin V/PI assay using flow cytometry was
performed. As seen in Fig. 3, the
total percentage of apoptotic cells was 11.2±2.5% for oridonin,
17.4±2.8% for VPA, but 53.1±4.5% for the combination for 24 h. In
the co-treatment for 48 h, the percentage of apoptotic cells
increased to 16.3±2.1, 19.6±2.4 and 63.8±6.6%, respectively. These
findings suggest that combination of oridonin and VPA exerted a
synergistic effect on the induction of apoptosis in HL-60
cells.
Activation of caspase is important for
combination treatment-triggered cell apoptosis
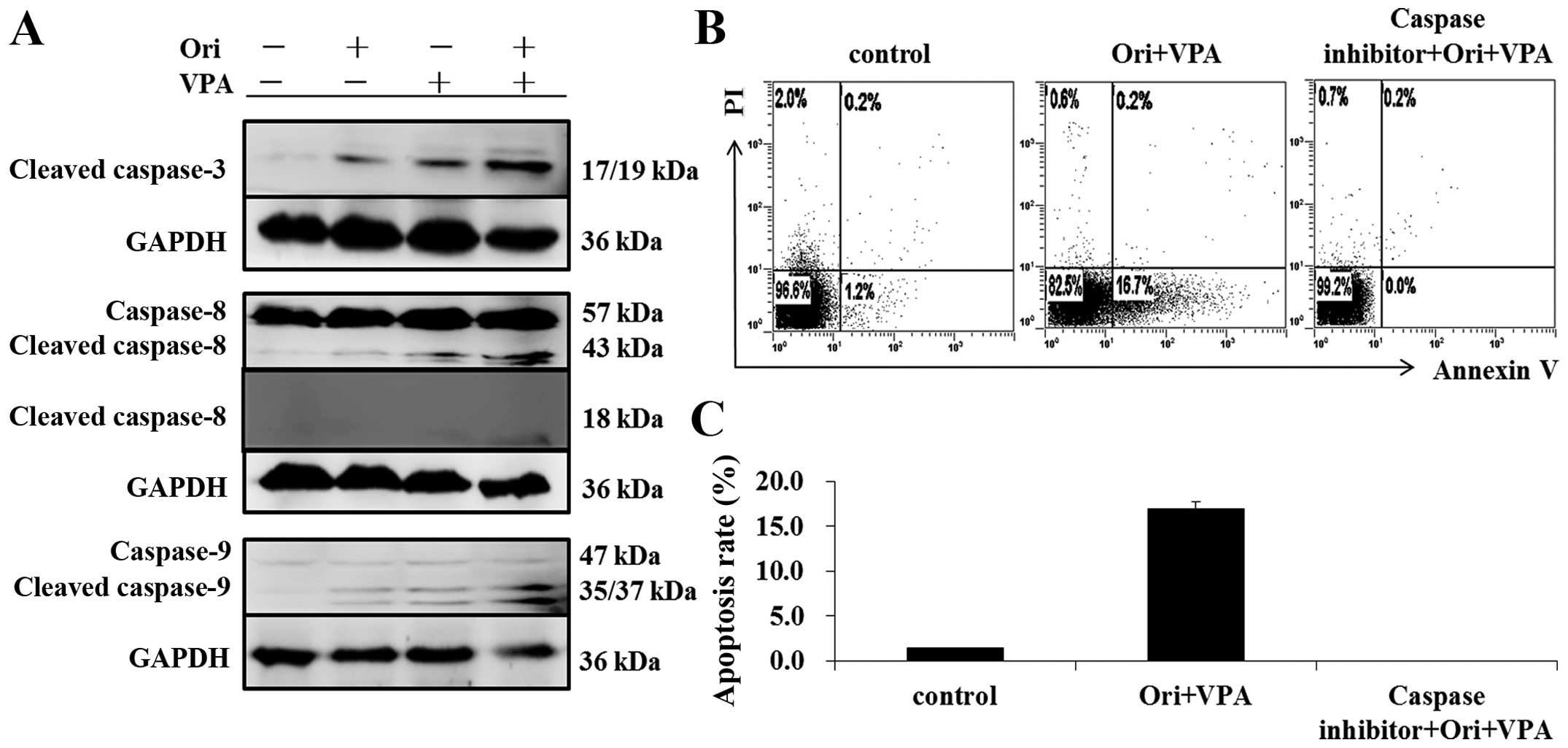
To clarify the mechanism involved in apoptosis
induced by oridonin and VPA, we detected the protein expression of
caspase-8, caspase-9 and cleaved-caspase-3 by western blot
analysis. As shown in Fig. 4A, the
protein expression of cleaved caspase-3, -8, and -9 were
significantly elevated after the combination of oridonin and VPA,
more obviously than that of either agent alone. The results implied
that these caspase family proteins may be involved in the apoptosis
induced by oridonin plus VPA. Furthermore, pretreatment with
caspase-inhibitor (Z-VAD-fmk) completely blocked the
apoptosis-induced by combination treatment, indicating that the
synergistic induction of apoptosis by oridonin and VPA is
caspase-dependent (Fig. 4B and
C).
Combination of oridonin with VPA
triggered the mitochondrial apoptotic pathway in HL-60 cells
Combination treatment induced the activation of
caspase-9, suggesting that intrinsic pathway is involved. As
confirmation, cytosolic cytochrome c was monitored by
western blot analysis. As shown in Fig. 5B, an evident increase of cytochrome
c protein was observed after combined treatment with
oridonin and VPA, suggesting that cytochrome c was released
from mitochondria into cytosol. This result may well account for
the activation of caspase-9.
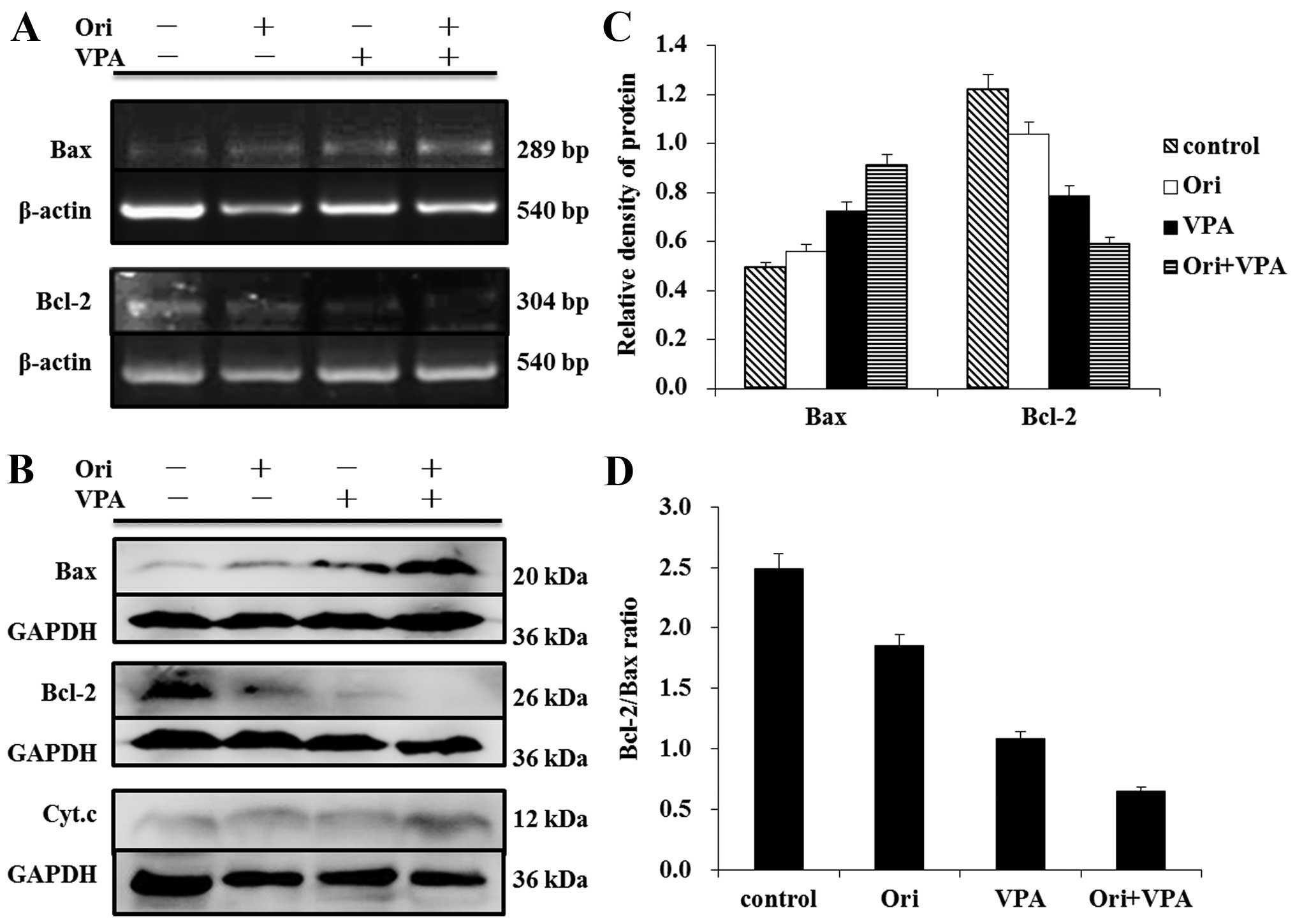
The Bcl-2 family proteins could regulate the release
of cytochrome c from mitochondria (25). To further confirm that the
mitochondrial pathway is involved in oridonin/VPA-induced
apoptosis, the expression of pro-apoptotic factor Bax and
anti-apoptotic factor Bcl-2 were detected at the transcriptional
and post-transcriptional level by RT-PCR and western blot analysis.
As shown in Fig. 5, treatment with
either 6 μM oridonin or 1 mM VPA for 24 h upregulated the
expression of Bax at mRNA and protein levels. Moreover, evident
augmentation was observed in the combination group as compared with
single agent. In contrast, the expression of Bcl-2 decreased more
clearly in combination treated cells than in single agent-treated
cells (Fig. 5A). Consequently, the
ratio of Bcl-2/Bax markedly declined (Fig. 5C). Together, these results
indicated that treatment of HL-60 cells with oridonin plus VPA
resulted in increased activation of the intrinsic mitochondrial
apoptotic pathway.
Combined treatment-induced apoptosis is
mediated through the Fas-mediated pathway
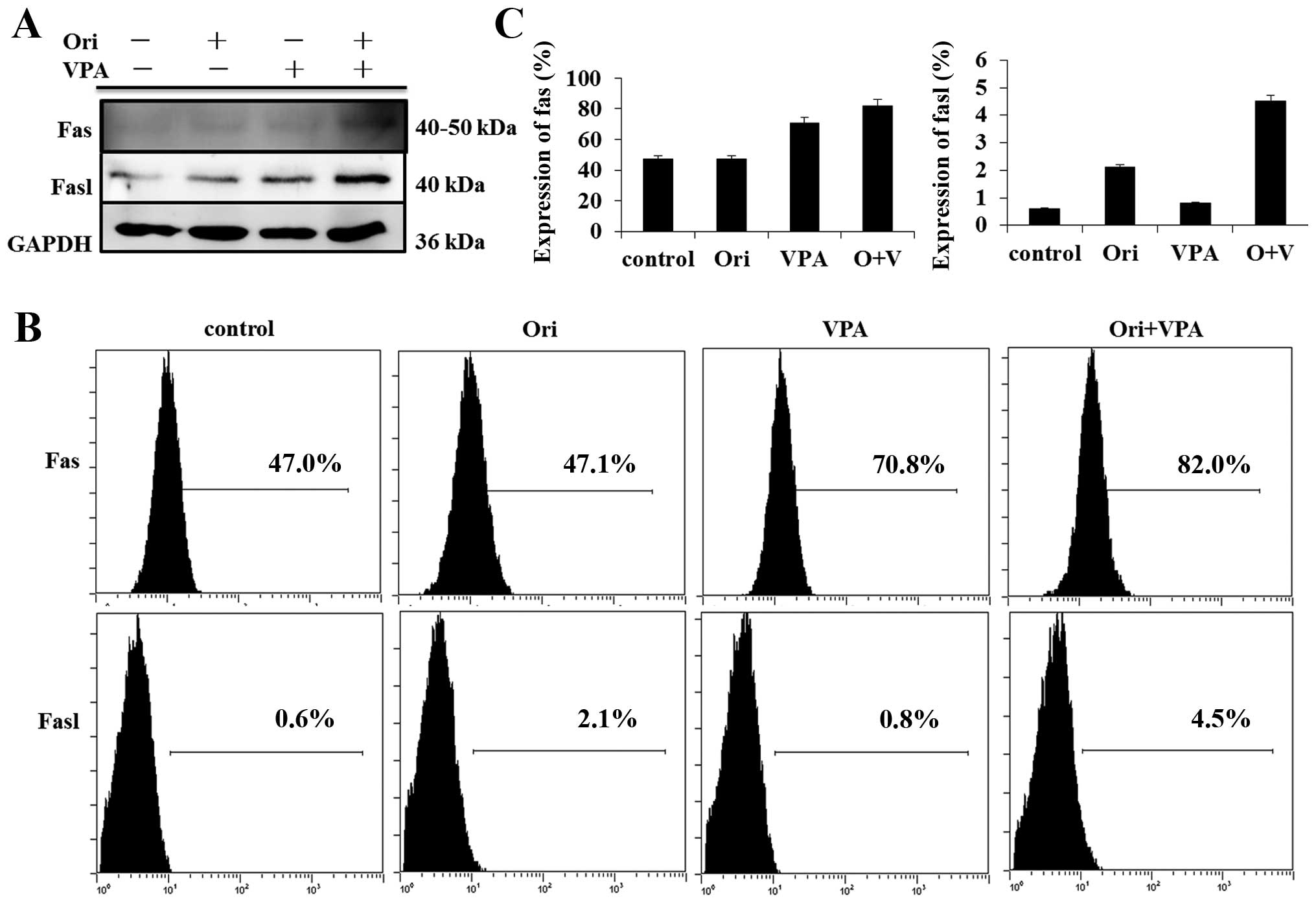
To characterize the role of the extrinsic pathway in
oridonin plus VPA-induced apoptosis, we detected Fas and FasL
protein by western blot analysis. The results showed that exposure
to oridonin or VPA alone triggered the Fas and FasL expression and
combination treatment led to stronger increase of their expression
(Fig. 6B). In parallel, the
expression of Fas and FasL was assessed by FCM. The data presented
herein suggest that activation of the extrinsic Fas-related pathway
plays a major role in the enhanced apoptosis observed in oridonin
plus VPA-treated cells.
The MAPK signaling pathway is involved in
apoptosis of HL-60 cells induced by oridonin plus VPA
To further elucidate the intracellular mechanisms
modulated by oridonin combined with VPA in the apoptosis of HL-60
cells, key proteins involved in MAPK signal pathway were examined
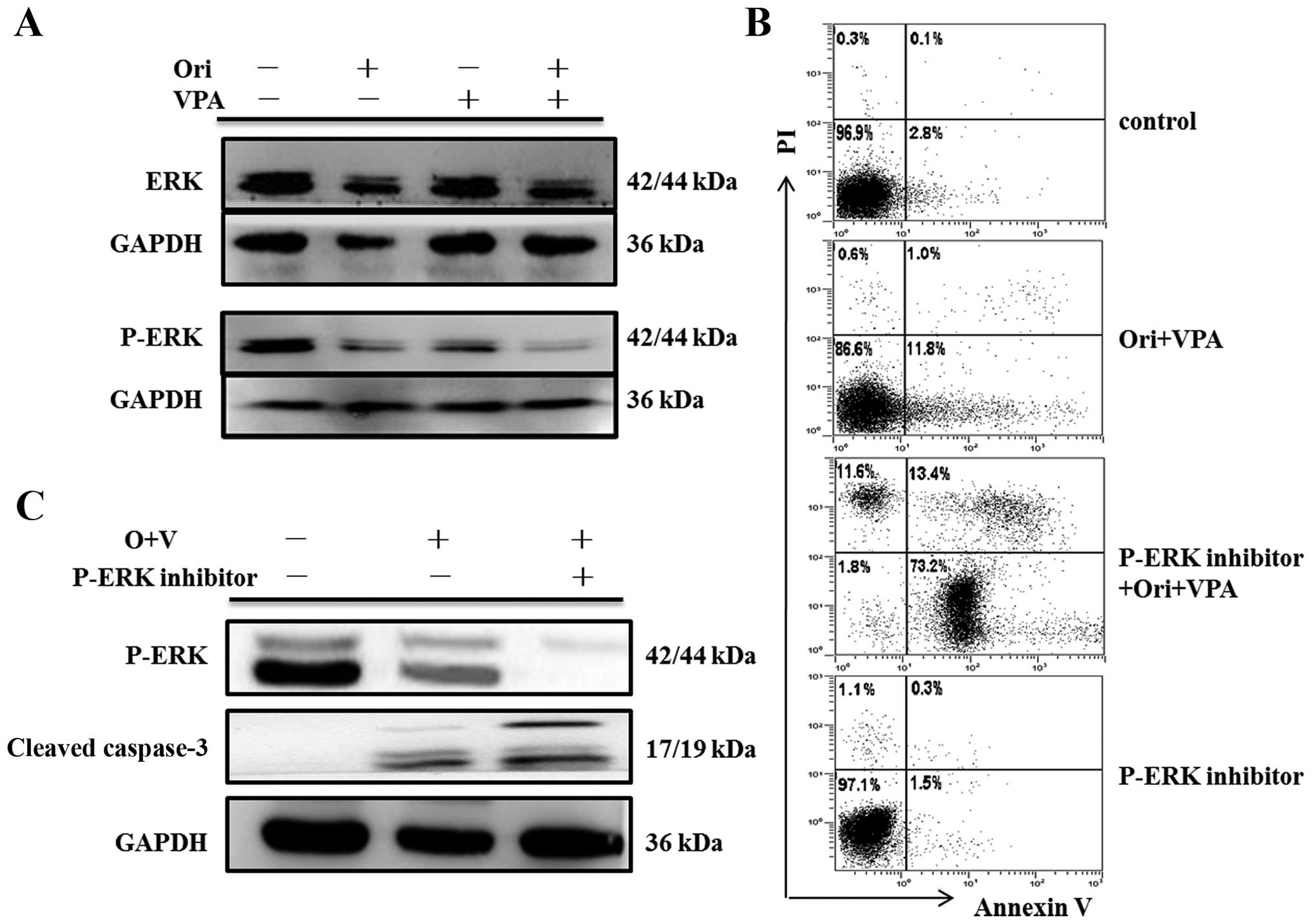
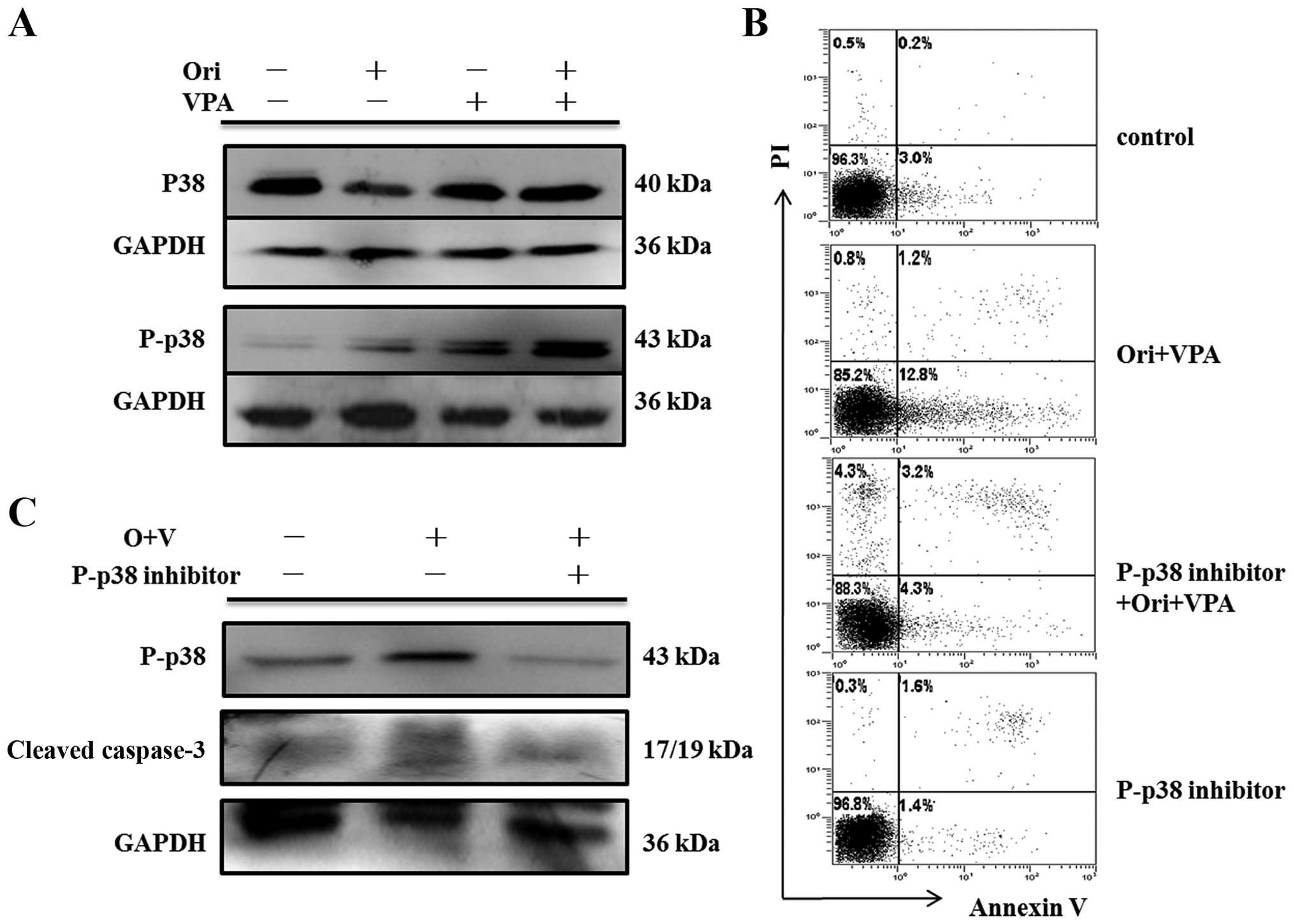
by western blot assay. As shown in Figs. 7Figure 8–9, there were no detectable changes in
expression of total ERK, p38, JNK and phosphorylated-JNK protein.
In contrast, phosphorylation of ERK was reduced, and
phosphorylation of p38 was increased.
To further confirm that the apoptotic effect of
combination therapy on AML cells was associated with the MAPK
pathway, HL-60 cells were pre-treated with the specific inhibitors
SP600125 (JNK inhibitor), SB203580 (p38 inhibitor) and PD98059 (ERK
inhibitor) respectively, for 30 min to block the three pathways,
and then treated with 6 μM oridonin and 1 mM VPA for 24 h. Then,
apoptosis analysis and western blot analysis for detecting the
expression of cleaved caspase-3 were conducted. As seen in Fig. 7C, PD98059 attenuated levels of
phospho-ERK in oridonin/VPA-treated cells, but enhanced apoptosis
induced by oridonin/VPA (Fig. 7B),
concomitant with elevated expression of cleaved caspase-3 (Fig. 7C). As expected, SB203580 blocked
expression of phospho-p38 and protected HL-60 cells from
combination-induced apoptosis, accompanied by downregulation of
cleaved caspase-3 (Fig. 8B and C).
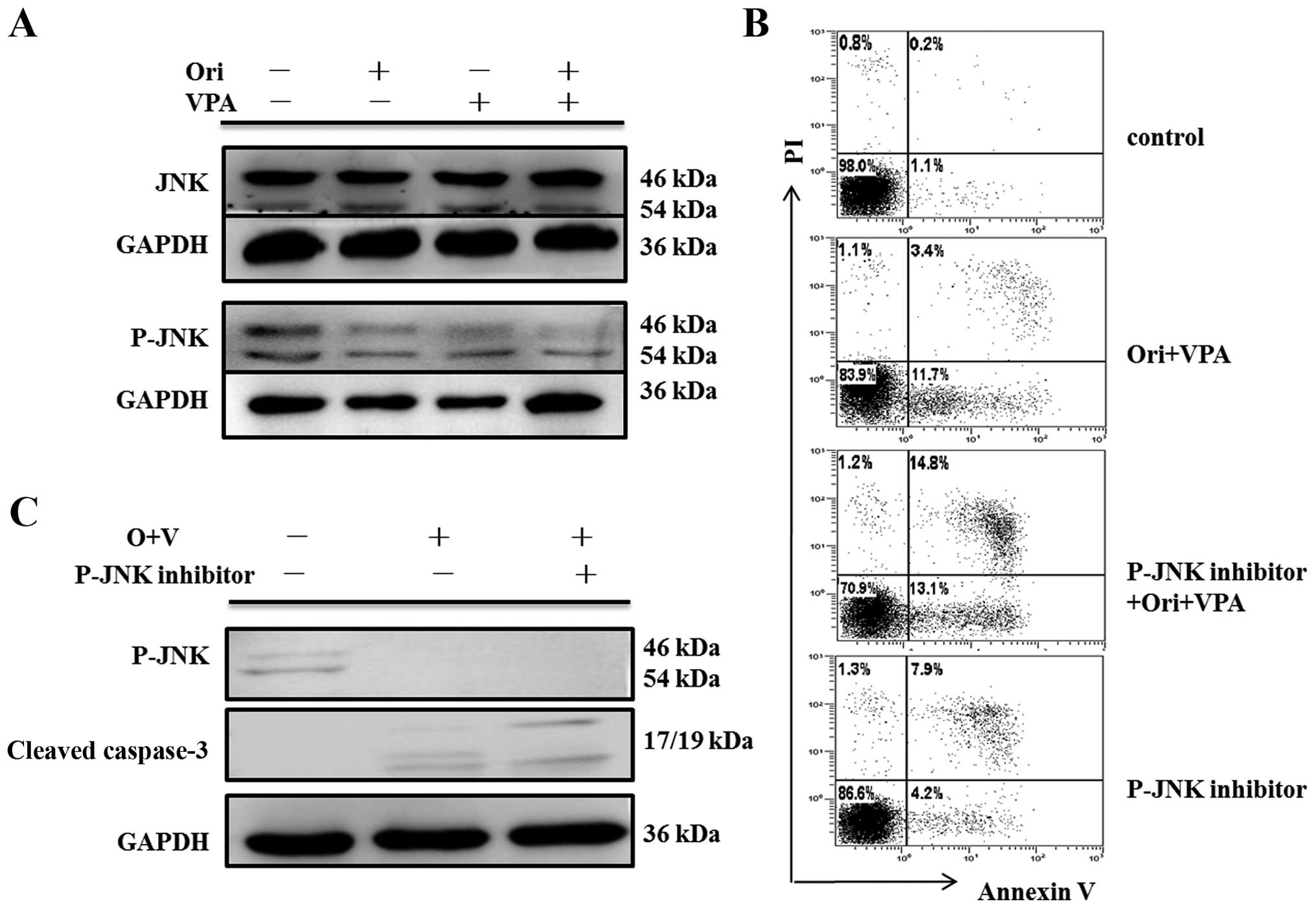
While no change of apoptosis after pre-treatment with SP600125 was
observed though JNK signaling pathway blocking (Fig. 9B), neither was there any activity
of caspase-3 (Fig. 9C). These
findings strongly indicated that ERK and P38 MAPK may control
oridonin/VPA-induced apoptosis as upstream regulators (Figs. 7Figure 8–9).
Effects of oridonin combined with VPA in
vivo
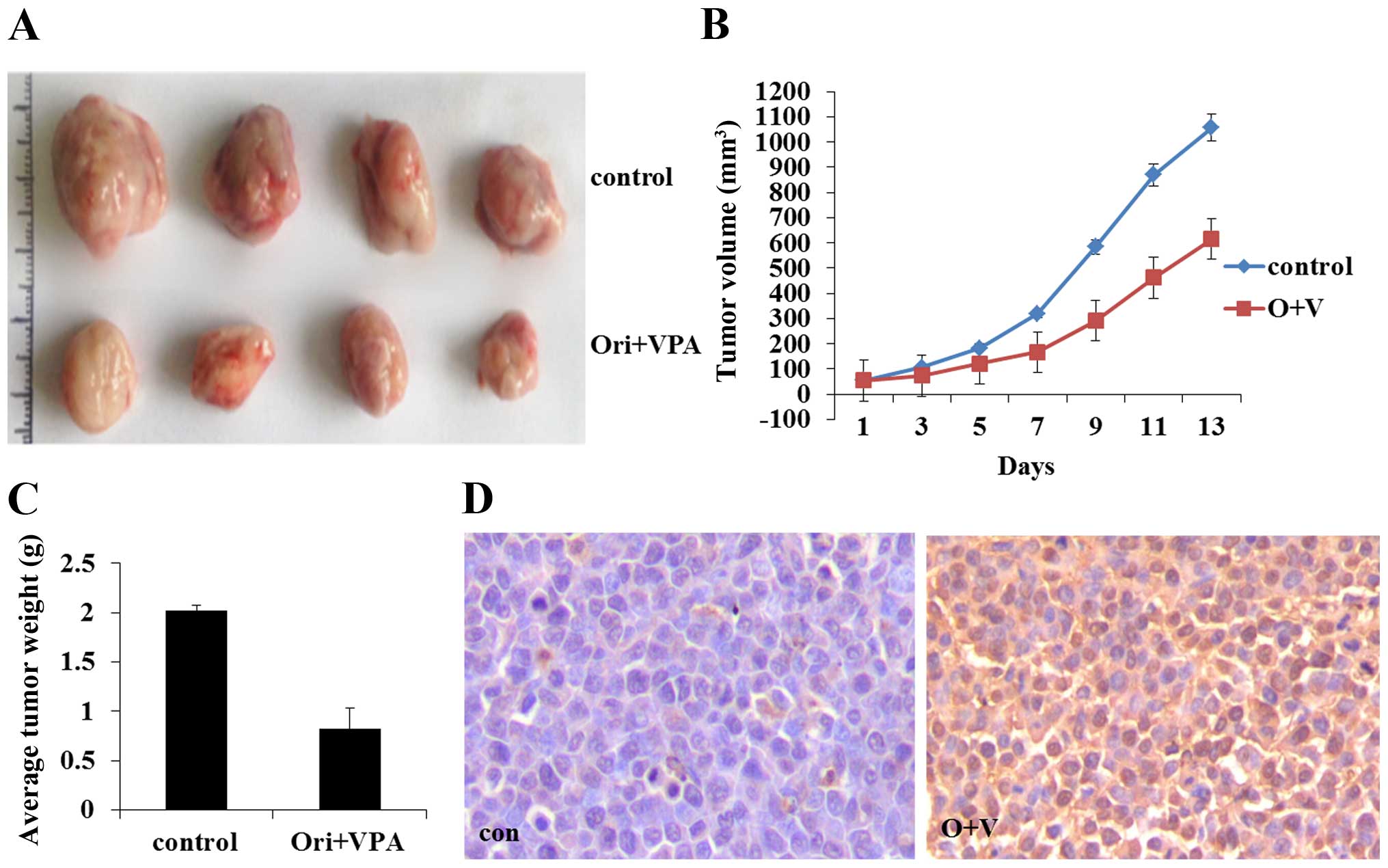
All nude mice were inoculated subcutaneously with
HL-60 cells and developed palpable tumor after a mean of 10 days.
Then the mice were treated with oridonin and VPA. Tumor volume and
tumor weight were measured and are presented as the mean ± SE. As
shown in Fig. 10A, compared with
the control group, combination treatment significantly reduced the
tumors size and the tumor weight (P<0.05) (Fig. 10). The results indicated that
combined treatment with oridonin and VPA also exerted a significant
effect on proliferation inhibition in vivo. TUNEL assay
revealed that combination of oridonin and VPA could induce
apoptosis of leukemic cells, in accord with the results acquired
in vitro (Fig. 10D).
Discussion
Due to the side effects and frequent acquisition of
drug resistance, the clinical outcome for AML remains discouraging
despite standard treatment. Therefore, novel therapeutic approaches
that act more selectively and more potently are urgently needed. It
has been well documented that either oridonin or VPA exerts
significant anti-leukemia activity (13,14,20–22).
In addition, each of them has been proved to enhance the anticancer
effect of other anti-neoplastic agents (13,14,17).
Nevertheless, there is no report on the activity and mechanism of
the combination of oridonin and VPA in AML cells. In the present
study, we demonstrated that the combined exposure of human leukemia
HL-60 cells to relatively low dose of oridonin and the histone
deacetylase inhibitor VPA exerts a synergistic effect on
proliferation inhibition, caspase activation, and apoptosis.
Moreover, the study revealed that the combination treatment-induced
apoptosis is associated with inhibition of ERK signaling as well as
activation of p38 MAPK signaling pathway. Finally, in vivo
studies demonstrated that tumor growth in a mouse model could be
inhibited by the combination therapy.
Classical apoptosis may occur by two major pathways:
the intrinsic (mitochondrial-mediated) and the extrinsic
(receptor-mediated) pathway (26).
The intrinsic pathway is characterized by change of mitochondrial
membrane, resulting in the release of cytochrome c and
activation of caspase-9 and downstream effectors caspase-3 and (or)
-7. The extrinsic pathway involves the binding of death ligands
such as Fas ligand or TNF-α to their corresponding death receptor,
the cleavage of caspase-8 and then the activation of downstream
effectors caspase-3 and (or) -7.
Previous studies demonstrated that oridonin induces
caspase-3 activation and apoptosis via mitochondrial pathway in the
gastric cancer cell line HGC-27 (26) and in gallbladder cancer cells lines
(27). Consistent with these
reports, we also observed increased expression of cleaved caspase-3
and -9 in HL-60 cells after exposure to oridonin for 24 h. Just as
a preceding report (28),
caspase-3 and caspase-9 were both activated after HL-60 cells were
treated with VPA alone. Moreover, co-administration of oridonin
with VPA led to increased activation of caspase-3 and -9. The
findings suggested that mitochondrial pathway may contribute to
enhanced apoptosis induced by combination therapy. Moreover, we
observed elevated expression of Bax and reduced expression of
Bcl-2, consequently downregulation of the Bcl-2/Bax ratio, together
with upregulation of cytochrome c in the cytosol. These
results further confirmed that oridonin/VPA-induced apoptosis is
associated with the activation of mitochondrial pathway.
In parallel, we found that oridonin/VPA markedly
induced the expression of cleaved caspase-8 fragments, concomitant
with upregulation of Fas and FasL proteins. Hence, we consider that
combination treatment induces apoptosis of HL-60 cells by the
specific activation of Fas signaling pathway.
Despite pivotal role of caspases in apoptosis, new
data also implicate that apoptosis can occur in the absence of
caspase activation (25). To
verify the role of caspase activation on combined treatment-induced
apoptosis, HL-60 cells were treated with oridonin and VPA in the
presence or absence of the pan-caspase inhibitor Z-VAD-FMK. The
results indicate that the pan-caspase inhibitor completely
prevented apoptosis induced by oridonin/VPA, suggesting that
combination treatment induced apoptosis in a caspase-dependent
manner.
The MAPK pathway is implicated to play an important
role in proliferation, differentiation, development, transformation
and apoptosis (29). In mammals,
the MAPK family is divided into three major subfamilies, namely
ERK, JNK and p38 (30). Indeed,
constitutive ERK1/2 activation has been claimed to play an
important role in the progression of tumorigenesis in AML (31). Inhibition of phosphate-ERK will
induce acute myeloid leukemia apoptosis (32). Previous studies have revealed that
oridonin is able to inhibit ERK signaling pathway in osteosarcoma
cells (33), while activating ERK
signaling pathway in HepG2 cells (35). In the present study, we found that
oridonin inhibited the expression of p-ERK in HL-60 cells. The
difference of the effect of oridonin on ERK signaling pathway may
be attributed to the specificity of cell type. Moreover, combined
treatment with oridonin and VPA could more significantly suppress
the phosphorylation of ERK1/2 in apoptosis induction. Finally,
pretreatment with ERK inhibitor enhanced apoptosis and activity of
caspase-3 induced by oridonin/VPA, which suggested that ERK acts
upstream of caspase-3 in the apoptotic process induced by
oridonin/VPA. Since activation of ERK indirectly allowed Bcl-2 to
form homo-dimers to produce an anti-apoptotic effect (35) and herein Bcl-2 was found to be
significantly downregulated, we speculated that ERK signaling may
participate in apoptosis of HL-60 cells through downregulating the
expression of Bcl-2 protein. It has been demonstrated that p38 and
JNK are more sensitive to stress stimuli ranging from osmotic shock
to inflammatory cytokines and are mostly activated during
drug-induced apoptosis of leukemia cell lines (36). Evidence has been shown that
oridonin is able to activate p38 MAPK and JNK signaling pathways in
osteosarcoma cells (34) or HepG2
cells (34). One recent report has
demonstrated that oridonin induces apoptosis in SW1990 pancreatic
cancer cells via caspase-dependent induction of p38 MAPK (37). The activation of p38 was
demonstrated to affect apoptotic pathway through inhibiting the
expression and phosphorylation of Bcl-2 protein in human hepatoma
cells (38). In the present study
we found p38 MAPK also participated in oridonin/VPA-induced
apoptosis, which was supported by upregulation of phosphorylation
of p38 and complete block of apoptosis by p38 inhibitor. In
addition, downregulation Bcl-2 may account for the mechanism by
which p38 MAPK signaling affects apoptotic procedure. These
findings may suggest that combination of VPA and oridonin can
enhance the apoptosis induced by oridonin through p38 pathway.
While our data showed that no remarkable change in P-JNK was
observed. Similarly, no appreciable effect on the apoptosis and
cleaved caspase-3 fragment was detected after pretreatment with JNK
inhibitor SP600125, which confirmed that apoptosis herein was not
associated with JNK pathways.
To further confirm the effect of oridonin plus VPA
on proliferation and apoptosis, our present study highlighted the
synergistic anti-leukemia effect of oridonin in combination with
VPA in vivo models in AML. The results indicated that
treatment with oridonin and VPA resulted in significant reduction
of tumor size and tumor weight of HL-60 xenograft mice, accompanied
by cell apoptosis in tumor tissue.
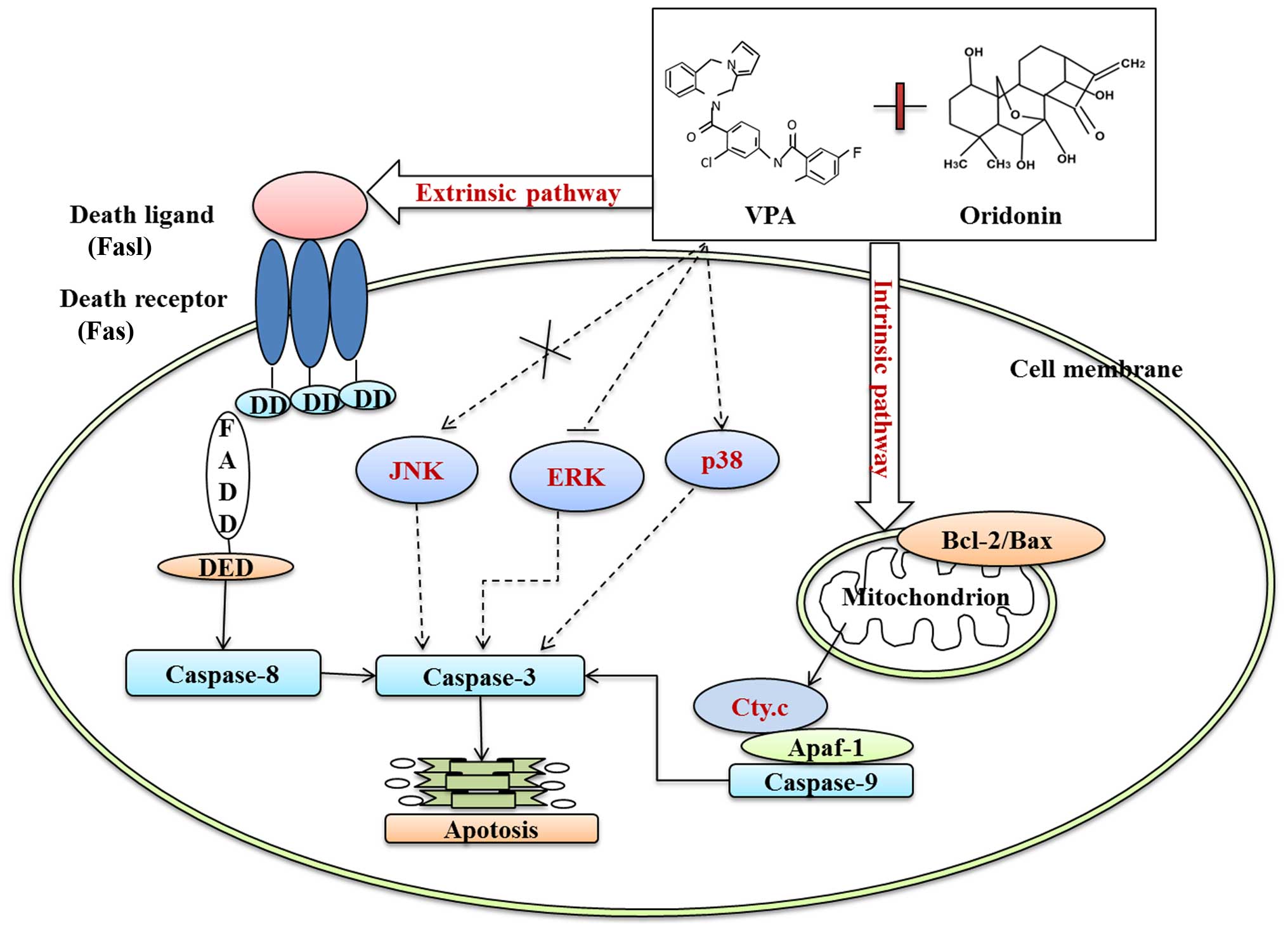
In conclusion, oridonin plus VPA exerted more
synergistic effect on inhibition of proliferation and apoptosis
than that of each of them alone. Mechanically, oridonin plus VPA
induced obvious caspase-dependent apoptosis by activation of the
intrinsic apoptosis pathway, as evidenced by the downregulation of
Bcl-2/Bax ratio, cytochrome c release and caspase-9
activation, as well as through the extrinsic apoptosis pathway by
triggering Fas/FasL and caspase-8 activation. In addition,
downregulation of p-ERK and upregulation of p-p38 also participated
in enhanced apoptosis of HL-60 cells induced by oridonin plus VPA
(Fig. 11). The results presented
herein indicate that combination treatment with oridonin and VPA
may be a potent strategy for targeted treatment of AML.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (nos. 81101605, 81573467), the ‘Twelfth
Five-Year’ National Science and Technology Support Program
(2013BAI07B02), the Natural Science Foundation of Shandong Province
of China (ZR2011HL045, ZR2015YL028, 2015ZRC03102) and the Project
for Laureate of Taishan Scholar.
References
|
1
|
Lengfelder E, Hofmann WK and Nolte F:
Management of elderly patients with acute promyelocytic leukemia:
Progress and problems. Ann Hematol. 92:1181–1188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Z, Chen GQ, Shen ZX, Chen SJ and Wang
ZY: Treatment of acute promyelocytic leukemia with arsenic
compounds: In vitro and in vivo studies. Semin Hematol. 38:26–36.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang ME, Ye YC, Chen SR, Chai JR, Lu JX,
Zhoa L, Gu LJ and Wang ZY: Use of all-trans retinoic acid in the
treatment of acute promyelocytic leukemia. Blood. 72:567–572.
1988.PubMed/NCBI
|
|
4
|
Zhang L and Zhu X: Epidemiology, diagnosis
and treatment of acute promyelocytic leukemia in children: The
experience in china. Mediterr J Hematol Infect Dis. 4:e20120122012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tallman MS and Nabhan C: Management of
acute promyelocytic leukemia. Curr Oncol Rep. 4:381–389. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Douer D: Advances in the treatment of
relapsed acute promyelocytic leukemia. Acta Haematol. 107:1–17.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tallman MS, Nabhan C, Feusner JH and Rowe
JM: Acute promyelocytic leukemia: Evolving therapeutic strategies.
Blood. 99:759–767. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanz MA, Martín G and Lo Coco F: Choice of
chemotherapy in induction, consolidation and maintenance in acute
promyelocytic leukaemia. Best Pract Res Clin Haematol. 16:433–451.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nasr R, Lallemand-Breitenbach V, Zhu J,
Guillemin MC and de Thé H: Therapy-induced PML/RARA proteolysis and
acute promyelocytic leukemia cure. Clin Cancer Res. 15:6321–6326.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tomita A, Kiyoi H and Naoe T: Mechanisms
of action and resistance to all-trans retinoic acid (ATRA) and
arsenic trioxide (As2O3) in acute
promyelocytic leukemia. Int J Hematol. 97:717–725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hillestad LK: Acute promyelocytic
leukemia. Acta Med Scand. 159:189–194. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu JJ, Wu XY, Peng J, Pan XL and Lu HL:
Antiproliferation effects of oridonin on HL-60 cells. Ann Hematol.
83:691–695. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao F, Tang Q, Yang P, Fang Y, Li W and Wu
Y: Apoptosis inducing and differentiation enhancement effect of
oridonin on the all-trans-retinoic acid-sensitive and -resistant
acute promyelocytic leukemia cells. Int J Lab Hematol. 32(1p1):
e114–e122. 2010. View Article : Google Scholar
|
|
14
|
Zhou GB, Kang H, Wang L, Gao L, Liu P, Xie
J, Zhang FX, Weng XQ, Shen ZX, Chen J, et al: Oridonin, a
diterpenoid extracted from medicinal herbs, targets AML1-ETO fusion
protein and shows potent antitumor activity with low adverse
effects on t(8;21) leukemia in vitro and in vivo. Blood.
109:3441–3450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao FH, Hu XH, Li W, Liu H, Zhang YJ, Guo
ZY, Xu MH, Wang ST, Jiang B, Liu F, et al: Oridonin induces
apoptosis and senescence in colorectal cancer cells by increasing
histone hyperacetylation and regulation of p16, p21, p27 and c-myc.
BMC Cancer. 10:6102010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen G, Wang K, Yang BY, Tang B, Chen JX
and Hua ZC: Synergistic antitumor activity of oridonin and arsenic
trioxide on hepatocellular carcinoma cells. Int J Oncol.
40:139–147. 2012.
|
|
17
|
Marks PA, Richon VM and Rifkind RA:
Histone deacetylase inhibitors: Inducers of differentiation or
apoptosis of transformed cells. J Natl Cancer Inst. 92:1210–1216.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Konopleva M, Contractor R, Kurinna SM,
Chen W, Andreeff M and Ruvolo PP: The novel triterpenoid CDDO-Me
suppresses MAPK pathways and promotes p38 activation in acute
myeloid leukemia cells. Leukemia. 19:1350–1354. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Phiel CJ, Zhang F, Huang EY, Guenther mg,
Lazar MA and Klein PS: Histone deacetylase is a direct target of
valproic acid, a potent anticonvulsant, mood stabilizer, and
teratogen. J Biol Chem. 276:36734–36741. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cimino G, Lo-Coco F, Fenu S, Travaglini L,
Finolezzi E, Mancini M, Nanni M, Careddu A, Fazi F, Padula F, et
al: Sequential valproic acid/all-trans retinoic acid treatment
reprograms differentiation in refractory and high-risk acute
myeloid leukemia. Cancer Res. 66:8903–8911. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang R, Faussat AM, Majdak P, Perrot JY,
Chaoui D, Legrand O and Marie JP: Valproic acid inhibits
proliferation and induces apoptosis in acute myeloid leukemia cells
expressing P-gp and MRP1. Leukemia. 18:1246–1251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu J, Bi G, Wen P, Yang W, Ren X, Tang T,
Xie C, Dong W and Jiang G: Down-regulation of CD44 contributes to
the differentiation of HL-60 cells induced by ATRA or HMBA. Cell
Mol Immunol. 4:59–63. 2007.PubMed/NCBI
|
|
24
|
Shimizu S, Narita M and Tsujimoto Y: Bcl-2
family proteins regulate the release of apoptogenic cytochrome c by
the mitochondrial channel VDAC. Nature. 399:483–487. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Constantinou C, Papas KA and Constantinou
AI: Caspase-independent pathways of programmed cell death: The
unraveling of new targets of cancer therapy? Curr Cancer Drug
Targets. 9:717–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun KW, Ma YY, Guan TP, Xia YJ, Shao CM,
Chen LG, Ren YJ, Yao HB, Yang Q and He XJ: Oridonin induces
apoptosis in gastric cancer through Apaf-1, cytochrome c and
caspase-3 signaling pathway. World J Gastroenterol. 18:7166–7174.
2012. View Article : Google Scholar
|
|
27
|
Bao R, Shu Y, Wu X, Weng H, Ding Q, Cao Y,
Li M, Mu J, Wu W, Ding Q, et al: Oridonin induces apoptosis and
cell cycle arrest of gallbladder cancer cells via the mitochondrial
pathway. BMC Cancer. 14:2172014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawagoe R, Kawagoe H and Sano K: Valproic
acid induces apoptosis in human leukemia cells by stimulating both
caspase-dependent and -independent apoptotic signaling pathways.
Leuk Res. 26:495–502. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramos S: Cancer chemoprevention and
chemotherapy: Dietary polyphenols and signalling pathways. Mol Nutr
Food Res. 52:507–526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lunghi P, Tabilio A, Dall'Aglio PP, Ridolo
E, Carlo-Stella C, Pelicci PG and Bonati A: Downmodulation of ERK
activity inhibits the proliferation and induces the apoptosis of
primary acute myelogenous leukemia blasts. Leukemia. 17:1783–1793.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu J, Wong WW, Khosravi F, Minden MD and
Penn LZ: Blocking the Raf/MEK/ERK pathway sensitizes acute
myelogenous leukemia cells to lovastatin-induced apoptosis. Cancer
Res. 64:6461–6468. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jin S, Shen JN, Wang J, Huang G and Zhou
JG: Oridonin induced apoptosis through Akt and MAPKs signaling
pathways in human osteosarcoma cells. Cancer Biol Ther. 6:261–268.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang H, Ye Y, Chui JH, Zhu GY, Li YW, Fong
DW and Yu ZL: Oridonin induces G2/M cell cycle arrest and apoptosis
through MAPK and p53 signaling pathways in HepG2 cells. Oncol Rep.
24:647–651. 2010.PubMed/NCBI
|
|
35
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar
|
|
36
|
Henson ES and Gibson SB: Surviving cell
death through epidermal growth factor (EGF) signal transduction
pathways: Implications for cancer therapy. Cell Signal.
18:2089–2097. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bu HQ, Liu DL, Wei WT, Chen L, Huang H, Li
Y and Cui JH: Oridonin induces apoptosis in SW1990 pancreatic
cancer cells via p53- and caspase-dependent induction of p38 MAPK.
Oncol Rep. 31:975–982. 2014.
|
|
38
|
Chiu CC, Chen JY, Lin KL, Huang CJ, Lee
JC, Chen BH, Chen WY, Lo YH, Chen YL, Tseng CH, et al: p38 MAPK and
NF-kappaB pathways are involved in naphtho[1,2-b] furan-4,5-dione
induced anti-proliferation and apoptosis of human hepatoma cells.
Cancer Lett. 295:92–99. 2010. View Article : Google Scholar : PubMed/NCBI
|