Introduction
Epidemiological studies have indicated that salivary
adenoid cystic carcinoma (SACC) is one of the most common types of
salivary gland cancers in China, accounting for 11% of epithelial
tumours and 27% of malignant tumours (1). Clinical data have shown that the low
long-term survival rate of SACC is associated with perineural
invasion, local recurrence and distant metastasis (2). Chemotherapy is a necessary adjuvant
to surgery; however, ~30% of new cases and 70% of recurrent cases
have platinum-based chemoresistance (3). Thus, there is a growing interest in
determining the mechanistic basis for SACC chemoresistance and
invasive ability to identify potential therapeutic targets.
GPX1 belongs to the glutathione peroxidase (GPxs)
family, the members of which protect the cell membrane from
oxidative DNA damage and maintain the body's balance of
intracellular redox systems by removing excess reactive oxygen
species (ROS) (4). GPX1 modulates
many pathophysiologic processes, and overexpression of GPX1 can
promote cell invasion, migration and cisplatin resistance in
breast, lung, bladder and prostate cancers (5–7).
Nuclear factor-kappaB (NF-κB) may regulate GPX1 transcription and
expression by combining with the GPX1 promoter region (8–10).
However, few studies have reported the tumour-promoting role of
GPX1 in head and neck cancers.
1,25-dihydroxyvitamin (1,25D3) is the active form of
vitamin D, which acts as the steroid hormone calcitriol and carries
out multiple cellular functions, and 1,25D3 regulates numerous
cellular pathways related to cancer risk and prognosis (11). Clinical studies have suggested that
vitamin D deficiency increases the risk of developing cancer and
that abundant vitamin D can reduce cancer incidence and improve
cancer prognosis and outcome (12,13).
Furthermore, it was reported that 1,25D3 could inhibit NF-κB
expression in B lymphocytes, oral squamous cell carcinoma, prostate
cancer and melanoma (14–17).
In the preseent study, we first investigated the
biological effects of GPX1 on SACC cell lines. Next, we detected
whether the NF-κB pathway was involved in these effects. Finally,
we explored whether 1,25D3 alleviates SACC progression by
suppressing GPX1 expression through the NF-κB signalling
pathway.
Materials and methods
The present research was conducted in accordance
with the Declaration of Helsinki and the Guide for the Care and Use
of Laboratory Animals as adopted and promulgated by the United
National Institutes of Health. It has also been approved by the
authors' institutional review board.
Cell lines and cell cultures
SACC cell lines (ACC-M, SACC-83 and ACC-2) were
purchased from the Cell Bank of the Experimental Animal Center (Sun
Yat-Sen University, Guangzhou, China). The cells were cultured in
RPMI-1640 medium (Gibco-BRL, Grand Island, NY, USA) supplemented
with 10% fetal bovine serum (FBS) in a 37°C humidified incubator
containing 5% CO2.
Reagents and chemicals
The vitamin D metabolite 1,25D3 (Sigma, St. Louis,
MO, USA) was dissolved at a concentration of 400 μM in anhydrous
alcohol (AA) for preservation. Immediately prior to use, the stock
was diluted to a final concentration of 30 nM in culture medium. An
NF-κB inhibitor (BAY 11-7082) was purchased from Beyotime Institute
of Biotechnology (Shanghai, China).
Small interfering RNA (siRNA) and
overexpression vector
The GPX1 overexpression vector, the GPX1 antisense,
and the scrambled siRNA were designed and synthesized by Invitrogen
(Carlsbad, CA, USA). The GPX1 siRNA sequences used are listed in
Table I. Full-length GPX1 coding
sequences were PCR-amplified and cloned into a pcDNA3.1 expression
vector (Invitrogen) according to the manufacturer's guidelines. DNA
sequencing was used to verify the constructs. siRNA transfection
was performed using Lipofectamine 2000 reagent (Invitrogen)
according to the manufacturer's instructions. Subsequent real-time
polymerase chain reaction or western blot analysis was performed to
verify changes in GPX1 expression. In the present study, the
experimental group was transfected with the antisense GPX1 siRNA or
GPX1 overexpression vector, whereas the control group was
transfected with a corresponding scrambled sequence.
 | Table IGPX1 siRNA sequences. |
Table I
GPX1 siRNA sequences.
| Sequence | Forward | Reverse |
|---|
| GPX1 antisense |
5′-GGUACUACUUAUCGAGAAUTT-3′ |
5′-AUUCUCGAUAAGUAGUACCTT-3′ |
| GPX1 NC |
5′-UUCUCCGAACGUGUCACGUTT-3′ |
5′-ACGUGACACGUUCGGAGAATT-3′ |
RNA extraction and PCR
Total RNA was extracted using TRIzol™ reagent
(Sigma-Aldrich, Arklow, Ireland) according to the manufacturer's
instructions and was then reverse-transcribed into cDNA using a
PrimeScript RT reagent kit (Takara Bio Inc., Shiga, Japan). The
newly synthesized cDNA was then used as a template for
detection.
Quantitative PCR was carried out using the
SYBR-Green method (Takara Bio) in a CFX96 Real-Time PCR Detection
system (Bio-Rad Laboratories, Hercules, CA, USA). The primers
(Table II) were purchased from
Takara Bio, and GAPDH was used as an endogenous control.
Amplification was performed according to the manufacturer's
protocol (Takara Bio).
 | Table IIPrimer sequences for qPCR. |
Table II
Primer sequences for qPCR.
| Gene | Forward primer | Reverse primer |
|---|
| GPX1 |
5′-GCGGGGCAAGGTACTACTTA-3′ |
5′-CTCTTCGTTCTTGGCGTTCT-3′ |
| GADPH |
5′-GCACCGTCAAGGCTGAGAAC-3′ |
5′-TGGTGAAGACGCCAGTGGA-3′ |
Protein extraction and western blot
analysis
For protein extraction, cells were washed twice with
cold phosphate-buffered saline (PBS), harvested by scraping and
lysed in lysis buffer (Beyotime Institute of Biotechnology).
Following centrifugation, the supernatant was collected, and
protein concentration was determined using a BCA protein assay kit
(Pierce™, no. 23227).
For western blotting, 20 μl of protein was loaded
and separated using 10% sodium dodecyl sulphate polyacrylamide gel
electrophoresis (SDS-PAGE) (Beyotime Institute of Biotechnology);
the protein bands were then transferred to Immobilon-P transfer
membranes (PVDF) (Beyotime Institute of Biotechnology). The
membranes were blocked with 5% non-fat milk in Tris-buffered saline
(TBS) containing 0.1% Tween-20 for 1 h at room temperature. The
blots were probed using antibodies against GPX1 (1:2,000; Abcam),
NF-κB P65 (P65, 1:1,000; Cell Signaling Technology, Danvers, MA,
USA), phospho-NF-κB P65 (P-p65, 1:1,000; Cell Signaling
Technology), urokinase (uPA, 1:1,000; GeneTex, Inc., Irvine, CA,
USA) and MMP-2 (1:1,000; GeneTex). Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH, 1:2000; Cell Signaling Technology) was used
as a loading control. After incubation with a horseradish
peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G
secondary antibody, an enhanced chemiluminescence detection method
(Pierce ECL Western Blotting Substrate; Thermal Form &
Function, Beverly, MA, USA) was used to visualize the proteins on
the blots.
Cell proliferation assay
Cells were plated at a density of 5×103
cells/well in 96-well plates, incubated overnight and counted using
Cell Counting kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan).
The medium in each well was removed, and a mixture of 10 μl CCK-8
and 90 μl RPMI-1640 medium was added. The plates were incubated for
an additional 2.5 h, and absorbance was measured at 450 nm using a
microplate spectrophotometer (Thermo Fisher Scientific, Pittsburgh,
PA, USA).
For cisplatin sensitivity testing, cells
(7.5×103) were seeded into 96-well plates. After
overnight incubation, the cells were treated with various
concentrations of cisplatin (0, 2.5, 5, 10, 20 and 40 μmol/l), and
a CCK-8 assay was performed to examine the cytotoxicity of
cisplatin after 48 h of treatment.
Flow cytometry apoptosis assay
After 24 h of transfection, cells were plated in
6-well plates and incubated with 7.5 μmol/l cisplatin for 48 h at
37°C. The cells were collected and washed with PBS, and cell
apoptosis was analysed by Annexin V/fluorescein isothiocyanate and
propidium iodide staining (Nanjing Keygen Biotech., Co., Ltd.,
Nanjing, China) using a BD FACSCalibur flow cytometer.
In vitro migration and invasion
assays
To assay invasion, 3×105 transfected
cells were seeded into the upper chamber of a polycarbonate
Transwell plate (8-mm pore size; Corning Incorporated, Corning, NY,
USA) that was pre-coated with Matrigel (Becton-Dickinson, Bedford,
MA, USA). RPMI-1640 containing 20% FBS was used as a
chemoattractant and was added to the lower chamber. After a 24-h
incubation at 37°C, the cells on the upper surface of the filter
were removed. Cells that had traversed the membrane were fixed in
methanol at 4°C for 20 min and then stained with 0.1% crystal
violet for 20 min. To microscopically quantify cell migration, the
cells were counted in 3 random fields (magnification, ×200). To
assay migration, a method similar to that used in the invasion
assay was employed, with the following modifications: cells were
seeded at 1.0×105/chamber into plates with no Matrigel
coating and incubated in 600 μl RPMI-1640 medium with 10% FBS in
the lower chamber for 18 h.
ELISA assay for uPA and MMP-2
The supernatant of the culture medium of the ACC-2
cells was collected and centrifuged at 1,000 × g for 20 min at room
temperature. The concentrations of uPA and MMP-2 in the supernatant
were quantified using an uPA ELISA kit (Cloud-Clone Corp., Houston,
TX, USA) and an MMP-2 ELISA kit (Shanghai ExCell, Biology, Inc.,
Shanghai, China) according to the recommended protocols. The
detection limits of the assays were 15.6 pg/ml (uPA) and 0.6 ng/ml
(MMP-2).
In vivo tumorigenicity assay
To explore the effects of 1,25D3 on tumour growth
in vivo, ACC-2 cells were treated for 3 days with 30 nM
1,25D3, AA and culture medium in the experimental group, control
group and blank group, respectively. Next, 2.0×106 ACC-2
cells were subcutaneously implanted into the right upper backs of 5
BALB/c nude mice, and the mice were reared for 24 days. The tumours
were measured every 3 to 4 days for tumour volume (mm3),
which equalled length × width2 × 0.5. All mice were
subsequently sacrificed, and pieces of tumour tissues were used to
establish orthotopic implant models. When tumour volume reached ~50
mm3, 30 nM 1,25D3, AA and PBS were mixed with 5 μM
cisplatinum and then intraperitoneally injected into the three
groups. Tumour formation was observed and tumour growth curves were
constructed. The mice were sactificed 24 days after the injection
of the ACC-2 cells, and the tumours were harvested, weighed and
frozen or paraffin-embedded. Finally, tissue protein was extracted
for analysis.
Immunohistochemistry
Immunohistochemistry was performed on
paraffin-embedded tissue sections from tumorigenicity assays. The
indicated antibodies (GPX1, 1:50; P65, 1:100; uPA, 1:50; MMP-2,
1:50; and Ki-67, 1:100; GeneTex) were used according to the
EnVision HRP detection system (Dako, Carpinteria, CA, USA). After
deparaffinization, antigen retrieval was conducted using 10 mM
sodium citrate buffer (pH 8.0) in a pressure cooker at full power
for 5 min. Tissue sections were then treated with 3% hydrogen
peroxide for 10 min. The primary antibodies were diluted with a
background-reducing diluent (Dako) according to the manufacturer's
specifications and were incubated at 4°C overnight. Next, slides
were incubated with the EnVision reagent for 30 min at 37°C. The
slides were then developed with 3,3′-diaminobenzidine for 3 min,
counterstained with Meyer's haematoxylin and mounted. The samples
were rinsed with phosphate-buffered saline (PBS) between each
step.
Statistical analyses
Statistical analyses were performed with SPSS 22.0
software (IBM Inc., Armonk, NY, USA). P-values <0.05 were
considered to indicate a statistically significant result. Images
were created with the Adobe Photoshop CS5 and the GraphPad Prism
5.
Results
Overexpression of GPX1 promotes cell
proliferation, invasion, migration and cisplatin resistance and
increases apoptosis in SACC cells
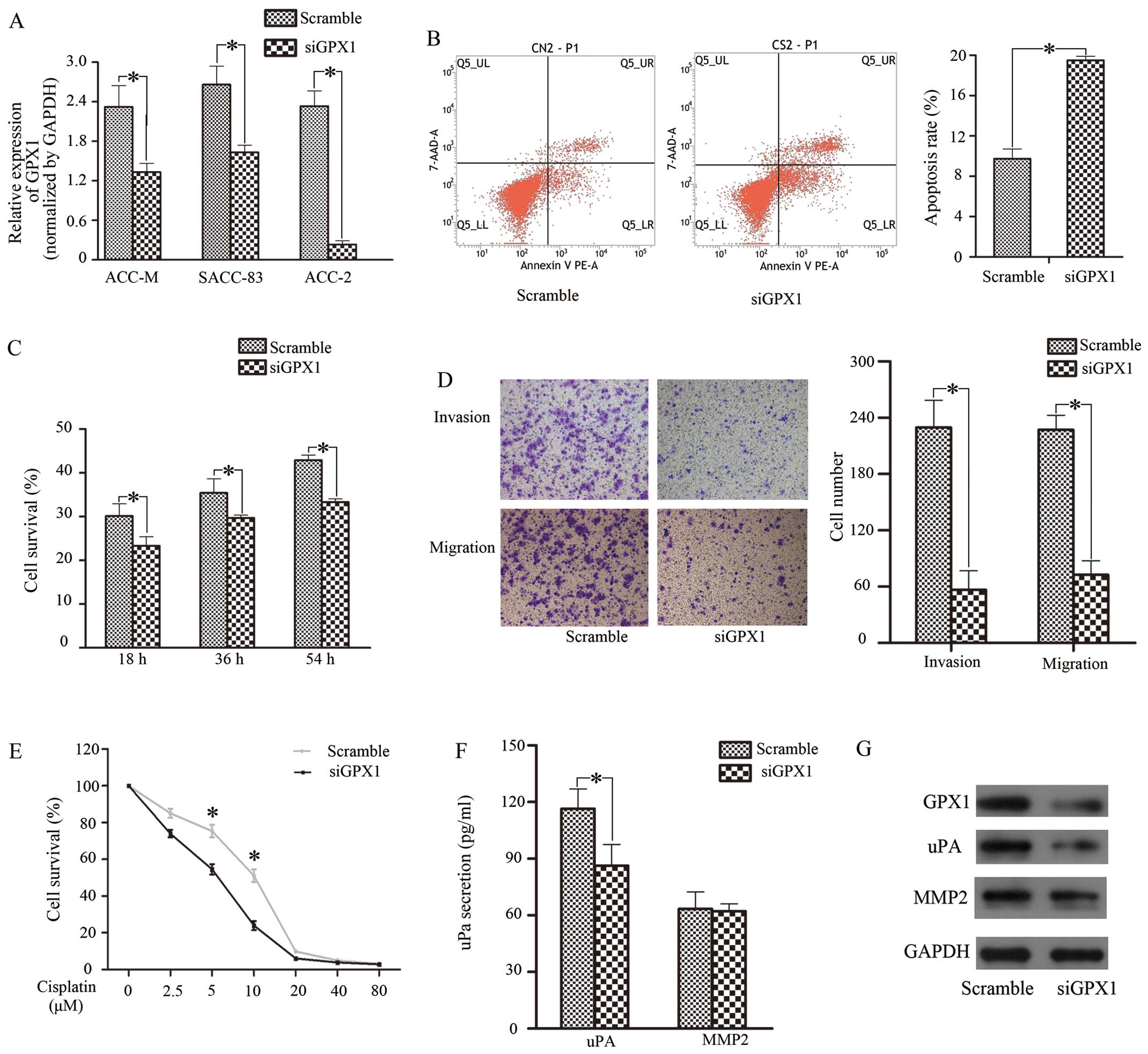
We transfected siGPX1 into 3 SACC cell lines; ACC-2
cells showed relatively effective silence GPX1, with an 80% GPX1
reduction compared with other cells (Fig. 1A and G). Therefore, ACC-2 cells
were selected for further experimentation. When we further
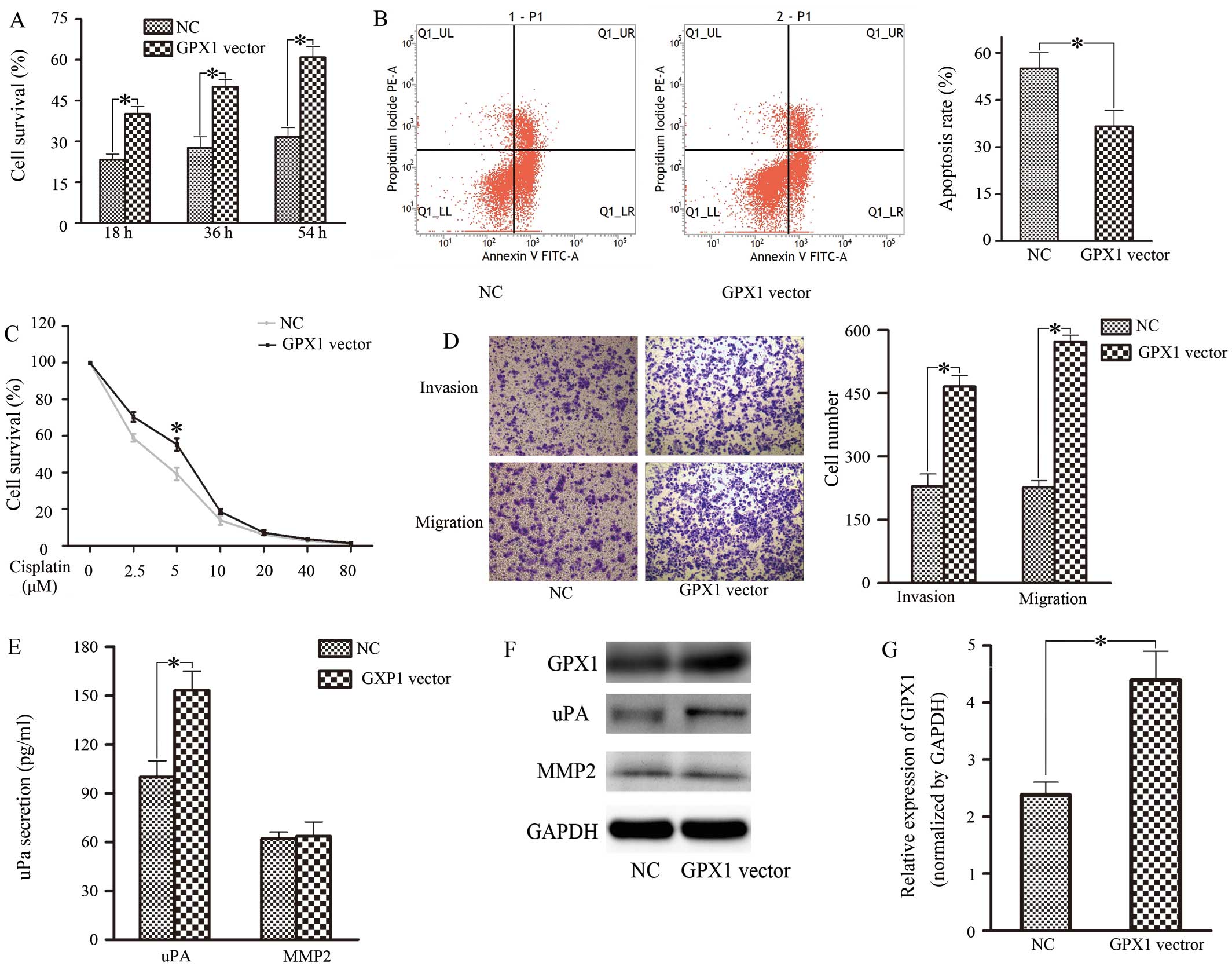
transfected cells with GPX1 vector and negative control (NC)
sequences, GPX1 increased by 40%. Twenty-four hours after
transfection, we performed CCK-8 assays to detect the effect of
GPX1 on ACC-2 cell proliferation at three time-points (18, 36 and
54 h). Cell proliferative capacity was reduced in the siGPX1 group
(Fig. 1C). As for cisplatin
resistance, as the expression of GPX1 decreased, the cells
displayed the same trend, particularly at concentrations of 5 and
10 μM (Fig. 1E). Next, we selected
a 5 μM concentration of cisplatin for use in a flow cytometry
apoptosis assay and found that the apoptosis rate in the siGPX1
cells (19.50%) was higher than that in the control (9.74%)
(Fig. 1B). Next, a Transwell assay
was performed; as shown in Fig.
1D, siGPX1 cells displayed weak invasive and migratory
abilities. In contrast, upregulation of GPX1 promoted proliferation
(Fig. 2A), cisplatin resistance
(Fig. 2C), invasion and migration
(Fig. 2D) but decreased apoptosis
(Fig. 2B) in ACC-2 cells. Taken
together, these results indicated that GPX1 overexpression in ACC-2
cells could be responsible for enhanced cell proliferation,
cisplatin resistance, invasion and migration.
GPX1-enhanced invasion and migration is
associated with uPA
We speculated that the contribution of GPX1 to SACC
motility might involve the downstream factors MMP-2 or uPA
(18,19). At 24 h after transfection, the
expression and secretion of uPA and MMP-2 were tested (Figs. 1F and G, and 2E and F). The results showed that uPA
secretion was dramatically reduced, but MMP-2 remained stabile when
GPX1 was reduced. Meanwhile, uPA secretion increased when cells
were transfected with the GPX1 overexpression vector. Based on
these results, we considered that GPX1-enhanced invasion and
metastasis in ACC-2 cells is associated with uPA.
The NF-κB pathway is related to
GPX1-mediated SACC biological effects
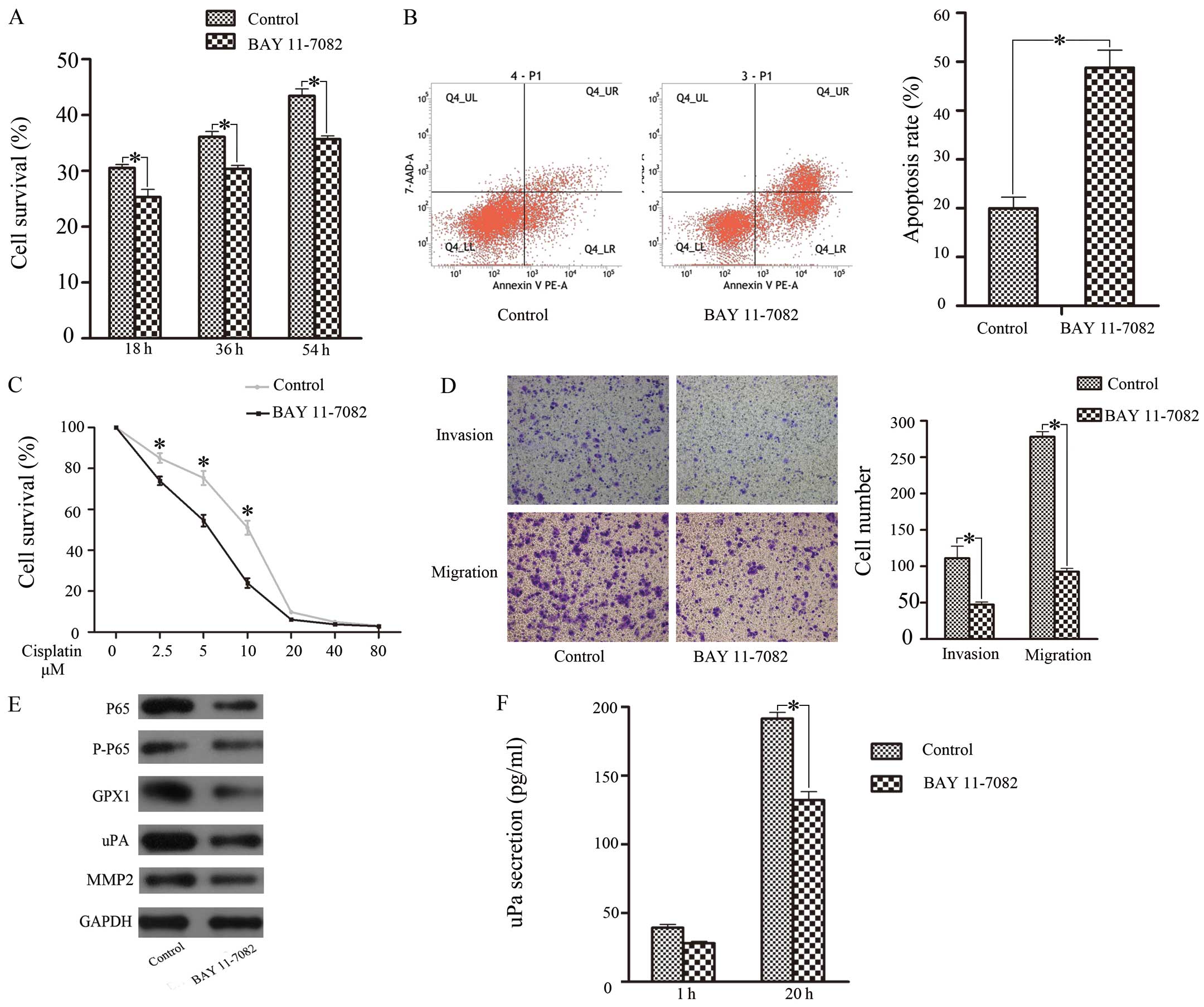
To clarify the mechanism by which GPX1 exerts its
effects, we investigated the role of the NF-κB pathway (20). Twenty-four hours after treatment
with BAY 11-7082, western blotting showed that NF-κB (P65)
expression was significantly downregulated (Fig. 3E). Cell proliferation (Fig. 3A), cisplatin resistance (Fig. 3B), and invasive and migratory
ability (Fig. 3D) were all
reduced; correspondingly, apoptosis increased when the
concentration of cisplatin reached 5 μM (Fig. 3C). GPX1 expression and uPA
expression were downregulated, but MMP-2 expression was sustained
(Fig. 3E). Next, we performed
ELISA to detect uPA and MMP-2 secretion. The results showed that
uPA secretion was reduced, but MMP-2 showed no change (Fig. 3F). These findings suggested that
the NF-κB pathway was related to proliferation, cisplatin
resistance, invasion, migration and apoptosis in SACC via
positively regulating GPX1 expression and uPA activation.
1,25D3 regulates SACC cell biological
effects through the NF-κB pathway
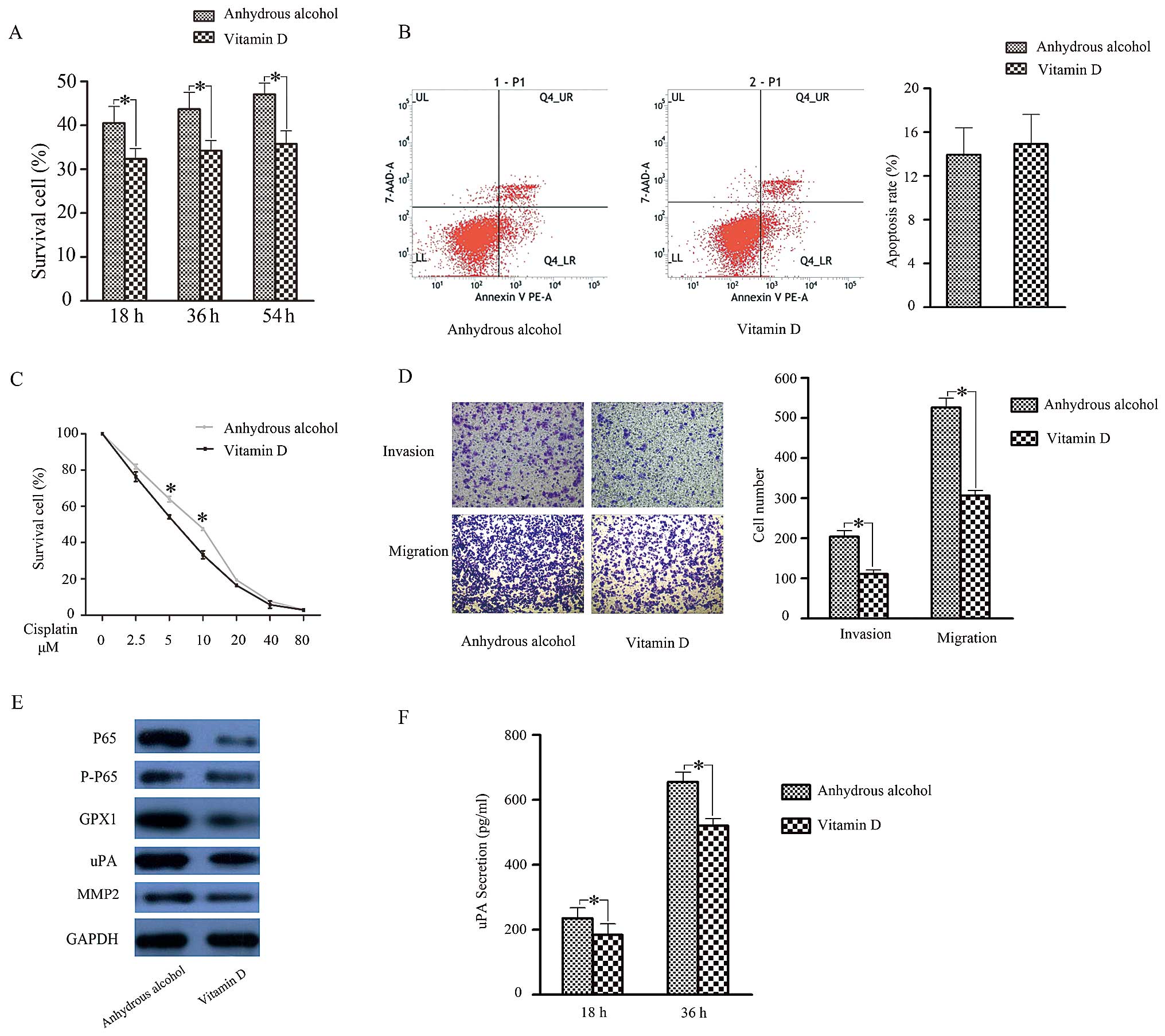
We further investigated the biological role of
1,25D3 in SACC. After preprocessing with 1,25D3 for 3 days, the
proliferative capacity (Fig. 4A)
and cisplatin resistance (Fig. 4C)
of ACC-2 cells were reduced. As for their invasive and migratory
capacities, cells treated with 1,25D3 displayed weaker motility
compared to controls (Fig. 4D).
Cell apoptosis assays showed no significant difference (Fig. 4B) after 1,25D3 treatment. These
results illustrated that 1,25D3 was able to reduce cell
proliferation, cisplatin resistance and motility in ACC-2
cells.
We next examined if the reduced biological function
of 1,25D3-treated ACC-2s was related to activation of NF-κB
signalling and subsequent downregulation of GPX1 expression. To
test this hypothesis, we examined NF-κB and GPX1 expression in
ACC-2 cells after 1,25D3 treatment. As anticipated, NF-κB, GPX1 and
uPA expression was inhibited (Fig.
4E). Moreover, ELISA results showed that uPA secretion was
reduced in 1,25D3-treated ACC-2 cells, whereas MMP-2 showed no
change (Fig. 4F). Taken together,
these results indicate that 1,25D3 may inhibit GPX1 expression to
regulate biological functions in SACC cells through the NF-κB
pathway.
In vivo, 1,25D3 alleviates SACC
progression by inhibiting GPX1 expression through the NF-κB
pathway
To further explore the potential function and
mechanism of 1,25D3 in vivo, we performed tumorigenicity
assays. On day 24, the mean tumour weight of the experimental group
was lower than those of the two control groups (Fig. 5A). According to the tumour growth
curves (Fig. 5B), obvious tumour
nodules formed in the control and blank groups on day 4 after
injection with ACC-2 cells, whereas in the experimental group this
occurred on day 5. The surfaces of the tumours in the experimental
group began to visibly fester on day 18 after injection with ACC-2
cells, and the tumours stopped growing. In contrast, tumours began
to visibly fester on day 20 in the control groups, and the tumours
continued to grow until day 24. H&E staining of the tumours
revealed that the experimental group had more significant necrotic
areas than the control groups (Fig.
5D). Furthermore, the expression of GPX1, P65, P-P65 and uPA in
the experimental group was lower than that in the control and blank
groups (Fig. 5C). Additionally,
immunohistochemical analysis of the inoculated tumours revealed
that the tumours of the experimental group expressed significantly
lower levels of Ki-67, uPA and P65, with no change in MMP-2
expression and increased GPX1 expression (exclusive cytoplasmic
expression; no nuclear expression) (Fig. 5E).
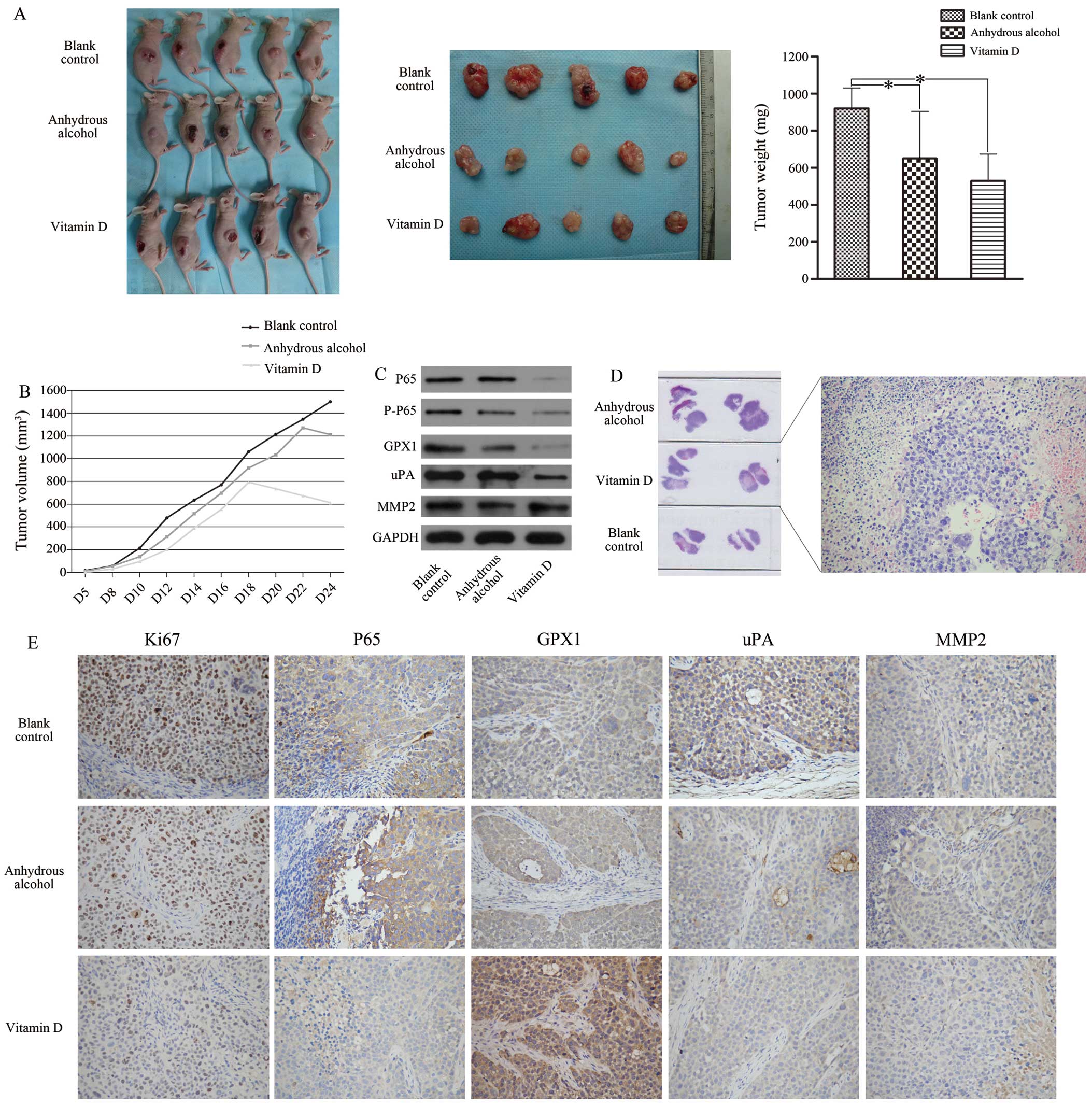 | Figure 51,25VD3 reduces the malignancy of
ACC-2 cells in vivo. (A) The volume and weight of the
transplanted tumours were significantly decreased in the vitamin D.
(B) The tumour growth curve showed that vitamin D group had the
slowest growth rate, and tissue necrosis appeared from the 18th
day. H&E-stained slices of transplanted tumours formed by ACC-2
cells also suggested that 1,25VD3 promoted cell necrosis (D,
magnification, ×200). The expression levels of GPX1, P65, P-P65 and
uPA were significantly decreased in vitamin D group compared with
the other two control groups (C). Immunohistochemistry staining
indicated that the levels of P65, uPA and Ki-67 were downregulated
in vitamin D group. No difference in MMP-2 expression was noted
among these groups. GPX1 showed only cytoplasmic staining and was
highly expressed in the experimental group (E, magnification,
×200). *P<0.05 compared with the blank control
group. |
Discussion
SACC is a malignant tumour arising from secretory
epithelial cells in the salivary glands of the head and neck. The
5-year disease-free survival rate is ≤90%; however, the survival
rate is reduced to 40% after 15 years (2). SACC has a poor prognosis, primarily
owing to its insidious invasion into adjacent tissues and
haematogenous spread to distant organs (lungs, bone and liver)
(21–23). Therefore, it is necessary to
identify and understand the mechanism behind SACC chemoresistance
and metastasis to improve treatment strategies for SACC
patients.
Recent studies have found that upregulation of GPx
expression leads cancer cells to develop drug resistance by
reducing ROS produced by platinum-based chemotherapy drugs, which
promotes tumourigenesis (24,25).
In the present study, we found that GPX1 acts as a key factor in
regulating SACC progression, as GPX1 suppression reduced ACC-2 cell
proliferation, cisplatin resistance and motility while increasing
cell apoptosis. Upregulation of GPX1 shows the corresponding
opposite effect. It has been reported that the generation of MMP-2
and uPA promotes tumour invasion and metastasis in squamous cell
carcinoma of the head and neck (18,19).
Following GPX1 downregulation, we detected a reduction of uPA
expression and secretion in ACC-2 cells, whereas MMP-2 remained
stable. In contrast, uPA secretion and activation increased when
GPX1 was overexpressed.
In malignant tumours, NF-κB expression can regulate
downstream protein expression, thereby regulating tumour
chemoresistance and invasion (8,26–28).
In the present study inhibiting the NF-κB pathway reduced ACC-2
cell growth, cisplatin resistance, and motility, while promoting
apoptosis. To further analyse the role of NF-κB-regulated factors,
the expression of GPX1 was decreased, and uPA secretion and
expression showed the same trend. Thus, we established a
correlation between NF-κB and GPX1 in ACC-2 cells and confirmed
that GPX1-induced uPA secretion is mediated by an NF-κB-dependent
pathway, which modulates SACC chemoresistance and invasion.
Vitamin D has widespread actions throughout the
human body, and supplementation may be a strategy for preventing
cancer incidence and/or tumour progression (29,30).
Mechanistically it has been suggested that vitamin D regulates a
wide range of factors, including interleukin-8, NF-κB, HBp17 and
miR98, which subsequently inhibit tumour development (12,14–17,31,32).
As expected, we found that the active metabolite of vitamin D,
1,25D3, inhibited ACC-2 cell proliferation, cisplatin resistance,
invasion and migration and alleviated SACC progression in
vivo. We analysed the mechanism by which 1,25D3 reduced the
extent of SACC malignancy. After treatment with 1,25D3, NF-κB and
GPX1 activation were responsively decreased in ACC-2 cells, and the
secretion and expression of uPA were also reduced. Tumorigenicity
assays confirmed that pre-applied 1,25D3 could inhibit the
progression of SACC tumours; subsequent immunohistochemical
examination also detected signal alterations among NF-κB, GPX1 and
uPA. Our combined in vitro and in vivo experiments
revealed that GPX1 is a target of 1,25D3, which alleviates SACC
progression by suppressing GPX1 expression through the NF-κB
pathway. This provides a theoretical basis for vitamin D
supplementation in cancer management. Avoiding vitamin D deficiency
and adding supplements may be an economical and effective way to
reduce cancer incidence and improve cancer prognosis.
In conclusion, in the present study, we demonstrated
that downregulation of GPX1 can suppress SACC cell proliferation,
cisplatin-resistance, migration, and invasion and promote apoptosis
through the NF-κB pathway and uPA activation. 1,25D3 achieved its
antineoplastic function via the abovementioned regulators.
Collectively, establishing 1,25D3 as a modifier of NF-κB/GPX1/uPA
expression provides a novel therapeutic strategy for the treatment
of SACC.
Acknowledgements
The present study was supported by grants from the
Key Laboratory of Malignant Tumor Molecular and Translational
Medicine of Guangzhou Bureau of Science and Information Technology
(no. [2013]163), the Key Laboratory of Malignant Tumor Gene
Regulation and Target Therapy of Guangdong Higher Education
Institutes (no. KLB09001). It was also supported by the National
Natural Science Foundation of China (no. 81101592), the Fundamental
Research Funds for the Central Universities (no. 13ykpy26) and the
Guangdong Province Natural Science Foundation (no.
S2013010014794).
Abbreviations:
|
1,25D3
|
1,25-dihydroxyvitamin D3
|
|
GPX1
|
glutathione peroxidase-1
|
|
GPxs
|
glutathione peroxidase
|
|
ROS
|
reactive oxygen species
|
|
NF-κB
|
nuclear factor-kappa B
|
|
SACC
|
salivary adenoid cystic carcinoma
|
|
VDR
|
vitamin D receptor
|
|
PCR
|
polymerase chain reaction
|
|
FBS
|
fetal bovine serum
|
|
CCK-8
|
Cell Counting kit-8
|
|
siRNA
|
small interfering RNA
|
|
ELISA
|
enzyme linked immunosorbent assay
|
|
TBS
|
Tris-buffered saline
|
|
H&E
|
haematoxylin and eosin
|
|
AA
|
anhydrous alcohol
|
|
NC
|
negative control
|
|
uPA
|
urokinase-type plasminogen
activator
|
|
MMP-2
|
matrix metalloproteinase-2
|
References
|
1
|
Thompson L: World Health Organization
classification of tumours: Pathology and genetics of head and neck
tumours. Ear Nose Throat J. 85:742006.PubMed/NCBI
|
|
2
|
Fordice J, Kershaw C, El-Naggar A and
Goepfert H: Adenoid cystic carcinoma of the head and neck:
Predictors of morbidity and mortality. Arch Otolaryngol Head Neck
Surg. 125:149–152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colevas AD: Chemotherapy options for
patients with metastatic or recurrent squamous cell carcinoma of
the head and neck. J Clin Oncol. 24:2644–2652. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brigelius-Flohé R: Tissue-specific
functions of individual glutathione peroxidases. Free Radic Biol
Med. 27:951–965. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lubos E, Loscalzo J and Handy DE:
Glutathione peroxidase-1 in health and disease: From molecular
mechanisms to therapeutic opportunities. Antioxid Redox Signal.
15:1957–1997. 2011. View Article : Google Scholar :
|
|
6
|
Fu TY, Hou YY, Chu ST, Liu CF, Huang CH,
Chen HC, Hsiao M, Lu PJ, Wang JS and Ger LP: Manganese superoxide
dismutase and glutathione peroxidase as prognostic markers in
patients with buccal mucosal squamous cell carcinomas. Head Neck.
33:1606–1615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schumacker PT: Reactive oxygen species in
cancer cells: Live by the sword, die by the sword. Cancer Cell.
10:175–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gong G, Méplan C, Gautrey H, Hall J and
Hesketh JE: Differential effects of selenium and knock-down of
glutathione peroxidases on TNFα and flagellin inflammatory
responses in gut epithelial cells. Genes Nutr. 7:167–178. 2012.
View Article : Google Scholar :
|
|
9
|
Schreck R, Albermann K and Baeuerle PA:
Nuclear factor kappa B: An oxidative stress-responsive
transcription factor of eukaryotic cells (Review). Free Radic Res
Commun. 17:221–237. 1992. View Article : Google Scholar
|
|
10
|
Vibet S, Goupille C, Bougnoux P, Steghens
JP, Goré J and Mahéo K: Sensitization by docosahexaenoic acid (DHA)
of breast cancer cells to anthracyclines through loss of
glutathione peroxidase (GPx1) response. Free Radic Biol Med.
44:1483–1491. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feldman D, Krishnan AV, Swami S,
Giovannucci E and Feldman BJ: The role of vitamin D in reducing
cancer risk and progression. Nat Rev Cancer. 14:342–357. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ting HJ, Messing J, Yasmin-Karim S and Lee
YF: Identification of microRNA-98 as a therapeutic target
inhibiting prostate cancer growth and a biomarker induced by
vitamin D. J Biol Chem. 288:1–9. 2013. View Article : Google Scholar :
|
|
13
|
Krishnan AV, Swami S and Feldman D: The
potential therapeutic benefits of vitamin D in the treatment of
estrogen receptor positive breast cancer. Steroids. 77:1107–1112.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bao BY, Yao J and Lee YF: 1alpha,
25-dihydroxyvitamin D3 suppresses interleukin-8-mediated prostate
cancer cell angiogenesis. Carcinogenesis. 27:1883–1893. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tse AK, Zhu GY, Wan CK, Shen XL, Yu ZL and
Fong WF: 1alpha,25-Dihydroxyvitamin D3 inhibits transcriptional
potential of nuclear factor kappa B in breast cancer cells. Mol
Immunol. 47:1728–1738. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosli SN, Shintani T, Hayashido Y,
Toratani S, Usui E and Okamoto T: 1α,25OH2D3 down-regulates
HBp17/FGFBP-1 expression via NF-κB pathway. J Steroid Biochem Mol
Biol. 136:98–101. 2013. View Article : Google Scholar
|
|
17
|
Janjetovic Z, Brozyna AA, Tuckey RC, Kim
TK, Nguyen MN, Jozwicki W, Pfeffer SR, Pfeffer LM and Slominski AT:
High basal NF-κB activity in nonpigmented melanoma cells is
associated with an enhanced sensitivity to vitamin D3 derivatives.
Br J Cancer. 105:1874–1884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang Z, Huang H, Li H, Chen W and Pan C:
EMMPRIN expression in tongue squamous cell carcinoma. J Oral Pathol
Med. 38:518–523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Z, Tan N, Guo W, Wang L, Li H, Zhang
T, Liu X, Xu Q, Li J and Guo Z: Overexpression of EMMPRIN isoform 2
is associated with head and neck cancer metastasis. PLoS One.
9:e915962014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ghashghaeinia M, Toulany M, Saki M,
Bobbala D, Fehrenbacher B, Rupec R, Rodemann HP, Ghoreschi K,
Röcken M, Schaller M, et al: The NFκB pathway inhibitors Bay
11-7082 and parthenolide induce programmed cell death in anucleated
erythrocytes. Cell Physiol Biochem. 27:45–54. 2011. View Article : Google Scholar
|
|
21
|
Kim KH, Sung MW, Chung PS, Rhee CS, Park
CI and Kim WH: Adenoid cystic carcinoma of the head and neck. Arch
Otolaryngol Head Neck Surg. 120:721–726. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsuba HM, Spector GJ, Thawley SE,
Simpson JR, Mauney M and Pikul FJ: Adenoid cystic salivary gland
carcinoma. A histopathologic review of treatment failure patterns.
Cancer. 57:519–524. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sung MW, Kim KH, Kim JW, Min YG, Seong WJ,
Roh JL, Lee SJ, Kwon TK and Park SW: Clinicopathologic predictors
and impact of distant metastasis from adenoid cystic carcinoma of
the head and neck. Arch Otolaryngol Head Neck Surg. 129:1193–1197.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takata Y, Kristal AR, Santella RM, King
IB, Duggan DJ, Lampe JW, Rayman MP, Blount PL, Reid BJ, Vaughan TL,
et al: Selenium, selenoenzymes, oxidative stress and risk of
neoplastic progression from Barrett's esophagus: Results from
biomarkers and genetic variants. PLoS One. 7:e386122012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao M, Mu X, Jiang C, Yang G, Chen H and
Xue W: Single-nucleotide polymorphisms of GPX1 and MnSOD and
susceptibility to bladder cancer: A systematic review and
meta-analysis. Tumour Biol. 35:759–764. 2014. View Article : Google Scholar
|
|
26
|
Dibra D, Mishra L and Li S: Molecular
mechanisms of oncogene-induced inflammation and
inflammation-sustained oncogene activation in gastrointestinal
tumors: An under-appreciated symbiotic relationship. Biochim
Biophys Acta. 1846:152–160. 2014.PubMed/NCBI
|
|
27
|
Harte MT, Gorski JJ, Savage KI, Purcell
JW, Barros EM, Burn PM, McFarlane C, Mullan PB, Kennedy RD, Perkins
ND, et al: NF-κB is a critical mediator of BRCA1-induced
chemoresistance. Oncogene. 33:713–723. 2014. View Article : Google Scholar :
|
|
28
|
Wang H, Khor TO, Yang Q, Huang Y, Wu TY,
Saw CL, Lin W, Androulakis IP and Kong AN: Pharmacokinetics and
pharmacodynamics of phase II drug metabolizing/antioxidant enzymes
gene response by anticancer agent sulforaphane in rat lymphocytes.
Mol Pharm. 9:2819–2827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murphy AB, Nyame Y, Martin IK, Catalona
WJ, Hollowell CM, Nadler RB, Kozlowski JM, Perry KT, Kajdacsy-Balla
A and Kittles R: Vitamin D deficiency predicts prostate biopsy
outcomes. Clin Cancer Res. 20:2289–2299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grant WB: Vitamin D status: Ready for
guiding prostate cancer diagnosis and treatment? Clin Cancer Res.
20:2241–2243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gilmore TD: The Rel/NF-kappaB signal
transduction pathway: Introduction. Oncogene. 18:6842–6844. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thota C, Laknaur A, Farmer T, Ladson G,
Al-Hendy A and Ismail N: Vitamin D regulates contractile profile in
human uterine myometrial cells via NF-κB pathway. Am J Obstet
Gynecol. 210:347.e1–347.e10. 2014. View Article : Google Scholar
|