Introduction
All-trans retinoic acid (ATRA) induces a
dramatic response in the treatment of patients with acute
promyelocytic leukemia (APL), leading to complete remission, but
most patients will eventually experience a relapse. The combination
of ATRA and anthracyclines results in an overall remission rate of
up to 95% and a cure rate that now exceeds 80% (1–4). A
recent study revealed that the combination of ATRA and arsenic
trioxide may be superior to the combination of ATRA and
chemotherapy for the treatment of patients with
low-to-intermediate-risk APL (5).
However, hematologic toxicity (acute cytopenia with the resulting
infections) was observed in the ATRA/anthracycline group and liver
toxicity and long Q-T syndrome were observed in patients in the the
ATRA/arsenic trioxide group. Since anthracyclines and arsenic
compounds are genotoxic and carcinogenic (6), long-term treatment with these drugs
should be avoided as much as possible. Although recent therapy
against APL has a high cure rate, further improvements are
needed.
To identify a compound that would be useful for APL
therapy when combined with ATRA, we have screened various compounds
that are less toxic and clinically available, such as inhibitors of
signal transduction pathways and physiologically active molecules.
As a result, the most effective agent we identified was tamoxifen.
We found that tamoxifen effectively enhances the
differentiation-inducing and growth-inhibitory effects of ATRA in
APL cells. Tamoxifen is a selective estrogen receptor modulator
that is used as the first-line treatment for estrogen
receptor-positive breast cancer. However, multiple non-estrogen
receptor-mediated mechanisms have been implicated in the antitumor
effects induced by tamoxifen in estrogen receptor-negative tumors
(7). There seems to be a
consistent relationship between higher doses of tamoxifen and
longer survival in patients with largely inoperable and recurrent
malignant glioma (8). Therefore,
in the present study, we sought to clarify the combined effects of
ATRA and tamoxifen on the growth and differentiation of human
myeloid leukemia cells including APL cells.
Materials and methods
Materials
RPMI-1640 medium, nitroblue tetrazolium (NBT), ATRA,
tamoxifen and α-tocopherol were purchased from Sigma-Aldrich Co.
(St. Louis, MO, USA). Dimethyl sulfoxide (DMSO), 1α,25-dihydroxy
vitamin D3 (VD3), and sodium butyrate were purchased
from Wako Chemicals (Osaka, Japan). Fluorescein isothiocyanate
(FITC)-labeled anti-CD11b antibody was obtained from
Becton-Dickinson Immunocytometry Systems (San Jose, CA, USA).
BMS195614 was purchased from Tocris Bioscience, R&D System Co.
(Minneapolis, MN, USA). PD98059 was obtained from Calbiochem (La
Jolla, CA, USA).
Leukemia patient
Leukemic bone marrow specimen was collected at
diagnosis, after the patient gave his written informed consent for
sample collection in accordance with institutional policy. A
45-year-old Japanese male was admitted to our hospital, presenting
with purpura. Laboratory data on admission were WBC 8,720/μl,
blasts 63.5%, and platelets 15,000/μl. Bone marrow aspiration
revealed hyperplastic marrow cells (nucleated cell count
1,232,000/μl), 94.4% of which were blasts. APL was diagnosed on the
basis of bone marrow morphology and the standard cytogenetic
translocation, t(15;17), in 97% of cells by FISH analysis.
Cells and cell culture
The HL-60 cell line, derived from an AML patient,
and NB4 and HT93 promyelocytic leukemia cells were maintained in
RPMI-1640 medium supplemented with 10% fetal bovine serum and 80
μg/ml gentamicin at 37°C in a humidified atmosphere of 5%
CO2 in air (9).
Heparinized bone marrow aspirations were diluted
with RPMI-1640 medium supplemented with 10% fetal bovine serum,
overlaid on 15 ml of Ficoll-Paque Plus (Amersham Biosciences,
Uppsala, Sweden) and centrifuged at 500 × g for 30 min. Only
specimens that contained ≥80% leukemia cells were studied. The
mononuclear cells were washed twice and suspended in RPMI-1640
medium supplemented with 10% fetal bovine serum, plated in culture
dishes at 5–12×105 cells/ml, and incubated at 37°C in a
humidified atmosphere of 5% CO2 in air.
Assay of cell growth and properties of
differentiated cells
Suspensions of cells (5×104 cells/ml) in
2 ml of culture medium were incubated with or without the test
compounds in multidishes. Cell numbers were counted in a Model Z1
Coulter Counter (Beckman Coulter, Tokyo, Japan).
Superoxide-generating oxidase was determined by the ability of the
cells to reduce NBT upon exposure to 12-O-tetradecanoyl
phorbol-13-acetate (9). Cells were
incubated in 1 ml of RPMI-1640 medium containing NBT (1 mg/ml) and
12-O-tetradecanoyl phorbol-13-acetate (100 ng/ml) at 37°C
for 50 min. The reaction was stopped by the addition of 5 M HCl (1
M, final concentration). The suspension was kept at room
temperature for 20 min and then centrifuged. Formazan deposits were
solubilized in dimethyl sulfoxide, and the absorption of the
formazan solution at 560 nm/107 cells was measured in a
spectrophotometer. Morphological changes were examined in cell
smears stained with May-Grünwald-Giemsa solution. Surface
expression of CD11b was determined by monoclonal antibody labeling
and flow cytometry using a FACScan (Becton-Dickinson, Mountain
View, CA, USA), as described previously (10).
Colony-forming assay
NB4 cells (3×103 per dish) were plated
into 3 ml of a semisolid medium with 0.8% methylcellulose and 20%
fetal bovine serum in triplicate multiwell plates (6 wells, 9.6
cm2 growth area/well) for 8 days. A solution of 0.1 ml
of PBS containing various concentrations of drugs was added to the
semisolid medium. Colonies were photographed under an inverted
microscope. Colonies in enlarged photographs were measured and
those >0.4 mm in diameter were counted.
Bone marrow plugs from a single femur of three
Balb/c mice were pooled in RPMI-1640 medium. A single-cell
suspension was prepared by vigorous pipetting. Nucleated cells
(1,000 cells/ml/dish) were placed in 1 ml of a semi-solid medium
containing haematopoietic growth factors (MethoCult® GF
M3434, Stem Cell Technology Inc., Vancouver, BC, Canada) in a
24-well plate and incubated for 8 days (11).
Western blot analysis
Cells were packed after being washed with cold PBS,
and then lysed at 1.5×107 cells/ml in sample buffer [63
mM Tris-HCl (pH 6.8), 15% glycerol, 2% sodium dodecyl sulfate
(SDS), 5% 2-mercaptoethanol and 0.005% bromophenol blue]. The
resultant lysates were resolved on 10% SDS-polyacrylamide gels. The
proteins were transferred electrically from the gel to an
Immobilon-P membrane (Millipore, Bedford, MA, USA) and
immunoblotted with antibody. All antibodies were purchased from
Cell Signaling Technology Japan (Tokyo). Horseradish peroxidase
(HRP)-conjugated antibody (Cell Signaling Technology, CA, USA) was
used as a secondary antibody (1:2,000 dilution). The bands were
developed by treatment with the Immun-Star HRP Chemiluminescent kit
(Bio-Rad Laboratories, Danvers, MA, USA) for 5 min at room
temperature, and detected using a Fuji Lumino Image Analyzer
LAS-4000 system (Fuji Film, Tokyo, Japan).
Statistical analysis
The results are expressed as means ± standard
deviation (SD). Pairs of data were compared using Student's t-test.
Significant differences were considered to exist for probabilities
<5% (P<0.05).
Results
Effects of tamoxifen on the
differentiation and growth of myeloid leukemia cells
Various compounds induce human myeloid leukemia
HL-60 cells to undergo differentiation into granulocytes and
monocytes (12). We examined the
effects of tamoxifen on the differentiation and growth of HL-60
cells. Tamoxifen concentration-dependently inhibited the
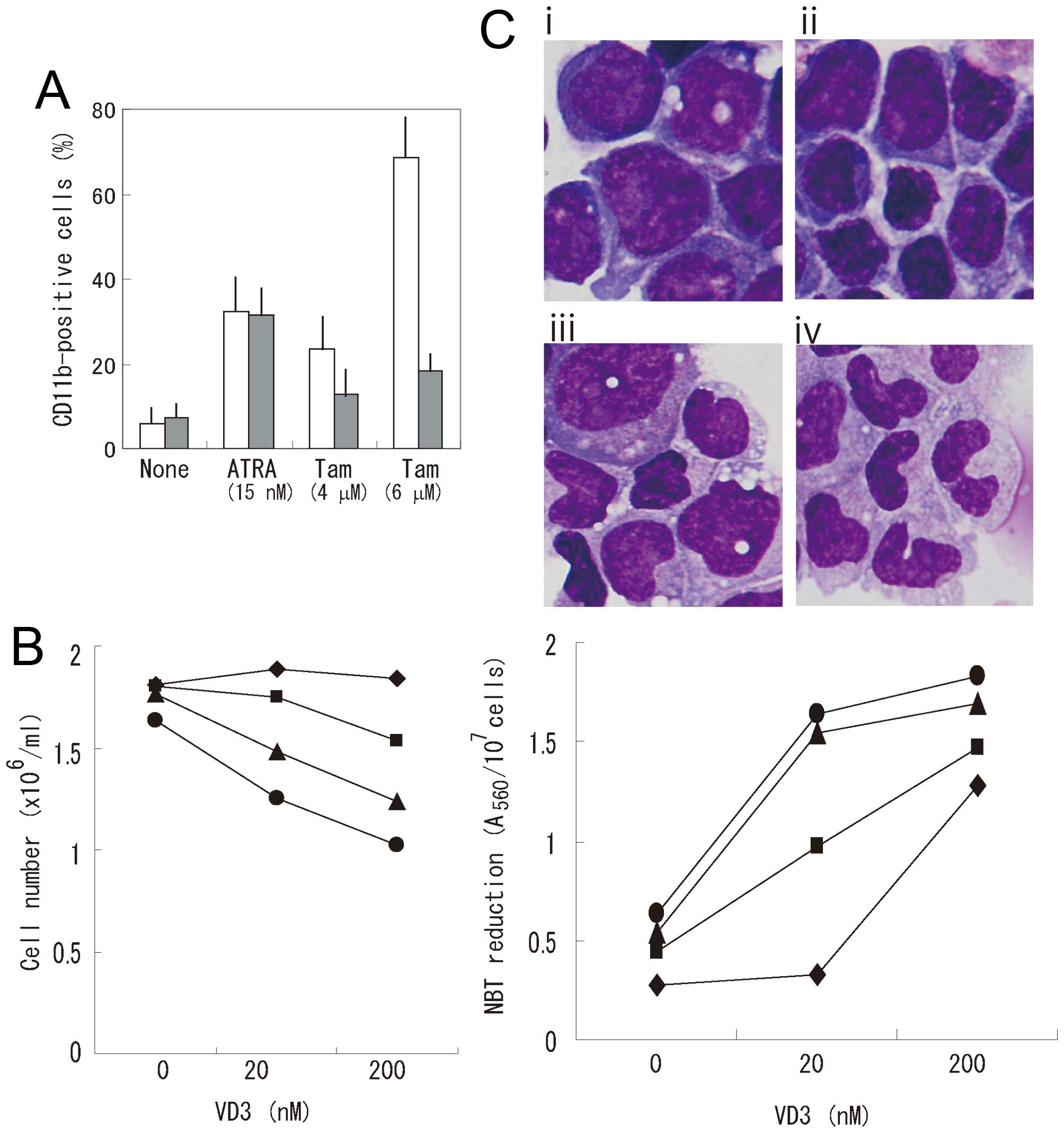
proliferation of HL-60 cells and induced the expression of CD11b, a
surface marker of myelomonocytic differentiation (Fig. 1A). Since our previous report
indicated that α-tocopherol prevents the growth-inhibitory effect
of tamoxifen in pancreatic cancer cells (11), we examined the effect of
α-tocopherol on CD11b expression in HL-60 cells (Fig. 1A). α-tocopherol significantly
suppressed tamoxifen-induced CD11b expression, but had little
effect on ATRA-induced CD11b expression. Tamoxifen also induced the
reduction of NBT, a functional marker of differentiation (Fig. 1B). However, tamoxifen scarcely
affected morphologic changes (Fig.
1C). These results indicate that tamoxifen is not a potent
inducer of differentiation. Tamoxifen effectively enhanced the
growth-inhibitory and NBT-reducing effects of VD3 in HL-60 cells
(Fig. 1B). Tamoxifen also enhanced
the morphologic differentiation of HL-60 cells induced by VD3
(Fig. 1C). Next, we examined the
effects of tamoxifen on the growth-inhibitory and NBT-reducing
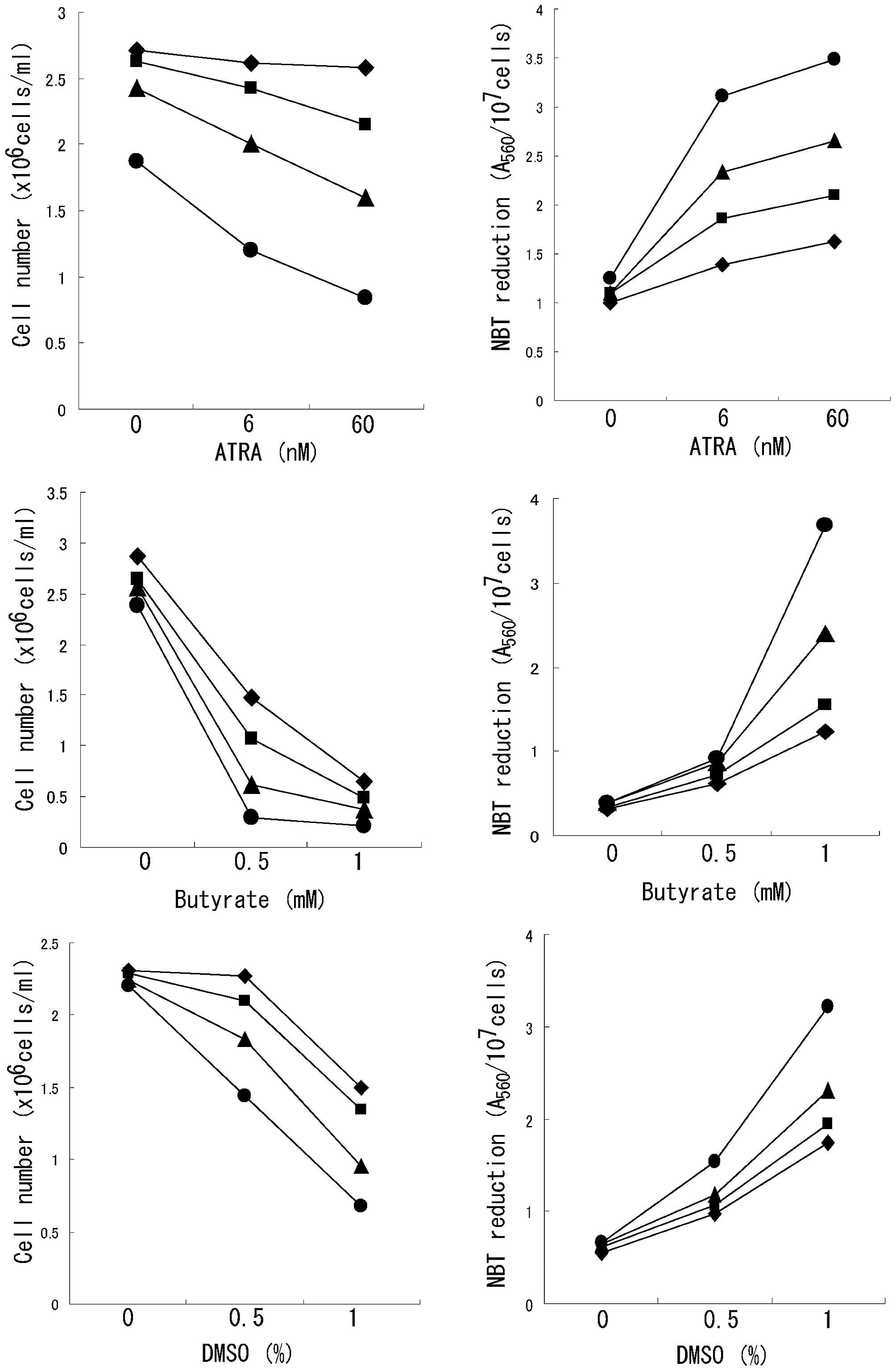
effects of ATRA, butyrate, and DMSO (Fig. 2). Tamoxifen enhanced ATRA-induced
NBT reduction in the APL cell line NB4 (Fig. 3A). Although ATRA significantly
inhibited the proliferation of HT93 cells, viable cells were still
observed among HT93 cells treated with 900 nM ATRA, as judged by
the MTT assay (Fig. 3B). Tamoxifen
and ATRA co-operatively inhibited the viability of HT93 cells, and
no viable cells were observed in a culture with a combination of 6
μM tamoxifen and 900 nM ATRA for 6 days.
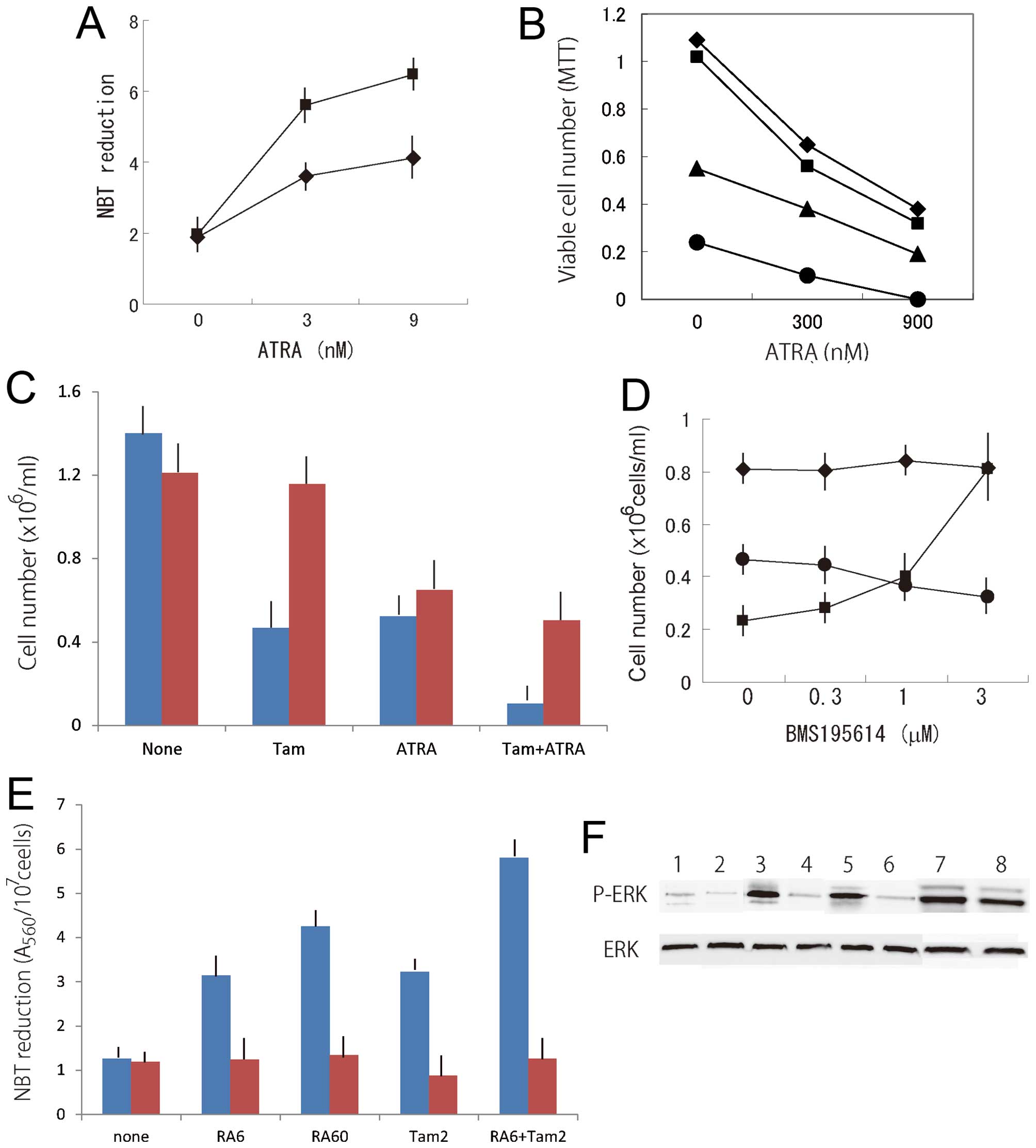 | Figure 3(A) Combined effects of ATRA and
tamoxifen on NBT reduction in NB4 cells. Cells were cultured with
(black square) or without (black rhombus) 2 μM tamoxifen in the
presence of ATRA for 4 days. Means of four determinations. (B)
Combined effects of ATRA and tamoxifen on the proliferation of HT93
cells. Cells were cultured with 0 (black rhombus), 2 μM (black
square), 4 μM (black trinagle), or 6 μM (black circle) tamoxifen in
the presence of ATRA for 4 days. Viable cell numbers were
determined by the MTT assay. Means of four determinations. (C)
Effect of α-tocopherol on the proliferation of NB4 cells treated
with tamoxifen and/or ATRA. Cells were cultured with (red square)
or without (blue square) 200 μM α-tocopherol in the presence or
absence of 8 μM tamoxifen (Tam) or 30 nM ATRA (ATRA) for 4 days.
Means ± SD of three determinations. (D) Effect of BMS195614 on the
proliferation of NB4 cells treated with tamoxifen or ATRA. Cells
were cultured with 4 μM tamoxifen (black circle) or 6 nM ATRA
(black square) for 4 days. Black rhombus, untreated control. Means
± SD of three determinations. (E) Effect of PD98059 on NBT
reduction in HL-60 cells treated with ATRA and/or tamoxifen. Cells
were cultured with (red square) or without (blue square) 1 μM
PD98059 in the presence of 6 nM ATRA (RA6), 60 nM ATRA (RA60), 2 μM
tamoxifen (Tam2) or 6 nM ATRA plus 2 μM tamoxifen (RA6+Tam2) for 4
days. Means ± SD of three determinations. (F) Effect of PD98059 on
the induction of ERK activity in HL-60 cells treated with ATRA
and/or tamoxifen. Cells were cultured without (1, 3, 5, and 7) or
with 1 μM PD98059 (2, 4, 6, and 8) in the absence (1 and 2) or
presence of 4 μM tamoxifen (3 and 4), 30 nM ATRA (5 and 6) or 4 μM
tamoxifen plus 30 nM ATRA (7 and 8) for 2 days. |
The growth-inhibitory effect of tamoxifen on NB4
cells was prevented by treatment with α-tocopherol, even in the
presence of ATRA (Fig. 3C).
ATRA-induced inhibition of the growth of NB4 cells was
concentration-dependently counteracted by a selective antagonist of
retinoic acid receptor-α, BMS195614, while this antagonist hardly
affected the growth-inhibitory effect of tamoxifen (Fig. 3D).
Role of ERK activation in the combined
effects of ATRA and tamoxifen
Several inducers including ATRA activate ERK in
leukemia cells before inducing myelomonocytic differentiation, and
this activation is needed to elicit differentiation and growth
arrest (13,14). Treatment with PD98059, a specific
inhibitor of MAPK kinase (MEK) phosphorylation and activation
(15), resulted in a loss of ERK
activity from leukemia cells and blockade of the differentiation of
leukemia cells (16). ATRA
significantly increased ERK activity and this increased ERK
activity was reduced by pretreatment with PD98059 (13). This inhibitor also suppressed the
NBT reduction induced by ATRA, suggesting that ERK activation is
required for ATRA-induced differentiation (13). PD98059 also inhibited
tamoxifen-induced NBT reduction in HL-60 cells (Fig. 3E), and kinase activity was enhanced
in tamoxifen-treated cells (Fig.
3F). Fig. 3E shows that
treatment with PD98059 reduced the NBT reduction induced by ATRA
plus tamoxifen. These results suggest that synergism between ATRA
and tamoxifen in the inhibition of proliferation and induction of
differentiation of myeloid leukemia cells is, at least in part, due
to ERK activation by tamoxifen.
Combined effects of tamoxifen and ATRA on
colony formation by NB4 cells and normal mouse bone marrow
cells
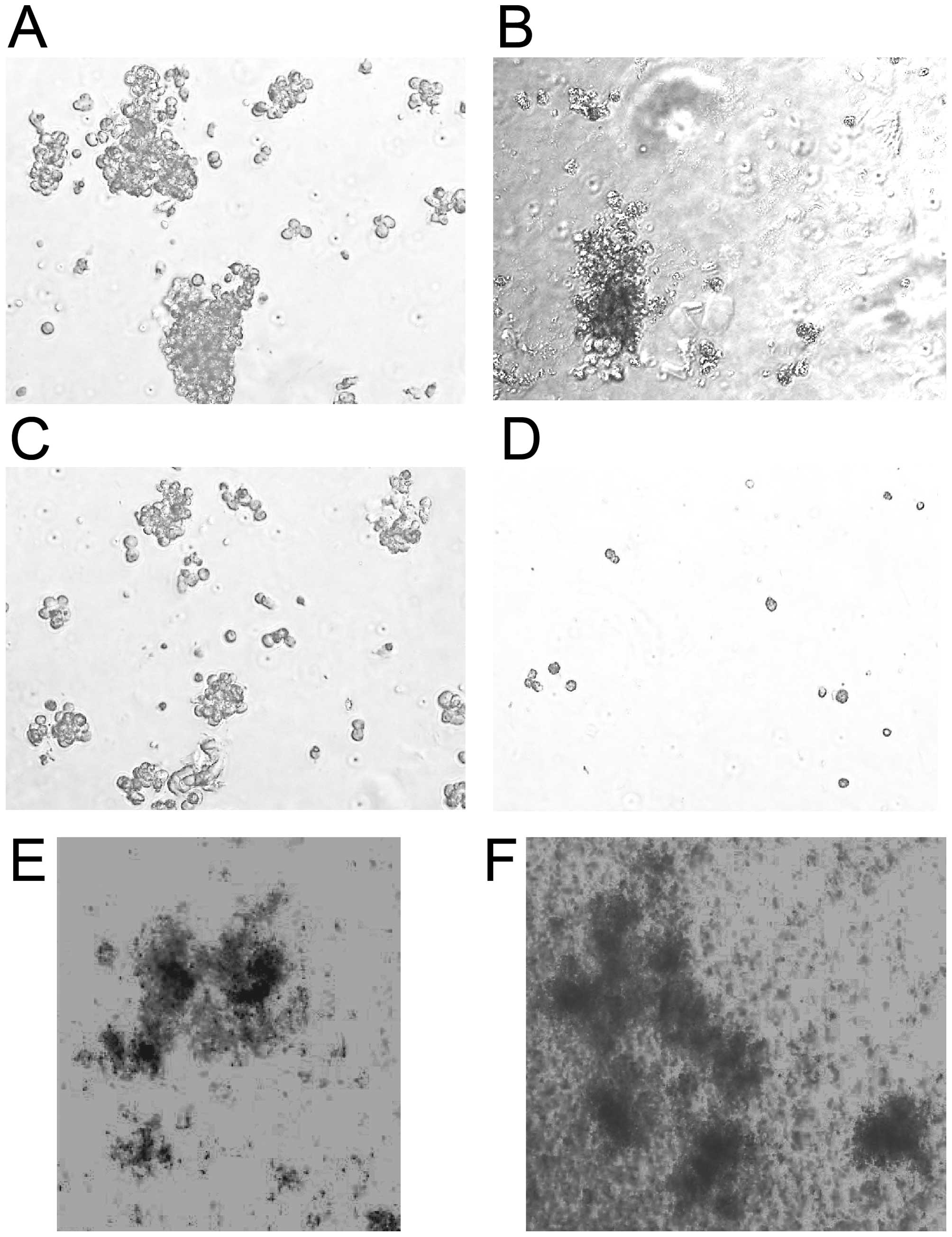
A colony-forming assay indicated that, while ATRA
effectively inhibited the formation of large colonies, 30 nM ATRA
could not completely eliminate small colonies (Fig. 4). The mean colony numbers in
untreated and ATRA-, tamoxifen-, and ATRA plus tamoxifen-treated
cultures were 476.5±51.6, 218.1±23.2, 132.3±22.6, and 0.6±0.1 (±
SD), respectively. Combined treatment with ATRA and tamoxifen
completely suppressed colony formation by NB4 cells, although
several clusters (<0.1 mm in diameter) were observed in cultures
treated with this combination (Fig.
4D), suggesting that the combined treatment was not cytotoxic.
Next, the combined effects of ATRA and tamoxifen on colony
formation in mouse bone marrow cells were compared to those in NB4
cells. The combination of 30 nM ATRA plus 6 μM tamoxifen
significantly suppressed colony formation by NB4 cells but hardly
affected colony formation by normal mouse bone marrow cells. Both
the number of colonies and the colony size in the treated cultures
were similar to those in untreated cultures (Fig. 4E and F). These results suggest that
APL cells were more sensitive to combined treatment with ATRA and
tamoxifen than normal hematopoietic cells.
Combined effects of ATRA and tamoxifen on
cell viability and differentiation of APL cells in primary
culture
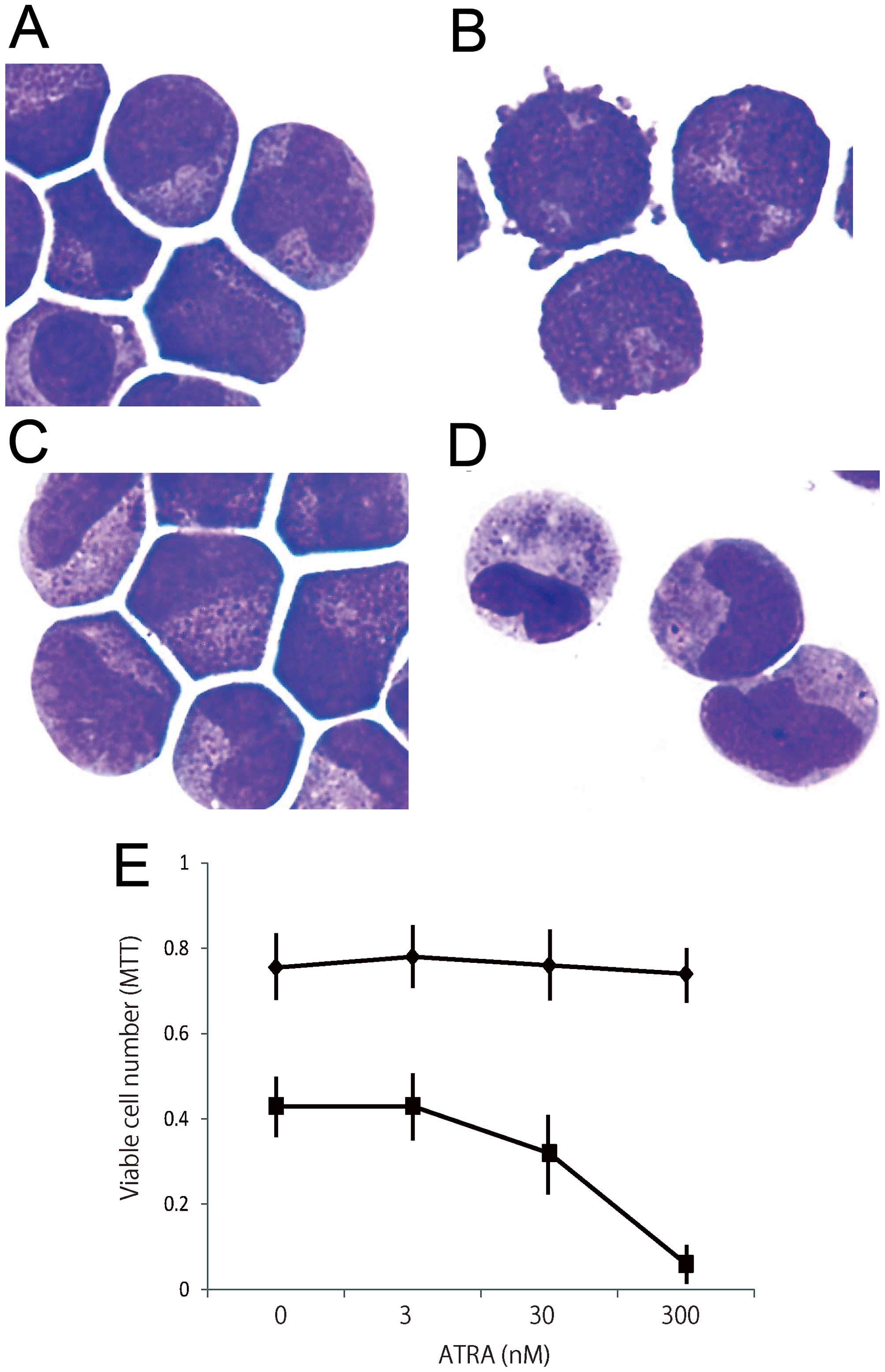
APL cells were freshly isolated and incubated with
various concentrations of ATRA and tamoxifen for 3 days. We
analyzed morphological changes in APL cells in primary culture.
Most untreated APL cells were still blastic after 3 days of culture
(Fig. 5A). Tamoxifen did not
affect the morphological changes in APL cells (Fig. 5B). Although ATRA at 300 nM induced
APL cells to differentiate into myelocytes and metamyelocytes, 9 nM
ATRA did not significantly affect the morphology of APL cells
(Fig. 5C). Combined treatment with
9 nM ATRA and 2 μM tamoxifen significantly induced the granulocytic
differentiation of APL cells (Fig.
5D), suggesting that tamoxifen enhances the granulocytic
differentiation of APL cells induced by ATRA.
Under treatment with ATRA for 3 days, although
malignant cells did not grow, most of the cells were still viable
even at a high concentration (300 nM). In the presence of 6 μM
tamoxifen, ATRA concentration-dependently reduced the numbers of
viable cells (Fig. 5E).
Discussion
Tamoxifen has been extensively tested and is widely
used. It is inexpensive and has a low side-effect profile, and thus
represents a very attractive therapeutic option. The
growth-inhibitory activity of tamoxifen in human APL cells seems to
be estrogen receptor-independent. 17β-estradiol did not affect the
growth and differentiation of leukemia cells treated with or
without ATRA. An antagonist specific to retinoic acid receptor-α
did not affect the growth-inhibitory effect of tamoxifen. On the
contrary, α-tocopherol, a membrane stabilizer, effectively
counteracted the effects of tamoxifen on leukemia cells. Tamoxifen
elevated lipid peroxidation and induced membrane damage via a
mitochondria-dependent pathway (11,17).
However, we cannot exclude the possibility that unique estrogen
receptor(s) might be involved in the action of tamoxifen. Kauss
et al reported that HL-60 cells expressed a membrane
receptor for estrogen that modulated ATRA-induced cell
differentiation (18).
Although tamoxifen does not have potent
differentiation-inducing and growth-inhibitory effects in APL
cells, it effectively enhanced the effects of ATRA on APL cells.
This combined treatment did not significantly affect colony
formation by normal mouse bone marrow cells, suggesting that some
system helps normal cells recover from membrane damage induced by
tamoxifen. This difference between normal and leukemia cells may
contribute to a scheme for modulating lipid peroxidation in the
membrane as a strategy to selectively kill leukemia cells.
Several inducers such as ATRA and VD3 activate ERK
in leukemia cells before inducing myelomonocytic differentiation,
and this activation is needed to elicit differentiation and growth
arrest (13,14). Tamoxifen also activated ERK in
leukemia cells, and combined treatment with ATRA and tamoxifen
further enhanced the phosphorylation of ERK. These results suggest
that tamoxifen induces membrane damage via lipid peroxidation and
then causes the activation of ERK signaling.
A phase I trial of tamoxifen with daunorubicin has
been performed in patients with relapsed or refractory acute
leukemia (19). Plasma levels of
tamoxifen approached 7 μM in patients treated with high-dose
tamoxifen (550 or 700 mg/day, p.o., for 7 days). No severe hepatic,
cardiac or retinal toxicity was noted. The toxicity of the
combination of tamoxifen and daunorubicin was comparable to that
seen with daunorubicin alone. This study suggested that plasma
concentrations of tamoxifen that are high enough to enhance the
differentiation and growth arrest of APL cells can be approached
with an acceptable toxicity profile. The combination of ATRA and
tamoxifen should be considered for the treatment of APL patients in
whom it is difficult to apply arsenic trioxide or
anthracyclines.
References
|
1
|
Wang ZY and Chen Z: Acute promyelocytic
leukemia: From highly fatal to highly curable. Blood.
111:2505–2515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tallman MS, Andersen JW, Schiffer CA,
Appelbaum FR, Feusner JH, Woods WG, Ogden A, Weinstein H, Shepherd
L, Willman C, et al: All-trans retinoic acid in acute promyelocytic
leukemia: Long-term outcome and prognostic factor analysis from the
North American Intergroup protocol. Blood. 100:4298–4302. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asou N, Kishimoto Y, Kiyoi H, Okada M,
Kawai Y, Tsuzuki M, Horikawa K, Matsuda M, Shinagawa K, Kobayashi
T, et al: Japan Adult Leukemia Study Group: A randomized study with
or without intensified maintenance chemotherapy in patients with
acute promyelocytic leukemia who have become negative for
PML-RARalpha transcript after consolidation therapy: The Japan
Adult Leukemia Study Group (JALSG) APL97 study. Blood. 110:59–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adès L, Guerci A, Raffoux E, Sanz M,
Chevallier P, Lapusan S, Recher C, Thomas X, Rayon C, Castaigne S,
et al: European APL Group: Very long-term outcome of acute
promyelocytic leukemia after treatment with all-trans retinoic acid
and chemotherapy: The European APL Group experience. Blood.
115:1690–1696. 2010. View Article : Google Scholar
|
|
5
|
Lo-Coco F, Avvisati G, Vignetti M, Thiede
C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona
E, et al: Gruppo Italiano Malattie Ematologiche dell'Adulto;
German-Austrian Acute Myeloid Leukemia Study Group; Study Alliance
Leukemia: Retinoic acid and arsenic trioxide for acute
promyelocytic leukemia. N Engl J Med. 369:111–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ratnaike RN: Acute and chronic arsenic
toxicity. Postgrad Med J. 79:391–396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mandlekar S and Kong ANT: Mechanisms of
tamoxifen-induced apoptosis. Apoptosis. 6:469–477. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Avgeropoulos NG and Batchelor TT: New
treatment strategies for malignant gliomas. Oncologist. 4:209–224.
1999.PubMed/NCBI
|
|
9
|
Niitsu N, Ishii Y, Matsuda A and Honma Y:
Induction of differentiation of acute promyelocytic leukemia cells
by a cytidine deaminase-resistant analogue of
1-β-D-arabinofuranosylcytosine,
1-(2-deoxy-2-methylene-β-D-erythro-pentofuranosyl)cytidine. Cancer
Res. 61:178–185. 2001.PubMed/NCBI
|
|
10
|
Takahashi T, Kawakami K, Mishima S,
Akimoto M, Takenaga K, Suzumiya J and Honma Y: Cyclopamine induces
eosinophilic differentiation and upregulates CD44 expression in
myeloid leukemia cells. Leuk Res. 35:638–645. 2011. View Article : Google Scholar
|
|
11
|
Miyake T, Honma Y, Urano T, Kato N and
Suzumiya J: Combined treatment with tamoxifen and a fusicoccin
derivative (ISIR-042) to overcome resistance to therapy and to
enhance the antitumor activity of 5-fluorouracil and gemcitabine in
pancreatic cancer cells. Int J Oncol. 47:315–324. 2015.PubMed/NCBI
|
|
12
|
Honma Y: Cotylenin A - a plant growth
regulator as a differentiation-inducing agent against myeloid
leukemia. Leuk Lymphoma. 43:1169–1178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yen A, Roberson MS, Varvayanis S and Lee
AT: Retinoic acid induced mitogen-activated protein
(MAP)/extracellular signal-regulated kinase (ERK) kinase-dependent
MAP kinase activation needed to elicit HL-60 cell differentiation
and growth arrest. Cancer Res. 58:3163–3172. 1998.PubMed/NCBI
|
|
14
|
Wang X and Studzinski GP: Activation of
extracellular signal-regulated kinases (ERKs) defines the first
phase of 1,25-dihydroxyvitamin D3-induced
differentiation of HL60 cells. J Cell Biochem. 80:471–482. 2001.
View Article : Google Scholar
|
|
15
|
Dudley DT, Pang L, Decker SJ, Bridges AJ
and Saltiel AR: A synthetic inhibitor of the mitogen-activated
protein kinase cascade. Proc Natl Acad Sci USA. 92:7686–7689. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishii Y, Kiyota H, Sakai S and Honma Y:
Induction of differentiation of human myeloid leukemia cells by
jasmonates, plant hormones. Leukemia. 18:1413–1419. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nazarewicz RR, Zenebe WJ, Parihar A,
Larson SK, Alidema E, Choi J and Ghafourifar P: Tamoxifen induces
oxidative stress and mitochondrial apoptosis via stimulating
mitochondrial nitric oxide synthase. Cancer Res. 67:1282–1290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kauss MA, Reiterer G, Bunaciu RP and Yen
A: Human myeloblastic leukemia cells (HL-60) express a membrane
receptor for estrogen that signals and modulates retinoic
acid-induced cell differentiation. Exp Cell Res. 314:2999–3006.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berman E, McBride M, Lin S, Menedez-Botet
C and Tong W: Phase I trial of high-dose tamoxifen as a modulator
of drug resistance in combination with daunorubicin in patients
with relapsed or refractory acute leukemia. Leukemia. 9:1631–1637.
1995.PubMed/NCBI
|