Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common cancer and the third leading cause of cancer-related deaths
worldwide. Most HCC patients are diagnosed with the tumor at
medium- or late-stage because of poor diagnosis (1–4).
Transcatheter arterial chemoembolization (TACE) is considered as an
important palliative therapy for late-stage HCC patients (5–7).
Ideally, during the TACE treatment, iodine oil should achieve
complete embolization and thus tumor necrosis. However, TACE
treatment does not attain complete tumor necrosis in the majority
of HCC patients, while the hypoxia status after TACE treatment
plays a key role in HCC recurrence and metastasis (8,9).
Previous studies have shown that low oxygen
environments are prevalent in tumors (10–12).
Hypoxia-inducible factor (HIF) is a transcription factor
upregulated in response to hypoxia. It is composed of HIF-1α and
HIF-1α subunit, and the HIF-1α unit is vital to HIF-1 stability and
expression level (13). HIF-1α is
involved in transcriptional regulation of a variety of target genes
in the growth of various types of cancers. TACE is a common
therapeutic approach. However, it might aggravate hypoxia and thus
affects HIF-1α protein expression, promoting growth, invasion and
metastasis of residual cancer, which is probably the main reason
influencing the long-term curative effects for TACE (14–16).
Cyclooxygenase (COX) plays a key role in
physiological and pathological regulation (17,18).
It consists of two subunits: COX-1 and COX-2. COX-2 is not
expressed in physiological condition, while its expression is
induced by pathological stimuli, such as cancer, endotoxin and
hormones (19,20). It is well documented that COX-2
accelerates cancer invasion and metastasis through inhibiting
cancer cell apoptosis and promoting tumor angiogenesis (21–23).
High expression of COX-2 is closely correlated with poor prognosis
for various types of cancers (24–26).
The role of high expression of HIF-1α and COX-2 in clinical
prognosis of HCC patients after TACE remains to be completely
elucidated.
Epithelial-to-mesenchymal transition (EMT) is a
process in which epithelial cells are transmitted to mesenchymal
cells that have higher ability of migration (27,28).
EMT is regulated by HIF-1α and COX-2, playing an important role in
tumor occurrence and metastasis (29,30).
The relationship between EMT and HIF-1α, COX-2 protein expression
in HCC tissue after TACE surgery is hardly investigated. In this
study, we detected HIF-1α and COX-2 protein expression in HCC
tissues after TACE surgery and in HepG2 cells treated with hypoxia
in vitro. In addition, the relationship between EMT and
HIF-1 protein expression was evaluated. This study aimed to provide
clues to increasing survival time for HCC patients post TACE.
Materials and methods
Clinical characteristics and medical
records
This study complied with the Declaration of Helsinki
and the ethics committee of the Third Affiliated Hospital, Sun
Yat-Sen University approved the study protocol. Informed consent
from all participants was obtained before the initial coronary
angiography.
Paired cancer tissues and adjacent normal liver
tissues from HCC patients undergoing TACE surgery were
prospectively collected between November 2006 and September 2013 at
the Third Affiliated Hospital, Sun Yat-Sen University. A total of
51 HCC patients were enrolled in this study, including 48 males and
3 females, and their age ranged from 29 to 72 years (median, 50.5
years). All HCC specimens were procured and processed following
standard procedures. The tissue was fixed using 10% formaldehyde
solution, embedded within paraffin, and then sectioned into 5-μm
slices. The general information of all patients including name,
gender and age was collected. Other clinical characteristics
including AFP level before surgery, liver function and medical
imaging data (CT or MRI), tumor size, tumor number, BCLC stage of
cancer, presence of cancer capsule, the presence of vascular
invasion and distant metastasis were also recorded. Cancer tissues
from 61 HCC patients without TACE surgery were collected and served
as controls. Postoperative follow-up was carried out regularly
through telephone and letter. The mean postoperative follow-up
period was 36.0 months (range, 3.0–75.0 months). Overall survival
was calculated from the date of surgery to the date of death or the
last follow-up.
Culture of HepG2 cells and hypoxia
treatment
HepG2 cells were cultured in DMEM medium with 10%
fetal calf serum (FBS, Gibco, USA) at 37°C in a humidified
atmosphere containing 5% CO2. Different concentrations
of CoCl2 (Sigma, USA) was added into culture medium to
mimic the hypoxic microenvironment (31).
CCK-8 assay
HepG2 cells were detached with trypsin and seeded on
a 96-well plate (1×104 cells/well). After culture in
DMEM culture medium supplemented with 10% FBS to allow cells become
adherent, cells were treated with 0, 100, 200 and 300 μM
CoCl2 diluted in DMEM/10% FBS for 24 h. Cells without
CoCl2 treatment served as controls. Then 10 μl CCK-8
solution was added into each well and the plate was incubated for
additional 1 h. Afterwards, the absorbance at 450 nm was measured
using a microplate reader. Wells without addition of CCK-8 solution
were taken as the blank. The cell viability was calculated using
the formula: Cell viability (100%) = (mean OD of CoCl2
group - mean OD of blank group)/mean OD of control group - mean OD
of blank group) × 100. We performed 3 parallel repeats in each
group. This experiment was carried out independently 3 times.
Quantitative reverse transcription PCR
(qRT-PCR) analysis
HepG2 cells (105 cell/ml) were seeded in
a 6-well plate and treated with 0, 100, 200 and 300 μM
CoCl2 diluted in DMEM/10% FBS for 12 and 24 h. Total RNA
was extracted using TRIzol (Invitrogen, USA) according to the
manufacturer's instructions. Total RNA (1 μg) was reverse
transcribed into cDNA with a reverse transcription kit (Promega,
USA). The PCR reaction was carried out in a total of 20 μl reaction
mixture using Invitrogen PCR kit and the reactions were performed
as follows, 50°C for 2 min, 95°C for 2 min, followed by 40 cycles
of 95°C for 15 sec and 60°C for 32 sec. β-actin was used as the
endogenous reference to measure the relative expression levels of
HIF-1α and COX-2. The primer sequences are shown in Table I.
 | Table IThe primer sequences. |
Table I
The primer sequences.
| Forward
(5′→3′) | Reverse
(5′→3′) |
|---|
| HIF-1α |
AAGCACTAGACAAAGCTCACCTG |
TTGACCATATCGCTGTCCAC |
| COX-2 |
TTGACCATATCGCTGTCCAC |
TTGACCATATCGCTGTCCAC |
| β-actin |
TTGACCATATCGCTGTCCAC |
TCGGCCACATTGTGAACTTT |
Western blot detection
Cells were lyzed in RIPA buffer and centrifuged to
extract total protein. Protein concentration of samples was
determined using the Bradford assay method. Sample protein (30 μg)
was separated in 8% SDS-PAGE and transferred to PVDF membrane. The
membrane was blocked with 5% skim milk in TBST, and then incubated
with primary antibodies overnight at 4°C using mouse anti-rat
HIF-1α polyclonal antibody (Santa Cruz, USA, dilution, 1:300),
mouse anti-rat COX-2 polyclonal antibody (Santa Cruz, 1:500) and
mouse anti-rat β-actin polyclonal antibody (Santa Cruz, 1:2,000).
The next day, after 3 washes in TBST, the membrane was incubated
with goat anti-mouse HRP-labeled secondary antibody (Santa Cruz,
1:5,000) for 1 h at room temperature (RT). Bands were developed
with an ECL chemiluminescence reagent system (Beyotime, China).
Invasion assay
Melting Matrigel (50 mg/l, BD, USA) was diluted 1:6
with pre-cooled serum-free DMEM culture medium, which was then used
to coat the upper membrane surface of the top chamber. Then the
Transwell plate was incubated at 37°C for 1.5 h. HepG2 cells were
digested using trypsin, centrifuged at 130 × g for 5 min and
re-suspended in serum-free DMEM containing 0 or 200 μM
CoCl2. Cells/well (1×105) were seeded into
the top chamber. DMEM (500 μl)/10% FBS was added in the bottom
chamber. After 24-h incubation, the top chamber was taken out and
fixed using 4% paraformaldehyde solution for 30 min. After 3 washes
with PBS, cells on the upper membrane surface were removed using a
cotton swab. Cells on the bottom membrane surface were stained with
Giemsa for 8 min followed by washing with PBS, 3 times for 5 min.
The number of cells on bottom membrane surface (invading cells) was
calculated randomly under a microscope in highly magnified field
(q). The experiment was repeated 3 times.
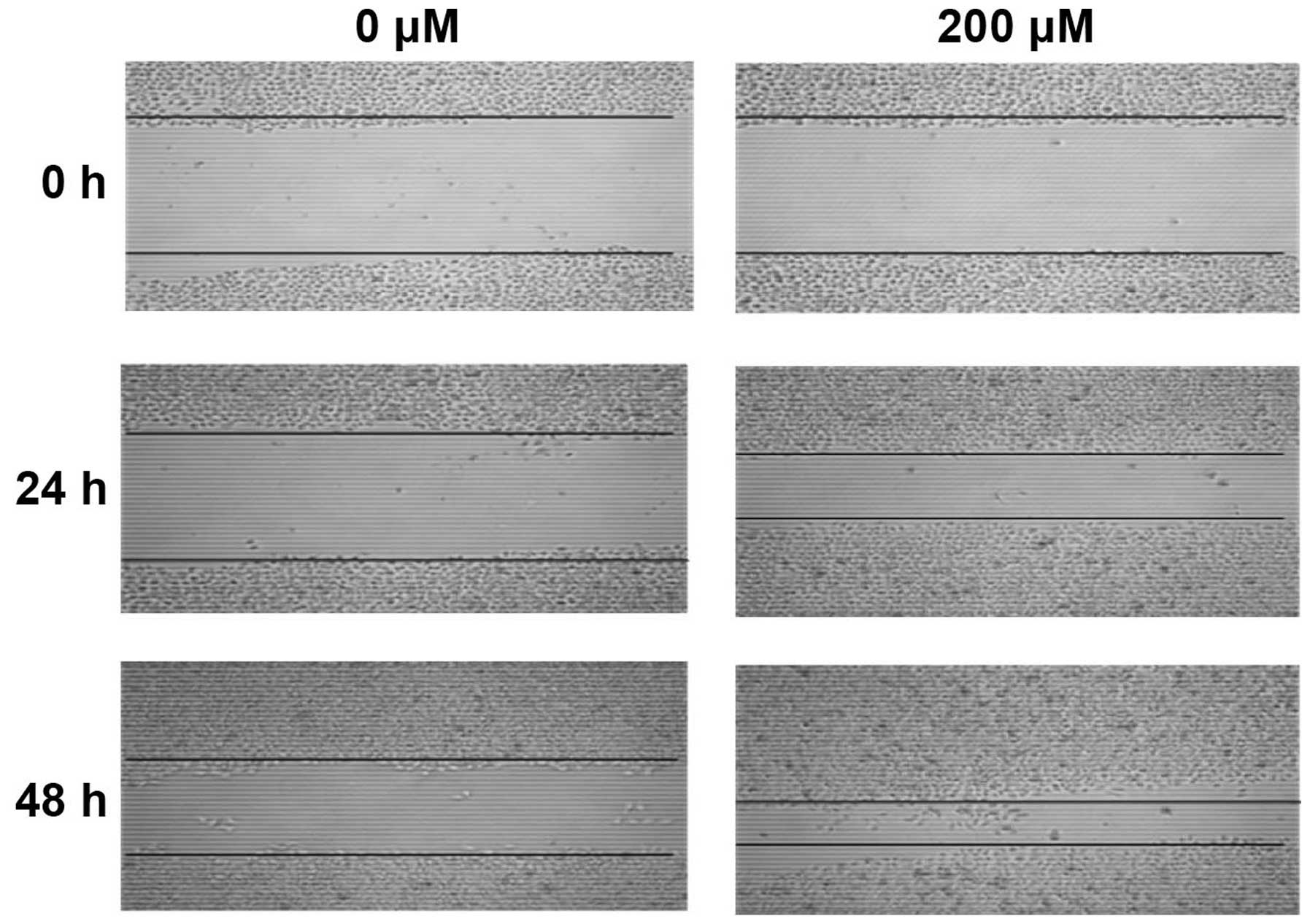
Wound-healing assay
Cells (1 ml) at a density of 4×105
cells/ml were seeded into each well of a 6-well plate and cultured
to 100% confluence. The monolayer of cells was then scratched with
a 10-μl pipette tip to create a wound gap. After 3 washes with PBS
to remove suspended cells, the plate was placed back into an
incubator for incubation for an additional 24 and 48 h. Images were
taken at 0, 24 and 48 h after the wound was induced to observe the
healing of the wound gap.
Immunohistochemistry detection
Tissue slices (4 μm) were deparaffinized using
dimethylbenzene, 2 times for 5 min and rehydrated using gradient
ethanol. Then slices were soaked in deionized water for 5 min and
dried using absorbent paper. Antigen retrieval was carried out by
heating the sample in a microwave oven. After cooled down, slices
were treated with 3% H2O2 at room temperature
for 10 min. Then slices were washed with PBS and blocked with 3%
BSA in PBS for 1 h. After blocking, slices was incubated with
primary antibody (1:50–200) at 4°C overnight, and subsequently
incubated with HRP-labeled secondary antibody (Dako, Canada) at
37°C for 1 h. The slices were then treated with a chromogen,
3,3′-diaminobenzidine tetrahydrochloride (DAB) and counterstained
with hematoxylin for 45 sec. Finally, slices were washed with
ultrapure water, dehydrated with gradient ethanol, deparaffinized
using dimethylbenzene for 10 min, dried and sealed.
Statistical analysis
Data were analyzed using SPSS 13.0 software.
Quantity data were expressed as mean ± SD. The Student's t-test was
used when 2 groups were compared. Fisher's exact test was used to
analyzed the difference between HIF-1α, COX-2 and clinical
pathological data. Spearman correlation coefficient was used to
determine the relationship between HIF-1α and COX-2 protein
expression. Survival analysis was performed using Kaplan-Meier
estimate. The survival curves between different groups were
analyzed using log-rank test. After the univariate analysis for 14
factors, only variables with P-value <0.05 were included in the
multivariate analysis using the Cox proportional hazards model to
identify the independent prognostic factors for overall survival.
P<0.05 was considered statistically significant.
Results
Increased expression of HIF-1α and COX-2
protein in HCC tissue after TACE surgery
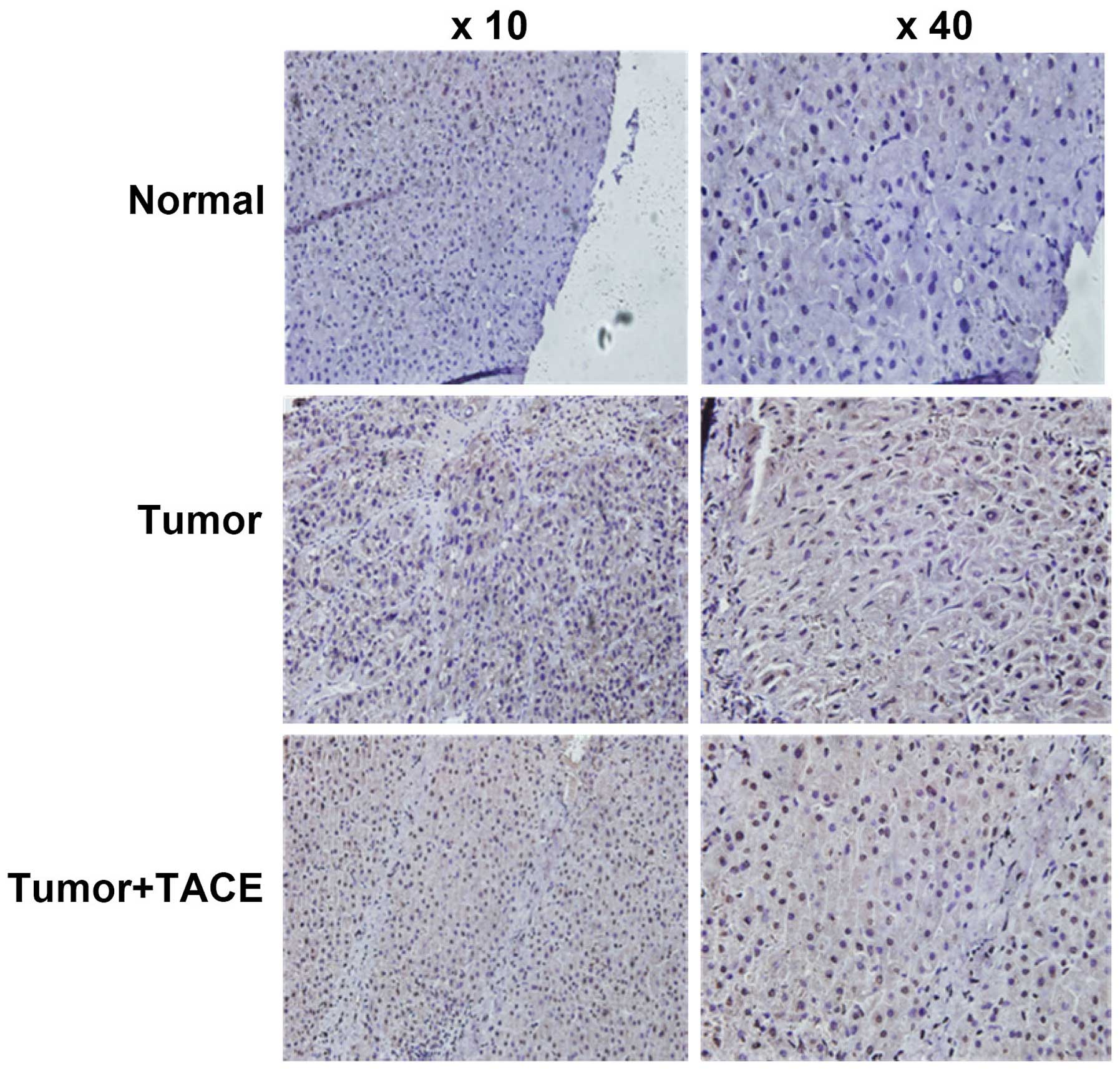
Immunohistochemistry experimental results showed
that HIF-1α protein expression in liver cancer tissue significantly
increased in 51 cases of HCC patients post TACE, compared with
corresponding adjacent noncancerous liver tissue (Fig. 1 and Table II, P=0.001). The positive rate of
HIF-1α protein expression in liver cancer tissue from HCC patients
without TACE surgery was significantly lower than that in liver
cancer tissue from HCC patients post TACE (Fig. 1 and Table II, P=0.013).
 | Table IIHIF-1α and COX-2 protein expression
in cancer tissues from HCC patients after TACE surgery. |
Table II
HIF-1α and COX-2 protein expression
in cancer tissues from HCC patients after TACE surgery.
| Positive rate of
HIF-1α | Positive rate of
COX-2 |
|---|
| Liver cancer tissue
of HCC patient after TACE surgery | 49.0% (26/51) | 56.8% (29/51) |
| Adjacent normal
liver tissue of HCC patient after TACE surgery | 21.6% (11/51) | 25.5% (13/51) |
| Liver cancer tissue
in HCC patient without TACE surgery | 34.4% (21/61) | 47.5% (23/61) |
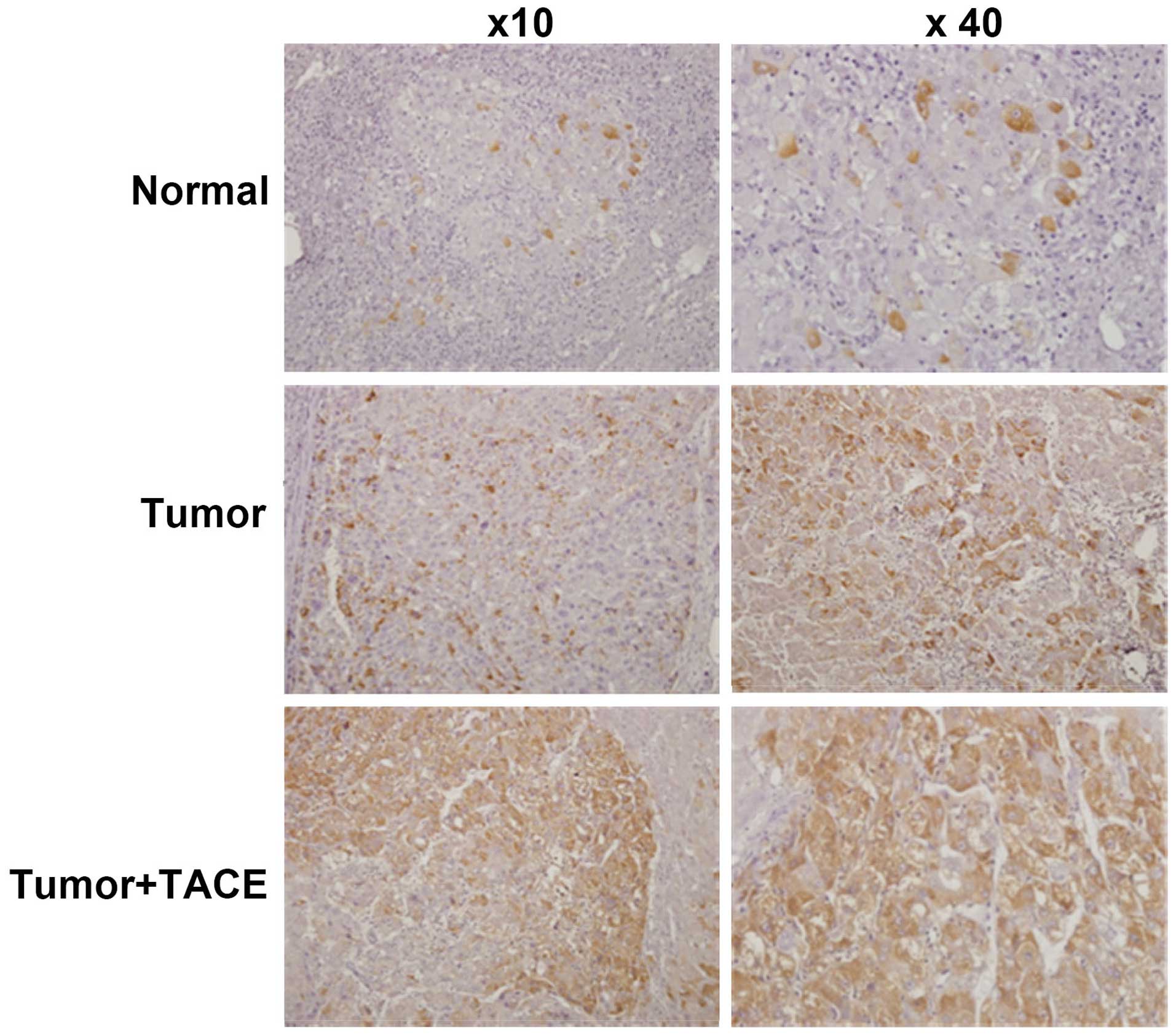
In Fig. 1 and
Table II, the
immunohistochemistry results showed that COX-2 protein expression
in liver cancer tissue of 51 cases of HCC patients post TACE was
higher than that in corresponding adjacent noncancerous liver
tissue (Fig. 2 and Table II, P=0.001). The positive rate of
COX-2 protein expression in liver cancer tissue from HCC patients
without TACE surgery was significantly lower than that in liver
cancer tissue from HCC patients post TACE (Fig. 2 and Table II, P=0.034).
The relationship between HIF-1α, COX-2
and clinicopathologic variables
After analysis by Fisher's exact test, our data
showed that HIF-1α protein level was significantly higher in groups
of BCLC stage A and ALT before surgery ≥40 U/l than groups of BCLC
stage B+C and ALT before surgery <40 U/l, respectively (P=0.011
and 0.028, respectively). However, increased protein expression of
HIF-1α had no significant difference in groups in terms of age,
gender, Childs classification, AFP level, tumor size, tumor number
and presence of cancer capsule (Table III). COX-2 protein expression
significantly increased in HCC patient with vascular invasion and
intrahepatic metastasis (P=0.021 and 0.048, respectively), while it
had no significant difference in groups in terms of gender, age,
Childs classification, AFP level, tumor size, tumor number and
presence of cancer capsule (Table
IV, P>0.05).
 | Table IIIHIF-1α expression was statistically
different between HCC patients in BCLC stage A and BCLC stage B+C,
and patients with high and low level of ALT before surgery
(P=0.011, P=0.028, respectively). |
Table III
HIF-1α expression was statistically
different between HCC patients in BCLC stage A and BCLC stage B+C,
and patients with high and low level of ALT before surgery
(P=0.011, P=0.028, respectively).
| | HIF-1α | |
|---|
| |
| |
|---|
| Clinical
characteristics | No. of
patients | Positive | Negative | P-value |
|---|
| Age | 51 | | | |
| >60 | 13 | 11 | 2 | 0.103 |
| ≤60 | 38 | 14 | 24 | |
| Gender | 51 | | | |
| Male | 48 | 22 | 26 | 0.110 |
| Female | 3 | 3 | 0 | |
| Tumor size | 51 | | | |
| ≥50 mm | 27 | 15 | 12 | 0.239 |
| <50 mm | 24 | 10 | 14 | |
| Vascular
invasion | 51 | | | |
| Yes | 19 | 10 | 8 | 0.346 |
| No | 32 | 15 | 18 | |
| Capsular
infiltration | 51 | | | |
| Yes | 8 | 4 | 4 | 0.626 |
| No | 43 | 21 | 22 | |
| AFP before surgery
(ng/ml) | 51 | | | |
| ≥200 | 29 | 14 | 15 | 0.564 |
| <200 | 22 | 11 | 11 | |
| Tumor number | 51 | | | |
| Single | 27 | 13 | 14 | 0.559 |
| Multiple | 24 | 12 | 12 | |
| BCLC stage | 51 | | | |
| A | 17 | 4 | 13 |
0.011a |
| B+C | 34 | 21 | 13 | |
| GCT level before
surgery | 51 | | | |
| ≥54 | 32 | 15 | 17 | 0.457 |
| <54 | 19 | 10 | 9 | |
| ALT level before
surgery | 51 | | | |
| ≥40 | 31 | 19 | 12 |
0.028a |
| <40 | 20 | 6 | 14 | |
| Intrahepatic
metastasis | 51 | | | |
| Yes | 31 | 17 | 14 | 0.228 |
| No | 20 | 8 | 12 | |
 | Table IVCOX-2 expression was statistically
different between HCC patients with vascular invasion and without
vascular invasion, and HCC patients with intrahepatic metastasis
and without intrahepatic metastasis (P=0.021 and P=0.048,
respectively). |
Table IV
COX-2 expression was statistically
different between HCC patients with vascular invasion and without
vascular invasion, and HCC patients with intrahepatic metastasis
and without intrahepatic metastasis (P=0.021 and P=0.048,
respectively).
| | COX-2 | |
|---|
| |
| |
|---|
| Clinical
characteristics | No. of
patients | Positive | Negative | P-value |
|---|
| Age | 51 | | | |
| >60 | 13 | 11 | 2 | 0.059 |
| ≤60 | 38 | 18 | 20 | |
| Gender | 51 | | | |
| Male | 48 | 27 | 21 | 0.604 |
| Female | 3 | 2 | 1 | |
| Tumor size | 51 | | | |
| ≥50 mm | 27 | 18 | 9 | 0.112 |
| <50 mm | 24 | 11 | 13 | |
| Vascular
invasion | 51 | | | |
| Yes | 19 | 20 | 8 |
0.021a |
| No | 32 | 9 | 14 | |
| Capsular
infiltration | 51 | | | |
| Yes | 8 | 6 | 2 | 0.233 |
| No | 43 | 23 | 20 | |
| AFP before surgery
(ng/ml) | 51 | | | |
| ≥200 | 29 | 16 | 13 | 0.503 |
| <200 | 22 | 13 | 9 | |
| Tumor number | 51 | | | |
| Single | 27 | 16 | 11 | 0.467 |
| Multiple | 24 | 13 | 11 | |
| BCLC stage | 51 | | | |
| A | 17 | 7 | 10 | 0.097 |
| B+C | 34 | 22 | 12 | |
| GCT level before
surgery | 51 | | | |
| ≥54 | 32 | 18 | 14 | 0.572 |
| <54 | 19 | 11 | 8 | |
| ALT level before
surgery | 51 | | | |
| ≥40 | 31 | 20 | 11 | 0.139 |
| <40 | 20 | 9 | 11 | |
| Intrahepatic
metastasis | 51 | | | |
| Yes | 31 | 21 | 10 |
0.048a |
| No | 20 | 8 | 12 | |
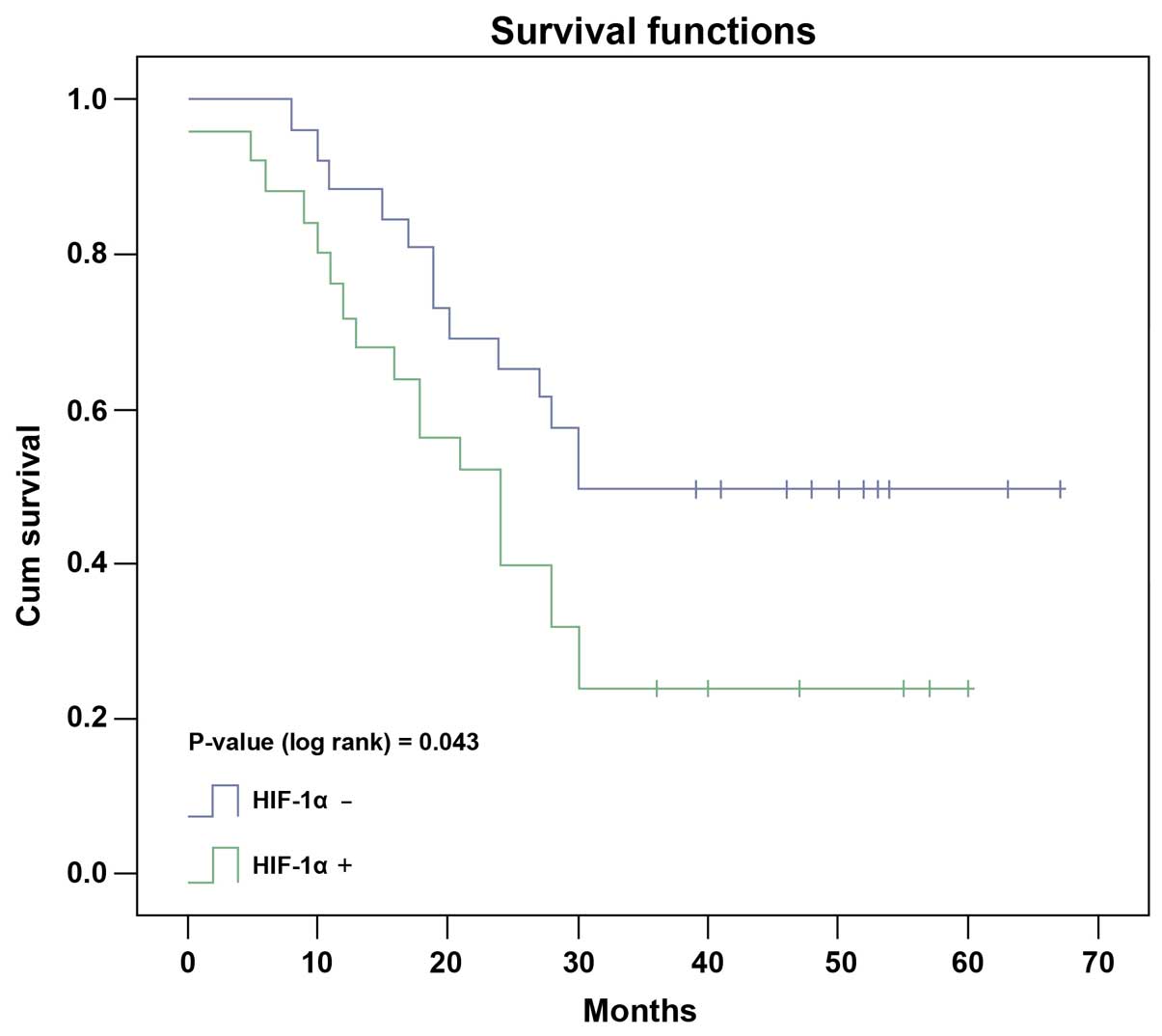
Reduced survival time in HCC patients
with positive expression of HIF-1α and COX-2 protein
Kaplan-Meier test showed that the median survival
time of HCC patients with positive HIF-1α protein detection was
27.0 months, significantly shorter than that of 43.0 months in
patients with negative HIF-1α protein detection (Fig. 3, P=0.043, log-rank test). In
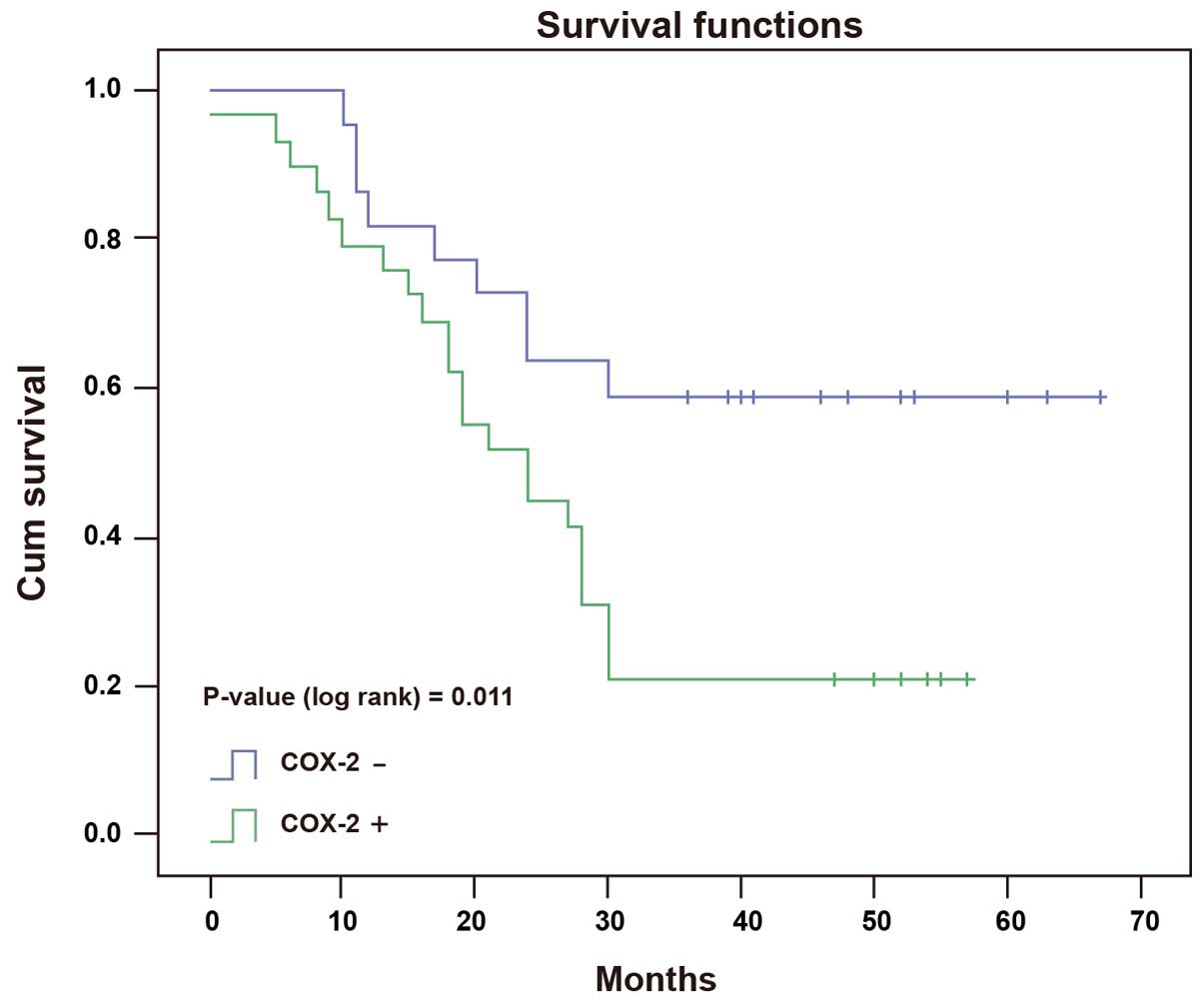
parallel, the median survival time in COX-2 positive patients was
26.0 months, which was significantly shorter than that of 46.0
months in COX-2 negative patients (Fig. 4, P=0.011, log-rank test).
Intrahepatic metastasis, tumor size and
COX-2 protein expression was associated with prognosis of HCC
patients
Univariate analysis showed that several parameters
were associated with poor prognosis of HCC, including tumor size,
intrahepatic metastasis, HIF-1α expression, COX-2 expression and
HIF-1α/COX-2 double expression (Table
V). Furthermore, multivariate Cox's proportional hazard
regression analysis showed these parameters were independent
prognostic factors for HCC patients (P=0.011), indicating that
tumor size, intra-hepatic metastasis and COX-2 protein expression
were closely associated with poor prognosis of HCC patients
(Table VI).
 | Table VUnivariate analysis showed that tumor
size, intrahepatic metastasis, vascular invasion, positive
expression of HIF-1α and COX-2α and double-positive expression of
HIF-1α plus COX-2 were correlated with prognosis of HCC
patients. |
Table V
Univariate analysis showed that tumor
size, intrahepatic metastasis, vascular invasion, positive
expression of HIF-1α and COX-2α and double-positive expression of
HIF-1α plus COX-2 were correlated with prognosis of HCC
patients.
| Clinical
characteristics | RR | 95% CI | P-value |
|---|
| Gender | 0.2 | 1.910–2.756 | 0.650 |
| Age | 1.66 | 1.829–2.836 | 0.204 |
| Tumor size | 9.99 | 1.250–2.290 |
0.002a |
| Vascular
invasion | 2.168 | 1.210–2.290 | 0.141 |
| Capsular
infiltration | 1.44 | 1.731–2.936 | 0.230 |
| AFP | 0.9 | 1.341–2.659 | 0.342 |
| BCLC stage | 1.503 | 1.527–2.473 | 0.220 |
| Tumor number | 1.55 | 2.249–2.750 | 0.213 |
| GCT | 0.29 | 2.242–2.758 | 0.588 |
| ALT | 1.4 | 2.136–2.863 | 0.228 |
| Intrahepatic
metastasis | 7.2 | 1.296–2.205 |
0.007a |
| HIF-1α | 4.35 | 1.675–2.325 |
0.037a |
| COX-2 | 6.4 | 1.271–2.729 |
0.011a |
| HIF-1α+COX-2 | 12.07 | 0.928–2.072 |
0.001a |
 | Table VIMultivariate analysis showed that
tumor size, intrahepatic metastasis and COX-2 expression were the
most important risk factors for prognosis of HCC patients. |
Table VI
Multivariate analysis showed that
tumor size, intrahepatic metastasis and COX-2 expression were the
most important risk factors for prognosis of HCC patients.
| Clinical
characteristics | RR | 95% CI | P-value |
|---|
| Tumor size | 0.385 | 0.165–0.899 |
0.027a |
| Intrahepatic
metastasis | 0.300 | 0.094–0.954 |
0.041a |
| Vascular
invasion | 2.264 | 0.816–6.279 | 0.116 |
| HIF-1α | 0.284 | 0.057–1.419 | 0.125 |
| COX-2 | 0.242 | 0.070–0.839 |
0.025a |
| HIF-1α+ COX-2 | 3.157 | 0.515–24.695 | 0.198 |
The positive correlation between HIF-1α
and COX-2 protein expression
The positive rate of COX-2 protein expression in HCC
patients with HIF-1α positive expression was 72.7% (18/25), which
was higher than that of 42.3% (11/26) in HIF-1α negative HCC
patients. Whereas, the positive rate of HIF-1α protein expression
in HCC patients with COX-2 positive expression was higher than that
of HCC patients without detectable COX-2 expression [62.1% (18/29)
and 31.8% (7/22), respectively]. Spearman rank correlation analysis
showed that there was a positive correlation between HIF-1α and
COX-2 protein expression (Table
VII, P=0.033).
 | Table VIIHIF-1α protein expression was
positively correlated with COX-2 protein expression (P=0.033). |
Table VII
HIF-1α protein expression was
positively correlated with COX-2 protein expression (P=0.033).
| HIF-1α | |
|---|
|
| |
|---|
| COX-2 | Positive | Negative | P-value |
|---|
| Positive | 18 | 11 | 0.033 |
| Negative | 7 | 15 | |
HIF-1α is positively correlated with
Snail protein positive expression, while it is negatively
correlated with Vimentin protein positive expression
The positive rates of Snail, Vimentin and E-cadherin
protein expression in HCC patients with HIF-1α positive expression
were 68% (17/25), 72% (18/25) and 16% (4/25), respectively. In
contrast, the positive rate of these 3 proteins was 30.8% (8/26),
34.6% (9/26) and 80.7% (21/26), respectively, in patients without
detectable HIF-1α expression. Spearman rank correlation analysis
showed that HIF-1α expression was positively correlated with Snail
and Vimentin protein expression (Table VIII, P=0.007), while it was
negatively correlated with E-cadherin expression (Table VIII, P=0.001).
 | Table VIIIHIF-1α protein expression was
positively correlated with Snail and Vimentin protein expression
(P=0.001 and 0.007, respectively), while it was negatively
correlated with E-cadherin protein expression (P=0.001). |
Table VIII
HIF-1α protein expression was
positively correlated with Snail and Vimentin protein expression
(P=0.001 and 0.007, respectively), while it was negatively
correlated with E-cadherin protein expression (P=0.001).
| HIF-1α | | |
|---|
|
| | |
|---|
| Snail | Positive | Negative | R value | P-value |
|---|
| Positive | 17 | 8 | 0.451 | 0.001 |
| Negative | 8 | 18 | | |
|
| HIF-1α | | |
|
| | |
| Vimentin | Positive | Negative | R value | P-value |
|
| Positive | 18 | 9 | 0.347 | 0.007 |
| Negative | 7 | 17 | | |
|
| HIF-1α | | |
|
| | |
| E-cadherin | Positive | Negative | R value | P-value |
|
| Positive | 4 | 21 | −0.491 | 0.001 |
| Negative | 21 | 5 | | |
CoCl2 decreases HepG2 cell
viability
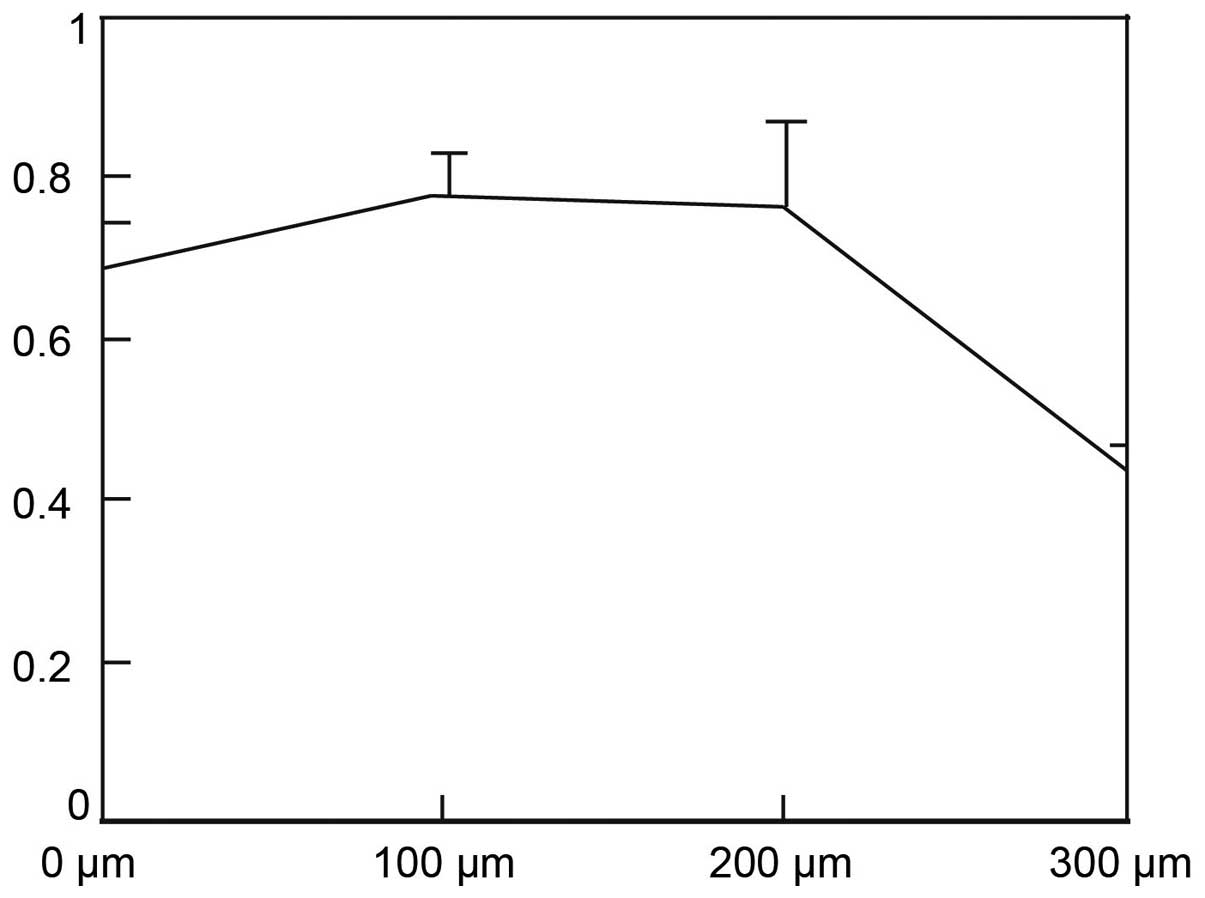
After treated with CoCl2 at concentration
of 100 and 200 μM for 24 h, HepG2 cell viability had no significant
difference compared with control group (treated with 0 μM
CoCl2, Fig. 5,
P>0.05). After treated with 300 μM CoCl2 for 24 h,
cell viability significantly decreased, compared with control group
(Fig. 5, P=0.001).
CoCl2 treatments promote HepG2
cell invasion and migration in vitro
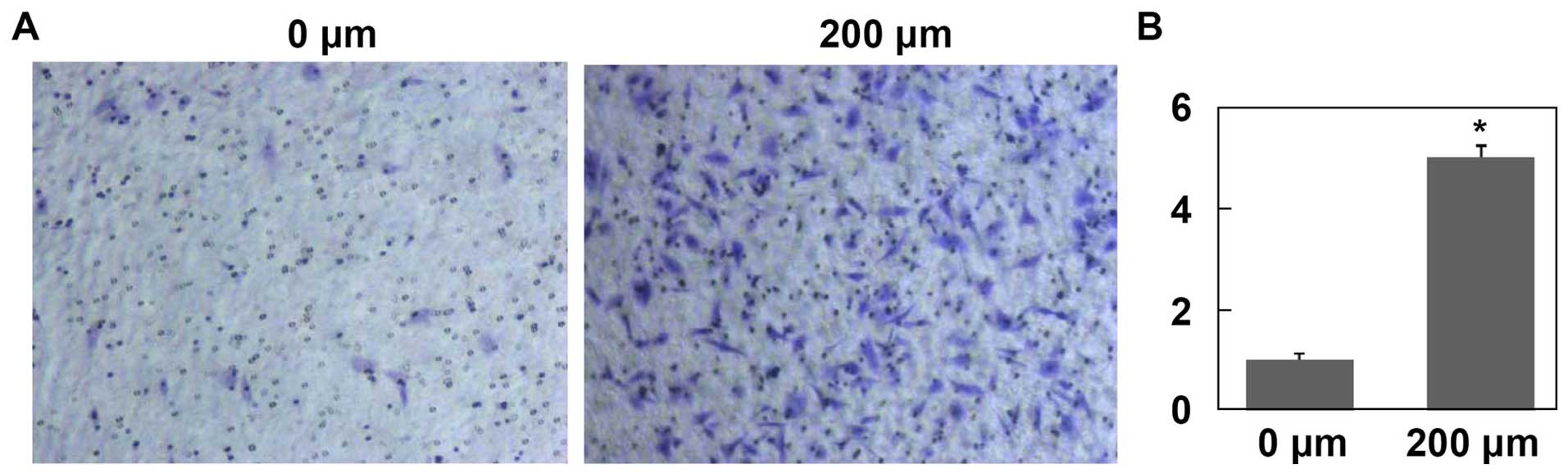
After treated with CoCl2 for 24 h, the
mean number of HepG2 cells migrated through Matrigel was
significantly higher than that of control group (Fig. 6, P<0.001). Moreover, after
treated with CoCl2 for 24 and 48 h, HepG2 cell migration
was significantly enhanced in a time-dependent manner (Fig. 7, P<0.001).
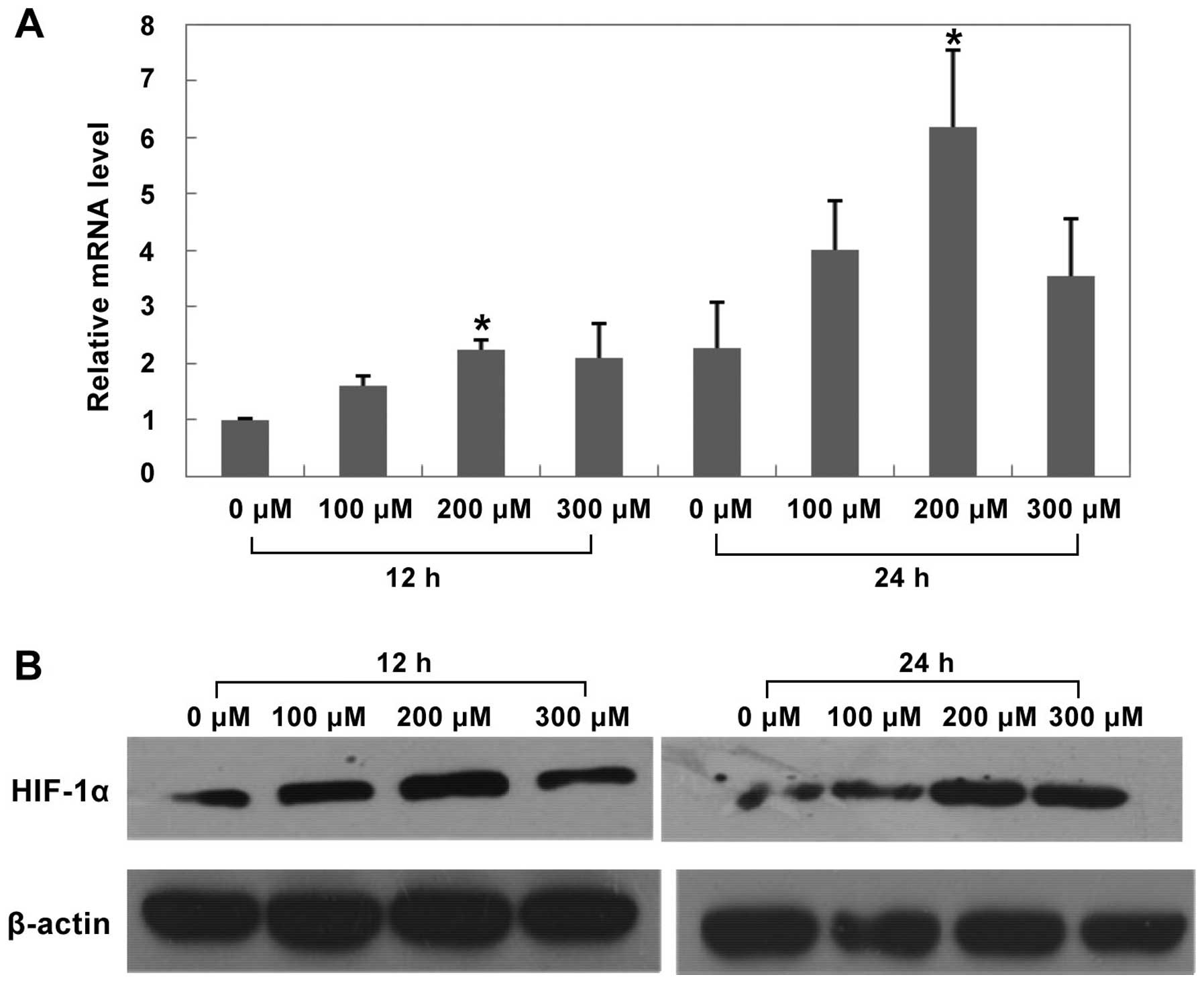
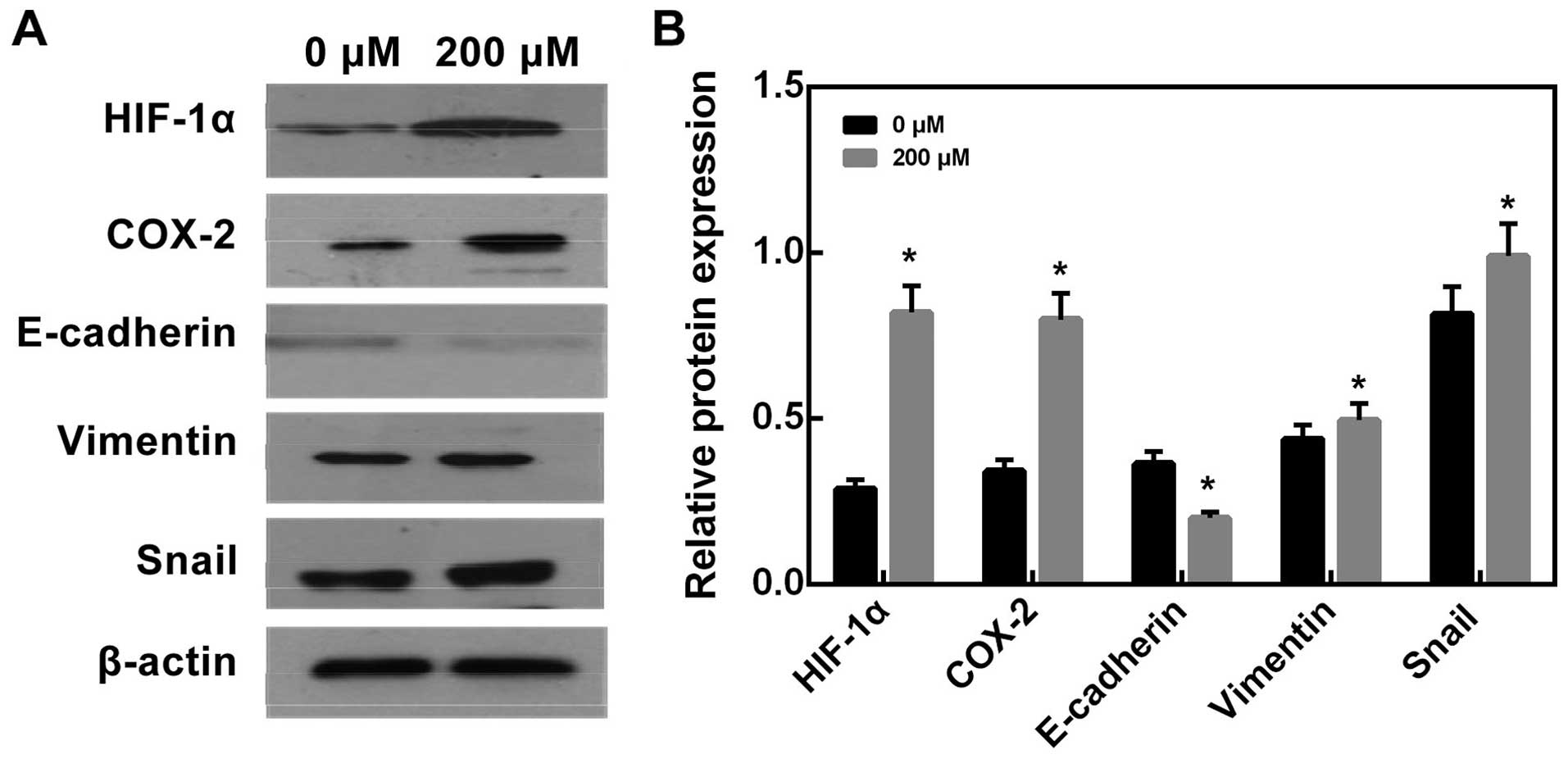
Effects of CoCl2 treatment on
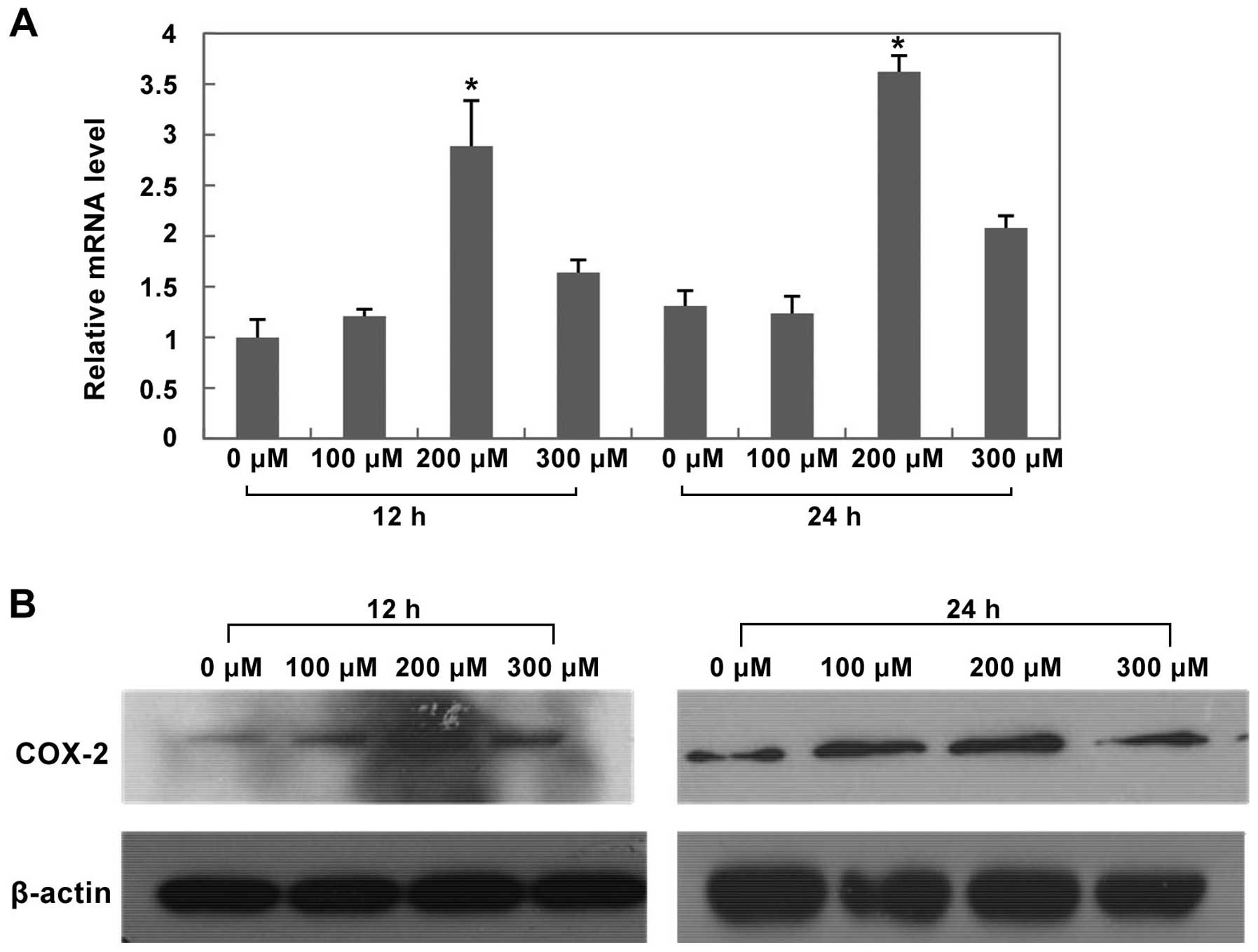
HIF-1α, COX-2 and EMT protein expression in HepG2 cells
After treated with CoCl2 at different
concentrations for 12 h and 24 h, both mRNA and protein expression
of HIF-1α and COX-2 significantly increased in the 200 μM
CoCl2 treatment group (Figs. 8 and 9, P<0.05 versus control group).
Furthermore, after treated with 200 μM CoCl2 for 24 h,
we found that E-cadherin protein expression significantly increased
compared with control group (Fig.
10, P<0.05), while Vimentin and Snail protein expression
decreased compared with control group (Fig. 10, P<0.05).
Discussion
The clinical significance of HIF-1α,
COX-2 and EMT in HCC patients after TACE surgery
TACE is considered as an important interventional
therapeutic approach to treat patients with hepatocellular
carcinoma (HCC). It has several advantages, e.g., it is a minimal
invasive procedure rendering short recovery time for patients, and
it is tumor-targeted causing fewer side effects. However, in many
cases tumor cells can adapt to the highly anaerobic
microenvironment resulted from TACE through the negative feed-back
response (32). HIF-1α, induced by
hypoxia and found to be expressed in many solid tumors, regulates
an array of genes to adapt to hypoxic environment, which promotes
tumor growth and metastasis. Increased HIF-1α protein expression
has been found in prostate cancer, head and neck cancer, ovarian
cancer, breast cancer and hepatocellular carcinoma (16,33,34).
COX-2 gene whose expression can be induced by HIF-1α has been shown
to be upregulated in a variety of malignant tumors and associated
with tumor growth and development (20). In this study, we found that the
protein levels of HIF-1α and COX-2 in HCC tissue from patients
after TACE surgery was significantly higher than that of adjacent
normal liver tissue and cancer tissue from HCC patients without
TACE surgery. Moreover, after TACE surgery HCC patients with HIF-1α
and COX-2 expression had shorter survival time than HCC patients
who were HIF-1α and COX-2 negative, indicating that both protein
detections might be helpful for the evaluation of prognosis of HCC
patients who had TACE. Further analysis also suggested that the
prognosis of HCC patients post TACE was related to tumor size,
intrahepatic metastasis and COX-2 expression, which were found to
be independent prognostic factors for HCC patients. We were unable
to conclude that HIF-1α was the most important prognostic factor
for HCC patients, which might be due to the small sample size of
this study. It is known that TACE triggers inflammatory reactions
that we speculated might be the most important prognostic factor,
which merits further studies.
In recent years, studies have found that COX-2 is
probably a downstream gene in HIF-1α signaling pathway, and its
expression is modulated by HIF-1α through the regulation of the
specific HRE sequence in the COX-2 promoter (35,36).
Both HIF-1α and COX-2 play a key role in the adaptation of hypoxic
environment for cancer cells. Our study also showed that HIF-1α
expression was positively correlated to COX-2 expression,
suggesting that HIF-1α might regulate COX-2 expression and
subsequently affect the prognosis of HCC patients after TACE. The
details of the regulatory mechanism remains to be explored.
EMT is considered as a key mechanism for tumor
recurrence and metastasis. Decreased expression of E-cadherin in
the EMT process leads to reduced cell adhesion and thus enhanced
tumor cell migration (37). Snail,
a key inducing factor of EMT, enhances tumor cell invasion through
downregulation of E-cadherin and upregulation of some other
related-proteins (38,39). Vimentin, a biomarker of EMT, also
is involved in tumor invasion and metastasis and its increased
expression is associated with poor prognosis of tumor patients
(40). In this study, we found
that HIF-1α was positively correlated to Snail and Vimentin
expression while negatively correlated to E-cadherin expression.
Our data suggested that HIF-1α expression might promote cancer cell
EMT, regulate cell motility and migration, and thus affect
prognosis of HCC patients after TACE surgery.
Effects of hypoxia on HIF-1α, COX-2
expression and EMT alteration in HepG2 cells
We found that HIF-1α and COX-2 expression increased
in tumor tissues from HCC patients after TACE surgery. We further
established a hypoxic cell model in vitro induced by
CoCl2 treatment. The results showed that both mRNA and
protein expression of HIF-1α and COX-2 in HepG2 cell increased
after CoCl2 treatment. EMT plays an important role in
tumor development and metastasis (27). Studies have shown that both cancer
invasion and migration were related to the EMT process (30,41).
Consistent with our clinical study results, in vitro study
showed that deceased E-cadherin expression and increased expression
of Vimentin and Snail were accompanied by enhanced HIF-1α and COX-2
expression induced by hypoxia. Moreover, hypoxia induced by
CoCl2 increased the ability of tumor invasion and
migration. Our data indicated that increased expression of HIF-1α
and COX-2 resulted from hypoxia promoted cancer cell EMT alteration
and thus its ability of invasion and migration, which probably is
an important factor contributing to the recurrence of HCC after
TACE surgery.
In conclusion, taken together, our clinical and
in vitro data suggest that TACE produced-hypoxic environment
induces HIF-1α expression upregulating COX-2 to promote cancer
angiogenesis, inhibiting cancer cell apoptosis and enhancing cancer
invasion and migration (20). In
addition, increased HIF-1α expression induces EMT alteration, which
enhances cancer cell migration and invasion. All these factors
might contribute to the poor prognosis of HCC patients post
TACE.
Acknowledgements
We thank Professor Zhao-Xing Pan for his kindly
statistical advice to this manuscript. This study has received
funding by the National Natural Science Foundation of China
(81172193 and 81430041).
References
|
1
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases. Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Curado M, Edwards B and Shin H: Cancer
Incidence in Five Continents. IARC Sci Publ; Lyon: 2007
|
|
3
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
4
|
Okuda K: Hepatocellular carcinoma. J
Hepatol. 32(Suppl): 225–237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cammà C, Schepis F, Orlando A, Albanese M,
Shahied L, Trevisani F, Andreone P, Craxì A and Cottone M:
Transarterial chemoembolization for unresectable hepatocellular
carcinoma: Meta-analysis of randomized controlled trials.
Radiology. 224:47–54. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Llovet JM, Real MI, Montaña X, Planas R,
Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al;
Barcelona Liver Cancer Group. Arterial embolisation or
chemoembolisation versus symptomatic treatment in patients with
unresectable hepatocellular carcinoma: A randomised controlled
trial. Lancet. 359:1734–1739. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maluccio MA, Covey AM, Porat LB, Schubert
J, Brody LA, Sofocleous CT, Getrajdman GI, Jarnagin W, Dematteo R,
Blumgart LH, et al: Transcatheter arterial embolization with only
particles for the treatment of unresectable hepatocellular
carcinoma. J Vasc Interv Radiol. 19:862–869. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saharinen P, Eklund L, Pulkki K, Bono P
and Alitalo K: VEGF and angiopoietin signaling in tumor
angiogenesis and metastasis. Trends Mol Med. 17:347–362. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu W, Kwon JH, Moon YH, Kim YB, Yu YS, Lee
N, Choi KY, Kim YS, Park YK, Kim BW, et al: Influence of
preoperative transcatheter arterial chemoembolization on gene
expression in the HIF-1α pathway in patients with hepatocellular
carcinoma. J Cancer Res Clin Oncol. 140:1507–1515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai CX, Gao Q, Qiu SJ, Ju MJ, Cai MY, Xu
YF, Zhou J, Zhang BH and Fan J: Hypoxia-inducible factor-1 alpha,
in association with inflammation, angiogenesis and MYC, is a
critical prognostic factor in patients with HCC after surgery. BMC
Cancer. 9:4182009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Semenza GL: Hypoxia-inducible factor 1:
Oxygen homeostasis and disease pathophysiology. Trends Mol Med.
7:345–350. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valencak J, Kittler H, Schmid K, Schreiber
M, Raderer M, Gonzalez-Inchaurraga M, Birner P and Pehamberger H:
Prognostic relevance of hypoxia inducible factor-1 alpha expression
in patients with melanoma. Clin Exp Dermatol. 34:e962–e964. 2009.
View Article : Google Scholar
|
|
13
|
Hirota K: Hypoxia-inducible factor 1, a
master transcription factor of cellular hypoxic gene expression. J
Anesth. 16:150–159. 2002. View Article : Google Scholar
|
|
14
|
Klatte T, Seligson DB, Riggs SB, Leppert
JT, Berkman MK, Kleid MD, Yu H, Kabbinavar FF, Pantuck AJ and
Belldegrun AS: Hypoxia-inducible factor 1 alpha in clear cell renal
cell carcinoma. Clin Cancer Res. 13:7388–7393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyoshi A, Kitajima Y, Ide T, Ohtaka K,
Nagasawa H, Uto Y, Hori H and Miyazaki K: Hypoxia accelerates
cancer invasion of hepatoma cells by upregulating MMP expression in
an HIF-1 alpha-independent manner. Int J Oncol. 29:1533–1539.
2006.PubMed/NCBI
|
|
16
|
Wu XZ, Xie GR and Chen D: Hypoxia and
hepatocellular carcinoma: The therapeutic target for hepatocellular
carcinoma. J Gastroenterol Hepatol. 22:1178–1182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marnett LJ: Cyclooxygenase mechanisms.
Curr Opin Chem Biol. 4:545–552. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith WL, DeWitt DL and Garavito RM:
Cyclooxygenases: Structural, cellular, and molecular biology. Annu
Rev Biochem. 69:145–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang D and Dubois RN: Prostaglandins and
cancer. Gut. 55:115–122. 2006. View Article : Google Scholar
|
|
20
|
Yang Y, Zhu J, Gou H, Cao D, Jiang M and
Hou M: Clinical significance of Cox-2, Survivin and Bcl-2
expression in hepatocellular carcinoma (HCC). Med Oncol.
28:796–803. 2011. View Article : Google Scholar
|
|
21
|
Erdem H, Gündogdu C and Sipal S:
Correlation of E-cadherin, VEGF, COX-2 expression to prognostic
parameters in papillary thyroid carcinoma. Exp Mol Pathol.
90:312–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Raspollini MR, Amunni G, Villanucci A,
Boddi V, Baroni G, Taddei A and Taddei GL: Expression of inducible
nitric oxide synthase and cyclooxygenase-2 in ovarian cancer:
Correlation with clinical outcome. Gynecol Oncol. 92:806–812. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi H, Xu JM, Hu NZ and Xie HJ: Prognostic
significance of expression of cyclooxygenase-2 and vascular
endothelial growth factor in human gastric carcinoma. World J
Gastroenterol. 9:1421–1426. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karray-Chouayekh S, Trifa F, Khabir A,
Boujelbene N, Sellami-Boudawara T, Daoud J, Frikha M, Gargouri A
and Mokdad-Gargouri R: Methylation status and overexpression of
COX-2 in Tunisian patients with ductal invasive breast carcinoma.
Tumour Biol. 32:461–468. 2011. View Article : Google Scholar
|
|
25
|
Lim SC, Lee TB, Choi CH, Ryu SY, Min YD
and Kim KJ: Prognostic significance of cyclooxygenase-2 expression
and nuclear p53 accumulation in patients with colorectal cancer. J
Surg Oncol. 97:51–56. 2008. View Article : Google Scholar
|
|
26
|
Shamma A, Yamamoto H, Doki Y, Okami J,
Kondo M, Fujiwara Y, Yano M, Inoue M, Matsuura N, Shiozaki H, et
al: Up-regulation of cyclooxygenase-2 in squamous carcinogenesis of
the esophagus. Clin Cancer Res. 6:1229–1238. 2000.PubMed/NCBI
|
|
27
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Copple BL: Hypoxia stimulates hepatocyte
epithelial to mesenchymal transition by hypoxia-inducible factor
and transforming growth factor-beta-dependent mechanisms. Liver
Int. 30:669–682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miladi-Abdennadher I, Abdelmaksoud-Dammak
R, Ayed-Guerfali DB, Ayadi L, Khabir A, Amouri A, Frikha F, Tahri
N, Ellouz S, Frikha M, et al: Expression of COX-2 and E-cadherin in
Tunisian patients with colorectal adenocarcinoma. Acta Histochem.
114:577–581. 2012. View Article : Google Scholar
|
|
31
|
Zhang YB, Wang X, Meister EA, Gong KR, Yan
SC, Lu GW, Ji XM and Shao G: The effects of CoCl2 on
HIF-1α protein under experimental conditions of autoprogressive
hypoxia using mouse models. Int J Mol Sci. 15:10999–11012. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ and
Cao GW: Increased expression of vascular endothelial growth factor
in hepatocellular carcinoma after transcatheter arterial
chemoembolization. Acta Radiol. 49:523–529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Semenza GL: Hypoxia-inducible factors:
Mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang
SY, Liu CJ, Teng SC and Wu KJ: Direct regulation of TWIST by HIF-1
alpha promotes metastasis. Nat Cell Biol. 10:295–305. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaidi A, Qualtrough D, Williams AC and
Paraskeva C: Direct transcriptional up-regulation of
cyclooxygenase-2 by hypoxiainducible factor (HIF)-1 promotes
colorectal tumor cell survival and enhances HIF-1 transcriptional
activity during hypoxia. Cancer Res. 66:6683–6691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schmedtje JF Jr, Ji YS, Liu WL, DuBois RN
and Runge MS: Hypoxia induces cyclooxygenase-2 via the NF-kappaB
p65 transcription factor in human vascular endothelial cells. J
Biol Chem. 272:601–608. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hashiguchi M, Ueno S, Sakoda M, Iino S,
Hiwatashi K, Minami K, Ando K, Mataki Y, Maemura K, Shinchi H, et
al: Clinical implication of ZEB-1 and E-cadherin expression in
hepatocellular carcinoma (HCC). BMC Cancer. 13:5722013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu S, Kumar SM, Martin JS, Yang R and Xu
X: Snail1 mediates hypoxia-induced melanoma progression. Am J
Pathol. 179:3020–3031. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Woo HY, Min AL, Choi JY, Bae SH, Yoon SK
and Jung CK: Clinicopathologic significance of the expression of
Snail in hepatocellular carcinoma. Korean J Hepatol. 17:12–18.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shirahata A, Sakata M, Sakuraba K, Goto T,
Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, Sanada Y, et
al: Vimentin methylation as a marker for advanced colorectal
carcinoma. Anticancer Res. 29:279–281. 2009.PubMed/NCBI
|
|
41
|
Shi Y, Wu H, Zhang M, Ding L, Meng F and
Fan X: Expression of the epithelial-mesenchymal transition-related
proteins and their clinical significance in lung adenocarcinoma.
Diagn Pathol. 8:892013. View Article : Google Scholar : PubMed/NCBI
|