Introduction
Indoleamine-2,3-dioxygenase (IDO) is known to be an
immunosuppressive enzyme. IDO was initially characterized in terms
of its catalysis of the first and rate-limiting step in the
kynurenine pathway of tryptophan catabolism (1–3). It
has since been reported that most tumors express IDO (4) and that IDO can contribute to
tumor-induced immunosuppression by starving natural killer (NK)/T
cells, which are sensitive to tryptophan deficiency (4–7). In
this situation, tumor cells can escape immune surveillance via the
action of IDO. Malignant tumors are also reportedly stimulated by
proinflammatory mediators, such as interferons and other cytokines,
to produce IDO (8,9). However, the regulatory mechanisms
related to IDO and malignancy remain largely uncharacterized.
Hepatocyte growth factor (HGF) is a heterodimeric
molecule that plays a key role in the regulation of migration,
invasion and angiogenesis in cancer (10–13).
It is composed of an α-chain containing the N-terminal hairpin
domain and 4 kringle domains, and a serine protease-like β-chain
(14). Therefore, NK4 is a variant
form of HGF, comprising the N-terminal and subsequent 4 kringle
domains of HGF (15). As NK4
retains binding capacity to the HGF receptor c-Met, NK4 competes
with HGF and inhibits the function of HGF (15,16).
Thus, NK4 functions as an antagonist of HGF. Numerous reports have
shown that NK4 inhibits growth, invasion, dissemination and
angiogenesis in malignant tumors (16–24).
Furthermore, NK4 reportedly inhibits the function of angiogenesis
factors such as VEGF and bFGF, regardless of HGF-c-Met signaling
(16,17).
Recently, it has been reported that NK4 expression
by gene transfer (at the tumor site) enhances tumor-specific
cytotoxic T lymphocyte (CTL) activation, resulting in complete
murine colon cancer cell line (CT26) tumor regression in
vivo (22). While IDO is not
specifically mentioned in the present study (22), it has been reported in other
studies that CT26 can produce IDO (25). These results suggest the
possibility that NK4 may exert potent antitumor activity, at least
partially, by enhancing the host's tumor immunity via the
regulation of IDO expression.
We conducted the experiments described below in an
effort to investigate the hypothesis that NK4 regulates IDO and to
characterize the signaling mechanism involved.
Materials and methods
Cell lines and culture
The human ovarian cancer cell line SKOV-3 (26) (American Type Culture Collection,
Manassas, VA, USA) was cultured in RPMI-1640 medium (Gibco, Grand
Island, NY, USA) containing 10% inactivated fetal calf serum
(Sigma, St. Louis, MO, USA), 100 U/ml penicillin, and 100 μg/ml
streptomycin (Gibco) at 37°C in a 5% CO2 atmosphere for
no longer than 8 weeks after recovery from frozen stocks.
The NK cell line KHYG-1 (27) was purchased from the Japanese
Collection of Research Bioresources (JCRB; Osaka, Japan). Cells
were cultured in RPMI1-640 medium supplemented with 100 nM of human
interleukin-2 (R&D Systems, Inc., Minneapolis, MN, USA) and 10%
inactivated fetal calf serum (Sigma) at 37°C in a 5% CO2
atmosphere for no longer than 8 weeks after recovery from frozen
stocks.
Antibodies and inhibitors
Anti-human-IDO monoclonal antibody was prepared and
utilized as previously reported (8). Anti-human-actin(Sigma),
anti-mouse-CD49b(R&DSystems), anti-HGF-α (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), anti-phospho-c-Met,
anti-c-Met, anti-phospho-AKT, anti-AKT, anti-phospho-ERK, anti-ERK,
anti-phospho-STAT3 and anti-STAT3 (Cell Signaling Technology, Inc.,
Danvers, MA, USA) antibodies were purchased and utilized according
to the manufacturer's instructions.
The c-Met tyrosine kinase inhibitor
PHA-665752((3Z)-5-[(2,6-dichlorobenzyl)sulfonyl]-3-[(3,5-dimethyl-4-{[(2R)-2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl]carbonyl}-1H-pyrrol-2-yl)methylene]-1,3-dihydro-2H-indol-2-one;
Merck KGaA, Darmstadt, Germany) (28), the phosphatidylinositol 3-kinase
(PI3K) inhibitor LY294002
(2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one; Cell Signaling
Technology) (29), the MEK1/2
inhibitor U0126 (1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]
butadiene; Cell Signaling Technology) (30), and the STAT3 inhibitor WP1066
((2E)-3-(6-Bromo-2-pyridinyl)-2-cyano-N-[(1S)-1-phenylethy]-2-propenamide
P; Santa Cruz Biotechnology) (31)
were purchased and were utilized according to the corresponding
manufacturer's instructions.
Experimental and control cell lines
The NK4, PTEN and luciferase (LUC) expression
plasmid vectors that were used in the present study have been
previously described (18,32–35).
These vectors were transfected into SKOV-3 using Lipofectamine-LTX
and Plus reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions. The cells were selected using 10 μg/ml
blasticidin S hydrochloride (Funakoshi Co., Ltd., Tokyo, Japan).
Resistant clones were obtained after 4 weeks as SKOV-3/NK4,
SKOV-3/PTEN and SKOV-3/LUC (control). The cells were subsequently
maintained in the presence of 10 μg/ml blasticidin S
hydrochloride.
Exposure to inhibitors
Before protein extraction for western blotting,
SKOV-3 cells (5×105/well) were seeded into 6-well plates
and cultured in RPMI-1640 medium containing 10% fetal calf serum
with varying concentrations of inhibitors (0, 1 or 10 μM)
overnight.
Western blotting
Ten micrograms of protein extracted from a
homogenate of cultured cells or 10 μl of culture supernatants were
mixed with 2X SDS-PAGE sample buffer [120 mM Tris-HCl (pH 6.8), 4%
SDS, 20% glycerol, 0.004% bromophenol blue and 10%
2-mercaptoethanol]. The resulting preparations were incubated at
95°C for 2 min and electrophoresed on a 0.1% SDS-5 or 10%
polyacrylamide gel, prior to blotting onto a polyfluorovinylidene
membrane. These membranes were then blocked with Non-Protein
Blocking Agent (ATTO Corp., Tokyo, Japan) at room temperature for 1
h and incubated with antibodies described above for 1 h at room
temperature. The membranes were washed with phosphate-buffered
saline (PBS)-Tween-20 three times, and incubated with several
horse-radish peroxidase-conjugated secondary antibodies. Signals
were detected by chemiluminescence (ECL Kit; Amersham Biosciences,
Piscataway, NJ, USA) via X-ray film.
In vitro cell growth kinetics
SKOV-3/NK4 and SKOV-3/LUC cells (500 of each line)
were seeded into the wells of 96-well plates and cultured in
RPMI-1640 medium containing 10% fetal calf serum. Every 24 h, cells
were counted using a colorimetric assay in conjunction with the
Cell Proliferation kit II (XTT) (Boehringer Mannheim GmbH
Biochemica, Mannheim, Germany) and a growth curve was derived from
these results.
Sensitivity of transfectants to NK cells
in vitro
The sensitivity of SKOV-3/NK4 and SKOV-3/LUC cells
to NK cells was investigated by colorimetric assay using XTT.
SKOV-3/NK4 and SKOV-3/LUC cells (500 of each line) were seeded into
a 96-well plate and co-cultured with KHYG-1 cells (0, 500, 1,000,
2,000 or 4,000 cells) in RPMI-1640 medium containing 10% fetal calf
serum for 72 h. After three washes with PBS to exclude KHYG-1 cells
completely, the viable cell count was determined by colorimetric
assay and calculated as the percent of control cells (cultured
without KHYG-1 cells).
Experimental animals
Four- to six-week-old female BALB/c nude mice (Japan
Clea Laboratories, Tokyo, Japan) were used. All animal experiments
were conducted according to the institutional and national
guidelines for animal experiments.
Subcutaneous tumor growth in vivo
SKOV-3/NK4 and SKOV-3/LUC cells (5×106
cells of each line) were inoculated subcutaneously into the backs
of mice to induce tumor growth. The tumor volume [(long diameter) ×
(short diameter)2 × 1/2] was measured twice a week and
used to obtain the tumor growth curves.
Immunohistochemical staining
At 1 week after subcutaneous tumor cell inoculation,
mice were sacrificed under isoflurane anesthesia and the tumor was
removed. After formalin fixation, paraffin sections were prepared,
deparaffinized, and treated with hydrogen peroxide for 30 min to
block endogenous peroxidase. The sections were then reacted with a
1:10 dilution (5 μg/ml) of anti-mouse CD49b primary antibody for 16
h at room temperature, washed three times with PBS, and then
incubated with enzyme-conjugated streptavidin for 30 min. The
sections were again washed with PBS 3 times, and color was
developed using the diaminobenzidine method. The number of stained
NK cells was counted under high-power magnification (x200).
Statistical analysis
Significance testing between the 2 groups was
performed using Student's t-test. A P-value of <0.05 was
considered significant.
Results
Establishing an NK4-expressing cell line
and determining its IDO expression
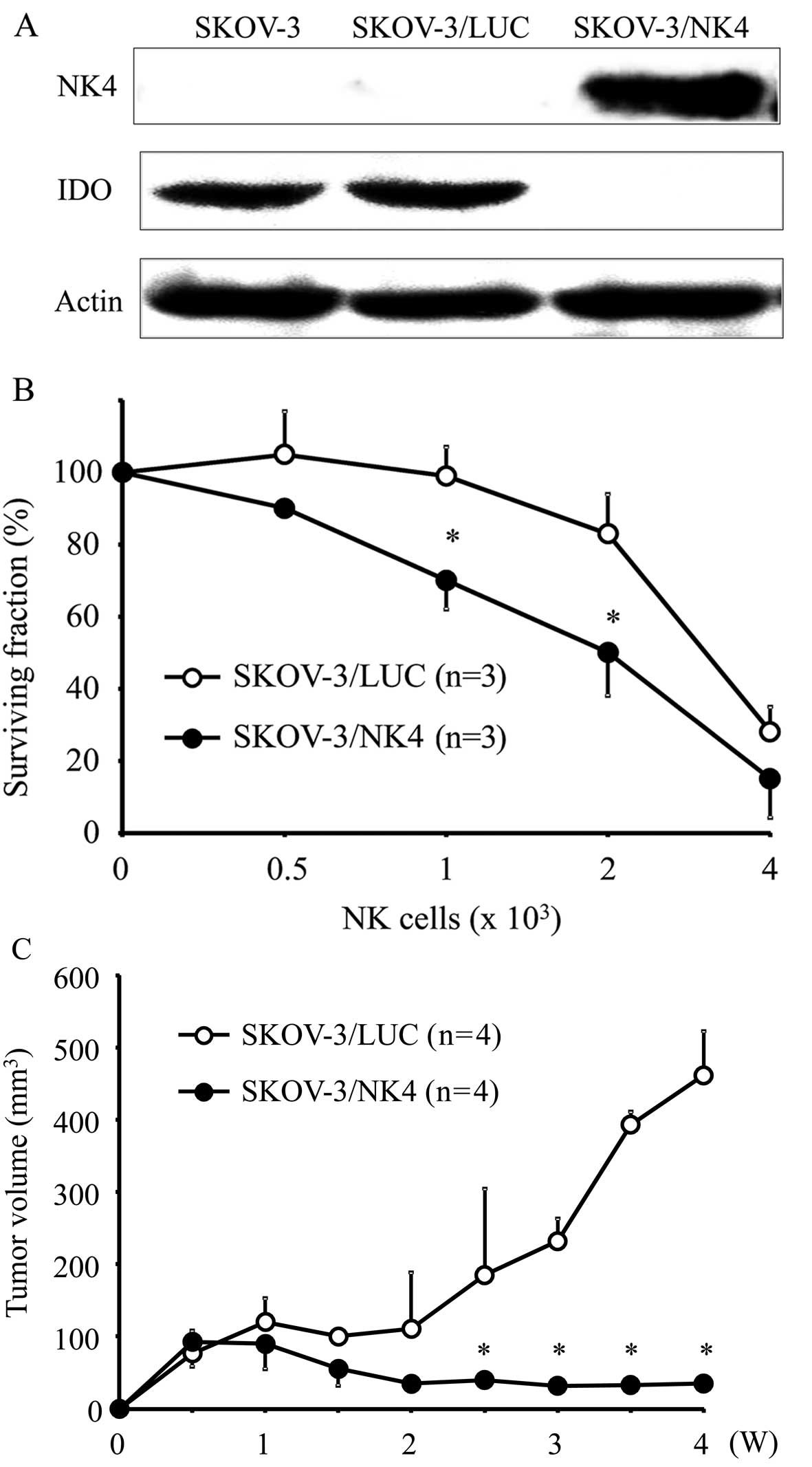
NK4 expression was detected by western blotting at
the position corresponding to a molecular weight of 67 kDa in
SKOV-3/NK4 culture supernatant samples. No NK4 expression was
detected in the culture supernatant of parent SKOV-3 or SKOV-3/LUC.
In contrast, parental SKOV-3 and SKOV-3/LUC showed evident IDO
expression, while SKOV-3/NK4 did not show IDO expression (Fig. 1A). These results suggest that the
expression of NK4 inhibits IDO expression.
In vitro cell growth kinetics
Growth curve analyses of SKOV-3/NK4 and SKOV-3/LUC
cells showed no significant differences between the two groups,
suggesting that the expression of NK4 did not affect cell growth
in vitro (data not shown).
Sensitivity of transfectants to NK cells
in vitro
The proportion of viable tumor cells co-cultured
with NK cells is shown in Fig. 1B.
The percent survival of SKOV-3/NK4 cells was significantly lower
than that of the control cells, indicating that the expression of
NK4 enhanced the sensitivity of tumor cells against NK cells.
Tumor growth in vivo
Both SKOV-3/NK4 and control cells formed small
nodules 1 week after inoculation (Fig.
1C). Subsequently, the tumors in the control group became
enlarged, whereas those in the SKOV-3/NK4 group were barely
increased in size, suggesting that the expression of NK4 inhibited
tumor growth in vivo.
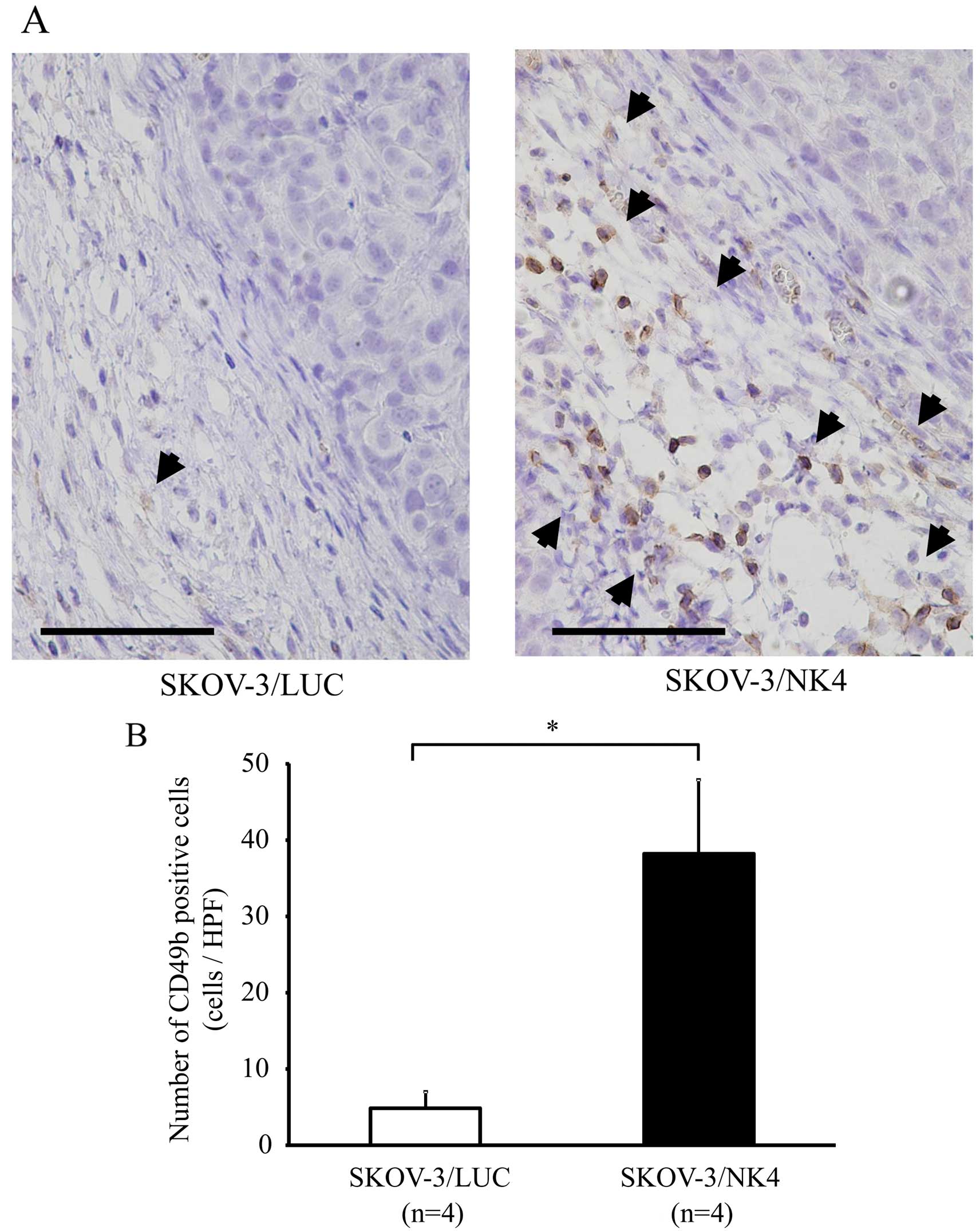
Number of NK cells in the tumor
stroma
Immunostaining of NK cells revealed accumulation of
NK cells in the stroma of SKOV-3/NK4 and control subcutaneous
tumors (Fig. 2A). The number of NK
cells (38±10) that accumulated in the SKOV-3/NK4 tumors was
significantly higher than that (5±2) in the control tumors
(P<0.01) (Fig. 2B). These
results suggest that the expression of NK4 promoted NK cell
accumulation around the tumor.
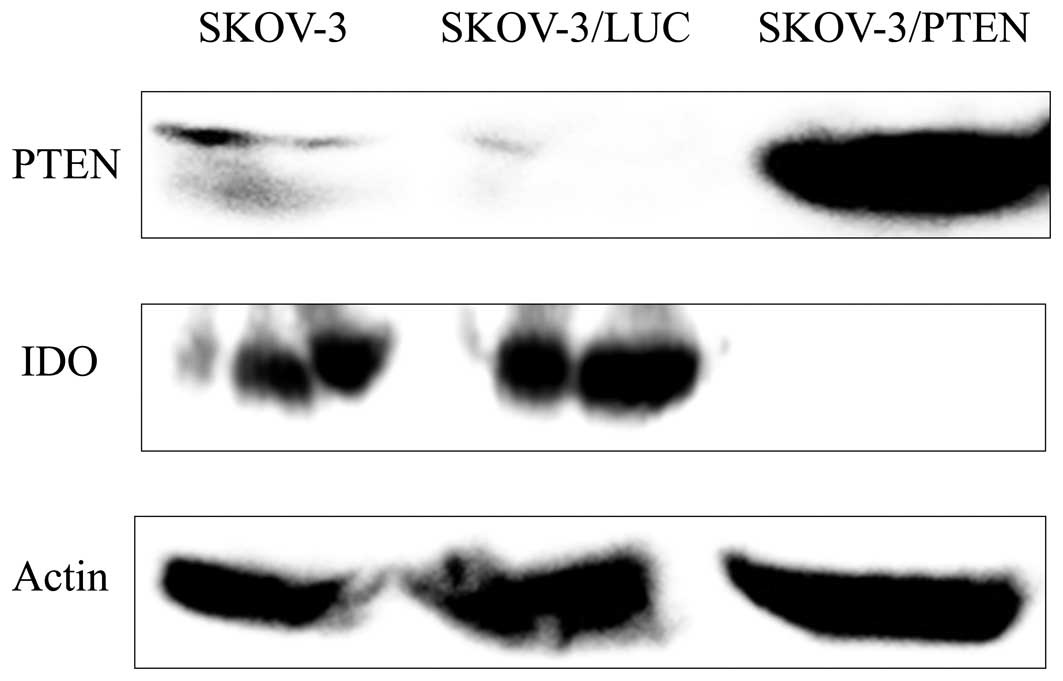
Establishing a PTEN-overexpressing cell
line and determining its IDO expression
Strong PTEN expression was detected by western
blotting at the position corresponding to a molecular weight of 55
kDa in SKOV-3/PTEN, while only weak PTEN expression was detected in
parent SKOV-3 and SKOV-3/LUC cells. In contrast, parental SKOV-3 or
SKOV-3/LUC showed evident IDO expression, while SKOV-3/PTEN did not
show IDO expression. These results suggest that overexpression of
PTEN inhibits IDO expression (Fig.
3).
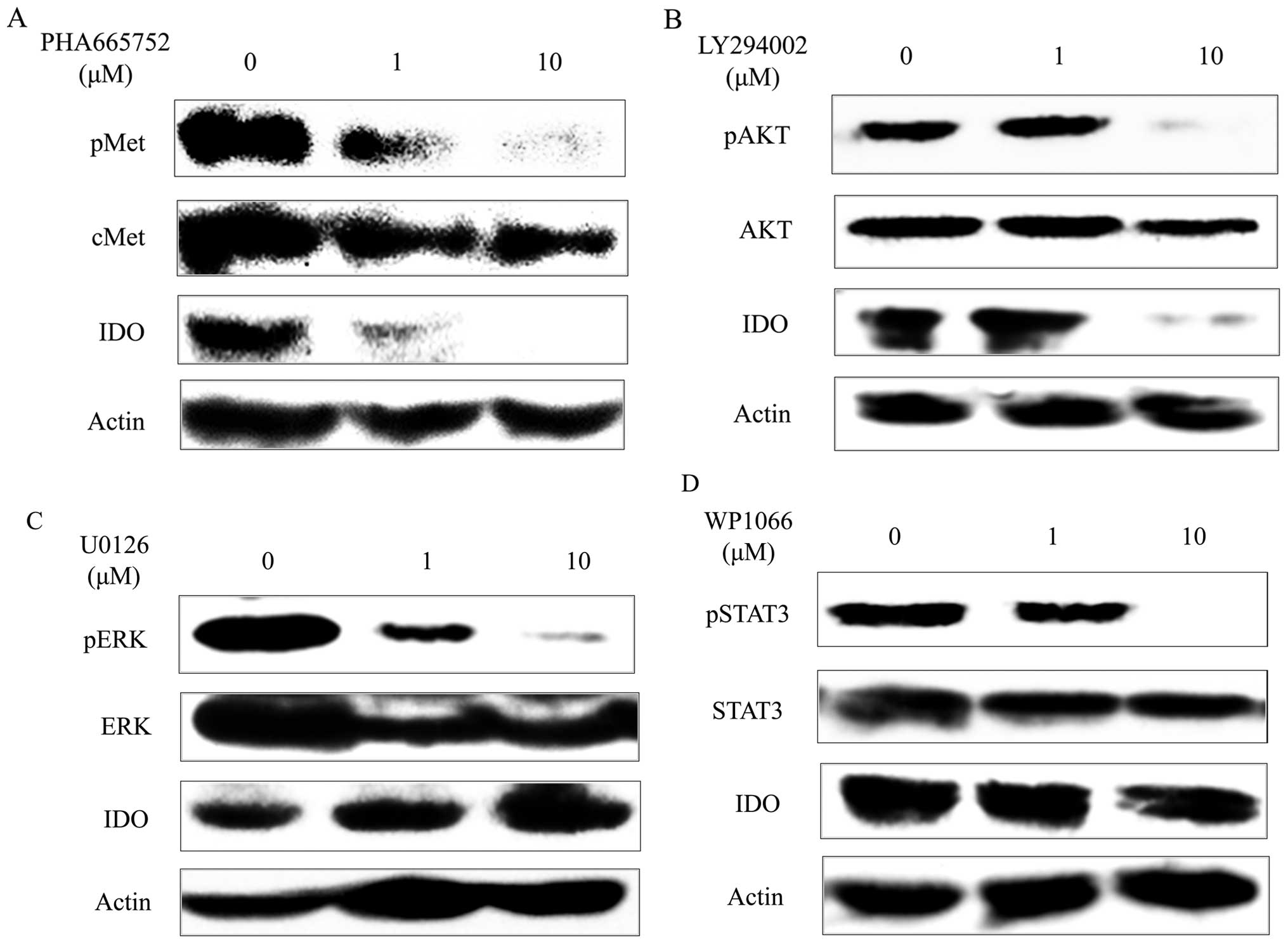
Inhibitors and IDO expression
As shown in Fig.
4A, the c-Met tyrosine kinase inhibitor PHA-665752 inhibited
c-Met phosphorylation of SKOV-3 in a concentration-dependent
manner. Similarly, it suppressed IDO expression of SKOV-3 in a
concentration-dependent manner. The PI3K inhibitor LY294002
inhibited AKT phosphorylation of SKOV-3 at the concentration of 10
μM. Similarly, it suppressed IDO expression of SKOV-3 (Fig. 4B) at the concentration of 10 μM. On
the other hand, although the MEK1/2 inhibitor U0126 inhibited ERK
(which is a downstream signaling factor of MEK) phosphorylation of
SKOV-3 in a concentration-dependent manner and the STAT3 inhibitor
WP1066 inhibited STAT3 phosphorylation of SKOV-3 at the
concentration of 10 μM, they did not affect IDO expression of
SKOV-3 (Fig. 4C and D).
Discussion
The experiments described above aimed to investigate
the hypothesis that NK4 influences IDO expression, and to clarify
the signaling pathway involved. First, we transfected an NK4
expression vector into a human ovarian cancer cell line
constitutively expressing IDO in order to examine the relationship
between NK4 expression and IDO expression. We found that the
NK4-expressing cell line did not express IDO. This indicates that
NK4 suppressed the expression of IDO by these cells. Furthermore,
experimentation using this NK4-expressing cell line yielded results
similar to those of a previous study, which used an
IDO-downregulated cell line transfected with a short hairpin RNA
vector targeting IDO (36).
Therefore, NK4 expression did not influence cancer cell growth
in vitro, but controlled tumor growth in vivo. In
addition, it enhanced the sensitivity of cancer cells to NK cells
in vitro and promoted NK cell accumulation in the tumor
stroma in vivo. These findings indicate that NK4 can inhibit
cancer growth in vivo by promoting NK cell accumulation via
the inhibition of IDO expression in tumors, suggesting that NK4
represents a potentially useful immunotherapeutic anticancer
agent.
In order to clarify the mechanism by which NK4
controls IDO, we performed an investigation utilizing various
biochemical inhibitors. It has been reported that while NK4 is
known to function in the c-Met signaling pathway as an antagonist
of HGF, it may also have other unknown functions unrelated to the
c-Met signaling pathway (16,17).
In order to investigate the known function, we utilized the c-Met
tyrosine kinase inhibitor PHA-665752. While PHA-665752 inhibited
c-Met phosphorylation, it also suppressed IDO expression. These
results suggest that NK4 suppresses IDO expression via the c-Met
signaling pathway. The PI3K-AKT, MAPK/ERK, and the JAK-STAT
pathways are known as signal pathways downstream of c-Met. In the
present study, while the PI3K inhibitor LY294002 inhibited AKT
phosphorylation of cancer cells, it also suppressed IDO expression.
In addition, enhanced expression of PTEN, which suppresses tumor by
negatively regulating the PI3K-AKT pathways (37,38),
inhibited IDO expression of cancer cells. Conversely, while the
MEK1/2 inhibitor U0126 and the STAT3 inhibitor WP1066 both
inhibited ERK and STAT3 phosphorylation, neither affected IDO
expression. These results suggest that NK4 inhibits IDO expression
via the c-Met-PI3K-AKT signaling pathway.
Recently, it has been reported that imatinib
mesylate, a small-molecule inhibitor of KIT and BCR-ABL tyrosine
kinase, inhibits IDO expression of gastrointestinal stromal tumors
via the PI3K-AKT pathway (39).
Results from both that study and the present suggest the
possibility that various tyrosine kinase receptors control IDO
expression in malignancies through the PI3K-AKT signaling pathway.
Furthermore, the collective results suggest that various
molecularly-targeted therapeutic agents that suppress tyrosine
kinase may function to enhance the cancer immunity of the host via
the inhibition of IDO.
Acknowledgements
The present study was supported by a JSPC KAKENHI
(grant no. 25462606) from Grant-in-Aid for Scientific Research from
the Ministry of Education, Culture, Sports, Science and Technology
of Japan (Y.S.).
References
|
1
|
Higuchi K and Hayaishi O: Enzymic
formation of D-kynurenine from D-tryptophan. Arch Biochem Biophys.
120:397–403. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamamoto S and Hayaishi O: Tryptophan
pyrrolase of rabbit intestine. D- and L-tryptophan-cleaving enzyme
or enzymes. J Biol Chem. 242:5260–5266. 1967.PubMed/NCBI
|
|
3
|
Shimizu T, Nomiyama S, Hirata F and
Hayaishi O: Indoleamine 2,3-dioxygenase. Purification and some
properties. J Biol Chem. 253:4700–4706. 1978.PubMed/NCBI
|
|
4
|
Uyttenhove C, Pilotte L, Théate I,
Stroobant V, Colau D, Parmentier N, Boon T and Van den Eynde BJ:
Evidence for a tumoral immune resistance mechanism based on
tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med.
9:1269–1274. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Munn DH, Zhou M, Attwood JT, Bondarev I,
Conway SJ, Marshall B, Brown C and Mellor AL: Prevention of
allogeneic fetal rejection by tryptophan catabolism. Science.
281:1191–1193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Della Chiesa M, Carlomagno S, Frumento G,
Balsamo M, Cantoni C, Conte R, Moretta L, Moretta A and Vitale M:
The tryptophan catabolite L-kynurenine inhibits the surface
expression of NKp46- and NKG2D-activating receptors and regulates
NK-cell function. Blood. 108:4118–4125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nonaka H, Saga Y, Fujiwara H, Akimoto H,
Yamada A, Kagawa S, Takei Y, Machida S, Takikawa O and Suzuki M:
Indoleamine 2,3-dioxygenase promotes peritoneal dissemination of
ovarian cancer through inhibition of natural killer cell function
and angiogenesis promotion. Int J Oncol. 38:113–120. 2011.
|
|
8
|
Takikawa O, Kuroiwa T, Yamazaki F and Kido
R: Mechanism of interferon-gamma action. Characterization of
indoleamine 2,3-dioxygenase in cultured human cells induced by
interferon-gamma and evaluation of the enzyme-mediated tryptophan
degradation in its anticellular activity. J Biol Chem.
263:2041–2048. 1988.PubMed/NCBI
|
|
9
|
Fujigaki S, Saito K, Sekikawa K, Tone S,
Takikawa O, Fujii H, Wada H, Noma A and Seishima M:
Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is
mediated dominantly by an IFN-gamma-independent mechanism. Eur J
Immunol. 31:2313–2318. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsumoto K, Date K, Shimura H and
Nakamura T: Acquisition of invasive phenotype in gallbladder cancer
cells via mutual interaction of stromal fibroblasts and cancer
cells as mediated by hepatocyte growth factor. Jpn J Cancer Res.
87:702–710. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura T, Matsumoto K, Kiritoshi A, Tano
Y and Nakamura T: Induction of hepatocyte growth factor in
fibroblasts by tumor-derived factors affects invasive growth of
tumor cells: In vitro analysis of tumor-stromal interactions.
Cancer Res. 57:3305–3313. 1997.PubMed/NCBI
|
|
12
|
Bussolino F, Di Renzo MF, Ziche M,
Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer
A and Comoglio PM: Hepatocyte growth factor is a potent angiogenic
factor which stimulates endothelial cell motility and growth. J
Cell Biol. 119:629–641. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grant DS, Kleinman HK, Goldberg ID,
Bhargava MM, Nickoloff BJ, Kinsella JL, Polverini P and Rosen EM:
Scatter factor induces blood vessel formation in vivo. Proc Natl
Acad Sci USA. 90:1937–1941. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakamura T, Nishizawa T, Hagiya M, Seki T,
Shimonishi M, Sugimura A, Tashiro K and Shimizu S: Molecular
cloning and expression of human hepatocyte growth factor. Nature.
342:440–443. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Date K, Matsumoto K, Shimura H, Tanaka M
and Nakamura T: HGF/NK4 is a specific antagonist for pleiotrophic
actions of hepatocyte growth factor. FEBS Lett. 420:1–6. 1997.
View Article : Google Scholar
|
|
16
|
Matsumoto K and Nakamura T: Mechanisms and
significance of bifunctional NK4 in cancer treatment. Biochem
Biophys Res Commun. 333:316–327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuba K, Matsumoto K, Date K, Shimura H,
Tanaka M and Nakamura T: HGF/NK4, a four-kringle antagonist of
hepatocyte growth factor, is an angiogenesis inhibitor that
suppresses tumor growth and metastasis in mice. Cancer Res.
60:6737–6743. 2000.PubMed/NCBI
|
|
18
|
Saga Y, Mizukami H, Suzuki M, Urabe M,
Kume A, Nakamura T, Sato I and Ozawa K: Expression of HGF/NK4 in
ovarian cancer cells suppresses intraperitoneal dissemination and
extends host survival. Gene Ther. 8:1450–1455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomioka D, Maehara N, Kuba K, Mizumoto K,
Tanaka M, Matsumoto K and Nakamura T: Inhibition of growth,
invasion, and metastasis of human pancreatic carcinoma cells by NK4
in an orthotopic mouse model. Cancer Res. 61:7518–7524.
2001.PubMed/NCBI
|
|
20
|
Son G, Hirano T, Seki E, Iimuro Y, Nukiwa
T, Matsumoto K, Nakamura T and Fujimoto J: Blockage of HGF/c-Met
system by gene therapy (adenovirus-mediated NK4 gene) suppresses
hepatocellular carcinoma in mice. J Hepatol. 45:688–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du W, Hattori Y, Yamada T, Matsumoto K,
Nakamura T, Sagawa M, Otsuki T, Niikura T, Nukiwa T and Ikeda Y:
NK4, an antagonist of hepatocyte growth factor (HGF), inhibits
growth of multiple myeloma cells: Molecular targeting of angiogenic
growth factor. Blood. 109:3042–3049. 2007.
|
|
22
|
Kubota T, Taiyoh H, Matsumura A, Murayama
Y, Ichikawa D, Okamoto K, Fujiwara H, Ikoma H, Nakanishi M, Kikuchi
S, et al: Gene transfer of NK4, an angiogenesis inhibitor, induces
CT26 tumor regression via tumor-specific T lymphocyte activation.
Int J Cancer. 125:2879–2886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki Y, Sakai K, Ueki J, Xu Q, Nakamura
T, Shimada H, Nakamura T and Matsumoto K: Inhibition of Met/HGF
receptor and angiogenesis by NK4 leads to suppression of tumor
growth and migration in malignant pleural mesothelioma. Int J
Cancer. 127:1948–1957. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsumoto G, Omi Y, Lee U, Kubota E and
Tabata Y: NK4 gene therapy combined with cisplatin inhibits tumour
growth and metastasis of squamous cell carcinoma. Anticancer Res.
31:105–111. 2011.PubMed/NCBI
|
|
25
|
Koblish HK, Hansbury MJ, Bowman KJ, Yang
G, Neilan CL, Haley PJ, Burn TC, Waeltz P, Sparks RB, Yue EW, et
al: Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase
potently suppress systemic tryptophan catabolism and the growth of
IDO-expressing tumors. Mol Cancer Ther. 9:489–498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fogh J, Wright WC and Loveless JD: Absence
of HeLa cell contamination in 169 cell lines derived from human
tumors. J Natl Cancer Inst. 58:209–214. 1977.PubMed/NCBI
|
|
27
|
Yagita M, Huang CL, Umehara H, Matsuo Y,
Tabata R, Miyake M, Konaka Y and Takatsuki K: A novel natural
killer cell line (KHYG-1) from a patient with aggressive natural
killer cell leukemia carrying a p53 point mutation. Leukemia.
14:922–930. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Christensen JG, Schreck R, Burrows J,
Kuruganti P, Chan E, Le P, Chen J, Wang X, Ruslim L, Blake R, et
al: A selective small molecule inhibitor of c-Met kinase inhibits
c-Met-dependent phenotypes in vitro and exhibits cytoreductive
antitumor activity in vivo. Cancer Res. 63:7345–7355.
2003.PubMed/NCBI
|
|
29
|
Guo M, Joiakim A and Reiners JJ Jr:
Suppression of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-mediated
aryl hydrocarbon receptor transformation and CYP1A1 induction by
the phosphatidylinositol 3-kinase inhibitor
2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002).
Biochem Pharmacol. 60:635–642. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Favata MF, Horiuchi KY, Manos EJ, Daulerio
AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F,
et al: Identification of a novel inhibitor of mitogen-activated
protein kinase kinase. J Biol Chem. 273:18623–18632. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iwamaru A, Szymanski S, Iwado E, Aoki H,
Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, et al: A
novel inhibitor of the STAT3 pathway induces apoptosis in malignant
glioma cells both in vitro and in vivo. Oncogene. 26:2435–2444.
2007. View Article : Google Scholar
|
|
32
|
Saga Y, Mizukami H, Suzuki M, Kohno T,
Urabe M, Ozawa K and Sato I: Overexpression of PTEN increases
sensitivity to SN-38, an active metabolite of the topoisomerase I
inhibitor irinotecan, in ovarian cancer cells. Clin Cancer Res.
8:1248–1252. 2002.PubMed/NCBI
|
|
33
|
Saga Y, Mizukami H, Takei Y, Ozawa K and
Suzuki M: Suppression of cell migration in ovarian cancer cells
mediated by PTEN overexpression. Int J Oncol. 23:1109–1113.
2003.PubMed/NCBI
|
|
34
|
Takei Y, Saga Y, Mizukami H, Takayama T,
Ohwada M, Ozawa K and Suzuki M: Overexpression of PTEN in ovarian
cancer cells suppresses i.p. dissemination and extends survival in
mice. Mol Cancer Ther. 7:704–711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Urabe M, Hasumi Y, Ogasawara Y, Matsushita
T, Kamoshita N, Nomoto A, Colosi P, Kurtzman GJ, Tobita K and Ozawa
K: A novel dicistronic AAV vector using a short IRES segment
derived from hepatitis C virus genome. Gene. 200:157–162. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang D, Saga Y, Mizukami H, Sato N, Nonaka
H, Fujiwara H, Takei Y, Machida S, Takikawa O, Ozawa K, et al:
Indoleamine-2,3-dioxygenase, an immunosuppressive enzyme that
inhibits natural killer cell function, as a useful target for
ovarian cancer therapy. Int J Oncol. 40:929–934. 2012.
|
|
37
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cantley LC and Neel BG: New insights into
tumor suppression: PTEN suppresses tumor formation by restraining
the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA.
96:4240–4245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Balachandran VP, Cavnar MJ, Zeng S,
Bamboat ZM, Ocuin LM, Obaid H, Sorenson EC, Popow R, Ariyan C,
Rossi F, et al: Imatinib potentiates antitumor T cell responses in
gastrointestinal stromal tu mor through the inhibition of Ido. Nat
Med. 17:1094–1100. 2011. View
Article : Google Scholar : PubMed/NCBI
|